The Effects of Toluene Mineralization under Denitrification Conditions on Carbonate Dissolution and Precipitation in Water: Mechanism and Model
Abstract
:1. Introduction
2. Materials and Methods
2.1. Model Construction
2.1.1. Mechanism Model Construction
2.1.2. Mathematical Model Construction
2.2. Model Validation
2.2.1. The Validation Experiment
- (1)
- Toluene biodegradation enrichment cultures
- (2)
- Physical and chemical parameter analyses
2.2.2. The Validation Method
3. Results and Discussion
3.1. Mechanism Model
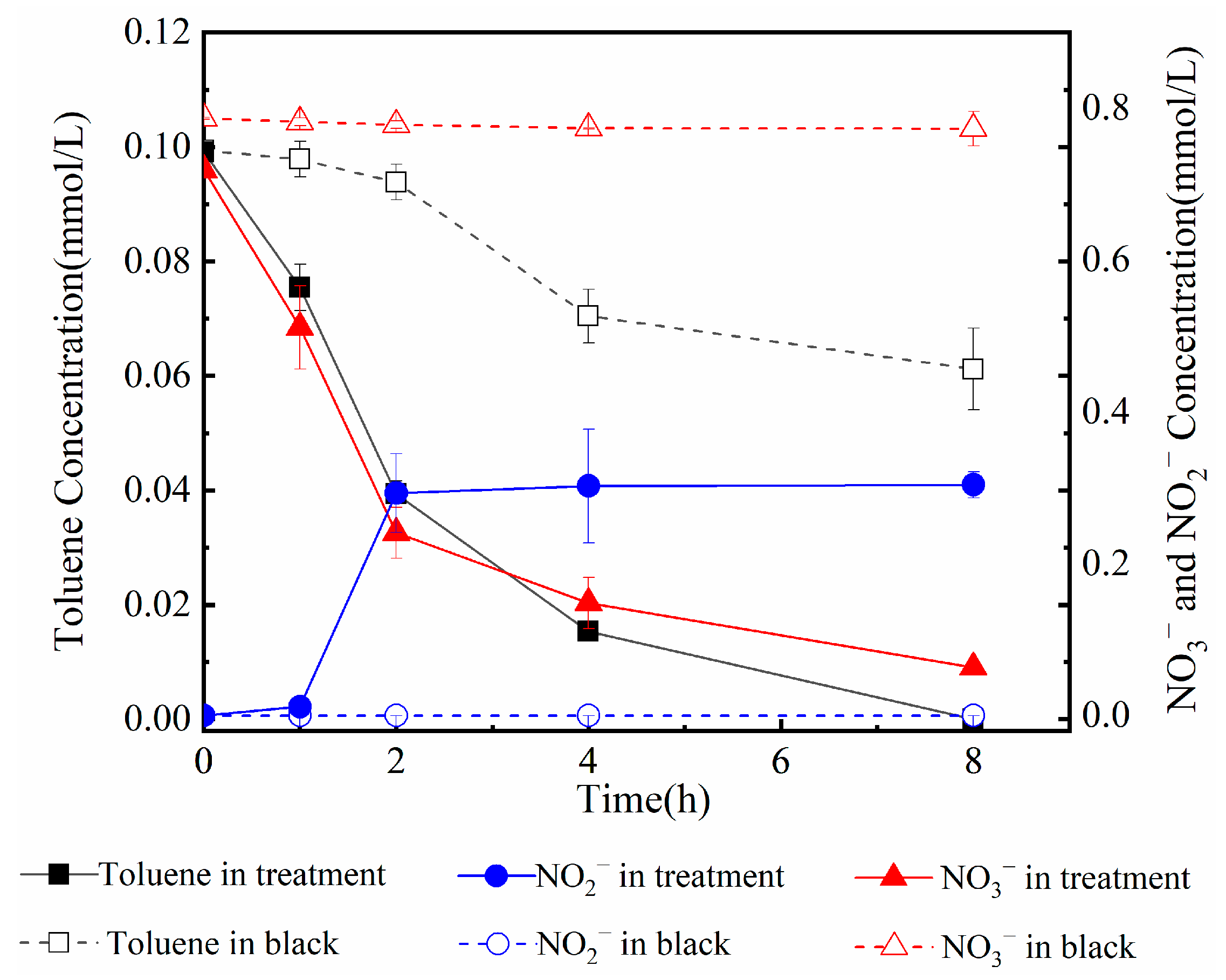
3.1.1. Response of H+ to Toluene Mineralization
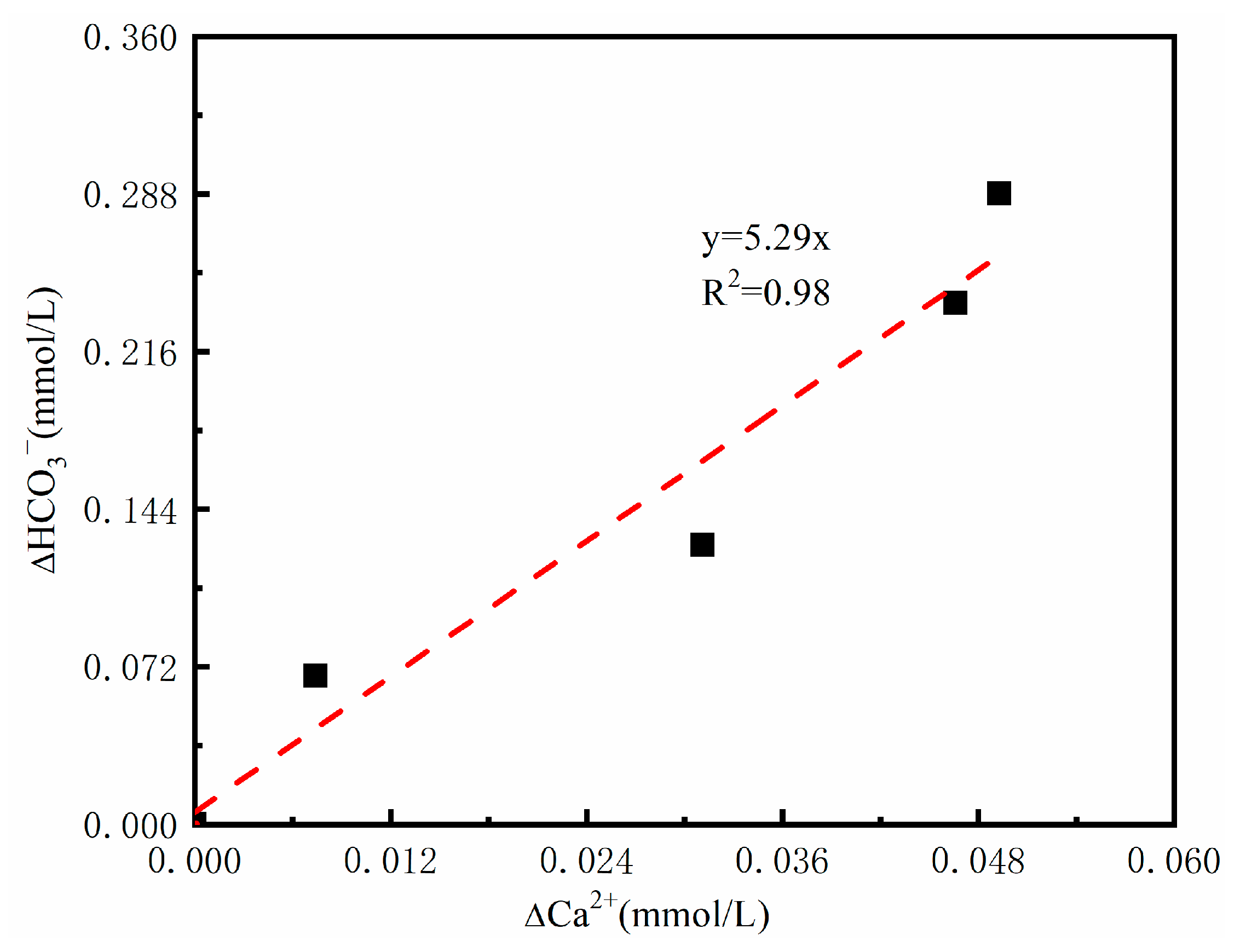
3.1.2. Response of Ca2+ and DIC to Toluene Mineralization
3.2. Mathematical Model
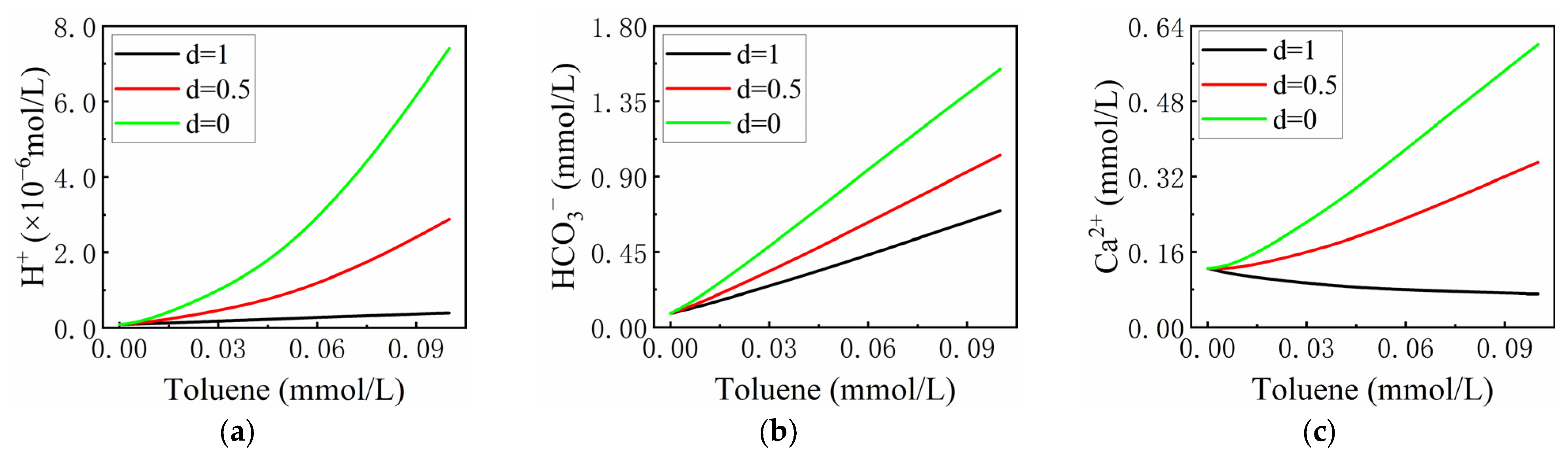
3.2.1. The Effect of the Proportion of Nitrate Consumed by the Complete Reduction of Nitrogen to the Total Amount Consumed (d)
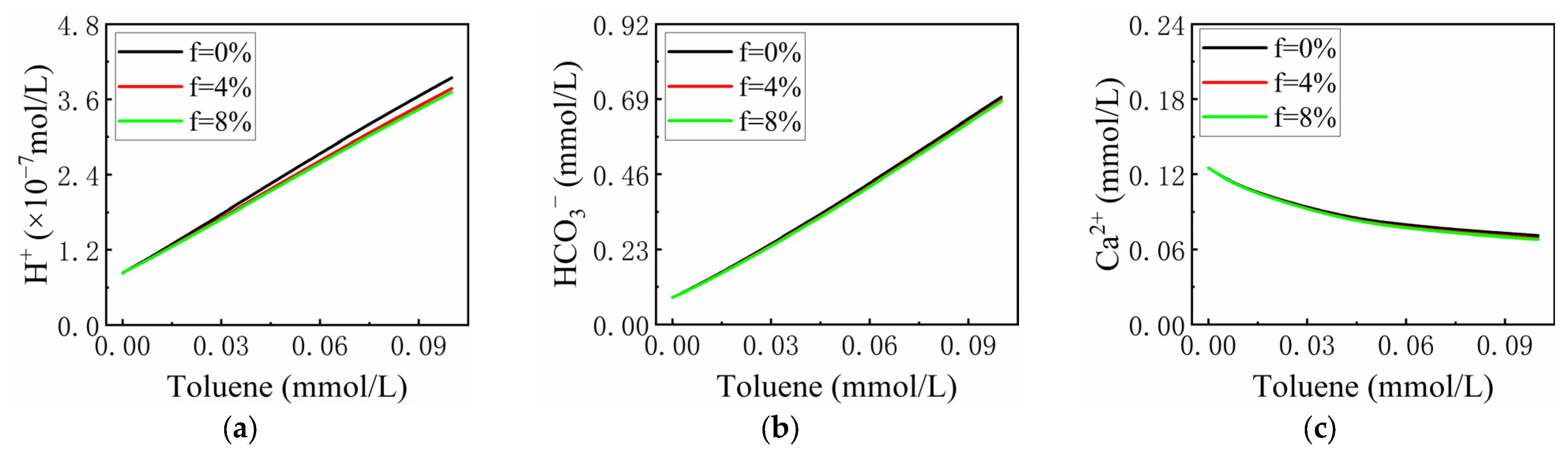
3.2.2. The Effect of the Proportion of Toluene Consumed by Microbial Growth on the Total Toluene Content (f)
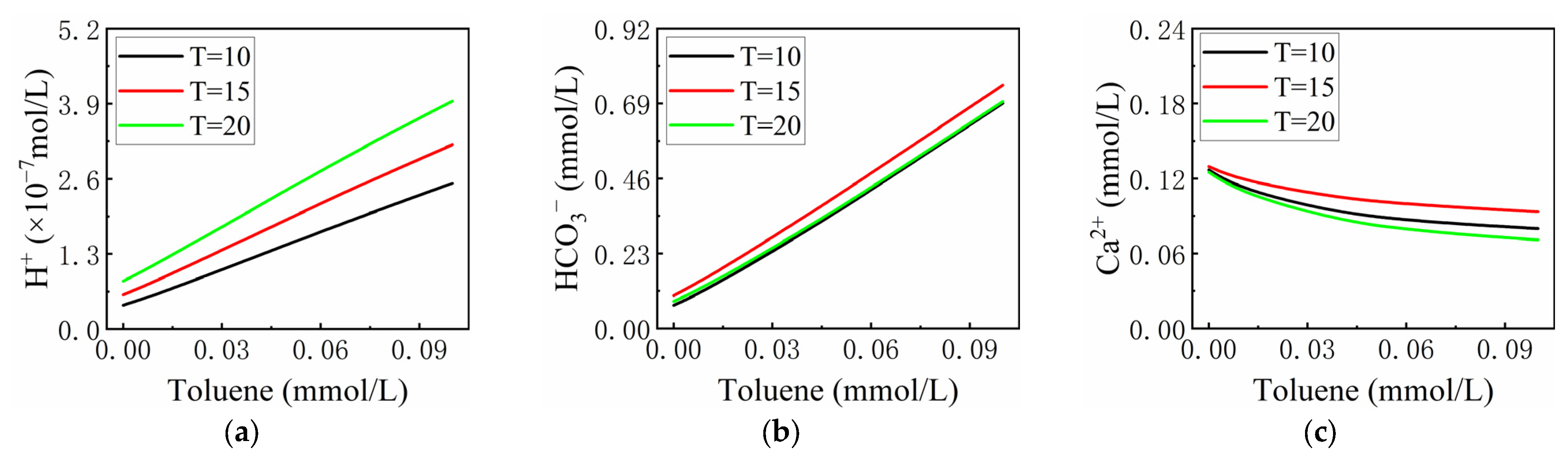
3.2.3. The Effect of Temperature (T)
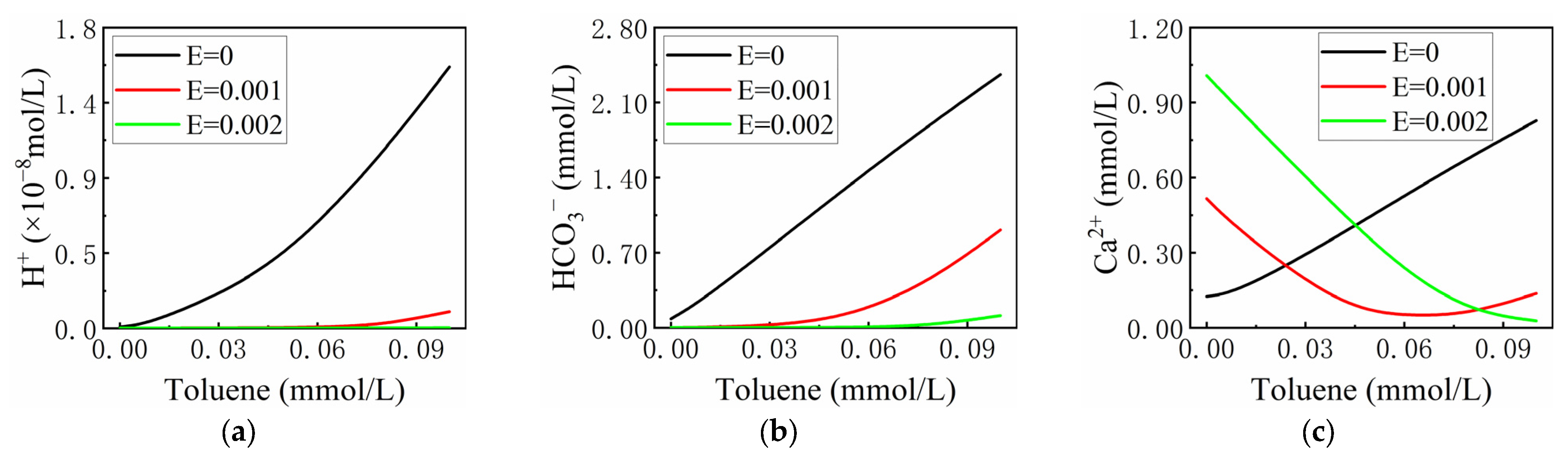
3.2.4. The Effect of the Milligram Equivalent of Other Ions (E)
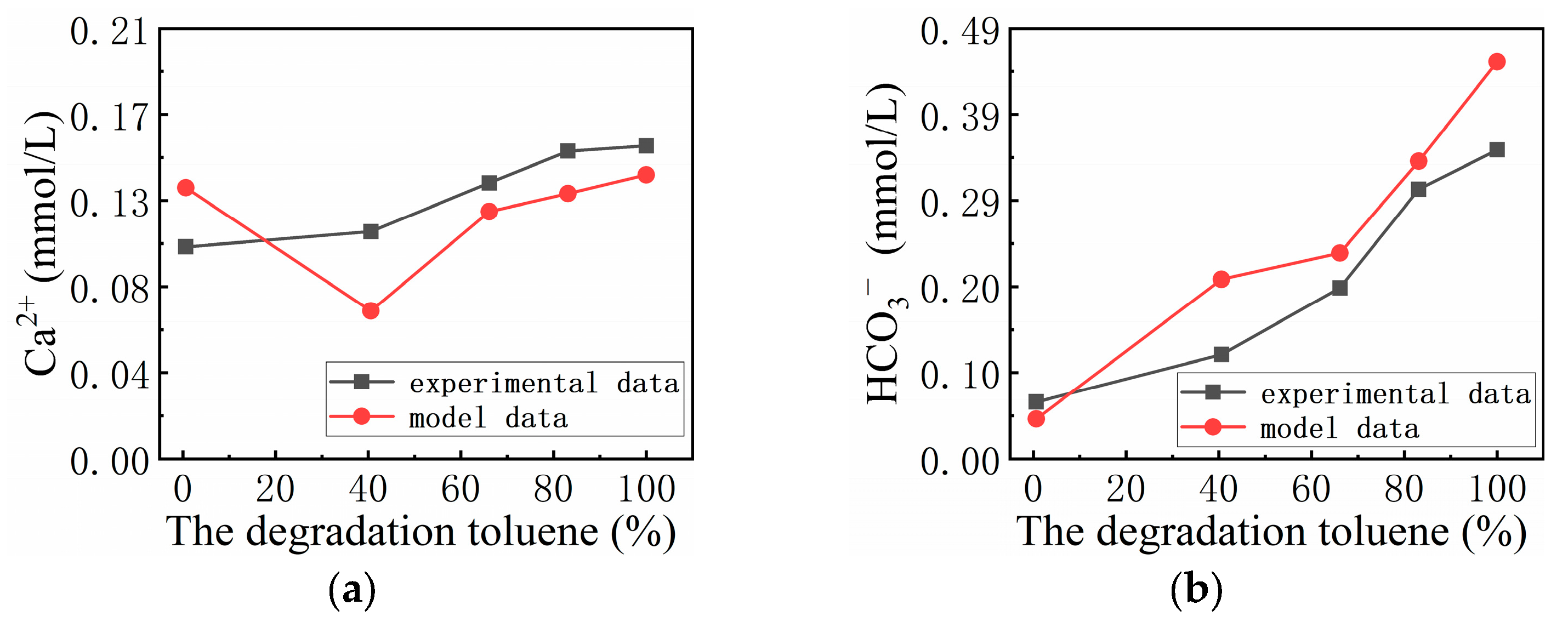
3.3. Fitting Results
3.4. Implications
