Impact of Peri-Implant Inflammation on Metabolic Syndrome Factors: A Systematic Review
Abstract
:1. Introduction
- Waist circumference (WC) ≥102 cm for males or ≥99 cm for females.
- High-density lipoprotein cholesterol (HDL-C) <1.0 mmol/L (40 mg/dL) for males or <1.3 mmol/L (50 mg/dL) for females.
- Triglyceride ≥ 1.69 mmol/L (150 mg/dL).
- Fasting plasma glucose ≥ 110 mg/dL (6.1 mmol/L).
- Blood pressure ≥ 130/85 mmHg.
2. Materials and Methods
2.1. Focused Question
- Population: Patients with osseointegrated dental implants.
- Intervention (exposure): Patients affected by peri-implantitis.
- Comparison: Patients with healthy peri-implant conditions.
- Outcome: Systemic metabolic factors in relation to MetS and its components.
2.2. Eligible Criteria and Study Selection
2.3. Information Source and Search Strategy
2.4. Data Management, Selection Process, and Data Synthesis
2.5. Quality and Risk of Bias Assessment
3. Results
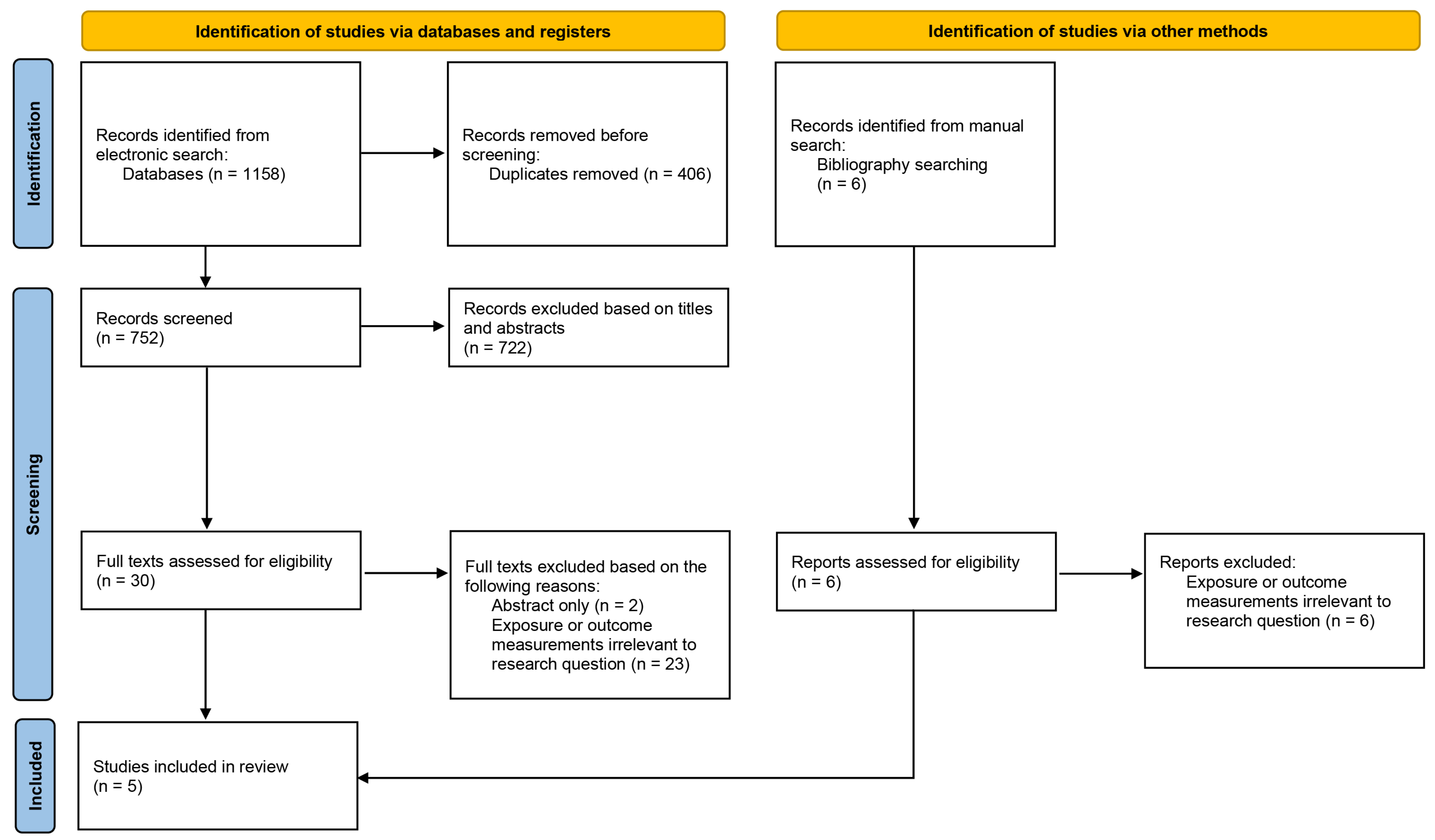
3.1. Study Selection
3.2. Study Characteristics
3.3. Peri-Implantitis and Dyslipidemia
3.4. Peri-Implantitis and Hyperglycemia
3.5. Peri-Implantitis and BMI
3.6. Peri-Implantitis and Hypertension
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Branemark, P.I.; Adell, R.; Breine, U.; Hansson, B.O.; Lindstrom, J.; Ohlsson, A. Intra-osseous anchorage of dental prostheses. I. Experimental studies. Scand. J. Plast. Reconstr. Surg. 1969, 3, 81–100. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2017, 73, 7–21. [Google Scholar] [CrossRef] [PubMed]
- Jivraj, S.; Chee, W. Rationale for dental implants. Br. Dent. J. 2006, 200, 661–665. [Google Scholar] [CrossRef] [PubMed]
- Elani, H.W.; Starr, J.R.; Da Silva, J.D.; Gallucci, G.O. Trends in Dental Implant Use in the U.S., 1999-2016, and Projections to 2026. J. Dent. Res. 2018, 97, 1424–1430. [Google Scholar] [CrossRef]
- Schwarz, F.; Derks, J.; Monje, A.; Wang, H.L. Peri-implantitis. J. Clin. Periodontol. 2018, 45 (Suppl. S20), S246–S266. [Google Scholar] [CrossRef] [PubMed]
- Lee, C.T.; Huang, Y.W.; Zhu, L.; Weltman, R. Prevalences of peri-implantitis and peri-implant mucositis: Systematic review and meta-analysis. J. Dent. 2017, 62, 1–12. [Google Scholar] [CrossRef]
- Koldsland, O.C.; Scheie, A.A.; Aass, A.M. Prevalence of peri-implantitis related to severity of the disease with different degrees of bone loss. J. Periodontol. 2010, 81, 231–238. [Google Scholar] [CrossRef]
