First-Principles Prediction of Structure and Properties of the Cu2TeO6 Monolayer
Abstract
1. Introduction
2. Materials and Methods
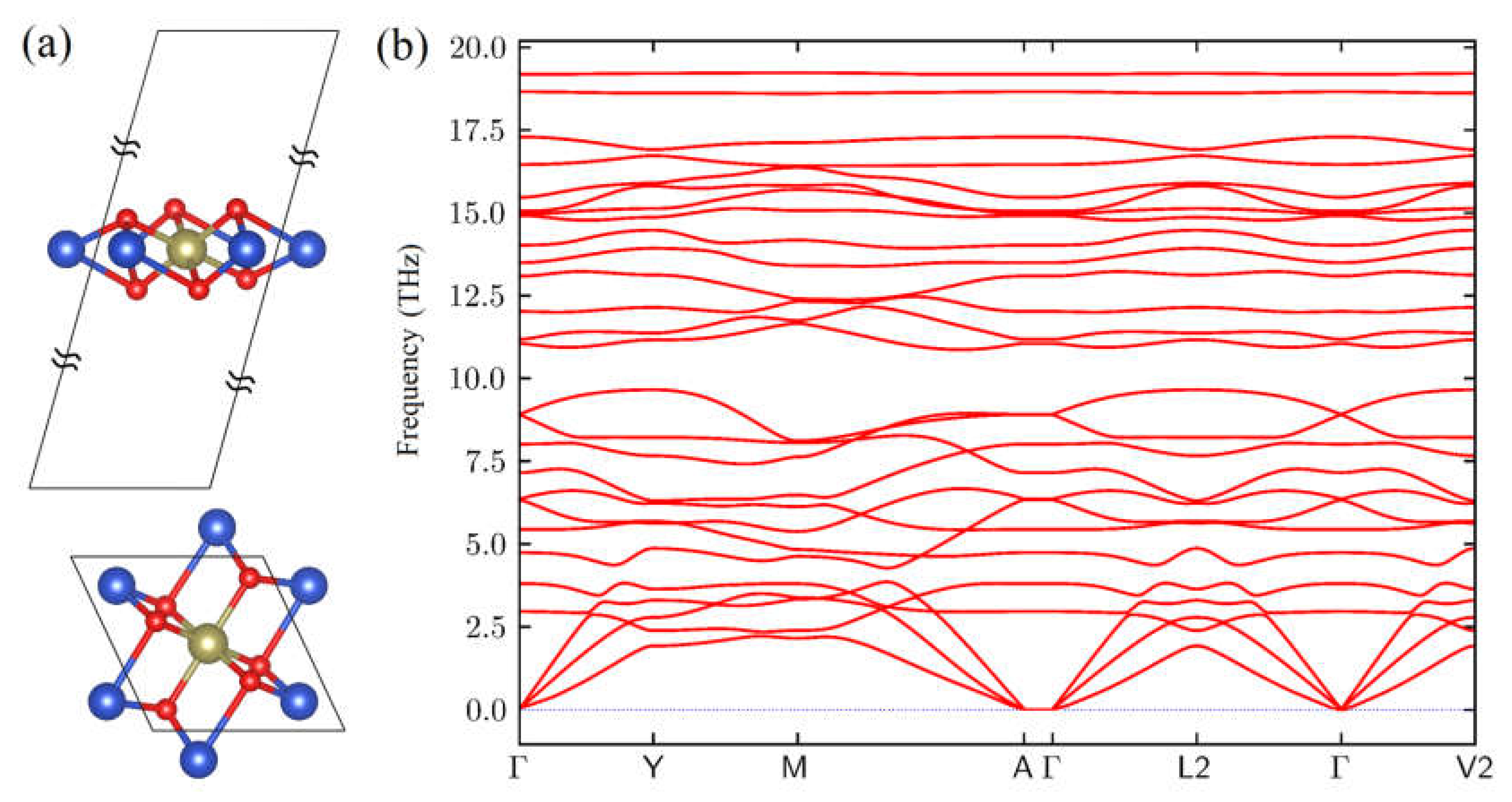
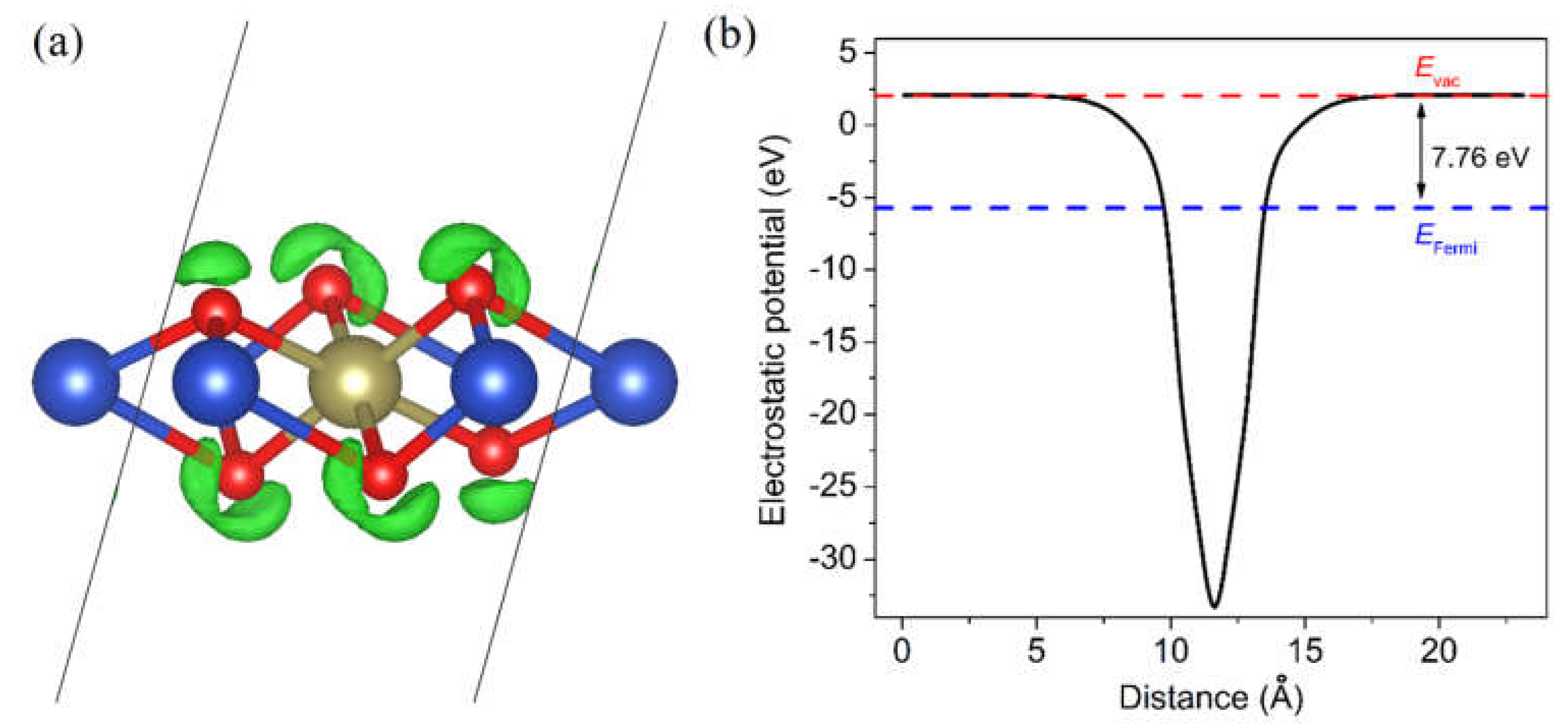
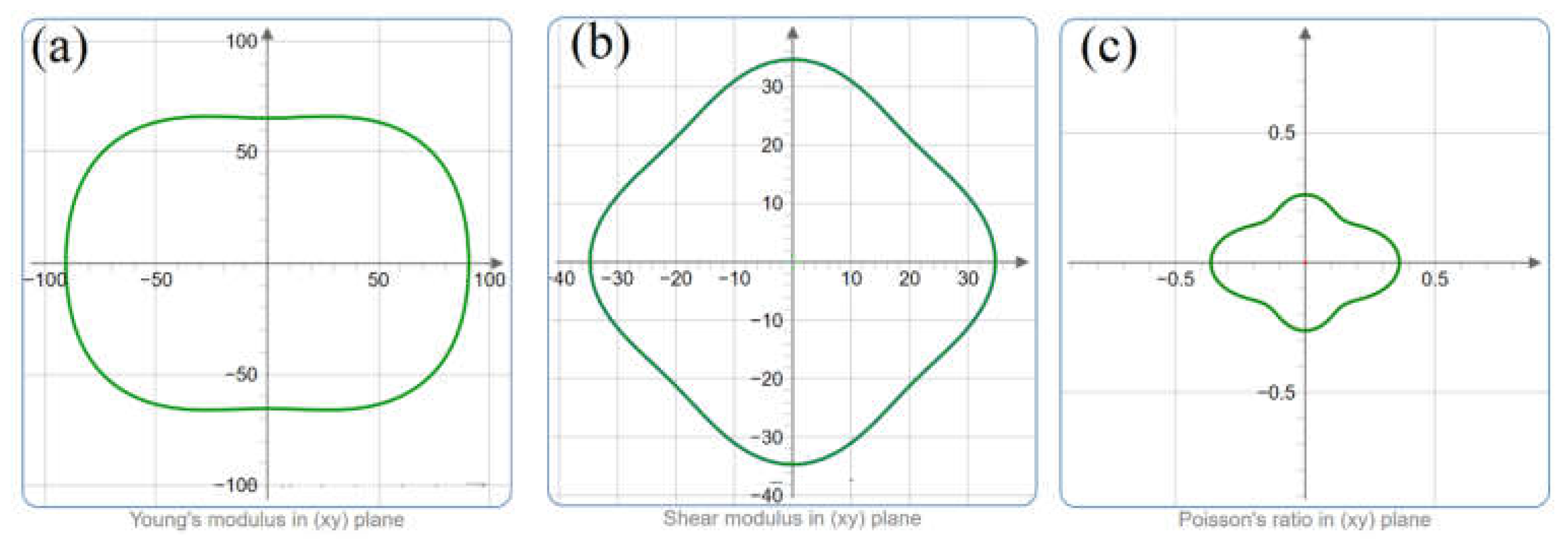
3. Results
4. Discussion and Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Xiao, L.; Zhengchen, W.; Wenbin, Y.; Liting, Y.; Renchao, C. Self-assembly MXene-rGO/CoNi film with massive continuous heterointerfaces and enhanced magnetic coupling for superior microwave absorber. Nano-Micro Lett. 2022, 14, 73. [Google Scholar]
- Xu, J.; Assoud, A.; Soheilnia, N.; Derakhshan, S.; Cuthbert, H.; Greedan, J.; Whangbo, M.; Kleinke, H. Synthesis, structure, and magnetic properties of the layered copper(II) oxide Na2Cu2TeO6. Inorg. Chem. 2005, 44, 5042–5046. [Google Scholar] [CrossRef] [PubMed]
- Golberg, D.; Bando, Y.; Tang, C.; Zhi, C. Boron nitride nanotubes. Adv. Mater. 2007, 19, 2413–2432. [Google Scholar] [CrossRef]
- Liu, Z.; Song, L.; Zhao, S.; Huang, J.; Ma, L.; Zhang, J.; Lou, J.; Ajayan, P.M. Direct growth of graphene/hexagonal boron nitride stacked layers. Nano Lett. 2011, 11, 2032–2037. [Google Scholar] [CrossRef] [PubMed]
- Mahatha, S.; Patel, K.; Menon, K. Electronic structure investigation of MoS2 and MoSe2 using angle-resolved photoemission spectroscopy and ab initio band structure studies. J. Phys. Condens. Matter. 2012, 24, 475504. [Google Scholar] [CrossRef]
- Pumera, M.; Loo, A. Layered transition-metal dichalcogenides (MoS2 and WS2) for sensing and biosensing. TrAC—Trends Anal. Chem. 2014, 61, 49–53. [Google Scholar] [CrossRef]
- Gogotsi, Y.; Anasori, B. The Rise of MXenes. ACS Nano 2019, 13, 8491–8494. [Google Scholar] [CrossRef]
- Chabi, S.; Kadel, K. Two-dimensional silicon carbide: Emerging direct band gap semiconductor. Nanomaterials 2020, 10, 2226. [Google Scholar] [CrossRef]
- Lei, S.; Ge, L.; Liu, Z.; Najmaei, S.; Shi, G.; You, G.; Lou, J.; Vajtai, R.; Ajayan, P. Synthesis and photoresponse of large GaSe atomic layers. Nano Lett. 2013, 13, 2777–2781. [Google Scholar] [CrossRef]
