Protective Effects on Neuronal SH-SY5Y Cells and Antioxidant Activity of Enzymatic Hydrolyzate from Silkworms Fed the Leaves of Cudrania tricuspidata
Abstract
1. Introduction
2. Materials and Methods
2.1. Enzymatic Preparation of Silkworm Hydrolyzate
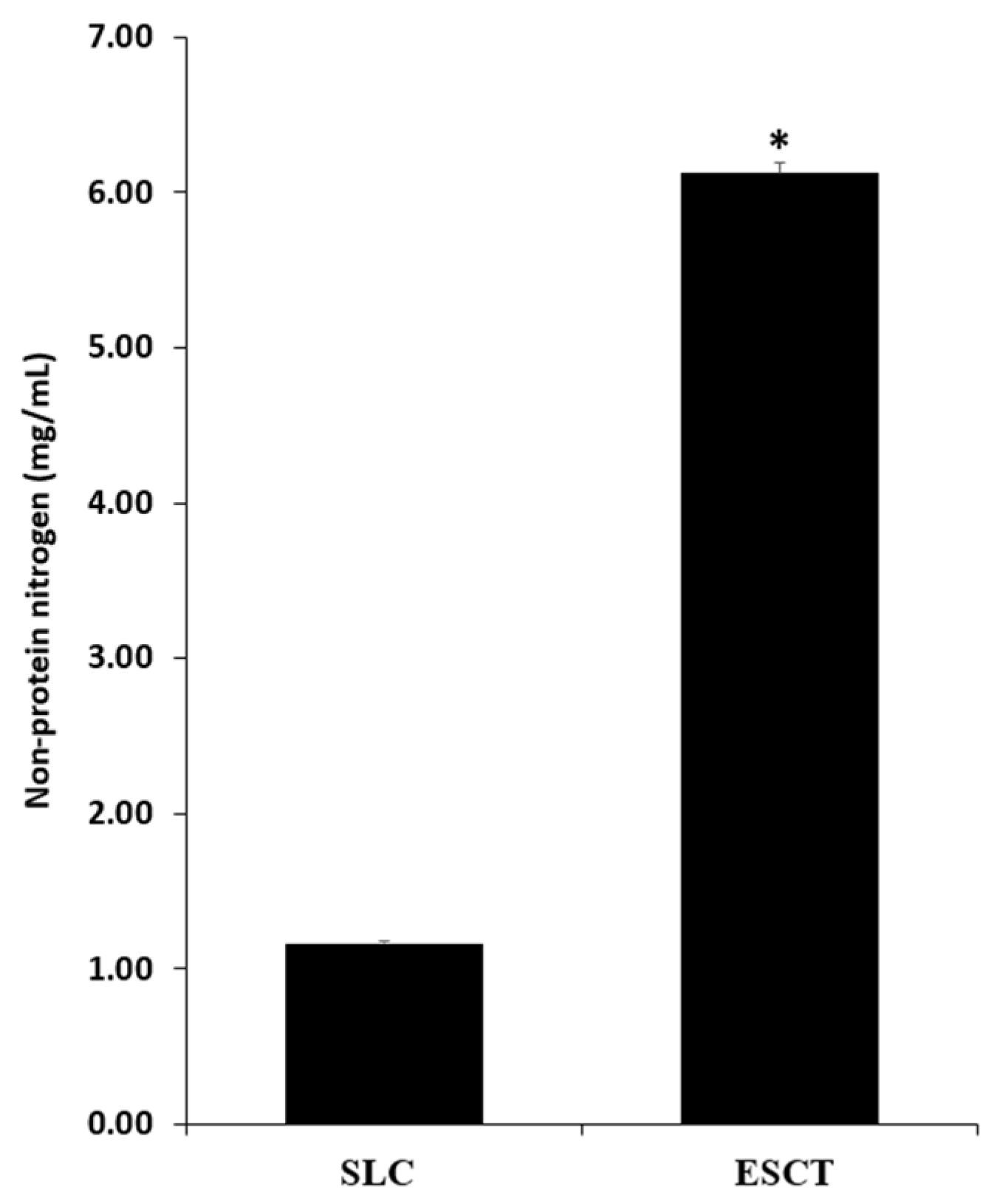
2.2. Non-Protein Nitrogen (NPN)
2.3. Determination of the Amino Acid Composition of Silkworm Hydrolyzate
2.4. Determination of Flavonoid Concentration in Hydrolyzate from Silkworms
2.5. Determination of Polyphenol Concentration in Hydrolyzate from Silkworms
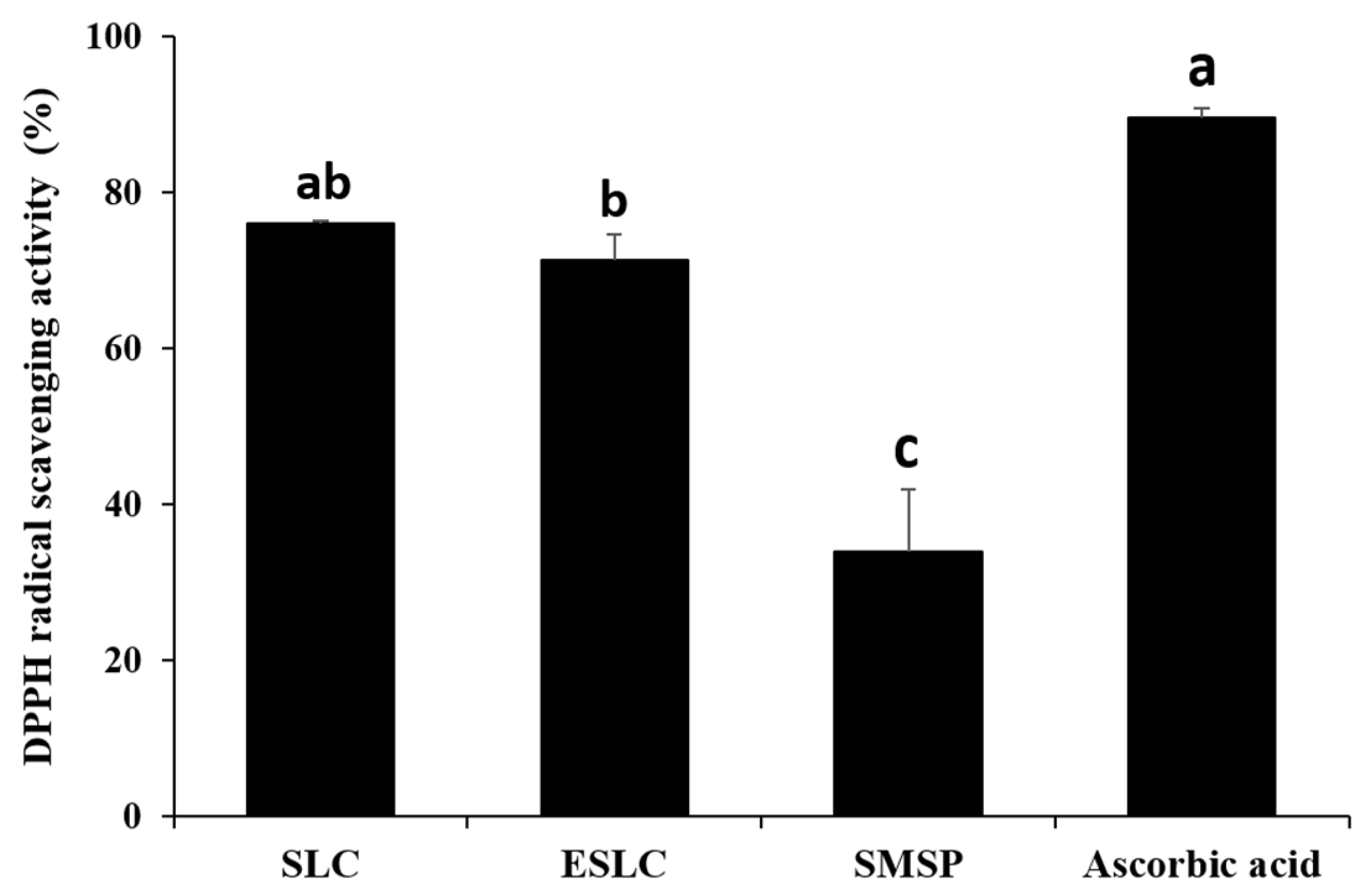
2.6. The 2,2-diphenyl-1-picrylhydrazyl (DPPH) Radical Scavenging
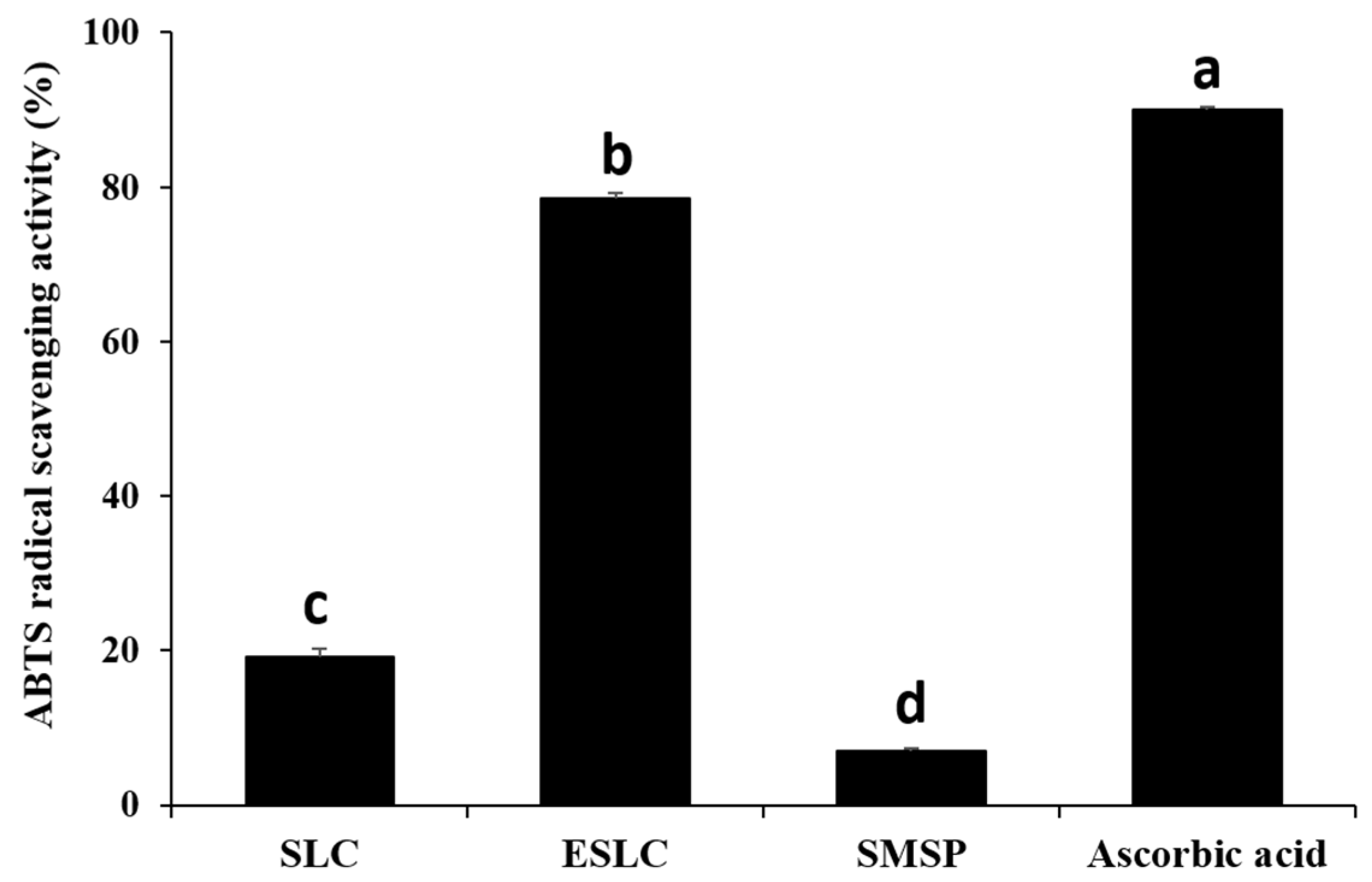
2.7. 2,2′-Azino-bis(3-ethylbenzothiazoline-6-sulfonicacid) Diammonium Salt (ABTS) Radical Scavenging
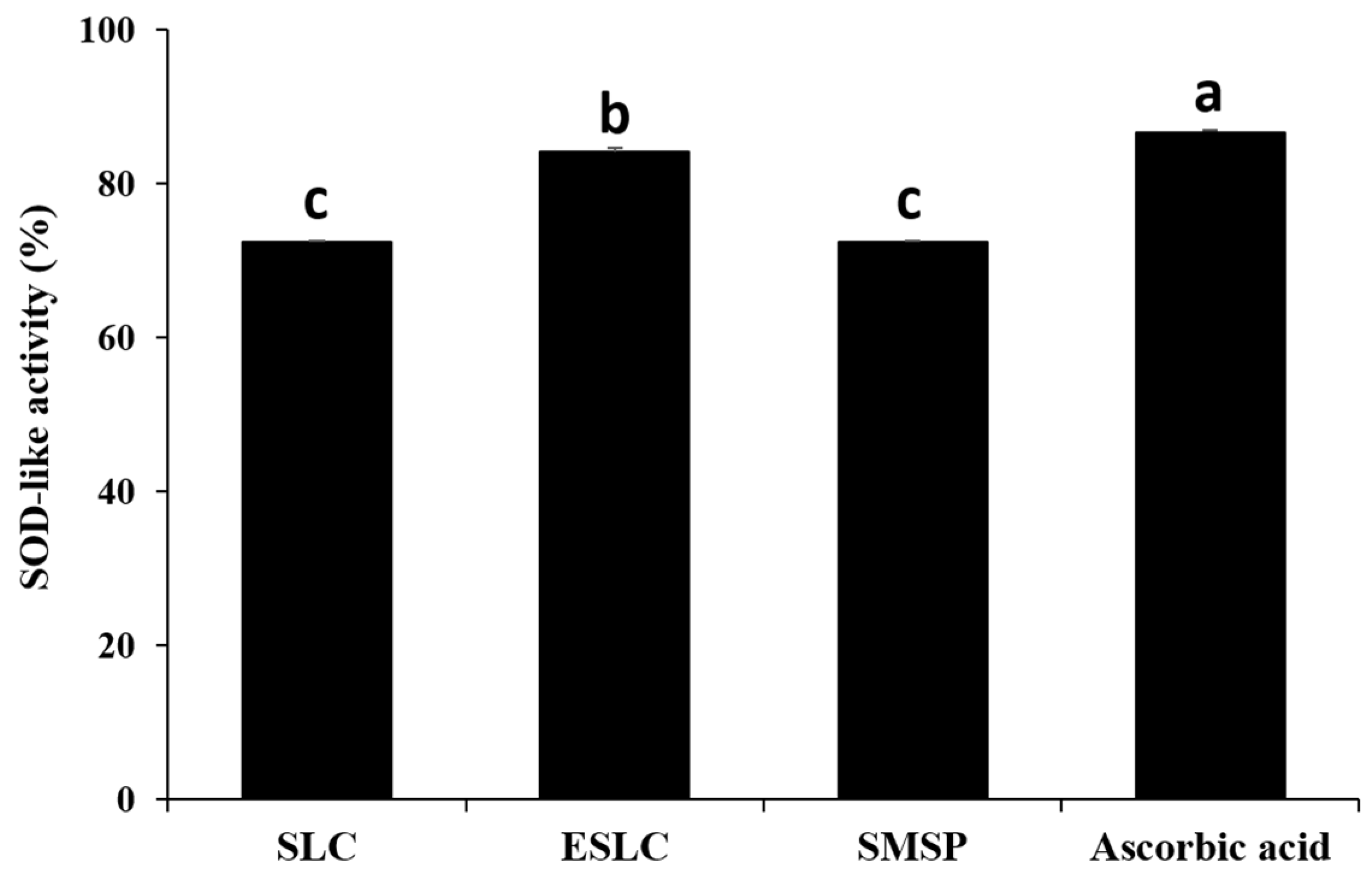
2.8. Superoxide Dismutase (SOD)-Like Activity
2.9. SH-SY5Y Cell Treatment and Cell Viability
2.10. Antioxidant Activity
2.11. Western Blot
2.12. Statistical Analysis
3. Results
3.1. NPN
3.2. Composition of Amino Acid
3.3. Total Flavonoid and Polyphenol Contents
3.4. DPPH Radical Scavenging Activity
3.5. ABTS Radical Scavenging Activity
3.6. SOD-like Activity
3.7. Neuroprotective Effect of ESLC on H2O2-Induced Oxidative Stress in SH-SY5Y Cells
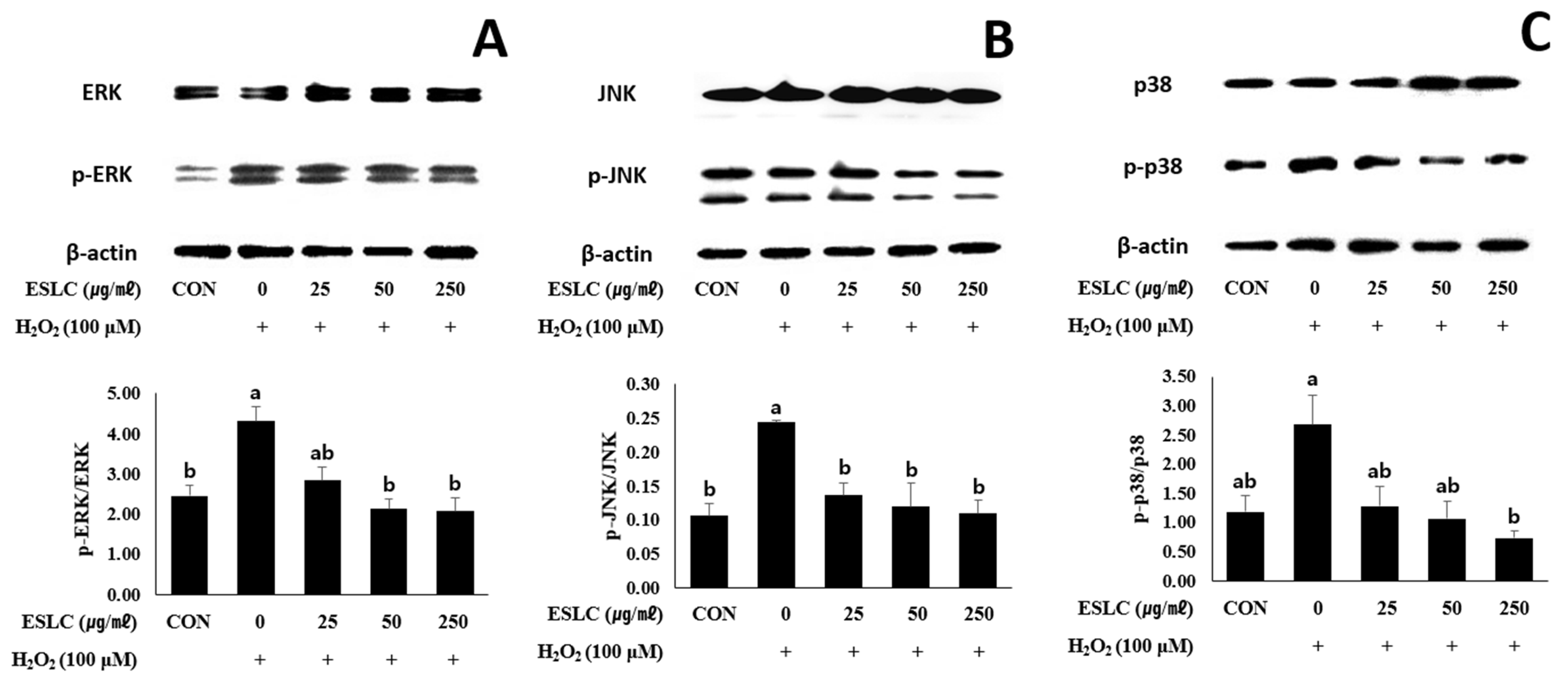
3.8. The Impact of ESLC on the ERK, JNK, and p38 Pathways in SH-SY5Y Cells Stimulated with H2O2
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Alston, J.M.; Beddow, J.M.; Pardey, P.G. Agricultural research, productivity, and food prices in the long run. Science 2009, 325, 1209–1210. [Google Scholar] [CrossRef]
- Song, M.H.; Han, M.H.; Lee, S.H.; Kim, E.S.; Park, K.H.; Kim, W.T.; Choi, J.Y. A field survey on edible insect farms in Korea. J. Life Sci. 2017, 27, 702–707. [Google Scholar] [CrossRef]