- Derks, J.; Tomasi, C. Peri-implant health and disease. A systematic review of current epidemiology. J. Clin. Periodontol. 2015, 42 (Suppl. S16), S158–S171. [Google Scholar] [CrossRef] [PubMed]
- Renvert, S.; Aghazadeh, A.; Hallstrom, H.; Persson, G.R. Factors related to peri-implantitis—A retrospective study. Clin. Oral. Implant. Res. 2014, 25, 522–529. [Google Scholar] [CrossRef] [PubMed]
- Ardakani, M.S.Z.; Salari, A.M.; Nasre-Esfahani, M. The relationship between cardiovascular diseases and peri-implantitis in patients with failed dental implants; a retrospective study. J. Dent. Med. 2020, 33, 152–157. [Google Scholar]
- Krennmair, S.; Weinlander, M.; Forstner, T.; Krennmair, G.; Stimmelmayr, M. Factors affecting peri-implant bone resorption in four Implant supported mandibular full-arch restorations: A 3-year prospective study. J. Clin. Periodontol. 2016, 43, 92–101. [Google Scholar] [CrossRef] [PubMed]
- Ustaoglu, G.; Erdal, E. Relationship between risk markers for cardiovascular disease and peri-implant diseases. Int. J. Implant. Dent. 2020, 6, 73. [Google Scholar] [CrossRef] [PubMed]
- Abduljabbar, T.; Al-Sahaly, F.; Kellesarian, S.V.; Kellesarian, T.V.; Al-Anazi, M.; Al-Khathami, M.; Javed, F.; Vohra, F. Comparison of peri-implant clinical and radiographic inflammatory parameters and whole salivary destructive inflammatory cytokine profile among obese and non-obese men. Cytokine 2016, 88, 51–56. [Google Scholar] [CrossRef] [PubMed]
- Huang, X.; Yu, T.; Ma, C.; Wang, Y.; Xie, B.; Xuan, D.; Zhang, J. Macrophages Play a Key Role in the Obesity-Induced Periodontal Innate Immune Dysfunction via Nucleotide-Binding Oligomerization Domain-Like Receptor Protein 3 Pathway. J. Periodontol. 2016, 87, 1195–1205. [Google Scholar] [CrossRef] [PubMed]
- Alasqah, M.N.; Al-Shibani, N.; Al-Aali, K.A.; Qutub, O.A.; Abduljabbar, T.; Akram, Z. Clinical indices and local levels of inflammatory biomarkers in per-implant health of obese and nonobese individuals. Clin. Implant Dent. Relat. Res. 2019, 21, 80–84. [Google Scholar] [CrossRef] [PubMed]
- Alkhudhairy, F.; Vohra, F.; Al-Kheraif, A.A.; Akram, Z. Comparison of clinical and radiographic peri-implant parameters among obese and non-obese patients: A 5-year study. Clin. Implant Dent. Relat. Res. 2018, 20, 756–762. [Google Scholar] [CrossRef]
