Abstract
This study investigated the memory-improving effects and mechanisms of action of hydrolysate from silkworm fed Cudrania tricuspidata leaves (HSCT) in rats with scopolamine-induced memory impairment. Thirty-two rats were categorized into 4 groups and the experiment was conducted for 6 weeks. The experimental diet groups are as follows: control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg); HSCT, HSCT diet + scopolamine (0.7 mg/kg); and donepezil, control diet + scopolamine (0.7 mg/kg) + donepezil (1.0 mg/kg). Except for the control group, the experimental groups received scopolamine 0.7 mg/kg intraperitoneally to induce decline in memory. Animal behavioral tests such as the Barnes maze, novel object recognition test, and Y-maze were performed to investigate the effects of HSCT on memory improvement. In addition, brain acetylcholine (ACh) concentration and acetylcholinesterase (AChE) activity were assessed to investigate the mechanism of memory improvement. Results of the animal behavior test suggested that the rats administered HSCT displayed improved memory behavior compared to those administered scopolamine (p < 0.05). The concentration of Ach was increased in the HSCT group compared to the scopolamine group (p < 0.05), and the activity of AChE was significantly reduced (p < 0.05). These results suggest that HSCT effectively improves memory by reducing AChE activity and increasing ACh content in the brains of rats with scopolamine-induced memory impairment. As a natural compound, HSCT has the potential to be used as a material to improve memory.
1. Introduction
The proportion of the elderly population aged 65 years and older in Korea has been increasing annually. According to a report by Statistics Korea in 2021, their proportion in Korea is currently 17.0%, entering a super-aged society, and is estimated to increase to 43.9% by 2060 [1,2]. In particular, Korea is aging the fastest among the Organization for Economic Cooperation and Development countries, and chronic diseases such as dementia, hypertension, and diabetes are reported to increase with age [3]. Dementia, the most common form of aging, is expected to affect 1 million people by 2030 and 2 million by 2050 [4]. Dementia is a clinical syndrome characterized by a progressive decline in cognition and the ability to live and function independently [5], resulting in a decline in the proper functioning ability of the brain related to memory, thinking, behavior, and daily activities [6,7]. In particular, Alzheimer’s disease (AD) is the most common degenerative brain disease that causes dementia and is characterized by a decline in brain function and impairment of cognitive functions, including memory [8]. One of the main causes of memory decline in Alzheimer’s disease is the decreased activity of cholinergic substances such as acetylcholine (ACh) in the brain tissue [9]. In the nerve cells of the brain tissue, acetyl CoA and choline are converted into ACh, which helps cells interact with each other, thus helping with memory and attention [10,11]. In contrast, acetylcholinesterase (AChE) secreted by nerve cells breaks down synthesized ACh into choline and acetate, reducing the concentration of ACh, which can lead to memory decline [12,13]. Drugs for the treatment of AD that restore brain function are still underdeveloped, and the currently available drugs for AD only temporarily alleviate or delay symptoms [14,15]. Additionally, some drugs used to restore brain function impaired by AD do not have proven efficacy or safety issues [16,17,18,19]. Therefore, there is a growing interest in the development of natural product-derived food ingredients that can minimize side effects, ensure safety, and continuously improve and manage the symptoms of AD.
Silkworm extract, a natural product, has excellent nutritional value owing to its high protein content [20] and has been utilized as a functional food ingredient in the food industry. Various studies have shown that it has several functions such as antioxidant effects [21], blood sugar improvement effects [22], liver protection effects [23], and modulation of the gut microbiota composition [24]. In addition, recent studies have reported that it improves memory by modulating the cholinergic nervous system and antioxidant defense system in animal models of memory impairment, making it a natural substance that can improve brain function impaired by AD [25,26].
Morus alba (commonly known as white mulberry) belongs to the family Moraceae, and the mulberry leaves are commonly known to serve as food for silkworms. These leaves are rich in nutrients such as proteins, vitamins, and minerals, contain a large amount of dietary fiber, and have a high content of various bioactive substances such as flavones, steroids, and triterpenes [27,28]. In addition, the nutritional and functional components of mulberry leaves have very important effects on the growth and quality of silkworms [29]. Cudrania tricuspidata (commonly called mandarin melon berry, silkworm thorn, etc.) belongs to the family Moraceae, and its leaves are rich in bioactive substances and possess excellent antioxidant activity compared to Morus alba [30,31,32], especially the content of the neurotransmitter gamma-aminobutyric acid, which has been reported to be effective in improving memory [33]. However, there are no studies on the functional effects of silkworms that are fed Cudrania tricuspidata leaves.
Enzymatic hydrolysis breaks down proteins into smaller units, and the resulting peptides and small molecule proteins can be used as new bioactive compounds because of their diverse functionalities [34]. You et al. [35] reported that the antioxidant activity of protein hydrolysates produced by treatment with enzymes such as Protamex increased with an increasing degree of hydrolysis. Kim et al. [36] reported that pig skin gelatin hydrolysate not only increased neuroprotective effects, but also inhibited AChE activity, which can cause AD. Therefore, this study was conducted to confirm the memory-improving effect of a hydrolysate produced from silkworms that were fed Cudrania tricuspidata in an experimental animal model of memory impairment and to determine its potential as a functional food ingredient.
2. Materials and Methods
2.1. Sample Preparation
Silkworms fed Cudrania tricuspidata leaves were ground with water to a weight of 20%, adjusted to pH 7.0, and hydrolyzed at 50 °C for 5 h by adding 1.5% and 0.5% of the proteolytic enzymes bromelain and alcalase, respectively. The hydrolysate was then readjusted to pH 5.5, and viscozyme was added for hydrolysis at 50 °C for 2 h. The hydrolysate was then subjected to enzyme inactivation at 100 °C for 15 min, concentrated under reduced pressure, mixed with maltodextrin at a ratio of 6:4, and then dried by spray drying to prepare a hydrolysate from silkworms fed Cudrania tricuspidata leaves (HSCT).