- van Huis, A. Potential of insects as food and feed in assuring food security. Annu. Rev. Entomol. 2013, 58, 563–583. [Google Scholar] [CrossRef]
- Nakagaki, B.J.; Defoliart, G.R. Comparison of diets for mass-rearing acheta domesticus (Orthoptera, Gryllidae) as a novelty food, and comparison of food conversion efficiency with values reported for livestock. J. Econ. Entomol. 1991, 84, 84891–84896. [Google Scholar] [CrossRef]
- Oonincx, D.G.; van Itterbeeck, J.; Heetkamp, M.J.W.; van den Brand, H.; van Loon, J.J.A.; van Huis, A. An exploration on greenhouse gas and ammonia production by insect species suitable for animal or human consumption. PLoS ONE 2010, 5, e14445. [Google Scholar] [CrossRef]
- Yoo, J.M.; Hwang, J.S.; Goo, T.W.; Yun, E.Y. Comparative analysis of nutritional and harmful components in Korean and Chinese mealworms (Tenebrio molitor). J. Korean Soc. Food Sci. Nutr. 2013, 42, 249–254. [Google Scholar] [CrossRef]
- Hwang, D.S.; Lim, C.H.; Lee, S.H.; Yun, E.Y. Activation plan for the edible insect industry by improving perception. Food Sci. Nutr. 2022, 55, 128–139. [Google Scholar] [CrossRef]
- Baek, M.; Hwang, J.S.; Kim, M.A.; Kim, S.H.; Goo, T.W.; Yun, E.Y. Comparative analysis of nutritional components of edible insects registered as novel foods. J. Life Sci. 2017, 27, 334–338. [Google Scholar] [CrossRef]
- Noh, J.H.; Yun, E.Y.; Park, H.; Jung, K.J.; Hwang, J.S.; Jeong, E.J.; Moon, K.S. Subchronic oral dose toxicity of freeze-dried power of Allomyrina dichotoma larvae. Toxicol. Res. 2015, 31, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Han, S.R.; Lee, B.S.; Jung, K.J.; Yu, H.J.; Yun, E.Y.; Hwang, J.S.; Moon, K.S. Safety assessment of freeze-dried powdered Tenebrio molitor larvae (yellow mealworm) as novel food source: Evaluation of 90-day toxicity in Sprague-Dawley rats. Regul. Toxicol. Pharmacol. 2016, 77, 206–212. [Google Scholar] [CrossRef] [PubMed]
