Abstract
Lactic acid bacteria (LAB) have long been used as starters in non-dairy cereal fermentation, as they aid in the production of products such as yoghurt and cheese. Broken rice milk is a plant-based milk alternative that is high in carbs and low in fat, providing excellent nutritional value to human users. The current study intends to ferment broken rice milk supplemented with 6% skim milk using three Lactobacillus strains for the development of yoghurt products, as well as to evaluate the growth, changes in physio-chemical properties, and sensory qualities of the yoghurt produced. Lactobacillus bulgaricus, Lactobacillus casei, Lactobacillus acidophilus, and a commercial yoghurt culture consortium fermented broken rice milk after 8 h. Rather than employing L. acidophilus or a commercial yogurt culture consortia, L. bulgarics was the most efficient starter for yoghurt manufacturing, followed by L. casei. L. bulgaricus had the highest viability counts of 8.5 Log CFU/mL, 0.18 specific growth rate, and 3.78 doubling time. Furthermore, it produces a significant reduction in pH to 4.3 and increases total titratable acidity to 0.09 percent with high overall acidity values of 1.4 mg/L of acetic and lactic acid contents. The maximum acidification rate (Vmax) was 0.2125, the maximum acidification time (Tmax) was 4 h, and the time to reach pH 4.6 (Te) was 5 to 8 h. As a result, L. bulgaricus was chosen as the most efficient isolate for the production of fermented rice milk yoghurt. More research is needed, however, to investigate the new rice-based yoghurt product’s sensory qualities as well as its toxicological effects on normal and malignant human cells.
1. Introduction
Plant-based foods, which include legumes, nuts, seeds, cereals, and pseudo-cereals, are regarded as one of the most important sources of milk alternatives [1]. Egypt is Africa’s largest rice producer, as it produced 600,000,000 tons of rice in 2014. From 2011 to 2014, Egypt was the world’s second-largest rice producer, with a production yield of 3.86 tons per acre [2]. Rice nutritional facts show that it is an important nutritional source because it contains carbohydrates, lipids, proteins, fibers, minerals, and vitamins [3]. Milling grades, percentage (%) of the rice’s head, chalkiness, and red-stripe grains, particle size distribution, and other physical parameters were used to assess the quality of crushed rice grains. High grade rice should have contained no more than 3.34 to 5.32% broken rice grains. Rice’s chalkiness, on the other hand, is a major concern in rice production because it expresses the breaking of grains during milling, determining its price and quality [4].
Rice milk is a new vegan alternative source of milk based on consuming free lactose boiled rice; this type of rice provides a healthy recipe low in fats and calories for obese people, containing approximately 140 calories and 3 g of fat per one cup [5]. Fermentation with different strains of LAB (Lactobacilli and Bifidobacteria) is used to improve the bioavailability of many important nutrients such as calcium, potassium, and iron micronutrients as they degrade carbohydrates to many intermediate metabolic compounds like alcohols, acetic and lactic acids as well as production of carbon dioxide [6,7].
LAB play critical roles in immune system enhancement, tumor prevention, blood cholesterol reduction, as well as LAB have antimicrobial activity against pathogenic microorganisms. L. bulgaricus and S. thermophilus are well-known for their ability to produce amylases, which leads to the saccharification of rice starch [8]. The goal of this study was to ferment broken rice milk with LAB while also measuring the pH and organic acids produced; moreover, determination of the chemical properties of fermented rice milk yoghurt.