- Brignardello-Petersen, R. Patients who are obese probably have worse peri-implant outcomes than those who are not, but there is not sufficient evidence that the implant survival is different. J. Am. Dent. Assoc. 2019, 150, e123. [Google Scholar] [CrossRef] [PubMed]
- Elsadek, M.F.; Almoajel, A. Assessment of clinical and radiographic peri-implant parameters in the obese and non-obese, along with destructive pro-inflammatory cytokines IL-1beta—And IL-6 in patients treated with Photodynamic therapy. Photodiagnosis Photodyn. Ther. 2022, 39, 102844. [Google Scholar] [CrossRef] [PubMed]
- Vohra, F.; Alkhudhairy, F.; Al-Kheraif, A.A.; Akram, Z.; Javed, F. Peri-implant parameters and C-reactive protein levels among patients with different obesity levels. Clin. Implant Dent. Relat. Res. 2018, 20, 130–136. [Google Scholar] [CrossRef]
- Dreyer, H.; Grischke, J.; Tiede, C.; Eberhard, J.; Schweitzer, A.; Toikkanen, S.E.; Glockner, S.; Krause, G.; Stiesch, M. Epidemiology and risk factors of peri-implantitis: A systematic review. J. Periodontal Res. 2018, 53, 657–681. [Google Scholar] [CrossRef]
- Monje, A.; Catena, A.; Borgnakke, W.S. Association between diabetes mellitus/hyperglycaemia and peri-implant diseases: Systematic review and meta-analysis. J. Clin. Periodontol. 2017, 44, 636–648. [Google Scholar] [CrossRef]
- Al-Sowygh, Z.H.; Ghani, S.M.A.; Sergis, K.; Vohra, F.; Akram, Z. Peri-implant conditions and levels of advanced glycation end products among patients with different glycemic control. Clin. Implant Dent. Relat. Res. 2018, 20, 345–351. [Google Scholar] [CrossRef]
- Dioguardi, M.; Cantore, S.; Quarta, C.; Sovereto, D.; Zerman, N.; Pettini, F.; Muzio, L.L.; Cosola, M.D.; Santacroce, L.; Ballini, A. Correlation Between Diabetes Mellitus and Peri-implantitis: A Systematic Review. Endocr. Metab. Immune Disord Drug Targets 2022, 23, 596–608. [Google Scholar] [CrossRef] [PubMed]
- Coelho, P.G.; Pippenger, B.; Tovar, N.; Koopmans, S.J.; Plana, N.M.; Graves, D.T.; Engebretson, S.; van Beusekom, H.M.M.; Oliveira, P.; Dard, M. Effect of Obesity or Metabolic Syndrome and Diabetes on Osseointegration of Dental Implants in a Miniature Swine Model: A Pilot Study. J. Oral. Maxillofac. Surg. 2018, 76, 1677–1687. [Google Scholar] [CrossRef] [PubMed]
- Seki, K.; Hasuike, A.; Hagiwara, Y. Clinical Evaluation of the Relationship Between Systemic Disease and the Time of Onset of Peri-Implantitis: A Retrospective Cohort Study. J. Oral. Implant. 2023, 49, 55–61. [Google Scholar] [CrossRef] [PubMed]