- Joshua, E.G. Progress, challenges, and opportunities in two-dimensional materials beyond graphene. ACS Nano 2014, 7, 2898–2962. [Google Scholar]
- Xu, Y.; Sprick, R.; Brownbill, N.; Blanc, F.; Li, Q.; Ward, J.; Ren, S.; Cooper, A. Bottom-up wet-chemical synthesis of a two-dimensional porous carbon material with high supercapacitance using a cascade coupling/cyclization route. J. Mater. Chem. A 2021, 9, 3303–3308. [Google Scholar] [CrossRef]
- Hamzan, N.B.; Goh, B.T.; Yeoh, K.H.; Chew, K.H. Size-controllable synthesis of 2D Mn3O4 triangular-shaped nanosheets by thermal chemical vapor deposition. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 142, 115273. [Google Scholar] [CrossRef]
- Coleman, J.N. Liquid exfoliation of layered materials. Science 2013, 334, 72–75. [Google Scholar]
- Xie, Y. Ultrathin black phosphorus nanosheets for efficient singlet oxygen generation. J. Am. Chem. Soc. 2015, 137, 11376–11382. [Google Scholar]
- Ma, R.; Sasaki, T. Two-dimensional oxide and hydroxide nanosheets: Controllable high-quality exfoliation, molecular assembly, and exploration of functionality. Acc. Chem. Res. 2015, 48, 136–143. [Google Scholar] [CrossRef]
- Al-Ghiffari, A.D.; Ludin, N.A.; Davies, M.L.; Yunus, R.M.; Suait, M.S. Systematic review of molybdenum disulfide for solar cell applications: Properties, mechanism and application. Mater. Today Commun. 2022, 32, 104078. [Google Scholar] [CrossRef]
- Ji, W.; Zhao, G.; Hu, G.; Fan, L.; Deng, H.; Fu, Q. The enhancement in dielectric properties for PVDF based composites due to the incorporation of 2D TiO2 nanobelt containing small amount of MWCNTs. Compos. Part A Appl. Sci. 2021, 149, 106493. [Google Scholar] [CrossRef]
- Zhang, Y.; Xu, W.; Liu, G.; Zhang, Z.; Zhu, J.; Li, M. Bandgap prediction of two-dimensional materials using machine learning. PLoS ONE 2021, 16, e0255637. [Google Scholar] [CrossRef]
- Hanaor, D.A.H.; Assadi, M.H.N.; Li, S.; Yu, A.; Sorrell, C.C. Ab initio study of phase stability in doped TiO2. Comput. Mech. 2012, 50, 185–194. [Google Scholar] [CrossRef]