2.2. Sodium Dodecyl Sulfate-Polyacrylamide Gel Electrophoresis (SDS-PAGE)
SDS-PAGE was performed using a 14% gel to determine the extent of hydrolysis with and without enzyme treatment. A total of 20 μg per sample was loaded after quantification of proteins by the Bradford method. After electrophoresis at 80 V for approximately 1 h, the gels were stained using 0.1% Coomassie Brilliant Blue (Bio-Rad, Hercules, CA, USA), and viewed using ChemiDoc Imager (Bio-Rad, Hercules, CA, USA), and proteolysis was assessed using a molecular weight maker (Life Technologies, Carlsbad, CA, USA).
2.3. Animal Studies
This study was approved by the Sahmyook University Animal Ethics Committee (SYUIACUC2022-002). Animal procedures were performed in strict accordance with the guidelines of the National Research Council and Institutional Animal Care and Use Committee (Seoul, Republic of Korea). This study was carried out in the animal facility of the Sahmyook University (Seoul, Republic of Korea), and all efforts were made to minimize suffering of animals. The rats and feed were purchased from Koatech (Gyeonggi-do, Republic of Korea) and Duyeol Biotech (Seoul, Republic of Korea), respectively. Thirty-two Sprague-Dawley male rats (12 weeks old) were housed singly in stainless steel cages in a room maintained at 50 ± 10% humidity and 22 ± 2 °C with a 12 h light–dark cycle. The rats were acclimatized for one week, during which period they consumed a basal diet and water ad libitum. After adaptation, the rats were randomly allocated to one of four diet groups (n = 8) during the six weeks of the study.
The standard diet AIN-93G (Envigo, Indianapolis, IN, USA) was used as the control diet. The HSCT diet was prepared by adding HSCT (5%) to the control diet. The experimental diet groups were as follows: control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i. p.); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i. p.); and donepezil, control diet + scopolamine (0.7 mg/kg, i. p.) + donepezil (1.0 mg/kg, i. p.). To induce memory impairment in rats, all experimental groups except the control group received scopolamine (Sigma, St. Louis, MO, USA) at a concentration of 0.7 mg/kg intraperitoneally, and the positive control group received an additional 1.0 mg/kg intraperitoneal dose of donepezil (Sigma, St. Louis, MO, USA), which has been shown to improve memory. Animal behavioral experiments were performed at the sixth week, and behavioral patterns of the rats were recorded and analyzed using video tracking software (Ethovision XT ver. 16; Noldus Information Technology, Wageningen, The Netherlands). The rats were anesthetized with CO2 gas and sacrificed by cervical decapitation, and the brain, heart, kidney, and liver were recovered and weighed.
2.4. Barnes Maze Test
The Barnes maze test is a method that is used to assess memory through spatial learning. The Barnes maze model is a flat, circular platform (92 cm in diameter) with 20 holes (5 cm in diameter) at regular intervals (7.5 cm). The platform is placed 100 cm above the floor, and a black escape box is placed in one of the holes. The experiment was conducted in bright light (300 lux) and with 80 dB of noise to elicit an escape response of the rats to the escape box. Visual cues with distinctive patterns and shapes were placed on the walls of the test chamber. Between each trial, the platform and escape box were cleaned with 70% ethanol to remove olfactory cues. One day prior to the start of a training trial, the animals were allowed to acclimatize to the platform and target box for 3 min, and the training trials were conducted once a day for 4 days. The animal was placed in the center of the platform and allowed to explore the escape box for 180 s. If the animal did not reach the escape box within 3 min, it was gently guided to the escape hole and we measured the latency time, distance, and number of errors it took the rats to find the escape route.
2.5. Novel Object Recognition Test (NORT)
NORT is an experiment to assess recognition memory; it was conducted in a box (50 × 50 × 50 cm) made of polyvinyl plastic. The animals were allowed to explore the box freely for 10 min on the day before the test to get acclimatized to the box. Identical objects (A, A′) were presented at equal distances from both vertices of the box, and the rats were allowed to explore them freely for 5 min. Sixty minutes later, one of the identical objects was replaced by another objects (A, B) to assess short-term memory, and the rats were allowed to explore freely for 5 min. To assess long-term memory, 24 h after exploration, the objects were replaced with different objects (A and C), and the rats were allowed to explore them freely for 5 min. During the 5 min exploration period, we measured the number of times the animals exhibited exploratory behaviors such as touching, sniffing, and licking familiar and novel objects. Object preference and discrimination scores were calculated using the following equation:
Object preference (%) = [number of times new objects or existing objects were explored/(number of times new objects were explored + number of times existing objects were explored)] × 100
Discrimination index = [number of times new or existing objects were explored—number of times existing objects were explored/(number of times new objects were explored + number of times existing objects were explored)] × 100
Higher discrimination scores indicate higher recognition of novel objects.
2.6. Y-Maze Test
The Y-maze consists of three branches made of black polyvinyl plastic sheets, with lengths, widths, heights, and angles of 45 cm, 10 cm, 35 cm, and 120°, respectively. In our study, the areas of each branch were labeled A, B, and C. Each animal was placed on a branch and allowed to move freely for 8 min to record its movement. The number of entries was recorded as the number of times the animal entered a branch completely up to its tail. The number of entries into the three different branches in turn (ABC, BCA, CAB, etc.) was recorded as a spontaneous alternation, and one point was awarded. The spontaneous alteration, which is an indicator of concentration and spatial cognition, was calculated as follows:
Spontaneous alteration (%) = (actual change/highest change) × 100
2.7. ACh Content
ACh content in the brain tissue of the experimental animals was measured according to the manufacturer’s protocol using the Choline/Acetylcholine Assay kit (ab65345, Abcam, Cambridge, UK). In a 96-well plate, 50 μL of brain tissue samples and standard solution were added, followed by 50 μL of reaction mixture, and the plate was incubated for 30 min at room temperature. Absorbance was measured at 570 nm using a multifunction microplate reader (MMR SPARK®, Tecan, Switzeriand). The ACh content in the brain was calculated using an Ach solution as a standard.