- de Oliveira, P.G.F.P.; Bonfante, E.A.; Bergamo, E.T.P.; de Souza, S.L.S.; Riella, L.; Torroni, A.; Benalcazar Jalkh, E.B.; Witek, L.; Lopez, C.D.; Zambuzzi, W.F.; et al. Obesity/Metabolic Syndrome and Diabetes Mellitus on Peri-implantitis. Trends Endocrinol. Metab. 2020, 31, 596–610. [Google Scholar] [CrossRef] [PubMed]
- Samson, S.L.; Garber, A.J. Metabolic syndrome. Endocrinol. Metab. Clin. N. Am. 2014, 43, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.L. A comprehensive definition for metabolic syndrome. Dis. Model. Mech. 2009, 2, 231–237. [Google Scholar] [CrossRef]
- Expert Panel on Detection, Evaluation; Treatment of High Blood Cholesterol in Adults. Executive Summary of the Third Report of the National Cholesterol Education Program (NCEP) Expert Panel on Detection, Evaluation, and Treatment of High Blood Cholesterol in Adults (Adult Treatment Panel III). JAMA 2001, 285, 2486–2497. [Google Scholar] [CrossRef]
- Cameron, A.J.; Shaw, J.E.; Zimmet, P.Z. The metabolic syndrome: Prevalence in worldwide populations. Endocrinol. Metab. Clin. N. Am. 2004, 33, 351–375, table of contents. [Google Scholar] [CrossRef] [PubMed]
- Saklayen, M.G. The Global Epidemic of the Metabolic Syndrome. Curr. Hypertens. Rep. 2018, 20, 12. [Google Scholar] [CrossRef]
- Wilson, P.W.; Meigs, J.B. Cardiometabolic risk: A Framingham perspective. Int. J. Obes. 2008, 32 (Suppl. S2), S17–S20. [Google Scholar] [CrossRef] [PubMed]
- Wilson, P.W.; D′Agostino, R.B.; Parise, H.; Sullivan, L.; Meigs, J.B. Metabolic syndrome as a precursor of cardiovascular disease and type 2 diabetes mellitus. Circulation 2005, 112, 3066–3072. [Google Scholar] [CrossRef] [PubMed]
- Smits, M.M.; Ioannou, G.N.; Boyko, E.J.; Utzschneider, K.M. Non-alcoholic fatty liver disease as an independent manifestation of the metabolic syndrome: Results of a US national survey in three ethnic groups. J. Gastroenterol. Hepatol. 2013, 28, 664–670. [Google Scholar] [CrossRef] [PubMed]
- McCracken, E.; Monaghan, M.; Sreenivasan, S. Pathophysiology of the metabolic syndrome. Clin. Dermatol. 2018, 36, 14–20. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, R.; Azevedo, I. Chronic inflammation in obesity and the metabolic syndrome. Mediat. Inflamm. 2010, 2010, 289645. [Google Scholar] [CrossRef]