- Noh, J.H.; Jeong, J.S.; Park, S.J.; Yun, E.Y.; Hwang, J.S.; Kim, J.S.; Jung, K.J.; Park, H.J.; Son, H.Y.; Moon, K.S. Toxicological safety evaluation of freeze-dried Protaetia brevitarsis larva powder. Toxicol. Rep. 2018, 5, 695–703. [Google Scholar] [CrossRef]
- Banday, M.T.; Adil, S.; Sheikh, I.U.; Hamadani, H.; Qadri, F.I.; Sahfi, M.E.; Sait, H.S.A.W.; Abd El-Mageed, T.A.; Salem, H.M.; Taha, A.E.; et al. The use of silkworm pupae (Bombyx mori) meal as an alternative protein source for poultry. Worlds Poult. Sci. J. 2023, 79, 119–134. [Google Scholar] [CrossRef]
- Ji, S.D.; Shin, K.H.; Ahn, D.K.; Cho, S.Y. The mass production technology and pharmaceutical effect of silkworm cordyceps (peacilomyces tenuipes). Food Sci. Ind. 2003, 36, 38–48. [Google Scholar]
- Yoon, J.W.; Rhee, S.K.; Lee, K.B. Effects of silkworm extract powder on plasma lipids and glucose in rats. J. Korean Soc. Food Sci. Nutr. 2005, 18, 140–145. [Google Scholar]
- Hu, Y.; Shi, Q.; Ying, S.; Zhu, D.; Chen, H.; Yang, X.; Xu, J.; Xu, F.; Tao, F.; Xu, B. Effects of compound Caoshi silkworm granules on stable COPD patients and their relationship with gut microbiota. A randomized controlled trial. Medicine 2020, 99, e20511. [Google Scholar] [CrossRef]
- Cermeno, M.; Bascon, C.; Amigo-Benavent, M.; Felix, M.; FitzGerald, R.J. Identification of peptides from edible silkworm pupae (Bombyx mori) protein hydrolyzates with antioxidant activity. J. Funct. Foods 2022, 92, 105052. [Google Scholar] [CrossRef]
- Lee, Y.S.; Rho, J.O. A study on quality characteristics of Kimchi with added mulberry leaves extracts. J. East. Asian Soc. Diet. Life 2014, 24, 827–836. [Google Scholar] [CrossRef]
- Son, H.K.; Han, J.H.; Lee, J.J. Anti-diabetic effect of the mixture of mulberry leaf and green tea powder in rats with streptozotocin-induced diabetes. Korean J. Food Preser. 2014, 21, 549–559. [Google Scholar] [CrossRef][Green Version]