2.8. AChE Activity
AChE activity in the brain tissue of the experimental animals was measured using the Acetylcholinesterase Assay Kit (ab138871, Abcam, Cambridge, UK) according to the manufacturer’s protocol. In a 96-well plate, 50 μL of brain tissue samples and standard solutions were added, followed by 50 μL of the reaction mix, and the plate was incubated for 30 min at room temperature. Absorbance was measured at 410 nm using a multifunctional microplate reader (MMR SPARK®, Tecan, Switzerland). The AChE content in the brain was calculated using an AChE solution as a standard.
2.9. Statistical Analysis
The results are presented as mean ± standard error of the mean (SEM). The significance of the differences among the groups was determined using SAS/PROC GLM software (SAS v.9.1; SAS Institute Inc., Cary, NC, USA). The statistical significance of all data was analyzed using the Student’s t-test compared with the scopolamine group.
3. Results
3.1. SDS-PAGE
SDS-PAGE analysis was performed to determine the change in the molecular weight of HSCT after enzymatic treatment (Figure 1). The molecular weight of proteins of silkworms fed Cudrania tricuspidata leaves (SCT) obtained without enzymatic hydrolysis showed a distribution of low-molecular-weight proteins (between 17–20 kDa) and high-molecular-weight proteins (between 35–48 kDa). In contrast, the proteins treated with protease in the HSCT group were effectively hydrolyzed into a low-molecular-weight protein of approximately 5 kDa.
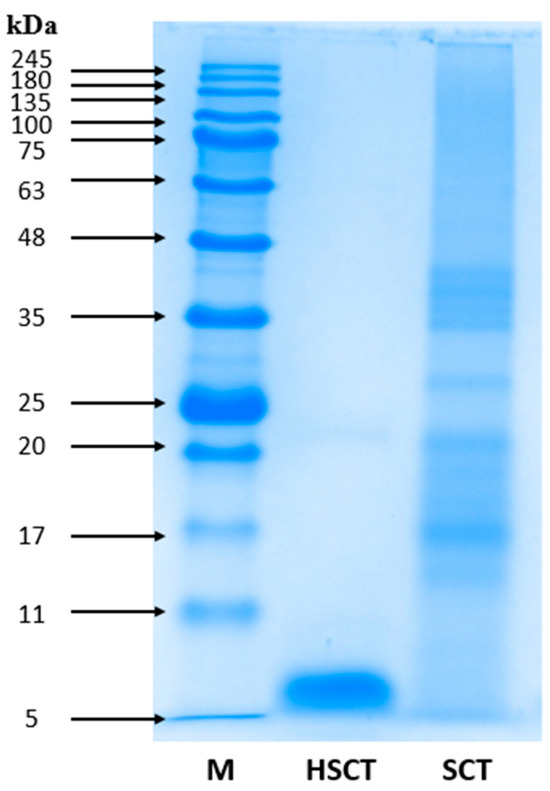
Figure 1.
SDS polyacrylamide gel electrophoresis profile of HSCT and SCT. M, molecular weight marker; HSCT, hydrolysate from silkworms fed Cudrania tricuspidata leaves; SCT, silkworms fed Cudrania tricuspidata leaves.
3.2. Organ Weight
To analyze the effects of scopolamine on organ tissue damage in rats, we weighed the brain, heart, kidneys, and liver after the animal experiments. These weights were not significantly different between the groups (Table 1).

Table 1.
Comparison of organ weights of rats after the 6 weeks of animal study.
3.3. Barnes Maze
The Barnes maze test was performed to evaluate spatial learning and memory in the experimental animals (Figure 2). During the training period, the distance explored to locate the escape box was not significantly different in the other groups except for the control group until day 3; however, on day 4, it was significantly decreased in the control, HSCT, and donepezil groups than in the scopolamine group (p < 0.05). Furthermore, the time to locate the escape box was also significantly decreased in the control, HSCT, and donepezil groups (p < 0.05).
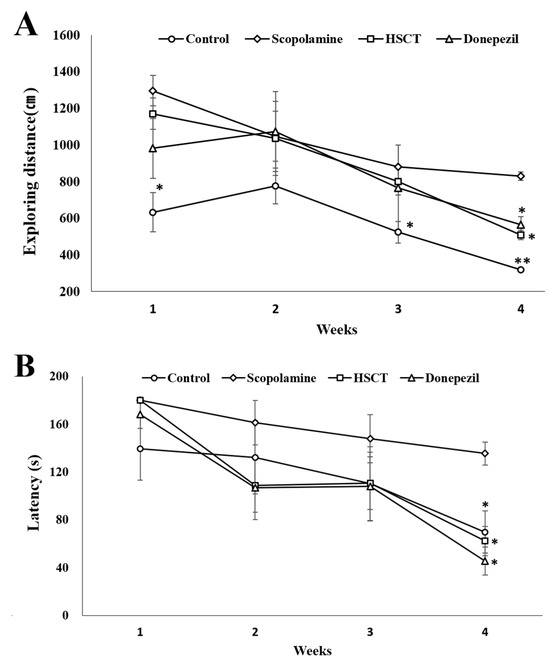
Figure 2.
Effect of HSCT on rats as observed in the Barnes maze test. Assessment of (A) distance and (B) latency time in a 4-day training trial. Values are means ± SEM; (A,B) were analyzed using Student’s t-test; * p < 0.05 or ** p < 0.01 compared with the scopolamine group. control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i. p.); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i. p.); donepezil, control diet + scopolamine (0.7 mg/kg, i. p.) + donepezil (1.0 mg/kg, i. p.); i. p., intraperitoneal injection.