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhao, B.; Huang, F.; Zhang, C.; Huang, G.; Xue, Q.; Liu, F. Pollution characteristics of aromatic hydrocarbons in the groundwater of China. J. Contam. Hydrol. 2020, 233, 103676. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Wu, W.-M.; Bolan, N.S.; Tsang, D.C.W.; Li, Y.; Qin, M.; Hou, D. Environmental fate, toxicity and risk management strategies of nanoplastics in the environment: Current status and future perspectives. J. Hazard. Mater. 2021, 401, 123415. [Google Scholar] [CrossRef] [PubMed]
- Busygina, T.; Rykova, V. Scientometric analysis and mapping of documentary array on the issue “Oil and petroleum products in soil and groundwater”. Environ. Sci. Pollut. Res. 2020, 27, 23490–23502. [Google Scholar] [CrossRef] [PubMed]
- Hu, S.; Xiao, C.; Liang, X.; Cao, Y.; Wang, X.; Li, M. The influence of oil shale in situ mining on groundwater environment: A water-rock interaction study. Chemosphere 2019, 228, 384–389. [Google Scholar] [CrossRef] [PubMed]
- Singh, S.; Gupta, V.K. Biodegradation and bioremediation of pollutants: Perspectives strategies and applications. Int. J. Pharmacol. Biol. Sci. 2016, 10, 53. [Google Scholar]
- Wu, Z.; Liu, G.; Ji, Y.; Li, P.; Yu, X.; Qiao, W.; Wang, B.; Shi, K.; Liu, W.; Liang, B.; et al. Electron acceptors determine the BTEX degradation capacity of anaerobic microbiota via regulating the microbial community. Environ. Res. 2022, 215, 114420. [Google Scholar] [CrossRef] [PubMed]
- Huang, H.; Jiang, Y.; Zhao, J.; Li, S.; Schulz, S.; Deng, L. BTEX biodegradation is linked to bacterial community assembly patterns in contaminated groundwater ecosystem. J. Hazard. Mater. 2021, 419, 126205. [Google Scholar] [CrossRef]
