Abstract
The testosterone–cortisol ratio is a concept in human biology that refers to the balance between testosterone, the main anabolic steroid, and cortisol, another steroid hormone. The two hormones are said to be habitually positively “coupled”. Increases or decreases in testosterone tend to be associated with corresponding increases or decreases in cortisol, and vice versa. The present study explored hormone coupling and its relationship to stress levels in the sport performances of an elite women’s volleyball team. (1) Aim: to assess the testosterone–cortisol concentration dynamic over 16 weeks and its link to sport performance in elite female volleyball players (height: 1.8 ± 0.1 m; 24.2 ± 2.7 years; playing experience 15 ± 2.8 years; years played at elite level 4.2 ± 2.2; testosterone–cortisol index time 1: 3.9 vs. time 2: 4.3) (n = 11). (2) Methods: blood samples (hormones among other biochemical parameters) and sports performance measurements (aerobic and anaerobic power among other tests) were taken from members of an elite women’s volleyball team over 16 weeks of competition. (3) Results: female volleyball players showed patterns of hormonal change and adaptation to stress. (4) Conclusions: the current investigation demonstrated that elite female volleyball players have higher basal levels of testosterone and cortisol than normal healthy women. The impact of training and competition is clearly reflected in the levels of T. Cortisol levels increase at the beginning of training and remain elevated throughout the season, but without significant changes.
1. Introduction
To excel in competitive sports, athletes must maintain their maximum capabilities throughout the competition to achieve peak performance [1]. Ways of managing and adapting to the stressful demands of competition are vitally important in terms of performance [2,3,4,5]. Participation in elite level volleyball is associated with an improvement in physical conditioning components [6,7,8,9]. The intermittent and explosive sport of volleyball involves a lot of running, jumping, and hitting motions [10,11]. Given the number of stressful scenarios that it offers as a team sport, decision-making is significantly more crucial for team performance than it is in non-team sports [12]. It is important to mention that our selection of volleyball as the sport of choice was not based on physical fitness levels alone. Rather, we chose volleyball over strictly aerobic sports, which are known to promote better adaptation to other stressors, due to the wide range of psychological as well as social stress factors present in the sport [13].
To be able to perform optimally in volleyball, training is planned by adapting the most appropriate training load with the aim of achieving sport-specific adaptation through super compensation, which consequently results in improved athletic performance [14]. Additionally, stress caused by competition is a set of sophisticated physiological and psychological processes [15] that significantly influences results, particularly in team sports such as volleyball [16].
Additionally, carefully selected training loads influence athletes’ biochemical and physiological features in a positive way. However, if you work out frequently and intensely, you can find that you perform worse and get tired faster [17]. During the competitive phase of volleyball, training loads are primarily concentrated on enhancing strength-endurance, as well as game technique and tactics [18]. Training loads must constantly be adapted to the specific adaptive capability of the athlete to achieve a gain in performance [19].
To measure and evaluate such stress and its effect on the performance [20] required by this type of sport [21,22], testosterone (T) and cortisol (C) hormone levels are proposed as reliable biomarkers for these processes [23].
T plays a crucial role in sport by facilitating nitrogen fixation and promoting tissue-specific protein synthesis [24]. Regardless of differences in circulation levels between men and women, numerous studies Updated reference [25,26,27] have shown that T appears to exert similar effects on exercise behaviour [28].
On the contrary, due to their catabolic, anti-inflammatory and homeostatic functions in affecting electrical and metabolic balance, C is regarded as a biomarker of stress response [29], as physical activity, particularly high-intensity physical activity, is often associated with a decrease in the hypothalamic–pituitary–adrenal axis, although the basal level of C is often higher than normal, reflecting a chronic response to training [21]. During active stress responses, this steroid hormone has a significant impact on both physiological and psychological aspects (i.e., functional, and dysfunctional impulsivity and motivation), influencing human behaviour towards desirable (high approach motivation) or undesirable (low avoidance motivation) outcomes [30]. The reference values for T in women are 15 to 70 ng/dL or 0.5 to 2.4 nmol/L [31] and for C, 5 to 25 mcg/dL or 140 to 690 nmol/L [32]. It has also been observed that C concentrations are significantly reduced, even below basal resting values, within 24 h of recovery from exhaustive physical activity [33].
Numerous studies have investigated the impact of physical activity on T and C levels in the body, especially when the T and C order is altered, reorienting in the opposite direction (catabolic and anabolic, respectively), demonstrating that physical activity causes an imbalance between catabolic hormones of an adrenal origin and anabolic hormones from a testicular source [31]. However, published data on this issue in women are far from conclusive, to the best of the author’s knowledge.
Despite ongoing research, there is currently no definitive sign that can accurately pinpoint stress and/or overtraining problems [32,34,35], although in recent decades the relationship between T and C (T:C) has been proposed as a possible diagnostic index [36,37]. The T:C ratio has been reported to be a sign of athletes’ anabolic states [38], acute training responses and effects [39,40,41], as well as psychophysiological responses such as stress and motivation [38,41,42].
For this reason, it has been argued that, in training processes, monitoring the T:C ratio can be used to check the response of our hormones to the intensity of our training to prevent stress and overtraining syndrome [43]. Both factors are associated with decreased performance, psychological changes, and neuroendocrine disruption [44].
Low T levels may be a sign of men’s poor overall health [45], and extreme amounts of circulating androgens, whether high or low, may be harmful to women’s health [46]. Exercise-induced amenorrhea and chronic hypercortisolism have been linked in women [47]. This has been connected to the female athlete triad’s “premature bone loss associated with decreased levels of progesterone and oestradiol” [48].
At the onset of physical activity, both T and C production is stimulated [49,50] but, after the end of physical activity, high circulating C levels negatively affect T synthesis [51]. Because a single isolated and punctual measurement could provide a misleading image depending on the sampling phase, it is crucial to analyse the dynamics of both hormones throughout a specific period rather than only acutely. Even though numerous research has evaluated the size and direction of the shift in both hormones, some of them have focused on concentrations during exercise or immediately after the end of exercise either acutely [52,53] or after 24 h [54,55,56,57].
Even though this information is valuable, it is necessary to monitor the hormone concentrations for a longer period to determine how long it takes the neuroendocrine system to return to a homeostatic resting state [58] and the extent to which they are linked to variations in performance while engaged in sports competition [59,60,61,62]. For coaches and sport scientists, this knowledge could be crucial in determining the number of recovery days needed after intense activity, such as that which might occur during competition. In volleyball, no previous studies have described these parameters through the season, based on the author’s knowledge.
To gain a deeper knowledge of the relationship between T and C levels and sport performance in elite female volleyball players, the major goal of this study is to examine the dynamics of C and T concentration across a 16-week competition period.
2. Materials and Methods
2.1. Participants
In the 2018–2019 season, a group of eleven female volleyball players (Table 1) were evaluated twice: first in September 2018 (pre-season), and again in January 2019 (during the first competitive break). The players were competing at the elite level in the Iberdrola League under the Royal Spanish Volleyball Federation.

Table 1.
General characteristics of participants.
The typical work week averaged 22.5 (Figure 1) hours of training (excluding competition). It included three double training sessions, 150 min of technical–tactical work (46.15%) (morning) and 180 min of physical work (53.85%) (afternoon).
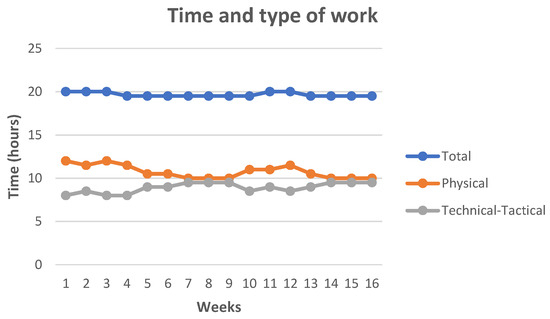
Figure 1.
Time and type of work.
All the participants involved in the data collection had no record of injuries, allergies, or hormonal disturbances. Additionally, none of them were allowed to take any illegal drugs or medications affecting their body mass. Prior to testing, they were instructed to refrain from engaging in strenuous physical activity for at least 24 h. Written informed consent forms, signed by parents of minor participants, were submitted. Due to the nature of the research, anonymity could not be guaranteed, but all data obtained were treated with scientific rigor and utmost discretion and were solely used for the research endeavour.
