Staining Susceptibility of Microhybrid and Nanohybrid Composites on Exposure to Different Color Solutions
Abstract
:1. Introduction
2. Materials and Methods
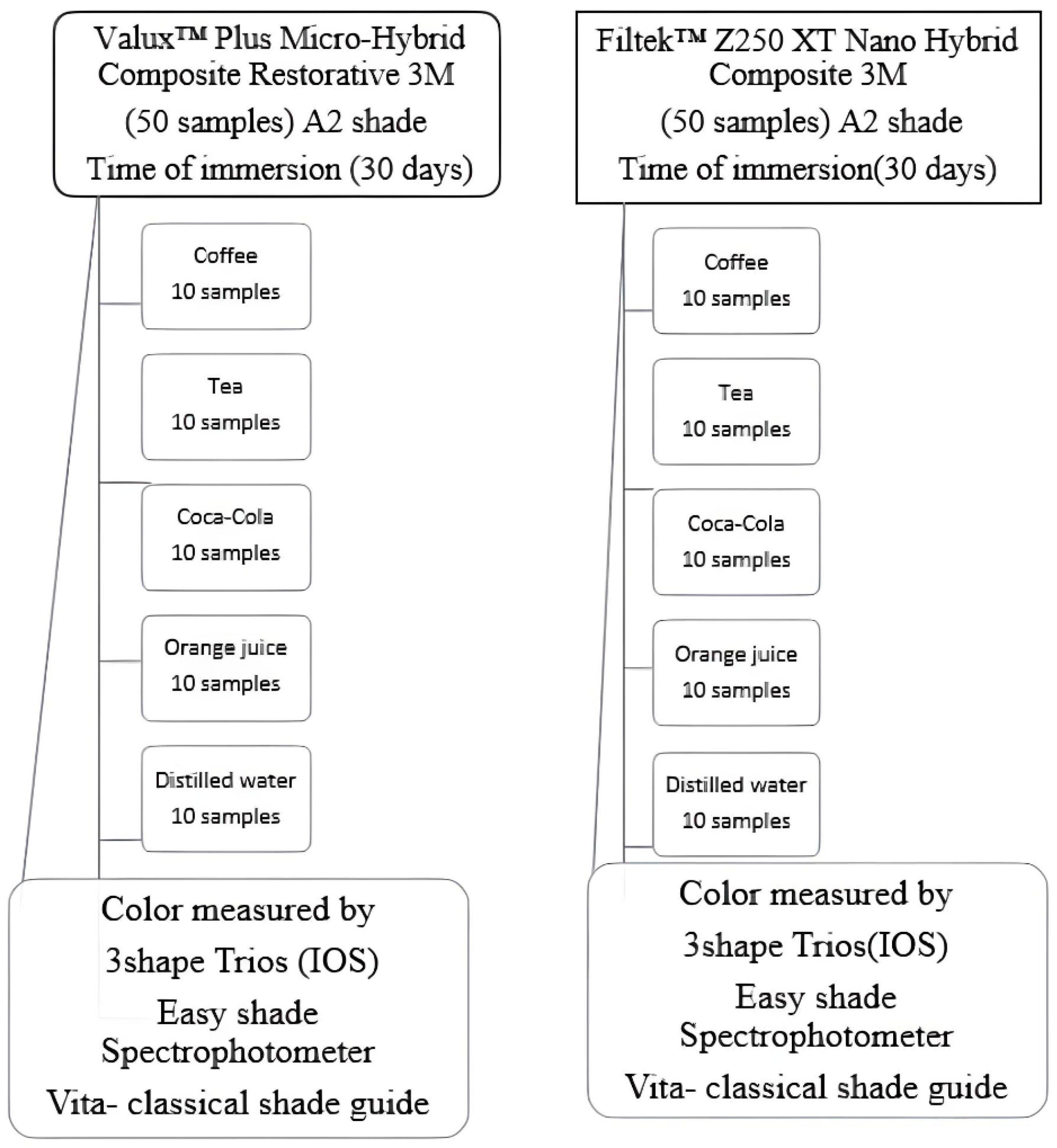
2.1. Sampling Preparation
2.2. Staining Solution Preparation
2.3. Color Assessment
- Easyshade spectrophotometer (Vita Easyshade, 4.0, Vita Zahnfabrik, Bad Sackingen, Germany): After the device was adjusted in accordance with the manufacturer’s guidelines prior to each test, color measurement was done after the spectrophotometer was positioned on the specimen at the same angle (90°) in the sample’s middle [32].
- Intraoral scanner (IOS) 3Shape Trios (SoftwareTrios 4, version 19.2.5, Copenhagen, Denmark): The shade calibration was performed in accordance with the manufacturer’s instructions [32]. The color assessment was immediately created after specimen scanning. The intraoral scanner gave two different measures: Vita classical and 3D Master Guide. The device recorded both measurements, and we converted them to L, a, and b values to compare results using a conversion table.
- Vita classical shade guide (Vita Zahnfabrik, Germany). Three calibrated dentists were asked to categorize the specimens from lightest to darkest, grouping specimens of comparable hues in that order according to their personal criteria. The shade value of the Vita classical shade guide was converted into numerical data using a conversion table [32].