- Davidson, C.J.; Hannigan, J.H.; Bowen, S.E. Effects of inhaled combined Benzene, Toluene, Ethylbenzene, and Xylenes (BTEX): Toward an environmental exposure model. Environ. Toxicol. Pharmacol. 2021, 81, 103518. [Google Scholar] [CrossRef]
- Cheng, L.; Wei, W.; Guo, A.; Zhang, C.; Sha, K.; Wang, R.; Wang, K.; Cheng, S. Health risk assessment of hazardous VOCs and its associations with exposure duration and protection measures for coking industry workers. J. Clean. Prod. 2022, 379, 134919. [Google Scholar] [CrossRef]
- Alenezi, R.A. BTEX and nitrogen oxides fume assessments in fuel dispense stations and residential areas. J. Eng. Res. 2022, 10, 50–59. [Google Scholar] [CrossRef]
- Alenezi, R.A.; Aldaihan, N. Impact of fuel dispensing stations in the vicinity residential homes on the indoor and outdoor air quality. Int. J. Environ. Sci. Technol. 2019, 16, 2783–2796. [Google Scholar] [CrossRef]
- Péquin, B.; Cai, Q.; Lee, K.; Greer, C.W. Natural attenuation of oil in marine environments: A review. Mar. Pollut. Bull. 2022, 176, 113464. [Google Scholar] [CrossRef] [PubMed]
- Haider, F.U.; Ejaz, M.; Cheema, S.A.; Khan, M.I.; Zhao, B.; Liqun, C.; Salim, M.A.; Naveed, M.; Khan, N.; Nunez-Delgado, A. Phytotoxicity of petroleum hydrocarbons: Sources, impacts and remediation strategies. Environ. Res. 2021, 197, 111031. [Google Scholar] [CrossRef] [PubMed]
- Kharangate-Lad, A.; D’Souza, N.C. Current Approaches in Bioremediation of Toxic Contaminants by Application of Microbial Cells; Biosurfactants and Bioemulsifiers of Microbial Origin. In Rhizobiont in Bioremediation of Hazardous Waste; Springer: Singapore, 2021; pp. 217–263. [Google Scholar] [CrossRef]
