Biomonitoring of Potentially Toxic Elements in an Abandoned Mining Region Using Taraxacum officinale: A Case Study on the “Tsar Asen” Mine in Bulgaria
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents
2.2. Sampling of Plant and Soil Samples
2.3. Determination of pH and Cation Exchange Capacity (CEC)
2.4. Sample Preparation
2.5. Analytical Procedure for PTEs Determination
2.6. Analytical Characteristics of the Method
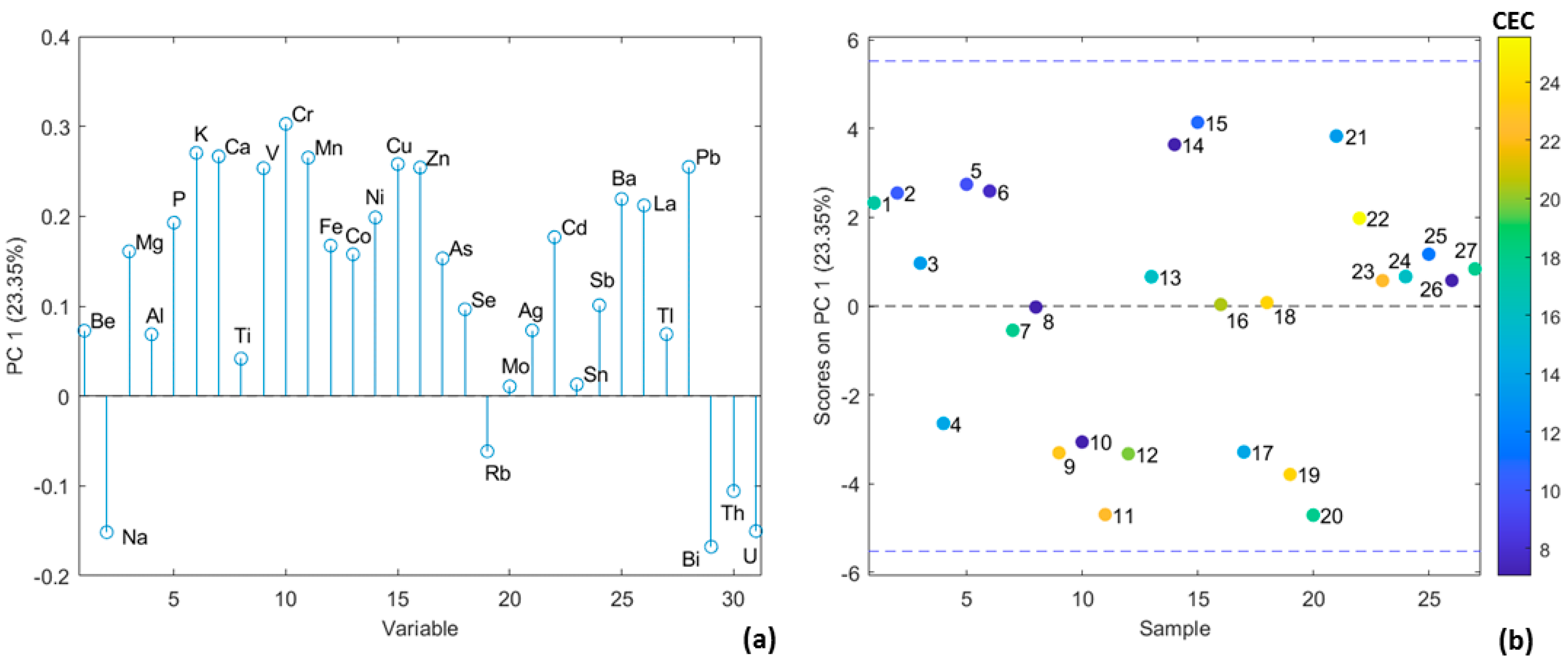
2.7. Multivariate Data Analysis
3. Results
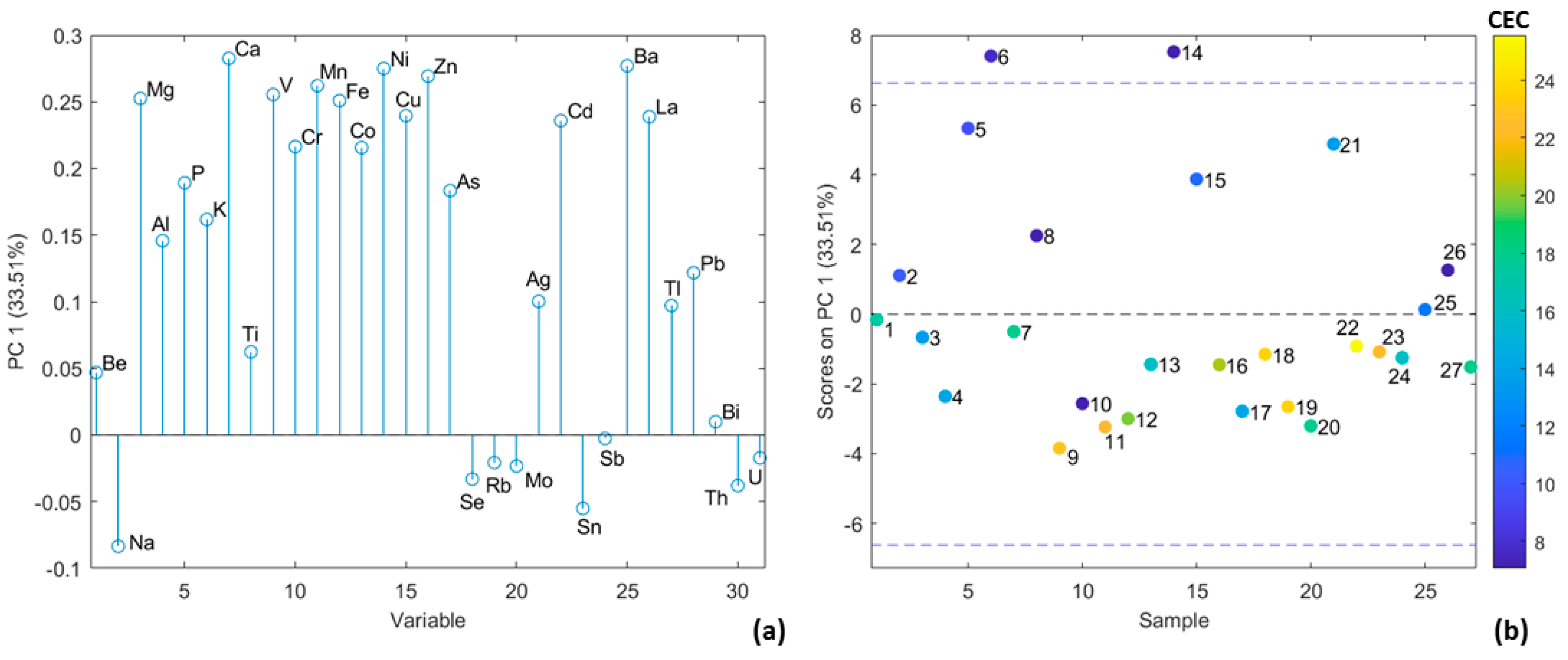
3.1. Determination of pH and Cation Exchange Capacity of Soil Samples
3.2. Total Element Concentration of Soil and Plant Samples
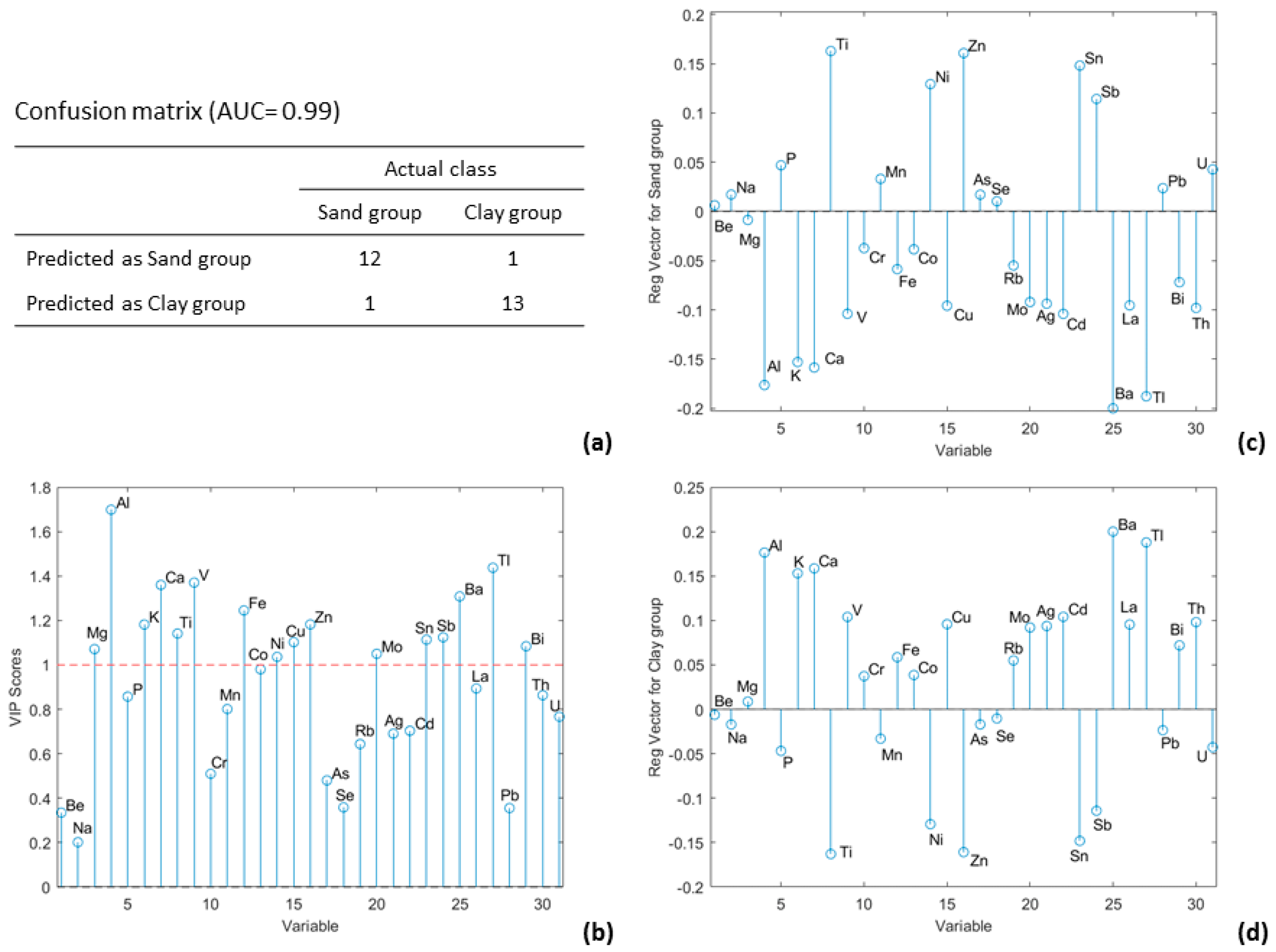
3.3. Partial Least Squares Discriminant Analysis
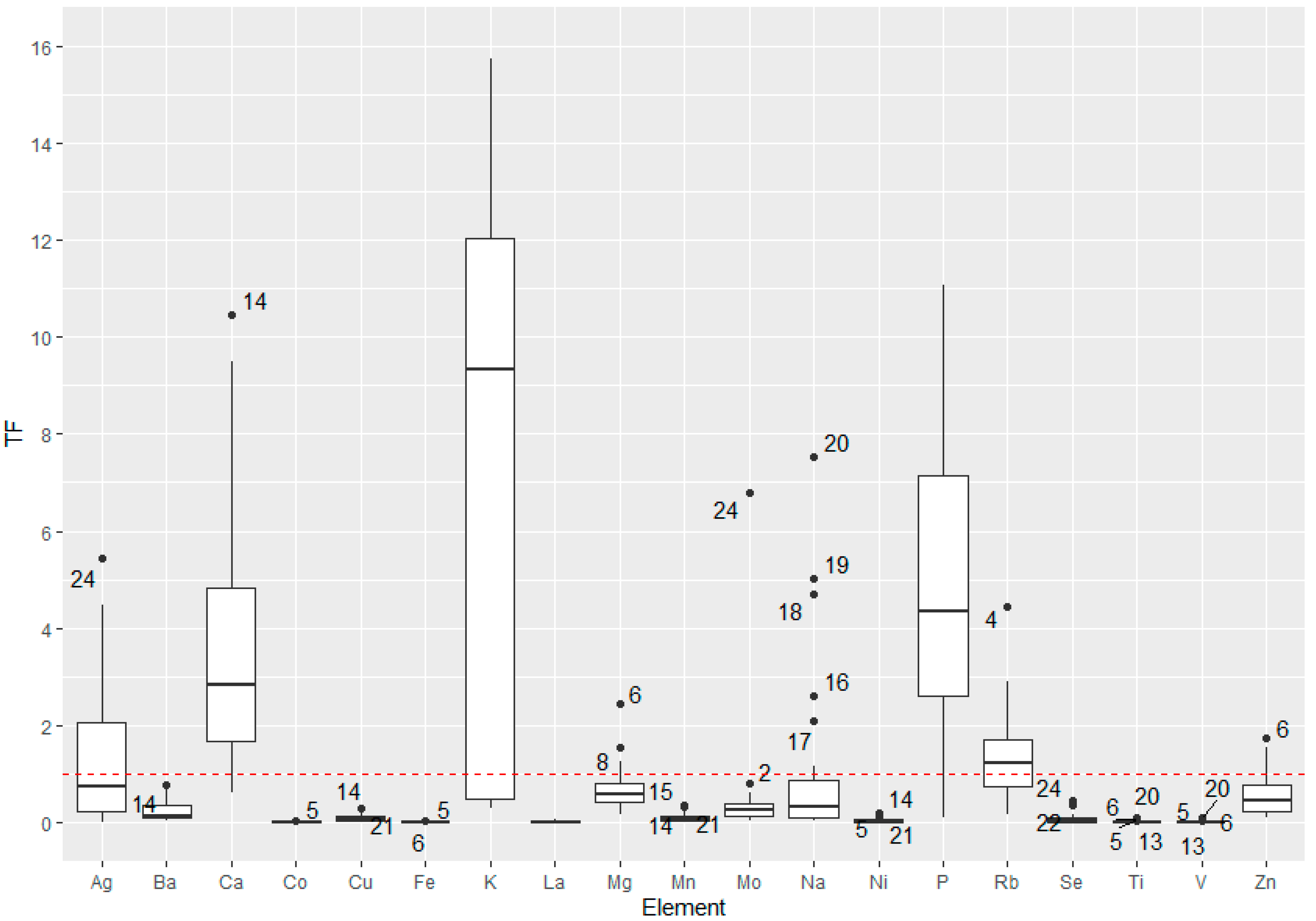
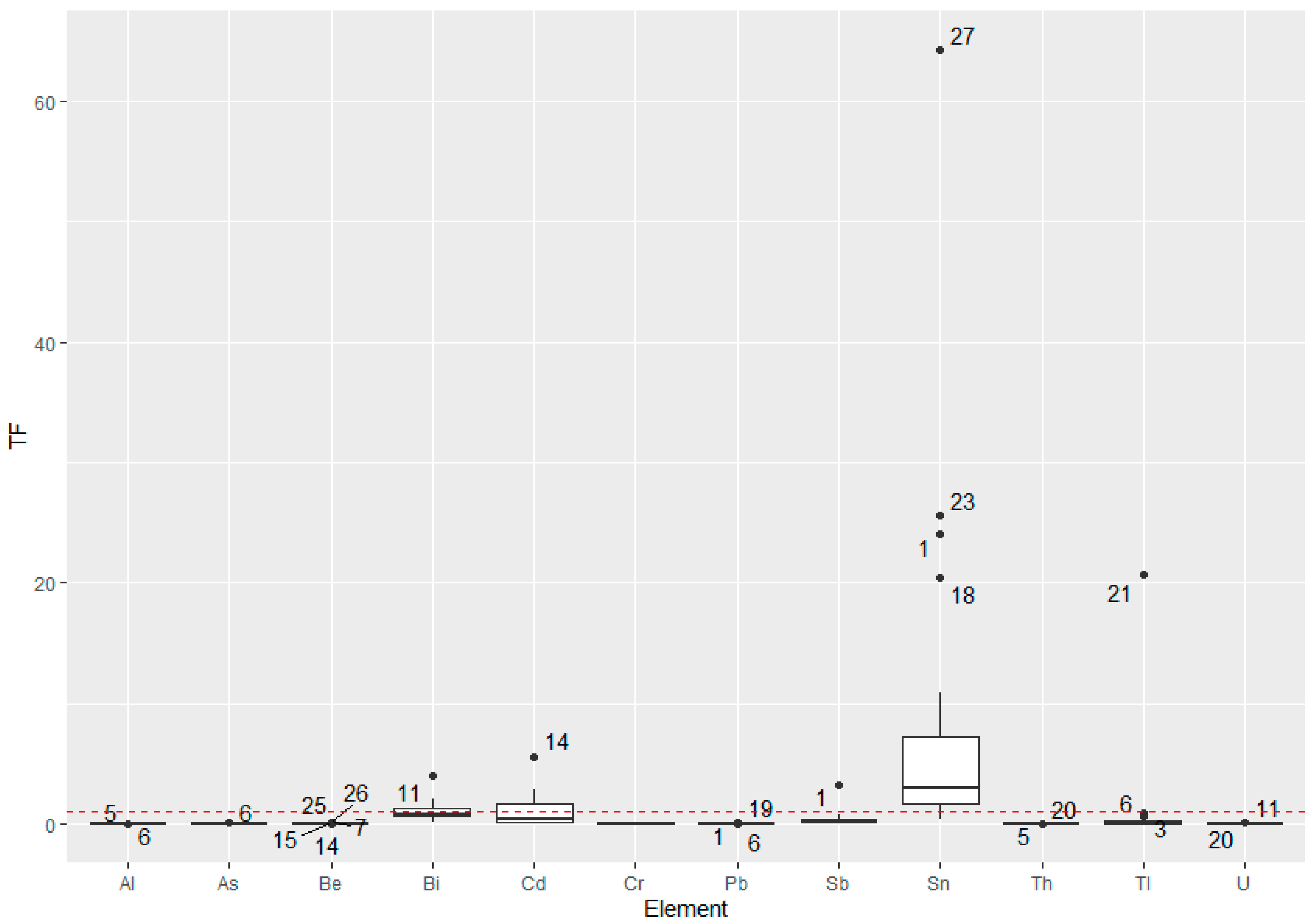
3.4. Soil-to-Plant Transfer Factor (TF) of the Elements
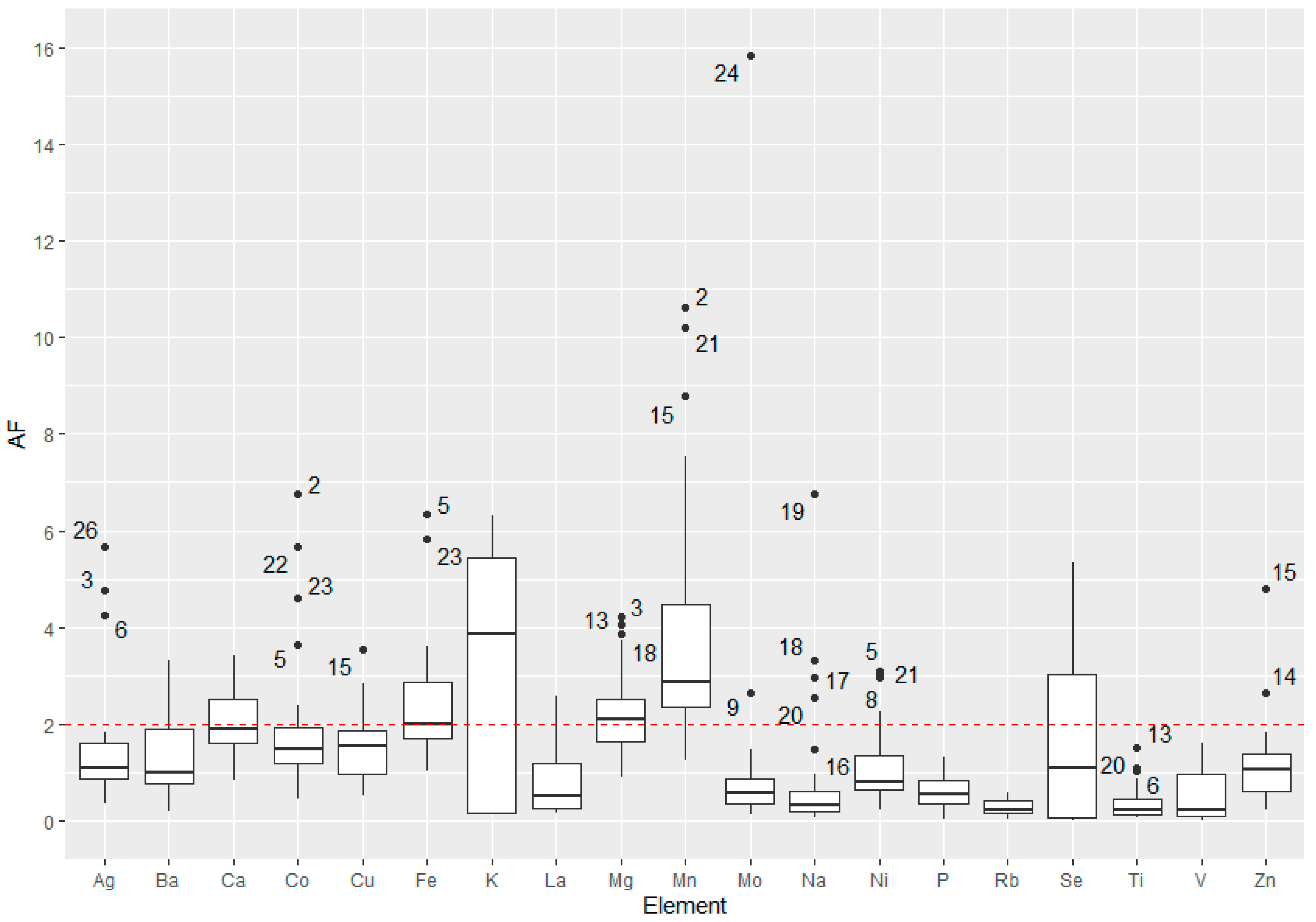
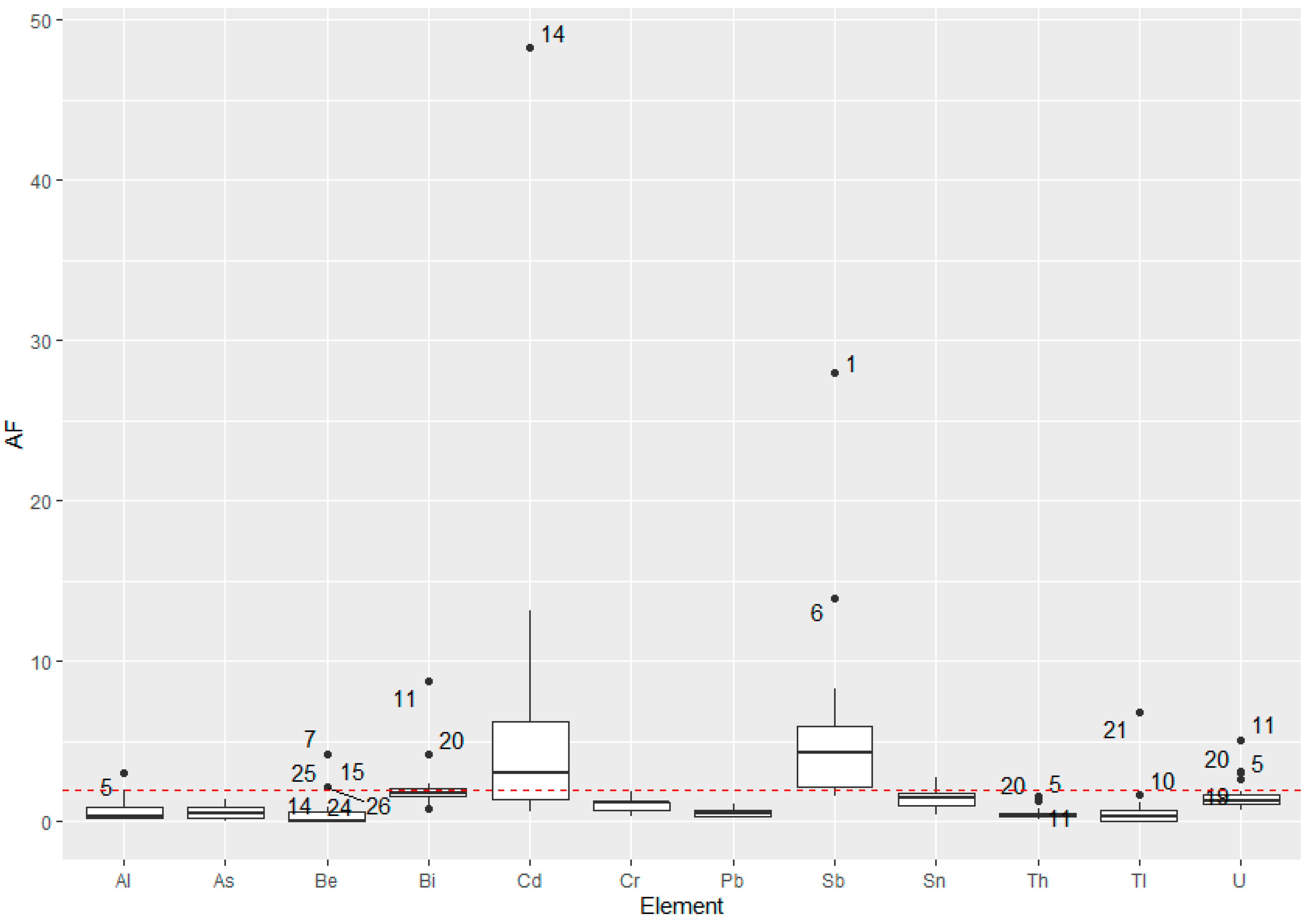
3.5. Accumulation Factor of the Elements
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Orosun, M.M.; Tchokossa, P.; Nwankwo, L.I.; Lawal, T.O.; Bello, S.A.; Ige, S.O. Assessment of heavy metal pollution in drinking water due to mining and smelting activities in Ajaokuta. Niger. J. Technol. Dev. 2016, 13, 30–38. [Google Scholar] [CrossRef]
- Alamgir, M.; Islam, M.; Hossain, N.; Kibria, M.G.; Rahman, M.M. Assessment of heavy metal contamination in urban soils of Chittagong city, Bangladesh. Int. J. Plant Soil Sci. 2015, 7, 362–372. [Google Scholar] [CrossRef]