2.4. Calculation of the Color Difference
2.5. Statistical Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Martinez-Ccahuana, L.; Álvarez-Vidigal, E.; Arriola-Guillén, E.; Aguilar-Gálvez, D. Effect of pediatric mouthwashes on the color stability of dental restorations with composite resins. In vitro comparative study. J. Clin. Exp. Dent. 2022, 14, e897–e902. [Google Scholar] [CrossRef]
- Bagheri, R.; Fani, M.; Ghasrodashti, A.B.; Yadkouri, N.N.; Mousavi, S. Effect of a Home Bleaching Agent on the Fracture Toughness of Resin Composites, Using Short Rod Design. J. Dent. 2014, 15, 74–80. [Google Scholar]
- Malekipour, M.R.; Sharafi, A.; Kazemi, S.; Khazaei, S.; Shirani, F. Comparison of color stability of a composite resin in different color media. Dent. Res. J. 2012, 9, 441–446. [Google Scholar]
- Khatri, C.A.; Stansbury, J.W.; Schultheisz, C.R.; Antonucci, J.M. Synthesis, characterization and evaluation of urethane derivatives of Bis-GMA. Dent. Mater. 2003, 19, 584–588. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, M.; Rajeshkumar, L.N.; Srinivasan, N.; Kumar, D.V.; Balaji, D. Influence of filler material on properties of fiber-reinforced polymer composites: A review. e-Polymers 2022, 22, 898–916. [Google Scholar] [CrossRef]
- Blackham, J.T.; Vandewalle, K.S.; Lien, W. Properties of Hybrid Resin Composite Systems Containing Prepolymerized Filler Particles. Oper. Dent. 2009, 34, 697–702. [Google Scholar] [CrossRef] [PubMed]
- Yousef, M.; Abo, E.N.A. Effect of different bleaching methods on stained composites. J. Dent. Oral. Hyg. 2015, 7, 22–27. [Google Scholar] [CrossRef]
- Silva, T.M.; Sales, A.L.; Pucci, C.R.; Borges, A.B.; Torres, C.R. The combined effect of food-simulating solutions, brushing and staining on color stability of composite resins. Acta Biomater. Odontol. Scand. 2017, 3, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Özdemir, O.S.; Karaman, E.; Tuncer, D.; Firat, E.; Karahan, S. Influence of Different Staining Beverages on Color Stability, Surface Roughness and Microhardness of Silorane and Methacrylate-based Composite Resins. J. Contemp. Dent. Pr. 2014, 15, 319–325. [Google Scholar] [CrossRef]
- Ertas, E.; Güler, A.U.; Yücel, A.; Köprülü, H.; Güler, E. Color Stability of Resin Composites after Immersion in Different Drinks. Dent. Mater. J. 2006, 25, 371–376. [Google Scholar] [CrossRef] [PubMed]