- Zhang, M.; Guo, C.; Shi, C.; Ning, Z.; Chen, Z. A quantitative redox zonation model for developing natural attenuation-based remediation strategy in hydrocarbon-contaminated aquifers. J. Clean. Prod. 2021, 290, 125743. [Google Scholar] [CrossRef]
- Budania, R.; Dangayach, S. A comprehensive review on permeable reactive barrier for the remediation of groundwater contamination. J. Environ. Manag. 2023, 332, 117343. [Google Scholar] [CrossRef] [PubMed]
- Weelink, S.A.B.; van Eekert, M.H.A.; Stams, A.J.M. Degradation of BTEX by anaerobic bacteria: Physiology and application. Rev. Environ. Sci. Bio/Technol. 2010, 9, 359–385. [Google Scholar] [CrossRef]
- Dordević, D.; Jančíková, S.; Vítězová, M.; Kushkevych, I. Hydrogen sulfide toxicity in the gut environment: Meta-analysis of sulfate-reducing and lactic acid bacteria in inflammatory processes. J. Adv. Res. 2021, 27, 55–69. [Google Scholar] [CrossRef]
- Chen, X.; Sheng, Y.; Wang, G.; Guo, L.; Zhang, H.; Zhang, F.; Yang, T.; Huang, D.; Han, X.; Zhou, L. Microbial compositional and functional traits of BTEX and salinity co-contaminated shallow groundwater by produced water. Water Res. 2022, 215, 118277. [Google Scholar] [CrossRef]
- Sohrabi, T.; Shakiba, M.; Mirzaei, F.; Pourbabaee, A. BTEX biodegradation using Bacillus sp. in a synthetic hypoxic aquatic environment: Optimization by Taguchi-based design of experiments. Int. J. Environ. Sci. Technol. 2022, 19, 5571–5578. [Google Scholar] [CrossRef]