Prior to the commencement of the study, the experimental procedures, associated risks and benefits were clearly explained to each athlete. Each player provided written consent before participating, in accordance with the ethical guidelines outlined in the Helsinki Declaration of the World Medical Association (2013) [63] for medical research on human subjects. The project was approved by the Ethics Committee for Research Involving Human Subjects of the University of the Basque Country (number: M10_2017_216). The study was conducted in accordance with Organic Law 15/1999 of 13 December on the Protection of Personal Data.
2.2. Experimental Design of the Problem
The current study (Figure 2) was carried out in non-experimental settings to guarantee ecological validity. As such, the coaching staff and participants did not receive any input or intervention from the research team. The coaching staff provided the team with information on training data, competition schedules and match results [64].
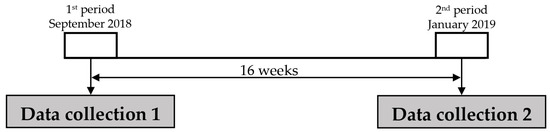
Figure 2.
Study design.
2.3. Evaluation Plan
Measurements were taken at two points during the season, specifically in September 2018 (T1) and January 2019 (T2), just prior to a training session. These times were chosen because the monitored parameters were expected to vary significantly during these times [7] (Figure 2).
To rule out any potential environmental or biological influences that might have an impact on the results, the measurements were conducted in the sports facility where the players practiced and competed [65]. The evaluations were conducted at the Municipal Sports Centre “El Ferial” in Haro, La Rioja. The humidity and temperature differences between T1 (humidity 42%, temperature 28.1 °C) and T2 (humidity 39%, temperature 14.9 °C) were 3% and 13.2 °C, respectively.
To ensure accurate evaluation, the participants were instructed not to engage in physical exercise for 24 h prior to the analysis. They were also advised not to consume any solid or liquid food within 4 h prior to the test and to maintain proper hydration. In addition, the participants were asked to urinate and/or defecate 30 min prior to data collection [66].
2.4. Blood Analysis
In each of the two periods, a biochemical blood analysis was carried out before each session of the physical tests. For these biochemical analyses, a university graduate in nursing was available for the extraction, labelling and packaging of the blood samples. The blood samples were collected using the Vacutainer vacuum system with anticoagulant. It is essential that the labelling of the sample tubes is carried out correctly before the patient leaves, leaving a record of who has collected the sample. These data must always be checked against the list of players previously provided by the club.
For the transportation of the samples to the laboratory, the tubes were tightly closed before moving them so that shaking or moving them abruptly during transport was avoided, as this could haemolyse the blood; therefore, all the samples were transported in the corresponding refrigerator in test tube racks. Whole blood samples with anticoagulant were transported refrigerated between +4 and +8 °C, with cold accumulators for sample transport, and arrived at the laboratory within 24 h after collection.
The laboratory where the analyses were carried out is a health service provider specialised around clinical analysis, dedicated to analysing the composition of biological fluids in the human organism to obtain information that contributes to better prevention, diagnosis, treatment, and monitoring of diseases. It is made up of a multidisciplinary team of healthcare professionals, including Medical Analysts, University Graduates in Nursing, Laboratory Technicians and Administrative Staff. It has a quality management system that complies with the ISO 9001:2008 standard and was previously used in this comprehensive research project [67].
2.5. Physical Performance Tests
To evaluate the physical capacities of the volleyball players, several fitness tests were conducted, which represented different aspects of volleyball performance. The tests were held in the afternoon, around 5 pm, on the respective days at the sports hall mentioned previously, under the same humidity and temperature conditions. Prior to the tests, a standardized warm-up of 20 min was performed to ensure that the athletes were suitably prepared for the various assessments, including tests for jumping, strength and agility [68].
There was a 5 min rest break between each attempt and throughout all the activities, as per previous recommendations [69]. The selected exercises for the test were chosen based on their demonstrated efficacy in improving performance in the discipline of volleyball [70]. Prior to each exercise, a specific description and justification were provided for the athletes to ensure that they were well-informed and understood the purpose of each test.
The tests were conducted in a specific order (Figure 3), beginning with the squat jump (SJ), followed by the countermovement jump (CMJ), the Abalakov jump (ABK), the drop jump (DJ), the medicine ball throw (using a 3 kg ball) and concluding with the Illinois agility test with change of direction (COD).
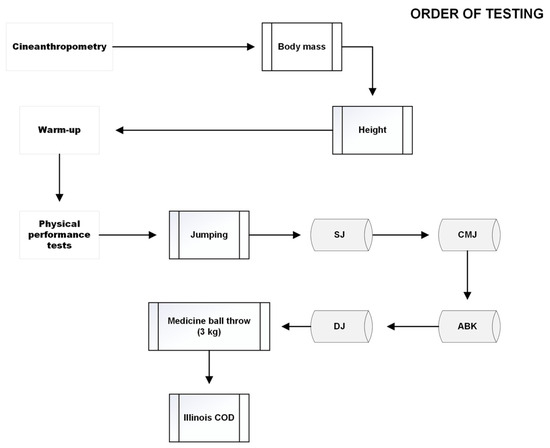
Figure 3.
Order of tests.
2.5.1. Warm-Up
Prior to the sports performance tests, a standardized 20-min warm-up session was conducted. The warm-up consisted of a 5 min jog forwards and backwards, as well as various exercises including hopping on one leg (forward and backward, on both the right and left sides), hip opening and closing, arm circumduction and heel running (forward and backward). The warm-up also included acceleration and injury prevention exercises such as hand planks from a standing position, jumps with trot from midfield, sprint from prone, lunge with trunk twist, squats with jump, explosive push-ups, hamstring stretches on trot, forward strides, zigzag running, and pyramid stretches from a quadruped position [71].
2.5.2. Performance Tests
- Jumping (SJ, CMJ, ABK, DJ)
The data for the fitness tests were collected using a Chronojump® Bosco System DIN-A1 (Chronojump Association for the Research and Dissemination of Technology Applied to Physical Activity and Sport, Barcelona, Spain) contact platform [72], which was controlled by a chronometric device responsible for timing the state changes of the detection device. The microcontroller error was 0.1%, resulting in a validity of 0.95 (CCI). The Chronopic 3 device was validated by the ACSM [73] for the entire spectrum studied, with an average error of 0.04 ± 0.18% for low signals (corresponding to contact time in a jump), and 0.05 ± 0.19% for high signals (time of flight) [74].
- Squat jump (SJ) [75]:
This exercise evaluates the lower limbs’ maximum concentric dynamic strength [76]. The SJ is a jump where the test participants rest on the jump platform with the feet slightly apart and with a 90° flexion. The arms are at the side of the body, to cancel the effects of the push-off. On command, the test person takes off as quickly as possible from the ground, looking for height. Remember that this jump cancels out the eccentric phase, which is always present in plyometric actions. This test has extremely high test–retest reliability (variation coefficients of 3.0%), with a high reliability (α = 0.97 and 0.98). This test has a high mean inter-test correlation (0.91) and a high intra-class correlation coefficient (0.97). Intra-subject variation in this test ranges between 2.4 and 4.6% [75].
- Countermovement jumping (CMJ) [77]:
For the vertical jump tests, the participants started in a standing position with their feet together and hands on their hips. They were instructed to jump as high as possible with a rapid countermovement. The flight time was used to calculate the change in the height of the body’s centre of gravity [76]. The calculation of jump height assumed that the take-off and landing positions of the body’s centre of gravity were the same [78]. Each participant was allowed two trials, with a 1 min recovery period between each trial, and the best score was recorded. The arms go to the side of the body during this jump. In this case, the athlete starts the movement from the upright position, then goes down and comes back up as fast as possible. In this case, the “eccentric–isometric–concentric” chain, present in plyometric actions, is respected. The CMJ is distinguished by having a relatively low coefficient of variation (3.0%) between tests [75]. The CMJ test had the highest factorial validity and the strongest correlation with the explosive power factor (r = 0.87). Based on these findings, it can be said that the CMJ is one of the most accurate and dependable field tests for determining the explosive power of the lower extremities [75].
- Abalakov [79]:
The Abalakov jump test was utilized to evaluate the explosive strength and maximum power of the lower body, with the goal of estimating the “reflex–elastic–explosive” manifestation [80]. Given the execution time of the Abalakov jump test and considering that around 50% of this time is damping, mainly eccentric, it is observed that the stretch reflex is triggered during this phase and not during the acceleration phase [67]. The percentage difference between the heights reached in the Abalakov and the countermovement jump (CMJ) was used to quantify the contribution of the arms to the jump height, which is referred to as the arm utilization index [79]. The Abalakov jump test was found to have a high correlation coefficient (0.969–0.995) and a low coefficient of variation (1.54–4.82%). A factor analysis revealed that the Abalakov jump test accounted for 82.90–95.79% of the variance of all jumping tests [81]. The Abalakov jump test exhibited a high correlation coefficient (0.969–0.995) and low coefficient of variation (1.54–4.82%). A factor analysis revealed that the Abalakov jump test accounted for 82.90–95.79% of the variance of all jumping tests [77].