- Kabiraj, A.; Jain, T.; Mahapatra, S. Massive Monte Carlo simulations-guided interpretable learning of two-dimensional Curie temperature. Patterns 2022, 3, 100625. [Google Scholar] [CrossRef]
- Li, Y.; Yu, C.; Gan, Y.; Jiang, P.; Yu, J.; Ou, Y.; Zou, D.F.; Huang, C.; Wang, J.; Jia, T.; et al. Mapping the elastic properties of two-dimensional MoS2 via bimodal atomic force microscopy and finite element simulation. NPJ Comput. Mater. 2018, 4, 49. [Google Scholar] [CrossRef]
- Pramchu, S.; Srisakonsub, P.; Sucharitakul, S.; Jaroenjittichai, A.P.; Laosiritaworn, Y. First-principles prediction of strain-induced Dirac semimetal state and negative Poisson’s ratio in TiZrB4 monolayer. Comput. Condens. Matter. 2022, 31, e00679. [Google Scholar] [CrossRef]
- Mellaerts, S.; Meng, R.; Menghini, M.; Afanasiev, V.; Seo, J.W.; Houssa, M.; Locquet, J.P. Two dimensional V2O3 and its experimental feasibility as robust room-temperature magnetic Chern insulator. NPJ 2D Mater. Appl. 2021, 5, 65. [Google Scholar] [CrossRef]
- Xiao, H.; Liang, S.; Xu, L.; Wang, R.; Yang, C. 2D high temperature ferromagnetic Co2Ti2Sn2 monolayer with tunable magnetic anisotropy and superior mechanical flexibility: A first-principles and Monte Carlo study. Phys. E Low-Dimens. Syst. Nanostruct. 2022, 135, 114939. [Google Scholar] [CrossRef]
- Sun, W.; Li, Y.; Wang, B.; Jiang, X.; Katsnelson, M.; Korzhavyi, P.; Eriksson, O.; Marco, I. A new 2D monolayer BiXene, M2C (M = Mo, Tc, Os). Nanoscale 2016, 8, 15753–15762. [Google Scholar] [CrossRef]
- Bandyopadhyay, A.; Frey, N.C.; Jariwala, D.; Shenoy, V.B. Engineering magnetic phases in two-dimensional non-van der Waals transition-metal oxides. Nano Lett. 2019, 19, 7793–7800. [Google Scholar] [CrossRef]
- Puthirath Balan, A.; Radhakrishnan, S.; Woellner, C.F.; Sinha, S.K.; Deng, L.; Reyes, C.D.L. Exfoliation of a non-van der Waals material from iron ore hematite. Nat. Nanotechnol. 2018, 13, 602–609. [Google Scholar]
