Efficacy of Cold Atmospheric Plasma on Pathogenicity of Oral Microcosm Biofilms
Abstract
1. Introduction
2. Materials and Methods
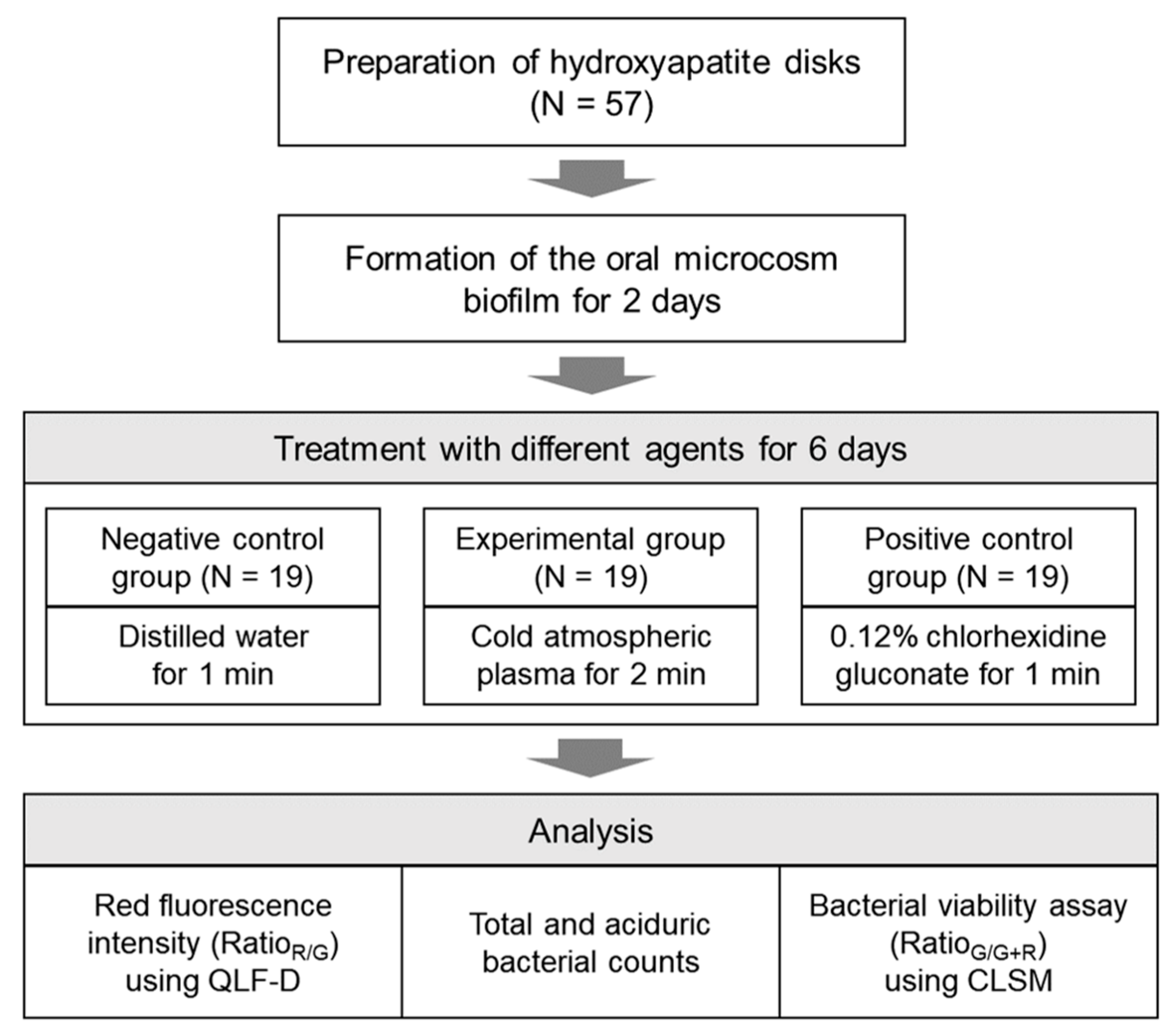
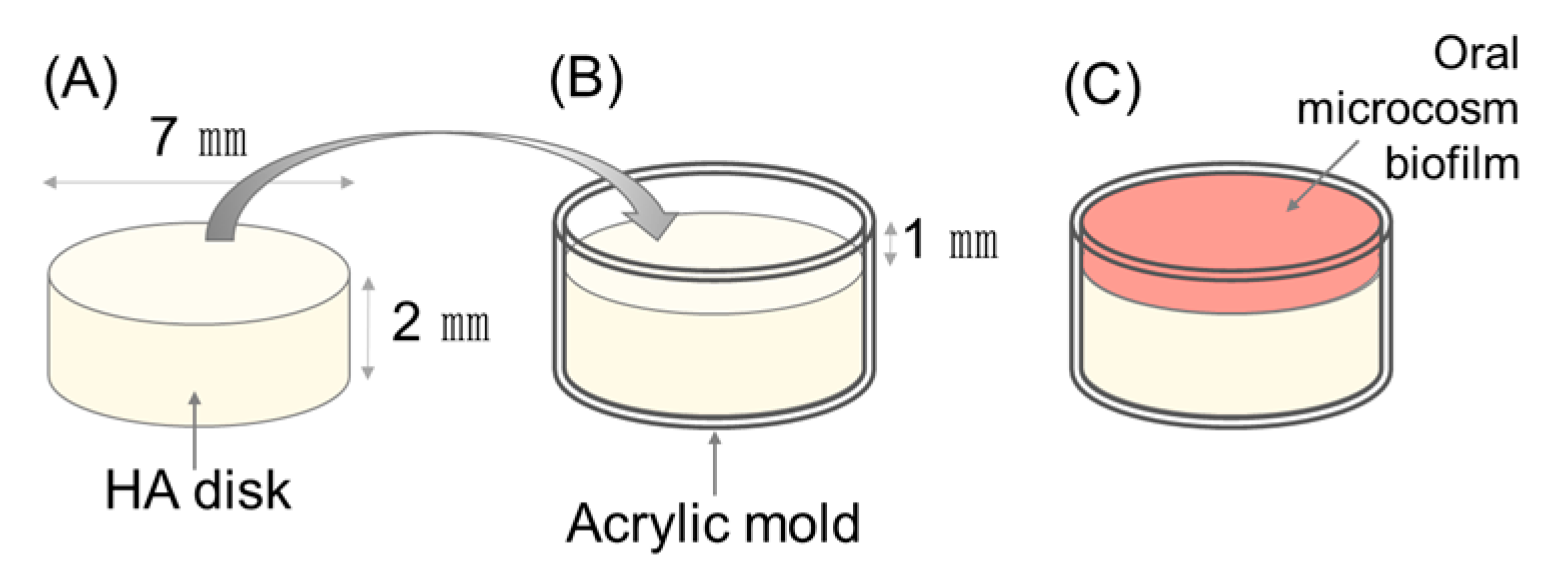