- Soares-Geraldo, D.; Scaramucci, T.; Steagall, W., Jr.; Braga, S.R.M.; Sobral, M.A.P. Interaction between staining and degradation of a composite resin in contact with colored foods. Braz. Oral Res. 2011, 25, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Ardu, S.; Duc, O.; Di Bella, E.; Krejci, I.; Daher, R. Color stability of different composite resins after polishing. Odontology 2018, 106, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Angerame, D.; De Biasi, M. Do Nanofilled/Nanohybrid Composites Allow for Better Clinical Performance of Direct Restorations Than Traditional Microhybrid Composites? A Systematic Review. Oper. Dent. 2018, 43, E191–E209. [Google Scholar] [CrossRef]
- Al Kheraif, A.A.; Qasim, S.S.; Ramakrishnaiah, R.; ur Rehman, I. Effect of different beverages on the color stability and degree of conversion of nano and microhybrid composites. Dent. Mater. J. 2013, 32, 326–331. [Google Scholar] [CrossRef] [PubMed]
- Korać, S.; Ajanović, M.; Džanković, A.; Konjhodžić, A.; Hasić-Branković, L.; Gavranović-Glamoč, A.; Tahmiščija, I. Color Stability of Dental Composites after Immersion in Beverages and Performed Whitening Procedures. Acta Stomatol. Croat. 2022, 56, 22–32. [Google Scholar] [CrossRef]
- Kalita, T.; Kalita, C.; Das, L.; Kataki, R.; Boruah, L.C.; Anija, R.; Mahanta, P.; Sr, P.M.; Saikia, A. Comparative Evaluation of Colour Stability and Surface Roughness of Nanohybrid Composite Resins in Mouth Rinse and Colouring Beverages. Cureus 2023, 15, e35303. [Google Scholar] [CrossRef] [PubMed]
- Şişmanoğlu, S.; Sengez, G. Effects of Acidic Beverages on Color Stability of Bulk-Fill Composites with Different Viscosities. Odovtos Int. J. Dent. Sci. 2022, 24, 90–99. [Google Scholar] [CrossRef]
- Chu, S.J.; Trushkowsky, R.D.; Paravina, R.D. Dental color matching instruments and systems. Review of clinical and research aspects. J. Dent. 2010, 38, e2–e16. [Google Scholar] [CrossRef] [PubMed]
- Paravina, R.D.; Powers, J.M. Esthetic Color Training in Dentistry, 1st ed.; Elsevier: Amsterdam, The Netherlands, 2004. [Google Scholar]
- Rutkūnas, V.; Dirsė, J.; Bilius, V. Accuracy of an intraoral digital scanner in tooth color determination. J. Prosthet. Dent. 2020, 123, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Fernández Millán, D.; Gallas Torreira, M.; Alonso de la Peña, V. Using a repositioning splint to determine reproducibility in the color registers of a dental spectrophotometer. J. Esthet. Restor. Dent. 2020, 32, 19–25. [Google Scholar] [CrossRef] [PubMed]
- Yamanel, K.; Caglar, A.; Özcan, M.; Gulsah, K.; Bagis, B. Assessment of Color Parameters of Composite Resin Shade Guides Using Digital Imaging versus Colorimeter. J. Esthet. Restor. Dent. 2010, 22, 379–388. [Google Scholar] [CrossRef] [PubMed]
- Backes, C.N.; França, F.M.G.; Turssi, C.P.; Amaral, F.L.B.D.; Basting, R.T. Color stability of a bulk-fill composite resin light-cured at different distances. Braz. Oral Res. 2020, 34, e119. [Google Scholar] [CrossRef]
