Abstract
Nanotechnology is a discipline of science and engineering that emphasizes developing, modifying, characterizing, and using nanoscale components in a variety of applications. Owing to their multiple advantages, including adhesion strength, surface hardness, long-term and extra-high-temperature corrosion resistance, improvement of interfacial behavior, etc., nanocoatings are efficiently utilized to minimize the influence of a corrosive environment. Additionally, nanocoatings are often applied in thinner and finer concentrations, allowing for greater versatility in instrumentation and reduced operating and maintenance costs. The exemplary physical coverage of the coated substrate is facilitated by the fine dimensions of nanomaterials and the significant density of their grounded boundaries. For instance, fabricated self-healing eco-sustainable corrosion inhibitors including PAC/CuONPs, PAC/Fe3O4NPs, and PAC/NiONPs, with uniform distributions and particulate sizes of 23, 10, and 43 nm, correspondingly, were effective in producing PAC/MONPs nanocomposites which exhibited IE% of 93.2, 88.1, 96.1, and 98.6% for carbon steel corrosion in 1M HCl at the optimum concentration of 250 ppm. Therefore, in this review, further steps are taken into the exploration of the significant corrosion-mitigation potential and applications of nanomaterial-based corrosion inhibitors and nano-modified coatings, including self-healing nanocoatings, natural source-based nanocoatings, metal/metallic ion-based nanocoatings, and carbon allotrope-based nanocoatings, to generate defensive film and protection against corrosion for several metals and alloys. These have been illuminated through the in-depth discussion on characterization techniques such as scanning electron microscopy (SEM), electrochemical impedance spectroscopy (EIS), potentiodynamic polarization (PDP), atomic force microscopy (AFM), energy dispersive spectroscopy (EDS), etc. After providing a general summary of the various types of nanomaterials and their protective mechanisms in wide corrosive media, we subsequently present a viewpoint on challenges and future directions.
1. Introduction
1.1. Corrosion and Its Adverse Impacts: An Economic Aspect
Corrosion is defined to be the degradation of a substance as a result of chemical interactions with its nearby surroundings. Corrosion of metallic structures has a significant influence on the world economy. According to estimates from the World Corrosion Organization (WCO), the current worldwide cost of corrosion (COC) is larger than $2.5 trillion, or around 3.4% of the world’s GDP [1]. Furthermore, according to a study by the National Association of Corrosion Engineers (NACE), corrosion costs around the globe are estimated to be roughly $255 billion [2]. Corrosion costs to the US economy annually, both indirect and direct, are projected to be $552 billion, or 6% of GDP [3]. Corrosion has a significant influence on the price of maintaining and implementing restorations on vehicles, consumer electronics, aircraft, overpasses, and industrial sites, such as those that generate and transmit energy, manufacture pharmaceuticals, desalinate water, process petrochemicals, and so forth [4,5,6]. The impact of indirect corrosion is associated with a specific loss of production due to delays and interruptions as well as taxation and overhead expenses imposed by corrosion, among other things. The survey from NACE highlights the transformation in corrosion strategic framework and utilization of cutting-edge innovation in the automotive sector as among the most notable great successes in corrosion prevention [7,8]. Before 1975, the automotive industry was losing billions of dollars a year to corrosion because automobile manufacturers were using few protection approaches. As of 1975, automobile manufacturers have been implementing corrosion strategic methods, which has resulted in a yearly reduction in corrosion-related production and execution expenses of automobiles of 9.6 billion US dollars, or 52%, from 1975 to 1999 [9]. As a consequence, the COC per unit of a fresh automobile was 44% lower in 1999 than it was in 1975 [10]. To preserve the performance and quality of components from environmental deterioration, corrosion-resistant films are sprayed on a variety of substrates. In 2018, the marketplace for preventive coatings was estimated to be worth EUR 26.5 billion [9]. The IRL group’s assessment predicted a quantity of 7.4 million tonnes in 2018 and development of more than 2 million tonnes by 2023. With the highest utilization of these coatings’ methods, Asia-Pacific is accountable for the greatest quantity growth, accounting for 84% of worldwide quantity (6.2 million metric tonnes) in 2018 [9,11]. Europe devoured 0.4 million tonnes last year, while the American region used 0.5 million tonnes (7% of the overall worldwide quantity) [12,13,14,15]. In 2023, a minor increase is anticipated for both industries, as per the IRL organization and the European Council of the Paint, Printing Ink, and Artist’s Colours Industry [16]. Consumption is closely tied to government spending on infrastructure and the rise in demand from the electricity industry, as well as from the automobile, transportation, and oil and gas industries. As a result, during 2020 and 2021, the worldwide paints and coatings industry grew at a world-level increase rate of 8.5% [17]. Surface preparation constitutes about 45% of the expense of a routine operation, whereas the expense of coating chemicals varies from 5 to 21% of the entire operation [18,19]. While investing in excellent anticorrosive coatings materials, such as high-performance zinc or urethane and epoxy polyamide systems, might seem costly, when the operational duration is longer than 10 years, benefits of about 40% might well be realized [20].
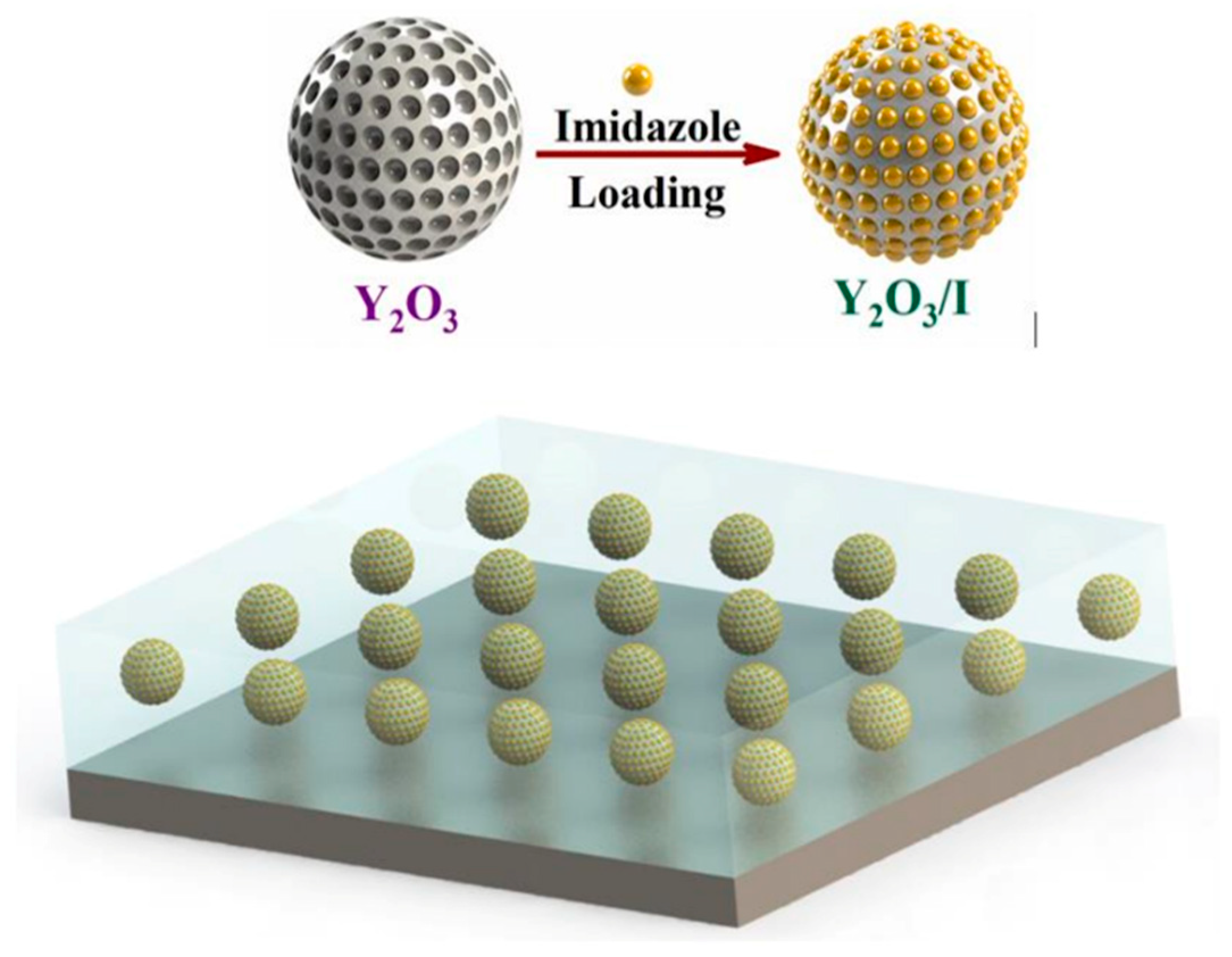
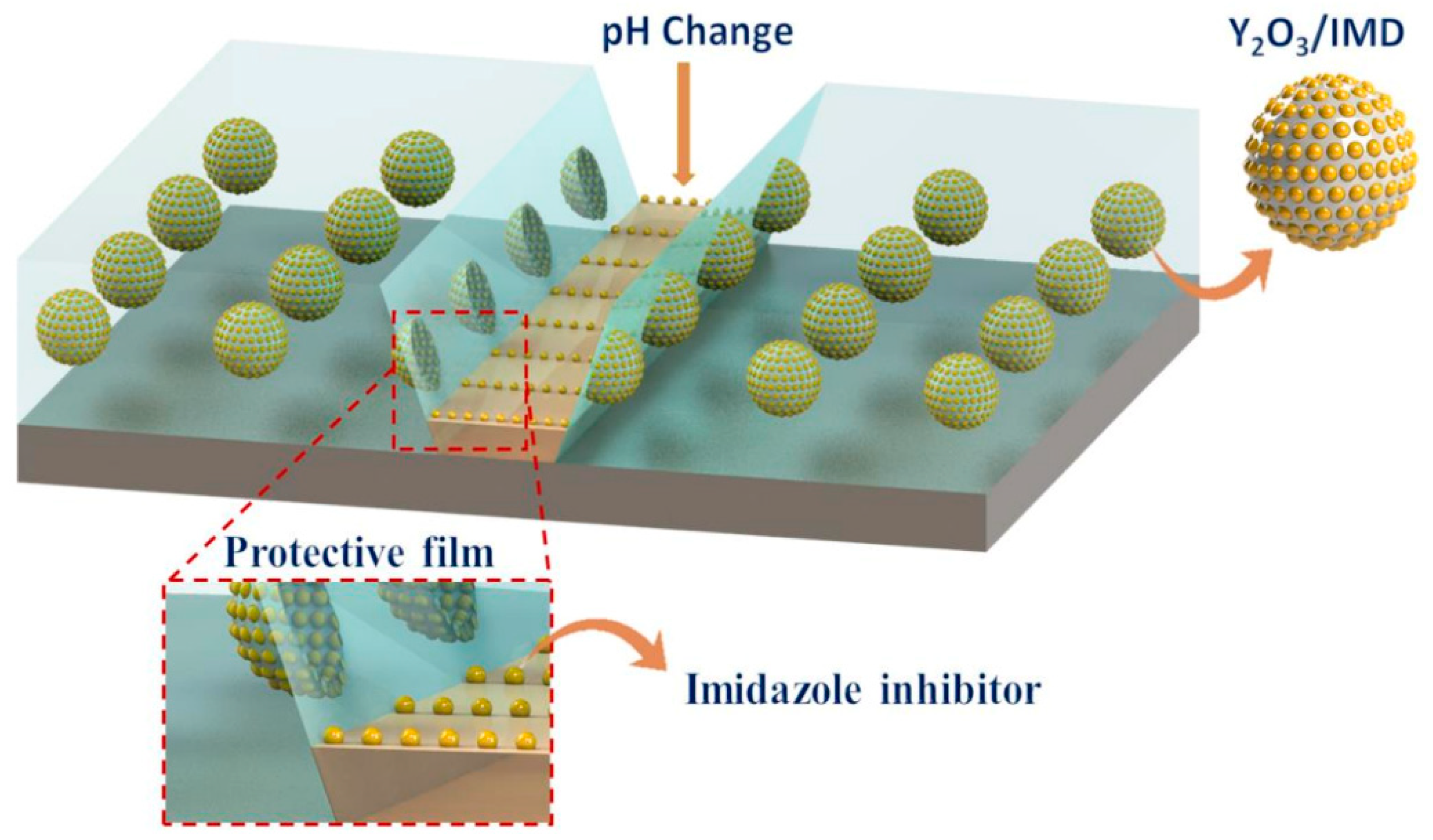
Numerous anticorrosive coatings are proven to be effective in developing protection against the harmful impacts of corrosion. Corrosion coatings are divided into water- (H2O), solvent-, and powder-oriented categories by dispersive medium categorization, along with other significant coating compositions. Since laws and guidelines governing the emission of volatile organic compounds (VOCs) through solvent-oriented coverings have been enacted and effectively implemented, H2O-borne coverings have grown significantly as a part of the industry [21,22,23,24,25]. Thus, corrosion mitigation techniques have been devised and widely applied in the automotive, stream, manufacturing, and marine enterprises for corrosion lessening and eradication. These techniques include organic/polymer composites, cathodic and anodic prevention, and corrosion-preventing elements. However, efficient barriers are designed to influence low-cost methods. Furthermore, hydrophobic films and inorganic–organic combinatorial films have extended the life span of a variety of substances in conditions that are susceptible to corrosion [26,27,28,29,30,31,32,33,34]. Nano-modified coverings or coverings relying on the incorporation of nanoparticles (NPs) have traditionally been used to prevent the corrosion of metal components. These coatings are primarily used as a physical barrier against corrosive substances like H+ and O2−. The major factors of metallic deterioration are associated with atmospheric impacts, which are brought on by metal susceptibility to weathering variables and immensely influenced by common climatological elements such as periods of moistness, humidity levels, and acidification, as well as by temperature, other pollutants’ actions, and acidity. For instance, in an experiment, to improve material protection, Nawaz et al. [35] created polymeric nanocomposite coatings that were altered with NP-based corrosion inhibitors (CIs). In this study, yttrium oxide NPs containing CIs (imidazole) were added to the epoxy composition before being deposited on the steel surface. Yttrium oxide NPs are permeable and have a large surface region. So, in this experiment, 3.0 weight % of Y2O3 NPs were added to 10 mg/mL IMD H2O, and the composition was then sonicated at ambient temperature for 30 min. The mixture was then placed on a heated surface at 70 °C for 24 h while being moderately stirred. The surplus solvent was evaporated, and crisped doped Y2O3 NPs were produced. The pictorial representation of infusing Y2O3 using imidazole is shown in Figure 1.
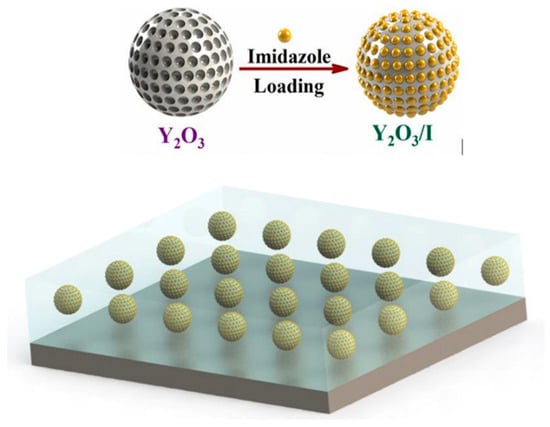
Figure 1.
The pictorial representation of infusing Y2O3 using imidazole in the epoxy coating. Reprinted with permission from Ref. [35]. MDPI (2021).
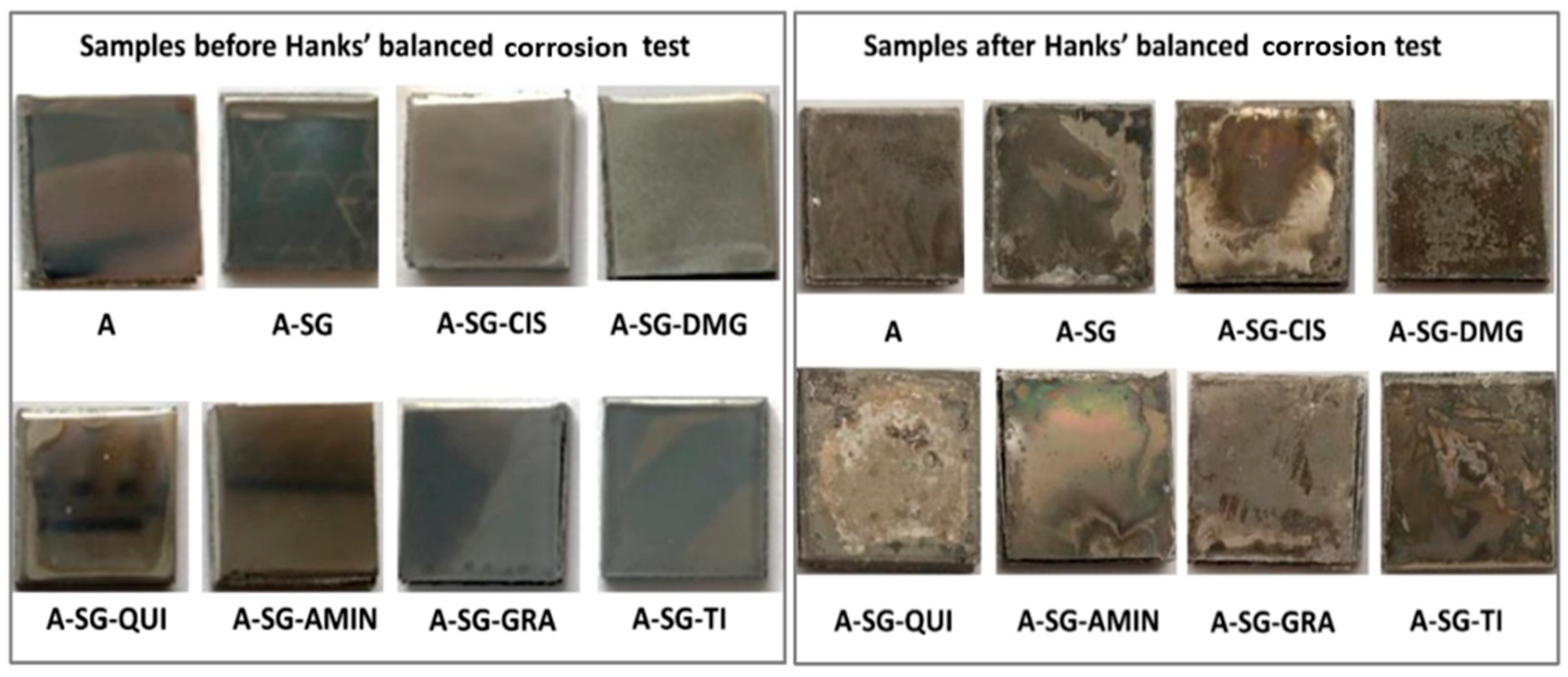
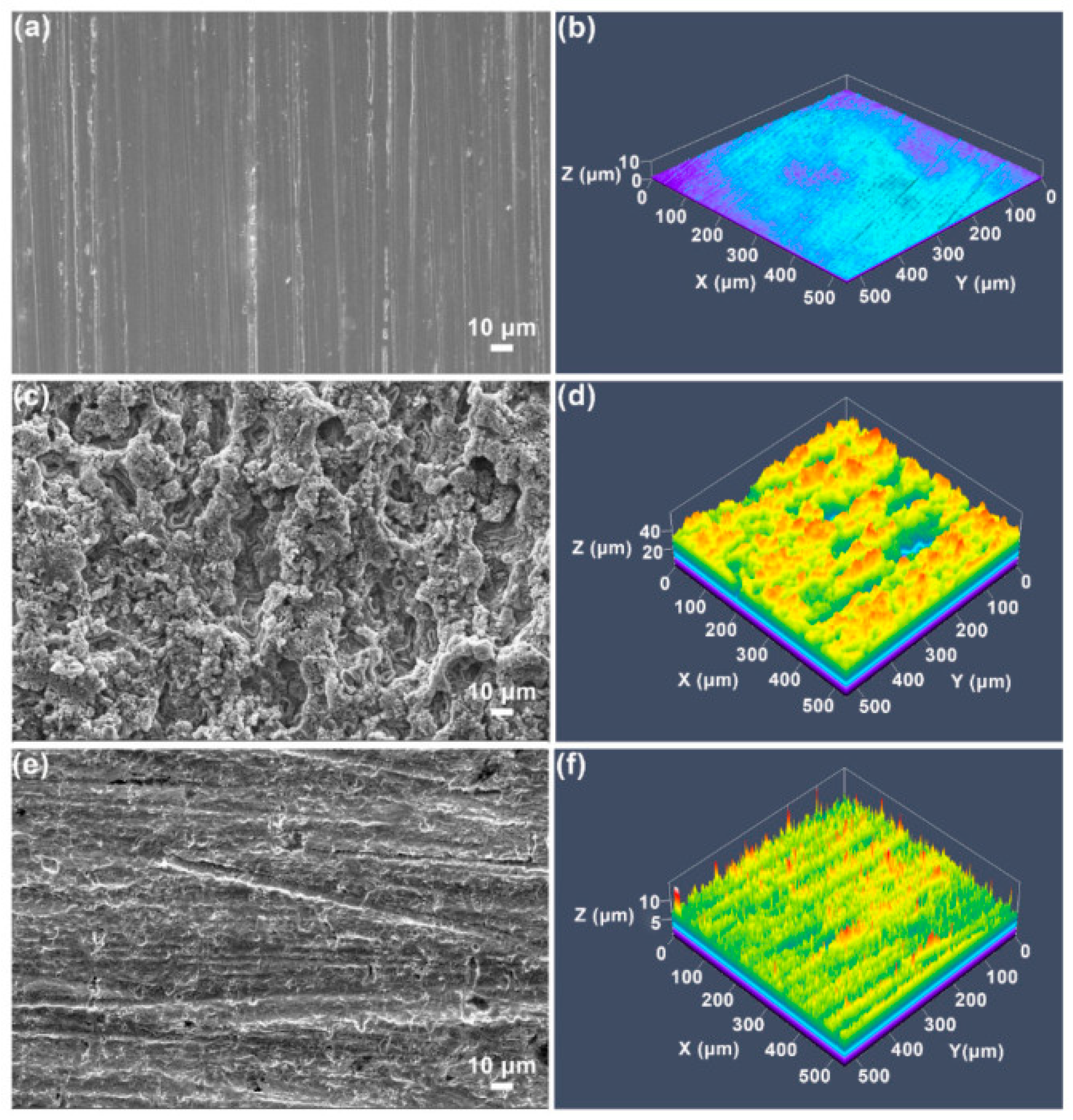
Similarly, to prevent corrosion of AZ61 Mg alloys, Alonso et al. [36] created inorganic–organic hybrids nano-loaded containing a variety of safe, non-hazardous, and eco-friendly CIs using the sol–gel method. Using pH testing and SEM in a Hanks’ balanced salt medium throughout a typical corrosion experiment, the coating’s efficacy was assessed. According to the findings, adding an inhibitor to the sol–gel coating considerably increased its ability to prevent corrosion, making it a superb obstacle for L-cysteine-doped hybrid sol–gel coatings. Images of the dried specimen substrate taken following the HBSS corrosion experiment are shown in Figure 2. It is evident how the corrosion negatively impacted the coating’s exterior stratum morphologically and, in some circumstances, the alloy’s interior.