2.1. Establishing the Number of Samples and Preparation of Specimens
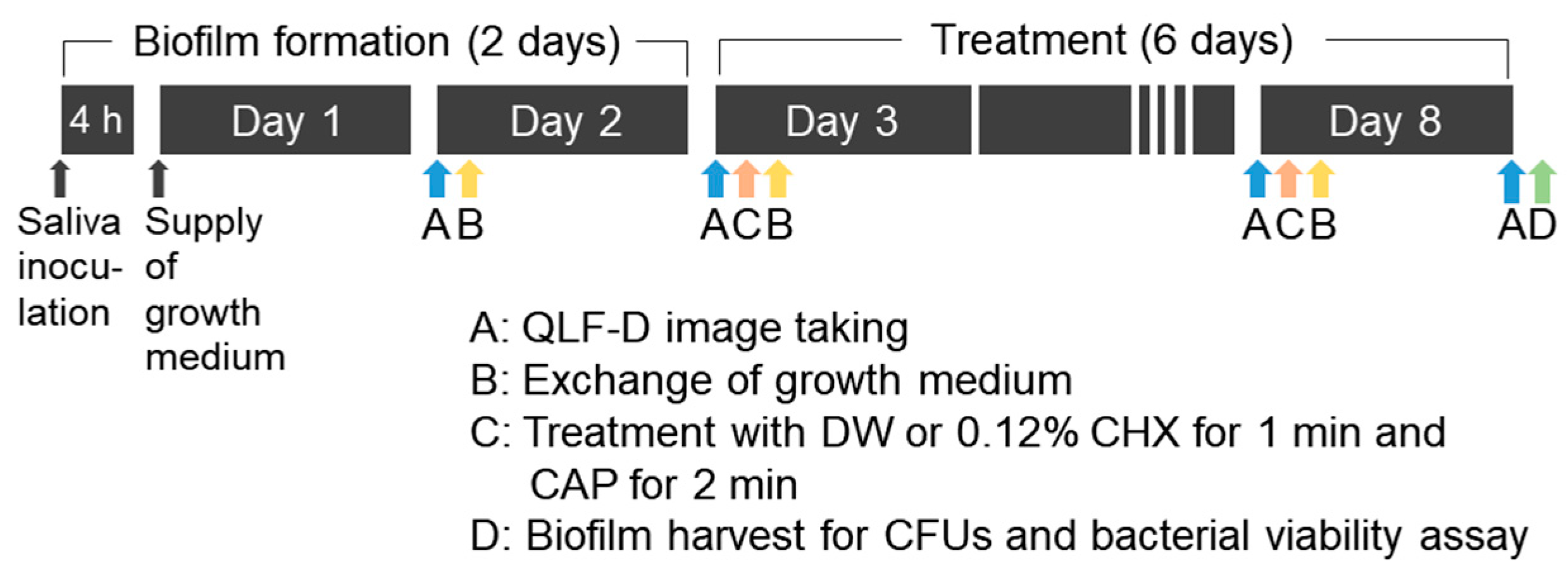
2.2. Development of the Oral Microcosm Biofilm
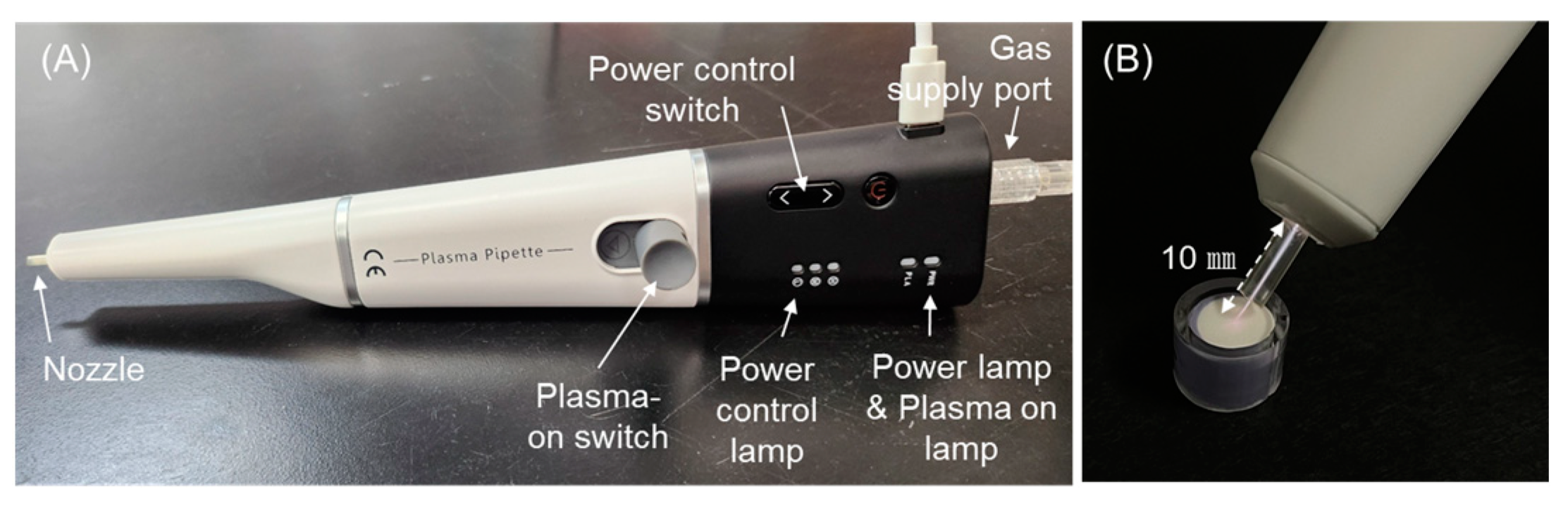
2.3. Plasma Treatment