- Ahamed, S.A.S.; Raheel, S.A.; Ajmal, M.B.; Kaur, M.; Alqahtani, N.M.; Tasleem, R.; Bahamdan, G.K.; Hegde, M.; Bhavikatti, S.K. Evaluation of Color Stability of Composite Resin Used to Characterize Acrylic Teeth—An In Vitro Study. Appl. Sci. 2023, 13, 1498. [Google Scholar] [CrossRef]
- Thaliyadeth, L.B.; Chakravarthy, D.; Neelamurthy, P.S.; Selvapandiane, V.; Jayadevan, A.; Dimple, N. Comparative Evaluation of Color Stability of Nanohybrid Direct and Indirect Resin-based Composites to Indian Spices: An In Vitro Study. J. Contemp. Dent. Pr. 2019, 20, 1071–1076. [Google Scholar] [CrossRef]
- Kumari, R.V.; Nagaraj, H.; Siddaraju, K.; Poluri, R.K. Evaluation of the Effect of Surface Polishing, Oral Beverages and Food Colorants on Color Stability and Surface Roughness of Nanocomposite Resins. J. Int. Oral Health JIOH 2015, 7, 63–70. [Google Scholar]
- Yaman, B.C.; Dörter, C.; Erdilek, D.; Efes, B.; Gömeç, Y.; Büyükgökçesu, S. The effects of halogen and light-emitting diode light curing on the depth of cure and surface microhardness of composite resins. J. Conserv. Dent. 2011, 14, 136. [Google Scholar] [CrossRef] [PubMed]
- Khalaj, K.; Soudi, A.; Tayefi-Nasrabadi, M.; Keshvad, M. The evaluation of surface sealants’ effect on the color stability of Nano-hybrid composite after polishing with One-Step system (in-vitro). J. Clin. Exp. Dent. 2018, 10, e927–e932. [Google Scholar] [CrossRef] [PubMed]
- Awliya, W.Y.; Al-Alwani, D.J.; Gashmer, E.S.; Al-Mandil, H.B. The effect of commonly used types of coffee on surface microhardness and color stability of resin-based composite restorations. Saudi Dent. J. 2010, 22, 177–181. [Google Scholar] [CrossRef] [PubMed]
- Valizadeh, S.; Asiaie, Z.; Kiomarsi, N.; Kharazifard, M.J. Color stability of self-adhering composite resins in different solutions. Dent. Med. Probl. 2020, 57, 31–38. [Google Scholar] [CrossRef]
- Darabi, F.; Seyed-Monir, A.; Mihandoust, S.; Maleki, D. The effect of preheating of composite resin on its color stability after immersion in tea and coffee solutions: An in-vitro study. J. Clin. Exp. Dent. 2019, 11, e1151. [Google Scholar] [CrossRef] [PubMed]
- Sirintawat, N.; Leelaratrungruang, T.; Poovarodom, P.; Kiattavorncharoen, S.; Amornsettachai, P. The Accuracy and Reliability of Tooth Shade Selection Using Different Instrumental Techniques: An In Vitro Study. Sensors 2021, 21, 7490. [Google Scholar] [CrossRef] [PubMed]
- Chami, V.D.; Gebert, F.; Assaf, D.D.; Centeno, A.C.; Ferrazzo, V.A.; Durand, L.B.; Marquezan, M. Color stability of resin composites for orthodontic attachments: An in vitro study. Dent. Press J. Orthod. 2022, 27, e2220432. [Google Scholar] [CrossRef] [PubMed]