- Yamamoto, K.; Yamada, N.; Endo, S.; Kurogi, K.; Sakakibara, Y.; Suiko, M. Novel silkworm (Bombyx mori) sulfotransferase swSULT ST3 is involved in metabolism of polyphenols from mulberry leaves. PLoS ONE 2022, 17, e0270804. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.J.; Do, J.R.; Kwon, J.H.; Kim, H.K. Physiological activities of extracts from different parts of Cudrania Tricuspidata. J. Korean Soc. Food Sci. Nutr. 2011, 40, 942–948. [Google Scholar] [CrossRef]
- Choi, J.H.; Nam, M.J.; Ryu, G.H.; Jeon, J.W.; Yun, S.S. Quantitative analysis of chemical components of hydrolyzate from silkworm fed with Cudrania tricuspidata Leaves. Biomed. Sci. Lett. 2022, 28, 322–326. [Google Scholar] [CrossRef]
- Byun, E.B.; Jang, B.S.; Sung, N.Y.; Byun, E.H. Immunomodulatory activity of crude polysaccharide separated from Cudrania tricuspidata leaf. J. Korean Soc. Food Sci. Nutr. 2016, 45, 1099–1106. [Google Scholar] [CrossRef]
- Kim, Y.E.; Cho, E.J.; Byun, E.H. Antioxidant and neuroprotective effects of crude polysaccharide fractions from Cudrania tricuspidata fruits. Korean J. Food Sci. Technol. 2018, 50, 543–548. [Google Scholar] [CrossRef]
- Azm, N.A.E.; Fleita, D.; Rifaat, D.; Mpingirika, E.Z.; Amleh, A.; El-Sayed, M.M.H. Production of bioactive compounds from the sulfated polysaccharides extracts of ulva lactuca: Post-extraction enzymatic hydrolysis followed by ion-exchange chromatographic fractionation. Molecules 2019, 24, 2132. [Google Scholar] [CrossRef]
- You, L.; Zhao, M.; Cui, C.; Zhao, H.; Yang, B. Effect of degree of hydrolysis on the antioxidant activity of loach (Misgurnus anguillicaudatus) protein hydrolyzates. Innov. Food Sci. Emerg. Technol. 2009, 10, 235–240. [Google Scholar] [CrossRef]
- Cho, D.H.; Kim, M.J.; Sim, E.Y.; Jeon, Y.H.; Lee, C.K.; Woo, K.S. Effect of carbohydrase treatments on phenolics content and antioxidant activity of maize flour. Korean J. Food Sci. Technol. 2018, 50, 132–137. [Google Scholar] [CrossRef]