3.4. NORT
NORT was performed in rats with induced memory impairment to determine the effects of HSCT on cognitive function and memory. Object preference and discrimination scales were calculated by analyzing the number of explorations of old and new objects. As shown in Figure 3, results of the experiment evaluating short-term memory showed that, compared to those in the scopolamine group, the number of times the rats in control and HSCT groups explored new objects significantly increased (p < 0.05), and the number of times they explored previously recognized objects significantly decreased (p < 0.05). As shown in Figure 4, results of the experiment evaluating long-term memory showed that the number of new objects explored and the discrimination scale were significantly higher in the donepezil group than in the scopolamine group (p < 0.05). Moreover, and the number of previously recognized objects was significantly decreased (p < 0.05).
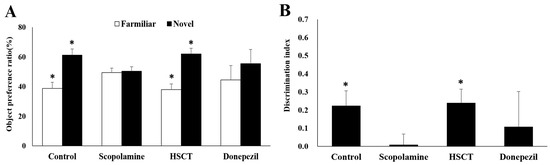
Figure 3.
Effect of HSCT on short-term memory of rats as observed in the Novel Object Recognition Test (NORT). Comparison of (A) object preference and (B) discrimination index during the retention trial of NORT. Values are means ± SEM; (A,B) were analyzed using Student’s t-test; * p < 0.05 compared with the scopolamine group. control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i.p); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i.p); donepezil, control diet + scopolamine (0.7 mg/kg, i.p) + donepezil (1.0 mg/kg, i.p); i. p., intraperitoneal injection.
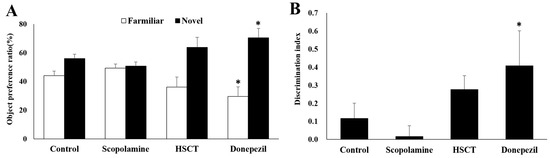
Figure 4.
Effect of HSCT on long-term memory of rats as observed in NORT. Comparison of (A) object preference and (B) discrimination index during the retention trial of NORT. Values are means ± SEM; (A,B) were analyzed using Student’s t-test; * p < 0.05 compared with the scopolamine group. control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i. p.); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i. p.); donepezil, control diet + scopolamine (0.7 mg/kg, i. p.) + donepezil (1.0 mg/kg, i. p.); i. p., intraperitoneal injection.
3.5. Y-Maze
A Y-maze test was performed on rats with scopolamine-induced memory impairment to confirm the improvement in memory and learning impairment following HSCT intake (Figure 5). Compared to those observed in the scopolamine group (49.28%), spontaneous alternations in the control group, HSCT group, and donepezil group were significantly higher (62.64%, 55.96%, and 53.87%, respectively) (p < 0.05).
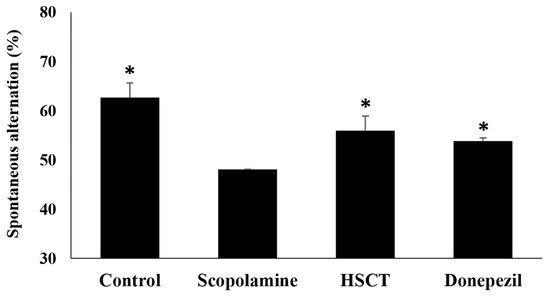
Figure 5.
Effect of HSCT on rats observed in the Y-maze test. Values are means ± SEM. Mean values were analyzed by Student’s t-test; * p < 0.05 compared with the scopolamine group. control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i. p.); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i. p.); donepezil, control diet + scopolamine (0.7 mg/kg, i. p.) + donepezil (1.0 mg/kg, i. p.); i. p., intraperitoneal injection.
3.6. ACh Content
The results of the investigation of the effect of the HSCT diet on ACh production in rats are shown in Figure 6. The ACh concentration in the scopolamine group was the lowest at 150.00 ± 20.23 pmol, and the ACh concentrations in the control group, HSCT group, or donepezil group were 495.33 ± 26.67 pmol, 366.49 ± 27.71 pmol, or 365.87 ± 19.00 pmol, respectively, which were significantly higher than those in the scopolamine group (p < 0.01).
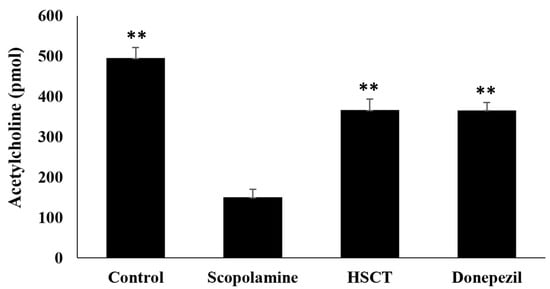
Figure 6.
Effect of HSCT on acetylcholine contents of brains of scopolamine-induced memory impairment in the rats. Mean values were analyzed using Student’s t-test; ** p < 0.01 compared with the scopolamine group. control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i. p.); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i. p.); donepezil, control diet + scopolamine (0.7 mg/kg, i. p.) + donepezil (1.0 mg/kg, i. p.); i. p., intraperitoneal injection.
3.7. AChE Activity
Results of analysis of AChE activity in mice fed HSCT diets are shown in Figure 7. The AChE activity in the scopolamine group was 402.42 ± 14.62 mU/mL, which was the highest among all the treated groups. Compared to that in the scopolamine group, AChE activity in the control, HSCT, and donepezil groups, at 254.45 ± 6.69 mU/mL, 338.03 ± 11.26 mU/mL, and 299.44 ± 12.10 mU/mL, respectively, were significantly lower (p < 0.05).
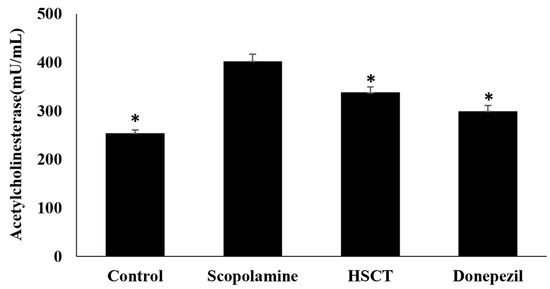
Figure 7.