- Balan, A.P.; Radhakrishnan, S.; Kumar, R.; Neupane, R.; Sinha, S.K.; Deng, L. A Non-van der Waals two-dimensional material from natural titanium mineral ore ilmenite. Chem. Mater. 2018, 30, 5923–5931. [Google Scholar] [CrossRef]
- The Materials Project. Materials Data on Cu2TeO6 by Materials Project; Lawrence Berkeley National Laboratory: Berkeley, CA, USA, 2019. [Google Scholar] [CrossRef]
- Jain, A.; Ong, S.P.; Hautier, G.; Chen, W.; Richards, W.D.; Dacek, S.; Cholia, S.; Gunter, D.; Skinner, D.; Ceder, G.; et al. Commentary: The Materials Project: A materials genome approach to accelerating materials innovation. APL Mater. 2013, 1, 11002. [Google Scholar] [CrossRef]
- Togo, A.; Tanaka, I. First-principles phonon calculations in materials science. Scr. Mater. 2015, 108, 1–5. [Google Scholar] [CrossRef]
- Henkelman, G.; Uberuaga, B.P.; Jonsson, H. A Climbing image nudged elastic band method for finding saddle points and minimum energy paths. J. Chem. Phys. 2000, 113, 9901. [Google Scholar] [CrossRef]
- Kresse, G.; Furthmuller, J. Efficient iterative schemes for ab initio total-energy calculations using a plane-wave basis set. Phys. Rev. B 1996, 54, 11169. [Google Scholar] [CrossRef]
- Perdew, J.P.; Burke, K.; Ernzerhof, M. Generalized gradient approximation made simple. Phys. Rev. Lett. 1996, 77, 3865–3868. [Google Scholar] [CrossRef] [PubMed]
- Kistanov, A.A.; Shcherbinin, S.A.; Botella, R.; Davletshin, A.; Cao, W. Family of two-dimensional transition metal dichlorides: Fundamental properties, structural defects, and environmental stability. J. Phys. Chem. Lett. 2022, 13, 2165–2172. [Google Scholar] [CrossRef]
- Shcherbinin, S.A.; Zhou, K.; Dmitriev, S.V.; Korznikova, E.A.; Davletshin, A.R.; Kistanov, A.A. Two-dimensional black phosphorus carbide: Rippling and formation of nanotubes. J. Phys. Chem. C 2018, 124, 10235–10243. [Google Scholar] [CrossRef]
- Sun, P.-P.; Kripalani, D.R.; Bai, L.; Chi, W.; Zhou, K. A highly efficient electron transport layer for perovskite solar cells. J. Phys. Chem. C 2021, 125, 5372–5379. [Google Scholar] [CrossRef]
