1. Introduction
Art conservation is essential for preserving cultural heritage by ensuring that artworks maintain their original physical and aesthetic condition as much as possible. One fundamental process in paintings conservation is “cleaning”, which encompasses a number of activities including removing embedded grime, eliminating or thinning aged varnishes, and cleaning away discolored overpaint from artwork surfaces [
1,
2,
3,
4,
5,
6]. To accomplish this delicate task, art conservators often use carefully tailored solvent mixtures delivered on cotton swabs to manipulate solubility parameters and solvent contact time so as to solubilize or swell only the unwanted components of dirt, varnish, and overpaint [
1,
2,
3,
4]. In the process, these obfuscating coatings can be removed by the gentle mechanical action of the swab, while minimizing the impact on the original painted surface1 [
1,
2,
3,
4,
7,
8]. Cleaning of artwork surfaces is incredibly complicated and highly dependent on oftentimes not fully known quantities, like the exact composition of the material to be removed, as well as the material to be retained. In simple terms, however, cleaning mixtures usually include a solvent known to have weaker solubilizing power for the coatings, sometimes called a “restrainer” in the older conservation literature, which acts to modify the stronger solubility characteristics of a more potent solvent for the material being removed. The weaker solvent is usually unable to successfully clean the dirt, varnish, or overpaint on its own, while the strong solvent might be too aggressive to be used directly on the artwork for fear that it will remove delicate glazes or swell the underlying paint to the point of deformation or pigment loss [
1,
2,
3,
4,
7,
8]. Choosing the proper balance of the two solvents allows careful tuning of the overall solubility parameters of the mixture to stay within the safe zone outside of the solubility window of the underlying paint. A long-held concern, however, is that differential evaporation rates could lead to changes in the cleaning solution’s composition, possibly reducing the weak solvent and leaving an overly aggressive residual liquid on the painted surface, or conversely, preferentially depleting the strong solvent, thereby leaving the solution less effective in cleaning [
4,
8,
9,
10].
Alternative cleaning solutions have been proposed to address these issues by utilizing azeotropic solvent blends whose specific compositions seem appropriate for conservation cleaning [
8,
9,
10,
11,
12,
13]. Azeotropic mixtures incorporating two or more solvents at very precise concentrations act as a single solvent, evaporating at a constant composition in the liquid and the gas phase [
14,
15]. Azeotropes can confound industrial solvent purification by distillation, and so hundreds of tabulated azeotropic mixtures are listed at their boiling point compositions in the CRC Handbook as well as the literature [
16,
17,
18,
19,
20,
21].
Augerson significantly developed the use of azeotropes in conservation by reviewing binary solvent pairs from the CRC Handbook that gave calculated solubility parameters that should be effective replacements for aromatics in the removal of varnish from oil paint [
8]. After testing several potential candidates, the azeotrope chosen was 19% v/v isopropanol (strong solvent) in n-hexane (weak solvent) with a boiling point of 62.7 °C. In his application of cleaning shellac varnish from a painted 16th century sleigh in the collection of the Coach Museum at Versailles, Augerson noted that in addition to offering evaporation at constant composition, the azeotrope has a higher vapor pressure than either pure solvent (a ‘positive azeotrope’), providing yet another tool for safeguarding the artifact by limiting contact time with the oil paint surface or by reducing absorption into cracks and fissures in the paint [
8]. The same azeotrope proved useful in other treatments of sleighs from the Coach Museum [
11,
12]. Stavroudis [
9] and Saunders [
10] have further described the use of azeotropes in paintings conservation. Saunders utilized the azeotrope of 38% v/v 2-butanone in cyclohexane, which boils at 71.8 °C, to reform and thin a synthetic resin varnish on an oil painting and to remove rubber cement from a 17th C Peruvian devotional painting [
12].
While these conservation treatments reported in the literature were undoubtedly highly successful, doubts arise over the actual evaporation behavior of these boiling point azeotrope compositions when utilized at the room temperature conditions typical of conservation treatments. Azeotropes are both temperature and pressure sensitive [
14,
15], with the azeotropic composition changing across wide ranges in either parameter. The present study employs various instrumental methodologies to experimentally determine the room temperature azeotropic composition of the isopropanol:n-hexane and 2-butanone:cyclohexane systems. The techniques used encompass vapor pressure determinations, gravimetric analyses, refractive index measurements, and directly evaluating the composition of the liquid phase in evaporating mixtures through gas chromatography. The investigation hopes to provide more clarity to the conservation community on the possible role of azeotropes in conservation cleaning while highlighting the potential for false expectations of solvent mixtures that are not azeotropes at room temperature conditions.