- Han, K.; Hong, U.; Park, S.; Kwon, S.; Kim, Y. In situ field method for evaluating biodegradation potential of BTEX by indigenous heterotrophic denitrifying microorganisms in a BTEX-contaminated fractured-rock aquifer. Environ. Technol. 2021, 42, 1326–1335. [Google Scholar] [CrossRef]
- Sra, K.S.; Ponsin, V.; Kolhatkar, R.; Hunkeler, D.; Thomson, N.R.; Madsen, E.L.; Buscheck, T. Sulfate Land Application Enhances Biodegradation in a Petroleum Hydrocarbon Smear Zone. Groundw. Monit. Remediat. 2023, 43, 44–59. [Google Scholar] [CrossRef]
- Zhu, H.H.; Chao, J.I.A.; Xu, Y.L.; Yu, Z.M.; Yu, W.J. Study on numerical simulation of organic pollutant transport in groundwater northwest of Laixi. J. Groundw. Sci. Eng. 2018, 6, 293–305. [Google Scholar] [CrossRef]
- Yang, L.-Z.; Liu, C.-H. Study on the characteristics and causes of carbon tetrachloride pollution of karst water in eastern suburbs of Jinan. J. Groundw. Sci. Eng. 2015, 3, 331–341. [Google Scholar] [CrossRef]
- Jiang, H.; Chen, D.; Zheng, D.; Xiao, Z. Anaerobic mineralization of toluene by enriched soil-free consortia with solid-phase humin as a terminal electron acceptor. Environ. Pollut. 2023, 317, 120794. [Google Scholar] [CrossRef] [PubMed]
- He, S.; Li, P.; Su, F.; Wang, D.; Ren, X. Identification and apportionment of shallow groundwater nitrate pollution in Weining Plain, northwest China, using hydrochemical indices, nitrate stable isotopes, and the new Bayesian stable isotope mixing model (MixSIAR). Environ. Pollut. 2022, 298, 118852. [Google Scholar] [CrossRef] [PubMed]