Effect of HSCT on acetylcholinesterase activity of brains of scopolamine-induced memory impairment in the rats. Mean values were analyzed using Student’s t-test; * p < 0.05 compared with the scopolamine group. control, control diet; scopolamine, control diet + scopolamine (0.7 mg/kg, i. p.); HSCT, HSCT diet + scopolamine (0.7 mg/kg, i. p.); donepezil, control diet + scopolamine (0.7 mg/kg, i. p.) + donepezil (1.0 mg/kg, i. p.); i. p., intraperitoneal injection.
4. Discussion
In this study, commercially available enzymes such as bromelain, alcalase, and viscozyme were used to hydrolyze SCT. The hydrolysis pattern of HSCT was checked using SDS-PAGE, and it was confirmed that the proteins of SCT were effectively hydrolyzed by the enzyme treatment. Next, to confirm the effect of the developed HSCT on improvement of memory, we induced memory impairment in rats via intraperitoneal administration of scopolamine. One of the important causes of memory impairment in AD is decreased activity of the cholinergic system in the brain tissue [37,38]. Scopolamine, which exerts anti-muscarinic effects by acting as a muscarinic ACh receptor antagonist, increases the activity of AChE, which degrades ACh at neuronal junctions and reduces memory in the short term [39]. Because of these properties, scopolamine is often used to induce memory impairment to study memory improvement in animals [40]. When comparing the results of the scopolamine-untreated control group and scopolamine-treated group, we also found that intraperitoneal administration of scopolamine effectively induced memory decline in rats. There were no significant differences in the intake of feed and body weight changes in rats due to intraperitoneal administration of scopolamine, and no significant differences were observed in organ weights among the scopolamine-treated groups. These results were similar to those reported by Kim et al. [41], who induced memory impairment by administering scopolamine to rats.
Rats can recognize their position in a confined space or maze, and with repeated training, they can find an escape route or new areas [42]. Using these characteristics, animal behavioral tests, such as the Barnes maze test, NORT, and Y-maze test, are commonly performed to investigate memory in rats [43]. The Barnes maze test assesses memory by measuring the distance and time a rat takes to navigate in a limited space to find an escape location [44]. The NORT is a method for assessing the memory that utilizes a rat’s ability to navigate towards a new object [45], and the Y-maze test is a method for assessing the memory that utilizes a rat’s ability to navigate towards a new, previously unvisited area [46]. In the current study, we investigated the effect of HSCT consumption on memory improvement by performing Barnes maze, NORT, and Y-maze tests in rats whose memory was impaired by scopolamine. The results of the Barnes maze test showed that on the 4th day of the training period, the exploration distance and exploration time to find the escape route were significantly decreased in the HSCT and donepezil groups than in the scopolamine group (Figure 2). The donepezil group received an additional dose of donepezil as a positive control. Donepezil has been reported to be effective against severe AD by inhibiting AChE and was approved by the US Food and Drug Administration in 2010 [47]. In animal studies, donepezil has been used as a positive control to investigate its memory-enhancing effects, although its side effects may be dose dependent and are mostly transient and mild [48,49]. In the NORT study, short-term memory was significantly improved in the HSCT group compared with the scopolamine group (Figure 3); however, there was no statistically significant difference in long-term memory between the HSCT and scopolamine groups (Figure 4). Although there are many different mechanisms involved in memory improvement and this study had limitations in analyzing these mechanisms, the results of the NORT study suggest that HSCT was effective in improving short-term memory rather than long-term memory. In the Y-maze test, the spontaneous alternation value of the HSCT group was significantly higher than that of the scopolamine group (Figure 5); the spontaneous alternation value evaluates short-term memory of rats in the Y-maze test [50].
Animal behavioral experiments confirmed that HSCT consumption improved the memory of rats whose memory was impaired by scopolamine, and the mechanism of memory improvement was investigated by examining ACh content and AChE activity in the rat brain. As shown in Figure 6 and Figure 7, the ACh content in the brains of HSCT-treated rats was significantly increased and AChE activity was significantly decreased compared to that in the brains of scopolamine-treated rats. ACh is a neurotransmitter that transmits chemical messages released by neurons and is synthesized under the action of acetyl CoA and cholineacetytransferase enzymes, and plays an important role in the cholinergic system related to memory [10]. ACh is broken down into acetate and choline by AChE. Scopolamine administration increases the activity of AChE and accelerates the breakdown of ACh, resulting in decreased ACh concentrations in the brain [51]. Memory decline is thought to be directly or indirectly associated with cholinergic dysfunction in the hippocampus. For example, in the hippocampus of patients with AD, ACh synthesis is reduced, and AChE activity is increased [41]. This is because a decreased concentration of ACh, a neurotransmitter that plays an important role in memory and learning in the brain, can lead to memory impairment [37]. Therefore, to treat memory loss in AD, AChE inhibitors such as donepezil are administered to reduce AChE activity and increase the brain ACh content, thereby alleviating the symptoms of AD [52]. Based on these results, HSCT is expected to improve memory impairment induced by increased AChE activity and decreased brain ACh concentration following scopolamine administration. In addition, the memory improvement effect of HSCT in animal behavioral experiments may be due to an increase in brain ACh concentration caused by a decrease in AChE activity. Currently, drugs used clinically to reduce AChE activity have temporary effects, and their use is limited because they cause side effects such as cardiac bradycardia, gastrointestinal disorders, and liver toxicity when consumed over a long period [41]. Therefore, it is necessary to develop natural product-derived materials, which can be consumed over a long period of time and do not have any side effects, to improve memory decline caused by AD. HSCT has the potential to be utilized as a natural substance to improve memory.