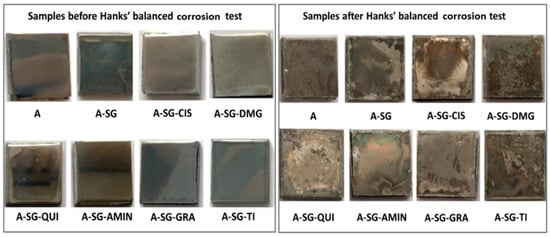
Figure 2.
Morphological scans of the surface (3 mm × 3 cm) of the Mg-Al sample; undipped and dipped in an HBSS following 14 days for specimens. Reprinted with permission from Ref. [36]. MDPI (2022).
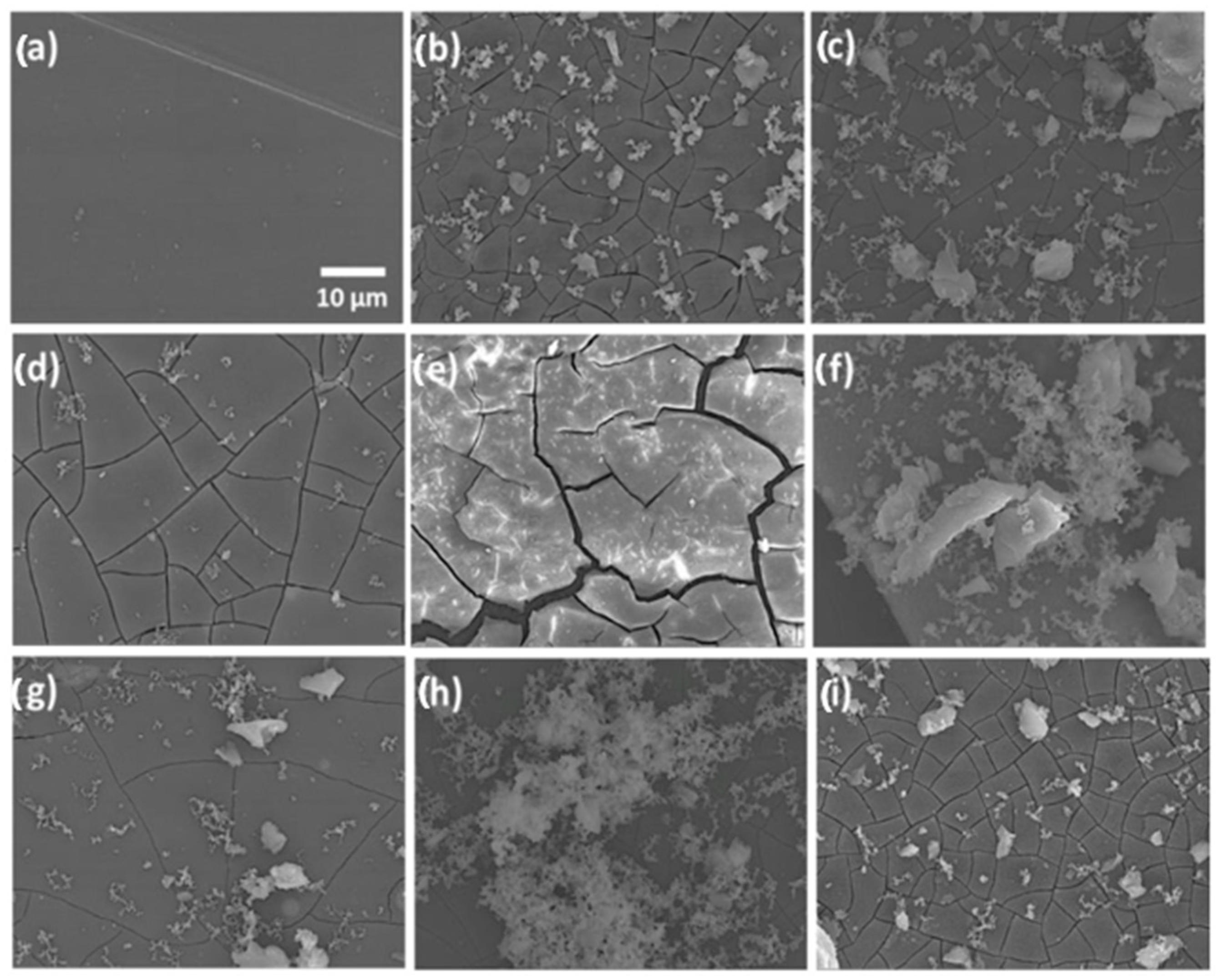
Continuing ahead, Figure 3 shows the SEM observations of the surface geometries of the specimens. According to electron microscopy, Specimen 0, which represented the AZ61 alloy as obtained, had a clean appearance containing small blemishes that were probably the result of surface cleaning (Figure 3a). Specimen A’s exterior (Figure 3b) contained white spots and fissures all across it, offering it a degraded aspect. The SEM pictures of specimen A-SG revealed a surface that was fractured and covered with a lot of contaminants (Figure 3c). Compared to the untreated specimen, the fracture width in this specimen seemed to be less. Specimen A-SG-CIS displayed a uniformly fractured texture containing minor white stains but no dissociation from the underlying alloy (Figure 3d). SEM scans of specimen A-SG-DMG showed that the substrate was fractured, however, the covering was still attached to the surface. In comparison to the prior specimen, the fractures appeared to be thicker and the distance among them was bigger (Figure 3e). Specimen A-SG-QUI displayed patches of the slightly disintegrated covering and yellowish aggregates of insoluble salts of Hanks’ medium on its surfaces (Figure 3f). The substrate of specimen A-SG-AMIN (Figure 3g) was uniform and had fewer tiny fractures than the specimen. The SEM pictures of specimen A-SG-GRA revealed consistent surface corrosion, minimal fissures, and substantial white salt accumulation (Figure 3h). The overlaying sol–gel covering of Specimen A-SG-TI, which contains TiO2 NPs as CIs, revealed a fractured exterior containing white granules. This sample’s fracture width resembled that of an untreated, deteriorated alloy (Figure 3i). Researchers verified that all coverings eroded to some degree from the statistics acquired herein, in contrast to the deterioration of the AZ61 alloy throughout the Hanks’ medium experiment performed.
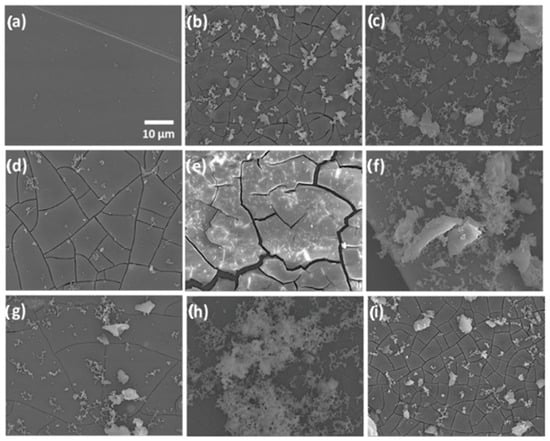
Figure 3.
SEM scans of the substrates: (a) specimen 0, untreated AZ61 alloy; (b) specimen A, deteriorated AZ61 alloy following the HBSS investigation; (c) deteriorated specimen A-SG; (d) deteriorated specimen A-SG-CIS; (e) deteriorated specimen A-SG-DMG; (f) deteriorated specimen A-SG-QUI; (g) deteriorated specimen A-SG-AMIN; (h) deteriorated specimen A-SG-GRA; and (i) deteriorated specimen A-SG-TI. Reprinted with permission from Ref. [36]. MDPI (2022).
1.2. Nanomaterials/Nano-Modified Coatings as Corrosion Inhibitors
Today, nanotechnology is a rapidly expanding subject with uses in academia. Due to their outstanding and distinctive basic physicochemical characteristics, including greater external region, mechanical characteristics, optical activity, and chemical reactivity, NPs with sizes ranging from 1 to 100 nm have been used in a variety of production, ecological, health sciences, agricultural, and food applications [37,38,39]. The most significant use of NPs in the industrial sector is their ability to preserve metal from corrosion in distinct predicaments. Metals are employed as basic materials or fundamental materials in enterprises and several other fields due to their many appealing features, including electrical and thermal conductance, increased boiling and melting ranges, greater compressive strength, greater mass-to-volume fraction, and flexibility. Electrochemical interactions involving metals and their surroundings occur when metals engage with their environment [40,41]. A variety of methods are employed to slow the corrosion rate (CR), including metallic surface treatment, environmental alterations, the application of CIs, and changes in pH and utilization of coatings. As compared to their typical macroscopic counterparts, nanomaterials (NMs) and their additives have a high surface-to-volume ratio, making them effective CIs [42,43]. By obstructing the active surface area on the metallic substrate, NMs restrict the pace of corrosion and halt surface reactions. They also give toughness, flatness, endurance, optical properties, and heat resistance. These substances are eco-sustainable and biodegradable. This review article’s goal is to examine novel and effective methods for employing NP-based coatings/nano-modified coatings to generate more potent CIs and corrosion mitigation for several metals and alloys. Over the past decades, various nanomaterials have been used by numerous research teams based on their suitability for anti-corrosion purposes. Here, some of them are examined together with their additives and exceptional anticorrosive properties.
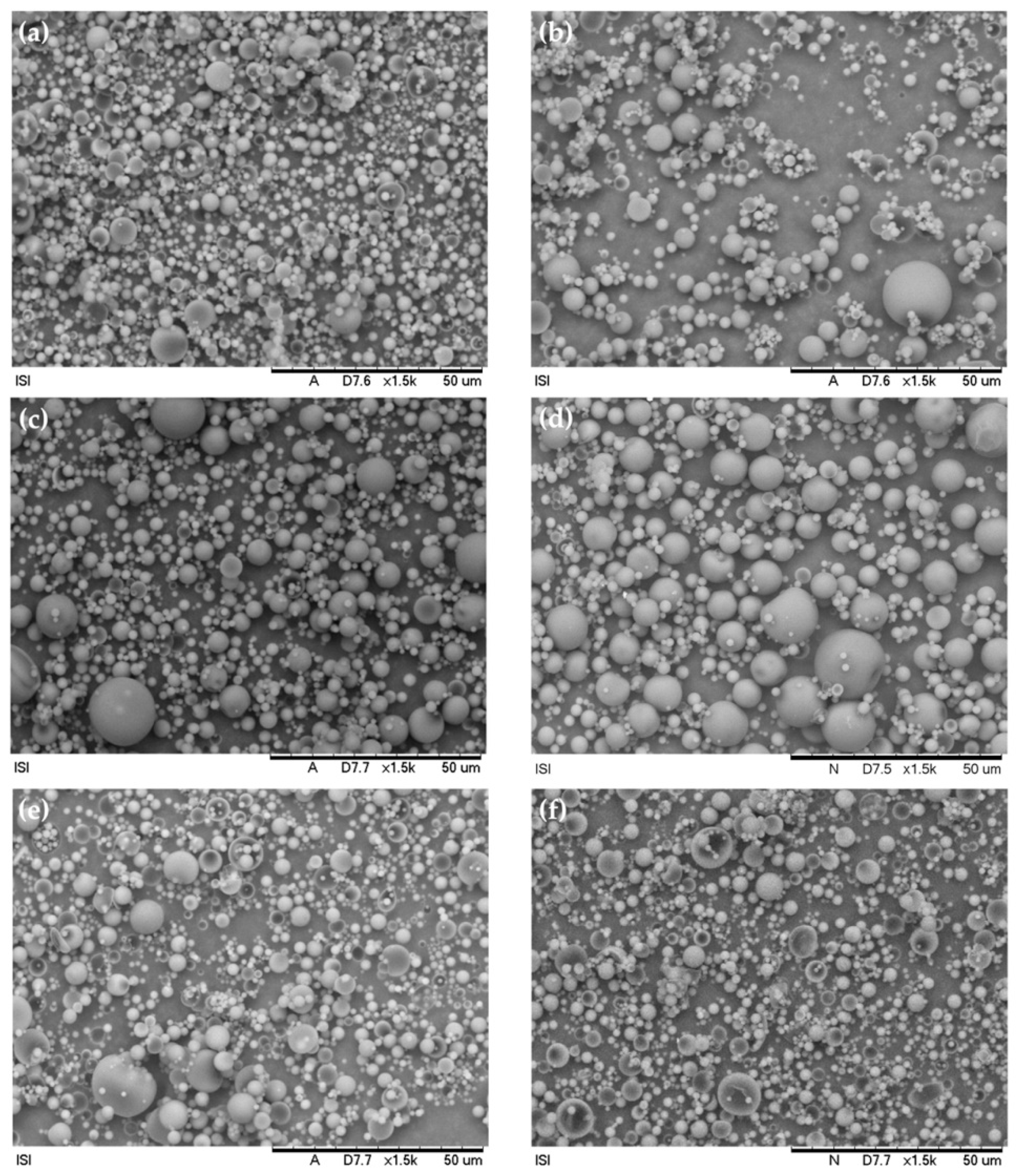
In an experiment, to utilize nano-modified coatings in defensive coverings to avail CIs in the long term, Calegari et al. [18] created sodium carboxymethylcellulose (CMC-Na) microparticles carrying benzotriazole (BTA) as CIs. The produced biopolymeric CMC-Na microparticles were morphologically evaluated using SEM, and their discharge characteristic was investigated by UV–Vis. The findings demonstrate that the microparticles that were created were uniform, circular, and of a matrix sort of character. SEM morphologies of the spray-dried particulates produced under various testing circumstances are shown in Figure 4. It is clear to see that, independent of the empirical conditions, all microparticles exhibited a spherical form with a flawless substrate as their most distinguishing feature. The dearth of coatings or incrustations on the microspheres’ substrates together with their uniform texture indicates that the CIs were disseminated throughout the biopolymeric matrix. Spherical-shaped particulates with homogeneous substrates were frequently produced when this family of biopolymers was used. The suitable proportion (1.25:1) amongst the encapsulated substance and the encasing polymeric matrices, together with the usage of poly (vinyl alcohol) (PVA) as a plasticizer in this study, helped to generate homogenous particulates. A preferred approach to improve the elasticity, hardness, and impact strength of polymeric coatings while reducing instability is the inclusion of plasticizers. Thus, it is probable that PVA reduced the intermolecular contacts in the CMC-Na matrix, increased polymeric chain motion, and, as a consequence, enhanced the physical characteristics of the resultant particulates and enhanced the corrosion mitigation of the nano-modified coatings.
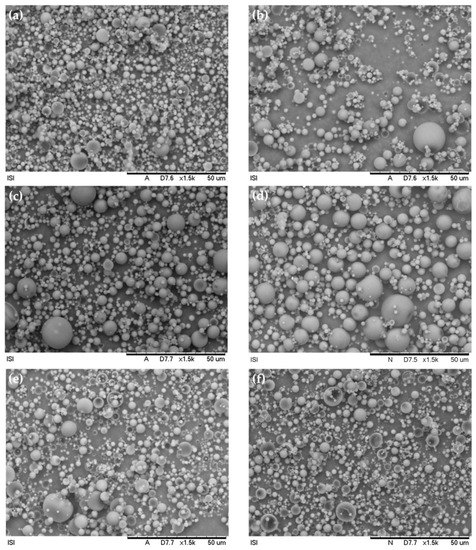
Figure 4.
SEM scans of CMC.BTA microspheres attained by the technique of spray-drying in distinctive empirical situations: (a) 440 L/h and 170 °C; (b) 600 L/h and 170 °C; (c) 440 L/h and 180 °C; (d) 600 L/h and 180 °C; (e) 440 L/h and 190 °C; and (f) 600 L/h and 190 °C. Reprinted with permission from Ref. [18]. MDPI (2022).
Moreover, it was found that the spray flow volume and input temperature had a big impact on the procedure outputs and discharge patterns. The optimal variables to generate greater processing outputs, better encapsulating rates, and superior releasing qualities were determined from the various process variables examined to be an intake pumping frequency of 2.5 mL/min, a temperature of 170 °C, and a drying air-flow frequency of 440 L/h.
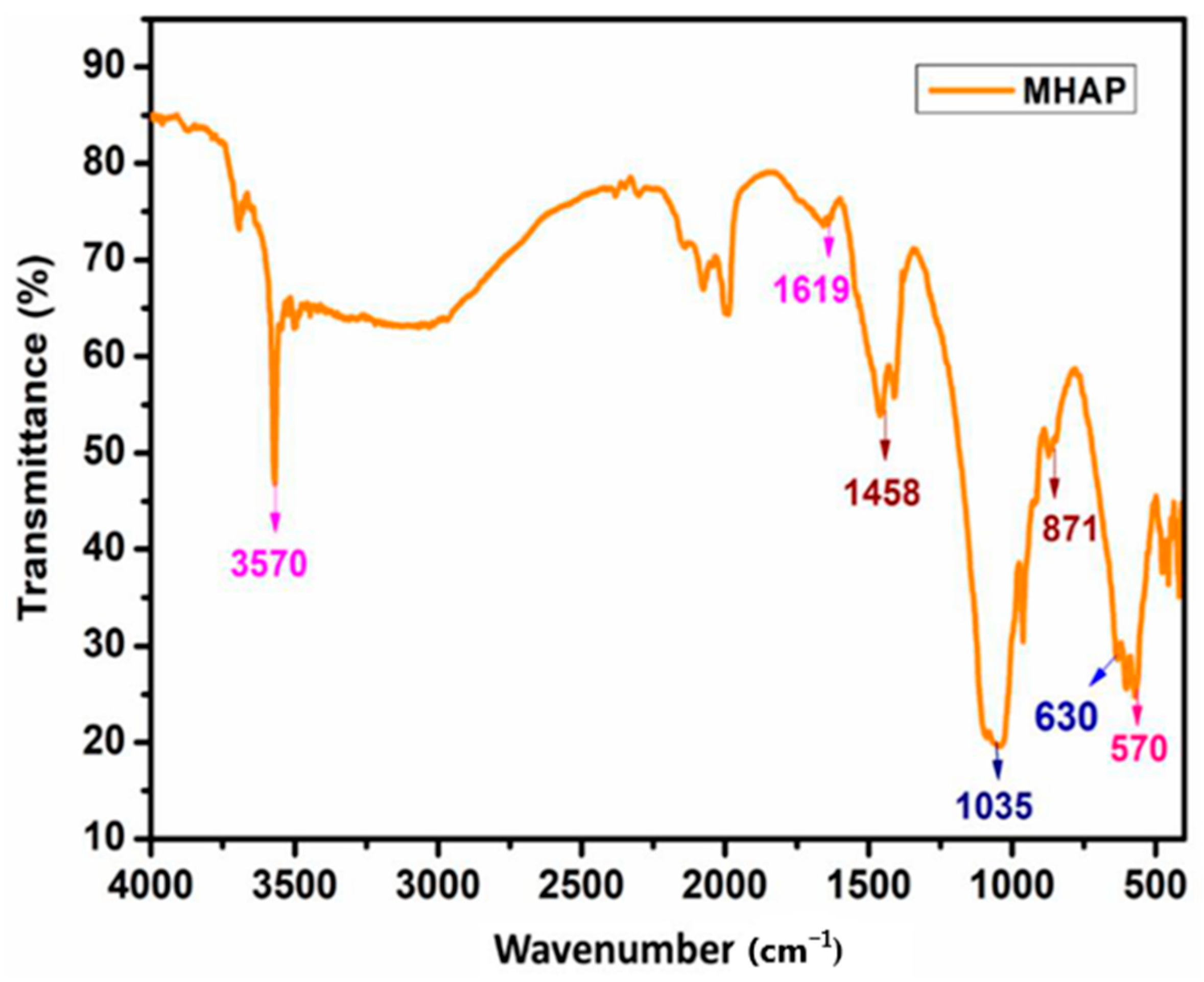
Furthermore, in their demonstration, Asif et al. [44] utilized a biodegradable polyol based on cardanol to generate nanocomposite polyurethane (PU) coverings. In order to create nanocomposite anticorrosive coverings, magnetic hydroxyapatite NPs (MHAP) were distributed 1–5% in PU compositions. As measured using submerged and electrochemical techniques, MHAP’s anticorrosive efficacy improved with increasing intensity. Figure 5 shows the FT-IR spectra of MHAP NPs. The -OH bending vibration was responsible for the maximum absorption at 3570 cm−1. While the phosphate moiety displayed absorption at 630 and 1035 cm−1, the stretching vibrations of carbonyl were recorded at 871 and 1458 cm−1. At 570 and 1619 cm−1, the stretching oscillation of the Fe-O bond took place. This demonstrated that the formation of MHAP NPs was robust.