2. Materials and Methods
2.1. LAB Strains
Three bacterial strains including L. bulgaricus, L. casei, and L. acidophilus were collected from the Microbial unit in Riyadh, Saudi Arabia. Twenty-five yogurt samples (starters) were obtained from a local market in Riyadh governorate, Saudi Arabia. de Man, Rogosa, and Sharpe (MRS) agar were used to maintain all LAB strains [9]. Its composition is as follows (g/L): protease peptone, 10; yeast extract, 5.00; dextrose, 20.00; polysorbate 80, 1.00; ammonium citrate, 2.00; sodium acetate, 5.00; magnesium sulphate, 0.10; manganese sulphate, 0.05; dipotassium phosphate, 2.00; agar, 15 and adjusted pH (at 25 °C) to 6.5 ± 0.2. MRS broth medium has the same composition of solid MRS agar medium except for adding agar. All media used were purchased from Oxoid, all chemicals used are laboratory grade.
2.2. Standard Inoculum
For all LAB strains preparation, 50 mL of MRS broth medium was prepared in 250 mL Erlenmeyer flask and sterilized by autoclaving at 120 °C for 20 min. After sterilization, all media were inoculated individually by a loopful of strain agar slant cultures and incubated statically at 37 °C for 72 h. The standard inoculum was collected when inoculum density reaches 3.6 × 10 7 CFU/mL for each microbial strain.
2.3. Rice Milk Preparation
Rice (Oryza sativa L.) packages were purchased from the local market in Riyadh governorate, Saudi Arabia, and transported to the laboratory of biofertilizers unit at King Saud University, Riyadh governorate, Saudi Arabia, for further studies. Briefly, 750 g of rice was washed with tap water thrice. Washed rice was then heated after the addition of 1 L of tap water and cooked for 1 h till the development of rice soup budding. Thereafter, 6% skim milk powder was added to the hot rice budding for increasing its total solids. The blending of hot rice budding was carried out till fine product was obtained and then all budding soup was packed in sterile glass jars. Each jar contains 100 mL of the final product, then heated at a temperature of 95 °C for 10 min [10]. All jars were cooled to the appropriate inoculation fermentation temperature at 37 °C.
2.4. Fermented Rice Milk Production
The three starters of LAB and the commercial yogurt culture (control treatment) were prepared for inoculating the previously prepared rice milk. First of all, the LAB cultures were grown on MRS broth for 72 h then 3 mL of each culture was transferred into 100 mL of sterilized skim milk broth (10%). Thereafter, 10 mL of each culture were taken individually and transferred into rice milk jars aseptically. After inoculation, all rice milk jars were incubated at 37 °C for 8 h. To determine the cell’s viability and changes in chemical parameters (pH and total acidity), pH, and total acidity, during fermentation process, random jars samples were taken at hourly intervals until the pH dropped down to 4.6 and coagulation of fermented rice milk yoghurt. All experimental trials were carried out in triplicates.
2.5. LAB Cell Viability
To determine LAB cell viability, the pour plate technique was performed using MRS agar and incubated at 37 °C for 48 h. Microaerophilic spindle-shaped and spherical colonies were counted, and the total count of LAB was expressed as Log colony-forming unit (Log10 CFU/mL) [11].
2.6. LAB Cell Kinetics
Time against LAB cell viability was plotted during the logarithmic phase, specific growth rate (μG), doubling time (td), and multiplication rate (MR) were calculated using the following equations [12]: Specific growth rate (μG/h) = (ln S − n S0)/(T − T0) Where: S = Amount of growth after t time (t) and S0 = Amount of growth at the beginning time (T0). Doubling time (td) = Ln (2)/μG. Multiplication rate (MR) = 1/td.
2.7. Chemical Properties of Fermented Rice Milk Yoghurt
2.7.1. PH and Titratable Acidity
For measuring pH values during the 8 h, fermentation of the previously prepared rice milk which were incubated at 37 °C, random jars were taken in triplicates and pH was measured using a digital pH meter (Adwa, Szeged 1000, Hungary). Total titratable acidity (TA) was determined using a titratable assay. Ten grams of fermented rice samples were collected randomly at hourly intervals and transferred into an Erlenmeyer flask. Three drops of phenolphthalein indicator were then added and titrated with 0.1 mL of NaOH until the development of the lightest pink color. TA was calculated as a percentage production of acetic acid (%) as follows according to Banu et al. [13].