- Drop jump [82]:
The drop jump test involves jumping after falling from a specified height, beginning in a position with the legs extended and executing a downward movement. The movement must be performed continuously with the hands on the hips and the trunk kept straight. This test is used to evaluate the “reflex–elastic–explosive” manifestation and to verify and assess strength [80]. The reliability of the drop jump test has been demonstrated by an intraclass ratio coefficient of 0.70–0.92, a standard error of measurement of 8.5–18.4 milliseconds (ms) and a coefficient of variation of 3.6–6.4% [80].
- b.
- Medicine Ball Throwing Test (3 kg) [83]
Using the medicine ball throwing test, upper body strength was evaluated [84]. The starting position is without prior impulse, from a standing position, with the legs comfortably apart, the feet in a symmetrical position and the ball held with both hands above and behind the head. In this way, the ball is thrown forward with as much force as possible, so that it falls within the throwing sector. The reliability obtained [85] through a test–retest is r = 0.98, and the validity of the drop jump test was evaluated using the intraclass correlation coefficient (ICC), which yielded a score of 0.98, indicating consistency between the means of each throwing mode (standing, kneeling, sitting, one-handed). The correlation coefficient was also calculated, resulting in a score of r = 0.49, with a significant p-value of <0.01 [67].
- c.
- Agility Test with Change of Direction, Illinois COD Agility Test (IAGT CDD)
MicroGate® Witty Wireless Training Timer photoelectric cells (Microgate Srl, Bolzano, Italy) were utilized in the study. These cells have a minimum resolution of 0.125 Ms and an event delay of 1 ms. They also incorporate a redundant code with information accuracy check and self-correction, with a pulse transmission accuracy of 0.4 Ms.
- Illinois agility test with change of direction:
The Illinois agility test consists of the following steps: To begin the Illinois agility test, assume a face-down position on the starting cone-marker, with your hands placed at shoulder level. When the assistant indicates and activates the stopwatch, the athlete must get up as quickly as possible, and run the entire course drawn by the cones, in the shortest time. The Illinois agility test [86] is widely regarded as a standard agility test [87] thanks to its strong validation and reproducibility [88,89]. As a result, it has become the go-to test for measuring changes in directional skill, making it a reliable tool for athletes and trainers alike [90]. According to research, the intraclass correlation coefficient and standard error of measurement for the Illinois agility test are 0.96 (95% CI, 0.85–0.98) and 0.19 s, respectively. In addition, the IAGT CDD has been found to be a reliable and valid test, with a correlation coefficient of 0.31 (95% CI, 0.24–0.39) and a significance level of p < 0.05 [59]. These findings suggest that the IAGT CDD is a trustworthy tool for assessing agility.
2.6. Statistical Analysis
All data are presented as mean ± SD. The normality of the variables was assessed using the Shapiro–Wilk normality test, since the sample size was less than 30. The parametric Student’s t-test for repeated or paired measures was used, assuming a normal distribution of the data. The percentage change (Δ%) from T1 to T2 in the outcome variables was calculated using the formula [(T2 − T1) /T1] × 100. To determine the effect size between participants, partial eta squared (η2p) was calculated. However, since this measure is prone to overestimation of the effect size, the following interpretation was used [91]: no effect if 0 ≤ η2p < 0.05; minimal effect if 0.05 ≤ η2p < 0.26; moderate effect if 0.26 ≤ η2p < 0.64 and strong effect if η2p ≥ 0.64. Pearson’s correlation analysis and Hopkins’ correlation magnitude were also conducted [92]. The magnitude of correlation coefficients was determined as trivial (r < 0.1), small (0.1 < r < 0.3), moderate (0.3 < r < 0.5), high (0.5 < r < 0.7), very high (0.7 < r < 0.9), near perfect (r > 0.9) and perfect (r = 1) [92]. In turn, the effect size variation is indicated providing a convenient (though not exhaustive) representation of the required sample size change [93] and a more intuitive and practical approach to probabilistic inference [94] based only on the ambiguity surrounding the statistic’s actual value. At the level of results, it has been considered at a general level that the significance of the difference obtained is in some way significant when it deviates from what is expected in a way that cannot be assumed to be produced by chance alone, since in this way, as we have said, it is unlikely that the result is produced by chance or random fluctuation. Therefore, those results that indicate a correlation coefficient greater than 0.5 will be considered. Following established guidelines [95], BMI was used to determine the prevalence of being overweight in the study sample. An Excel spreadsheet was used to record the values of the measurements collected for later statistical analysis in Windows SPSS® 25.0 software (Inc., Chicago, IL, USA). P 0.05 was the threshold for significance.
3. Results
3.1. Hormones
In Table 2, we can observe the percentage and absolute changes between the initial test (T1) and the final test (T2) for all hormone measures and the index that relates them. The main changes observed were a decrease (−3.470%) in C levels and an increase in T levels (2.945%). These changes imply that the T:C ratio has improved by 10.574%, as is illustrated graphically in Figure 4 and in Table 2. Although, as can be seen, the p-values are greater than 0.05, there is no statistically significant difference.

Table 2.
Absolute and percentage changes.
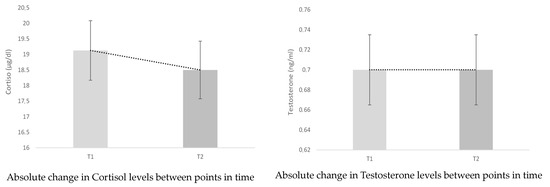
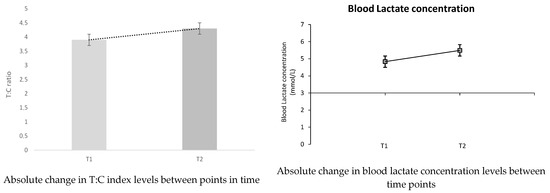
Figure 4.
Significant and absolute percentage changes.
3.2. Performance Tests
Table 3 displays the % and absolute changes between pre-test (T1) and post-test (T2) for all performance measures for the elite female volleyball players. An improvement was observed in the most-used jumps in volleyball and a slight decrease in agility. On a statistical level, it can be observed that there is a significant difference in the CMJ and ABK jumps, but not in the rest of the tests performed.

Table 3.
Variation in physical fitness scores.
Figure 4 shows the time-to-time changes of the hormones involved and their rate, as well as the most significant percentage change, in this case of blood lactate concentration (38.851%).
3.3. Relationship between Hormone Levels and Performance Changes
Figure 5 and Figure 6 show the bivariate correlations analysis between the levels of these hormones and some of the performance tests performed on the participants.
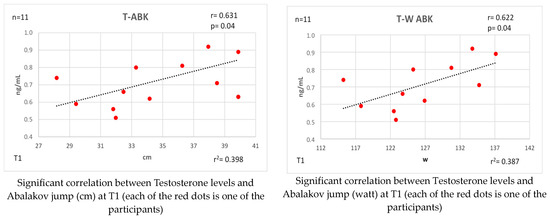
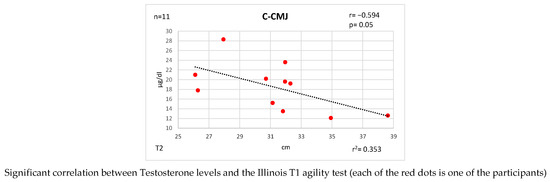
Figure 5.
Correlations established between T and physical fitness tests at T1.
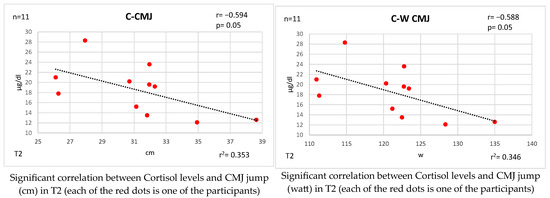
Figure 6.
Correlations established between T and C and physical fitness tests at T2.