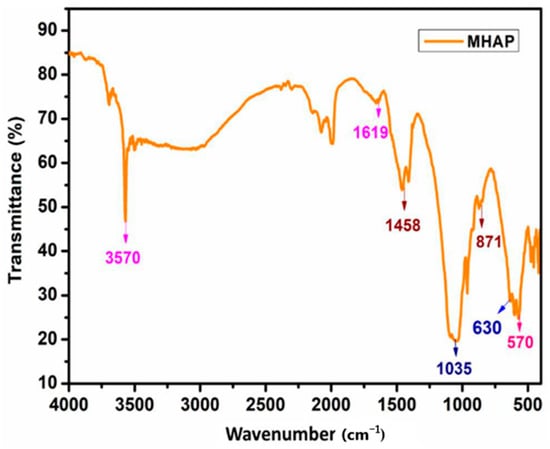
Figure 5.
FT-IR spectra of magnetic hydroxyapatite NPs. Reprinted with permission from Ref. [44]. MDPI (2022).
In terms of elasticity, cross-cut adherence, gloss, pencil stiffness, and chemical stability, the nanocomposite PU coverings demonstrated excellent coating characteristics. The coatings were further examined using SEM and TGA for surface morphology and thermal characteristics. The introduction of MHAP enhanced the hydrophobic properties of PU coatings, and a maximum result (105) was seen at a 3% dosage. The created coatings demonstrated their hydrophobicity with outstanding anticorrosive efficacy.
Using low-carbon steel (CS) surfaces, Halim et al. [45] developed Tin (Sn) matrices nanocomposite coverings including NiO and ZnO NPs, both separately and in combination. Investigating the impact of NPs’ reinforcements upon the microstructural geometry and density of Sn coverings, altering the interfacial barrier between the substrate and coating, and examining the corrosion protection of the low-CS sample were the main objectives. In this study, NiO and ZnO NPs of different types were employed. The thermal degradation of nickel acetate Ni((CH3COO)24H2O) at 500 °C for 2 h in a muffle furnace produced the nano-sized NiO powder. With the use of a ball milling method for 1 h at 600 rpm, the resulting NiO powder’s size was brought down to the nanoscale. The well-known up–down method was used to synthesize ZnO on the nanoscale. In short, 98% pristine ZnO powder (Adwic) was utilized as the precursor substance, and SS balls were utilized to grind the content for 24 h at 600 rpm while it was at atmospheric pressure and ambient temperature. The proportion of balls to powder was 10:1. Following grinding, a very granular nanopowder containing several nanometers was produced. Sn-coated low-CS surface and Sn-nanocomposite coverings comprising NiO and ZnO NPs were shown in the substrate’s cross-sectional nanostructure, which was responsible for the corrosion mitigation process.
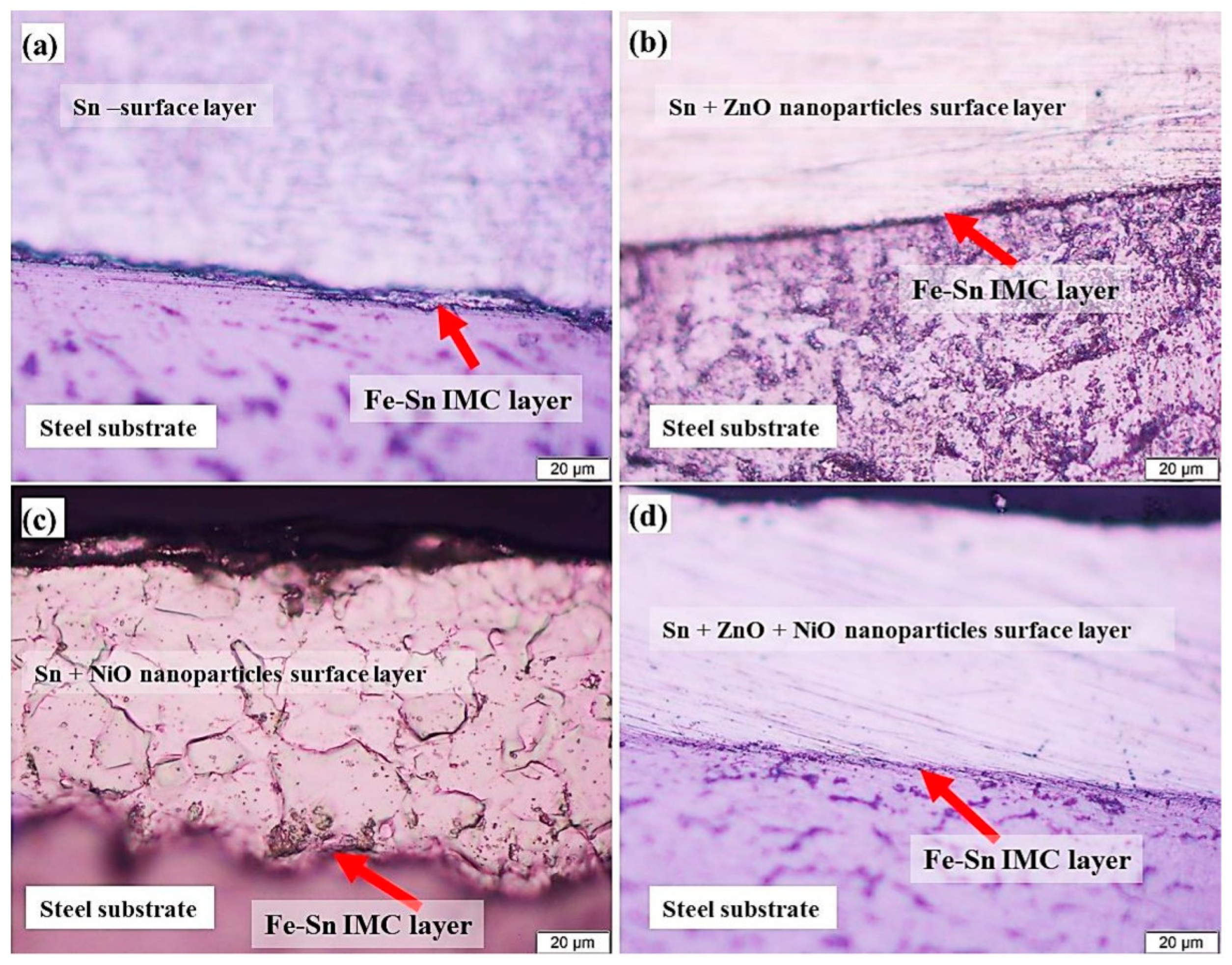
In the inter-metallic layering of Sn-composite coverings, Figure 6 depicts the shape of the protective coating, which is crucial to its production. Moreover, the inclusion of reinforced NP elements had a significant impact on the density of the interface and inter-metallic coatings of Sn-composite-coated films. Sn was the only coating applied to the low-CS surface, and it is an erratic, extremely thin coating having an extremely modest thickness (Figure 6a). Interestingly, Figure 6b depicts a reasonably thick covered film and the existence of ZnO NPs in the protective coating. It was intriguing to discover that the inclusion of NiO NPs in the protective coating (Figure 6c) exerts a significant impact upon the interfacial layer’s width, preventing it from thickening. Employing combined oxides of NiO and ZnO NPs results in a noticeable alteration in the coating thickness because it significantly thickens the interfacial film of protective coatings.
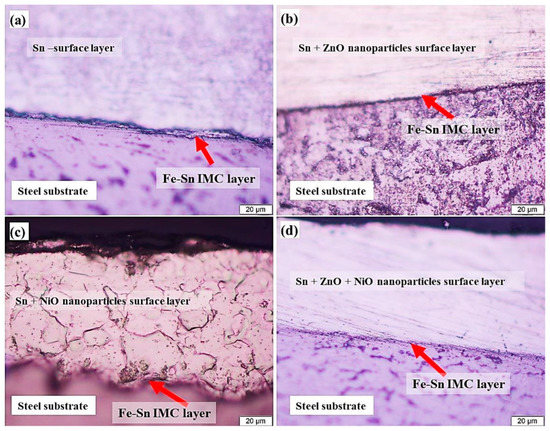
Figure 6.
Micrography of four distinct covered substrate and interfacial coatings of steel: (a) pristine Sn covering (S1); (b) Sn + ZnO NPs covering (S2); (c) Sn + NiO NPs covering (S3); and (d) Sn + ZnO + NiO NPs covering (S4). Reprinted with permission from Ref. [45] MDPI (2022).
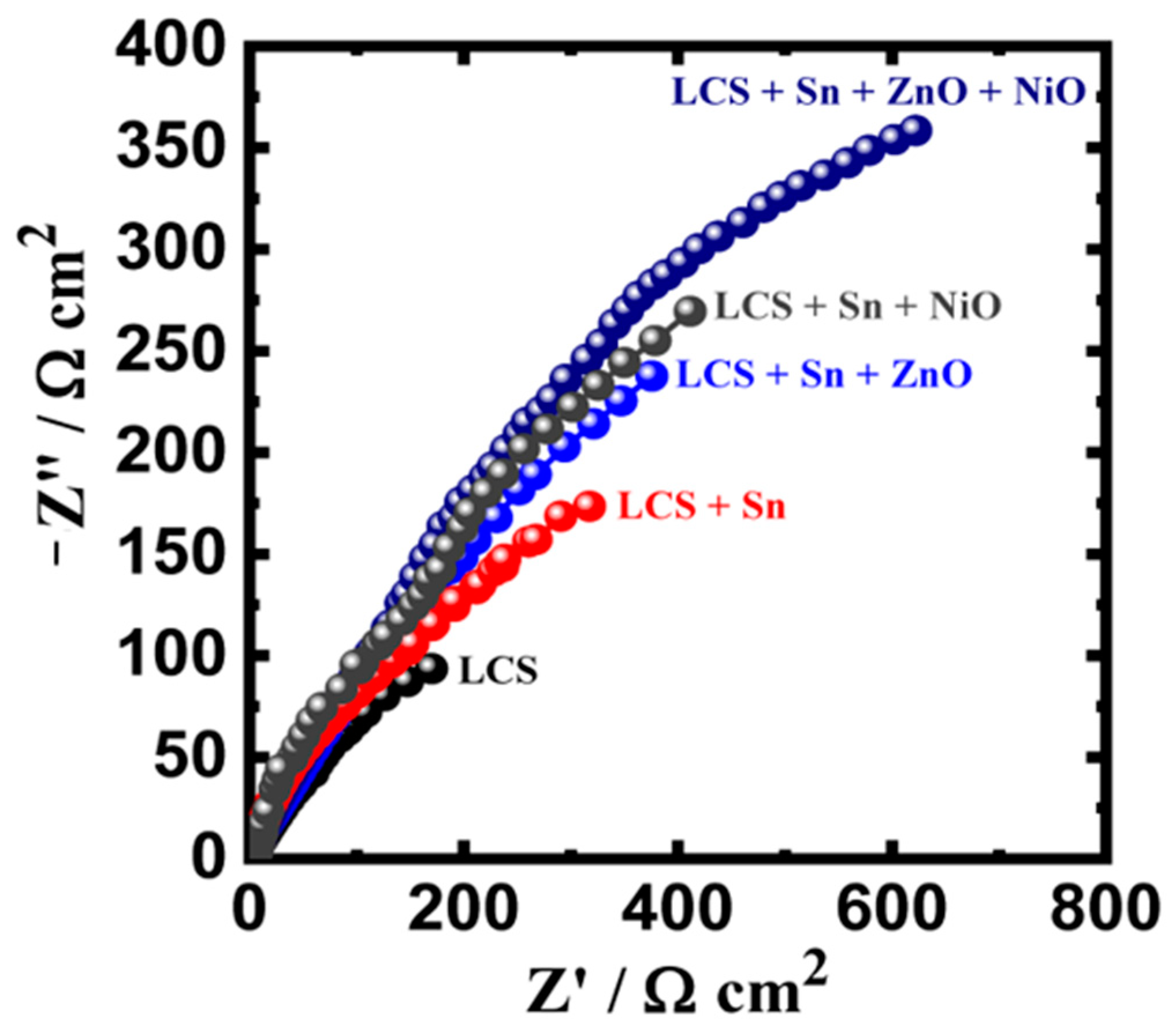
EIS evaluations were also performed in order to verify the information acquired from the PCP operations. When reporting on corrosion and its prevention for metals in a variety of corrosive conditions, the EIS approach could be effectively used. Following being submerged for 1 h in a 3.5% NaCl medium, Nyquist graphs were recorded for the LCS, LCS + Sn, LCS + Sn + ZnO, LCS + Sn + NiO, and LCS + Sn + ZnO + NiO specimens. Figure 7 illustrates how every spectrum only displays one semicircle, whose diameter is the shortest for the LCS specimen. The width of the semicircle increased after applying a Sn layer to the steel, demonstrating that the layer’s inclusion enhances resistance to corrosion. A broader semicircle resulted from the addition of 0.25% ZnO NPs to the Sn-coated film upon the substrate of the LCS specimen, while adding 0.25% NiO NPs to the Sn covering had a significantly greater impact. The steel specimen that contained both NiO and ZnO NPs together with the Sn covering offered the maximum semicircle radius. Following introducing NiO and ZnO, presumably separately or together, the resistance indices RS, RP1, and RP2 rose. Additionally, the existence of Q (with “n” values that ranged from 0.67 to 0.80) might be viewed as a double-layer capacitance with certain apertures to lessen the charged specimen interfaces. Furthermore, it was seen that the values of Cdl and YQ decreased with the addition of the Sn protective film. Significant declines were noted with the addition of NiO and ZnO NPs, with the LCS + Sn + ZnO + NiO specimen showing the lowest value. The EIS and PDP investigations both show that Sn covering upon steel improves corrosion protection in the chloride medium and that this action was increased by the addition of NiO and ZnO NPs.
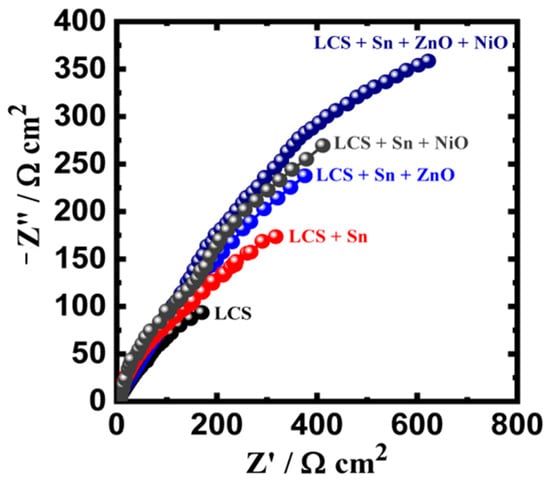
Figure 7.
Nyquist graph for LCS, LCS + Sn, LCS + Sn + ZnO, LCS + Sn + NiO, and LCS + Sn + ZnO + NiO specimens following 1 h dipping in 3.5% NaCl medium. Reprinted with permission from Ref. [45] MDPI (2022).
Various nanomaterials have been used by numerous research teams based on their suitability for anti-corrosion purposes as listed in Table 1.

Table 1.
Summary of some recently investigated nano-modified/nanomaterial-based corrosion inhibitors/coatings for several metals in various corrosive media.
2. Utilization of Various Nano-Modified Coatings for Corrosion Mitigation
Nanomaterials (NMs) are substances that demonstrate at least one morphological property at the nanoscale, or within 100 nm, including particulate size, architectural dimension, particle aspect, etc. [60]. These NMs can be found as 2D nanolayers, nanoplatelets or nanosheets as well as OD NPs, 1D nanotubes, nanorods, and nanowires. Particularly in the areas of electrical, photonic, thermal, mechanical, chemical, physical, and magnetic activity, NMs exhibit improved characteristics. Their very tiny dimensions that permit enhanced surface volume fractions, and wide surfaces for efficient interaction are primarily responsible for these behaviors. By applying nanocrystalline structured coatings upon metal substrates, NMs have the potential to reduce the extent of corrosion. Coatings using nano-modifications have been successfully used to lessen the negative impacts of corrosion [61,62]. The dimensions or sizes of NMs, in conjunction with their intense layering, provide strong adhesion and extremely suitable physical coverage of the protective film. Because there are many different kinds of coating substances and techniques for many different environmental factors and applications, the most widely used method for inhibiting, limiting, minimizing, or mitigating the impacts of corrosion is the nano-modified coating of stacked corrosion inhibitors. These coatings treat the inside or outside surfaces of a particular material at a variety of temperatures, enabling crucial advantages: notably extremely smooth substrates and effective interfacial permeability on the substrates. In the long term, nanocoatings are likely to be less expensive than they initially appear to be, notably when used on a massive scale. This is because of the significant savings that can be realized from greatly reduced repair costs, protection, the prevention of equipment failures, and other factors. Without modifying bulk substances, nano-modified coatings offer an efficient way to address corrosion, abrasion, and wear concerns through surface treatment. Nanostructured coatings are used in a variety of technological systems, such as maritime, aviation, automobile, medical (dental, orthopedic), athletic, architectural, energy, and packaging materials. Research predicts that the market for nanostructured coatings will grow by 20% between 2020 and 2030 [63].
Nanocoatings are created using a variety of synthetic techniques, based on the intended function. In addition to more conventional techniques, including chemical vapor deposition (CVD) and physical vapor deposition (PVD), new techniques like sol–gel and laser cladding processes are also utilized to create nanostructured coatings. Other methods include the spray-coating method and the electrodeposition method [64,65,66]. The CVD process entails the deposition of the covering over the preheated substratum after chemical interactions dissociate vaporous or gaseous material adjacent to it. Coating metallic or ceramic materials is a popular application for this technique. The substrate’s temperature is important since it may affect how well the coating adheres to the substrate. Owing to the intrinsic characteristics of the CVD process, multi-directional layering of coating substances on a substrate is conceivable. The CVD method is costly for covering wide surfaces because it requires elevated substrate temperatures and a slow pace of precipitation. The intended material is transformed into a gaseous state and then deposited using the PVD technique on a substrate region. The PVD process involves three stages: i) the intended substance evaporates; (ii) the evaporated substance is transported to the substratum; and (iii) the intended substance is deposited as a coating over the substratum. The spray-coating method involves passing the desired substance through a nozzle and depositing it over a substratum to form a coating. In the industrial sector, spray-coating methods are frequently employed to apply organic binders and lacquers onto erratically structured glass, fabrics, wood, and metals. Among the most popular spray-coating methods is thermal spraying. The coating substance is warmed or liquefied and sprayed over the substrate during this procedure. Typically, chemical or electrical techniques are used to warm the coating substance. In comparison to other traditional techniques such as PVD and CVD, thermal spraying allows for the faster coating of surfaces with vast surface regions. Increased toughness, reduced permeability, and improved adherence are all benefits of cold-sprayed coatings [67,68]. In this approach, a solvent with a chemically active constituent is used as a precursor, accompanied by polycondensation and hydrolysis to create a sol unit. A coating is created by drying and heating a gel that is created by gently polymerizing a sol. Coatings made using the sol–gel technique have consistent physical and chemical properties. The sol–gel technique’s ability to form a coating relies on a number of variables, including the catalyst’s chemistry, the primary substance used to make the sol, heating, density, pH, etc. The sol–gel method could be utilized to create coatings that are wear-resistant. Sol–gel technology has also been utilized to create coatings for nanocomposite materials that are microns thick. The electrodeposition process includes depositing a metal or alloy coating substance along a conductive substratum that is submerged in an electrolyte with a salt of the metal that is to be covered utilizing an electric current (electrolysis). By altering empirical variables including the electric voltage, current density, exposure duration, and content of the plating medium, the content, geometry, and smoothness of the coating can be changed. Utilizing a high-energy laser to shred granular or wire feedstock’s intended substance over the substratum, laser cladding is a technique for producing nanostructured coatings. Laser procedure variables like power input and scan rate significantly impact coating integrity. When contrasted to alternative technologies, laser cladding provides benefits including excellent cooling rates, minimal dispersion rates, and flexibility of mechanization. This technique could be utilized to layer coatings using a variety of powders. However, several drawbacks of the laser cladding method include non-uniform content, poor metallurgical integrity, and coating defects.
Additionally, a range of criteria, such as the needed nanoparticle size, surface properties, and levels of biodegradability, biocompatibility, and cytotoxicity, influence the choice of matrix materials. By incorporating organic or inorganic NPs, it is possible to change the physical characteristics of coating matrices [69,70,71]. For instance, a nanocomposite or nanocoating comprising some inorganic NPs could prevent cracks from forming and spreading, fill porosities, and begin fracture bridging, deflection, and bowing. Therefore, a competent design of a nanocomposite might create the composite material system with the desired physical characteristics by taking into account the intricate interplay between the matrix, interface, and NPs. In this section, the various types of nano-modified coatings including self-healing-based nanocoating, natural source-based nanocoating, metal/metallic oxide-incorporated nanocoating, and carbon allotrope-based nanocoating have been discussed with their excellent corrosion-inhibition efficiency and fabrication procedures for various nanocoatings.
2.1. Self-Healing-Based Nano-Modified Coating
Among the best ways to avoid corrosion of highly chemically reactive metals and their alloys is to coat and cover them. When corrosive media permeate the covering and contact with the alloy substrate, pristine coverings over metals created using conventional techniques such chemical conversion, electroless Ni, and plasma electrolytic oxidation (PEO) cannot continue to defend against corrosion [14,72]. To enhance protection against corrosion and extend the lifespan of a coating, CIs are physically applied to the sealant matrix. However, this has the downside of early inhibitor leaking preceding the commencement of corrosion, which reduces the effectiveness of corrosion inhibition. The unpredictable discharge of inhibitors is frequently addressed by the development of smart self-healing nanocoatings, which have gained significant interest in the field of corrosion research during the past ten years.