- De Araújo, S.N.; Ramos, S.J.; Martins, G.C.; Teixeira, R.A.; de Souza, E.S.; Sahoo, P.K.; Fernandes, A.R.; Gastauer, M.; Caldeira, C.F.; Souza-Filho, P.W.M.; et al. Copper mining in the eastern Amazon: An environmental perspective on potentially toxic elements. Environ. Geochem. Health 2022, 44, 1767–1981. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.Y.; Ali, B.N.M.; Tair, R.; Musta, B.; Abdullah, M.H.; Cleophas, F.; Isidore, F.; Nadzir, M.S.M.; Roselee, M.H.; Yusoff, I. Distance impacts toxic metals pollution in mining affected river sediments. Environ. Res. 2022, 214 Pt 1, 113757. [Google Scholar] [CrossRef]
- Rubinos, D.A.; Jerez, Ó.; Forghani, G.; Edraki, M.; Kelm, U. Geochemical stability of potentially toxic elements in porphyry copper-mine tailings from Chile as linked to ecological and human health risks assessment. Environ. Sci. Pollut. Res. Int. 2021, 28, 57499–57529. [Google Scholar] [CrossRef]
- Tchounwou, P.B.; Yedjou, C.G.; Patlolla, A.K.; Sutton, D.J. Heavy Metal Toxicity and the Environment. Exp. Suppl. 2012, 101, 133–164. [Google Scholar]
- Kierczak, J.; Pietranik, A.; Pędziwiatr, A. Ultramafic geoecosystems as a natural source of Ni, Cr, and Co to the environment: A review. Sci. Total Environ. 2021, 755 Pt 1, 142620. [Google Scholar] [CrossRef]
- Ettler, V.; Stepanek, D.; Mihaljevic, M.; Drahota, P.; Jedlicka, R.; Kribek, B.; Vanek, A.; Penizek, V.; Sracek, O.; Nyambe, I. Slag dusts from Kabwe (Zambia): Contaminant mineralogy and oral bioaccessibility. Chemosphere 2020, 260, 127642. [Google Scholar] [CrossRef]
- Eulises, C.S.J.; Gonzalez-Chavez, M.D.C.A.; Carrillo-Gonzalez, R.; Garcıa-Cue, J.L.; Fernandez-Reynoso, D.S.; Noerpeld, M.; Scheckeld, K.G. Bioaccessibility of potentially toxic elements in mine residue particles. Environ. Sci. Process. Impacts 2021, 23, 367–380. [Google Scholar] [CrossRef]
- Bačeva, K.; Stafilov, T.; Matevski, V. Bioaccumulation of heavy metals by endemic Viola species from the soil in the vicinity of the As-Sb-Tl mine “allchar” Republic of Macedonia. Int. J. Phytoremediat. 2014, 16, 347–365. [Google Scholar] [CrossRef]