* 60.05 is the molecular weight (g/mol) of acetic acid.
* 90.08 is the molecular weight (g/mol) of lactic acid.
2.7.2. Acidification Kinetics
To calculate acidification kinetics, a change in pH values (ΔpH) through 8 h of rice milk fermentation incubated at 37 °C was measured and ΔpH was calculated according to the following equation: ΔpH = pH value at zero time–pH value at stationary phase. Vmax is expressed as the ratio between pH values against incubation time (min), exhibited as (pH unit /min), and was calculated using the following equation Vmax = max (ΔpH Δt). Time (h) to reach maximum acidification rate (Tm) and Time (h) to reach pH 4.6 (Te) were recorded [14].
2.7.3. Nutritional Facts of Fermented Rice Milk Yoghurt by LAB Strains
Total soluble solids (TSS), moisture, ash, lipids, crude protein, crude fiber content, total carbohydrates, and energy density were determined according to AOAC, [15]. The equation of Buchholz and Schoeller [16] was used as follows: Total carbohydrates = Total Solids (TS) − (Fat − Protein + Ash). The energy density (E) = 4 [Protein (%) + Carbohydrates (%)] + 9 (Fat%), where E is energy density per 100 g of the product.
2.8. Shelf Life Time of Fermented Rice Milk Yogurt
All produced samples of fermented rice milk yogurt were stored at 5 °C for 12 days. Randomly selected jar samples were taken at weekly intervals to determine the changes of pH, total titratable acidity (%), and viability Log (CFU/mL). All experiments were carried out in triplicates.
2.9. Statistical Analysis
One-way ANOVA was performed using SPSS Statistics software version 19 and Tukey’s studentized range (HSD) test (p < 0.05) was used to determine the significant differences between means.
3. Results
3.1. Production of Fermented Rice Milk Yoghurt by LAB
3.1.1. LAB Cell Viability
Growth dynamics of viable cells of LAB starter cultures (L. bulgaricus, L. casei, and L. acidophilus) and commercial yogurt culture starters used to ferment the fortified broken rice milk with 6% skim milk was as shown below in Figure 1. LAB cell viabilities were exponentially increased as they significantly grow on broken rice milk (p ≤ 0.05) through eight hours of incubation with maximum growth of 4.5–8.5 Log CFU/ mL for L. bulgaricus, 4.6–8.5 Log CFU/ mL for L. casei, 4.6 to 7.9 Log CFU/ mL for L. acidophilus, and 4.5 to 7.9 Log CFU/ mL for commercial yogurt culture incubated at 37 °C with more than one-fold increase (1.88, 1.75, 1.66, and 1.71, respectively). The highest cell viability went to L. bulgaricus (8.5 Log CFU/mL) while L. acidophilus and yogurt culture recorded the lowest values of LAB cell viabilities at 7.9 Log CFU/mL with R2 of 0.929 and 0.9513.
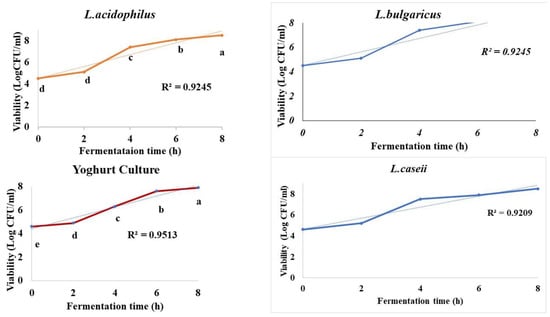
Figure 1.
Viability (Log CFU/mL) of the three LAB strains against commercially yogurt culture (control) during broken rice fermentation at 37 °C. Small letters (a–e) pointed to a significant difference (p < 0.05).