These coatings are considered to be “active” coatings with the ability to recover from an initial scratch or imperfection and so persist to provide structures underneath with corrosion prevention. The responsiveness of coatings to exterior stimuli is a characteristic shared by all systems [73]. A trigger’s reaction could be instantaneous or managed through evaluation of the triggers. They are typically coatings made of a top coat and a primer. The top layer might deteriorate and act as a trigger when exposed to UV light. A capsule’s ability to discharge an active ingredient is stimulated by light. The material can restore the top coat’s damage. A novel method for building pH-responsive smart nanocontainers (MSN-MBT@LDH) was provided by Ouyang et al. [66]. By using pH-regulated MSN-MBT@LDH nanocontainers comprising an MBT-loaded MSN core and a cover made of LDH nanosheets, they show how to fabricate a proactive SNAP covering over Mg alloy with greatly improved corrosion resistance. The mechanized MSN-MBT@LDH nanocontainers exhibited a pH-dependent liberation of MBT from MSN in acidic circumstances and were readily produced by a straightforward two-step sorption procedure. The SNAP coating incorporating MSN-MBT@LDH nanocontainers showed a considerable improvement and enhanced resilience in corrosion protection in NaCl medium as contrasted to plain SNAP covering. This was partly attributable to the trapping of hostile Cl− ions availing the benefit of the extra LDH nanoplates that were not deposited over the MSN interface, and mostly to the corrosion inhibition of MBT caused by flaws occurring at the coating interaction. Researchers presume the suggested approach will open the path to the thrilling development of an inhibitor-loaded nano reservoir as well as its comprehensive implementation for the preparedness of smart corrosion protection coating materials in the sector of corrosion science due to the reduced expense and ease of development of MBT-loaded and pH-responsive MSN-based nanocontainers in this work.
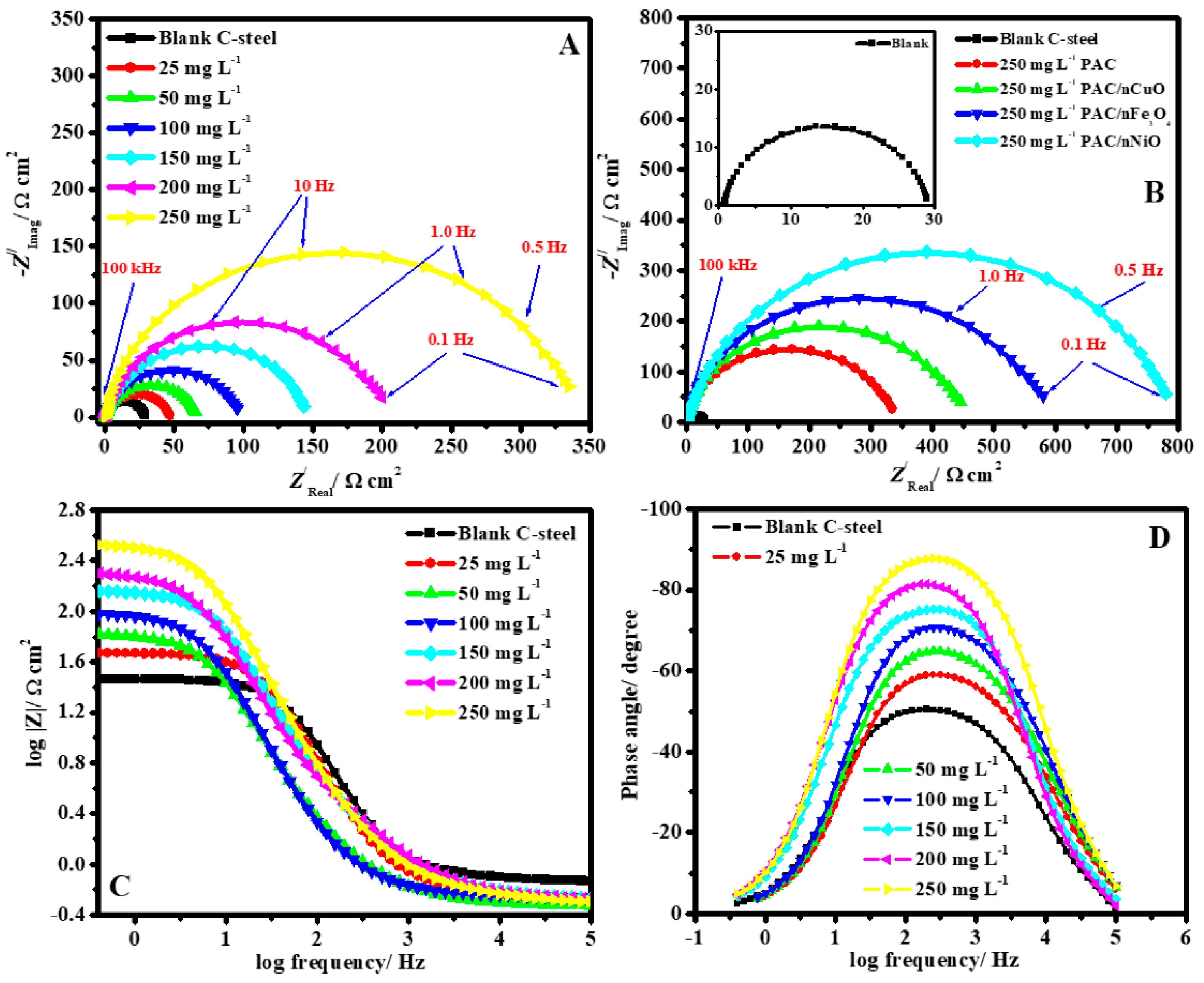
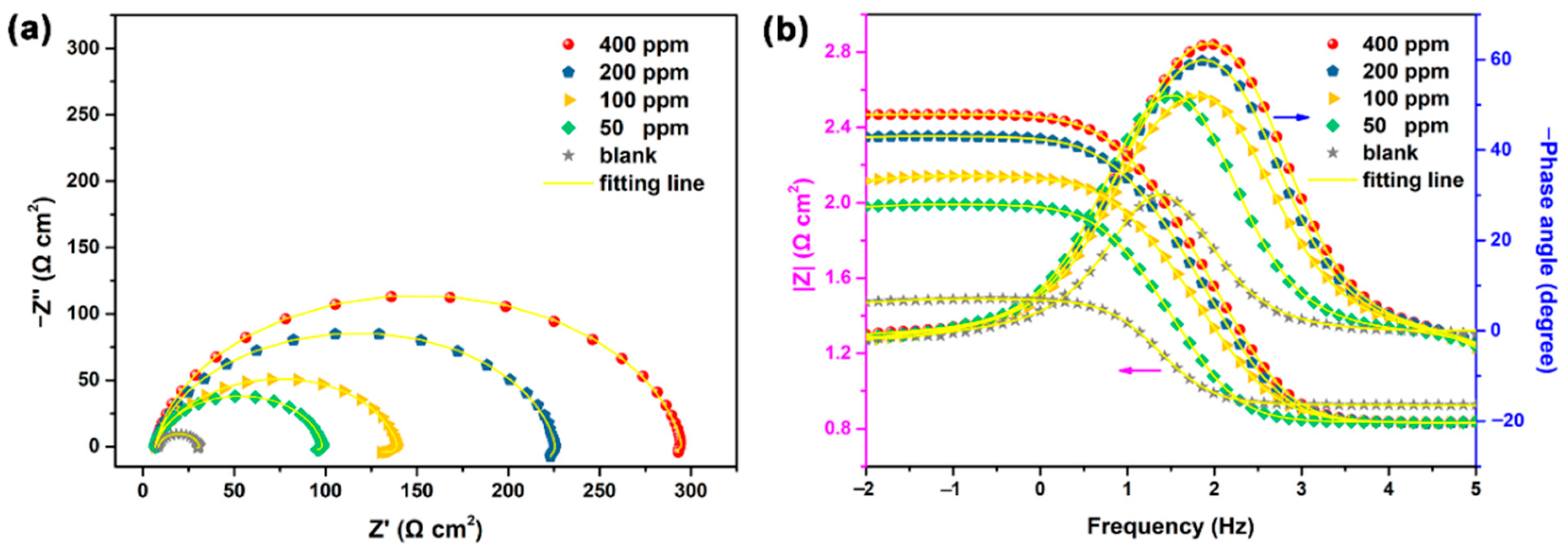
Similarly, incorporating nano-oxides of various metals (MONPs), such as CuONPs, Fe3O4NPs, and NiONPs, into primary alginate-modified cellulose (PAC), Gouda et al. [74] effectively produced unique self-healing eco-sustainable nano-modified coating. The results from spectroscopy showed that the synthesis of PAC/CuONPs, PAC/Fe3O4NPs, and PAC/NiONPs with uniform distributions and particulate sizes of 23, 10, and 43 nm, correspondingly, was effective in producing PAC/MONPs nanocomposites. By using gravimetric and electrochemical methods, the protective effect of the as-prepared PAC and PAC/MONPs nanocomposites for C-steel corrosion in 1M HCl was investigated. Using 250 ppm of PAC/Fe3O4NPs, PAC/CuONP, and PAC/NiONPs, the IE% was 93.2, 88.1, 96.1, and 98.6%, correspondingly. Figure 8 shows the impedance graphs for the working electrode in HCl media for the following conditions: (A) Nyquist in the lack and presence of numerous PAC doses; (B) Nyquist in the lack and presence of numerous nanocomposite inhibitors at 250 ppm; (C) Bode and (D) phase angle depictions in the lack and presence of numerous PAC doses at 323 K. The Nyquist curves clearly show that an increase in the quantity of nanocomposites causes a concomitant increase in the capacitance loops’ width. The Nyquist diagram’s non-ideal half-circle characteristic might be explained by considering surface roughness as a result of the formation of an adherent nanocomposite coating that was gathered alongside the corrosion byproduct. In both the untreated and treated media, the Nyquist curves show that there is just one capacitive looping and no inductive looping. The values obtained in the intermediate frequency region of the phase angle curve (Figure 8D) vary from 51.1 for the uninhibited metal to 87.5 with an increment in the PAC concentration. The maximum phase shift in the intermediate frequencies’ ranges was 90° in the case of an ideal capacitor. Therefore, the capacitive regimen in the presence of nanocomposites was improved by the phase angle strategy approaching the values of the angle 90° in the existence of PAC.
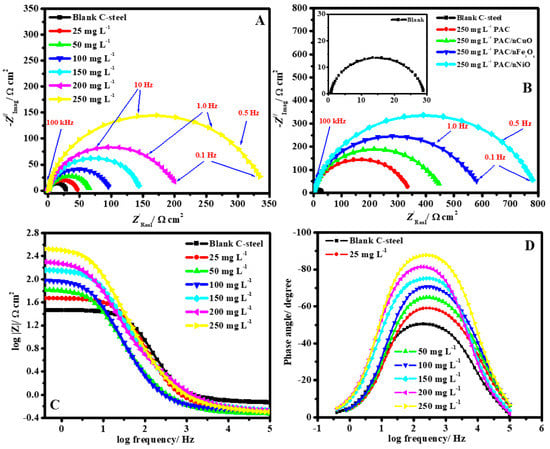
Figure 8.
Impedance curves for C-steel in acidic media: (A) Nyquist with the exclusion and inclusion of various dosages of PAC; (B) Nyquist with the exclusion and inclusion of 250 ppm of several nanocomposite inhibitors; and (C) Bode and (D) phase curve illustration with the exclusion and inclusion of various dosage of PAC at 50 °C. Reprinted with permission from Ref. [74]. MDPI (2021).
Similarly, in an attempt to assess the corrosion protection effectiveness for straight-to-coating materials, González et al. [75] added CeO2 NPs to aqueous adhesives with up to 70% biobased composition. In this, isobornyl methacrylate monomers and 2-octyl acrylate were batch miniemulsion-polymerized with a phosphate polymerizable surfactant (Sipomer PAM200), resulting in the creation of phosphate-functionalized elastomers. These adhesives might be applied directly to steel, allowing the functionalized polymer granules to react with the metals and form a thick phosphatization barrier across the polymer and metals to prevent rapid corrosion. As per the EIS studies, the in situ integration of the CeO2 NPs throughout the polymerization procedure resulted in their uniform dispersion in the finished polymer layer, producing exceptional anti-corrosion efficacy. Following 41 days (about 1000 h) of soaking in a NaCl H2O medium, steel substrates covered with the composite polymer coating (30–40 m thick) demonstrated decent barrier corrosion protection and active inhibitory capabilities because of the existence of CeO2 NPs. This research paves the way for the creation of environmentally friendly hybrid anticorrosion waterborne films. Kaseem et al. [76] showed that LDH films formed on the permeable interface of the PE film were functionalized using WO3 implanted onto the substrate. Owing to the excellent durability of the albumin-WO3 composite, the electrochemical analyses in a 3.5 wt.% NaCl medium demonstrated that the altered LDH layer (LDH-ALB-WO3 film) successfully collected the chloride anions throughout the corrosion experiment. It was discovered that the layer constructed from the PE coating became dense when the interface of the LDH layer was decorated with WO3 NP-functionalized albumin molecules. The composite covering created in this work was regarded as a smart-healing layer for actively preventing the corrosion of Mg alloys. In addition, the flake-like structure containing albumin and WO3 NPs obtained in this work is suggested to improve the functional characteristics of the AZ31 Mg alloy. By using Diels–Alder (DA) bonds to cross-link the PU prepolymer using furan-altered CeO2 and create a unique NIR-triggered self-healing anti-corrosion covering, Wang et al. [77] were able to successfully prevent mild steel (MS) from corroding when it was damaged. A variety of techniques were used to precisely characterize the produced NP and PU coatings. The generated covering (PU-DA-d@CeNPs5) showed an impedance value at 0.01 Hz of 1.29 × 10−9 Ω cm2, outstanding mechanical properties (38.89 ± 0.52 MPa), and noteworthy adhesive capabilities (12 MPa). Following 100 days of constant soaking, the impedance factor at 0.01 Hz was greater than 107 Ω cm2. Dopamine’s capacity for photothermal transformation also permitted the covering to self-heal in a matter of seconds when exposed to near-infrared light. Prior to and following self-healing, the coating’s tribological and mechanical resistance was essentially unaltered. This research offered a practical method for creating an anti-corrosion polyurethane covering featuring superior mechanical properties and a significant degree of self-healing.
Najjar et al. [78] fabricated self-healing covering of epoxy resin integrated with core–shell NPs of organic polysulfide@urea-formaldehyde resin as self-healing agents and PANI/ZnO nanocomposites. The self-healing efficacy and corrosion mitigation property of the scratched covering on the anodized 2024-T3 Al alloy substrates in 3.5% brine medium at 65 °C were examined. In this work, 0.045 g of ZnO NPs and 4.5 g (48 mmol) of aniline were combined in 200 mL of distilled water to create PANI/ZnO nanocomposites. To obtain a homogeneous suspension of ZnO, the liquid was aggressively agitated with magnetic stirring in an ice water bath for 4 h. After that, in the ZnO suspension, 1 g (3 mmol) of DBSA was vigorously agitated for 2 h at 0 °C. The PANI/ZnO nanocomposite initially appeared as a white precipitate, which was subsequently strained and repeatedly rinsed with methanol and distilled water. After 12 h of drying in a vacuum oven at 50 °C, the result yielded 3.49 g of PANI/ZnO nanocomposite (PZ1). Core–shell NPs comprising a healing agent were disseminated in epoxy resin and TEPA matrices together with the manufactured nanocomposite in proportions of 0, 5, 7.5, and 10 weight percent to create coating material. As revealed from the Bode graphs, in the scenario of a scratched covering without core–shell NPs (EPZ1), the coating resilience began to deteriorate immediately following 96 h of exposure to corrosive fluids. On the other hand, the Bode graphs of the coatings (EPZ1C5, EPZ1C7.5, and EPZ1C10) with different quantities of core–shell NPs reveal that, for all of the coatings, the resistance (Rc) was decreased upon scratching owing to the penetration of electrolytes via the scratches. Furthermore, the decrease in coating resistance over time was not as pronounced as it was for coatings without additional core–shell NPs, showing that these particulates had a protective impact against corrosion. The permeable oxide layer on anodized substrates improved the covering efficiency. The improvement in corrosion resistance of coatings was caused by the addition of 1.54 wt% of PANI/ZnO nanocomposite. Eventually, the EPZ1 coating on anodized aluminum that included 7.5 wt% core–shell NPs exhibited the best self-healing behavior.
2.2. Plant Extract and Natural Source-Based Nanocoatings
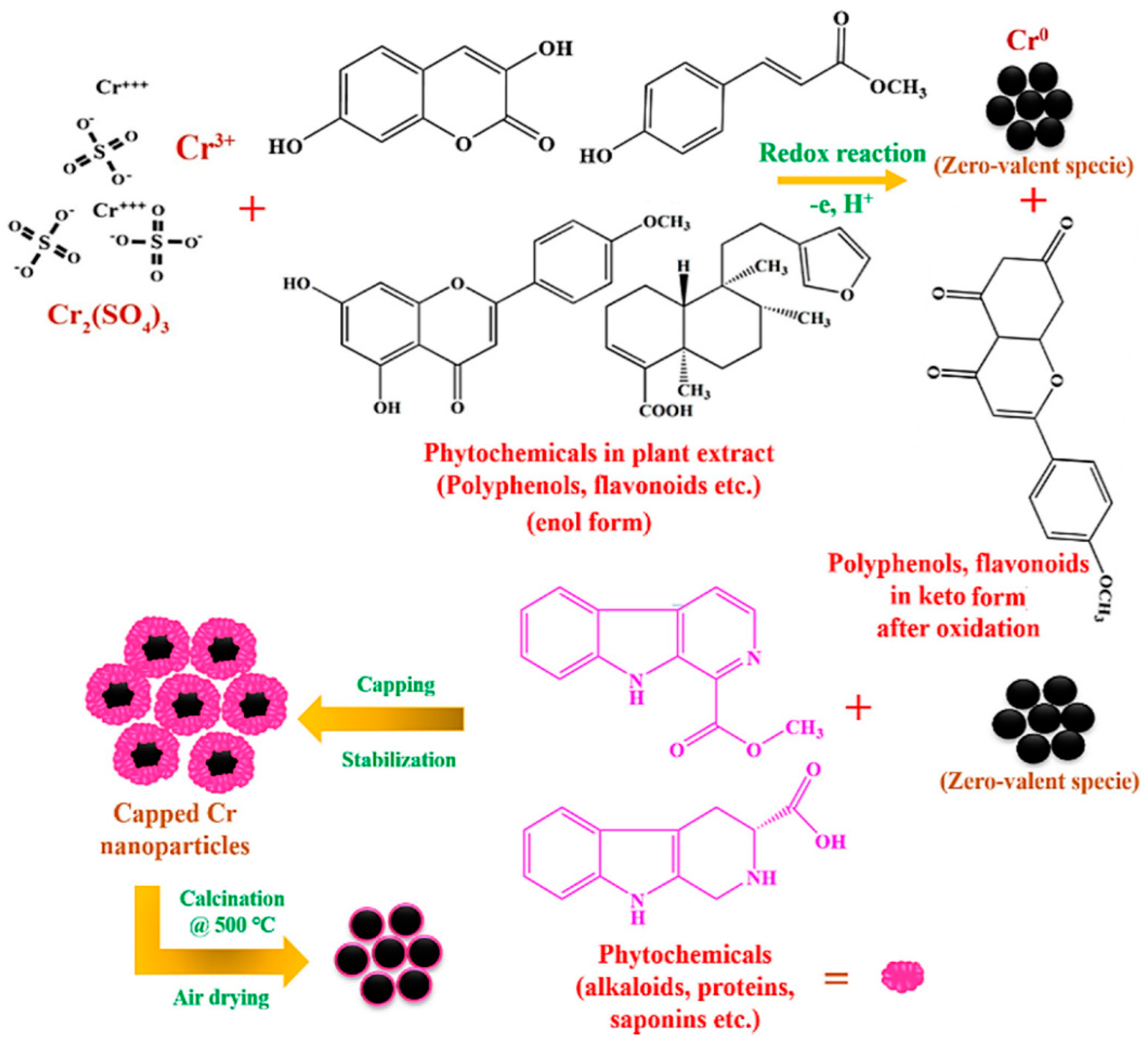
The examination of the utilization of nanotechnology in various biological phenomena is known as nanobiotechnology, which is the fusion of biology and nanotechnology [73,79]. Making biocompatible, environmentally safe, and biologically active NMs and NPs is another focus of nanobiotechnology. The production of natural and non-hazardous additives has proven crucial owing to the toxicity and expensive price of synthetic additives. The efficacy of nanocoatings is being improved by adding organic elements like plant extracts as “green” additions. Plant extracts are employed as coating framework additives because they are eco-benign, non-hazardous, inexpensive, effortlessly accessible, and heavy metal-free. They come from the plants’ stems, leaves, branches, fruit, and seeds [80,81,82]. They contain bioactive components with antioxidants that may aid in improving nanocoatings’ characteristics. In an experiment, as a capping and reducing reagent, Khan et al. [5] used an extract from the leaves of Abutilon indicum (L.) Sweet to create chromium oxide (Cr2O3) NPs. The NPs’ sizes varied between 17 and 42. Utilizing a live/dead staining approach and agar well penetration, the antibacterial and anticorrosion efficacy of the green-produced NPs was assessed using four distinct bacterial strains, including S. aureus, E. coli, B. subtilis, and B. bronchiseptica. By observing a color alteration after the introduction of a metal salt precursor to the leaf extract, the formation of Cr2O3 NPs was optically observed. The reaction mixture’s color shift from red to black signified the production of Cr2O3 NPs. The surface plasmon resonance (SPR) process on the NPs’ interface was responsible for this color change. To decrease and stabilize the metal ions to nano size, certain phytocompounds may function as ligands and establish chelates with various metal ions. After dissolving in H2O, chromium sulfate salt (Cr2(SO4)3) transforms into readily floating ions. Owing to a lack of electrons, the readily floating Cr3+ ions are drawn to the phytomolecules of the plant (polyphenols, etc.). As a consequence, metallic ions and phytomolecules within plants produce chelate complexes when O2 is used as the donor and Cr3+ as the acceptor in the donor–acceptor mechanism (Figure 9). This causes flavonoids, polyphenols, etc. to oxidize and change into the keto state (Figure 9). On either end, the adjacent tannins, alkaloids, flavonoids, and other plant phytomolecules stabilize Cr3+ while reducing it to the zero-valent isotope Cr0. They easily oxidize throughout air drying and carbonization to become Cr2O3 NPs covered in Abutilon Indicum phytomolecules (L.) Sweet leaf infusion.