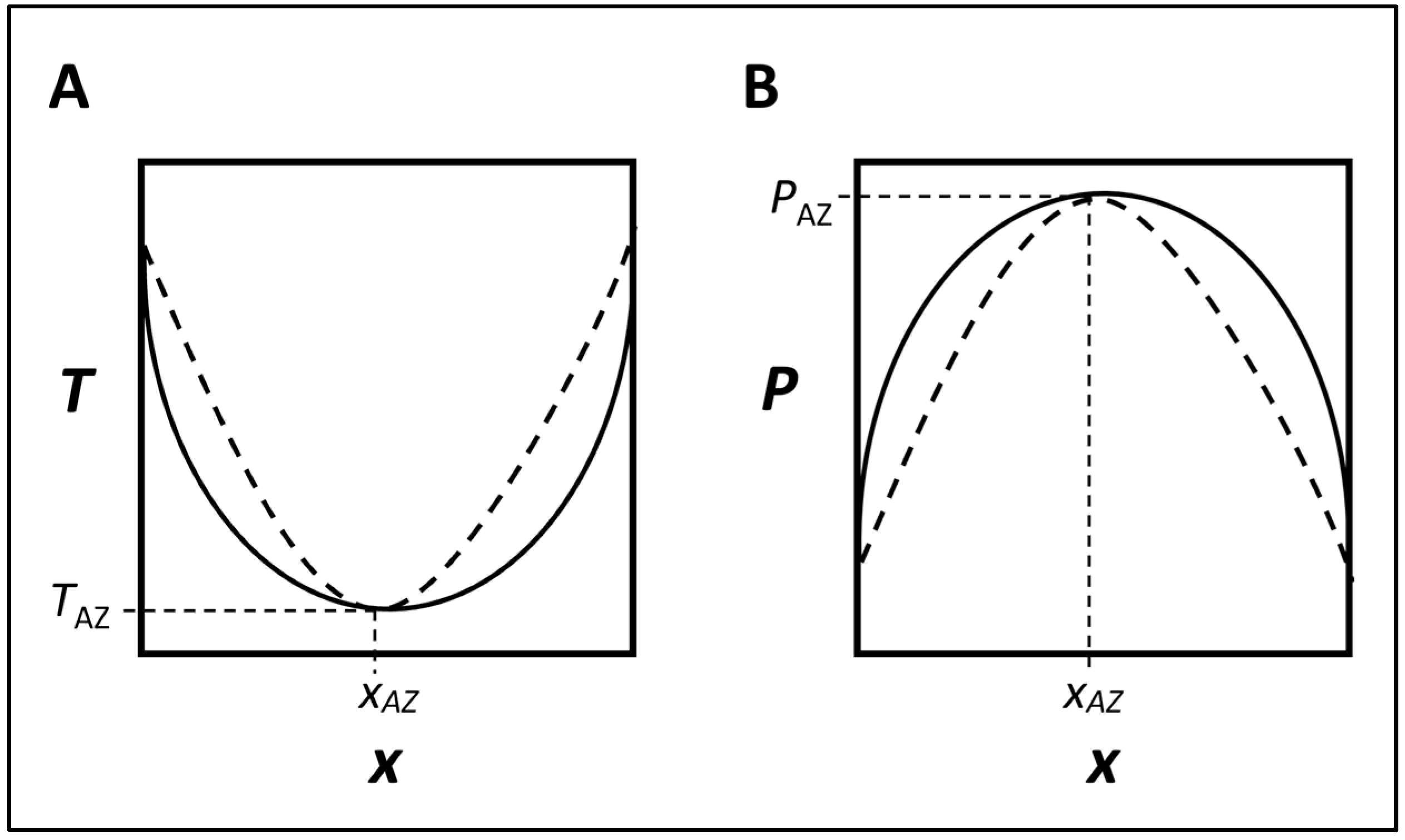
1.1. Characteristics of Homogeneous Pressure-Maximum Azeotrope in a Completely Miscible System
There are several types of azeotropes, each with specific characteristics. For art conservation, the common type has been pressure-maximum azeotropes that form homogeneous solutions with completely miscible components. This refers to a particular type of azeotrope where the mixture of two or more miscible liquids exhibits a single-phase behavior and has a maximum vapor pressure compared to other compositions at a given temperature. In such a system, the mixture’s components have uniform distribution throughout the solution, and their boiling points coincide to form a unique composition that evaporates as a single substance without altering the composition of the liquid or vapor phases [
14]. This type of azeotrope is also known as a ‘positive’ azeotrope, and it often occurs when the components of the mixture have similar molecular sizes and polarities, leading to stronger intermolecular interactions [
15].
Azeotropes are both temperature and pressure sensitive [
14,
15]. Typical equilibrium data plots for a homogeneous pressure-maximum azeotrope involve two primary graphs: a temperature–composition (T-x-y) plot and a pressure–composition (P-x-y) plot (
Figure 1). These plots illustrate the behavior of the azeotropic mixture in both the liquid and vapor phases under varying compositions and conditions [
14]. In these types of plots, x and y refer to the fraction of one of the mixture components in the liquid and gas phase, respectively.
In the temperature–composition (T-x-y) plot (
Figure 1A), the
x-axis represents the mole fraction of one component (usually designated as Component A) in the mixture, while the
y-axis represents the temperature. Two curves are plotted in this graph: a solid one for the liquid phase composition and a dashed one for the vapor phase composition. The point where both curves intersect, i.e., have the same compositions, represents the azeotropic point (x
AZ, T
AZ). At this point, the composition of the liquid and vapor phases is identical, and the azeotropic temperature is observed [
15].