- Sakiroff, L.M.; Chennell, P.; Yessaad, M.; Pereira, B.; Bouattour, Y.; Sautou, V. Evaluation of color changes during stability studies using spectrophotometric chromaticity measurements versus visual examination. Sci. Rep. 2022, 12, 8959. [Google Scholar] [CrossRef] [PubMed]
- Faris, T.M.; Abdulrahim, R.H.; Mahmood, M.A.; Mhammed Dalloo, G.A.; Gul, S.S. In vitro evaluation of dental color stability using various aesthetic restorative materials after immersion in different drinks. BMC Oral Health 2023, 23, 49. [Google Scholar] [CrossRef]
- Meshki, R.; Rashidi, M. Effect of natural and commercially produced juices on colour stability of microhybrid and nanohybrid composites. BDJ Open 2022, 8, 11. [Google Scholar] [CrossRef] [PubMed]
- Poggio, C.; Scribante, A.; Colombo, M.; Beltrami, R.; Chiesa, M. Surface discoloration of composite resins: Effects of staining and bleaching. Dent. Res. J. 2012, 9, 567. [Google Scholar] [CrossRef] [PubMed]
- Vichi, A.; Ferrari, M.; Davidson, C.L. Color and opacity variations in three different resin-based composite products after water aging. Dent. Mater. 2004, 20, 530–534. [Google Scholar] [CrossRef]
- Chittem, J. Spectrophotometric Evaluation of Colour Stability of Nano Hybrid Composite Resin in Commonly Used Food Colourants in Asian Countries. J. Clin. Diagn. Res. 2017, 11, ZC61. [Google Scholar] [CrossRef] [PubMed]
- Lee, Y.K.; Powers, J.M. Combined effect of staining substances on the discoloration of esthetic Class V dental restorative materials. J. Mater. Sci. Mater. Med. 2007, 18, 165–170. [Google Scholar] [CrossRef]
- Meenakshi, C.; Sirisha, K. Surface quality and color stability of posterior composites in acidic beverages. J. Conserv. Dent. 2020, 23, 57. [Google Scholar]
- Colombo, M.; Gallo, S.; Poggio, C.; Ricaldone, V.; Arciola, C.R.; Scribante, A. New Resin-Based Bulk-Fill Composites: In vitro Evaluation of Micro-Hardness and Depth of Cure as Infection Risk Indexes. Materials 2020, 13, 1308. [Google Scholar] [CrossRef]
- Cacciafesta, V.; Sfondrini, M.F.; Lena, A.; Scribante, A.; Vallittu, P.K.; Lassila, L.V. Flexural strengths of fiber-reinforced composites polymerized with conventional light-curing and additional postcuring. Am. J. Orthod. Dentofac. Orthop. 2007, 132, 524–527. [Google Scholar] [CrossRef] [PubMed]
- Pieniak, D.; Walczak, A.; Walczak, M.; Przystupa, K.; Niewczas, A.M. Hardness and Wear Resistance of Dental Biomedical Nanomaterials in a Humid Environment with Non-Stationary Temperatures. Materials 2020, 13, 1255. [Google Scholar] [CrossRef] [PubMed]
- Bansal, K.; Acharya, S.R.; Saraswathi, V. Effect of alcoholic and non-alcoholic beverages on color stability and surface roughness of resin composites: An in vitro study. J. Conserv. Dent. 2012, 15, 283–288. [Google Scholar] [CrossRef]