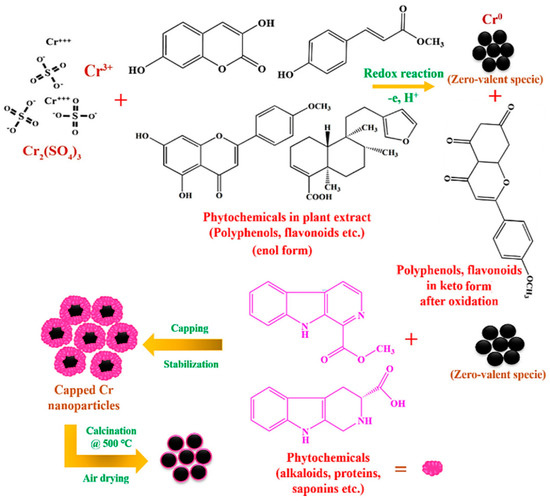
Figure 9.
Illustration for the green production of Cr2O3 NPs utilizing Abutilon indicum (L.) Sweet leaf extract. Reprinted with permission from Ref. [5]. MDPI (2021).
Sudha et al. [71] used Delonix elata leaf extract as a capping and reducing reagent to produce nickel oxide NPs (NiO NPs) in an environmentally friendly way utilizing ultrasonic waves. Utilizing SEM and Brunauer–Emmett–Teller examination, the phase formation, crystalline nature, physical and thermal durability, and surface area of the synthesized NiO NPs were examined. When contrasted to the other three electrolyte media, the NiO NP-coated Zn metal sheets demonstrated outstanding corrosion-inhibition behaviors. They demonstrated an 88.6% increase in resistance to corrosion in 1 M H2SO4, compared to improvements of 75.74%, 68.4%, and 56.8% in the corrosion resistance in 1 M HCl, 3.5% NaCl, and 6 M KOH media, correspondingly. Moreover, in 1 M HCl, 3.5% NaCl, 6 M KOH, and 1 M H2SO4 environments, NiO NP-coated Mg metal plates showed good corrosion-prevention efficacy of 71.9%, 61.1%, 55.9%, and 79.5%. The Tafel curve demonstrated that the generated large surface region and mesoporous NiO NPs effectively reduce the corrosive character of Mg and Zn sheets under all electrolytes, particularly in acidic media.
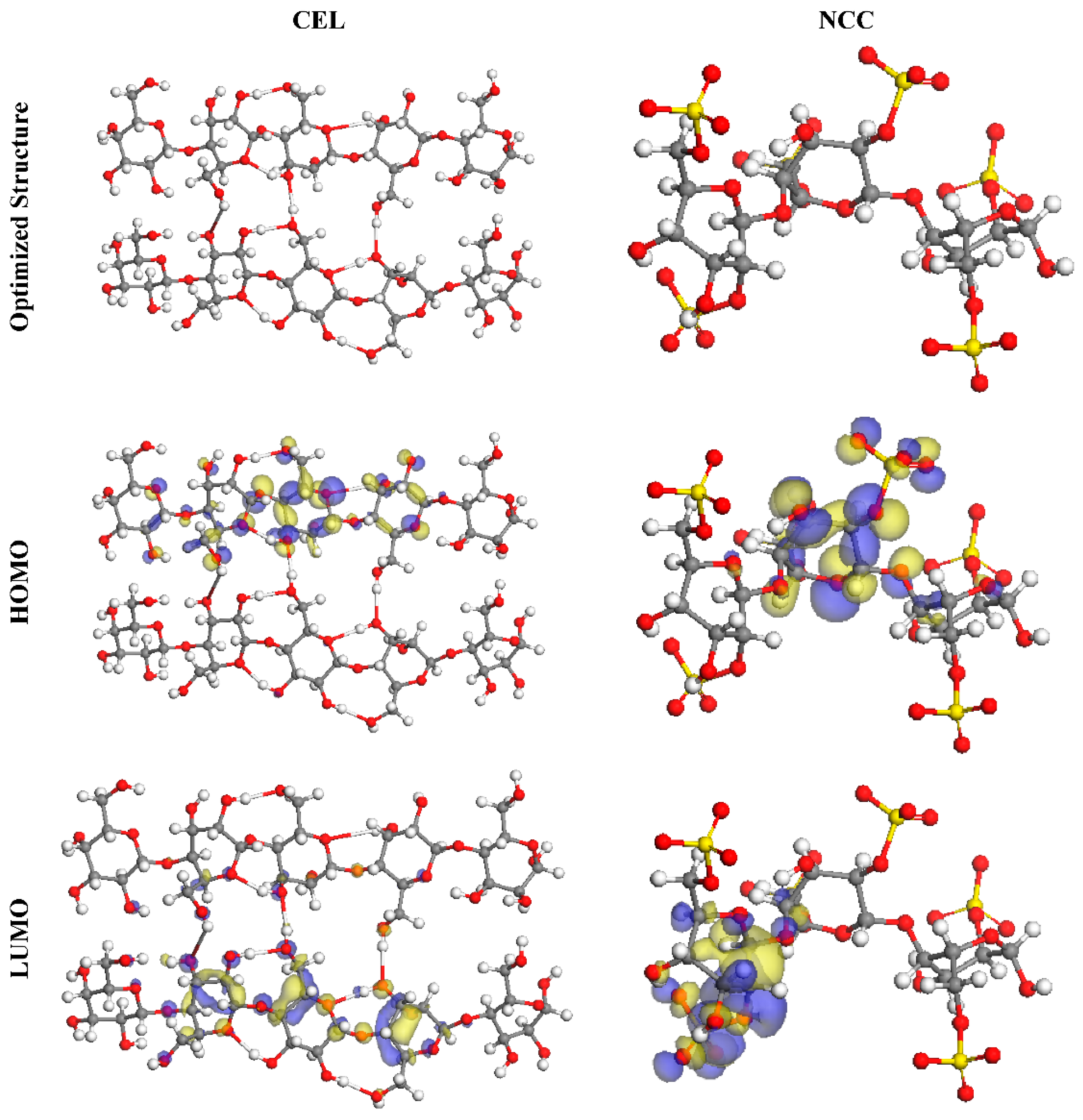
Toghan et al. [8] produced nanocrystalline cellulose (CEL) through the acidic hydrolysis of macrocrystalline cellulose (NCC). A morphological representation of NCC revealed a rod-shaped framework with a mean diameter of 10–25 nm, and a thickness and a width of 100–200 nm. Pristine NCC and CEL were discovered to have BET surface areas of 10.41 and 27 m2/g, correspondingly. DFT analyses were carried out to examine the degree of interactions between the active centers of NCC and CEL molecules on the substrate of the SS316 alloy. Figure 10 shows the optimal geometries for CEL and NCC’s HOMO and LUMO orbitals. As per the FMO concept, HOMO and LUMO energies determined whether an inhibitor molecule was acting as a donor or acceptor at the contact between the inhibitor and the metallic substrate. The compounds with higher EHOMO and reduced ELUMO values were regarded as very potent CIs as a result. In comparison to CEL’s maximal EHOMO value of 5.73 eV, the NCC’s is 5.45 eV. Figure 10 shows that the HOMO value was found on the hydroxy, pyran, and sulfate moieties of the inhibitor compounds, indicating that the sulfur and oxygen atoms were the preferred spots for electrophilic interactions on the Fe surface.
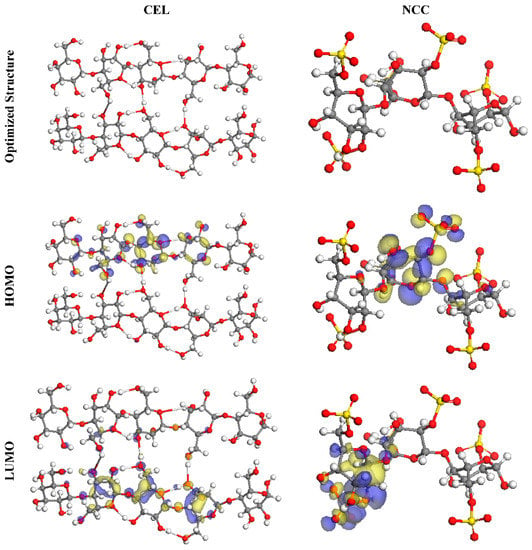
Figure 10.
The optimized molecular configurations, HOMO, and LUMO of both inhibitors using DMol3 array. Reprinted with permission from Ref. [8]. MDPI (2021).
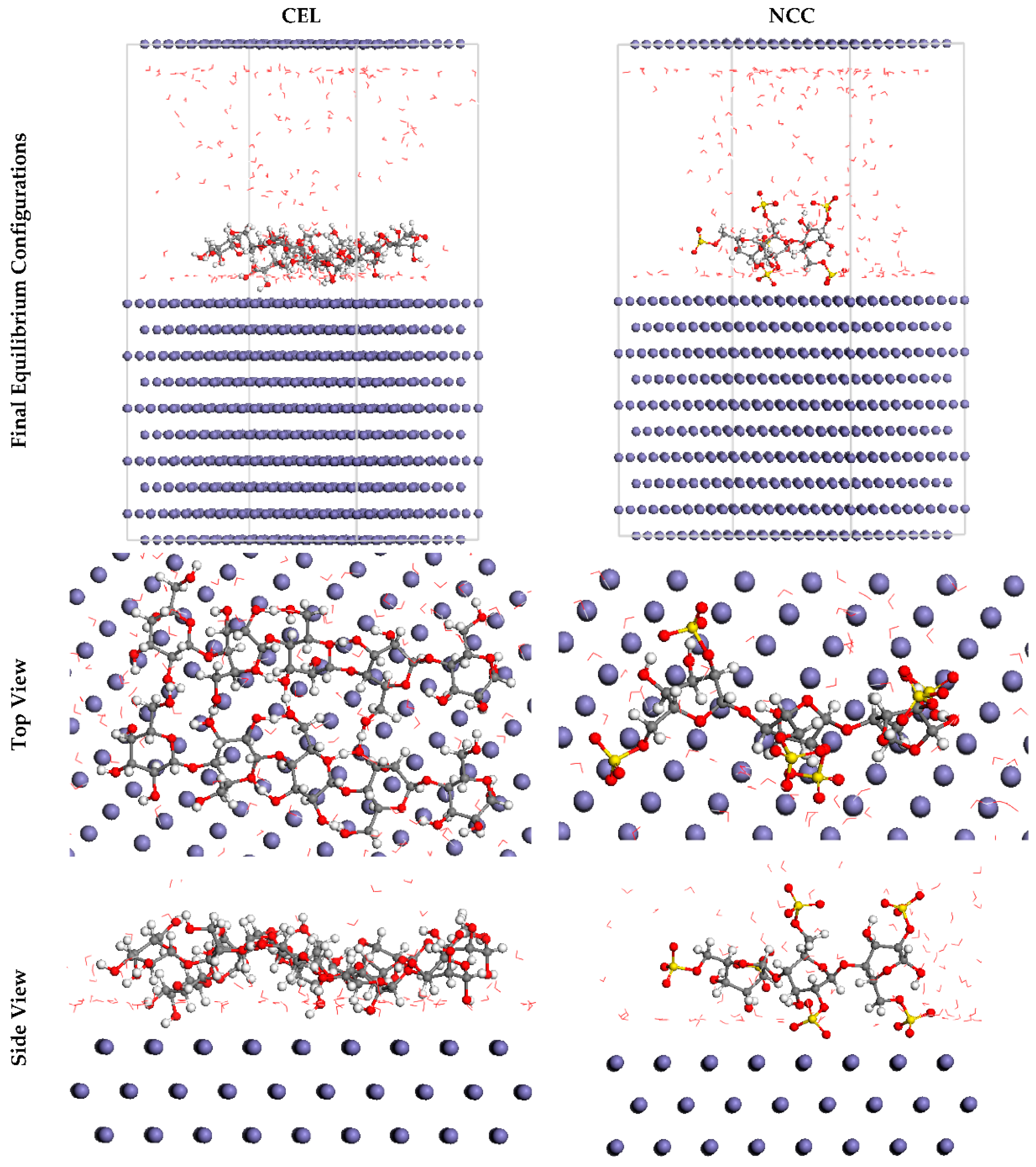
Furthermore, MC simulations were used to identify the associations between the inhibitory molecules and the SS316 alloy substrate as well as to propose a descriptive notion for the operation of adsorption. The broadest appropriate adsorption arrangements for the inhibitory molecules over the SS316 alloy substrate are shown in Figure 11 as a result. These layouts are produced by the adsorption locator unit and proffer basic plane frameworks, indicating an increment in the adsorption and the extremely high surface treatment. Additionally, the NCC (2831.49 kcal mol−1) exhibited a more elevated negative value of the absorption coefficient than the CEL (2133.54 kcal mol−1), which also supports the notion that NCC was aggressively adsorbed onto the substratum of the SS316 alloy, forming a staunchly adsorbed coating that prevents corrosion. These outcomes are consistent with the findings of the experiments. Furthermore, the adsorption energy values of NCC for the post-geometry optimal phase, i.e., relaxed (130.73 kcal mol−1), were also greater than CEL (61.39 kcal mol−1) and much more negative than CEL (2962.22 kcal mol−1) for the pre-geometry optimal phase, i.e., unrelaxed (2194.93 kcal mol−1).
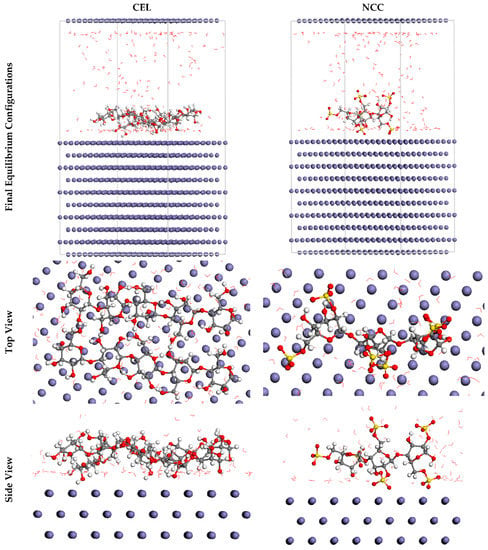
Figure 11.
The optimal arrangement for adsorption of the CEL and NCC over the Fe (1 1 0) surface attained through the adsorption locator array. Reprinted with permission from Ref. [8]. MDPI (2021).
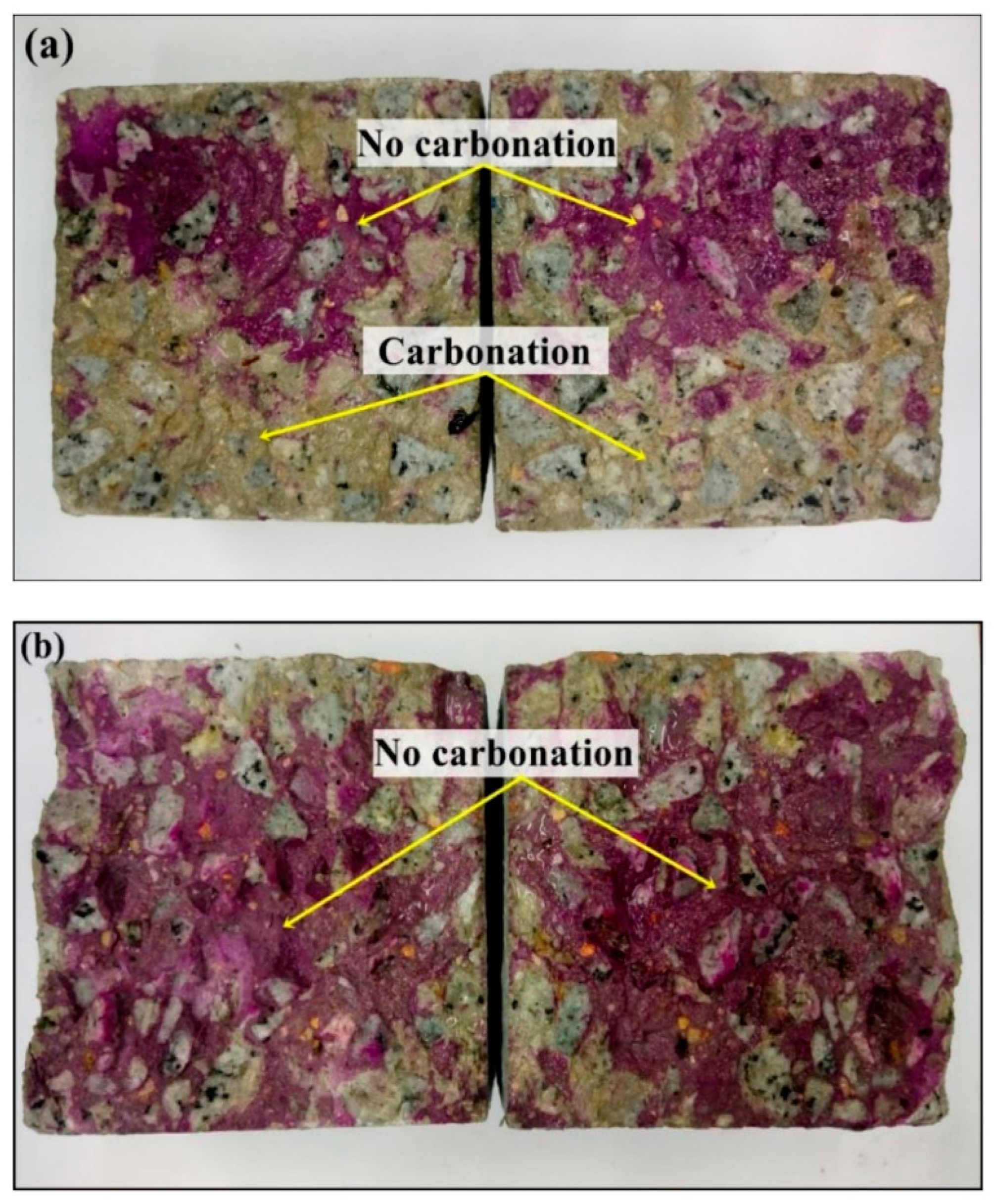
Similarly, Assad et al. [83] researched the ability of gum arabic-NPs (GA-NPs) to prevent corrosion in reinforced concrete after 180 days of exposure to a CO2 atmosphere. Utilizing a variety of accepted methods, the steel reinforcement of concrete was tested both with and without GA-NPs. Figure 12 shows the physical examinations of accelerating carbonated samples following 180 days of CO2 treatment (both with and without the GA-NP inhibitor). When a 3% GA-NP inhibitor was used, it could be observed that the cube concrete sample (cube) had shallower carbonation depths than the reference sample.
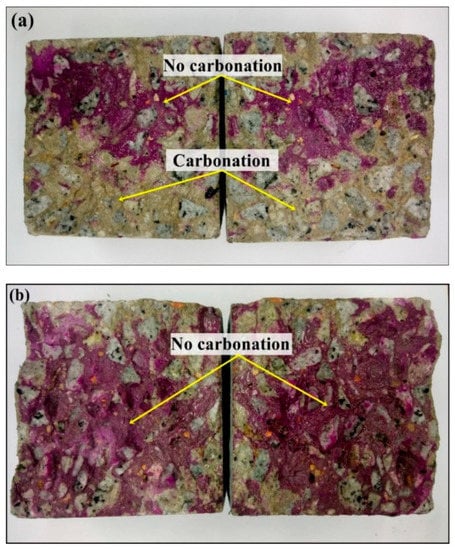
Figure 12.
Expedited carbonation for concrete samples: (a) regulated and (b) GA-NP inhibitor. Reprinted with permission from Ref. [83]. MDPI 2021.
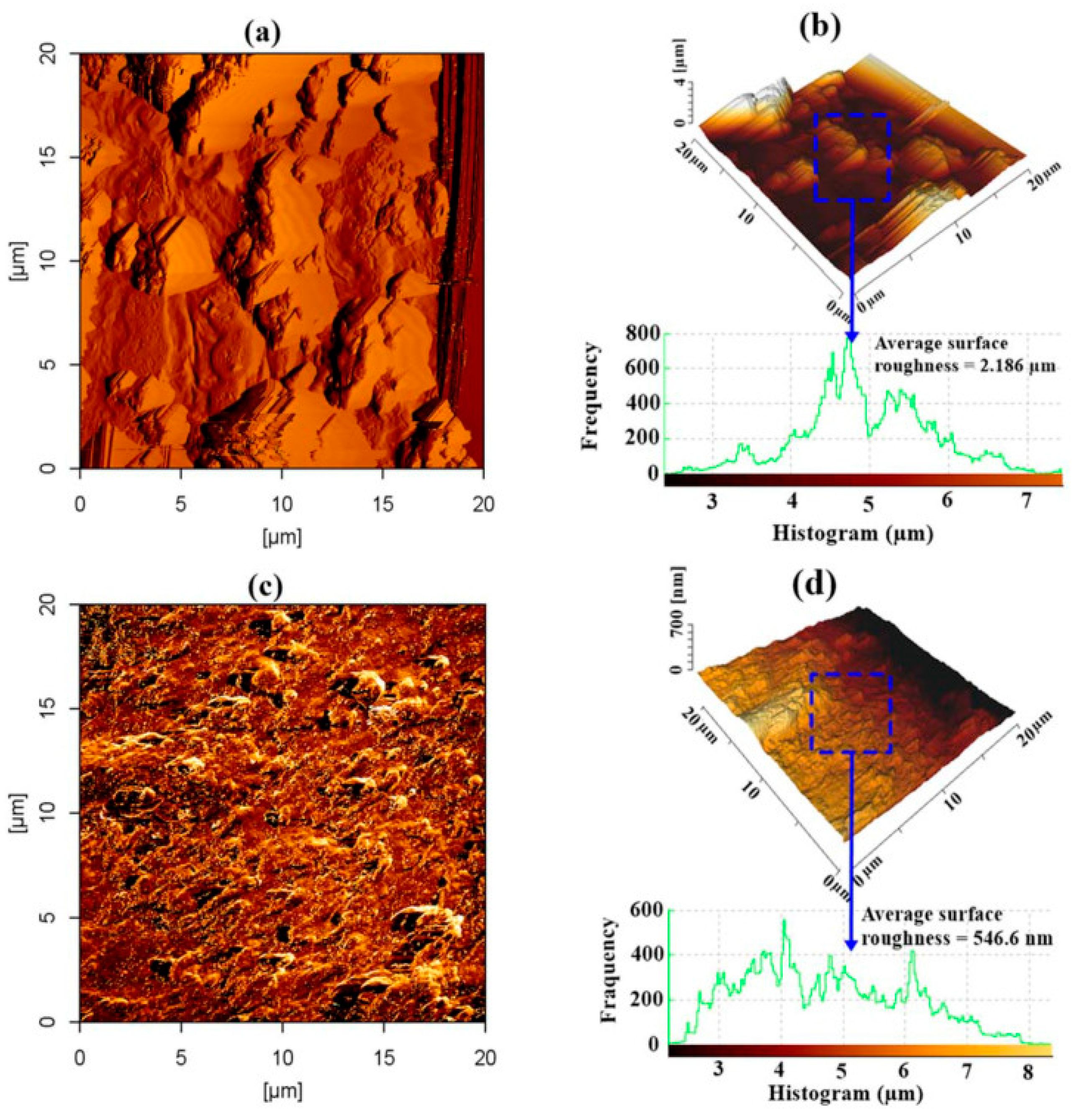
Figure 13 shows the 2D and 3D AFM geometries of the steel reinforcing substrate for treated and untreated concrete using the GA-NP inhibitor. Figure 13a,b shows that the exterior of the reinforced steel was badly eroded and severely damaged, exhibiting a surface roughness of 2.186 m, in the absence of GA-NP inhibitor. The production of reaction products, primarily iron (II) carbonate-FeCO3 and magnetite-Fe3O4, as a result of CO2 penetration could be attributed to the significant average surface imperfection. In contrast, the picture of the reinforced steel surfaces (Figure 13c,d) was greatly improved when GA-NP inhibitor was present, resulting in an increase of 75% over the reference steel reinforcement’s surface roughness of 546.6 nm (without inhibitor). This finding additionally demonstrated that the creation of a thin coating and the sorption of inhibitor prevented CO2 from penetrating the surface of the steel reinforcing.