In the pressure–composition (P-x-y) plot (
Figure 1B), the
x-axis again represents the mole fraction of one component (usually designated as Component A) in the mixture, and the
y-axis represents the pressure. The same two curves are plotted in this graph too. For a homogeneous pressure-maximum azeotrope, the vapor pressure of the azeotrope is higher than that of either of the pure components or any other compositions of the mixture at the given temperature. As before, the point where both curves intersect represents the azeotropic point (x
AZ, P
AZ), and the azeotropic pressure is observed at this intersection [
14].
These equilibrium data plots provide valuable information about the behavior of azeotropic mixtures and show that they are both temperature and pressure dependent. These graphs are crucial for understanding the properties and applications of such mixtures in various fields, including art conservation.
1.2. Binary System Azeotrope
Figure 2 presents a plot of equilibrium data for a ’positive’ azeotrope of the type used in the cleaning of paintings. In the plot, the horizontal axis represents the composition of the mixture. While commonly represented as mole fraction or % m/m, for consistency in discussing the azeotrope compositions as given in the conservation literature, the horizontal axis in this graph shows the concentration of Component A in the mixture as a volume percentage (% v/v). The vertical axis shows the temperature of the mixture. In
Figure 2, the lower point
X of the equilibrium data represents the composition and temperature at which the azeotrope exists. The value of % v/v
AZ is the volume percentage of Component A in the azeotrope. The value T
AZ represents the temperature at which the azeotrope boils at a given experimental pressure, which for conservation applications would be ambient pressure of 1 atm or 760 mmHg.
The equilibrium data plot delineates how different mixtures’ boiling or evaporation processes occur as shown in
Figure 2. For example, if a solvent mixture is prepared at a % v/v lower than the % v/v
AZ (shown in point a), it reaches its liquid/gas equilibrium state at point 1 when this solution heats up. As the mixture boils (or evaporates), the gas phase composition will approximate the azeotrope at point X. In contrast, the liquid phase moves along the lower liquid/gas curve towards point 2. If no more boiling (or evaporation) is allowed, the mixture that remained in the liquid phase will now have a % v/v equal to that at point c, meaning that the liquid is now less rich in Component A. Thus, as the original mix had a composition a, the remaining liquid will have a lower composition c after evaporation. At the same time, the gas phase will have a composition equal to the azeotrope.
On the other hand, if a mixture is prepared with a % v/v higher than the % v/v
AZ (shown as point b in
Figure 2), then as the solution heats up and boils (or evaporates) at point 3, the gas phase will move toward the azeotrope point X while the liquid phase composition will move towards point 4. If the boiling is stopped, the new liquid phase composition will have a higher % v/v in Component A at point d. Thus, the liquid phase will increase in concentration of Component A as the mixture is boiled. As a useful generalization, a zeotropic composition (i.e., non-azeotrope) will shift its liquid composition in the direction opposite to the azeotropic point (x
AZ) during evaporation.
1.3. Aims of the Study
Drawing upon the comprehension of equilibrium data diagrams, a series of experiments were designed to evaluate two azeotropic mixtures, namely isopropanol with n-hexane and 2-butanone in cyclohexane, since these purported azeotropes have been used previously in art conservation [
8,
10]. The objective was to ascertain their compositional attributes under ambient temperature conditions of evaporation and juxtapose these findings with the compositions conventionally reported at boiling points in chemical literature like the CRC Handbook [
16]. This approach elucidates the behavior and properties of these mixtures under conditions normally encountered in conservation practice. Because part of the motivation for this study was to create undergraduate chemistry laboratory exercises with an Arts focus as part of a science curriculum overhaul at Sam Houston State University, simple analytical instruments commonly deployed in these courses were used in the analysis.
2. Materials and Methods
2.1. Solvents
Although conservators often use commodity or laboratory-grade solvents to clean paintings, all experiments reported here were performed using high-purity solvents. Isopropanol, n-hexane, 2-butanone, and cyclohexane were acquired from vendors (e.g., Sigma Aldrich, St. Louis, MO, USA, Fisher Scientific, Pittsburgh, PA, USA) in purity exceeding 97%. It is worth cautioning that hexane is a known neurotoxin and should only be used under the strictest laboratory or studio precautions.
2.2. Vapor Pressure Measurements
Vapor pressure measurements were performed at room temperature (~21 °C) using gas pressure sensors and temperature probes from Vernier® Science Education company (Beaverton, OR, USA) and an associated tablet interface running LabQuest 2. Only the isopropanol:n-hexane mixture was assessed by this method. Pressure-maximum azeotropes (i.e., ‘positive’ azeotropes) should have their highest vapor pressure at the azeotropic composition. In the experimental setup, an empty 250 mL Erlenmeyer flask was interfaced with a 60-mL syringe and a pressure sensor utilizing gastight Luer-lock valved connectors. A vacuum condition was established by retracting the plunger of the 60 mL syringe and subsequently sealing the Luer-lock system. This action was repeated multiple times until the pressure sensor displayed a reading within the 245 to 255 mmHg range. Subsequently, a syringe containing 5 mL of the solvent mixture was attached to the remaining available Luer-lock connector, and its contents were injected into the Erlenmeyer flask. After a brief stabilization period, the internal chamber reached vapor saturation from the solvent mixture. The differential pressure, as measured by the sensor, was recorded as the vapor pressure of the liquid under investigation. All measurements were conducted in triplicate, and the observed standard error at the recorded pressures ranged from 1.5 to 7.7 mmHg for the 3 measurements.