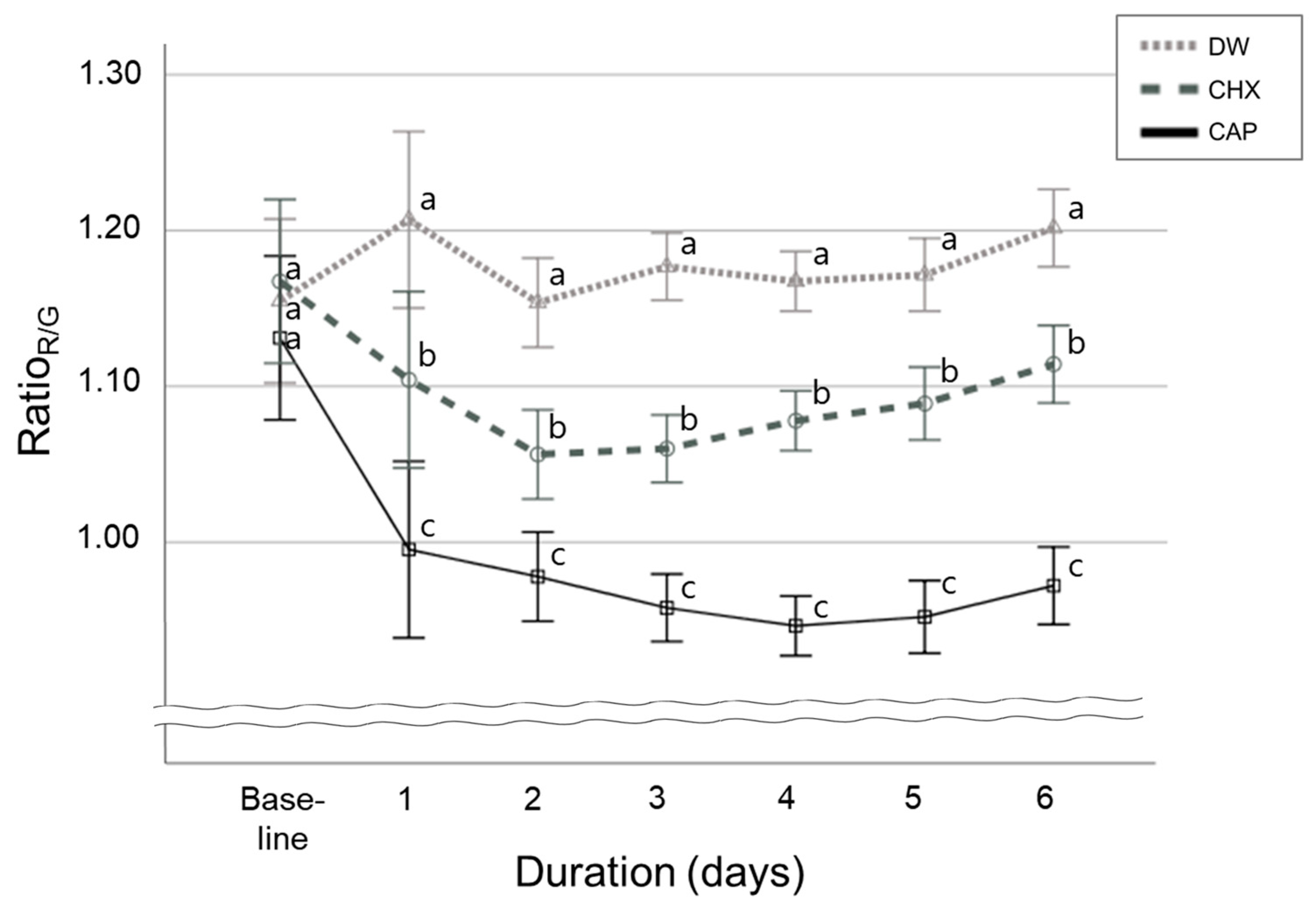
2.4. Evaluating the Intensity of Red Fluorescence
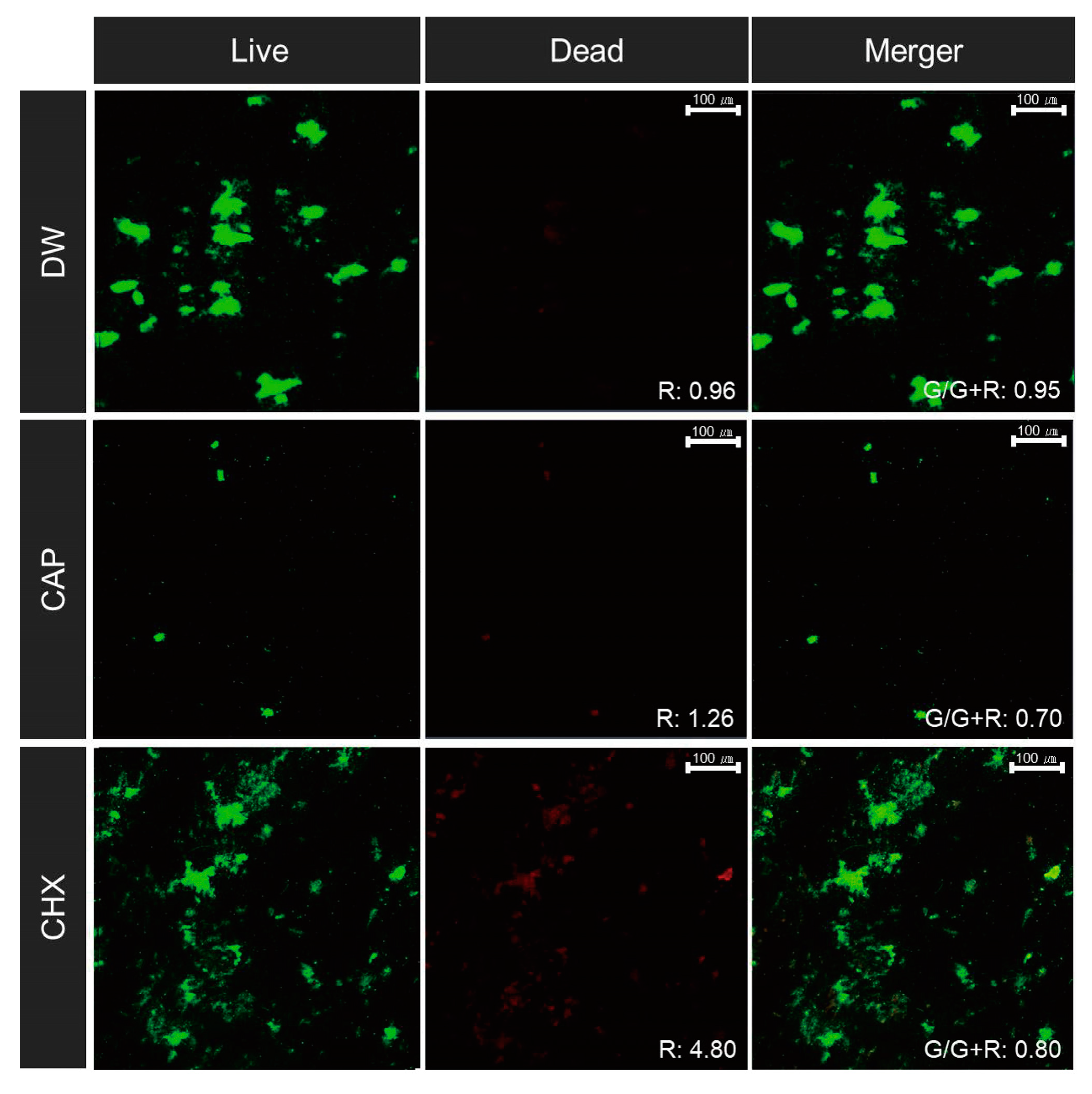
2.5. Bacterial Viability Assay
2.6. Evaluation of Colony Count
2.7. Statistical Analysis
3. Results
3.1. Red Fluorescence Signal Strength in Oral Microcosm Biofilms
3.2. Bacterial Viability
3.3. Bacterial Counts
4. Discussion
5. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- World Health Organization. Global Oral Health Status Report: Towards Universal Health Coverage for Oral Health by 2030; World Health Organization: Geneva, Switzerland, 2022; Available online: https://www.who.int/publications/i/item/9789240061484 (accessed on 17 January 2024).