3.1.2. LAB Growth Kinetics
The growth kinetics of LAB were calculated during the exponential phase of growth curve as represented in Figure 2. A logarithmic increase of LAB numbers was observed during the first four hours of incubation at 37 °C, and the stationary growth phase was recorded after 8 h of incubation at 37 °C. Figure 3 was plotted to illustrate the different growth parameters (μG, td, and MR) for the three LAB starter cultures and the commercial yoghurt starter during the logarithmic growth phase. μG was calculated as equal to 0.18, 0.11, 0.11, and 0.10 h−1 for L. bulgaricus, L. casei, and commercial yogurt culture, respectively. td was calculated as equal to 3.78, 6.20, 5.98, and 6.31 h for L. bulgaricus, L. casei, and commercial yogurt culture, respectively. MR was calculated to record 0.26, 0.16, 0.22, and 0.29 for L. bulgaricus, L. casei, and commercial yogurt culture, respectively with correlation coefficient (R2) of 0.99, 0.91, 1.0.99 for rice milk fermented by L. bulgaricus, L. casei, L. acidophilus, and yogurt culture, respectively.
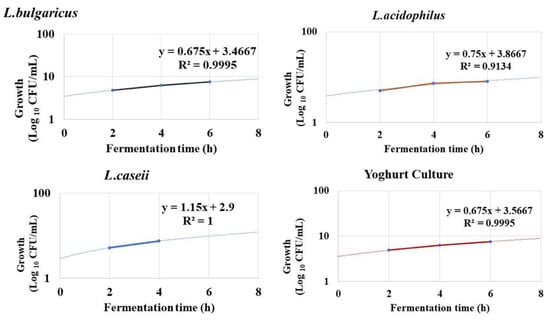
Figure 2.
Growth rates of the three LAB strains against commercially yogurt culture (control) through the logarithmic growth phase during eight hours of fermentation indicating the correlation coefficient R2.
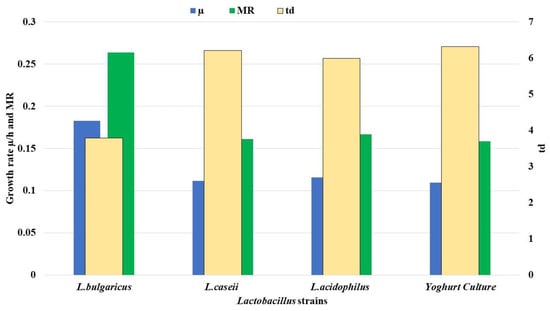
Figure 3.
Growth kinetics (μG, td, and MR) of the three LAB strains against commercial yogurt culture (control) through the logarithmic growth phase during four hours of fermentation where, μ/h = specific growth rate, td = doubling time, and MR = multiplication rate.
3.2. Chemical Properties of Rice Milk Yogurt Fermented with LAB
3.2.1. Determination of pH and TA
Changes in pH values and titratable acidity (TA) of fermented rice milk yoghurt product through 8 h of fermentation and incubation at 37 °C were recorded as illustrated in Figure 4. pH noted 6.0 at zero time and dropped down to 4.3 and 4.6 in all experimental trials. However, TA recorded a significant increase (p ≤ 0.05) through the eight hours of rice milk fermentation reaching 0.08 and 0.09 mg/L for L. bulgaricus, L. casei (with R2 reached from 0.9698–0.9383, respectively. L. acidophilus recorded TA of 0.08 mg/L with R2 reaching from 0.8338–0.9453. Yogurt culture recorded TA of 0.07% with R2 reaching 0.8787–0.9887. Acetic and lactic acids increased through 8 h of fermentation time to record the highest concentration by the end of the eighth hour. Lactic acid production was higher compared to acetic acid in all fermented yogurt treatments inoculated with L. bulgaricuss and L. casei. The minimum concentration of acetic and lactic acid was recorded by 0.075 mg/mL, Figure 5.