- Öztürk, E.; Güder, G. Correlation between three-dimentional surface topography and color stability of different nanofilled composites. Scanning 2015, 37, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Macedo, M.G.F.P.; Volpato, C.A.M.; Henriques, B.A.P.C.; Vaz, P.C.S.; Silva, F.S.; Silva, C.F.C.L. Color stability of a bis-acryl composite resin subjected to polishing, thermocycling, intercalated baths, and immersion in different beverages. J. Esthet. Restor. Dent. 2018, 30, 449–456. [Google Scholar] [CrossRef] [PubMed]
- Szczesio-Wlodarczyk, A.; Sokolowski, J.; Kleczewska, J.; Bociong, K. Ageing of Dental Composites Based on Methacrylate Resins—A Critical Review of the Causes and Method of Assessment. Polymers 2020, 12, 882. [Google Scholar] [CrossRef] [PubMed]
- Kolbeck, C.; Rosentritt, M.; Lang, R.; Handel, G. Discoloration of facing and restorative composites by UV-irradiation and staining food. Dent. Mater. 2006, 22, 63–68. [Google Scholar] [CrossRef] [PubMed]
- Bagheri, R.; Burrow, M.F.; Tyas, M. Influence of food-simulating solutions and surface finish on susceptibility to staining of aesthetic restorative materials. J. Dent. 2005, 33, 389–398. [Google Scholar] [CrossRef] [PubMed]
- Meireles, S.S.; Demarco, F.F.; Santos, I.S.; Dumith, S.C.; Bona, A.D.e.l.l.a. Validation and Reliability of Visual Assessment with a Shade Guide for Tooth-Color Classification. Oper. Dent. 2008, 33, 121–126. [Google Scholar] [CrossRef] [PubMed]
- Khashayar, G.; Dozic, A.; Kleverlaan, C.; Feilzer, A. Data Comparison Between Two Dental Spectrophotometers. Oper. Dent. 2012, 37, 12–20. [Google Scholar] [CrossRef] [PubMed]
- Piedra-Cascón, W.; Adhikari, R.R.; Özcan, M.; Krishnamurthy, V.R.; Revilla-León, M.; Gallas-Torreira, M. Accuracy assessment (trueness and precision) of a confocal based intraoral scanner under twelve different ambient lighting conditions. J. Dent. 2023, 134, 104530. [Google Scholar] [CrossRef]
- Parameswaran, V.; Anilkumar, S.; Lylajam, S.; Rajesh, C.; Narayan, V. Comparison of accuracies of an intraoral spectrophotometer and conventional visual method for shade matching using two shade guide systems. J. Indian Prosthodont. Soc. 2016, 16, 352. [Google Scholar] [CrossRef] [PubMed]
- Reyes, J.; Acosta, P.; Ventura, D. Repeatability of the human eye compared to an intraoral scanner in dental shade matching. Heliyon 2019, 5, e02100. [Google Scholar] [CrossRef] [PubMed]
| Solutions | Compositions | Brand |
|---|---|---|
| Tea | Caffeine, tannins, theophylline, vitamin, glucose. | Lipton yellow label tea, Dubai, UAE |