- Lahjouj, A.; Hmaidi, A.E.; Bouhafa, K. Spatial and statistical assessment of nitrate contamination in groundwater: Case of Sais Basin, Morocco. J. Groundw. Sci. Eng. 2020, 8, 143–157. [Google Scholar]
- Liang, C.; Li, C.; Zhu, Y.; Du, X.; Yao, C.; Ma, Y.; Zhao, J. Recent advances of photocatalytic degradation for BTEX: Materials, operation, and mechanism. Chem. Eng. J. 2023, 455, 140461. [Google Scholar] [CrossRef]
- Zainab, R.; Hasnain, M.; Ali, F.; Dias, D.A.; El-Keblawy, A.; Abideen, Z. Exploring the bioremediation capability of petroleum-contaminated soils for enhanced environmental sustainability and minimization of ecotoxicological concerns. Environ. Sci. Pollut. Res. 2023, 30, 104933–104957. [Google Scholar] [CrossRef]
- De Muynck, W.; De Belie, N.; Verstraete, W. Microbial carbonate precipitation in construction materials: A review. Ecol. Eng. 2010, 36, 118–136. [Google Scholar] [CrossRef]
- Fedorov, K.; Sun, X.; Boczkaj, G. Combination of hydrodynamic cavitation and SR-AOPs for simultaneous degradation of BTEX in water. Chem. Eng. J. 2021, 417, 128081. [Google Scholar] [CrossRef]
- Spence, M.J.; Bottrell, S.H.; Thornton, S.F.; Richnow, H.H.; Spence, K.H. Hydrochemical and isotopic effects associated with petroleum fuel biodegradation pathways in a chalk aquifer. J. Contam. Hydrol. 2005, 79, 67–88. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, B.; Agrawal, V.; Sharma, S.; Hakala, J.A.; Xiong, W. Effects of Carbonate Minerals on Shale-Hydraulic Fracturing Fluid Interactions in the Marcellus Shale. Front. Earth Sci. 2021, 9, 695978. [Google Scholar] [CrossRef]