At time T1, significant negative correlations of T with the agility test were observed (r = −0.596 [high]; p = 0.05). Negative relationships between the outcomes were also noted for these same tests with the T/C index: agility test (r = −0.598 [high]; p = 0.05). At this first moment, strong correlations of the T with the ABK jump longitudinal (r = 0.631 [high]; p = 0.04) and power watts (r = 0.622 [high]; p = 0.04) can be seen. Likewise, the T/C index with the CMJ jump: length (r = 0.598 [high]; p = 0.05) and watts (r = 0. 590 [high]; p = 0.05), the ABK jump: length (r = 0.646 [high]; p = 0.03) and power (r = 0.640 [high]; p = 0.03).
At time T2, negative correlations were found between CMJ and C, both in its longitudinal measure (r = −0.594 [high]; p = 0.05) and in its translation into watts of power (r = −0.588 [high]; p = 0.05). The same trend holds between SJ jump and T/C index: longitudinal (r = −0.624 [high]; p = 0.04) and power watts (r = −0.623 [high]; p = 0.04). Strong correlations are now established between the DJ jump and the T/C index: length (r = 0.604 [high]; p = 0.05) and watts (r = 0.602 [high]; p = 0.05).
The rest of the results are not shown in this section, as they are not significant at p > 0.05.
The percentage change for hormone levels (Table 2) are 2.945% for T, 3.470% for C and 10.574% for the T:C ratio. As for the performance results, the percentage changes (Table 3) are for the SJ jump—4.0% ± 11.3%, for the CMJ jump—3.0% ± 2.2%, for the ABK jump—2.2% ± 3.1%, for the DJ jump—3.5% ± 6.6%, for the MBT—0.7% ± 11.0% and for the agility test—1.8% ± 3.8%.
4. Discussion
The main objective of this study is to examine T and C concentration levels over a 16-week competitive period to gain a more comprehensive understanding of sporting performance in elite female volleyball players. The main results describe that serum total T concentrations were higher (0.7 ng/mL vs. 0.35 ng/mL), twice the ranges recorded in previous studies in female volleyball players [1] at both time points, although the values obtained are within the theoretically established ranges for serum total T of 0.3–0.9 ng/mL for women. The results also show that C levels were on average 4.7% (19.1 μg/dL vs. 18.2 μg/dL) higher at T1 and 1.57% (18.5 μg/dL vs. 18.2 μg/dL) according to these studies, ranges that are also within the theoretically established levels for basal C in women of 6–30 μg/dL. Of note, the hormone levels observed in this study were higher than those commonly used as a reference in clinical practice, which are derived from a “reference” population of healthy women [2]. Consistent results from different statistical methods suggest that young female athletes differ constitutively from the general female population in terms of their hormone levels [96].
Since the T concentration between moments has improved (2.945%) in the body of the participants, without being a significant change, this will tend to develop muscle mass more easily and therefore athletic performance will increase, as observed in some of the CMJ (3.0% ± 2.2%; p = 0.002) and ABK (2.2% ± 3.1%; p = 0.028) jumps, with significant changes between moments. Consequently, and knowing that muscles are the most energy-consuming tissue, participants will increase their energy expenditure the more physical activity they do, while developing their muscle mass according to their situation [3].
In the case of T, the more T that is secreted (within established theoretical levels) and the more physical exercise that is performed, the more muscle mass and strength will increase. Furthermore, as this hormone speeds up metabolism, it will help to lose body fat, while increasing muscle mass [3]. This will help participants to increase their level of jumping, as we have seen. We can see that, as mentioned above, participants have T concentration levels that are within the established theoretical parameters at both time points, with a slight percentage increase (2.945%) between time points. This indicates that, although not by much, the work performed between time points has been adequate.
The correlations between T and the agility test at T1 (high) showed the expected pattern of correspondence, with a considerable magnitude of change, indicating a high causal probability of the changes produced not being influenced by chance and that being negative indicates that the level of T influences this type of test inversely proportional to the reduction in test times. The Illinois agility test is considered a standard agility test [8] due to its high validation and reproducibility [9,10]. It is used to assess speed and agility abilities, including reaction time, acceleration, deceleration and change of direction [11], and it is used to quantify changes in directional ability [12]. The intraclass correlation coefficient and standard error of measurement values for the test are 0.96 (95% CI, 0.85–0.98) and 0.19 s, respectively. The validity of the COD IAGT has been assessed with the t-test, yielding a correlation coefficient of r = 0.31 (95% CI, 0.24–0.39) and a significant p-value < 0.05 [9]. These findings suggest that the COD IAGT is a reliable and valid test for assessing agility.
The measurement scales showed high significant correlations at T1 between T and the ABK jump (high), a significant disturbance suggesting a minimal probability of chance, because it is an action that requires explosive strength and coordination. This can be explained because T has statistically significant correlations with the development of speed and explosive strength [3].
At time T2, C presents significant negative correlations with CMJ jump (high), with a significant magnitude of change, indicating that there is a low probability that the changes are intervened by chance, and it can be concluded that the lower the level of C, and practically the same level of T, the lower the strength and explosiveness.
Aside from exercise intensity and duration, there are additional factors that can impact the production of C, including diet. When we consume complex carbohydrates after exercise, they increase insulin levels. This aspect has several metabolic effects, such as promoting glucose transport to skeletal muscle or suppressing the release of fatty acids from adipose tissue, while increasing fat storage by activating lipase [97,98], all aspects that help to reduce the high C levels that have appeared after training [21]. Another measure to avoid high C levels is to sleep and rest well, which enables cell renewal and recovery [22]. Therefore, it is necessary to observe the concentrations of these hormones not acutely but over a period to assess how they fluctuate and to know when the neuroendocrine system returns to equilibrium [58].
Both T and C have an acute/chronic effect on athletes, although, for the moment, the evidence is scarce in relation to the optimal hormonal response or environments for performance, inducing physical and psychological strategies that promote certain hormonal states that enhance performance in sporting competition [23]. Or, on the other hand, incorporating hormone monitoring to aid decision-making for stimulus planning and recovery may be important strategies for coaches [24]. To understand the benefits of T and C on sporting performance we must bear in mind that on a theoretical level, according to the principles of training, the level of preparation of elite athletes in general is increasing every year [25]. Conversely, it is observed that the level of preparation of athletes arriving at sporting events is decreasing and so any benefits, such as strategies to promote optimal levels of T or C concentrations, could be critical to sporting performance [3]. In this sense, the T-C ratio, the T:C ratio, is a ratio used to determine the anabolic versus catabolic state in the face of training. According to studies, alterations in the T:C ratio may be connected to an improvement in performance. This T:C ratio is considered a marker of physiological stress, mainly associated with overtraining [26]. Other authors argue that such a view seems to be a cursory interpretation of more complex adaptive processes [27]. In relation to the T:C ratio and its impact on athletic performance, the evidence is discrepant, but on the other hand, it is clear about the hormonal influences of T and C on anabolic processes, regarding sports preparation and the immediate reaction to exercise. Therefore, it makes sense to determine an optimal T and C response to develop appropriate strategies that generate a precise hormonal environment to enhance athletic performance [99].
In this case, we see that the T:C ratio increases as was predictable with the data provided for T and C. The T:C ratio represents the balance between anabolic and catabolic processes and has been suggested by various researchers as an indicator of the training load [28,29,30,31] and could be a useful tool for intervening in its planning, before pathological physiological alterations occur in athletes [29,30]. The increase in this ratio (10.574%) (Figure 4) indicates a predominance of anabolic processes, which lead to an increase in performance (overcompensation) [28,29,31,32]. It should be noted that there is some debate surrounding the T:C ratio and that it is a parameter that requires more research [34,35]. Some authors even consider that the T:C ratio does not indicate metabolic balance [36].
At T1, the T:C ratio correlates positively and significantly with CMJ and ABK jumps [38]. This relationship is repeated in the results obtained with the agility test. At time T2 it is observed that, although at a general level the performance results improve in comparison with the results obtained at T1, there is a significant negative correlation with the SJ jump but a significant positive correlation with the DJ jump, an aspect that could lead us to think that coordination improves as the T:C ratio increases [100]. This increase could reflect the decrease in stress as it was during the first competitive break and given the good position reached in the ranking at that time of the season [101].
Correlations between and within dimensions were in line with the expected relationships. Overall, negative correlations between the stress and recovery scales indicate the importance of considering them separately [39]. The negative correlations between the stress and recovery scales could be that since one scale focuses on mental aspects, the other focuses on physical aspects. This supports the theoretical differences between these two scales [40]. However, it is important to note that further research is needed to establish the long-term trends of these correlations through repeated measure tests and larger participant sample sizes, as these correlations may vary depending on the characteristics of the sample being studied. These aspects can be seen in Figure 5 and Figure 6. Moreover, it is necessary to individualise the work of each participant despite being a team discipline [102].