5. Conclusions
In summary, we investigated the memory-enhancing effects and mechanisms of HSCT in rats with memory impairment induced by scopolamine administration. In rats treated with scopolamine, HSCT decreased AChE activity and increased ACh concentration, which are important for learning and memory, and improved the memory of HSCT-treated rats in animal behavioral tests, such as the BM test, NORT, and Y-maze test. However, a limitation of our study is that the results were not confirmed at a higher dose than the dose of HSCT used in this study and mechanisms other than ACh and AChE activities related to memory improvement were not investigated. In addition, we did not compare the functionality of silkworm hydrolysate fed a different diet to silkworm hydrolysate fed Cudrania tricuspidata leaves. Therefore, it is necessary to investigate dose-dependent memory improvement effects of HSCT in subsequent studies to explore the optimal dose of HSCT and further study the various mechanisms involved in memory improvement. It is also necessary to conduct more detailed studies of the properties of hydrolysates to improve our understanding of the active substances. If these studies are conducted sequentially, HSCT might become a highly valuable natural functional agent that can be used to effectively improve memory decline caused by AD.
Author Contributions
Conceptualization, S.-S.Y. and K.-S.H.; methodology, G.-M.A., T.-H.J. and K.-S.H.; software, J.-H.C. and M.-J.N.; validation, M.-J.N. and S.-S.Y.; formal analysis, G.-M.A. and T.-H.J. investigation, G.-M.A. and J.-H.C.; data curation, G.-M.A., J.-H.C. and M.-J.N.; writing—original draft preparation, G.-M.A. and T.-H.J.; writing—review and editing, T.-H.J. and K.-S.H.; supervision, K.-S.H.; project administration, T.-H.J. and K.-S.H.; funding acquisition, S.-S.Y. and K.-S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by a grant of the Agricultural Technology Commercialization Support Project through the Rural Development Administration, Republic of Korea (Project number RS-2022-KO000997).
Institutional Review Board Statement
This study was approved by the Sahmyook University Animal Ethics Committee (SYUIACUC2022-002). Animal procedures were performed in strict accordance with the guidelines of the National Research Council and Institutional Animal Care and Use Committee (Seoul, Republic of Korea).
Informed Consent Statement
Not applicable.
Data Availability Statement
The data generated for this study are available upon reasonable request from the corresponding author.
Acknowledgments
We appreciate to the student researchers who assisted with the animal experiments in this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, E.J.; Lee, K.H. Knowledge, attitude, and performance of nurses in a tertiary hospital toward older adults. J. Korean Gerontol. Nurs. 2020, 22, 165–173. [Google Scholar] [CrossRef]
- Hwang, S.J. Population aging and generational conflict: Intergenerational equity over resource allocation. J. Soc. Sci. 2022, 33, 149–172. [Google Scholar] [CrossRef]
- Han, G.S.; Yan, E.J. Status of health and nutritional intake of the elderly in long-term care facilities: Focus on Gwangju Metropolitan City. J. Nutr. Health 2020, 53, 27–38. [Google Scholar] [CrossRef]
- Kim, Y.E.; Park, J.H. A study on risk factors for the prevalence of dementia: Geographically weighted regression. JKAIS 2021, 22, 662–670. [Google Scholar] [CrossRef]
- Livingston, G.; Sommerlad, A.; Orgeta, V.; Costafreda, S.G.; Huntley, J.; Ames, D.; Ballard, C.; Banerjee, S.; Burns, A.; Cohen-Mansfield, J. Dementia prevention, intervention, and care. Lancet 2017, 390, 2673–2734. [Google Scholar] [CrossRef]
- Livingston, G.; Huntley, J.; Sommerlad, A.; Ames, D.; Ballard, C.; Banerjee, S.; Brayne, C.; Burns, A.; Cohen-Mansfield, J.; Cooper, C.; et al. Dementia prevention, intervention, and care: 2020 report of the Lancet Commission. Lancet 2020, 396, 413–446. [Google Scholar] [CrossRef]
- Latimer, C.S.; Keene, C.D.; Flanagan, M.E.; Hemmy, L.S.; Lim, K.O.; White, L.R.; Montine, K.S.; Montine, T.J. Resistance to Alzheimer disease neuropathologic changes and apparent cognitive resilience in the Nun and Honolulu-Asia Aging Studies. J. Neuropathol. Exp. Neurol. 2017, 76, 458–466. [Google Scholar] [CrossRef]
- Fiest, K.M.; Roberts, J.I.; Maxwell, C.J.; Hogan, D.B.; Smith, E.E.; Frolkis, A.; Cohen, A.; Kirk, A.; Pearson, D.; Pringsheim, T.; et al. The prevalence and incidence of dementia due to Alzheimer’s disease: A systematic review and meta-analysis. Can. J. Neurol. Sci. 2016, 43, S51–S82. [Google Scholar] [CrossRef]
- Verma, S.; Kumar, A.; Tripathi, T.; Kumar, A. Muscarinic and nicotinic acetylcholine receptor agonists: Current scenario in Alzheimer’s disease therapy. J. Pharm. Pharmacol. 2018, 70, 985–993. [Google Scholar] [CrossRef] [PubMed]
- Decker, A.L.; Duncan, K. Acetylcholine and the complex interdependence of memory and attention. Curr. Opin. Behav. Sci. 2020, 32, 21–28. [Google Scholar] [CrossRef]