- Cai, P.; Ning, Z.H.; Zhang, N.; Zhang, M.; Guo, C.; Niu, M.; Shi, J. Insights into Biodegradation Related Metabolism in an Abnormally Low Dissolved Inorganic Carbon (DIC) Petroleum-Contaminated Aquifer by Metagenomics Analysis. Microorganisms 2019, 7, 412. [Google Scholar] [CrossRef] [PubMed]
- Laczi, K.; Erdeiné Kis, Á.; Szilágyi, Á.; Bounedjoum, N.; Bodor, A.; Vincze, G.E.; Kovács, T.; Rákhely, G.; Perei, K. New frontiers of anaerobic hydrocarbon biodegradation in the multi-omics era. Front. Microbiol. 2020, 11, 590049. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Yuan, Z.; Yu, Z.; Xue-song, F. BTEX in the environment: An update on sources, fate, distribution, pretreatment, analysis, and removal techniques. Chem. Eng. J. 2022, 435, 134825. [Google Scholar] [CrossRef]
- Jiang, W.; Sheng, Y.; Wang, G.; Shi, Z.; Liu, F.; Zhang, J.; Chen, D. Cl, Br, B, Li, and noble gases isotopes to study the origin and evolution of deep groundwater in sedimentary basins: A review. Environ. Chem. Lett. 2022, 20, 1497–1528. [Google Scholar] [CrossRef]
- Min, Z. Mechanism and Simulation of Response on GroundwaterChemical Compositions to Hydrodynamic Conditions. Min. Sci. Technol. 2009, 19, 435–440. [Google Scholar]
- ISO 10304-1:2007/Cor 1:2010; Water Quality—Determination Of Dissolved Anions By Liquid Chromatography Of Ions—Part 1: Determination Of Bromide, Chloride, Fluoride, Nitrate, Nitrite, Phosphate And Sulfate. ISO Copyright Office: Geneva, Switzerland, 2010.