2.3. Gas Chromatography
Calibration curves for gas chromatography response factors were generated employing a Mini GC Plus, an educational-grade gas chromatograph manufactured by Vernier® Science Education. The apparatus utilizes ambient air as the carrier gas and features software-controlled pressure regulation, adjustable within a range of 1–21 kPa above ambient pressure. The chromatographic separation was achieved using an 11-m Restek MXT-1 capillary column composed of 100% dimethylpolysiloxane. Detection was performed using a polymer-based MEMS (Micro-Electro-Mechanical Systems) Chemi-Capacitive detector. The chromatographic runs commenced at an initial temperature of 35 °C maintained for 2 min. Subsequently, the temperature was increased at 3 °C/min, concluding at 65 °C, under a constant pressure of 7.0 kPa. The total runtime for each analysis was 12 min, although most solvent separations were completed within the initial 4 min window. Calibration curves were designed to encompass the azeotropic compositions of the investigated mixturesas reported at their respective boiling points. These ranged from 5 to 30% v/v for isopropanol in n-hexane mixtures and 33 to 47% v/v for 2-butanone in cyclohexane mixtures. Sample injections involved volumes of 0.60 µL for isopropanol and n-hexane mixtures and 0.20 µL for 2-butanone and cyclohexane mixtures, introduced from the liquid phase of the evaporating mixtures. All measurements were performed in triplicate. The Mini GC Plus does not detect hydrocarbon solvents, so compositional analysis relied on quantification of the respective isopropanol or 2-butatone remaining in the mixture.
2.4. Refractive Index Measurements
For the solvent mixture of 2-butanone in cyclohexane, a refractive index calibration curve was generated using a Fisherbrand™ Handheld Digital Brix/Refractive Index Refractometer (Fisher Scientific, Pittsburgh, USA) with a refractive index range of 1.3330 to 1.5177. Each analysis employed approximately 0.50 mL of the liquid standard or the evaporating test mixture. The calibration curve was established using compositional variations ranging from 0 to 100% v/v of 2-butanone and cyclohexane. All measurements were performed in triplicate. Similar measurements were not performed on the isopropanol:n-hexane system due to the nearly identical refractive indices of these two solvents.
4. Discussion
To optimally interpret the outcomes pertaining to the solvent mixtures, it is imperative to scrutinize the equilibrium data plot for the systems in question, with particular emphasis on the impact of environmental pressure during the evaporation process. A wealth of azeotropic information under varying pressure conditions is documented in the literature (