- Papi, P.; Murro, B.D.; Pranno, N.; Bisogni, V.; Saracino, V.; Letizia, C.; Polimeni, A.; Pompa, G. Prevalence of peri-implant diseases among an Italian population of patients with metabolic syndrome: A cross-sectional study. J. Periodontol. 2019, 90, 1374–1382. [Google Scholar] [CrossRef]
- D′Aiuto, F.; Ready, D.; Tonetti, M.S. Periodontal disease and C-reactive protein-associated cardiovascular risk. J. Periodontal Res. 2004, 39, 236–241. [Google Scholar] [CrossRef]
- Fu, Y.W.; Li, X.X.; Xu, H.Z.; Gong, Y.Q.; Yang, Y. Effects of periodontal therapy on serum lipid profile and proinflammatory cytokines in patients with hyperlipidemia: A randomized controlled trial. Clin. Oral. Investig. 2016, 20, 1263–1269. [Google Scholar] [CrossRef]
- Pussinen, P.J.; Vilkuna-Rautiainen, T.; Alfthan, G.; Palosuo, T.; Jauhiainen, M.; Sundvall, J.; Vesanen, M.; Mattila, K.; Asikainen, S. Severe periodontitis enhances macrophage activation via increased serum lipopolysaccharide. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2174–2180. [Google Scholar] [CrossRef]
- Borgnakke, W.S. IDF Diabetes Atlas: Diabetes and oral health—A two-way relationship of clinical importance. Diabetes Res. Clin. Pract. 2019, 157, 107839. [Google Scholar] [CrossRef] [PubMed]
- Moher, D.; Shamseer, L.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; Group, P.-P. Preferred reporting items for systematic review and meta-analysis protocols (PRISMA-P) 2015 statement. Syst. Rev. 2015, 4, 1. [Google Scholar] [CrossRef] [PubMed]
- Borgnakke, W.S.; Ylostalo, P.V.; Taylor, G.W.; Genco, R.J. Effect of periodontal disease on diabetes: Systematic review of epidemiologic observational evidence. J. Clin. Periodontol. 2013, 40 (Suppl. S14), S135–S152. [Google Scholar] [CrossRef]
- Moskalewicz, A.; Oremus, M. No clear choice between Newcastle-Ottawa Scale and Appraisal Tool for Cross-Sectional Studies to assess methodological quality in cross-sectional studies of health-related quality of life and breast cancer. J. Clin. Epidemiol. 2020, 120, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Wells, G.A.; Shea, B.; O’Connell, D.; Peterson, J.; Welch, V.; Losos, M.; Tugwell, P. The Newcastle-Ottawa Scale (NOS) for Assessing the Quality of Nonrandomized Studies in Meta-Analyses. 2000. Available online: https://www.ohri.ca/programs/clinical_epidemiology/oxford.asp (accessed on 24 October 2023).