- Hasselmo, M.E. The role of acetylcholine in learning and memory. Curr. Opin. Neurobiol. 2006, 16, 710–715. [Google Scholar] [CrossRef]
- Choi, M.R.; Lee, M.Y.; Kim, J.E.; Hong, J.E.; Jang, K.H.; Lee, J.Y.; Chun, J.W.; Kim, T.H.; Shin, H.K.; Kim, E.J. Rubus Coreanus Miquel Improves on Impairment of Memory in Senescence-Accelerated Mouse (SAM). J. Korean Soc. Food Sci. Nutr. 2022, 41, 1253–1258. [Google Scholar] [CrossRef]
- Rastegar-Moghaddam, S.H.; Hosseini, M.; Alipour, F.; Rajabian, A.; Bideskan, A.E. The effects of vitamin D on learning and memory of hypothyroid juvenile rats and brain tissue acetylcholinesterase activity and oxidative stress indicators. Naunyn Schmiedebergs Arch. Pharmacol. 2022, 395, 337–351. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.Y. Past and Future of Drug Treatments for Alzheimer’s Disease. J. Korean Neuropsychiatr. Assoc. 2018, 57, 30–42. [Google Scholar] [CrossRef]
- Cagnin, A.; Brooks, D.J.; Kennedy, A.M.; Gunn, R.N.; Myers, R.; Turkheimer, F.E.; Jones, T.; Banati, D.R.B. In-vivo measurement of activated microglia in dementia. Lancet 2001, 358, 461–467. [Google Scholar] [CrossRef]
- Sano, M.; Bell, K.L.; Galasko, D.; Galvin, J.E.; Thomas, R.G.; van Dyck, C.H.; Aisen, P.S. A randomized, double-blind, placebo-controlled trial of simvastatin to treat Alzheimer disease. Neurology 2011, 77, 556–563. [Google Scholar] [CrossRef]
- Feldman, H.H.; Doody, R.S.; Kivipelto, M.; Sparks, D.L.; Waters, D.D.; Jones, R.W.; Schwam, E.; Schindler, R.; Hey-Hadavi, J.; Demicco, D.A.; et al. Randomized controlled trial of atorvastatin in mild to moderate Alzheimer disease: LEADe. Neurology 2010, 74, 956–964. [Google Scholar] [CrossRef]
- Bentham, P.; Gray, R.; Sellwood, E.; Hills, R.; Crome, P.; Raftery, J. Aspirin in Alzheimer’s disease (AD2000): A randomised open-label trial. Lancet Neurol. 2008, 7, 41–49. [Google Scholar] [CrossRef] [PubMed]
- Jaturapatporn, D.; Isaac, M.G.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012, 15, CD006378. [Google Scholar] [CrossRef]
- Banday, M.T.; Adil, S.; Sheikh, I.U.; Hamadani, H.; Qadri, F.I.; Sahfi, M.E.; Sait, H.S.A.W.; Abd El-Mageed, T.A.; Salem, H.M.; Taha, A.E.; et al. The use of silkworm pupae (Bombyx mori) meal as an alternative protein source for poultry. Worlds Poult. Sci. J. 2023, 79, 119–134. [Google Scholar] [CrossRef]
- Cermeno, M.; Bascon, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolysates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Yoon, J.W.; Rhee, S.K.; Lee, K.B. Effects of Silkworm Extract Powder on Plasma Lipids and Glucose in Rats. J. Korean Soc. Food Sci. Nutr. 2005, 18, 140–145. [Google Scholar]
- Ji, S.D.; Shin, K.H.; Ahn, D.K.; Cho, S.Y. The mass production technology and pharmaceutical effect of silkworm cordyceps (Peacilomyces tenuipes). Food Sci. Ind. 2003, 36, 38–48. [Google Scholar] [CrossRef]
- Hu, Y.; Shi, Q.; Ying, S.; Zhu, D.; Chen, H.; Yang, X.; Xu, J.; Xu, F.; Tao, F.; Xu, B. Effects of compound Caoshi silkworm granules on stable COPD patients and their relationship with gut microbiota. A randomized controlled trial. Medicine 2020, 99, e20511. [Google Scholar] [CrossRef] [PubMed]
- Baek, S.Y.; Li, F.Y.; Kim, J.H.; Ahn, C.W.; Kim, H.J.; Kim, M.R. Protein hydrolysate of silkworm pupa prevents memory impairment induced by oxidative stress in scopolamine-induced mice via modulating the cholinergic nervous system and antioxidant defense system. Prev. Nutr. Food Sci. 2020, 25, 389–399. [Google Scholar] [CrossRef]
- Zhou, Y.; Zhou, S.; Duan, H.; Wang, H.; Yan, W. Silkworm pupae: A functional food with health benefits for humans. Foods 2022, 11, 1594. [Google Scholar] [CrossRef]
- Lee, Y.S.; Rho, J.O. A study on quality characteristics of Kimchi with added mulberry leaves extracts. J. East Asian Soc. Diet. Life 2014, 24, 827–836. [Google Scholar] [CrossRef]
- Son, H.K.; Han, J.H.; Lee, J.J. Anti-diabetic effect of the mixture of mulberry leaf and green tea powder in rats with streptozotocin-induced diabetes. Korean J. Food Preserv. 2014, 21, 549–559. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, K.; Yamada, N.; Endo, S.; Kurogi, K.; Sakakibara, Y.; Suiko, M. Novel silkworm (Bombyx mori) sulfotransferase SWSULT ST3 is involved in metabolism of polyphenols from mulberry leaves. PLoS ONE 2022, 17, e0270804. [Google Scholar] [CrossRef]
- Do, G.P.; Lee, H.J.; Do, J.R.; Kim, H.K. Inhibition of adipogenesis in 3T3-L1 adipocytes with water and ethanol extracts of Cudrania tricuspidata Leaves. Korean J. Food Preserv. 2011, 18, 244–249. [Google Scholar] [CrossRef]
- Lee, H.J.; Do, J.R.; Kwon, J.H.; Kim, H.K. Physiological activities of extracts from different parts of Cudrania tricuspidata. J. Korean Soc. Food Sci. Nutr. 2011, 40, 942–948. [Google Scholar] [CrossRef]
- Cuong, T.V.; Chin, K.B. Evaluation of Cudrania tricuspidata leaves on antioxidant activities and physicochemical properties of pork patties. Korean J. Food Sci. Anim. Resour. 2018, 38, 889–900. [Google Scholar] [CrossRef]