- Bešter, P.K.; Lobnik, F.; Eržen, I.; Kastelec, D.; Zupan, M. Prediction of cadmium concentration in selected home-produced vegetables. Ecotoxicol. Environ. Saf. 2013, 96, 182–190. [Google Scholar] [CrossRef]
- Pinto, E.; Almeida, A.A.; Aguiar, A.A.R.M.; Ferreira, I.M.P.L.V.O. Changes in macrominerals, trace elements and pigments content during lettuce (Lactuca sativa L.) growth: Influence of soil composition. Food Chem. 2014, 152, 603–611. [Google Scholar] [CrossRef]
- Roivainen, P.; Makkonen, S.; Holopainen, T.; Juutilainen, J. Element interactions and soil properties affecting the soil-to-plant transfer of 6 elements relevant to radioactive waste in boreal forest. Radiat. Environ. Biophys. 2012, 51, 69–78. [Google Scholar] [CrossRef] [PubMed]
- Adamo, P.; Iavazzo, P.; Albanese, S.; Agrelli, D.; De Vivo, B.; Lima, A. Bioavailability and soil-to-plant transfer factors as indicators of potentially toxic element contamination in agricultural soils. Sci. Total Environ. 2014, 500–501, 11–22. [Google Scholar] [CrossRef] [PubMed]
- Lin, L.; Yu, B.; Yan, X.; Liao, X.; Zhang, Y. Accumulation of soil Cd, Cr, Cu, Pb by Panax notoginseng and its associated health risk. Acta Ecol. Sin. 2014, 34, 2868–2875. [Google Scholar]
- Wanat, N.; Joussein, E.; Soubrand, M.; Lenain, J.F. Arsenic (As), antimony (Sb), and lead (Pb) availability from Au-mine Technosols: A case study of transfer to natural vegetation cover in temperate climates. Environ. Geochem. Health 2014, 36, 783–795. [Google Scholar] [CrossRef] [PubMed]
- Alhogbi, B.G.; Al-Ansari, S.A.; El-Shahawi, M.S. A Comparative Study on the Bioavailability and Soil-to-Plant Transfer Factors of Potentially Toxic Element Contamination in Agricultural Soils and Their Impacts: A Case Study of Dense Farmland in the Western Region of Saudi Arabia. Processes 2023, 11, 2515. [Google Scholar] [CrossRef]