- Davis, W.B. Determination of flavanones in citrus fruits. Anal. Chem. 1947, 19, 476–478. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventos, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. Methods Enzymol. 1999, 299, 152–178. [Google Scholar] [CrossRef]
- Piao, X.M.; Jang, E.K.; Chung, J.W.; Lee, G.A.; Lee, H.S.; Sung, J.S.; Jeon, Y.A.; Lee, J.R.; Kim, Y.G.; Lee, S.Y. Variation in antioxidant activity and polyphenol content in tomato stems and leaves. Plant Breed. Biotech. 2013, 1, 366–373. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Marklund, S.; Marklund, G. Involvement of the superoxide anion radical in the autoxidation of pyrogallol and convenient assay for superoxide dismutase. Eur. J. Biochem. 1974, 47, 469–474. [Google Scholar] [CrossRef]
- Lee, P.H.; Park, S.Y.; Jang, T.H.; Yim, S.H.; Nam, S.H.; In, M.J.; Kim, D.C.; Chae, H.J. Effects of complex carbohydrase treatment on physiological activities of pear peel and core. J. Korean Soc. Food Sci. Nutr. 2014, 43, 404–410. [Google Scholar] [CrossRef]
- Kim, E.J.; Chol, J.Y.; Yu, M.; Kim, M.Y.; Lee, S.; Lee, B.H. Total polyphenols, total flavonoid contents, and antioxidant activity of Korean natural and medicinal plants. Korean J. Food Sci. Technol. 2012, 44, 337–342. [Google Scholar] [CrossRef]
- Arranz, S.; Calixto, F.S. Analysis of polyphenols in cereals may be improved performing acidic hydrolysis: A study in wheat flour and wheat bran and cereals of the diet. J. Cereal Sci. 2010, 51, 313–318. [Google Scholar] [CrossRef]
- Hwang, I.G.; Woo, K.S.; Kim, T.M.; Kim, D.J.; Yang, M.H.; Jeong, H.S. Change of physicochemical characteristics of Korean pear (Pyrus pyrifolia Nakai) juice with heat treatment conditions. Korean J. Food Sci. Technol. 2006, 38, 342–347. [Google Scholar]