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Al-Askar, M.; Ajlan, S.; Alomar, N.; Al-Daghri, N.M. Clinical and Radiographic Peri-Implant Parameters and Whole Salivary Interleukin-1beta and Interleukin-6 Levels among Type-2 Diabetic and Nondiabetic Patients with and without Peri-Implantitis. Med. Princ. Pract. 2018, 27, 133–138. [Google Scholar] [CrossRef] [PubMed]
- Blanco, C.; Linares, A.; Dopico, J.; Pico, A.; Sobrino, T.; Leira, Y.; Blanco, J. Peri-implantitis, systemic inflammation, and dyslipidemia: A cross-sectional biochemical study. J. Periodontal Implant. Sci. 2021, 51, 342–351. [Google Scholar] [CrossRef] [PubMed]
- Lucarini, G.; Zizzi, A.; Rubini, C.; Ciolino, F.; Aspriello, S.D. VEGF, Microvessel Density, and CD44 as Inflammation Markers in Peri-implant Healthy Mucosa, Peri-implant Mucositis, and Peri-implantitis: Impact of Age, Smoking, PPD, and Obesity. Inflammation 2019, 42, 682–689. [Google Scholar] [CrossRef] [PubMed]
- Sanchez-Siles, M.; Lucas-Azorin, J.; Salazar-Sanchez, N.; Carbonell-Meseguer, L.; Camacho-Alonso, F. Salivary Concentration of Oxidative Stress Biomarkers in a Group of Patients with Peri-Implantitis: A Transversal Study. Clin. Implant Dent. Relat. Res. 2016, 18, 1015–1022. [Google Scholar] [CrossRef]
- Papi, P.; Letizia, C.; Pilloni, A.; Petramala, L.; Saracino, V.; Rosella, D.; Pompa, G. Peri-implant diseases and metabolic syndrome components: A systematic review. Eur. Rev. Med. Pharmacol. Sci. 2018, 22, 866–875. [Google Scholar]
- Esposito, K.; Giugliano, D. The metabolic syndrome and inflammation: Association or causation? Nutr. Metab. Cardiovasc. Dis. 2004, 14, 228–232. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Murakami, M.; Shimazaki, Y.; Matsumoto, S.; Yamashita, Y. The extent of alveolar bone loss is associated with impaired glucose tolerance in Japanese men. J. Periodontol. 2006, 77, 392–397. [Google Scholar] [CrossRef]
- Saito, T.; Murakami, M.; Shimazaki, Y.; Oobayashi, K.; Matsumoto, S.; Koga, T. Association between alveolar bone loss and elevated serum C-reactive protein in Japanese men. J. Periodontol. 2003, 74, 1741–1746. [Google Scholar] [CrossRef] [PubMed]
- Pirih, F.Q.; Monajemzadeh, S.; Singh, N.; Sinacola, R.S.; Shin, J.M.; Chen, T.; Fenno, J.C.; Kamarajan, P.; Rickard, A.H.; Travan, S.; et al. Association between metabolic syndrome and periodontitis: The role of lipids, inflammatory cytokines, altered host response, and the microbiome. Periodontology 2021, 87, 50–75. [Google Scholar] [CrossRef]
- Assery, N.M.; Jurado, C.A.; Assery, M.K.; Afrashtehfar, K.I. Peri-implantitis and systemic inflammation: A critical update. Saudi Dent. J. 2023, 35, 443–450. [Google Scholar] [CrossRef] [PubMed]
- Gasmi Benahmed, A.; Gasmi, A.; Tippairote, T.; Mujawdiya, P.K.; Avdeev, O.; Shanaida, Y.; Bjorklund, G. Metabolic Conditions and Peri-Implantitis. Antibiotics 2023, 12, 65. [Google Scholar] [CrossRef]
- Papantonopoulos, G.; Gogos, C.; Housos, E.; Bountis, T.; Loos, B.G. Peri-implantitis: A complex condition with non-linear characteristics. J. Clin. Periodontol. 2015, 42, 789–798. [Google Scholar] [CrossRef] [PubMed]
- Preshaw, P.M.; Alba, A.L.; Herrera, D.; Jepsen, S.; Konstantinidis, A.; Makrilakis, K.; Taylor, R. Periodontitis and diabetes: A two-way relationship. Diabetologia 2012, 55, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Haire-Joshu, D.; Glasgow, R.E.; Tibbs, T.L. Smoking and diabetes. Diabetes Care 1999, 22, 1887–1898. [Google Scholar] [CrossRef]