- Antoniadis, V.; Shaheen, S.M.; Boersch, J.; Frohne, T.; Du Laing, G.; Rinklebe, J. Bioavailability and risk assessment of potentially toxic elements in garden edible vegetables and soils around a highly contaminated former mining area in Germany. J. Environ. Manag. 2017, 186, 192–200. [Google Scholar] [CrossRef]
- Nath, R. Health and Desease: Role of Micronutrients and Trace Elements; A.P.H. Publishing Corporation: New Delhi, India, 2000; p. 639. [Google Scholar]
- Takuchev, N. Contribution of air pollutants above the city of Stara Zagora, Bulgaria on non-oncological morbidity of the population in the region of skin and subcutaneous tissue diseases. In Proceedings of the International Scientific Conference, Gabrovo, Bulgaria, 18–19 November 2011. [Google Scholar]
- Popov, P.; Strashimirov, S.; Popov, K.; Petrunov, R.; Kanazirski, M.; Tzonev, D. Main features in geology and metallogeny of the Panagyurishte ore region. An. Univ. Min. Geol. 2003, 46, 119–125. [Google Scholar]
- Djingova, R.; Kuleff, I. Seasonal variations in the metal concentration in the leaves of Taraxacum officinale, Plantago major and Plantago lanceolata. Chem. Ecol. 1999, 16, 239–253. [Google Scholar] [CrossRef]
- Djingova, R.; Ivanova, J.; Wagner, G.; Korhammer, S.; Markert, B. Distribution of lanthanoids, Be, Bi, Ga, Te, Tl, Th and U, on the territory of Bulgaria using Populus nigra ‘Italica’ as an indicator. Sci. Total Environ. 2001, 280, 85–91. [Google Scholar] [CrossRef] [PubMed]
- Djingova, R.; Kuleff, I.; Andreev, N. Comparison of the ability of several vascular plants to reflect environmental pollution. Chemosphere 1993, 27, 1385–1396. [Google Scholar] [CrossRef]
- Djingova, R.; Kuleff, I.; Markert, B. Chemical fingerprinting of plants. Ecol. Res. 2004, 19, 3–11. [Google Scholar] [CrossRef]