- Sung, H.M.; Kim, S.H.; Kim, K.M.; Yun, S.K.; Jung, H.J.; Kim, T.Y.; Wee, J.H. Antioxidant activity of soy-sprout extracts prepared by enzyme and ultra-high pressure. J. Korean Soc. Food Sci. Nutr. 2014, 43, 1228–1235. [Google Scholar] [CrossRef]
- Jeong, H.J.; Park, S.B.; Kim, S.; Kim, H.K. Total polyphenol content and antioxidative activity of wild grape (Vitiscoignetiae) extracts depending on ethanol concentrations. J. Korean Soc. Food Sci. Nutr. 2007, 36, 1491–1496. [Google Scholar] [CrossRef]
- Jung, H.N.; Lee, K.U.; Hwang, K.T.; Kwak, H.K. Antioxidant properties of polyphenol fractions from cranberry powder in LPS-stimulated RAW264.7 cells. J. Korean Soc. Food Sci. Nutr. 2015, 44, 1241–1247. [Google Scholar] [CrossRef]
- Park, B.Y.; Yoon, K.Y. Conditions for hydrolysis of perilla seed meal protein for producing hydrolyzates and ultrafiltered peptides and their antioxidant activity. Korean J. Food Preserv. 2018, 25, 605–612. [Google Scholar] [CrossRef]
- Jang, H.R.; Liceaga, A.M.; Yoon, K.Y. Purification, characterisation and stability of an antioxidant peptide derived from sandfish (Arctoscopus japonicus) protein hydrolyzates. J. Funct. Foods 2016, 20, 433–442. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Y.; Liu, Y.; Ruan, R. Bioactive peptides derived from traditional Chinese medicine and traditional Chinese food: A review. Food Res. Int. 2016, 89, 63–73. [Google Scholar] [CrossRef] [PubMed]
- Balaban, R.S.; Nemoto, S.; Finkel, T. Mitochondria, oxidants, and aging. Cell 2005, 120, 483–495. [Google Scholar] [CrossRef] [PubMed]
- Valko, M.; Leibfritz, D.; Moncol, J.; Cronin, M.T.D.; Mazur, M.; Telser, J. Free radicals and antioxidants in normal physiological functions and human disease. Int. J. Biochem. Cell Biol. 2007, 39, 44–84. [Google Scholar] [CrossRef] [PubMed]