Table 2). Each condition is delineated with corresponding azeotropic pressure (P
AZ), azeotropic temperature (T
AZ), and composition, which is frequently reported in terms of mole ratio or weight percentage. In this research, compositions are rendered in volume percentage (% v/v) to facilitate ease of discussion. The azeotropic data documented in the literature exhibit variability even under identical boiling pressures at 760 mmHg.
As demonstrated in the data in
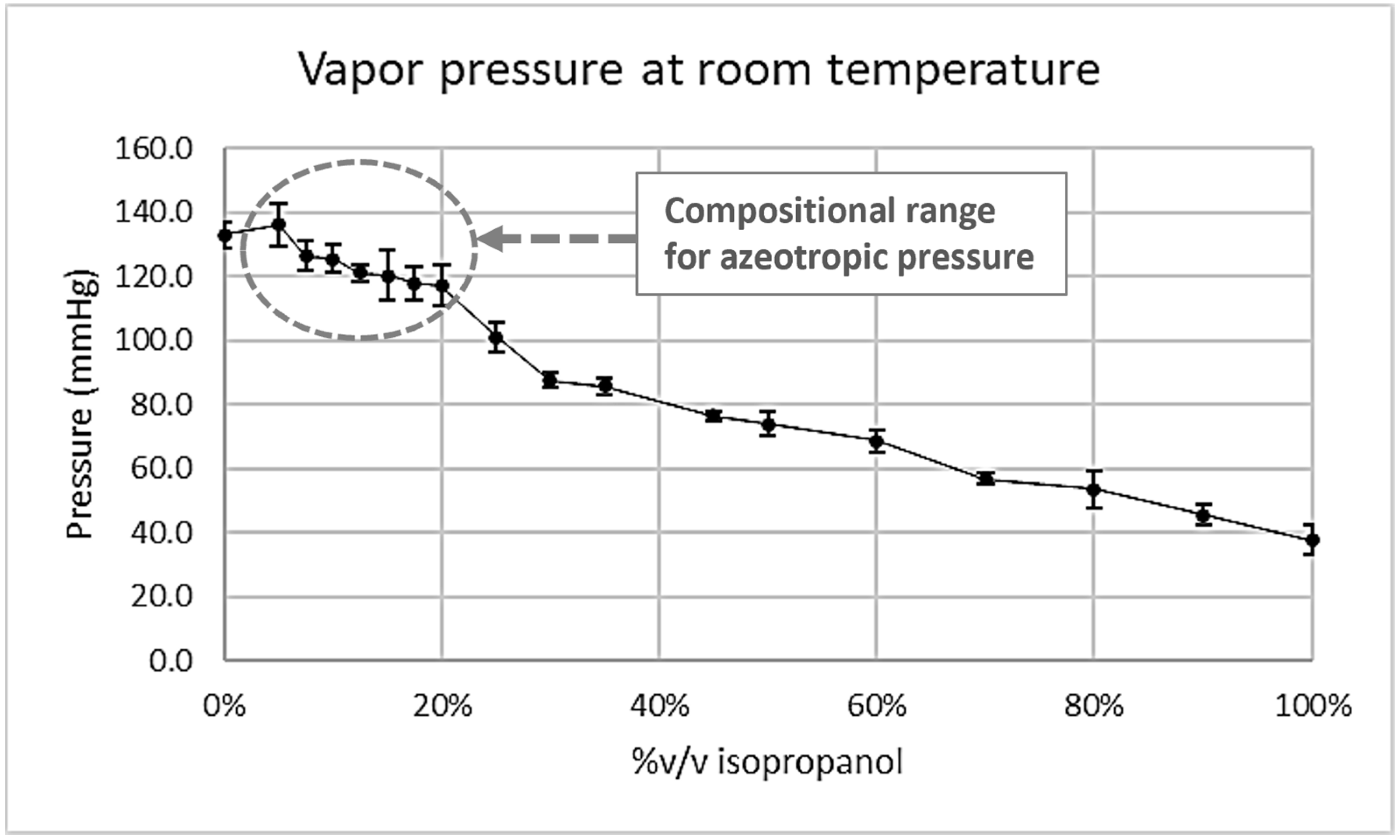
Table 2, the composition of the binary system isopropanol:n-hexane is contingent upon the system’s pressure, with a reported range spanning 199 to 845 mmHg. Consequently, this system is markedly influenced by environmental conditions, as the documented composition undergoes alteration within the range of 10 to 19.2% v/v over the recorded azeotropic pressures and temperatures. Within the realm of chemical industry literature, this phenomenon is designated a pressure-sensitive distillation system. With no documented data at ambient temperature and atmospheric pressure, the present investigation ascertained experimentally that the composition of the azeotrope approximates 10% v/v isopropanol, which just happens to match the data reported for 30 °C and 199 mmHg [
17]. As such, the true room temperature azeotrope contains only about half as much isopropanol, the stronger solvent in the conservation applications in which it was used, which may therefore be ineffective at the cleaning being attempted. Moreover, with evaporation at room temperature, the use of a 19% v/v isopropanol in n-hexane solution should increase in the concentration of isopropanol as the solution dries, which approximates the concern of differential evaporation that led to the exploration of azeotropes for picture cleaning originally.
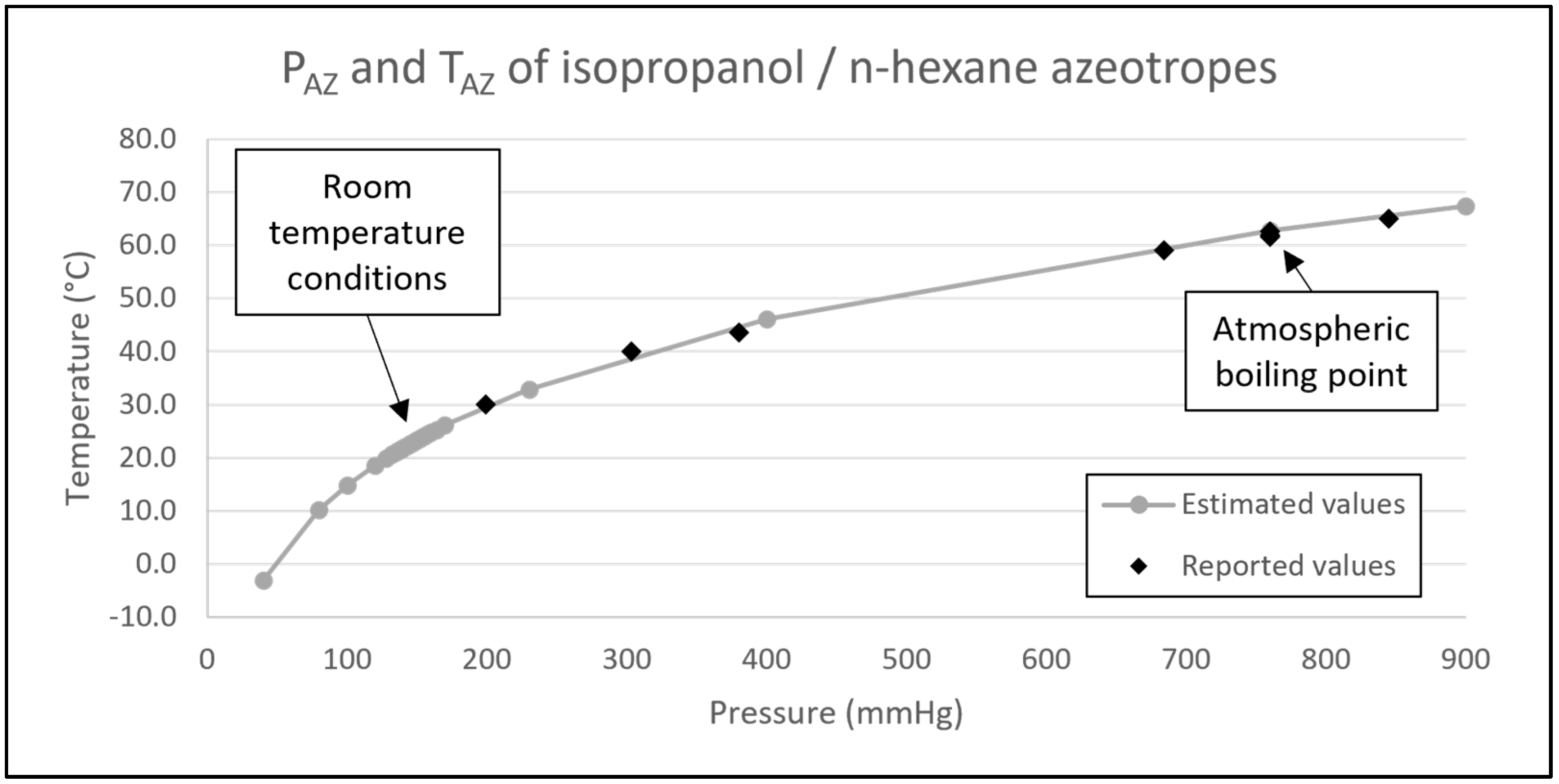
The room temperature azeotrope composition can also be estimated mathematically. Employing a methodology already described in the literature [
22], estimations were conducted for temperature (T
AZ), pressure (P
AZ), and composition (x
AZ) pertaining to the isopropanol and n-hexane system under various conditions. This theoretical calculation relies on empirical data pertinent to the system, including aspects such as enthalpy of vaporization for the respective solvents, vapor pressures of the pure solvents, and a singular dataset of reported azeotropic parameters comprising T
AZ, P
AZ, and x
AZ, and as such, the approach is susceptible to error. Using this estimation procedure from the literature [
22], the calculated T
AZ and x