- Charnowska, K.; Milewska, A. The content of heavy metals in an indicator plant (Taraxacum officinale) in Warsaw. Polish J. Environ. Stud. 2000, 9, 125–128. [Google Scholar]
- Keane, B.; Collier, M.H.; Rogstad, S.H. Pollution and genetic structure of North American populations of the common Dandelion (Taraxacum officinale). Environ. Monit. Assess. 2005, 105, 341–357. [Google Scholar] [CrossRef]
- Petrova, S.; Yurukova, L.; Velcheva, I. Taraxacum officinale as a biomonitor of metals and toxic elements (Plovdiv, Bulgaria). Bulg. J. Agric. Sci. 2013, 19, 241–247. [Google Scholar]
- Kleckerová, A.; Dočekalová, H. Dandelion Plants as a Biomonitor of Urban Area Contamination by Heavy Metals. Int. J. Environ. Res. 2014, 8, 157–164. [Google Scholar]
- Fröhlichová, A.; Száková, J.; Najmanová, J.; Tlustoš, P. An assessment of the risk of element contamination of urban and industrial areas using Taraxacum sect. Ruderalia as a bioindicator. Environ. Monit. Assess. 2018, 190, 150. [Google Scholar]
- Vanni, G.; Cardelli, R.; Marchini, F.; Saviozzi, A.; Guidi, L. Are the Physiological and Biochemical Characteristics in Dandelion Plants Growing in an Urban Area (Pisa, Italy) Indicative of Soil Pollution? Water Air Soil Pollut. 2015, 226, 124. [Google Scholar] [CrossRef]
- Bretzel, F.; Benvenuti, S.; Pistelli, L. Metal contamination in urban street sediment in Pisa (Italy) can affect the production of antioxidant metabolites in Taraxacum officinale Weber. Environ. Sci. Pollut. Res. Int. 2014, 21, 2325–2333. [Google Scholar] [CrossRef]
- Savinov, A.B.; Kurganova, L.N.; Shekunov, Y.I. Lipid peroxidation rates in Taraxacum officinale Wigg. and Vicia cracca L. from biotopes with different levels of soil pollution with heavy metals. Russ. J. Ecol. 2007, 38, 174–180. [Google Scholar] [CrossRef]