- Kistanov, A.A.; Cai, Y.; Zhou, K.; Srikanth, N.; Dmitriev, S.V.; Zhang, Y.-W. Exploring the charge localization and band gap opening of borophene: A first-principles study. Nanoscale 2018, 10, 1403–1410. [Google Scholar] [CrossRef]
- Grin, Y.; Savin, A.; Silvi, B. The Chemical Bond: Fundamental Aspects of Chemical Bonding; Frenking, G., Shaik, S., Eds.; Wiley-VCH: Weinheim, Germany, 2014; Volume 1, p. 345. [Google Scholar]
- Cai, Y.; Zhang, G.; Zhang, Y.-W. Layer-dependent band alignment and work function of few-layer phosphorene. Sci. Rep. 2014, 4, 6677. [Google Scholar] [CrossRef]
- Lee, E.J.H.; Balasubramanian, K.; Weitz, R.T.; Burghard, M.; Kern, K. Contact and edge effects in graphene devices. Nat. Nanotechnol. 2008, 3, 486–490. [Google Scholar] [CrossRef]
- Chen, C.; Jin, T.; Wei, L.; Yong, L.; Xiaodong, L.; Wang, Y.; Zhang, L.; Liao, C.; Hu, N.; Song, C.; et al. High-work-function metal/carbon nanotube/low-work-function metal hybrid junction photovoltaic device. NPG Asia Mater. 2015, 7, e220. [Google Scholar] [CrossRef]
- Zhao, M.; Xia, W.; Wang, Y.; Luo, M.; Tian, Z.; Guo, Y.; Hu, W.; Xue, J. Nb2SiTe4: A stable narrow-gap two-dimensional material with ambipolar transport and mid-infrared response. ACS Nano 2019, 13, 10705–10710. [Google Scholar] [CrossRef] [PubMed]
- Maździarz, M. Comment on ‘The Computational 2D Materials Database: High-throughput modeling and discovery of atomically thin crystals’. 2D Mater. 2019, 6, 48001. [Google Scholar] [CrossRef]
- Singh, S.; Langa, L.; Dovale-Farelo, V.; Herath, U.; Tavadze, P.; Coudert, F.-X.; Romero, A.H.A. MechElastic: Python library for analysis of mechanical and elastic properties of bulk and 2D materials. Comput. Phys. Commun. 2021, 267, 108068. [Google Scholar] [CrossRef]
- Andrew, R.C.; Mapasha, R.E.; Ukpong, A.M.; Chetty, N. Mechanical properties of graphene and boronitrene. Phys. Rev. B 2012, 85, 125428. [Google Scholar] [CrossRef]
- Zhao, T.; Zhang, S.; Guo, Y.; Wang, Q. TiC2: A new two-dimensional sheet beyond MXenes. Nanoscale 2016, 8, 233–242. [Google Scholar] [CrossRef]