AZ at various P
AZ for the system are depicted in
Figure 4 and
Figure 5. Additionally, eight values extracted from the literature [
16,
17,
18,
19,
20,
21] were integrated into these figures for the purpose of comparing experimentally derived values to the forecasted data. As demonstrated in
Figure 4, the analysis yielded a negligible discrepancy between the reported data points of T
AZ and P
AZ at both the lower and upper extremes of the pressure range. This suggests the calculation from the literature [
22] does a good job of approximating the true azeotropic values.
At a P
AZ of 760 mmHg, which equates to 1 atm and denotes the atmospheric boiling point, the T
AZ is situated at 62.7 °C as shown in
Figure 4. Conversely, when the temperature is at ambient levels (approximately 21 °C), the P
AZ undergoes a decrease to 138 mmHg. It is noteworthy that, at room temperature, the value of 138 mmHg exceeds the vapor pressure exhibited by the pure solvents (with isopropanol’s vapor pressure at 39 mmHg and n-hexane’s at 132 mmHg). This observation aligns with the expectations for a pressure-maximum (‘positive‘) azeotrope. Additionally, our empirical measurements, as depicted in
Figure 3, indicate that the vapor pressure of the azeotrope at room temperature approximates the range of 125 to 135 mmHg, which closely corresponds to the computed P
AZ at this temperature from
Figure 4.
The extrapolated values of T
AZ and x
AZ for the binary system, as visually represented in
Figure 5, manifest pronounced variations that are contingent upon the prevailing azeotropic pressure (P
AZ). The same reported x
AZ values are depicted in
Figure 5 for comparative analysis with the calculated approximations, and it is observed that these literature values are lower than the estimated values by a range of approximately 1–5%. These discrepancies highlight the necessity for empirical determination of the azeotropic composition under ambient temperature conditions. Based on the projected composition in
Figure 5, the azeotrope at an ambient temperature of 21 °C is anticipated to comprise 12.8% v/v isopropanol in the mixture. The value ascertained experimentally through our study approximates 10% v/v isopropanol, similarly lower than the calculated value just as the reported values are in
Figure 5. It is notable that both the calculated and experimental values are starkly less than the boiling point azeotrope composition of 19% v/v isopropanol used by conservators with the expectation that constant composition evaporation will occur [
8,
9,
10,
11,
12].
Utilizing the aforementioned methodology [
22], estimations were also made for the 2-butanone in cyclohexane system.