- Stoodley, P.; Sauer, K.; Davies, D.G.; Costerton, J.W. Biofilms as complex differentiated communities. Annu. Rev. Microbiol. 2002, 56, 187–209. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, N.; Nyvad, B. The role of bacteria in the caries process: Ecological perspectives. J. Dent. Res. 2011, 90, 294–303. [Google Scholar] [CrossRef]
- Nyvad, B.; Takahashi, N. Integrated hypothesis of dental caries and periodontal diseases. J. Oral Microbiol. 2020, 12, 1710953. [Google Scholar] [CrossRef]
- Jones, C.G. Chlorhexidine: Is it still the gold standard? Periodontol. 2000 1997, 15, 55–62. [Google Scholar] [CrossRef] [PubMed]
- Flötra, L.; Gjermo, P.; Rölla, G.; Waerhaug, J. Side effects of chlorhexidine mouth washes. Scand. J. Dent. Res. 1971, 79, 119–125. [Google Scholar] [CrossRef]
- Brookes, Z.; Teoh, L.; Cieplik, F.; Kumar, P. Mouthwash effects on the oral microbiome: Are they good, bad, or balanced? Int. Dent. J. 2023, 73 (Suppl. 2), S74–S81. [Google Scholar] [CrossRef]
- do Amaral, G.C.L.S.; Hassan, M.A.; Sloniak, M.C.; Pannuti, C.M.; Romito, G.A.; Villar, C.C. Effects of antimicrobial mouthwashes on the human oral microbiome: Systematic review of controlled clinical trials. Int. J. Dent. Hyg. 2023, 21, 128–140. [Google Scholar] [CrossRef]
- von Woedtke, T.; Laroussi, M.; Gherardi, M. Foundations of plasmas for medical applications. Plasma Sources Sci. Technol. 2022, 31, 054002. [Google Scholar] [CrossRef]
- Borges, A.C.; Kostov, K.G.; Pessoa, R.S.; de Abreu, G.M.; Lima, G.d.M.; Figueira, L.W.; Koga-Ito, C.Y. Applications of cold atmospheric pressure plasma in dentistry. Appl. Sci. 2021, 11, 1975. [Google Scholar] [CrossRef]
- Gherardi, M.; Tonini, R.; Colombo, V. Plasma in dentistry: Brief history and current status. Trends Biotechnol. 2018, 36, 583–585. [Google Scholar] [CrossRef]
- Sladek, R.E.; Filoche, S.K.; Sissons, C.H.; Stoffels, E. Treatment of Streptococcus mutans biofilms with a nonthermal at-mospheric plasma. Lett. Appl. Microbiol. 2007, 45, 318–323. [Google Scholar] [CrossRef]
- Lee, M.J.; Kwon, J.S.; Jiang, H.B.; Choi, E.H.; Park, G.; Kim, K.M. The antibacterial effect of nonthermal atmospheric pressure plasma treatment of titanium surfaces according to the bacterial wall structure. Sci. Rep. 2019, 9, 1938. [Google Scholar] [CrossRef] [PubMed]
- Carreiro, A.F.P.; Delben, J.A.; Guedes, S.; Silveira, E.J.D.; Janal, M.N.; Vergani, C.E.; Pushalkar, S.; Duarte, S. Low-temperature plasma on peri-implant–related biofilm and gingival tissue. J. Periodontol. 2019, 90, 507–515. [Google Scholar] [CrossRef] [PubMed]
- Jablonowski, L.; Fricke, K.; Matthes, R.; Holtfreter, B.; Schlüter, R.; von Woedtke, T.; Weltmann, K.D.; Kocher, T. Removal of naturally grown human biofilm with an atmospheric pressure plasma jet: An in-vitro study. J. Biophotonics 2017, 10, 718–726. [Google Scholar] [CrossRef] [PubMed]
- Blumhagen, A.; Singh, P.; Mustapha, A.; Chen, M.; Wang, Y.; Yu, Q. Plasma deactivation of oral bacteria seeded on hy-droxyapatite disks as tooth enamel analogue. Am. J. Dent. 2014, 27, 84–90. [Google Scholar] [PubMed]
- Koban, I.; Holtfreter, B.; Hübner, N.O.; Matthes, R.; Sietmann, R.; Kindel, E.; Weltmann, K.D.; Welk, A.; Kramer, A.; Kocher, T. Antimicrobial efficacy of non-thermal plasma in comparison to chlorhexidine against dental biofilms on titanium discs in vitro—Proof of principle experiment. J. Clin. Periodontol. 2011, 38, 956–965. [Google Scholar] [CrossRef] [PubMed]