5. Strengths
This study boasts several strengths, including (i) the extended duration of the follow-up with high-level volleyball athletes over a 16-week competitive season before a championship and (ii) the integrated approach used, which examined both physiological and performance parameters in both the athletes and the team.
6. Limitations
The scope of the current study and the controlled conditions of the tasks performed constrain the generalizability of our research findings. Furthermore, none of the participants in the study were non-athletes engaged in regular physical activity, which makes it unclear whether the observed changes were due to high levels of physical activity in general or to high-level sports performance specifically. Another limitation of the study is the lack of data on sleep or diet to help explain the different levels observed.
7. Future Research Lines
It is recommended that future research should compare these variations in hormonal responses with objective training load parameters and/or other hormonal modulators to examine their association with athletic performance. However, additional studies are necessary to determine the hormonal effects of sports activities more precisely.
8. Practical Applications
Based on the results obtained, it was found that monitoring hormonal responses during season planning can yield valuable information regarding the level of stress caused by sports training and competitions, as well as the ways in which athletes adapt to stress induced by sports activities. This information can be used to manage training load and plan training cycles more effectively throughout the competitive season.
9. Conclusions
This retrospective, longitudinal and observational study on hormonal changes in an elite women’s volleyball team revealed that highly trained players exhibit elevated baseline levels of T and C compared to those of healthy women without sports training. This finding could be indicative of the athletes’ physiological adaptation to stressors, as continuous exposure to stressors is known to promote positive adaptations to such factors. In summary, the impact of training and competition during a volleyball season is distinctly reflected in the levels of T. Furthermore, C levels were found to increase at the beginning of training and remain elevated throughout the season, but without any significant changes. T could be an indicator of the athletes’ balance and could even justify necessary interventions to optimise training loads on an individual basis.
Author Contributions
Conceptualization, Á.M.-O.; methodology, Á.M.-O., J.C.-G. and J.M.-A.; software, Á.M.-O.; validation, Á.M.-O., J.C.-G. and J.M.-A.; formal analysis, Á.M.-O., J.C.-G. and J.M.-A.; investigation, Á.M.-O., J.C.-G. and J.M.-A.; resources, Á.M.-O., J.C.-G. and J.M.-A.; data curation, Á.M.-O. and J.M.-A.; writing—original draft preparation, Á.M.-O.; writing—review and editing, Á.M.-O., J.F.-L. and J.C.-G.; visualization, Á.M.-O. and J.C.-G.; supervision, J.C.-G. and J.M.-A.; project administration, Á.M.-O. All authors have read and agreed to the published version of the manuscript.
Funding
The authors did not receive financial support from any organisation for the work presented.
Institutional Review Board Statement
The study was conducted in accordance with the ethical guidelines dictated in the Declaration of Helsinki of the World Medical Association (2014) for medical research on human beings and with the approval of the project of the Ethics Committee for Research on Human Beings of the University of the Basque Country, number M10_2017_216. It should be noted that the data obtained have been treated with the utmost confidentiality and scientific rigor; their use being restricted by the guidelines of the research projects following the scientific method required in each case, complying with Organic Law 15/1999, of 13 December, on the Protection of Personal Data (LOPD). The procedures used have respected the ethical criteria of the Responsible Committee for Human Experimentation (established by law 14/2007, published in the OSG number 159).
Informed Consent Statement
Informed consent was obtained from all subjects who participated in the study.
Data Availability Statement
To promote transparency of the data supporting the results reported in the article, the authors have established the data availability statement. The data associated with this article are not publicly available but are available from the corresponding author upon reasonable request.
Conflicts of Interest
All authors certify that they have no affiliation or involvement with any organisation or entity with financial or non-financial interest in the subject matter or materials covered in this manuscript.
References
- Arnold, R.; Fletcher, D.; Daniels, K. Demographic Differences in Sport Performers’ Experiences of Organizational Stressors. Scand. J. Med. Sci. Sports 2016, 26, 348–358. [Google Scholar] [CrossRef]
- Crocker, P.R.E.; Tamminen, K.A.; Gaudreau, P. Coping in Sport. In Contemporary Advances in Sport Psychology: A Review; Mellalieu, S., Hanton, S., Eds.; Routledge: London, UK, 2015; pp. 28–67. [Google Scholar]
- Lazarus, R.S. How Emotions Influence Performance in Competitive Sports. Sport Psychol. 2000, 14, 229–252. [Google Scholar]
- Nicholls, A.R.; Levy, A.R.; Carson, F.; Thompson, M.A.; Perry, J.L. The Applicability of Self-Regulation Theories in Sport: Goal Adjustment Capacities, Stress Appraisals, Coping, and Well-Being among Athletes. Psychol. Sport Exerc. 2016, 27, 47–55. [Google Scholar] [CrossRef]
- Schinke, R.J.; Battochio, R.C.; Dube, T.V.; Lidor, R.; Tenenbaum, G.; Lane, A.M. Adaptation Processes Affecting Performance in Elite Sport. J. Clin. Sport Psychol. 2012, 6, 180–195. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Calleja-González, J.; Clemente-Suárez, V.J.; Zourdos, M.C. Influence of Anthropometric Profile on Physical Performance in Elite Female Volleyballers in Relation to Playing Position | Influencia de La ComposiciÓn Corporal En El Rendimiento FÍsico de Jugadoras de Voleibol En FunciÓn de Su PosiciÓn de Juego. Nutr. Hosp. 2015, 31, 7658. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Urdampilleta, A.; Ostojic, S.M. Dietary Intake Habits and Controlled Training on Body Composition and Strength in Elite Female Volleyball Players during the Season. Appl. Physiol. Nutr. Metab. 2015, 40, 827–834. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Urdampilleta, A.; Calleja-Gonzalez, J.; Seco, J.; Cordova, A. Relationship of Long-Term Macronutrients Intake on Anabolic-Catabolic Hormones in Female Elite Volleyball Players. Nutr. Hosp. 2017, 34, 1155–1162. [Google Scholar] [CrossRef]
- Mielgo-Ayuso, J.; Zourdos, M.C.; Calleja-González, J.; Córdova, A.; Fernandez-Lázaro, D.; Caballero-García, A. Eleven Weeks of Iron Supplementation Does Not Maintain Iron Status for an Entire Competitive Season in Elite Female Volleyball Players: A Follow-Up Study. Nutrients 2018, 10, 1526. [Google Scholar] [CrossRef]
- Freitas, V.H.; Nakamura, F.Y.; Miloski, B.; Samulski, D.; Bara-Filho, M.G. Sensitivity of physiological and psychological markers to training load intensification in volleyball players. J. Sports Sci. Med. 2014, 13, 571. Available online: https://pubmed.ncbi.nlm.nih.gov/25177184/ (accessed on 26 July 2023).