Figure 6 shows the derived data for T
AZ and P
AZ. At ambient temperature, the calculated composition of the azeotrope ranges between 38.88 and 38.94% v/v 2-butanone, with an associated P
AZ spanning 107–121 mmHg. The value documented in the literature at boiling conditions (T
AZ 72 °C and P
AZ 760 mmHg) for this system is 40% v/v 2-butanone. Consequently, this azeotropic mixture does not exhibit significant pressure sensitivity, and the composition remains nearly invariant over a large range of pressures.
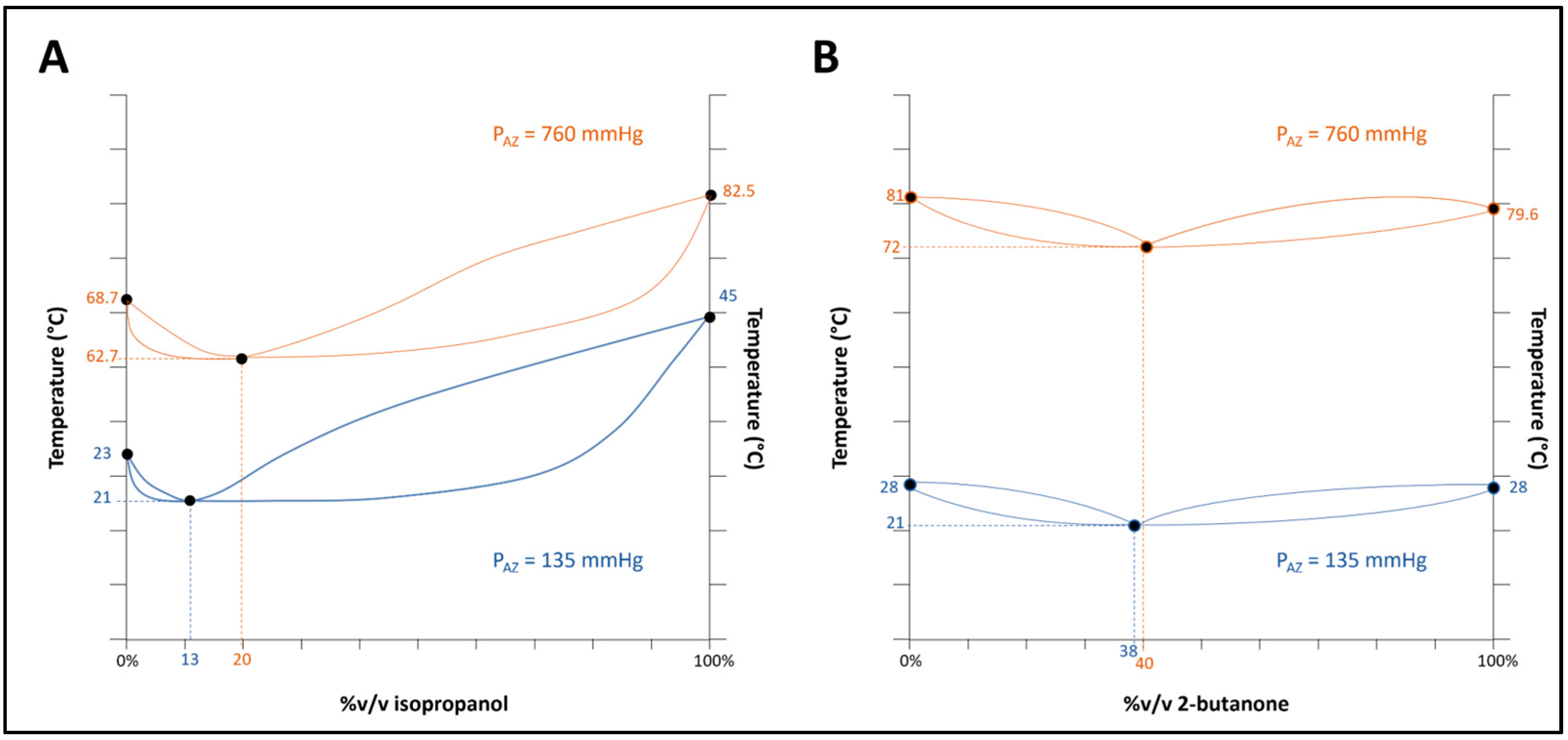
Considering the empirical data and estimated azeotrope compositions, we revised the temperature–composition equilibrium schematics to clarify these observations.
Figure 7 offers relevant illustrations of the systems under study. The equilibrium plots substantially aid in conceptualizing the influence of pressure on the azeotropic temperature and composition. In the instance of the isopropanol and n-hexane system (
Figure 7A), the alteration in the vapor pressure of the mixture from 760 mmHg (i.e., under boiling conditions) to a vapor pressure of 135 mmHg at ambient temperature results in a shift in the azeotropic composition of approximately 7%. Conversely, the 2-butanone and cyclohexane system (
Figure 7B) does not show a significant change in azeotropic composition within the 112 to 760 mmHg pressures range.