- Choi, J.H.; Nam, M.J.; Ryu, G.H.; Jeon, J.W.; Yun, S.S. Quantitative analysis of chemical components of hydrolysate from silkworm fed with Cudrania tricuspidata Leaves. Biomed. Sci. Lett. 2022, 28, 322–326. [Google Scholar] [CrossRef]
- Azm, N.A.E.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of bioactive compounds from the sulfated polysaccharides extracts of ulva lactuca: Post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Cui, C.; Zhao, H.; Yang, B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolysates. Innov. Food Sci. Emerg. Technol. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- Kim, D.W.; Park, K.; Ha, G.; Jung, J.R.; Chang, O.; Ham, J.S.; Jeong, S.G.; Park, B.Y.; Song, J.; Jang, A.R. Anti-oxidative and neuroprotective activities of pig skin gelatin hydrolysates. Korean J. Food Sci. Anim. Resour. 2013, 33, 258–267. [Google Scholar] [CrossRef]
- Kuhl, D.E.; Koeppe, R.A.; Minoshima, S.; Snyder, S.E.; Ficaro, E.P.; Foster, N.L.; Frey, K.A.; Kilbourn, M.R. In vivo mapping od cerebral acetylcholinesterase activity in aging and Alzheimer’s disease. Neurology 1999, 52, 691–699. [Google Scholar] [CrossRef]
- Kasa, P.; Papp, H.; Kasa, P.; Torok, I. Donepezil dose dependently inhibits acetylcholinesterase activity in various areas and in the presynaptic cholinergic and the postsynaptic cholinoceptive enzyme-positive structures in the human and rat brain. Neuroscience 2000, 101, 89–100. [Google Scholar] [CrossRef]
- Bartus, R.T. On neurodegenerative diseases, models, and treatment strategies: Lessons learned and lessons forgotten a generation following the cholinergic hypothesis. Exp. Neurol. 2000, 163, 495–529. [Google Scholar] [CrossRef]
- Ebert, U.; Kirch, W. Scopolamine model of dementia: Electroencephalogram findings and cognitive performance. Eur. J. Clin. Investig. 1998, 28, 944–949. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Noh, B.W.; Pang, Q.Q.; Lee, S.H.; Kim, J.H.; Cho, E.J. Protective mechanism of cirsium japonicum var. maackii against scopolamine-induced cognitive impairment. J. Agric. Life Environ. Sci. 2022, 34, 73–87. [Google Scholar] [CrossRef]
- Morris, R. Developments of a water-maze procedure for studying spatial learning in the rat. J. Neurosci. Methods 1984, 11, 47–60. [Google Scholar] [CrossRef]
- Sun, N.; Wang, H.; Wang, X.Y.; Yu, Q.; Han, J.Y.; Huang, Y.; Zhou, W.X. Deletion of AhR attenuates fear memory leaving other types of memory intact. Behav. Brain Res. 2023, 451, 114505. [Google Scholar] [CrossRef] [PubMed]
- Gawei, K.; Gibula, E.; Marszalek-Grabska, M.; Fliarowska, J.; Kotlinska, J.H. Assessment of spatial learning and memory in the Barnes maze task in rodents—Methodological consideration. Naunyn. Schmiedebergs Arch. Pharmacol. 2018, 392, 1–18. [Google Scholar] [CrossRef]
- Mihaylova, A.; Doncheva, N.; Zlatanova, H.; Delev, D.; Ivanovska, M.; Koeva, Y.; Murdjeva, M.; Kostadinov, L. Dopaminergic agonist pramipexole improves memory and increases IL-10 production in LPS-challenged rats. Iran. J. Basic Med. Sci. 2021, 24, 577–585. [Google Scholar] [CrossRef]
- Lee, J.H.; Park, S.H.; Lee, C.H.; Kim, H.J.; Jung, C.J.; Beik, G.Y.; Shin, J.G.; Jung, J.W. Investigating the effect of Crataegus pinnatifida, a functional food, on cognition and memory deficit. Korean J. Food Preserv. 2019, 26, 238–245. [Google Scholar] [CrossRef]
- Farlow, M.R.; Salloway, S.; Tariot, P.N.; Yardley, J.; Moline, M.L.; Wang, Q.; Brand-Schieber, E.; Zou, H.; Hsu, T.; Satlin, A. Effectiveness and tolerability of high-dose (23 mg/d) versus standard dose (10 mg/d) donepezil in moderate to severe Alzheimer’s disease: A 24-week, randomized, double-blind study. Clin. Ther. 2010, 32, 1234–1251. [Google Scholar] [CrossRef]
- Youn, H.C.; Jeong, H.G. Pharmacotherapy for dementia. J. Korean Med. Assoc. 2018, 61, 758–764. [Google Scholar] [CrossRef]
- Lee, S.E.; Park, S.B.; Kweon, H.Y.; Park, J.Y.; Lee, J.Y.; Jo, Y.Y.; Lee, J.H.; Jang, G.Y.; Choi, S.J.; Kim, D.H. Cognition improving effect of the compositions prepared with extracts of Wongam, Sorghum bicolor (L.) Moench and pupae of Bombyx mori L. Korean J. Food Preserv. 2021, 28, 989–999. [Google Scholar] [CrossRef]
- Prieur, E.A.K.; Jadavii, N.M. Assessing spatial working memory using the spontaneous alternation Y-maze test in aged male mice. Bio Protoc. 2019, 9, e3162. [Google Scholar] [CrossRef]
- Botton, P.H.; Costa, M.S.; Ardais, A.P.; Mioranzza, S.; Souza, D.O.; da Rocha, J.B.T.; Porciuncula, L.O. Caffeine prevents disruption of memory consolidation in the inhibitory avoidance and novel object recognition tasks by scopolamine in adult mice. Behav. Brain Res. 2010, 214, 254–259. [Google Scholar] [CrossRef] [PubMed]
- Pisani, S.; Mueller, C.; Huntley, J.; Aarsland, D.; Kempton, M.J. A meta-analysis of randomised controlled trials of physical activity in people with Alzheimer’s disease and mild cognitive impairment with a comparison to donepezil. Int. J. Geriatr. Psychiatry 2021, 36, 1471–1487. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).