- Ackova, D.G.; Kadifkova-Panovska, T.; Andonovska, K.B.; Stafilov, T. Evaluation of genotoxic variations in plant model systems in a case of metal stressors. J. Environ. Sci. Health B 2016, 51, 340–349. [Google Scholar] [CrossRef] [PubMed]
- Collier, M.H.; Keane, B.; Rogstad, S.H. Productivity differences between dandelion (Taraxacum officinale; Asteraceae) clones from pollution impacted versus nonimpacted soils. Plant Soil 2010, 329, 173–183. [Google Scholar] [CrossRef]
- Collier, M.H.; Boughter, S.A.; Dameron, M.P.; Gribbins, K.M.; Keane, B.; Shann, J.R.; Rogstad, S.H. Uptake and distribution of copper, lead, and zinc in dandelions (Taraxacum officinale; Asteraceae) sampled from polluted and nonpolluted soils. J. Torrey Bot. Soc. 2017, 144, 47–57. [Google Scholar] [CrossRef]
- ISO 10390:2021; Soil, Treated Biowaste and Sludge-Determination of pH. ISO: Geneva, Switzerland, 2021.
- ISO 23470:2018; Soil Quality—Determination of Effective Cation Exchange Capacity (CEC) and Exchangeable Cations Using a Hexamminecobalt(III)chloride Solution. ISO: Geneva, Switzerland, 2018.
- Mihaylova, V.; Lyubomirova, V.; Djingova, R. Optimization of sample preparation and ICP-MS analysis for determination of 60 elements for characterization of the plant ionome. Int. J. Environ. Anal. Chem. 2013, 93, 1441–1456. [Google Scholar] [CrossRef]
- Lyubomirova, V.; Mihaylova, V.; Djingova, R. Effects of soil properties and anthropogenic activity on the transfer of 52 elements in the system soil/Taraxacum officinale. J. Soils Sediments 2015, 15, 1549–1557. [Google Scholar] [CrossRef]
- US EPA 3051a. Method 3051a: Microwave Assisted Acid Digestion of Sediments, Sludges, Soils, and Oils. Available online: https://www.epa.gov/esam/us-epa-method-3051a-microwave-assisted-acid-digestion-sediments-sludges-and-oils (accessed on 1 January 2023).