Implications for Art Conservation
Several purportedly azeotropic solvent mixtures have been suggested as viable alternatives to aromatic solvents that exhibit moderate toxicity and have, in certain instances, demonstrated efficacy in the removal of dirt and overpaint or the reduction in aged, discolored varnish [
8,
9,
10,
11,
12]. These solvent mixtures have been drawn from the literature by noting azeotropes containing solvents whose combined solubility parameters seem like they would be effective at removing grime and aged organic coatings. However, the compositions of azeotropes are typically provided in the chemical literature at boiling temperatures far from the room temperature conditions where they are utilized in conservation studios.
As previously shown, in the context of the isopropanol and n-hexane system, the deviation in azeotropic composition between boiling and ambient temperature is substantial. This divergence, on the order of 10% v/v in isopropanol concentration, could have significant implications for art conservators depending on the specific nature of the materials being solubilized, or conversely the artwork components they wish to leave unscathed. For illustration, if a conservator operates assuming that the azeotropic composition is 19% v/v isopropanol at room temperature, the solution will experience an enrichment in isopropanol concentration as evaporation occurs. It is important to recognize that isopropanol is characterized as a more potent solvent in the context of solubilizing shellac, as corroborated by prior studies [
8,
11], and so the changing mixture will become a better solvent for those coatings as it evaporates. In contrast, utilizing an azeotropic mixture with an accurate isopropanol concentration of 10% v/v for the cleaning of artworks may yield an insufficiently robust solvent system to achieve the desired efficacy in its application, even if it will evaporate at constant composition, although this has not been tested by the authors. Regardless of the question of potency, the main argument for the use of azeotropes in conservation treatment, i.e., that they will remain constant in composition over the course of their evaporation, is questionable. With the isopropanol and n-hexane system, the boiling point azeotrope mixture would clearly behave zeotropically under conditions of room temperature evaporation; however, for the 2-butanone and cyclohexane system, the composition would remain somewhat consistent since there is not a significant dependence on temperature or pressure.
In pursuit of furnishing more insightful azeotropic data, we estimated in
Table 3 the azeotropic composition for an array of previously determined potential azeotropic mixtures that hold relevance in art conservation [
8] using the mathematical methodology previously indicated [
22]. The estimations encompass boiling, room temperature (~21 °C), and an arbitrary azeotropic pressure of 100 mmHg. Three principal facets warrant attention in this discussion. First, azeotropic mixtures composed of alcohol (e.g., isopropanol) and a hydrocarbon (i.e., n-hexane) tend to exhibit pressure sensitivity, leading to fluctuations in their azeotropic composition. It is imperative to recognize and account for these alterations, especially in scenarios where an elevated concentration of alcohol is necessitated for its ability to solubilize or swell a coating material during removal. Along similar lines, it is notable that azeotropic mixtures composed of two non-alcoholic solvents do not generally experience significant variations in their azeotropic composition under disparate pressure conditions. Consequently, these mixtures exhibit an attribute that is decidedly advantageous for art conservation purposes, as their concentration remains relatively consistent during evaporation at room temperature.
Secondly, the dynamics of solvent composition changes during evaporation are affected by the initial composition of the solvent mixture. Should a solvent mixture be used at ambient temperature with a concentration that is under the azeotropic composition, the ensuing evaporation will enrich the mixture in the more volatile solvent. Conversely, if a solvent mixture is prepared with a concentration that surpasses the azeotropic composition, the evaporation process will lead to an accumulation of the less volatile solvent. This phenomenon holds potential value for art conservators who may benefit from a solution that undergoes an alteration in potency over time, for instance becoming a “weaker” solvent as the solvent evaporates from the surface being cleaned.
Finally, what is often overlooked in advocating for the use of azeotropes in the cleaning of painted surfaces is the preferential absorption of one component of the azeotrope into the coating material itself, thus throwing off the azeotropic composition of the evaporating liquid. Dissolution of organic material into the azeotrope also has the potential of altering the evaporation process, just as solvents with dissolved organic polymers have been shown in conservation research to alter the solvent’s solubility characteristics [
23]. Since the chemical composition of dirt, yellowed varnishes, and aged overpaint are difficult to know, it may be impossible to predict the evaporation behavior of even an appropriately formulated room temperature azeotropic cleaning solution.
In an earlier cursory experiment performed by one of the authors, the room temperature evaporation of a 9.5% v/v isopropanol in n-hexane solution from the surface of a young cobalt blue hue linseed oil paint (Grumbacher) was compared to the evaporation of the same solution without an organic coating present [
24]. The headspace over the evaporating solution was depleted of isopropanol in the sample with the oil paint, consistent with the expectation that isopropanol would be rapidly absorbed into the paint film, thus breaking the azeotrope. This complicating factor may prove the use of even room temperature azeotropes as insufficient for controlling solvent strength during evaporation from painted surfaces, although their use might ensure stock solutions do not change over the course of their use or storage. Other potential issues include evaporative cooling of the paint surface, the use of exhaust systems during artwork treatment, and the tendency of water to adsorb into polar organic solvents, all of which might change the evaporation behavior of the cleaning mixture to some extent. Future research will explore more closely how the evaporation dynamics of room temperature azeotropic solutions vary in the presence of organic coatings or solutions.