- Costerton, J.W.; Stewart, P.S.; Greenberg, E.P. Bacterial biofilms: A common cause of persistent infections. Science 1999, 284, 1318–1322. [Google Scholar] [CrossRef]
- Filoche, S.K.; Soma, K.J.; Sissons, C.H. Caries-related plaque microcosm biofilms developed in microplates. Oral Microbiol. Immunol. 2007, 22, 73–79. [Google Scholar] [CrossRef] [PubMed]
- Sissons, C.H. Artificial dental plaque biofilm model systems. Adv. Dent. Res. 1997, 11, 110–126. [Google Scholar] [CrossRef]
- Marsh, P.D. Controlling the oral biofilm with antimicrobials. J. Dent. 2010, 38 (Suppl. 1), S11–S15. [Google Scholar] [CrossRef]
- Lee, J.; Cho, S.; Kim, H.E. Antimicrobial effects of non-thermal atmospheric pressure plasma on oral microcosm biofilms. Int. J. Environ. Res. Public Health 2023, 20, 2447. [Google Scholar] [CrossRef]
- Lee, E.S.; Kang, S.M.; Ko, H.Y.; Kwon, H.K.; Kim, B.I. Association between the cariogenicity of a dental microcosm biofilm and its red fluorescence detected by Quantitative Light-induced Fluorescence-Digital (QLF-D). J. Dent. 2013, 41, 1264–1270. [Google Scholar] [CrossRef] [PubMed]
- Hwang, H.Y.; Kim, H.E. Influence of a novel pH-cycling model using dental microcosm biofilm on the remineralizing efficacy of fluoride in early carious lesions. Clin. Oral Investig. 2021, 25, 337–344. [Google Scholar] [CrossRef]
- Kang, M.K.; Kim, H.E. Remineralizing efficacy of fluoride in the presence of oral microcosm biofilms. J. Dent. 2021, 115, 103848. [Google Scholar] [CrossRef]
- Lee, E.S.; de Josselin de Jong, E.D.J.; Kim, B.I. Detection of dental plaque and its potential pathogenicity using quantitative light-induced fluorescence. J. Biophotonics 2019, 12, e201800414. [Google Scholar] [CrossRef]
- Stocks, S.M. Mechanism and use of the commercially available viability stain, BacLight. Cytometry A 2004, 61, 189–195. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.R.; Kim, H.E. Red fluorescence threshold for assessing the lesion activity of early caries. Photodiagnosis Photodyn. Ther. 2020, 32, 102040. [Google Scholar] [CrossRef] [PubMed]
- Volgenant, C.M.; van der Veen, M.H.; de Soet, J.J.; ten Cate, J.M. Effect of metalloporphyrins on red autofluorescence from oral bacteria. Eur. J. Oral Sci. 2013, 121, 156–161. [Google Scholar] [CrossRef]
- de Josselin de Jong, E.; Higham, S.M.; Smith, P.W.; van Daelen, C.J.; van der Veen, M.H. Quantified light-induced fluores-cence, review of a diagnostic tool in prevention of oral disease. J. Appl. Phys. 2009, 105, 102031. [Google Scholar] [CrossRef]
- Lee, E.S.; de Josselin de Jong, E.D.J.; Jung, H.I.; Kim, B.I. Red fluorescence of dental biofilm as an indicator for assessing the efficacy of antimicrobials. J. Biomed. Opt. 2018, 23, 015003. [Google Scholar] [CrossRef]
- Kim, Y.S.; Lee, E.S.; Kwon, H.K.; Kim, B.I. Monitoring the maturation process of a dental microcosm biofilm using the Quantitative Light-induced Fluorescence-Digital (QLF-D). J. Dent. 2014, 42, 691–696. [Google Scholar] [CrossRef]
- Marsh, P.D. Dental plaque as a microbial biofilm. Caries Res. 2004, 38, 204–211. [Google Scholar] [CrossRef] [PubMed]
- Souza, J.G.S.; Costa Oliveira, B.E.C.; Costa, R.C.; Bechara, K.; Cardoso-Filho, O.; Benso, B.; Shibli, J.A.; Bertolini, M.; Barāo, V.A.R. Bacterial-derived extracellular polysaccharides reduce antimicrobial susceptibility on biotic and abiotic surfaces. Arch. Oral Biol. 2022, 142, 105521. [Google Scholar] [CrossRef] [PubMed]