- Lyubomirova, V.; Mihaylova, V.; Djingova, R. Determination of macroelements in potable waters with cell-based inductively-coupled plasma mass spectrometry. Spectrosc. Eur. 2020, 32, 18–21. [Google Scholar]
- Lyubomirova, V.; Djingova, R. Determination of Se in Bulgarian commercial flour and bread. Compt. Rend. Acad. Bulg. Sci. 2015, 68, 847–852. [Google Scholar]
- Venelinov, T.; Mihaylovam, V.; Peycheva, R.; Todorov, M.; Yotova, G.; Todorov, B.; Lyubomirova, V.; Tsakovski, S. Sediment Assessment of the Pchelina Reservoir, Bulgaria. Molecules 2021, 26, 7517. [Google Scholar] [CrossRef]
- Rodushkin, I.; Ruth, T.; Huhtasaari, A. Comparison of two digestion methods for elemental determinations in plant material by ICP techniques. Anal. Chim. Acta 1999, 378, 191–200. [Google Scholar] [CrossRef]
- Rodushkin, I.; Engstrom, E.; Sorlin, D.; Baxter, D. Levels of inorganic constituents in raw nuts and seeds on the Swedish market. Sci. Total Environ. 2008, 392, 290–304. [Google Scholar] [CrossRef] [PubMed]
- Ivanova, J.; Korhammer, S.; Djingova, R.; Heidenreich, H.; Markert, B. Determination of lanthanoids and some heavy and toxic elements in plant certified reference materials by inductively coupled plasma mass spectrometry. Spectrochim. Acta B 2001, 56, 3–12. [Google Scholar] [CrossRef]
- Platikanov, S.; Rodriguez-Mozaz, S.; Huerta, B.; Barceló, D.; Cros, J.; Batle, M.; Poch, G.; Tauler, R. Chemometrics quality assessment of wastewater treatment plant effluents using physicochemical parameters and UV absorption measurements. J. Environ. Manag. 2014, 140, 33–44. [Google Scholar] [CrossRef]
- Acquavita, A.; Aleffi, I.F.; Benci, C.; Bettoso, N.; Crevatin, E.; Milani, L.; Tamberlich, F.; Toniatti, L.; Barbieri, P.; Licen, S.; et al. Annual characterization of the nutrients and trophic state in a Mediterranean coastal lagoon: The Marano and Grado Lagoon (northern Adriatic Sea). Reg. Stud. Mar. Sci. 2015, 2, 132–144. [Google Scholar] [CrossRef]
- Yotova, G.; Lazarova, S.; Kudłak, B.; Zlateva, B.; Mihaylova, V.; Wieczerzak, M.; Venelinov, T.; Tsakovski, S. Assessment of the Bulgarian Wastewater Treatment Plants’ Impact on the Receiving Water Bodies. Molecules 2019, 24, 2274. [Google Scholar] [CrossRef]
- Mihaylova, V.; Yotova, G.; Kudłak, B.; Venelinov, T.; Tsakovski, S. Chemometric Evaluation of WWTPs’ Wastewaters and Receiving Surface Waters in Bulgaria. Water 2022, 14, 521. [Google Scholar] [CrossRef]
- Ministry of Health-Care; Ministry of Agriculture and Forestry. Regulation No. 3/2008 of the Ministry of Environment and Water, Ministry of Health-Care, Ministry of Agriculture and Forestry; Ministry of Health-Care: Sofia, Bulgaria; Ministry of Agriculture and Forestry: Sofia, Bulgaria, 2008. [Google Scholar]
- Tsukada, H.; Nakamura, Y. Transfer factors of 31 elements in several agricultural plants collected from 150 farm fields in Aomori, Japan. J. Radioanal. Nucl. Chem. 1998, 236, 123–131. [Google Scholar] [CrossRef]
- Lyubomirova, V.; Mihaylova, V.; Djingova, R. Changes in the ionome of Taraxacum officinale under different anthropogenic influences. Phytol. Balcan. 2014, 20, 247–255. [Google Scholar]
- Yotova, G.; Zlateva, B.; Ganeva, S.; Simeonov, V.; Kudlak, B.; Namiesnik, J.; Tsakovski, S. Phytoavailability of potentially toxic elements from industrially contaminated soils to wild grass. Ecotoxicol. Environ. Saf. 2018, 164, 317–324. [Google Scholar] [CrossRef]
- Romero-Baena, A.J.; Barba-Brioso, C.; Ross, A.; Gonzalez, I.; Aparicio, P. Mobility of potentially toxic elements in family garden soils of the Riotinto mining area. Appl. Clay Sci. 2021, 203, 105999. [Google Scholar] [CrossRef]
- Leng, Q.; Ren, D.; Wang, Z.; Zhang, S.; Zhang, X.; Chen, W. Assessment of Potentially Toxic Elements Pollution and Human Health Risks in Polluted Farmland Soils around Distinct Mining Areas in China—A Case Study of Chengchao and Tonglushan. Toxics 2023, 11, 574. [Google Scholar] [CrossRef] [PubMed]
- Bini, C.; Wahsha, M.; Fontana, S.; Maleci, L. Effects of heavy metals on morphological characteristics of Taraxacum officinale Web growing on mine soils in NE Italy. J. Geochem. Explor. 2012, 123, 101–108. [Google Scholar] [CrossRef]
- Kabata-Pendias, A.; Pendias, H. Trace Elements in Soils and Plants; CRC Press: Boca Raton, FL, USA, 1984. [Google Scholar]

| ICP-MS | Instrumental Conditions |
|---|---|
| Argon plasma gas flow | 15 L/min |
| Auxiliary gas flow | 1.20 L/min |
| Nebulizer gas flow | 0.90 L/min |
| Lens voltage | 6.00 V |
| ICP-RF power | 1100 W |
| Pulse stage voltage | 950 V |
| Dwell time | 50 ms |
| Acquisition mode | Peak hop |
| Peak pattern | One point per mass at maximum peak |
| Number of runs | 4 |