- Kim, Y.W.; Lee, A.J.; Han, D.H.; Lee, D.C.; Hwang, H.; Kim, Y.; Moon, S.; Youn, T.; Lee, M.; Jeon, W. Reliable high work-function molybdenum dioxide synthesis via template-effect-utilizing atomic layer deposition for next-generation electrode applications. J. Mater. Chem. C 2022, 10, 12957–12965. [Google Scholar] [CrossRef]
- Chun, J.-Y.; Han, J.-W.; Seo, D.-S. Application of high work function anode for organic light emitting diode. Mol. Cryst. Liq. Cryst. 2009, 514, 115–121. [Google Scholar] [CrossRef]
- Lajevardipour, A.; Neek-Amal, M.; Peeters, F.M. Thermomechanical properties of graphene: Valence force field model approach. J. Phys. Condens. Matter. 2012, 24, 175303. [Google Scholar] [CrossRef]
- Cadelano, E.; Palla, P.L.; Giordano, S.; Colombo, L. Elastic properties of hydrogenated graphene. Phys. Rev. B 2010, 82, 235414. [Google Scholar] [CrossRef]
- Miao, F.; Liang, S.-J.; Cheng, B. Straintronics with van der Waals materials. NPJ Quantum Mater. 2021, 6, 59. [Google Scholar] [CrossRef]


| a | 5.272 Å |
| b | 5.272 Å |
| α | 100.25 |
| β | 100.25 |
| γ | 112.43 |
| C11 | 101.18 N/m |
| C22 | 73.29 N/m |
| C12 | 27.26 N/m |
| C44 | 34.65 N/m |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Korznikova, E.A.; Bryzgalov, V.A.; Kistanov, A.A. First-Principles Prediction of Structure and Properties of the Cu2TeO6 Monolayer. Appl. Sci. 2023, 13, 815. https://doi.org/10.3390/app13020815
Korznikova EA, Bryzgalov VA, Kistanov AA. First-Principles Prediction of Structure and Properties of the Cu2TeO6 Monolayer. Applied Sciences. 2023; 13(2):815. https://doi.org/10.3390/app13020815
Chicago/Turabian StyleKorznikova, Elena A., Vladimir A. Bryzgalov, and Andrey A. Kistanov. 2023. "First-Principles Prediction of Structure and Properties of the Cu2TeO6 Monolayer" Applied Sciences 13, no. 2: 815. https://doi.org/10.3390/app13020815
APA StyleKorznikova, E. A., Bryzgalov, V. A., & Kistanov, A. A. (2023). First-Principles Prediction of Structure and Properties of the Cu2TeO6 Monolayer. Applied Sciences, 13(2), 815. https://doi.org/10.3390/app13020815