- Dumanovic, J.; Nepovimova, E.; Natic, M.; Kuca, K.; Jacevic, V. The significance of reactive oxygen species and antioxidant defense system in plants: A concise overview. Front. Plant Sci. 2021, 11, 552969. [Google Scholar] [CrossRef] [PubMed]
- Hasanuzzaman, M.; Raihan, R.H.; Masud, A.A.C.; Rahman, K.; Nowroz, F.; Rahman, M.; Nahar, K.; Fujita, M. Regulation of reactive oxygen species and antioxidant defense in plants under salinity. Int. J. Mol. Sci. 2021, 22, 9326. [Google Scholar] [CrossRef]
- Choi, S.Y.; Kim, J.H.; Lee, J.M.; Lee, S.H.; Cho, E.J. Protective effect of Acer okamotoanum from oxidative stress in C6 glial cells. J. Appl. Biol. Chem. 2017, 60, 141–147. [Google Scholar] [CrossRef]
- Cho, H.R.; Lee, S.O. Novel hepatoprotective peptides derived from protein hydrolyzates of mealworm (Tenebrio molitor). Food Res. Int. 2020, 133, 109194. [Google Scholar] [CrossRef] [PubMed]
- Xia, L.; Ng, T.B.; Fang, E.F.; Wong, J.H. Bioactive Constituents of the Silk Worm Bombyx mori. Antitumor Potential and Other Emerging Medicinal Properties of Natural Compounds; Springer: Berlin/Heidelberg, Germany, 2013; pp. 335–344. [Google Scholar] [CrossRef]
- Wada, T.; Penninge, J.M. Mitogen-activated protein kinases in apoptosis regulation. Oncogene 2004, 23, 2838–2849. [Google Scholar] [CrossRef]
- Soares, S.M.; Diniz, F.F.; Gomes, G.N.; Bahia, D. The mitogen-activated protein kinase (MAPK) pathway: Role in immune evasion by trypanosomatids. Front. Microbiol. 2016, 7, 183. [Google Scholar] [CrossRef]
- Tian, W.; Heo, S.; Kim, D.W.; Kim, I.S.; Ahn, D.C.; Tae, H.J.; Kim, M.K.; Park, B.Y. Ethanol extract of Maclura tricuspidata fruit protects SH-SY5Y neuroblastoma cells against H2O2-induced oxidative damage via inhibiting MAPK and NF-κB signaling. Int. J. Mol. Sci. 2021, 22, 6946. [Google Scholar] [CrossRef]


| Essential Amino Acid | Composition (%) | Non-Essential Amino Acid | Composition (%) | ||
|---|---|---|---|---|---|
| SLC (1) | ESLC (2) | SLC | ESLC | ||