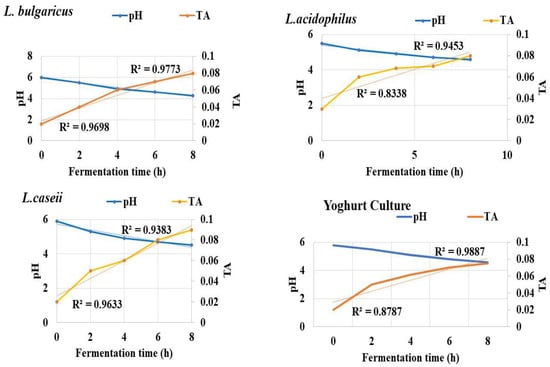
Figure 4.
Chemical properties (pH and TA) of broken rice milk and the three LAB strains against commercial yogurt culture (control) during eight hours of fermentation, incubated at 37 °C.
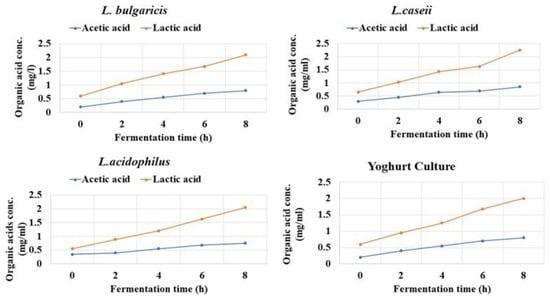
Figure 5.
Acetic and lactic acid values of broken rice milk and the three LAB strains against commercial yogurt culture (control) during eight hours of fermentation, incubated at 37 °C.
3.2.2. Acidification Kinetics
Vmax, Tmax, and Te were calculated for the fermented rice milk yoghurt product by the three studied LAB strains compared to commercial yogurt culture as a starter for rice milk yoghurt production. The highest Vmax was noticed by L. bulgaricus at 0.2125 pH U/min, followed by L. casei and L. acidophilus recording 0.1715 and 0.1125 pH U/min, respectively. The lowest Vmax observed by the commercial yoghurt culture reached 0.15 pH U/min. Tmax was reached after four hours of incubation for all trials. Te was 5 h by L. bulgaricus, 6 h by L. casei. It takes a long time to reach pH value of 4.5 by the commercial yoghurt starter and L. acidophilus recording 7 h and 8 h, respectively Table 1.

Table 1.
Kinetics parameters of acidification through fermentation of rice milk by the three lactic acid strains and the commercial yogurt culture.
3.3. Nutritional Facts of Fermented Rice Milk Yoghurt by LAB Strains
Fermented rice milk yogurt by the LAB strains varied in their chemical composition when compared to the non-fermented (control) rice milk products. Total solids were increased by the fermentation process. Protein content highly increased from 0.15% for the control treatment product to 3.22, 2.37, 2.35, and 3.22% for fermented products by L. bulgaricus, L. casei, L. acidophilus, and commercial yogurt culture, respectively. Ash content increased from 0.33% to record 0.76, 0.54, 0.50, and 0.75% for L. bulgaricus, L. casei, L. acidophilus, and commercial yogurt culture, respectively. The contents of total solid increased from 7.84% to 9.56, 9.18, 8.57, and 9.24% for L. bulgaricus, L. casei, L. acidophilus, and commercial yogurt culture, respectively.
Moisture, fat, and carbohydrate contents dropped down and moisture decreased from 93.20% to 90.55, 91.45, 91.32, and 90.87% for L. bulgaricus, L. casei, L. acidophilus, and commercial yogurt culture, respectively. Moreover, the fat content dropped down from 0.65% to 0.52% for L. bulgaricus and commercial yogurt culture inoculated samples, while L. casei and L. acidophilus samples recorded 0.65 and 0.50%, respectively Table 2.

Table 2.
Chemical properties of fermented rice milk yoghurt.