- Burland, S.M.; Edwards, E.A. Anaerobic benzene biodegradation linked to nitrate reduction. Appl. Environ. Microbiol. 1999, 65, 529–533. [Google Scholar] [CrossRef]
- Durand, S.; Guillier, M. Transcriptional and Post-transcriptional control of the nitrate respiration in bacteria. Front. Mol. Biosci. 2021, 8, 667758. [Google Scholar] [CrossRef]
- Song, T.; Zhang, X.; Li, J.; Wu, X.; Feng, H.; Dong, W. A review of research progress of heterotrophic nitrification and aerobic denitrification microorganisms (HNADMs). Sci. Total Environ. 2021, 801, 149319. [Google Scholar] [CrossRef]
- Mitchell, M.J.; Jensen, O.E.; Cliffe, K.A.; Maroto-Valer, M.M. A model of carbon dioxide dissolution and mineral carbonation kinetics. Proc. R. Soc. A Math. Phys. Eng. Sci. 2009, 466, 1265–1290. [Google Scholar] [CrossRef]
- Cordova, C.E. Plows, Plagues, and Petroleum: How Humans Took Control of Climate. Holocene 2010, 20, 653. [Google Scholar] [CrossRef]
- Niu, J.; Liu, Q.; Lv, J.; Peng, B. Review on microbial enhanced oil recovery: Mechanisms, modeling and field trials. J. Pet. Sci. Eng. 2020, 192, 107350. [Google Scholar] [CrossRef]
- Peña-Calva, A.; Olmos-Dichara, A.; Viniegra-González, G.; Cuervo-López, F.M.; Gómez, J. Denitrification in presence of benzene, toluene, and m-xylene. Appl. Biochem. Biotechnol. 2004, 119, 195–208. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, S.; Kumari, K.; Singh, D. Different strategies and bio-removal mechanisms of petroleum hydrocarbons from contaminated sites. Arab. Gulf J. Sci. Res. 2023. ahead of print. [Google Scholar] [CrossRef]
- Muccee, F.; Ejaz, S.; Riaz, N. Toluene degradation via a unique metabolic route in indigenous bacterial species. Arch. Microbiol. 2019, 201, 1369–1383. [Google Scholar] [CrossRef] [PubMed]
- Tsui, T.-H.; van Loosdrecht, M.C.; Dai, Y.; Tong, Y.W. Machine learning and circular bioeconomy: Building new resource efficiency from diverse waste streams. Bioresour. Technol. 2022, 369, 128445. [Google Scholar] [CrossRef]
- Tsui, T.-H.; Zhang, L.; Zhang, J.; Dai, Y.; Tong, Y.W. Engineering interface between bioenergy recovery and biogas desulfurization: Sustainability interplays of biochar application. Renew. Sustain. Energy Rev. 2022, 157, 112053. [Google Scholar] [CrossRef]
- Cao, S.; Du, R.; Zhou, Y. Coupling anammox with heterotrophic denitrification for enhanced nitrogen removal: A review. Crit. Rev. Environ. Sci. Technol. 2021, 51, 2260–2293. [Google Scholar] [CrossRef]
- Rassamee, V.; Sattayatewa, C.; Pagilla, K.; Chandran, K. Effect of oxic and anoxic conditions on nitrous oxide emissions from nitrification and denitrification processes. Biotechnol. Bioeng. 2011, 108, 2036–2045. [Google Scholar] [CrossRef]


| Compounds | Concentration (mg/L) |
|---|---|
| Biotin | 2.00 |
| Folic acid | 2.00 |
| Riboflavin | 5.00 |
| Nicotinic acid | 5.00 |
| p-Aminobenzoic acid | 5.00 |
| Thioctic acid | 5.00 |
| Calcium D-(+)-pantothenate | 5.00 |
| Pyridoxine hydrochloride | 10.00 |
| Compounds | Concentration (g/L) |
|---|---|
| FeSO4 4H2O | 1.80 |
| CoCl2 6H2O | 0.25 |
| CuCl2 2H2O | 0.01 |
| NiCl 6H2O | 0.01 |
| ZnCl2 | 0.10 |
| H3BO3 | 0.50 |
| EDTA | 2.50 |
| MnCl2 4H2O | 0.70 |
| (NH4)6Mo7O24 4H2O | 0.01 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gan, S.; Zhang, M.; Zhou, Y.; Guo, C.; Yang, S.; Xie, Y.; Wang, X.; Sun, L.; Ning, Z. The Effects of Toluene Mineralization under Denitrification Conditions on Carbonate Dissolution and Precipitation in Water: Mechanism and Model. Appl. Sci. 2023, 13, 11867. https://doi.org/10.3390/app132111867
Gan S, Zhang M, Zhou Y, Guo C, Yang S, Xie Y, Wang X, Sun L, Ning Z. The Effects of Toluene Mineralization under Denitrification Conditions on Carbonate Dissolution and Precipitation in Water: Mechanism and Model. Applied Sciences. 2023; 13(21):11867. https://doi.org/10.3390/app132111867
Chicago/Turabian StyleGan, Shuang, Min Zhang, Yahong Zhou, Caijuan Guo, Shuai Yang, Yan Xie, Xinzhe Wang, Lin Sun, and Zhuo Ning. 2023. "The Effects of Toluene Mineralization under Denitrification Conditions on Carbonate Dissolution and Precipitation in Water: Mechanism and Model" Applied Sciences 13, no. 21: 11867. https://doi.org/10.3390/app132111867
APA StyleGan, S., Zhang, M., Zhou, Y., Guo, C., Yang, S., Xie, Y., Wang, X., Sun, L., & Ning, Z. (2023). The Effects of Toluene Mineralization under Denitrification Conditions on Carbonate Dissolution and Precipitation in Water: Mechanism and Model. Applied Sciences, 13(21), 11867. https://doi.org/10.3390/app132111867