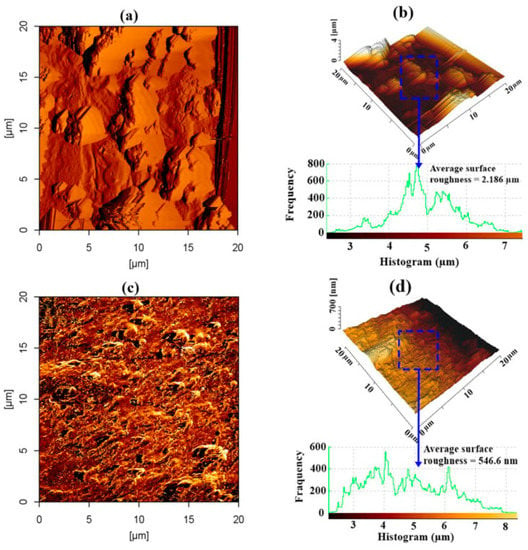
Figure 13.
AFM scans for steel reinforcement substrates subjected to CO2 for a duration of 180 days: (a,b) 2D, 3D scans for regulated and (c,d) 2D, 3D scans for inhibited samples including GA-NPs. Reprinted with permission from Ref. [83]. MDPI 2021.
The outcomes show that adding 3% GA-NP inhibitor into concrete prevented corrosion by causing inhibitor molecules to adhere to the substrate of the metal reinforcement, forming a defensive coating. As a result, it was discovered that the IE% increased up to 94.5% with a reduction in the CR of 0.57 × 10−3 mm/year. Additionally, the findings demonstrate the existence of the GA-NP inhibitor, which caused calcium hydroxide absorption and decreased the Ca/Si to 3.72% and 0.69%, correspondingly. As a result, the pH was raised by 12.5 and 9.27%, correspondingly, and C-S-H gel was created. It could be said that eco-friendly GA-NPs offer the substantial capability to suppress corrosion and enhance the carbonation resilience of the concrete matrices to produce reinforced concrete constructions with long-lasting architecture.
2.3. Metal/Metallic Oxide-Incorporated Nanocoatings
Contemporary human civilization has grown significantly as a result of the use of metals. They are necessary for everything from machinery to architecture to technology to consumables. Regrettably, the majority of naturally available and natively inexpensive metals are rarely appropriate for the prevalent industrial purposes in several fields. Owing to unfavorable operating conditions, they gradually lack vitality or crumble. Another of the biggest problems that cause disastrous device breakdown is still metal corrosion. It takes place in commercial facilities including those for electricity, chemical manufacturing, food manufacturing, and building, among others, presenting environmental and public health risks in addition to a heavy financial strain. For the mitigation of metallic corrosion, some studies used nanocoatings centered on metal or metallic oxide [20,84]. The chemical, physical, and thermal characteristics of a nanocoating are enhanced by the inclusion of metals. The metallic coating is created at the nanoscale, which essentially protects the covered substrate or greatly improves all of the coating’s qualities. Several pristine metals including nickel (Ni), phosphorous (P), cobalt (Co), tungsten (W), copper (Cu), zinc (Zn), and iron (Fe), etc., are used in metallic nanocoating. Utilizing a nanosized covering strengthens this improvement since NMs function differentially than micromaterials. For instance, when the thickness of the Zn covering needs to be enhanced to offer the necessary shielding, a finer nanocoating of Zn resolves both the surface integrity and weldability issues. Nevertheless, pristine Zn corrosion resilience is inadequate in extreme oxidizing circumstances and extreme temperatures, thus, it is alloyed with other metals to increase corrosion resistance. It has been demonstrated that Zn-Ni alloy coverings are harder than Zn and Cd individually. Additionally, due to Cd’s inadequate adhesive wear resilience, Zn-Ni alloys are a top contender to replace it in aeronautical applications.
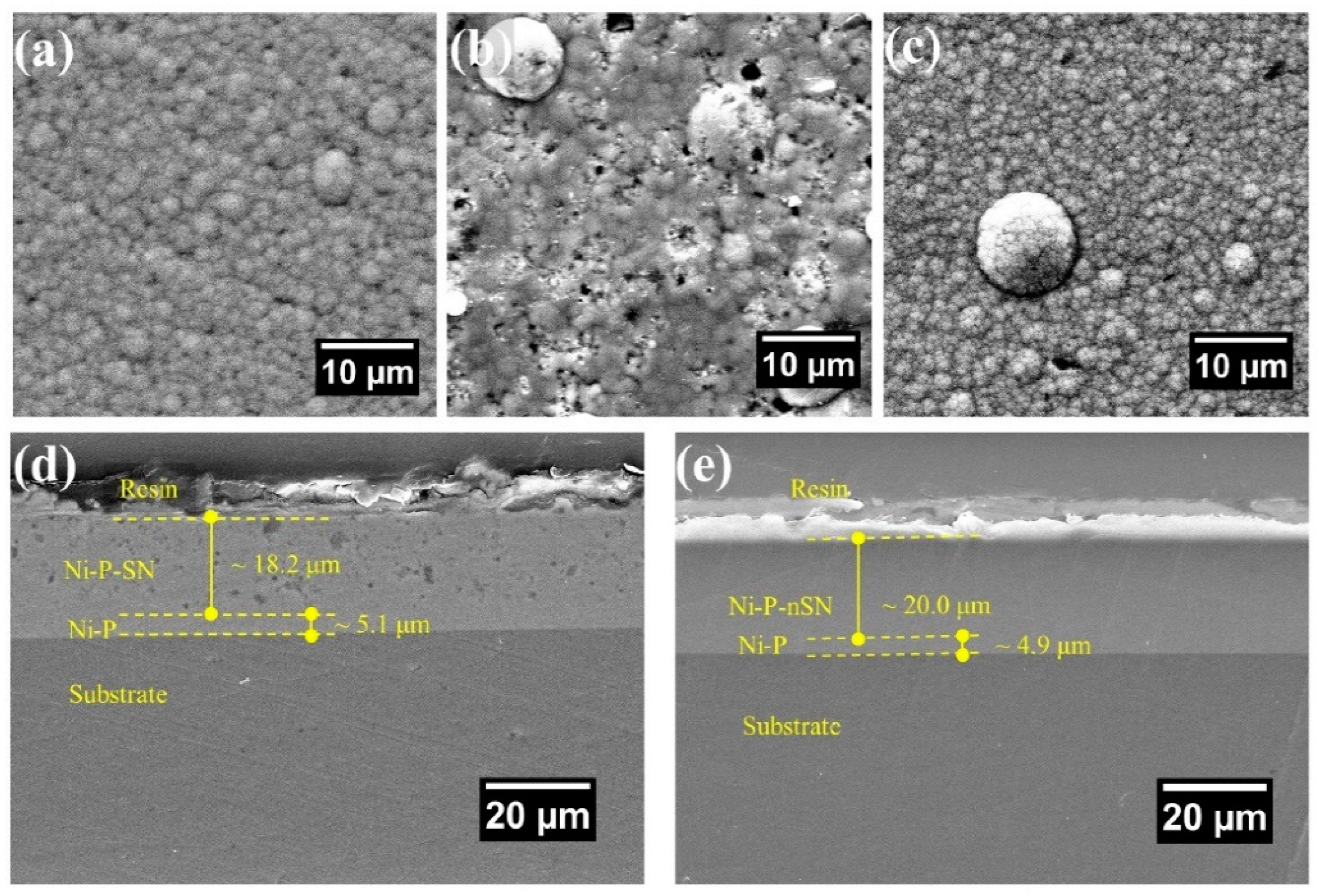
In order to manufacture Ni-P-Si3N4 composites using the electroless coating process, Dhakal et al. [85] included nano Si3N4 (mean diameter ~20 nm) and submicron Si3N4 (mean dimension 200 nm) granules when covering a Ni-P alloy in a low-CS surface. Minimal surface flaws including voids and micropores were present in the composite covering comprised of 20 nm Si3N4 NPs, however, following the addition of 200 nm Si3N4 NPs, these flaws were more noticeable. Figure 14 displays the SEM pictures of the coating’s interface and cross-section. In contrast to the Ni-P-SN, which seems to exhibit a coarser texture with voids and holes (Figure 14a), the Ni-P covering has a distinctively smoother surface shape (Figure 14b). Furthermore, the Ni-P-nSN coating’s uniform texture (Figure 14c) shows no discernible variations from the standard Ni-P covering (Figure 14a). The inherent covering process of the P and Ni atoms in the Ni-P-nSN sample has not been affected by the included nSN particulate, hence the coating surfaces exhibit considerably fewer surface flaws including holes, cavities, and uneven cluster formation.
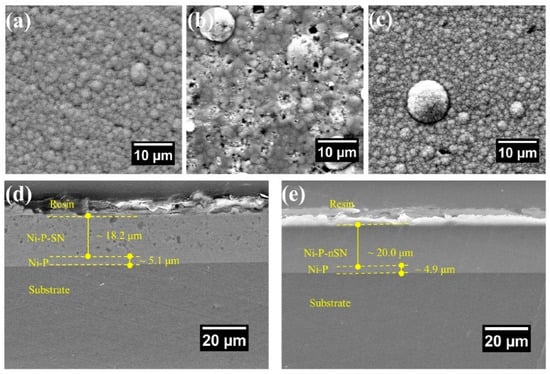
Figure 14.
Surface topologies of (a) Ni-P, (b) Ni-P-SN, (c) Ni-P-nSN composite, (d,e) Ni-P-SN and Ni-P-nSN composed of a coating (~5 µm). Reprinted with permission from Ref. [85]. MDPI (2022).
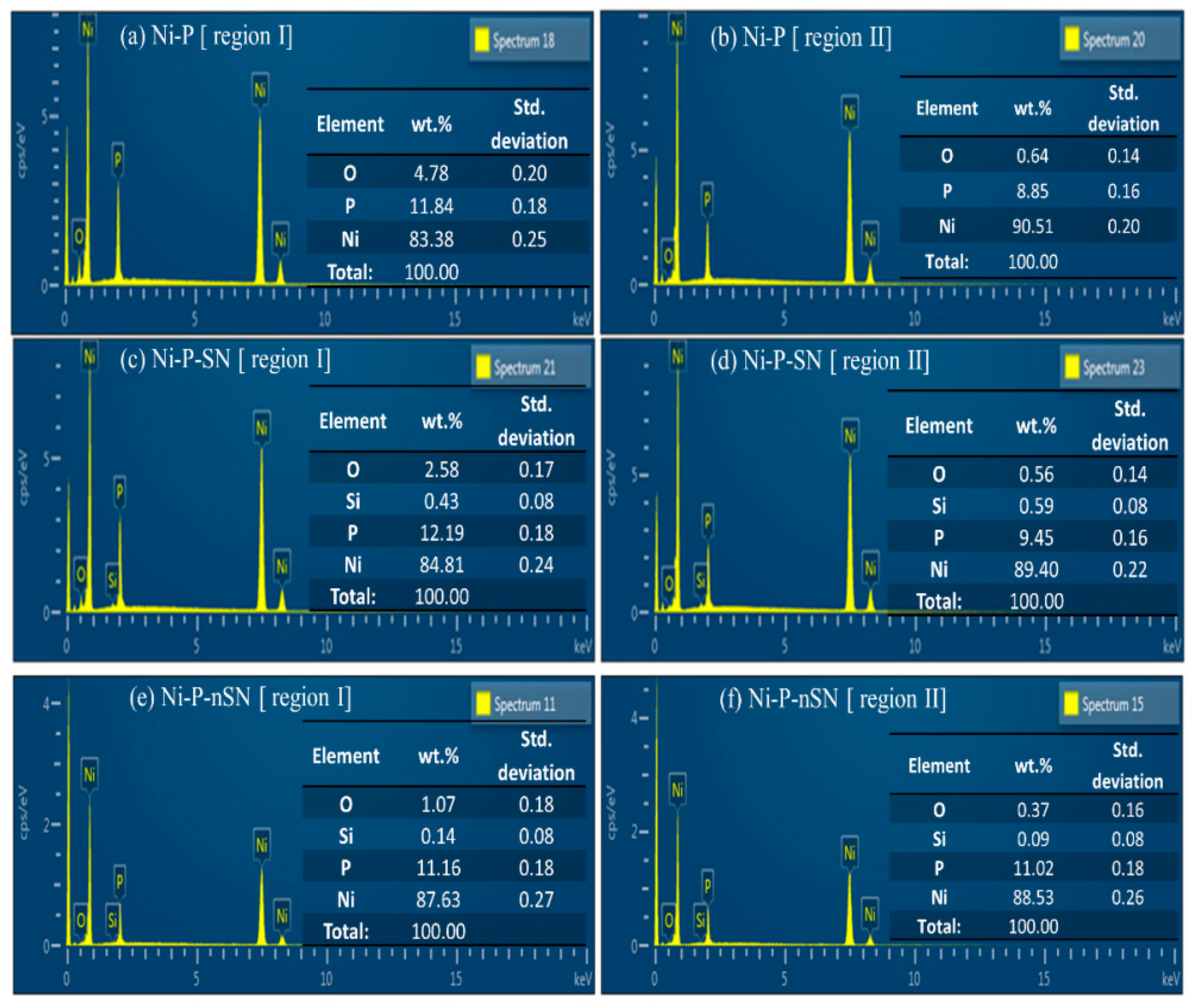
Figure 15 displays the EDS findings of the pristine and eroded substrates denoted by area “I” and zone “II” in all three samples. Ni-P coating’s oxygen level improved from 0.6 wt.% on the uncorroded substrate to 4.8 wt.% (Figure 15a,b). The severe dissolving of Ni, however, was indicated by the Ni content dropping from 90.5 wt.% in the initial substrate to 83.4 wt.% in the damaged area. Moreover, compared to the oxygen level of the Ni-P covering in its deteriorated area, the oxygen level was greatly decreased in the Ni-P-SN deteriorated substratum (Figure 15a) and even substantially reduced in Ni-P-nSN (Figure 15c,e). Figure 15e shows that the Ni content in Ni-P-corroded nSN’s interface (87.6 wt.%) was barely altered from the surface’s initial level (Figure 15f) (88.5 wt%). It implies that, relative to the other samples, Ni-P-nSN has a much lower rate of Ni disintegration. Ni-P coverings could also be classified as high-P coverings due to their mean P-contents of 9 wt.% (Figure 15b) and 11 wt. % (Figure 15d,f), and Ni-P-nSN and Ni-P-SN composite coatings, respectively. As all the circumstances remain similar for regular Ni-P and the composite coverings, the increase in P concentration of about 2 wt.% in both Ni-P-nSN and Ni-P-SN composite coverings has only been seen as a result of particle integration. The increase in P concentration in this research is a sign that the metallic Ni stage in the composite coverings is diminishing while the Ni-P alloy stage is increasing. It is important to note that the passive nature of Ni-P-based coverings is improved with raising P concentration. The composite coatings’ enhanced P concentration is advantageous for their corrosion-resistant characteristics. Furthermore, as both composites have almost similar P contents, the P addition to the corrosion protection capabilities of composite coverings should be identical. As a result, it is possible to consider that the added Si3N4 particles’ larger particle dimension is the only factor contributing to Ni-P-increased nSN’s anti-corrosion performance. The addition of Si3N4 NPs (20 nm) afforded the covering a more stable, defect-free interface morphology and served as excellent CIs for the Ni-P framework.
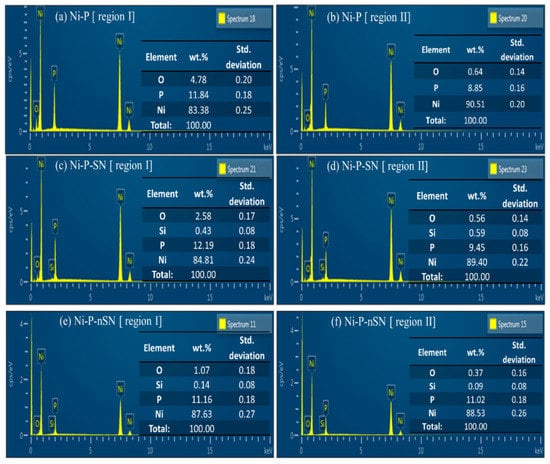
Figure 15.
EDS findings of the deteriorated substrate (region I) and original substrate (region II) of (a,b) Ni-P, (c,d) Ni-P-SN, and (e,f) Ni-P-nSN samples. Reprinted with permission from Ref. [85]. MDPI (2022).
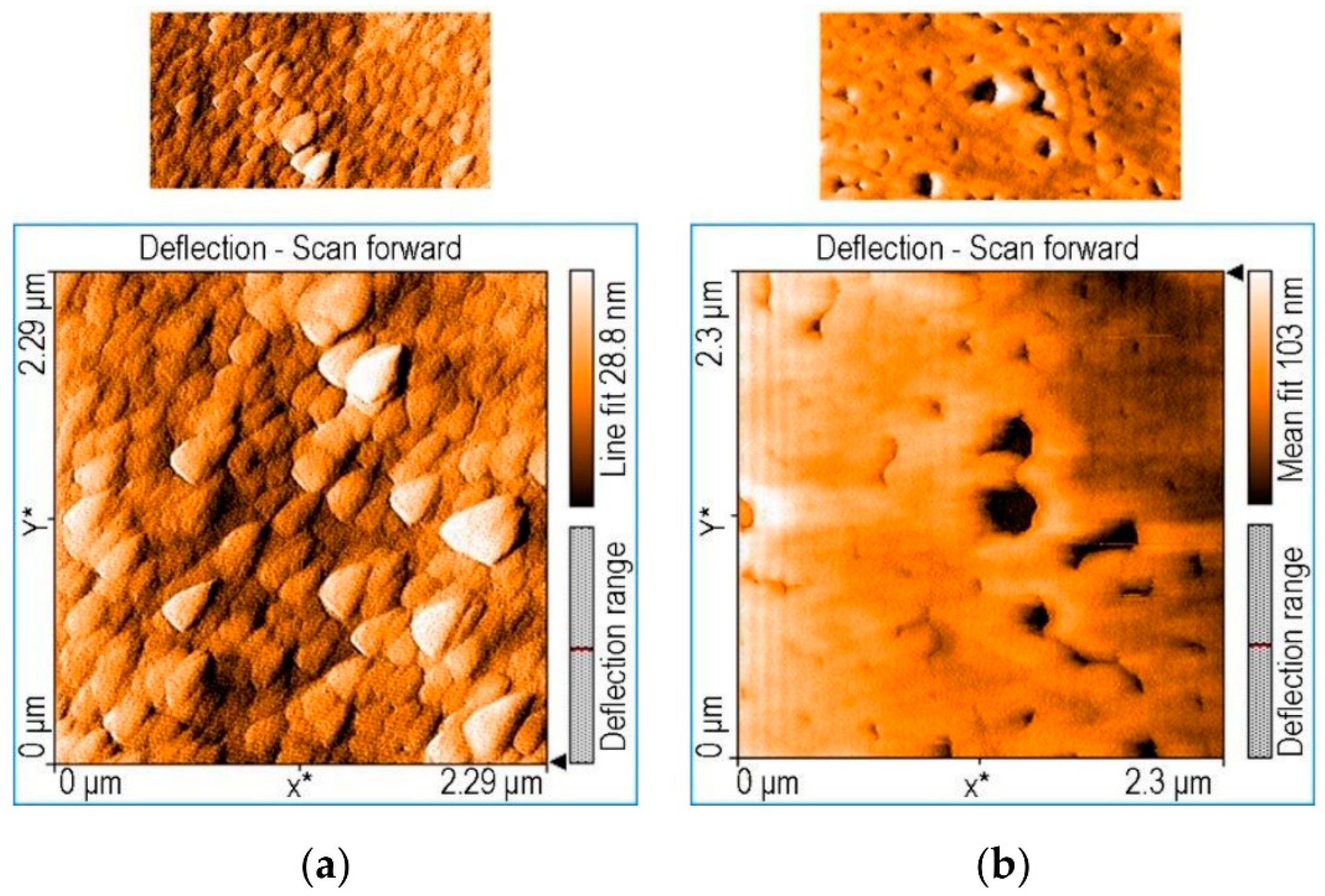
In order to increase the corrosion prevention of steel in corrosive conditions, Birdeanu et al. [86] reported the development of novel sandwich-type composites centered on 5,15-(4-carboxy-phenyl)-10,20-diphenylporphyrin or 5,10-(4-carboxy-phenyl)-15,20-(4-phenoxy-phenyl)-porphyrin and MnTa2O6. Using the drop-casting method, thinner coatings with a sandwich- or single-type configurations were produced on CS. By using electrochemical examinations including PDP and open circuit potential (OCP), the prevention of steel corrosion activity was assessed in corrosive environments of 0.1 M HCl. It was explained how changes in the anodic (βa) and cathodic (βc) Tafel curves might affect CR. The steel electrode coated using sandwich-type layers of 5,15-(4-carboxy-phenyl)-10,20-diphenylporphyrin on the bottom surface and MnTa2O6 on the upper achieved the highest corrosion prevention efficacy of 91.76%. The two bis-carboxyl-substituted porphyrins’ dense and adhesive coatings were hypothesized to provide a physical protective function that underlies how the inhibitors prevent the oxidation of CS. These create massive, chemically acid-resistant supramolecular frameworks (see Figure 16), preventing the acid from coming into direct contact with the steel. In the instance of trans-porphyrin (Figure 16a), as opposed to the cis-derivative scenario, the dense coatings of quadrilateral construction blocks accumulated both in J-type and H-type operations are more prominent, which results in the physical barrier impact (Figure 16b). Figure 16b shows how the cis-porphyrin is helicoidally accumulated over small rings that link collectively while providing scope for big holes and tiny apertures, which could serve as sites of interaction between the acid and metal and the commencement of pitting corrosion.