3.4. Shelf Life Properties of Rice Milk Yoghurt Product
All fermented rice milk products were stored at 5 °C for 12 days. pH and TA were determined through the storage process. A drop in pH values was observed from 4.3–4.6 in all treatments at zero time to 4.2, 4.1, 4.2, and 4.1 for L. bulgaricus, L. casei, L. acidophilus, and commercial yogurt culture products, respectively after 12 days of storage. While TA of all samples was markedly increased during the storage period to reach its maximum content at 0.083, 0.087, 0.081, 0.067 for L. bulgaricus, L. casei, L. acidophilus, and commercially yogurt culture products, respectively Table 3.

Table 3.
Viable cell count, pH, and titratable acidity for fermented rice yogurt during storage at 5 °C for 12 days.
4. Discussion
Rice is the seed of the grass species O. sativa and takes the second place in the list of economical cereal crops consumed worldwide. It has rich nutritional constituents such as carbohydrates, proteins, fats, minerals, fibers, and vitamins characterized by their high digestibility and high content of lysine amino acid among all the cereals [4]. Our study aimed to produce new plant-based yoghurt alternative by fermenting rice milk using three different LAB strains (L. bulgaricus, L. casei, and L. acidophilus) as well as using commercial yogurt culture through 8 h of incubation at 37 °C. Our results recorded the highest LAB cell viability and the best chemical composition depending on the recorded pH, TA, and total organic acids produced by L. bulgaricus. Indeed, more research is needed to study the new rice-based yoghurt product for their sensory properties as well as their toxicology effects on normal and tumor human cells.
According to the LAB cell viability results and the results published by Hammes, [17] it was found that L. bulgaricus and L. casei were the most favorable and efficient starters for making milk rice yoghurt. Moreover, de Mesquita et al., [11] noted that LAB specific growth rates and doubling time values range between 0.12 and 0.21 h−1 and 1.38 and 2.44 h, respectively.
pH and TA contents of the rice milk yoghurt were determined throughout the making process during 8 h. A drop in pH was observed due to the formation of some alcohols and the development of organic acids such as lactic and acetic ones, Park et al. [18,19]. On the other hand, a species of LAB named Bifidobacterium infantes 20088 was studied by El Tahir, [20] who noticed that pH values declined at maximum growth rate of 1.65 and 0.3 when used to ferment rice milk and skim milk, respectively. A study was carried out by Abou-Dobara et al., [10] and Mishra and Mishra, [21] where a mixture of cow milk and rice milk was fermented by using LAB, and showed the highest Vmax recorded 23.33 × 10−3p Hunits/min.
Supplementing rice milk products by skim milk was applied for enhancing the fermentation process due to its high content of lactose and casein that considered the carbon and nitrogen sources needed for cellular metabolism and energy formation. Padma et al. [4], found the same results by using probiotic bacteria to produce lactic acid from carbohydrates through fermentation leading to carbohydrate and fat reductions and a high increase in total protein content. Astuti et al. [22] explained the reasons for fat reduction in fermented yoghurt products and stated that the fat reduction happens due to the exo-production of lipases during fermentation process leading to the degradation of triacylglycerol into fatty acid and glycerol that are used as energy sources during metabolic activities. Increase in ash content (iron, calcium, and magnesium) in plant-based fermented products such as soy, and rice milk was recorded by Obadina et al. [23]. Fermented rice yogurt was characterized by their low pH values and high acidity due to the formation of organic fatty acids and glycerol [24,25,26].
As a conclusion, to meet the various flavor preferences, the dairy industry is focusing on the development of new production methods or active marketing strategies and consumers’ health-claim expectations, as well as to increase dairy product consumption per capita. The production of food products from rice milk is still in its early stages, and the effects on human health are being studied. We also considered that our study could serve as a guide for future research and starter culture selection for similar productions. Consumption of such products is steadily increasing as customers increasingly regard them as functional rather than traditional food products. The demand for single-serving convenience foods with no additional processing required for consumption is rapidly increasing. While the market for drinking fermented dairy products was developing, custom labelled products began to enter the market, bringing the competition to a head.