- Bowen, W.H.; Burne, R.A.; Wu, H.; Koo, H. Oral biofilms: Pathogens, matrix, and polymicrobial interactions in microenvi-ronments. Trends Microbiol. 2018, 26, 229–242. [Google Scholar] [CrossRef] [PubMed]
- Karygianni, L.; Ren, Z.; Koo, H.; Thurnheer, T. Biofilm matrixome: Extracellular components in structured microbial com-munities. Trends Microbiol. 2020, 28, 668–681. [Google Scholar] [CrossRef]
- Melvaer, K.L.; Helgeland, K.; Rölla, G. A charged component in purified polysaccharide preparations from Streptococcus mutans and Streptococcus sanguis. Arch. Oral Biol. 1974, 19, 589–595, IN25. [Google Scholar] [CrossRef]
- Tatevossian, A. The effects of heat inactivation, tortuosity, extracellular polyglucan and ion-exchange sites on the diffusion of [14C]-sucrose in human dental plaque residue in vitro. Arch. Oral Biol. 1985, 30, 365–371. [Google Scholar] [CrossRef]
- Razzokov, J.; Yusupov, M.; Bogaerts, A. Oxidation destabilizes toxic amyloid beta peptide aggregation. Sci. Rep. 2019, 9, 5476. [Google Scholar] [CrossRef]
- Razzokov, J.; Fazliev, S.; Kodirov, A.; AttrI, P.; Chen, Z.; Shiratani, M. Mechanistic insight into permeation of plas-ma-generated species from vacuum into water bulk. Int. J. Mol. Sci. 2022, 23, 6330. [Google Scholar] [CrossRef]
- Xu, Z.; Shen, J.; Cheng, C.; Hu, S.; Lan, Y.; Chu, P.K. In Vitro antimicrobial effects and mechanism of atmospheric-pressure He/O2 plasma jet on Staphylococcus aureus biofilm. J. Phys. D Appl. Phys. 2017, 50, 105201. [Google Scholar] [CrossRef]
- Yoo, E.M.; Choi, Y.R.; Kang, M.K. Antimicrobial efficacy of nitrogen-based non-thermal atmospheric pressure plasma jet on dental biofilm. Iran J. Sci. Technol. Trans. A Sci. 2020, 44, 1541–1547. [Google Scholar] [CrossRef]
- Du, T.; Shi, Q.; Shen, Y.; Cao, Y.; Ma, J.; Lu, X.; Xiong, Z.; Haapasalo, M. Effect of modified nonequilibrium plasma with chlorhexidine digluconate against endodontic biofilms in vitro. J. Endod. 2013, 39, 1438–1443. [Google Scholar] [CrossRef] [PubMed]
- Khosravi, S.; Jafari, S.; Zamani, H.; Nilkar, M. Inactivation of Staphylococcus aureus and Escherichia coli biofilms by air-based atmospheric-pressure DBD plasma. Appl. Biochem. Biotechnol. 2021, 193, 3641–3650. [Google Scholar] [CrossRef] [PubMed]

| Treatments | N | Total Bacteria | Aciduric Bacteria |
|---|---|---|---|
| Distilled water | 19 | 7.19 (0.52) a | 5.83 (0.48) a |
| Cold atmospheric plasma | 19 | 5.96 (0.66) b | 4.89 (0.60) b |
| Chlorhexidine gluconate | 19 | 6.44 (0.80) b | 4.61 (0.68) b |
| p-values † | <0.001 | <0.001 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, H.-E. Efficacy of Cold Atmospheric Plasma on Pathogenicity of Oral Microcosm Biofilms. Appl. Sci. 2024, 14, 1211. https://doi.org/10.3390/app14031211
Kim H-E. Efficacy of Cold Atmospheric Plasma on Pathogenicity of Oral Microcosm Biofilms. Applied Sciences. 2024; 14(3):1211. https://doi.org/10.3390/app14031211
Chicago/Turabian StyleKim, Hee-Eun. 2024. "Efficacy of Cold Atmospheric Plasma on Pathogenicity of Oral Microcosm Biofilms" Applied Sciences 14, no. 3: 1211. https://doi.org/10.3390/app14031211
APA StyleKim, H.-E. (2024). Efficacy of Cold Atmospheric Plasma on Pathogenicity of Oral Microcosm Biofilms. Applied Sciences, 14(3), 1211. https://doi.org/10.3390/app14031211