| Coffee | Zinc, copper, magnesium, potassium, caffeine. | Nescafe, Nestle, Vevey, Switzerland |
| Orange juice | Ascorbic acid, potassium, citric acid, and folic acid. | Almarai, 100% orange juice (natural), Riyadh, Saudi Arabian |
| Coca-Cola | Sugar, caramel, caffeine, orthophosphoric acid, water. | Coca-Cola, Dubai, UAE |
| Water | Distilled water |
| Easyshade Spectrophotometer | ||||||||
| L | a | b | ||||||
| (Composite) | (Media) | (Mean) | (SD) | (Mean) | (SD) | (Mean) | (SD) | (ΔE_ab) |
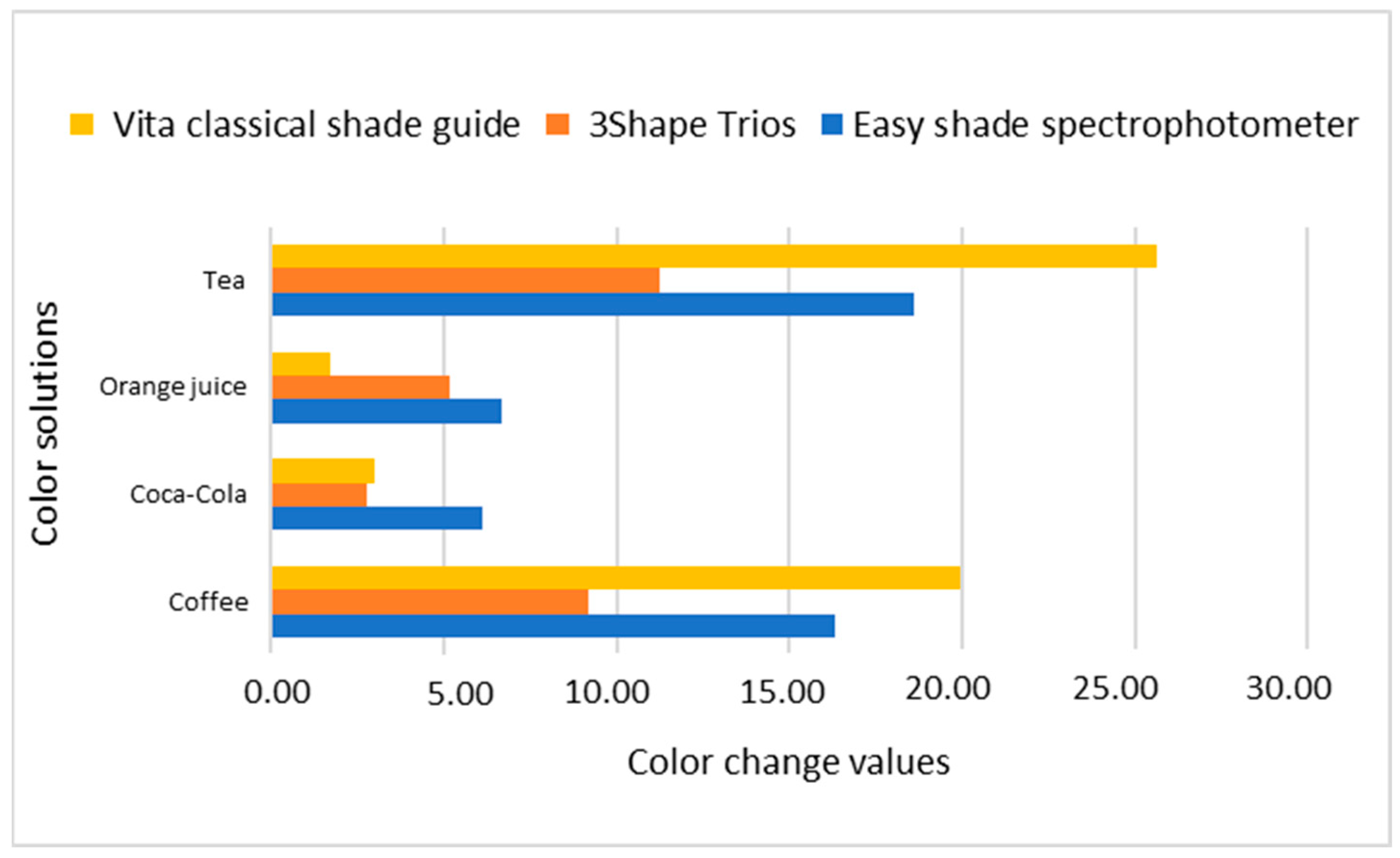
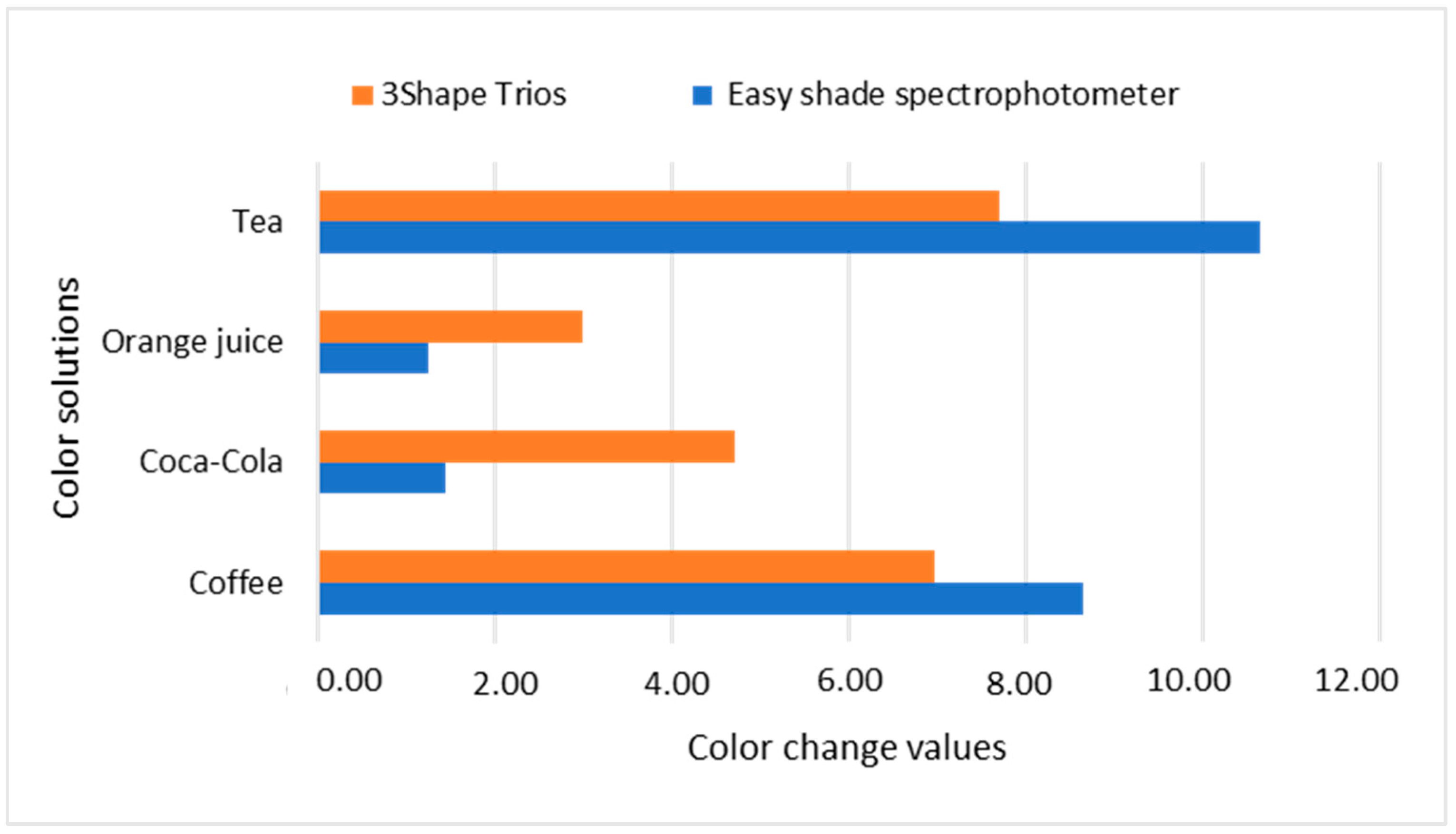
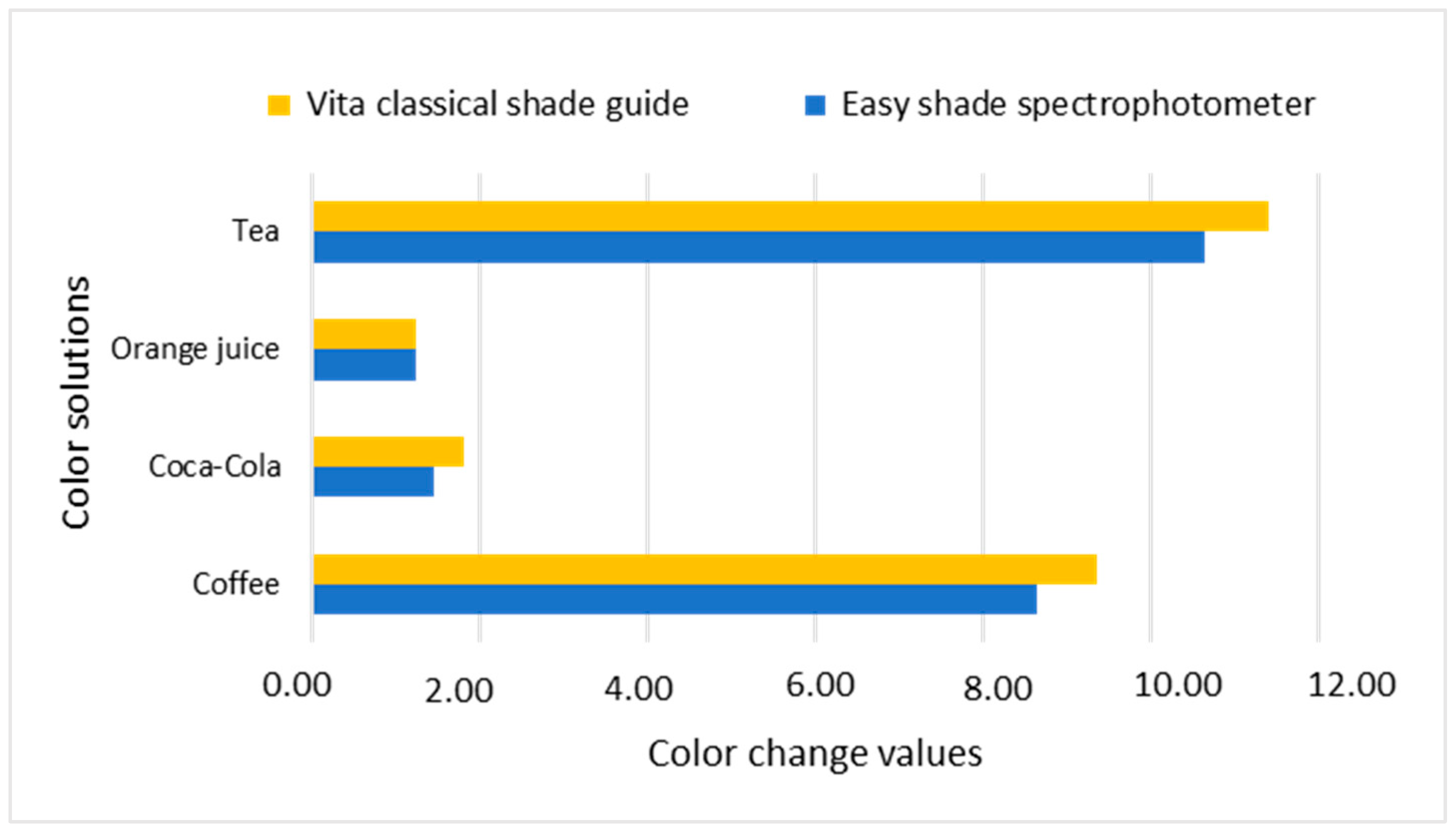
| Nanohybrid Filtek Z250 XT 3M ESPE | Coffee | 63.83 | 0.81 | 4.34 | 0.29 | 32.36 | 0.86 | 16.32 * |
| Coca-Cola | 81.98 | 0.62 | 2.20 | 0.07 | 27.64 | 0.39 | 6.11 * | |
| Orange | 82.18 | 0.66 | 2.01 | 0.14 | 28.36 | 0.78 | 6.69 * | |
| Tea | 61.30 | 0.95 | 5.07 | 0.26 | 32.37 | 0.50 | 18.61 * | |
| Water | 77.34 | 3.36 | 1.03 | 0.45 | 23.84 | 1.34 | ||
| Microhybrid Valux Plus 3M ESPE | Coffee | 72.47 | 0.80 | 1.35 | 0.11 | 27.08 | 1.27 | 8.65 * |
| Coca-Cola | 81.25 | 0.92 | 0.69 | 0.16 | 23.55 | 0.90 | 1.46 | |
| Orange | 80.14 | 1.16 | 0.60 | 0.07 | 24.15 | 0.55 | 1.24 | |
| Tea | 70.78 | 0.45 | 2.50 | 0.27 | 28.21 | 0.56 | 10.64 * | |
| Water | 80.73 | 3.29 | 1.62 | 0.54 | 24.55 | 4.06 | ||
| 3Shape Trios | ||||||||
| L | a | b | ||||||
| (Composite) | (Media) | (Mean) | (SD) | (Mean) | (SD) | (Mean) | (SD) | (ΔE_ab) |
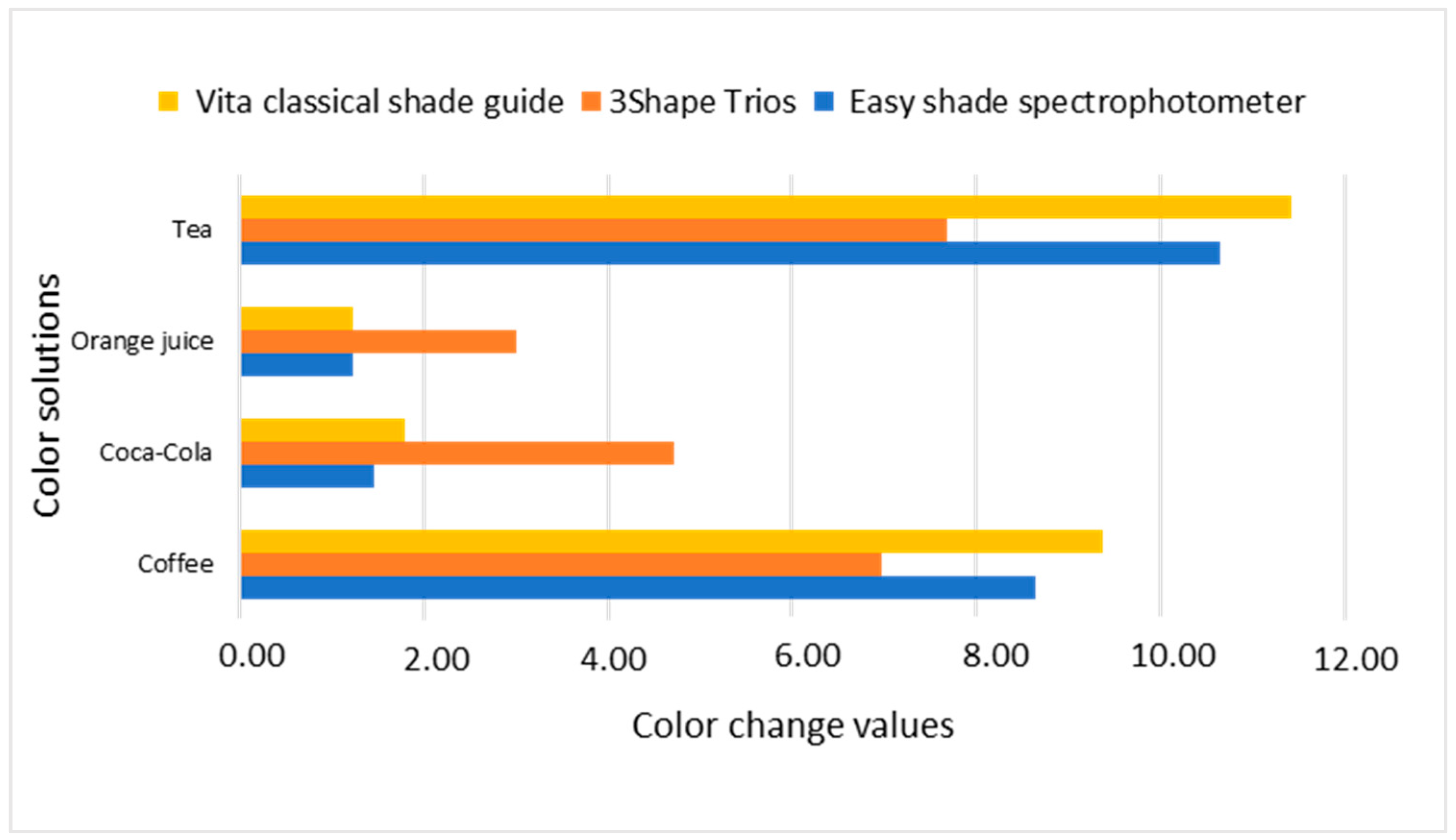
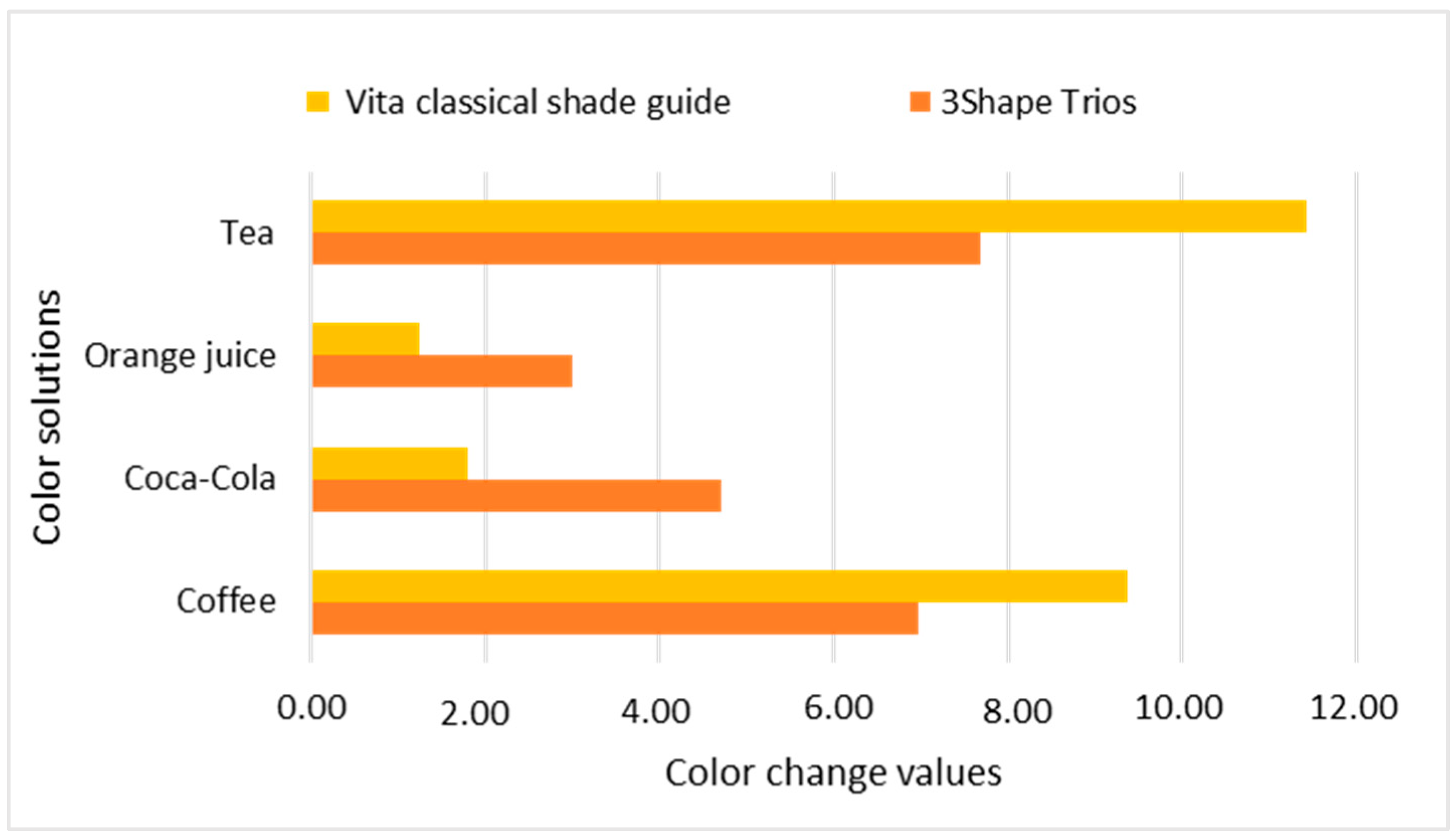
| Nanohybrid Filtek Z250 XT 3M ESPE | Coffee | 69.59 | 0.21 | 2.34 | 0.37 | 22.27 | 1.09 | 9.20 * |
| Coca-Cola | 76.27 | 1.16 | 0.98 | 0.00 | 17.97 | 0.00 | 4.71 * | |
| Orange | 76.09 | 2.33 | 1.11 | 0.23 | 20.40 | 1.71 | 5.14 * | |
| Tea | 66.84 | 1.91 | 3.40 | 0.53 | 22.13 | 0.66 | 11.24 * | |
| Water | 75.47 | 1.24 | 1.12 | 0.5 | 15.29 | 2.7 | ||
| Microhybrid Valux Plus 3M ESPE | Coffee | 69.43 | 0.05 | 2.66 | 0.04 | 18.88 | 3.06 | 6.97 * |
| Coca-Cola | 75.67 | 1.39 | 1.23 | 0.62 | 13.29 | 1.40 | 2.80 | |
| Orange | 78.80 | 0.00 | 0.97 | 0.00 | 19.34 | 0.00 | 3.00 | |
| Tea | 69.39 | 0.00 | 2.69 | 0.00 | 21.25 | 0.00 | 7.69 * | |
| Water | 76.13 | 1.53 | 0.98 | 0.00 | 17.97 | 0.00 | ||
| Vita Classical Shade Guide | ||||||||
| L | a | b | ||||||
| (Composite) | (Media) | (Mean) | (SD) | (Mean) | (SD) | (Mean) | (SD) | (ΔE_ab) |
| Nanohybrid Filtek Z250 XT 3M ESPE | Coffee | 40.61 | 6.08 | 7.01 | 0.24 | 12.88 | 0.01 | 19.95 * |
| Coca-Cola | 58.54 | 3.51 | 6.17 | 0.91 | 10.37 | 2.95 | 3.02 | |
| Orange | 60.09 | 0.59 | 6.43 | 0.72 | 10.91 | 2.00 | 1.71 | |
| Tea | 34.92 | 0.00 | 7.23 | 0.00 | 12.87 | 0.00 | 25.63 * | |