Author Contributions
Conceptualization, W.N.H. and D.H.M.A.; methodology, S.M.H.; software, S.M.H. and D.H.M.A.; validation, W.N.H., D.H.M.A. and S.M.H.; formal analysis, S.M.H.; investigation, W.N.H.; resources, D.H.M.A.; writing—original draft preparation, S.M.H. and W.N.H.; writing—review and editing, D.H.M.A.; visualization, S.M.H.; supervision, W.N.H.; project administration, D.H.M.A.; funding acquisition, D.H.M.A. All authors have read and agreed to the published version of the manuscript.
Funding
Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors acknowledge the support from Princess Nourah bint Abdulrahman University Researchers Supporting Project number (PNURSP2023R15), Princess Nourah bint Abdulrahman University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Tangyu, M.; Muller, J.; Bolten, C.J.; Wittmann, C. Fermentation of plant-based milk alternatives for improved flavour and nutritional value. Appl. Microbiol. Biotechnol. 2019, 103, 9263–9275. [Google Scholar] [CrossRef] [PubMed]
- FAO. Chemical, sensorial and rheological properties of a new organic rice bran beverage. Rice Sci. 2016, 16, 226–234. [Google Scholar]
- Zhao, M.; Lin, Y.; Chen, H. Improving nutritional quality of rice for human health. Theor. Appl. Genet. 2020, 133, 1397–1413. [Google Scholar] [CrossRef] [PubMed]
- Padma, M.; Rao, P.J.; Edukondalu, L.; Aparna, K.; Babu, G.R. Development of probiotic rice milk and its storage studies. Pharma Innov. 2021, 10, 902–906. [Google Scholar]
- Craig, W.J.; Fresán, U. International analysis of the nutritional content and a review of health benefits of non-dairy plant-based beverages. Nutrients 2021, 13, 842. [Google Scholar] [CrossRef]
- Achi, O.K.; Asamudo, N.U. Cereal-based fermented foods of Africa as functional foods. In Bioactive Molecules in Food; Springer: Cham, Switzerland, 2019; pp. 1527–1558. [Google Scholar]
- Anal, A.K. Quality ingredients and safety concerns for traditional fermented foods and beverages from Asia: A review. Fermentation 2019, 5, 8. [Google Scholar] [CrossRef]
- Ray, M.; Ghosh, K.; Singh, S.; Mondal, K.C. Folk to functional: An explorative overview of rice-based fermented foods and beverages in India. J. Ethn. Foods 2016, 3, 5–18. [Google Scholar] [CrossRef]
- Renschler, M.A.; Wyatt, A.; Anene, N.; Robinson-Hill, R.; Pickerill, E.S.; Fox, N.E.; McKillip, J.L. Using nitrous acid-modified de Man, Rogosa, and Sharpe medium to selectively isolate and culture lactic acid bacteria from dairy foods. J. Dairy Sci. 2020, 103, 1215–1222. [Google Scholar] [CrossRef]
- Abou-Dobara, M.I.; Ismail, M.M.; Refaat, N.M. Chemical composition, sensory evaluation and starter activity in cow, soy, peanut and rice milk. J. Nutr. Health Food Eng. 2016, 5, 00175. [Google Scholar]
- De Mesquita, A.R.C.; Costa, C.R.R.; Frutuoso, J.; Pinheiro, I.O.; Mota, A.; Franchitti, A.A.; Ximenes, E.A. Activity of metabolites produced by new strains of Lactobacillus in modified de Man, Rogosa and Sharpe (MRS) medium against multidrug-resistant bacteria. Afr. J. Microbiol. Res. 2017, 11, 345–355. [Google Scholar] [CrossRef]