- Sheppard, J.M.; Newton, R.U. Long-Term Training Adaptations in Elite Male Volleyball Players. J. Strength Cond. Res. 2012, 26, 2180–2184. [Google Scholar] [CrossRef]
- Keaney, L.C.; Kilding, A.E.; Merien, F.; Dulson, D.K. The Impact of Sport Related Stressors on Immunity and Illness Risk in Team-Sport Athletes. J. Sci. Med. Sport 2018, 21, 1192–1199. [Google Scholar] [CrossRef] [PubMed]
- Dziembowska, I.; Wójcik, M.; Hołyńska-Iwan, I.; Litwic-Kaminska, K.; Słomka, A.; Żekanowska, E. Female Volleyball Players Are More Prone to Cortisol Anticipatory Stress Response than Sedentary Women. Medicina 2019, 55, 258. [Google Scholar] [CrossRef] [PubMed]
- Filaire, E.; Bernain, X.; Sagnol, M.; Lac, G. Preliminary Results on Mood State, Salivary Testosterone:Cortisol Ratio and Team Performance in a Professional Soccer Team. Eur. J. Appl. Physiol. 2001, 86, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Pfister, H.P.; Muir, J.L. Prenatal Exposure to Predictable and Unpredictable Novelty Stress and Oxytocin Treatment Affects Offspring Development and Behavior in Rats. Int. J. Neurosci. 1992, 62, 227–241. [Google Scholar] [CrossRef]
- Hoffman, J.R.; Falk, B.; Radom-Isaac, S.; Weinstein, Y.; Magazanik, A.; Wang, Y.; Yarom, Y. The Effect of Environmental Temperature on Testosterone and Cortisol Responses to High Intensity, Intermittent Exercise in Humans. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 83–87. [Google Scholar] [CrossRef]
- Margonis, K.; Fatouros, I.G.; Jamurtas, A.Z.; Nikolaidis, M.G.; Douroudos, I.; Chatzinikolaou, A.; Mitrakou, A.; Mastorakos, G.; Papassotiriou, I.; Taxildaris, K.; et al. Oxidative Stress Biomarkers Responses to Physical Overtraining: Implications for Diagnosis. Free Radic. Biol. Med. 2007, 43, 901–910. [Google Scholar] [CrossRef]
- Radojewski, M.; Podgórski, T.; Pospieszna, B.; Kryaciak, J.; Sliwicka, E.; Karolkiewicz, J. Skeletal Muscle Cell Damage Indicators in Volleyball Players after the Competitive Phase of the Annual Training Cycle. J. Hum. Kinet. 2018, 62, 81–90. [Google Scholar] [CrossRef]
- Pereira, A.; Costa, A.M.; Santos, P.; Figueiredo, T.; João, P.V. Training Strategy of Explosive Strength in Young Female Volleyball Players. Medicina 2015, 51, 126–131. [Google Scholar] [CrossRef]
- Sgrò, P.; Romanelli, F.; Felici, F.; Sansone, M.; Bianchini, S.; Buzzachera, C.F.; Baldari, C.; Guidetti, L.; Pigozzi, F.; Lenzi, A.; et al. Testosterone Responses to Standardized Short-Term Sub-Maximal and Maximal Endurance Exercises: Issues on the Dynamic Adaptive Role of the Hypothalamic-Pituitary-Testicular Axis. J. Endocrinol. Investig. 2014, 37, 13–24. [Google Scholar] [CrossRef]
- Vaamonde, D.; Da Silva-Grigoletto, M.E.; Fernandez, J.M.; Algar-Santacruz, C.; García-Manso, J.M. Findings on Sperm Alterations and DNA Fragmentation, Nutritional, Hormonal and Antioxidant Status in an Elite Triathlete. Case Report. Rev. Andal. Med. Deporte 2014, 7, 143–148. [Google Scholar] [CrossRef][Green Version]
- Anderson, T.; Lane, A.R.; Hackney, A.C. Cortisol and Testosterone Dynamics Following Exhaustive Endurance Exercise. Eur. J. Appl. Physiol. 2016, 116, 1503–1509. [Google Scholar] [CrossRef]
- Fry, R.W.; Lawrence, S.R.; Morton, A.R.; Schreiner, A.B.; Polglaze, T.D.; Keast, D. Monitoring Training Stress in Endurance Sports Using Biological Parameters. Clin. J. Sport Med. 1993, 3, 6–13. [Google Scholar] [CrossRef]
- Chichinadze, K.; Chichinadze, N. Stress-Induced Increase of Testosterone: Contributions of Social Status and Sympathetic Reactivity. Physiol. Behav. 2008, 94, 595–603. [Google Scholar] [CrossRef]
- Bateup, H.S.; Booth, A.; Shirtcliff, E.A.; Granger, D.A. Testosterone, Cortisol, and Women’s Competition. Evol. Hum. Behav. 2002, 23, 181–192. [Google Scholar] [CrossRef]
- Mehta, P.H.; Jones, A.C.; Josephs, R.A. The Social Endocrinology of Dominance: Basal Testosterone Predicts Cortisol Changes and Behavior Following Victory and Defeat. J. Pers. Soc. Psychol. 2008, 94, 1078–1093. [Google Scholar] [CrossRef] [PubMed]
- Zyphur, M.J.; Narayanan, J.; Koh, G.; Koh, D. Testosterone–Status Mismatch Lowers Collective Efficacy in Groups: Evidence from a Slope-as-Predictor Multilevel Structural Equation Model. Organ. Behav. Hum. Decis. Process. 2009, 110, 70–79. [Google Scholar] [CrossRef]
- Liening, S.H.; Josephs, R.A. It Is Not Just About Testosterone: Physiological Mediators and Moderators of Testosterone’s Behavioral Effects. Soc. Personal. Psychol. Compass 2010, 4, 982–994. [Google Scholar] [CrossRef]
- Filaire, E.; Alix, D.; Ferrand, C.; Verger, M. Psychophysiological Stress in Tennis Players during the First Single Match of a Tournament. Psychoneuroendocrinology 2009, 34, 150–157. [Google Scholar] [CrossRef]
- Foster, J.D.; Trimm IV, R.F. On Being Eager and Uninhibited: Narcissism and Approach-Avoidance Motivation. Pers. Soc. Psychol. Bull. 2008, 34, 1004–1017. [Google Scholar] [CrossRef]
- Elloumi, M.; Maso, F.; Michaux, O.; Robert, A.; Lac, G. Behaviour of Saliva Cortisol [C], Testosterone [T] and the T/C Ratio during a Rugby Match and during the Post-Competition Recovery Days. Eur. J. Appl. Physiol. 2003, 90, 23–28. [Google Scholar] [CrossRef]
- Armstrong, L.E.; VanHeest, J.L. The Unknown Mechanism of the Overtraining Syndrome: Clues from Depression and Psychoneuroimmunology. Sports Med. 2002, 32, 185–209. [Google Scholar] [CrossRef] [PubMed]
- Anderson, D.E. The Impact of Feedback on Dietary Intake and Body Composition of College Women Volleyball Players over a Competitive Season. J. Strength Cond. Res. 2010, 24, 2220–2226. [Google Scholar] [CrossRef] [PubMed]
- Borresen, J.; Lambert, M.I. Autonomic Control of Heart Rate during and after Exercise: Measurements and Implications for Monitoring Training Status. Sports Med. 2008, 38, 633–646. [Google Scholar] [CrossRef] [PubMed]
- Smith, T.B.; Hopkins, W.G.; Lowe, T.E. Are There Useful Physiological or Psychological Markers for Monitoring Overload Training in Elite Rowers? Int. J. Sports Physiol. Perform. 2011, 6, 469–484. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Kuhn, C.M. Anabolic Steroids. Recent Prog. Horm. Res. 2002, 57, 411–434. [Google Scholar] [CrossRef] [PubMed]
- Christiansen, J.J.; Djurhuus, C.B.; Gravholt, C.H.; Iversen, P.; Christiansen, J.S.; Schmitz, O.; Weeke, J.; Jørgensen, J.O.L.; Møller, N. Effects of Cortisol on Carbohydrate, Lipid, and Protein Metabolism: Studies of Acute Cortisol Withdrawal in Adrenocortical Failure. J. Clin. Endocrinol. Metab. 2007, 92, 3553–3559. [Google Scholar] [CrossRef]
- Cook, C.J.; Crewther, B.T.; Kilduff, L.P. Are Free Testosterone and Cortisol Concentrations Associated with Training Motivation in Elite Male Athletes? Psychol. Sport Exerc. 2013, 14, 882–885. [Google Scholar] [CrossRef]
- Beaven, C.M.; Gill, N.D.; Cook, C.J. Salivary Testosterone and Cortisol Responses in Professional Rugby Players after Four Resistance Exercise Protocols. J. Strength Cond. Res. 2008, 22, 426–432. [Google Scholar] [CrossRef]
- Beaven, C.M.; Gill, N.D.; Ingram, J.R.; Hopkins, W.G. Acute Salivary Hormone Responses to Complex Exercise Bouts. J. Strength Cond. Res. 2011, 25, 1072–1078. [Google Scholar] [CrossRef]
- Arruda, A.F.S.; Aoki, M.S.; Miloski, B.; Freitas, C.G.; Moura, N.R.; Moreira, A. Playing Match Venue Does Not Affect Resting Salivary Steroids in Elite Futsal Players. Physiol. Behav. 2016, 155, 77–82. [Google Scholar] [CrossRef]