| Water | 60.55 | 0 | 6.99 | 0 | 12.46 | 0 | ||
| Microhybrid Valux Plus 3M ESPE | Coffee | 51.96 | 3.61 | 8.12 | 0.25 | 16.04 | 0.93 | 9.37 * |
| Coca-Cola | 62.13 | 0.78 | 6.53 | 0.62 | 11.74 | 1.66 | 1.80 | |
| Orange | 61.13 | 1.06 | 6.17 | 0.48 | 11.74 | 1.66 | 1.24 | |
| Tea | 49.66 | 2.28 | 8.44 | 0.17 | 15.59 | 0.35 | 11.42 * | |
| Water | 60.55 | 0.00 | 6.99 | 0.00 | 12.46 | 0.00 | ||
| Easyshade Spectro-Photometer (a) | IOS 3Shape Trios (b) | Vita Classical Shade Guide (c) | p-Value (a–b) | p-Value (a–c) | p-Value (b–c) | ||
|---|---|---|---|---|---|---|---|
| Nanohybrid Filtek Z250 XT 3M ESPE | Coffee | 16.32 (0.86) | 9.20 (1.09) | 19.95 (0.01) | 0.0007 | 0.2 | 0.0006 |
| Coca-Cola | 6.11 (0.39) | 4.71 (0.00) | 3.02 (2.95) | 0.0002 | 0.7 | 0.0002 | |
| Orange | 6.69 (0.78) | 5.14 (1.71) | 1.71 (2.00) | 0.0001 | 0.7 | 0.0001 | |
| Tea | 18.61 (0.50) | 11.24 (0.66) | 25.63 (0.00) | 0.0003 | 0.1 | 0.0001 | |
| Microhybrid Valux Plus 3M ESPE | Coffee | 8.65 (1.27) | 6.97 (3.06) | 9.37 (0.93) | 0.0006 | 0.1 | 0.0001 |
| Coca-Cola | 1.46 (0.90) | 2.80 (1.40) | 1.80 (1.66) | 0.0003 | 0.7 | 0.0001 | |
| Orange | 1.24 (0.55) | 3.00 (0.00) | 1.24 (1.66) | 0.0002 | 0.5 | 0.0004 | |
| Tea | 10.64 (0.56) | 7.69 (0.00) | 11.42 (0.35) | 0.0001 | 0.1 | 0.0002 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mohamad-Kharib, A.; Chamorro-Petronacci, C.; Pérez-Jardón, A.; Castelo-Baz, P.; Martin-Biedma, B.; Ginzo-Villamayor, M.J.; García-García, A. Staining Susceptibility of Microhybrid and Nanohybrid Composites on Exposure to Different Color Solutions. Appl. Sci. 2023, 13, 11211. https://doi.org/10.3390/app132011211
Mohamad-Kharib A, Chamorro-Petronacci C, Pérez-Jardón A, Castelo-Baz P, Martin-Biedma B, Ginzo-Villamayor MJ, García-García A. Staining Susceptibility of Microhybrid and Nanohybrid Composites on Exposure to Different Color Solutions. Applied Sciences. 2023; 13(20):11211. https://doi.org/10.3390/app132011211
Chicago/Turabian StyleMohamad-Kharib, Azheen, Cintia Chamorro-Petronacci, Alba Pérez-Jardón, Pablo Castelo-Baz, Benjamín Martin-Biedma, María José Ginzo-Villamayor, and Abel García-García. 2023. "Staining Susceptibility of Microhybrid and Nanohybrid Composites on Exposure to Different Color Solutions" Applied Sciences 13, no. 20: 11211. https://doi.org/10.3390/app132011211
APA StyleMohamad-Kharib, A., Chamorro-Petronacci, C., Pérez-Jardón, A., Castelo-Baz, P., Martin-Biedma, B., Ginzo-Villamayor, M. J., & García-García, A. (2023). Staining Susceptibility of Microhybrid and Nanohybrid Composites on Exposure to Different Color Solutions. Applied Sciences, 13(20), 11211. https://doi.org/10.3390/app132011211