- Renvert, S.; Persson, G.R.; Pirih, F.Q.; Camargo, P.M. Peri-implant health, peri-implant mucositis, and peri-implantitis: Case definitions and diagnostic considerations. J. Periodontol. 2018, 89 (Suppl. S1), S304–S312. [Google Scholar] [CrossRef]
| No. | Query |
|---|---|
| 1 | (peri-implant disease* OR peri-implant mucositis OR peri-implantitis OR peri-implant infection OR peri-implant inflammation).mp. |
| 2 | exp peri-implantitis/ |
| 3 | 1 OR 2 |
| 4 | exp metabolic syndrome/ |
| 5 | exp metabolic diseases/ |
| 6 | exp diabetes mellitus/OR exp diabetes/OR exp blood glucose/OR exp hyperglycaemia/ exp hyperglycemia/OR exp HbA1c/OR hyperglyc?emia.mp. OR diabetes.mp. |
| 7 | exp hypertension/OR exp blood pressure/ |
| 8 | exp obesity/OR exp obesity abdominal/OR exp central obesity/OR exp abdominal obesity/OR abdominal obesity.mp. OR exp body mass index/ |
| 9 | exp triglycerides/OR high triglycerides.mp. |
| 10 | exp dyslipidemia/OR exp Cholesterol/OR exp Cholesterol, HDL/OR exp HDL/OR low HDL.mp. OR exp LDL/OR LDL.mp. |
| 11 | exp cardiovascular diseases/OR chronic heart disease*.mp. OR peripheral arterial disease.mp. OR exp cerebrovascular disease/OR cerebrovascular disease*.mp. OR cardiovascular event*.mp. OR exp stroke/ |
| 12 | metaboli*.mp. |
| 13 | OR/4-12 |
| 14 | 3 AND 13 |
| Study | Participant Population | Exclusion Criteria | Group Settings | Diagnostic Criteria | Outcome Parameters | Results |
|---|---|---|---|---|---|---|
| Sanchez-Siles et al. 2016 [47] Cross-sectional | Participants were classified into three groups: patients with implants affected by peri-implantitis, patients with healthy implants, and healthy subjects without implants or any periodontal diseases. | Peri-implant mucositis; periodontal diseases; age over 70 years; antioxidant-based dietary supplements. | Peri-implantitis (PI, n = 30) Peri-implant health (PH, n = 30) Control without implants (C, n = 10) | Based on the consensus report of the sixth European workshop on periodontology | BMI (mean ± SD) | PI: 27.47 ± 4.05 PH: 27.52 ± 4.81 C: 25.47 ± 2.34 p = 0.378 |
| Al-Askar et al. 2018 [44] Cross-sectional | Type-2 diabetic and nondiabetic patients with and without peri-implantitis All male population | Systemic diseases; antibiotic and/or steroid intake within the previous 3 months; alcohol consumption; periodontal treatment within the previous 3 months; tobacco smoking for at least 12 months. | Nondiabetic with peri-implantitis (NP, n = 39) Nondiabetic without peri-implantitis (NH, n = 52) Type-2 diabetic with peri-implantitis (DP, n = 35) Type-2 diabetic without peri-implantitis (DH, n = 45) | Peri-implantitis: PPD ≥ 5 mm, MBL ≥ 2 mm | HbA1c (%, mean ± SD) | NP: 5.2 ± 0.1 NH: 4.3 ± 0.3 p < 0.001 DP: 9.3 ± 1.5 DH: 4.7 ± 0.1 p < 0.001 |
| Lucarini et al. 2019 [46] Cross-sectional | Patients with healthy implants as well as those with at least one implant in need of peri-implant treatment for inflammatory reasons. | Presence of an important disease (HIV+, HCV+, allergies, etc.); inadequate plaque control; administration of antibiotics or corticosteroids within previous 6 months; chronic periodontitis. | Peri-implantitis (PI, n = 16) Peri-implant mucositis (PM, n = 16) Peri-implant health (PH, n = 16) | Peri-implantitis: PPD ≥ 5 mm with evidence of MBL Peri-implant mucositis: Presence of BOP without evidence of MBL | BMI (mean ± SD) | PI: 33.94 ± 5.71 PM: 32.56 ± 5.88 PH: 29.44 ± 4.34 p = 0.61 |