- Cook, C.J.; Crewther, B.T. The Effects of Different Pre-Game Motivational Interventions on Athlete Free Hormonal State and Subsequent Performance in Professional Rugby Union Matches. Physiol. Behav. 2012, 106, 683–688. [Google Scholar] [CrossRef]
- Meeusen, R.; Duclos, M.; Foster, C.; Fry, A.; Gleeson, M.; Nieman, D.; Raglin, J.; Rietjens, G.; Steinacker, J.; Urhausen, A.; et al. Prevention, Diagnosis, and Treatment of the Overtraining Syndrome: Joint Consensus Statement of the European College of Sport Science and the American College of Sports Medicine. Med. Sci. Sports Exerc. 2013, 45, 186–205. [Google Scholar] [CrossRef]
- Adlercreutz, H.; Harkonen, M.; Kuoppasalmi, K.; Näveri, H.; Huhtaniemi, I.; Tikkanen, H.; Remes, K.; Dessypris, A.; Karvonen, J. Effect of Training on Plasma Anabolic and Catabolic Steroid Hormones and Their Response during Physical Exercise. Int. J. Sports Med. 1986, 7 (Suppl. S1), 27–28. [Google Scholar] [CrossRef]
- Araujo, A.B.; Dixon, J.M.; Suarez, E.A.; Murad, M.H.; Guey, L.T.; Wittert, G.A. Clinical Review: Endogenous Testosterone and Mortality in Men: A Systematic Review and Meta-Analysis. J. Clin. Endocrinol. Metab. 2011, 96, 3007–3019. [Google Scholar] [CrossRef]
- Davis, S.R.; Wahlin-Jacobsen, S. Testosterone in Women--the Clinical Significance. Lancet Diabetes Endocrinol. 2015, 3, 980–992. [Google Scholar] [CrossRef]
- Olson, B.R. Exercise-Induced Amenorrhea. Am. Fam. Phys. 1989, 39, 213–221. [Google Scholar]
- Drinkwater, B.L.; Nilson, K.; Ott, S.; Chesnut, C.H. Bone Mineral Density After Resumption of Menses in Amenorrheic Athletes. JAMA J. Am. Med. Assoc. 1986, 256, 380–382. [Google Scholar] [CrossRef]
- Bloom, S.R.; Johnson, R.H.; Park, D.M.; Rennie, M.J.; Sulaiman, W.R. Differences in the Metabolic and Hormonal Response to Exercise between Racing Cyclists and Untrained Individuals. J. Physiol. 1976, 258, 1–18. [Google Scholar] [CrossRef]
- Wilkerson, J.E.; Horvath, S.M.; Gutin, B. Plasma Testosterone during Treadmill Exercise. J. Appl. Physiol. 1980, 49, 249–253. [Google Scholar] [CrossRef]
- Cumming, D.C.; Quigley, M.E.; Yen, S.S.C. Acute Suppression of Circulating Testosterone Levels by Cortisol in Men. J. Clin. Endocrinol. Metab. 1983, 57, 671–673. [Google Scholar] [CrossRef]
- Hough, J.P.; Papacosta, E.; Wraith, E.; Gleeson, M. Plasma and Salivary Steroid Hormone Responses of Men to High-Intensity Cycling and Resistance Exercise. J. Strength Cond. Res. 2011, 25, 23–31. [Google Scholar] [CrossRef]
- Thorpe, R.; Sunderland, C. Muscle Damage, Endocrine, and Immune Marker Response to a Soccer Match. J. Strength Cond. Res. 2012, 26, 2783–2790. [Google Scholar] [CrossRef]
- Daly, W.; Seegers, C.A.; Rubin, D.A.; Dobridge, J.D.; Hackney, A.C. Relationship between Stress Hormones and Testosterone with Prolonged Endurance Exercise. Eur. J. Appl. Physiol. 2005, 93, 375–380. [Google Scholar] [CrossRef]
- Duclos, M.; Corcuff, J.B.; Rashedi, M.; Fougère, V.; Manier, G. Trained versus Untrained Men: Different Immediate Post-Exercise Responses of Pituitary Adrenal Axis. A Preliminary Study. Eur. J. Appl. Physiol. Occup. Physiol. 1997, 75, 343–350. [Google Scholar] [CrossRef]
- Aw, M.; Timmerman, S.; Kk, B.; Da, R.; Ac, H. Strenuous, Fatiguing Exercise: Relationship of Cortisol to Circulating Thyroid Hormones. Int. J. Endocrinol. Metab. 2005, 1, 18–24. [Google Scholar]
- Tremblay, M.S.; Copeland, J.L.; Van Helder, W. Influence of Exercise Duration on Post-Exercise Steroid Hormone Responses in Trained Males. Eur. J. Appl. Physiol. 2005, 94, 505–513. [Google Scholar] [CrossRef] [PubMed]
- Viru, A.; Viru, M. Biochemical Monitoring of Sport Training; Human Kinetics: Champaign, IL, USA, 2001. [Google Scholar]
- Calmeiro, L.; Tenenbaum, G.; Eccles, D.W. illia. Managing Pressure: Patterns of Appraisals and Coping Strategies of Non-Elite and Elite Athletes during Competition. J. Sports Sci. 2014, 32, 1813–1820. [Google Scholar] [CrossRef]
- Doron, J.; Martinent, G. Trajectories of Psychological States of Women Elite Fencers during the Final Stages of International Matches. J. Sports Sci. 2016, 34, 836–842. [Google Scholar] [CrossRef]
- Doron, J.; Martinent, G. Appraisal, Coping, Emotion, and Performance during Elite Fencing Matches: A Random Coefficient Regression Model Approach. Scand. J. Med. Sci. Sports 2017, 27, 1015–1025. [Google Scholar] [CrossRef]
- Gaudreau, P.; Nicholls, A.; Levy, A.R. The Ups and Downs of Coping and Sport Achievement: An Episodic Process Analysis of within-Person Associations. J. Sport Exerc. Psychol. 2010, 32, 298–311. [Google Scholar] [CrossRef]
- World Medical Association. World Medical Association Declaration of Helsinki: Ethical Principles for Medical Research Involving Human Subjects. Jama 2013, 310, 2191–2194. [Google Scholar] [CrossRef]
- Vilar, L.; Araújo, D.; Davids, K.; Button, C. The Role of Ecological Dynamics in Analysing Performance in Team Sports. Sports Med. 2012, 42, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Seebacher, F.; Franklin, C.E. Determining Environmental Causes of Biological Effects: The Need for a Mechanistic Physiological Dimension in Conservation Biology. Philos. Trans. R. Soc. B Biol. Sci. 2012, 367, 1607–1614. [Google Scholar] [CrossRef]
- Fletcher, G.F.; Ades, P.A.; Kligfield, P.; Arena, R.; Balady, G.J.; Bittner, V.A.; Coke, L.A.; Fleg, J.L.; Forman, D.E.; Gerber, T.C.; et al. Exercise Standards for Testing and Training: A Scientific Statement from the American Heart Association. Circulation 2013, 128, 873–934. [Google Scholar] [CrossRef] [PubMed]
- Miguel-Ortega, Á.; Calleja-González, J.; Mielgo-Ayuso, J. Comparison of Sports Performance and Kinanthropometric Profiles of Elite Female Basketball and Volleyball Players over the Course of a Competitive Season. Appl. Sci. 2023, 13, 8267. [Google Scholar] [CrossRef]
- Verhagen, E.; Vriend, I.; Gouttebarge, V.; Kemler, E.; De Wit, J.; Zomerdijk, D.; Nauta, J. Effectiveness of a Warm-up Programme to Reduce Injuries in Youth Volleyball Players: A Quasi-Experiment. Br. J. Sports Med. 2023, 57, 464–470. [Google Scholar] [CrossRef]
- Lopez-Samanes, A.; Del Coso, J.; Hernández-Davó, J.L.; Moreno- -Pérez, D.; Romero-Rodriguez, D.; Madruga-Parera, M.; Muñoz, A.; Moreno- -Pérez, V. Acute Effects of Dynamic versus Foam Rolling Warm-up Strategies on Physical Performance in Elite Tennis Players. Biol. Sport 2021, 38, 595. [Google Scholar] [CrossRef]
- González-Ravé, J.M.; Arija, A.; Clemente-Suarez, V. Seasonal Changes in Jump Performance and Body Composition in Women Volleyball Players. J. Strength Cond. Res. 2011, 25, 1492–1501. [Google Scholar] [CrossRef]
- McGowan, C.J.; Pyne, D.B.; Thompson, K.G.; Rattray, B. Warm-Up Strategies for Sport and Exercise: Mechanisms and Applications. Sports Med. 2015, 45, 1523–1546. [Google Scholar] [CrossRef]
- De Blas, X.; Padullés, J.M.; Del Amo, J.L.L.; Guerra-Balic, M. Creation and Validation of Chronojump-Boscosystem: A Free Tool to Measure Vertical Jumps. RICYDE Rev. Int. Cienc. Deporte 2012, 8, 334–356. [Google Scholar] [CrossRef]
- American College of Sport Medicine (ACSM). In Proceedings of the 56th Annual Meeting, Incheon, Republic of Korea, 2–5 May 2023; pp. 27–30. Available online: https://www.medscape.com/viewcollection/30326 (accessed on 25 July 2023).