| Valine | 5.15 ± 1.10 (3) | 5.28 ± 1.58 | Cysteine | 1.49 ± 0.23 | 2.39 ± 0.27 |
| Methionine | 1.45 ± 0.33 | 1.50 ± 0.95 | Aspartic acid | 7.56 ± 0.56 | 10.12 ± 3.49 |
| Isoleucine | 3.82 ± 0.10 | 4.03 ± 1.23 | Glutamic acid | 10.13 ± 0.70 | 12.04 ± 4.12 |
| Leucine | 5.41 ± 0.18 | 5.46 ± 1.74 | Serine | 9.31 ± 0.45 | 8.41 ± 2.39 |
| Phenylalanine | 3.67 ± 0.29 | 3.80 ± 1.21 | Glycine | 12.80 ± 0.27 | 9.38 ± 2.37 |
| Tryptophan | 0.75 ± 0.10 | 1.12 ± 0.50 | Proline | 5.34 ± 0.32 | 5.31 ± 1.58 |
| Lysine | 5.03 ± 0.68 | 5.62 ± 1.87 | Arginine | 13.13 ± 0.35 | 10.79 ± 3.03 |
| Threonine | 3.87 ± 0.09 | 4.07 ± 1.18 | Alanine | 2.64 ± 0.13 | 2.84 ± 0.73 |
| Histidine | 1.14 ± 0.12 | 1.02 ± 0.39 | Tyrosine | 7.31 ± 0.28 | 6.82 ± 2.26 |
| Total | 30.29 | 31.90 | Total | 69.71 | 68.10 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
An, G.-M.; Jung, T.-H.; Han, K.-S. Protective Effects on Neuronal SH-SY5Y Cells and Antioxidant Activity of Enzymatic Hydrolyzate from Silkworms Fed the Leaves of Cudrania tricuspidata. Appl. Sci. 2024, 14, 1733. https://doi.org/10.3390/app14051733
An G-M, Jung T-H, Han K-S. Protective Effects on Neuronal SH-SY5Y Cells and Antioxidant Activity of Enzymatic Hydrolyzate from Silkworms Fed the Leaves of Cudrania tricuspidata. Applied Sciences. 2024; 14(5):1733. https://doi.org/10.3390/app14051733
Chicago/Turabian StyleAn, Gyu-Mi, Tae-Hwan Jung, and Kyoung-Sik Han. 2024. "Protective Effects on Neuronal SH-SY5Y Cells and Antioxidant Activity of Enzymatic Hydrolyzate from Silkworms Fed the Leaves of Cudrania tricuspidata" Applied Sciences 14, no. 5: 1733. https://doi.org/10.3390/app14051733
APA StyleAn, G.-M., Jung, T.-H., & Han, K.-S. (2024). Protective Effects on Neuronal SH-SY5Y Cells and Antioxidant Activity of Enzymatic Hydrolyzate from Silkworms Fed the Leaves of Cudrania tricuspidata. Applied Sciences, 14(5), 1733. https://doi.org/10.3390/app14051733