| Ustaoglu et al. 2020 [12] Cross-sectional | Patients with healthy implants and those diagnosed with peri-implant mucositis and peri-implantitis | Systemic administration of antibiotics for the last 3 months; pregnancy or breast feeding; diabetes mellitus; history of malignancy, radiotherapy, chemotherapy, or immuno-deficiency within the last 4 years. | Peri-implantitis (PI, n = 58) Peri-implant mucositis (PM, n = 49) Peri-implant health (PH, n = 49) | Based on the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases Peri-implantitis: PPD ≥ 6 mm and/or MBL ≥ 3 mm Peri-implant mucositis: Presence of BOP and/or suppuration without evidence of MBL | T-C (mg/dL, mean ± SD) | PI: 213.4 ± 39.1 PM: 198.6 ± 44.3 PH: 202 ± 41.3 p = 0.221 † |
| Triglycerides (mg/dL, median, IQR) | PI: 148 (107.5) PM: 125 (93) PH: 95 (61) p < 0.001 † | |||||
| HDL-C (mg/dL, median, IQR) | PI: 47 (20.5) PM: 52 (27.2) PH: 54 (19) p = 0.081 † | |||||
| LDL-C (mg/dL, mean ± SD) | PI: 130.2 ± 31.4 PM: 119.5 ± 38.5 PH: 120.1 ± 37.5 p = 0.674 † | |||||
| Blanco et al. 2021 [45] Cross-sectional | Individuals diagnosed with peri-implantitis and subjects with peri-implant health as controls | Not specified. Patients identified as current smokers or had previous history of periodontitis were also included. | Peri-implantitis (PI, n = 16) Controls (n = 31) | Based on the 2017 World Workshop on the Classification of Periodontal and Peri-implant Diseases Peri-implantitis: PPD ≥ 6 mm and/or MBL ≥ 3 mm | T-C (mg/dL, mean) | PI: 212.0 Controls: 156.0 p < 0.001 * |
| Triglycerides (mg/dL, mean) | PI: 80.0 Controls: 66.0 p = 0.119 * | |||||
| HDL-C (mg/dL, mean) | PI: 61.0 Controls: 55.0 p = 0.112 * | |||||
| LDL-C (mg/dL, mean) | PI: 120.0 Controls: 91.0 p < 0.001 * | |||||
| Glucose (mg/dL, mean) | PI:83.0 Controls: 81.0 p = 0.420 * |
| Study | Selection (Max. 3 Stars) | Comparability (Max. 2 Stars) | Outcome (Max. 1 Star) | ||||
|---|---|---|---|---|---|---|---|
| Representativeness | Sample Size | Selection of Non-Exposed Group | Ascertainment of Exposure | Comparability of Exposed and Non-Exposed Groups | Outcome Measurement | Statistical Test | |
| Sanchez-Siles et al. 2016 [47] | * | * | * | * | * | * | |
| Al-Askar et al. 2018 [44] | * | * | * | * | * | * | |
| Lucarini et al. 2019 [46] | * | * | * | * | * | * | |
| Ustaoglu et al. 2020 [12] | * | * | * | ** | * | * | |
| Blanco et al. 2021 [45] | * | * | * | * | ** | * | * |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, Y.; Lu, E.M.-C.; Moyes, D.; Niazi, S.A. Impact of Peri-Implant Inflammation on Metabolic Syndrome Factors: A Systematic Review. Appl. Sci. 2023, 13, 11747. https://doi.org/10.3390/app132111747
Zhang Y, Lu EM-C, Moyes D, Niazi SA. Impact of Peri-Implant Inflammation on Metabolic Syndrome Factors: A Systematic Review. Applied Sciences. 2023; 13(21):11747. https://doi.org/10.3390/app132111747
Chicago/Turabian StyleZhang, Yuchen, Emily Ming-Chieh Lu, David Moyes, and Sadia Ambreen Niazi. 2023. "Impact of Peri-Implant Inflammation on Metabolic Syndrome Factors: A Systematic Review" Applied Sciences 13, no. 21: 11747. https://doi.org/10.3390/app132111747
APA StyleZhang, Y., Lu, E. M.-C., Moyes, D., & Niazi, S. A. (2023). Impact of Peri-Implant Inflammation on Metabolic Syndrome Factors: A Systematic Review. Applied Sciences, 13(21), 11747. https://doi.org/10.3390/app132111747