- De Blas, X.; González-Gómez, J.; Gómez, R. Validación de Chronopic 3. In Poster Presenting in American College of Sport Medicine (ACSM) 56th Annual Meeting; Medscape: Seattle, WA, USA, 2009; pp. 27–30. [Google Scholar]
- Markovic, G.; Dizdar, D.; Jukic, I.; Cardinale, M. Reliability and Factorial Validity of Squat and Countermovement Jump Tests. J. Strength Cond. Res. 2004, 18, 551–555. [Google Scholar] [CrossRef]
- Bosco, C.; Luhtanen, P.; Komi, P.V. A Simple Method for Measurement of Mechanical Power in Jumping. Eur. J. Appl. Physiol. Occup. Physiol. 1983, 50, 273–282. [Google Scholar] [CrossRef]
- Rodríguez-Rosell, D.; Mora-Custodio, R.; Franco-Márquez, F.; Yáñez-García, J.M.; González-Badillo, J.J. Traditional vs. Sport-Specific Vertical Jump Tests: Reliability, Validity, and Relationship with the Legs Strength and Sprint Performance in Adult and Teen Soccer and Basketball Players. J. Strength Cond. Res. 2017, 31, 196–206. [Google Scholar] [CrossRef] [PubMed]
- Ostojic, S.M.; Mazic, S.; Dikic, N. Profiling in Basketball: Physical and Physiological Characteristics of Elite Players. J. Strength Cond. Res. 2006, 20, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Bobbert, M.F.; Gerritsen, K.G.M.; Litjens, M.C.A.; Van Soest, A.J. Why Is Countermovement Jump Height Greater than Squat Jump Height? Med. Sci. Sports Exerc. 1996, 28, 1402–1412. [Google Scholar] [CrossRef]
- Borràs, X.; Balius, X.; Drobnic, F.; Galilea, P. Vertical Jump Assessment on Volleyball: A Follow-up of Three Seasons of a High-Level Volleyball Team. J. Strength Cond. Res. 2011, 25, 1686–1694. [Google Scholar] [CrossRef] [PubMed]
- Petway, A.J.; Freitas, T.T.; Calleja-González, J.; Leal, D.M.; Alcaraz, P.E. Training Load and Match-Play Demands in Basketball Based on Competition Level: A Systematic Review. PLoS ONE 2020, 15, e0229212. [Google Scholar] [CrossRef]
- Tenelsen, F.; Brueckner, D.; Muehlbauer, T.; Hagen, M. Validity and Reliability of an Electronic Contact Mat for Drop Jump Assessment in Physically Active Adults. Sports 2019, 7, 114. [Google Scholar] [CrossRef]
- Stockbrugger, B.A.; Haennel, R.G. Validity and Reliability of a Medicine Ball Explosive Power Test. J. Strength Cond. Res. 2001, 15, 431–438. [Google Scholar] [CrossRef]
- Ortiz-Cervera, V. Entrenamiento de Fuerza y Explosividad para la Actividad Física y el Deporte de Competición; INDE: Barcelona, Spain, 1999. [Google Scholar]
- De Guio, F.; Benoit-Cattin, H.; Davenel, A. Quantitative Study of Signal Decay Due to Magnetic Susceptibility Interfaces: MRI Simulations and Experiments. In Proceedings of the 2007 29th Annual International Conference of the IEEE Engineering in Medicine and Biology Society, Lyon, France, 22–26 August 2007; IEEE: Piscataway, NJ, USA, 2007; pp. 1607–1610. [Google Scholar] [CrossRef]
- Getchell, B. Physical Fitness: A Way of Life, 2nd ed.; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 1979. [Google Scholar]
- Horicka, P.; Hianik, J.; Šimonek, J. The Relationship between Speed Factors and Agility in Sport Games. J. Hum. Sport Exerc. 2014, 9, 49–58. [Google Scholar] [CrossRef]
- Hachana, Y.; Chaabène, H.; Nabli, M.A.; Attia, A.; Moualhi, J.; Farhat, N.; Elloumi, M. Test-Retest Reliability, Criterion-Related Validity, and Minimal Detectable Change of the Illinois Agility Test in Male Team Sport Athletes. J. Strength Cond. Res. 2013, 27, 2752–2759. [Google Scholar] [CrossRef] [PubMed]
- Negra, Y.; Chaabene, H.; Hammami, M.; Amara, S.; Sammoud, S.; Mkaouer, B.; Hachana, Y. Agility in Young Athletes: Is It a Different Ability From Speed and Power? J. Strength Cond. Res. 2017, 31, 727–735. [Google Scholar] [CrossRef] [PubMed]
- Tsitskaris, G.; Theoharopoulos, A.; Garefis, A. Speed, speed dribble and agility of male basketball players playing in different positions. J. Hum. Mov. Stud. 2003, 45, 21–30. Available online: https://www.researchgate.net/publication/290652138_Speed_speed_dribble_and_agility_of_male_basketball_players_playing_in_different_positions (accessed on 25 July 2023).
- Ferguson, C.J. An Effect Size Primer: A Guide for Clinicians and Researchers. Prof. Psychol. Res. Pract. 2009, 40, 532–538. [Google Scholar] [CrossRef]
- Hopkins, W.G.; Marshall, S.W.; Batterham, A.M.; Hanin, J. Progressive Statistics for Studies in Sports Medicine and Exercise Science. Med. Sci. Sports Exerc. 2009, 41, 3–12. [Google Scholar] [CrossRef]
- Cohen, J. A Power Primer. Psychol. Bull. 1992, 112, 155–159. [Google Scholar] [CrossRef]
- Batterham, A.M.; Hopkins, W.G. Making meaningful inferences about magnitudes. Int. J. Sports Physiol. Perform. 2006, 1, 50–57. [Google Scholar] [CrossRef]
- Cole, T.J.; Bellizzi, M.C.; Flegal, K.M.; Dietz, W.H. Establishing a Standard Definition for Child Overweight and Obesity Worldwide: International Survey. Br. Med. J. 2000, 320, 1240–1243. [Google Scholar] [CrossRef]
- Roli, L.; De Vincentis, S.; Rocchi, M.B.L.; Trenti, T.; De Santis, M.C.; Savino, G. Testosterone, Cortisol, HGH, and IGF-1 Levels in an Italian Female Elite Volleyball Team. Health Sci. Rep. 2018, 1, e32. [Google Scholar] [CrossRef]
- Sidossis, L.S.; Stuart, C.A.; Shulman, G.I.; Lopaschuk, G.D.; Wolfe, R.R. Glucose plus Insulin Regulate Fat Oxidation by Controlling the Rate of Fatty Acid Entry into the Mitochondria. J. Clin. Investig. 1996, 98, 2244–2250. [Google Scholar] [CrossRef]
- Wolfe, R.R. Metabolic Interactions between Glucose and Fatty Acids in Humans. Am. J. Clin. Nutr. 1998, 67 (Suppl. S3), 519S–526S. [Google Scholar] [CrossRef] [PubMed]
- Guest, N.S.; Horne, J.; Vanderhout, S.M.; El-Sohemy, A. Sport Nutrigenomics: Personalized Nutrition for Athletic Performance. Front. Nutr. 2019, 6, 8. [Google Scholar] [CrossRef] [PubMed]
- Lew, J.; Jones, S.L.; Caccese, C.; Orfi, I.; Little, C.; Botteron, K.N.; McCracken, J.T.; Nguyen, T.V. Developmental Variation in Testosterone:Cortisol Ratio Alters Cortical- and Amygdala-Based Cognitive Processes. J. Dev. Orig. Health Dis. 2022, 13, 310–321. [Google Scholar] [CrossRef]
- Schelling, X.; Calleja-González, J.; Terrados, N.; Mjaanes, J.; Benjamin, H.J. Testosterone And Cortisol With Relation To Training Volume And Playing Time In Professional Spanish Basketballers. Med. Sci. Sports Exerc. 2011, 43 (Suppl. S1), 857–858. [Google Scholar]
- Schelling, X.; Calleja-González, J.; Torres-Ronda, L.; Terrados, N. Using Testosterone and Cortisol as Biomarker for Training Individualization in Elite Basketball: A 4-Year Follow-up Study. J. Strength Cond. Res. 2015, 29, 368–378. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).