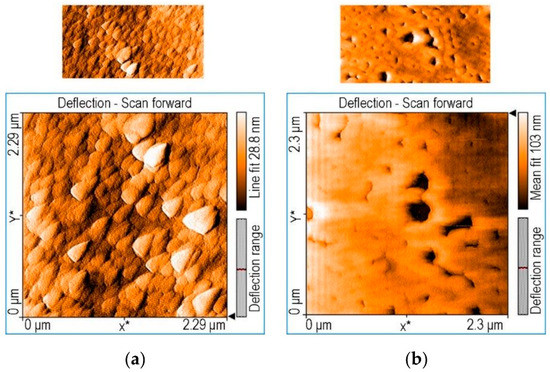
Figure 16.
AFM scans illustrating significant surface coverage shown by trans 5,15-(4-carboxy-phenyl)-10,20- diphenylporphyrin (a) in contrast to cis configuration, 5,10-(4-carboxy-phenyl)-15,20-(4-phenoxy- phenyl)-porphyrin (b). Reprinted with permission from Ref. [86]. MDPI 2021.
As channels for innate antioxidants, including phenolic, anthocyanins, flavonols, and pectin compounds, Khadar et al. [53] utilized extracts of dried flower petals, like lotus and rose petals, to create nano-manganese oxide using an economic and environmental process called ultrasonic wave-aided green formulation. The produced nano-manganese oxides were employed to improve MS (MS-corrosion-inhibitory)’s behavior in an acidic medium (1 M HCl). Through the use of TGA and FTIR analysis, functional groups and thermodynamic properties of the produced metal oxides were examined. By using BET surface area measurement, it was discovered that the samples made from the RP and LP extracts, accordingly, had specific surface dimensions of 27.914 and 39.438 m2g−1. Excellent electrochemical characteristics were seen for LPM, and in 1M HCl medium, RPM and LPM had greater corrosion prevention efficiencies (51.50% and 72.63%, respectively) than the bare MS plate.
Using a one-step electrospinning approach, Radwan et al. [87] created Polystyrene-nickel oxide (PS-NiO) superhydrophobic nanocomposite coverings on Al substrates. The polystyrene coating’s hydrophilicity, thermal properties, and surface roughness are significantly impacted by the introduction of NiO NPs. The produced coating’s geometry was assessed, and the results showed that a beaded-fiber architecture had formed. The micro/nanoscale pattern of the bead fibers combined with the coating’s already-improving surface ruggedness led to an improvement in WCA and a decrease in CAH. PSN-2 obtained the greatest corrosion protection in saltwater by recording the maximum WCA of 155° ± 2 and the minimum WCAH of 5° ± 3. However, raising the proportion of NiO over that point lowers the coating’s ability to defend against corrosion because NiO tends to aggregate on the coating substrate and because the hydrophilic characteristic of the metal oxides increases the hydrophilic nature. Similarly, AlTp, a MOF composed of aluminum terephthalate, and its two nanocomposites comprising iron oxide and graphene oxide (GO) were created by Mirzayi et al. [61]. The AM60B Mg alloy was subsequently shielded against corrosion by the produced nanocomposites in a 30% ethylene glycol medium including 0.5 M NaCl. The inhibitory properties of the constituents in the dosage region of 50–400 ppm were examined using the PDP and EIS. For AlTp/Fe2O3, AlTp, and AlTp/GO, correspondingly, the IE% determined from EIS data were 86.52%, 90.2%, and 90.8%. PDP demonstrated this pattern to be valid. The outcomes show that adding NPs to the AlTp architecture enhanced its ability to suppress.
Preethi et al. [88] created convenient, affordable, and environmentally acceptable silver NPs (AgNPs) using the edible native Macrolepiota mushroom. The characterized studies supported AgNPs of 20–50 nm in size with a spherical morphology. Corrosive bacteria Terribacillus aidingensis EN3, Bacillus thuringiensis EN2, and Bacillus oleronius EN9 were used to investigate the biocorrosion effectiveness of myco-synthesized AgNPs and the mushroom extract towards MS. In several exploratory conditions, the effectiveness of MS’s corrosion inhibition was assessed using weight reduction evaluation, EIS, and surface assessment. In both systems, II and IV, lower CR (0.07 mm/y, 0.14 mm/y), lower weight reduction (0.006 ± 2, 0.011 ± 2), and higher corrosion inhibition efficacy (59%, 18%) were found. In both surface characterization experiments (XRD and FTIR), peak strength decreased when mushroom extract and AgNPs were present. According to EIS tests, mushroom extract and AgNPs operate as a corrosion-inhibiting eco-friendly substance that adheres to MS substrates in cooling H2O stack systems, preventing corrosion.
2.4. Carbon Allotrope-Based Nanocoatings
Owing to their inexpensive, non-hazardous, readily accessible key ingredients, and simple manufacturing processes, carbon allotrope-based nanocoatings have attracted significant attention in the past ten years [70,89]. They are used in pharmacology, detectors, optoelectronics, catalyst supports, and cell imaging. Currently, carbon dots (CDs) are used as sustainable CIs and have shown encouraging outcomes. For instance, dopamine was used by Cui et al. [90] to create unique nitrogen-doped carbon dots (NCDs), which were then tested in a 1 M HCl medium as CIs for Q235 CS. The corrosion behavior of CS in 1 M HCl medium was then investigated using the EIS methodology in both the exclusion and inclusion of NCDs. Figure 17a shows the Nyquist graphs, which are constituted of a depressed and inductive capacitive curve. The semi-diameter of the capacitive curve grows as the intensity of NCDs rises, showing that the inclusion of NCDs successfully hinders the charge transfer operation at the steel-solution functionality. The finding that all Nyquist graphs exhibit the identical depressed form indicates that NCDs do not affect the steel corrosion in HCl media. Instead, this depressed curve is mostly due to corrosion-induced non-uniformity and deposited molecules over the metallic substrate. The impedance modulus ratio at the minimum frequency (10 Hz) in the addition of NCDs is larger than those in blank media in the context of the Bode graphs in Figure 17b, and it increases with rising NCD contents. The maximal impedance modulus measurement at 400 ppm NCDs is 295 Ωcm2, which is 10 times more than the value at zero concentration. Additionally, while NCDs are introduced to the HCl medium, the phase angle peak increases in size and becomes more pronounced as the quantity of NCDs increases. These results demonstrate substantial NCD binding over the steel substrate and enhanced NCD concentration-dependent corrosion inhibition.
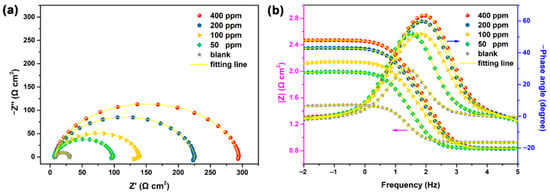
Figure 17.
EIS findings of Q235 CS in 1 M HCl medium in the exclusion and inclusion of various dosages of NCDs: (a) Nyquist graph and (b) Bode graph. Reprinted with permission from Ref. [90]. MDPI (2021).
The surface topography and hardness of the specimens were also examined using LSCM and SEM following 60 h of exposure. The steel substrate was pristine before it was exposed to the corrosive substance, and it only had sanded marks (Figure 18a). The steel had a surface irregularity of around 0.27 µm (Figure 18b). The steel substrate had been exposed to a 1 M HCl medium for 60 h when it was significantly rusted, turned weak, and then became permeable (Figure 18c). When contrasted to the corrosion-free steel, the substrate roughness of the steel significantly improved to 5.18 µm (Figure 18d). In contrast, the deterioration of the steel substrate was greatly reduced with the introduction of 400 ppm NCDs, but the refined flaws remained discernible (Figure 18e). The corrosion-damaged steel’s surface hardness was just about 1.0 µm at this time (Figure 18f). These findings show that the adsorbed NCDs successfully prevent acid steel corrosion.
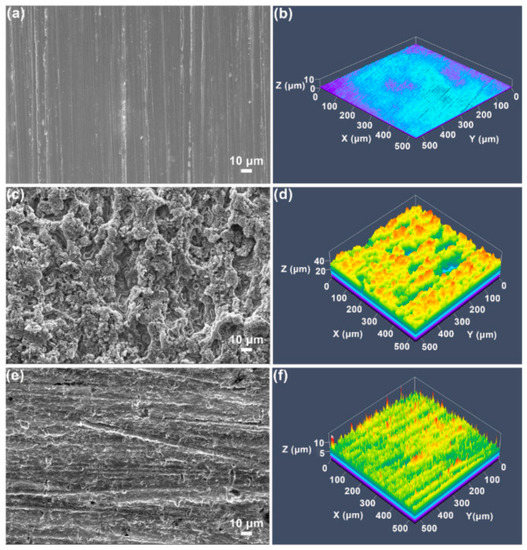
Figure 18.
LSCM and SEM scans of Q235 CS substrate following 60 h of subjection to 1 M HCl medium with the exclusion and inclusion of 400 ppm NCDs, (a,b) refined steel, (c,d) in blank media, (e,f) with the inclusion of 400 ppm NCDs. Reprinted with permission from Ref. [90]. MDPI (2021).
By attaching HMS to the GO substrate, Yan et al. [91] created novel dual-functional GO-based (GAS) NMs. The efficacy of GAS-passive covering’s corrosion prevention was improved by GAS NMs’ superior dispersal and solubility with epoxy resin. Following 40 days of exposure, |Z|0.01Hz of GAS-Coat persisted at 890 ΩM cm2, and Fb barely rose to 0.48. Furthermore, GAS-Coat only offered passive protection against corrosion while the covering had been degraded. It is significant to observe that by injecting and discharging the CIs’ MBT, GAS NMs offered extra proactive corrosion prevention for epoxy coating. In affected regions, GAS-Coat had accomplished self-healing and continued to have higher corrosion resistance over prolonged exposure. GAS-Coat offers a significant deal of possibility for useful anticorrosion operations because it performs both active and passive corrosive protection.
In order to create a proactive (inhibitive)/passive (barrier) anti-corrosion covering solution, Keshmiri et al. [62] created a sustainable MOF centered on cerium (Ce-MOF) on GO nanosheets (GO@Ce-MOF). The 1,3,5-benzene tricarboxylic acid used in the construction of the Ce-MOF NPs produced a dense configuration resembling a straw sheaf. The Ce-MOF NPs were then housed in the GO as a nanocontainer, and the resulting NPs were then introduced to the epoxy matrices. Raman spectroscopy and transmission electron microscopy (TEM) were used to validate the effective production of NPs. TGA and BET techniques were used to measure the NPs’ surface region and thermal behaviors. Utilizing the EIS approach and a salt spray experiment, the produced nanostructures’ passive and active anti-corrosion abilities were examined in epoxy matrices. GO@Ce-MOF/corrosion EP’s resistance was improved by about 500% over Blank/EP according to the EIS data from the damaged coverings after 48 h. Additionally, following 7 weeks of exposure to corrosive solutions, the GO@Ce-MOF/EP covering demonstrated better protective qualities, exhibiting low-frequency coefficients greater than 1010 Ωcm2. Similarly, unique N and S co-doped CDs (N, S-CD) with a significant S and N concentration of 19% and 17%, correspondingly, were created by Zhang et al. [92]. The Tafel data demonstrate that N, S-CDs are a highly effective mixed inhibitor, and the g ratio rises as N, S-CD levels increased. Since the polarization assessment and the EIS findings are in agreement, it could be concluded that a persistent N, S-CD adsorption coating could be created on the copper (Cu) substrate to substantially prevent Cu corrosion. The anti-corrosion capabilities of N, S-CDs were investigated in relation to temperature using the polarization curve. Though the corrosion procedure was accelerated by the temperature increase, Cu in a 0.5 M H2SO4 medium at 298–318 K was well protected by N, S-CDs. The novel NMs exhibit outstanding efficiency for Cu (99.88%) at a dose of 50 mg in 0.5 M H2SO4 medium; this research may offer a fresh perspective on the exploration of highly potent CIs for metal preservation that is also environmentally acceptable.
3. Effect of Grain Size and Corrosion Inhibition Mechanism
Noticeably, innate micro-architectural permeability, which exhibits weakness in impeding the permeation of superfluous entities deleterious to a particular surface, typically inhibits the effective utilization of nanocoatings for inhibiting or reducing corrosion, fouling, and scratching from self-healing functionality. The efficacy of nanocoatings against corrosion could be considerably impacted by grain size. It has been shown in numerous trials that the corrosion resistance of nanocoatings with greater grain size showed superior corrosion resistance when the grain size of the NPs was increased while annealing at greater temperatures. This trend might be a result of twins forming after annealing. It was frequently noted that when grain size grew, the density of the twins also grew. Low free energy is a characteristic of twin boundaries that increases resistance to corrosion. Therefore, although the annealed coatings exhibited a higher grain size, improved twin density after annealing improved the uniformity of the passive coating and decreased the sensitivity of the annealed coverings to pitting corrosion. The corrosion investigations of nanocrystalline nickel-tungsten (Ni-W) nanocoatings have also demonstrated that the corrosion rate enhances with grain size in an acidic medium (pH 3), while the corrosion rate reduces with an increment in grain size in an alkaline medium (pH 10) [93,94]. This indicates that the pH affects the nanocoatings’ corrosion behavior in addition to their grain size. The development of passive oxide coating, which relies on W concentration, and the active sites accessible for corrosion, which rely on grain boundary volume, were discovered to be two conflicting elements that affect how these nanocoatings (grain size: 5 to 63 nm) behave in media of varying pH levels. Therefore, the emergence of corrosion in an alkaline medium, where corrosion intensified with the reduction in grain size, was attributed to the increasing grain boundary volume. On the other hand, in an acidic saline condition, the rate of corrosion reduced as the grain size increased. In this instance, W concentration was the dominant factor in corrosion, and coatings with greater W concentrations demonstrated better corrosion resistance as a result of robust oxide layer development. As a result, nanocoatings demonstrated greater corrosion resistance in an alkaline medium than in an acidic medium. Furthermore, the NPs’ smaller size is more favorable since it allows them to enter extremely tiny pores, indentation patterns, and capillary regions both in the coating matrices and at the metal substrate.
The superior corrosion-mitigation tendency of nanocoatings has been centered on the high surface-to-volume ratio and light characteristics as a result of particle size as a major advancement in nanotechnology [67,95,96,97]. All metallic substrates, either electroplated or precoated, could be covered with nanocoatings using a variety of covering techniques, which might shield the metallic surface against corrosion impacts for multiple decades. Often, the coverings are translucent, and they also improve the surface’s aesthetic effect. The most crucial prerequisite for a potential adsorbent is, indeed, a broad specific surface region regarded as adsorption, which is a phenomenon that involves the accumulation of corrosion inhibitor molecules on an adsorbent’s surface. The permeability of the adsorbents defines their adsorption capabilities, whether adsorption is driven by long-range linkages (physisorption) or by chemical interactions, including ionic or covalent bonds (chemisorption). Since physical adsorption favors porosity with a size similar to the adsorbent’s molecular radius, substances such as nanomaterials that are frequently characterized by small mesopores and micropores are frequently regarded as effective adsorbents. The most common means of immobilization is physical adsorption which is easily initiated at mild temperatures. Considering a multilayer adsorbate over an adsorbent, physical adsorption has been shown to possess a minimal enthalpy of adsorption (<−40 kJ/mol), whereas chemical adsorption (chemisorption) entails a chemical reaction involving the adsorbate and adsorbent and typically results in the emergence of chemical bonds. It also has a greater enthalpy of adsorption, ranging from 80 to 240 kJ/mol [98,99]. To further explain the inhibition process of the nano-modified coatings, some researchers have documented the adsorption behavior of NP-based corrosion inhibitors or coatings. For instance, in an experiment to increase the effectiveness of inhibition for an St37 steel substrate, a proposed nano-modified coating created by inserting silver nanoparticles (AgNPs) formed in situ through reduction of AgNO3 utilizing organic honey in carboxymethyl cellulose (CMC) matrices was used by Solomon et al. [100]. When the system temperature was raised to 40, 50, and 60 °C, correspondingly, the IE% declined to 61.0%, 60.9%, and 60.8%. CMC molecules were physically adsorbed over the metallic substrate in the acid medium, according to the fluctuation of ղ with ΔGads0 value and temperature. Similarly, in order to explain the corrosion-inhibition mechanism of nanocoating, Nawaz et al. [35] created polymeric nanocomposite coatings that were altered to incorporate CIs. The CIs (imidazole)-loaded yttrium oxide NPs employed in this study were added to the epoxy composition before being deposited on the substrate material. According to EIS, the infused material (Y2O3/IMD) appeared to be pH-responsive, a characteristic that aids in the liberation of the inhibitor from the particulates and enhances corrosion mitigation. After the electrolyte first contacts metal, corrosion action caused a localized pH variation. Because the hydrolysis of Fe ions facilitated the CIs’ escape, it was anticipated that the pH at the damaged region would approach acidic levels in the anodic zones. As per the conceptual layout shown in Figure 19, the inhibitory impact reported for the Y2O3/IMD-modified covering was caused by the adsorption capability of imidazole over the surface of the steel.
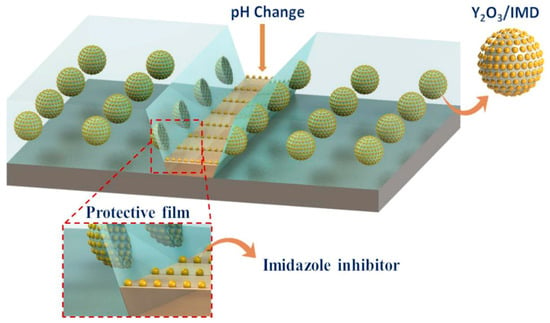
Figure 19.
Illustration displaying the inhibition mechanism of Y2O3/IMD-modified covering over the surface of the steel. Reprinted with permission from Ref. [35]. MDPI (2021).
For the generation of a preventative coating featuring enhanced corrosion protection and self-healing capabilities, Sazou et al. [101] studied conducting polyaniline (PANI)-based paint formulations. The regulated inhibitor discharge process suggested that corrosion-inhibitory dopant ions were discharged via the breakdown of PANI as a result of metal electrolysis at tiny holes or an ion-exchange mechanism. The dopant anions that were discharged created a second physical shield that prevented oxygen reduction or the transit of aggressive anions. These PANI-based coatings are often known as “smart coatings” because the dopant anion is discharged when needed, or more specifically when the coating is degraded and results in metallic corrosion and, therefore, a reduction in PANI. Along with the production of oxide, complexes comprising the metallic ions were generated on the metallic substrate, forming extensive anodic shielding. Furthermore, in these circumstances, PANI discharged the dopant anion (DBSA), which developed complexes with Fe and resulted in the passivation of steel. The lowering of PANI, which indicated its physical absorption over the metallic substrate, caused the discharge of inhibitory dopants and superior corrosion mitigation.