- Maier, R.M.; Pepper, I.L. Bacterial growth. In Environmental Microbiology; Academic Press: Cambridge, MA, USA, 2015; pp. 37–56. [Google Scholar]
- Banu, I.; Bumbac, G.; Bombos, D.; Velea, S.; Gălan, A.M.; Bozga, G. Glycerol acetylation with acetic acid over Purolite CT-275. Product yields and process kinetics. Renew. Energy 2020, 148, 548–557. [Google Scholar] [CrossRef]
- Donkor, O.N.; Henriksson, A.; Vasiljevic, T.; Shah, N.P. Effect of acidification on the activity of probiotics in yoghurt during cold storage. Int. Dairy J. 2006, 16, 1181–1189. [Google Scholar] [CrossRef]
- AOAC. Official Methods of Analysis, 17th ed.; Association of Official Analytical Chemists: Washington, DC, USA, 2000. [Google Scholar]
- Buchholz, A.C.; Schoeller, D.A. Is a calorie a calorie? Am. J. Clin. Nutr. 2004, 79, 899S–906S. [Google Scholar] [CrossRef]
- Hammes, W.P. Fermentation of non-dairy foods. Food Biotechnol. 1991, 5, 293–303. [Google Scholar] [CrossRef]
- Park, D.J.; Oh, S.; Ku, K.H.; Mok, C.; Kim, S.H.; Imm, J.Y. Characteristics of yogurt-like products prepared from the combination of skim milk and soymilk containing saccharified-rice solution. Int. J. Food Sci. Nutr. 2005, 56, 23–34. [Google Scholar] [CrossRef]
- Li, S.; Tao, Y.; Li, D.; Wen, G.; Zhou, J.; Manickam, S.; Chai, W.S. Fermentation of blueberry juices using autochthonous lactic acid bacteria isolated from fruit environment: Fermentation characteristics and evolution of phenolic profiles. Chemosphere 2021, 276, 130090. [Google Scholar] [CrossRef]
- El Tahir, L.H.M. Microbiological and Physiochemical Study on Bifidobacterium infantis 20088 Fermented Peanut Milk and Rice Milk and their Blends; Sudan University of Science and Technology: Khartoum, Sudan, 2015. [Google Scholar]
- Mishra, S.; Mishra, H.N. Effect of synbiotic interaction of fructooligosaccharide and probiotics on the acidification profile, textural and rheological characteristics of fermented soy milk. Food Bioprocess Technol. 2013, 6, 3166–3176. [Google Scholar] [CrossRef]
- Astuti, M.; Meliala, A.; Dalais, F.S.; Wahlqvist, M.L. Tempe, a nutritious and healthy food from Indonesia. Asia Pac. J. Clin. Nutr. 2000, 9, 322–325. [Google Scholar] [CrossRef]
- Obadina, A.; Akinola, O.; Shittu, T.; Bakare, H. Effect of natural fermentation on the chemical and nutritional composition of fermented soymilk nono. Niger. Food J. 2013, 31, 91–97. [Google Scholar] [CrossRef]
- Ikujenlola, A.V.; Adurotoye, E.A.; Adeniran, H.A. Chemical and sensory properties of probioticated drinks from blends of African yam bean, soybean and coconut milk analogues. Acta Univ. Cibiniensis. Ser. E Food Technol. 2019, 23, 147–156. [Google Scholar] [CrossRef]
- Kumari, A.; Ranadheera, C.; Prasanna, P.; Senevirathne, N.; Vidanarachchi, J. Development of a rice incorporated synbiotic yogurt with low retrogradation properties. Int. Food Res. J. 2015, 22, 2032. [Google Scholar]
- Gamage, G.; Adikari, A.; Nayananjalie, W.; Prasanna, P.; Jayawardena, N.; Wathsala, R. Physicochemical, microbiological and sensory properties of probiotic drinking yogurt developed with goat milk. Int. J. Sci. Res. Publ. 2016, 6, 203–208. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).