| Determined isotopes of major elements (RPa, V) | 27Al(0.015),42,43,44Ca(42Ca-0.014;43Ca-0.013; 44Ca-0.015),54,56,57Fe(54Fe-0.014;56Fe-0.015; 57Fe-0.013),23Na(0.017),24,25,26Mg(24Mg-0.016,25,26Mg-0.015),55Mn(0.015),39K(0.017),31P(0.013),46,47,48,49Ti (0.012) |
| Determined isotopes of micro- and trace elements *(Oxygen flow rate [mL/min]/RPq [V]) | 107Ag, 75As (1.0/0.7) *, 136,138Ba, 9Be, 112,114Cd, 59Co, 52Cr, 63,65Cu, 139La, 96,98Mo, 60,62Ni, 207,208Pb, 85,87Rb, 121,123Sb, 77,78Se (1.5/0.3) *, 80,82Se (0.9/0.3) *, 118,120Sn, 232Th, 205Tl, 238U, 51V, 66Zn |
| Soil Sample | CEC [meq] | pH (H2O) | pH (KCl) |
|---|---|---|---|
| 1 | 17.0 | 6.35 | 5.08 |
| 2 | 10.5 | 6.20 | 4.49 |
| 3 | 13.4 | 6.16 | 5.30 |
| 4 | 13.8 | 6.71 | 5.61 |
| 5 | 9.6 | 6.23 | 4.98 |
| 6 | 7.8 | 6.75 | 5.72 |
| 7 | 17.6 | 6.72 | 5.94 |
| 8 | 7.1 | 6.87 | 5.79 |
| 9 | 22.5 | 6.31 | 5.80 |
| 10 | 7.4 | 7.29 | 6.62 |
| 11 | 21.9 | 6.76 | 6.22 |
| 12 | 19.6 | 6.86 | 6.04 |
| 13 | 16.0 | 6.96 | 5.55 |
| 14 | 7.2 | 6.18 | 4.85 |
| 15 | 11.0 | 6.41 | 5.39 |
| 16 | 20.3 | 7.08 | 6.16 |
| 17 | 14.4 | 6.83 | 5.97 |
| 18 | 23.7 | 6.98 | 6.69 |
| 19 | 28.1 | 7.55 | 7.16 |
| 20 | 17.9 | 6.76 | 5.78 |
| 21 | 13.7 | 6.55 | 5.55 |
| 22 | 25.6 | 7.61 | 7.22 |
| 23 | 22.3 | 7.39 | 6.65 |
| 24 | 16.3 | 5.95 | 4.45 |
| 25 | 11.8 | 7.43 | 6.18 |
| 26 | 7.1 | 6.83 | 6.03 |
| 27 | 18.2 | 6.60 | 6.28 |
| Soil Samples | Plant Samples | |||||
|---|---|---|---|---|---|---|
| Element [mg/kg] | Mean | Median | (Min–Max) | Mean | Median | (Min–Max) |
| Ag | 0.62 | 0.21 | 0.002–8.89 | 0.19 | 0.14 | 0.044–0.71 |
| Al | 32,125 | 33,675 | 12,491–57,054 | 196 | 84 | 33.8–943 |
| As | 5.24 | 4.93 | 1.36–9.87 | 0.083 | 0.067 | 0.001–0.21 |
| Ba | 159 | 145 | 74–303 | 29.3 | 20.5 | 4.03–68.9 |
| Be | 1.23 | 1.33 | 0.36–2.68 | 0.022 | 0.001 | 0.001–0.15 |
| Bi | 0.53 | 0.52 | 0.24–1.10 | 0.50 | 0.42 | 0.18–2.10 |
| Ca | 8134 | 7882 | 1996–26,874 | 21,261 | 19,641 | 8848–35,366 |
| Cd | 0.97 | 0.70 | 0.14–6.25 | 0.62 | 0.33 | 0.061–5.31 |
| Co | 22.7 | 21.3 | 6.70–58.9 | 0.34 | 0.25 | 0.07–1.15 |
| Cr | 39.1 | 37.4 | 15.3–74.0 | 2.17 | 2.47 | 0.60–4.01 |
| Cu | 386 | 300 | 51.9–1288 | 18.2 | 17.8 | 6.14–40.8 |
| Fe | 41,069 | 41,387 | 13,989–97,297 | 334 | 267 | 138–854 |
| K | 3344 | 2957 | 1081–6076 | 33,444 | 39,417 | 1769–64,474 |
| La | 20.4 | 19.1 | 11.5–61.8 | 0.31 | 0.18 | 0.062–0.93 |
| Mg | 11,516 | 11,066 | 2670–25,999 | 6147 | 5689 | 2438–11,364 |
| Mn | 1321 | 1114 | 561–5461 | 109 | 80.1 | 35.5–298 |
| Mo | 3.92 | 2.57 | 0.99–14.6 | 1.58 | 0.73 | 0.15–19.8 |
| Na | 880 | 713 | 169–2493 | 447 | 164 | 35.2–3382 |
| Ni | 27.9 | 25.5 | 11.6–59.6 | 1.07 | 0.76 | 0.22–2.89 |
| P | 665 | 637 | 157–2148 | 2359 | 2171 | 198–5294 |
| Pb | 33.2 | 30.2 | 12.5–101 | 0.80 | 0.83 | 0.30–1.61 |
| Rb | 15.3 | 13.7 | 7.6–38.4 | 18.9 | 14.8 | 2.47–39.8 |
| Sb | 0.17 | 0.14 | 0.024–0.47 | 0.037 | 0.029 | 0.011–0.195 |
| Se | 6.04 | 5.08 | 0.003–21.6 | 0.29 | 0.19 | 0.001–0.91 |
| Sn | 0.66 | 0.49 | 0.020–2.83 | 1.43 | 1.46 | 0.39–2.75 |
| Th | 4.30 | 4.11 | 2.45–12.4 | 0.034 | 0.039 | 0.008–0.109 |
| Ti | 758 | 567 | 85.9–2199 | 6.96 | 4.11 | 1.54–28.9 |
| Tl | 0.08 | 0.09 | 0.009–0.21 | 0.018 | 0.007 | 0.001–0.205 |
| U | 1.06 | 0.86 | 0.57–3.40 | 0.031 | 0.026 | 0.013–0.100 |
| V | 112 | 107 | 36.0–185 | 0.40 | 0.19 | 0.004–1.31 |
| Zn | 133 | 127 | 36.4–340 | 61.1 | 55.5 | 12.4–249 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mihaylova, V.; Yotova, G.; Marinova, K.; Benderev, A.; Lyubomirova, V.; Tsakovski, S. Biomonitoring of Potentially Toxic Elements in an Abandoned Mining Region Using Taraxacum officinale: A Case Study on the “Tsar Asen” Mine in Bulgaria. Appl. Sci. 2023, 13, 11860. https://doi.org/10.3390/app132111860
Mihaylova V, Yotova G, Marinova K, Benderev A, Lyubomirova V, Tsakovski S. Biomonitoring of Potentially Toxic Elements in an Abandoned Mining Region Using Taraxacum officinale: A Case Study on the “Tsar Asen” Mine in Bulgaria. Applied Sciences. 2023; 13(21):11860. https://doi.org/10.3390/app132111860
Chicago/Turabian StyleMihaylova, Veronika, Galina Yotova, Kristina Marinova, Aleksey Benderev, Valentina Lyubomirova, and Stefan Tsakovski. 2023. "Biomonitoring of Potentially Toxic Elements in an Abandoned Mining Region Using Taraxacum officinale: A Case Study on the “Tsar Asen” Mine in Bulgaria" Applied Sciences 13, no. 21: 11860. https://doi.org/10.3390/app132111860
APA StyleMihaylova, V., Yotova, G., Marinova, K., Benderev, A., Lyubomirova, V., & Tsakovski, S. (2023). Biomonitoring of Potentially Toxic Elements in an Abandoned Mining Region Using Taraxacum officinale: A Case Study on the “Tsar Asen” Mine in Bulgaria. Applied Sciences, 13(21), 11860. https://doi.org/10.3390/app132111860