Moreover, the effects of incorporating various loading proportions of TiO2-NPs within pristine epoxy resin were examined as an aspect of preparation of epoxy-TiO2 nanocomposite coatings using the solvent intercalation technique with the use of sonication [102]. In this experiment, the epoxy polymeric resin was altered with polydimethylsiloxane (PDMS), and distinct stacking ratios of TiO2-NPs were presented to the altered polymer matrices to generate PDMS-reconfigured epoxy nanocomposite coating structures. These coating platforms were created to further enhance the corrosion-resistant effectiveness and to accomplish the hydrophobicity of the formed coated substrates. Only a mild flexion of the Bode graphs at the minimal frequency area and one semicircle in the Nyquist graphs indicated that the integration of distinct loading rates of TiO2-NPs within the epoxy polymer metrics had considerably improved the barrier characteristics of the nano-modified coatings. These results may be explained by the significant contribution of the integrated TiO2-NPs in varying the framework of the epoxy polymer matrices. The integrated NPs helped to reorganize the polymer metrics through physical interactions caused by Van Der Waal’s force, which enables closure of the pore spaces of the epoxy metrics and influences the infiltrated electrolyte to transit a greater length in an attempt to advance the surface layer sorption and result in the effective corrosion inhibition. To create active (inhibitive)/passive (barrier) anti-corrosion coating systems, Keshmiri et al. [62] created a sustainable MOF centered on cerium (Ce-MOF) on GO nanosheets (GO@Ce-MOF) in an aquatic medium. The 1,3,5-benzene tricarboxylic acid used in the fabrication of the Ce-MOF NPs produced a dense shape resembling a straw sheaf. The generated NPs were then incorporated into the epoxy matrices using the GO as a host for the Ce-MOF NPs in a nanocontainer. They concentrated on one of the most crucial aspects of employing nano-modified coatings to shield metals from corrosion: their adhesion strength following a mechanical flaw. The formation of OH− ions beneath the coating causes coating separation and adhesion reduction. Two events at the metal/coating substrate interface account for this: (1) a deterioration of interfacial adhesive bonds caused by the production of hydroxyl ions, and (2) an increase in pH. In this investigation, a cathodic disbonding analysis was used to explore the coatings’ wet adhesion intensity. The Blank/EP specimen was given the largest delamination diameter (11.39 mm), followed by GO/EP (9.69 mm), Ce-MOF/EP (6.6 mm), and GO@Ce-MOF/EP (5.88 mm). The outcomes demonstrate that Ce-MOF NPs considerably increased Ce-MOF/EP’s adherence over the metallic surface. The 1,3,5-BTC configuration had a significant number of electron donors, which helps strengthen the bonds between the metal and the coating. The local pH decreased as a result of a reaction between hydroxyl ions and cerium cations that resulted in the formation of insoluble cerium oxides/hydroxides at the cathodic regions. When GO@Ce-MOF/EP was contrasted to the Ce-MOF/EP composite, covering delamination occurred less frequently throughout the experimental period of 24 h due to the regulated discharge of Ce-MOF NPs. Another factor contributing to the GO@Ce-MOF nanoparticles’ remarkable barrier characteristics was their active involvement in fixing the micro flaws in the epoxy coating. These NPs might make it easier for corrosive substances to enter the coating/metal interface.
Furthermore, in order to generate corrosion inhibitors for the AM60B Mg alloy in a 30% ethylene glycol medium comprising 0.5 M NaCl, Mirzayi et al. [61] created an aluminum terephthalate (AlTp) MOF and its two nanocomposites comprising GO (AlTp/GO) and iron oxide (AlTp/Fe2O3). For the AlTp, AlTp/GO, and AlTp/Fe2O3, the values of ∆Gads0 were 26.92, 26.85, and 27.83 kJ mol−1, correspondingly. In this procedure, inhibitory molecules were adsorbed over the metallic substrate by electrostatic interaction. Contrastingly, ∆Gads0 values found here range within the two magnitudes mentioned, indicating that physisorption and chemisorption were two forms of interaction involved in the adsorption process of the inhibitors upon the AM60B substrate.
4. Challenges and Future Outlooks
This article provides a thorough analysis of the fabrication of nano-modified coatings (self-healing-based nanocoatings, natural source-derived nanocoatings, metal/metallic oxide-incorporated nanocoatings, and carbon allotrope-based nanocoatings), as well as their performance in terms of corrosion mitigation. The practical difficulties in the emergence and fabrication of nanostructured coatings for preferred corrosion inhibition effectiveness include: (i) choosing the precise nanocoating substance that is particularly suited for a given substrate and operational surroundings (e.g., the flexibility of accumulation, which becomes quite difficult for a substrate with complicated geometries and variability in volume, adhesion on the substratum, etc.); (ii) choosing the proper fill/reinforcement; (iii) chemical impediment in the manufacture of nanocoatings; (iv) ability to be applied upon wide surface region; and (v) protracted effectiveness.
In regard to improving the mechanical characteristics that might slow the dispersion of cracks in the covering, numerous underpinning pathways for interface reinforcement, including the engagement between NPs and the matrix, must be improved. This might lead to the creation of unique microstructures (for example, a refined scale lamellar framework). The weak tribological and mechanical capabilities of certain coating matrices, such as their low resilience to interface erosion and wear, might be effectively accounted for by NPs in this approach. Additionally, since the maximal packing capacity of NPs with a greater surface region is constrained, homogeneous dissemination of NPs in the matrix is essential to achieving superior mechanical characteristics (such as ductility and strength) of nanocomposite coverings. Moreover, competent preparatory and treatment techniques are required to produce excellent particle dispersion. Additionally, using methods like molecular assembly or atomic layer deposition (ALD), the interface between the NPs and the matrices could be accurately altered at the atomic or molecular scale to produce certain intriguing shapes, including core–shell hybrid NPs. Considering this, more research is still required to discover the mechanical characteristics of additional types of NPs using improved characterization approaches and established NP manufacturing processes. It is also important to provide statistical assessments of the mechanical characteristics of NPs in regard to size dependence and material influences, etc. Furthermore, it is crucial to have a greater understanding of the functions represented by NPs in the context of each application. It might, therefore, be highly beneficial to have direct visualizations of the interfacial behavior of NPs in operations at the nano, micro, and even atomic levels.
5. Conclusions
In this review, several nano-modified coatings including self-healing-based nanocoatings, natural source-derived nanocoatings, metal/metallic oxide-incorporated nanocoatings, and carbon allotrope-based nanocoatings have been discussed in terms of their successive corrosion mitigation. The attained results show that nano-modified coatings possess superior corrosion protection for metals as compared to conventional coatings due to the lack of holes and flaws in the covering and the creation of a shield against the infiltration of corrosive electrolytes. Owing to the tiny granular dimensions of nanocrystalline formations, which offer superior void occupancy and greater coating surface stability, they are preferable to microcrystalline frameworks for enhancing corrosion resistance. Among all these coatings, self-healing nanocoatings have shown better corrosion mitigation properties with the bonus of a self-healing nature. The mechanized nanocontainers involved in this coating can exhibit a pH-dependent liberation of the inhibitor from the specific core in a corrosive solution and could be readily produced through a straightforward two-step sorption procedure. Additionally, numerous researchers presume the self-healing nanocoatings could open the path to the thrilling development of an inhibitor-loaded nano reservoir as well as its comprehensive implementation for the preparedness of smart corrosion protection coating materials in the sector of corrosion science due to the reduced expense and ease of development of inhibitor-loaded and pH-responsive-based smart nano containers.
Additionally, research has thus far concentrated on the configuration of inhibitor stacking and coating preparation mechanisms with varying degrees of intricacy, such as the physical adsorption of the active agents in the shells and the physical packing of the pores with distinct chemical species, in order to advance the self-healing processes. These processes must be appropriately developed for the novel class of active agents, particularly eco-benign and bio-based agents with incredibly outstanding characteristics in comparison to existing conventional products utilized in various sectors, such as automobiles, construction, airspace, etc., which could be facilitated by adhering to the outlined research dynamics and viewpoints or establishing new groundbreaking strategies to utilize the extremely intriguing characteristics of nano-modified coatings. This will help to safeguard our history, culture, and future.
Author Contributions
Conceptualization, A.T.; methodology, A.T.; validation, A.K. and S.K.; investigation, A.K. and S.K.; data curation, A.K.; writing—original draft preparation, A.K.; funding acquisition, S.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maji, A.K.; Saha, G.; Das, S.; Basu, S.; Tavares, J.M.R. Lecture Notes in Networks and Systems 170, Proceedings of the International Conference on Computing and Communication Systems, Shillong, India, 28–30 April 2020; Springer: Singapore, 2021; Volume 203, ISBN 9789813340831. [Google Scholar]
- Abdeen, D.H.; El Hachach, M.; Koc, M.; Atieh, M.A. A Review on the Corrosion Behaviour of Nanocoatings on Metallic Substrates. Materials 2019, 12, 210. [Google Scholar] [CrossRef] [PubMed]
- Bahaskaran, R.; Palaniswamy, N.; Rengaswamy, N.S. A Review of Differing Approaches Used to Estimate the Cost of Corrosion, Anti-Corrosion Method and Materials. J. Appl. Sci. Res. 2013, 52, 29–41. [Google Scholar]
- Raghavendra, N. Expired Lorazepam Drug: A Medicinal Compound as Green Corrosion Inhibitor for Mild Steel in Hydrochloric Acid System. Chem. Afr. 2019, 2, 463–470. [Google Scholar] [CrossRef]
- Khan, S.A.; Shahid, S.; Hanif, S.; Almoallim, H.S.; Alharbi, S.A.; Sellami, H. Green Synthesis of Chromium Oxide Nanoparticles for Antibacterial, Antioxidant Anticancer, and Biocompatibility Activities. Int. J. Mol. Sci. 2021, 22, 502. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Fan, B.; Zhou, T.; Hao, H.; Yang, B.; Sun, H. Molecular Assembly between Weak Crosslinking Cyclodextrin Polymer and Trans-Cinnamaldehyde for Corrosion Inhibition towards Mild Steel in 3.5% NaCl Solution: Experimental and Theoretical Studies. Polymers 2019, 11, 635. [Google Scholar] [CrossRef]
- Gamal, H.A.; El-Feky, M.S.; Alharbi, Y.R.; Abadel, A.A.; Kohail, M. Enhancement of the Concrete Durability with Hybrid Nano Materials. Sustainability 2021, 13, 1373. [Google Scholar] [CrossRef]
- Toghan, A.; Gouda, M.; Shalabi, K.; El-Lateef, H.M.A. Preparation, Characterization, and Evaluation of Macrocrystalline and Nanocrystalline Cellulose as Potential Corrosion Inhibitors for Ss316 Alloy during Acid Pickling Process: Experimental and Computational Methods. Polymers 2021, 13, 2275. [Google Scholar] [CrossRef]
- Faccini, M.; Bautista, L.; Soldi, L.; Escobar, A.M.; Altavilla, M.; Calvet, M.; Domènech, A.; Domínguez, E. Environmentally Friendly Anticorrosive Polymeric Coatings. Appl. Sci. 2021, 11, 3446. [Google Scholar] [CrossRef]
- Peltzman, S. The Effects of Automobile Safety Regulation. J. Polit. Econ. 1975, 83, 677–725. [Google Scholar] [CrossRef]
- International Monetary Fund. World Economic Outlook: War Sets Back The Global Recovery; International Monetary Fund: Washington, DC, USA, 2022; ISBN 978-1-61635-942-3. [Google Scholar]
- Ates, M. A Review on Conducting Polymer Coatings for Corrosion Protection. J. Adhes. Sci. Technol. 2016, 30, 1510–1536. [Google Scholar] [CrossRef]
- Shi, X. On the Use of Nanotechnology to Manage Steel Corrosion. Recent Pat. Eng. 2010, 4, 44–50. [Google Scholar] [CrossRef]
- Xie, Z.H.; Shan, S. Nanocontainers-Enhanced Self-Healing Ni Coating for Corrosion Protection of Mg Alloy. J. Mater. Sci. 2018, 53, 3744–3755. [Google Scholar] [CrossRef]
- Thulasi, G.; Pragathiswaran, C.; Anusuya, N. Experimental Investigation and Analysis of Corrosion Inhibition of Titanium Modified by Chitosan Silver Nanomaterials and Its Applications. Mater. Today Proc. 2020, 37, 2780–2785. [Google Scholar] [CrossRef]
- Simoneau, C.; Raffael, B.; Garbin, S.; Hoekstra, E.; Mieth, A.; Lopes, J.A.; Reina, V. JRC Science for Policy Report: Non-Harmonised Food Contact Materials in the EU: Regulatory and Market Situation Baseline Study Final Report; Publications Office of the European Union: Luxembourg, 2016; ISBN 978-92-79-64632-4. [Google Scholar]
- Punmiya, V. Paint Sector. Nirmal Bang 2020, 1, 3–63. [Google Scholar]
- Calegari, F.; Sousa, I.; Ferreira, M.G.S.; Berton, M.A.C.; Marino, C.E.B.; Tedim, J. Influence of the Operating Conditions on the Release of Corrosion Inhibitors from Spray-Dried Carboxymethylcellulose Microspheres. Appl. Sci. 2022, 12, 1800. [Google Scholar] [CrossRef]
- Palanisamy, K.L.; Devabharathi, V.; Meenakshi Sundaram, N.; Olaseinde, O.A.; Olarenwaju, O.F.; Mohammed, S.T.; Mahvidi, S.; Gharagozlou, M.; Mahdavian, M.; Naghibi, S.; et al. Corrosion Inhibition Studies of Mild Steel with Carrier Oil Stabilized of Iron Oxide Nanoparticles Incorporated into a Paint. Int. J. ChemTech Res. 2018, 7, 1661–1664. [Google Scholar] [CrossRef][Green Version]
- Narenkumar, J.; Parthipan, P.; Madhavan, J.; Murugan, K.; Marpu, S.B.; Suresh, A.K.; Rajasekar, A. Bioengineered Silver Nanoparticles as Potent Anti-Corrosive Inhibitor for Mild Steel in Cooling Towers. Environ. Sci. Pollut. Res. 2018, 25, 5412–5420. [Google Scholar] [CrossRef]
- Kumar, A.; Thakur, A. Encapsulated Nanoparticles in Organic Polymers for Corrosion Inhibition; Elsevier Inc.: Amsterdam, The Netherlands, 2020; ISBN 9780128193594. [Google Scholar]
- Thakur, A.; Kumar, A. Recent Advances on Rapid Detection and Remediation of Environmental Pollutants Utilizing Nanomaterials-Based (Bio)Sensors. Sci. Total Environ. 2022, 834, 155219. [Google Scholar] [CrossRef]
- Thakur, A.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Anti-Corrosive Potential of the Sustainable Corrosion Inhibitors Based on Biomass Waste: A Review on Preceding and Perspective Research. J. Phys. Conf. Ser. 2022, 2267, 012079. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Sharma, S.; Ganjoo, R.; Assad, H. Materials Today: Proceedings Computational and Experimental Studies on the Efficiency of Sonchus Arvensis as Green Corrosion Inhibitor for Mild Steel in 0.5 M HCl Solution. Mater. Today Proc. 2022, 66, 609–621. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Vo, D.V.N.; Sharma, A. Suppressing Inhibitory Compounds by Nanomaterials for Highly Efficient Biofuel Production: A Review. Fuel 2022, 312, 122934. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Kumar, A. Recent Innovations in Nano Container-Based Self-Healing Coatings in the Construction Industry. Curr. Nanosci. 2021, 18, 203–216. [Google Scholar] [CrossRef]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Kumar, A. Experimental, DFT and MC Simulation Analysis of Vicia Sativa Weed Aerial Extract as Sustainable and Eco-Benign Corrosion Inhibitor for Mild Steel in Acidic Environment. Sustain. Chem. Pharm. 2022, 29, 100785. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A.; Kaya, S.; Marzouki, R.; Zhang, F.; Guo, L. Recent Advancements in Surface Modification, Characterization and Functionalization for Enhancing the Biocompatibility and Corrosion Resistance of Biomedical Implants. Coatings 2022, 12, 1459. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Corrosion Inhibition Activity of Pyrazole Derivatives on Metals and Alloys in Acidic Environment: A Review. J. Emerg. Technol. Innov. Res. 2019, 6, 339–343. [Google Scholar]
- Thakur, A.; Kumar, A. Potential of Weeds Extract As A Green Corrosion Inhibitor on Mild Steel: A Review. Think India J. 2019, 22, 3226–3240. [Google Scholar]
- Thakur, A.; Kaya, S.; Abousalem, A.S.; Sharma, S.; Ganjoo, R.; Assad, H.; Kumar, A. Computational and Experimental Studies on the Corrosion Inhibition Performance of an Aerial Extract of Cnicus Benedictus Weed on the Acidic Corrosion of Mild Steel. Process Saf. Environ. Prot. 2022, 161, 801–818. [Google Scholar] [CrossRef]
- Parveen, G.; Bashir, S.; Thakur, A.; Saha, S.K.; Banerjee, P.; Kumar, A. Experimental and Computational Studies of Imidazolium Based Ionic Liquid 1-Methyl-3-Propylimidazolium Iodide on Mild Steel Corrosion in Acidic Solution Experimental and Computational Studies of Imidazolium Based Ionic Liquid 1-Methyl-3-Propylimidazolium. Mater. Res. Express 2020, 7, 016510. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Sustainable Inhibitors for Corrosion Mitigation in Aggressive Corrosive Media: A Comprehensive Study. J. Bio-Tribo-Corrosion 2021, 7, 67. [Google Scholar] [CrossRef]
- Thakur, A.; Kumar, A. Potential of Natural Product Extract as Green Corrosion Inhibitors for Steel—A Review. J. Gujarat Res. Soc. 2019, 21, 200–212. [Google Scholar]
- Nawaz, M.; Naeem, N.; Kahraman, R.; Montemor, M.F.; Haider, W.; Shakoor, R.A. Effectiveness of Epoxy Coating Modified with Yttrium Oxide Loaded with Imidazole on the Corrosion Protection of Steel. Nanomaterials 2021, 11, 2291. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez-Alonso, L.; López-Sánchez, J.; Serrano, A.; de la Fuente, O.R.; Galván, J.C.; Carmona, N. Hybrid Sol-Gel Coatings Doped with Non-Toxic Corrosion Inhibitors for Corrosion Protection on AZ61 Magnesium Alloy. Gels 2022, 8, 34. [Google Scholar] [CrossRef]
- El-Kamel, R.S.; Ghoneim, A.A.; Fekry, A.M. Electrochemical, Biodegradation and Cytotoxicity of Graphene Oxide Nanoparticles/Polythreonine as a Novel Nano-Coating on AZ91E Mg Alloy Staple in Gastrectomy Surgery. Mater. Sci. Eng. C 2019, 103, 109780. [Google Scholar] [CrossRef] [PubMed]
- Al-Moubaraki, A.H.; Obot, I.B. Corrosion Challenges in Petroleum Refinery Operations: Sources, Mechanisms, Mitigation, and Future Outlook. J. Saudi Chem. Soc. 2021, 25, 101370. [Google Scholar] [CrossRef]
- Arvidsson, R.; Sandén, B.A. Carbon Nanomaterials as Potential Substitutes for Scarce Metals. J. Clean. Prod. 2017, 156, 253–261. [Google Scholar] [CrossRef]
- GMoodley, K. Advances in Corrosion Inhibition Materials and Technologies: A Review. Adv. Mater. Lett. 2019, 10, 231–247. [Google Scholar] [CrossRef]
- Tarzanagh, Y.J.; Seifzadeh, D.; Rajabalizadeh, Z.; Khodayari, A.; Sohrabnezhad, S. Sol-Gel/MOF Nanocomposite for Effective Protection of 2024 Aluminum Alloy against Corrosion. Surf. Coat. Technol. 2019, 380, 125038. [Google Scholar] [CrossRef]
- Lou, X.Y.; Li, Y.P.; Yang, Y.W. Gated Materials: Installing Macrocyclic Arenes-Based Supramolecular Nanovalves on Porous Nanomaterials for Controlled Cargo Release. Biotechnol. J. 2019, 14, 1800354. [Google Scholar] [CrossRef]
- Ashassi-Sorkhabi, H.; Moradi-Alavian, S.; Jafari, R.; Kazempour, A.; Asghari, E. Effect of Amino Acids and Montmorillonite Nanoparticles on Improving the Corrosion Protection Characteristics of Hybrid Sol-Gel Coating Applied on AZ91 Mg Alloy. Mater. Chem. Phys. 2019, 225, 298–308. [Google Scholar] [CrossRef]
- Asif, A.H.; Mahajan, M.S.; Sreeharsha, N.; Gite, V.V.; Al-Dhubiab, B.E.; Kaliyadan, F.; Nanjappa, S.H.; Meravanige, G.; Aleyadhy, D.M. Enhancement of Anticorrosive Performance of Cardanol Based Polyurethane Coatings by Incorporating Magnetic Hydroxyapatite Nanoparticles. Materials 2022, 15, 2308. [Google Scholar] [CrossRef]
- Steel, C.; Nanoparticles, N. Enhancement of Surface and Interface Properties of Low. Crystals 2022, 12, 332. [Google Scholar]
- Elbasuney, S.; Gobara, M.; Zoriany, M.; Maraden, A.; Naeem, I. The Significant Role of Stabilized Colloidal ZrO2 Nanoparticles for Corrosion Protection of AA2024. Environ. Nanotechnol. Monit. Manag. 2019, 12, 100242. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Mahdavian, M.; Naderi, R. Superior Corrosion Protection and Adhesion Strength of Epoxy Coating Applied on AZ31 Magnesium Alloy Pre-Treated by PEO/Silane with Inorganic and Organic Corrosion Inhibitors. Corros. Sci. 2021, 178, 109065. [Google Scholar] [CrossRef]
- Ye, X.; Jiang, Z.; Li, L.; Xie, Z.H. In-Situ Growth of NiAl-Layered Double Hydroxide on AZ31 Mg Alloy towards Enhanced Corrosion Protection. Nanomaterials 2018, 8, 411. [Google Scholar] [CrossRef]
- Khamis, E.A.; Hamdy, A.; Morsi, R.E. Magnetite Nanoparticles/Polyvinyl Pyrrolidone Stabilized System for Corrosion Inhibition of Carbon Steel. Egypt. J. Pet. 2018, 27, 919–926. [Google Scholar] [CrossRef]
- Badi, N.; Khasim, S.; Pasha, A.; Alatawi, A.S.; Lakshmi, M. Silver Nanoparticles Intercalated Polyaniline Composites for High Electrochemical Anti-Corrosion Performance in 6061 Aluminum Alloy-Based Solar Energy Frameworks. J. Bio-Tribo-Corrosion 2020, 6, 123. [Google Scholar] [CrossRef]
- Lakshmi, R.V.; Aruna, S.T.; Sampath, S. Ceria Nanoparticles Vis-à-Vis Cerium Nitrate as Corrosion Inhibitors for Silica-Alumina Hybrid Sol-Gel Coating. Appl. Surf. Sci. 2017, 393, 397–404. [Google Scholar] [CrossRef]
- Ren, B.; Chen, Y.; Li, Y.; Li, W.; Gao, S.; Li, H.; Cao, R. Rational Design of Metallic Anti-Corrosion Coatings Based on Zinc Gluconate@ZIF-8. Chem. Eng. J. 2020, 384, 123389. [Google Scholar] [CrossRef]
- Syed Khadar, Y.A.; Surendhiran, S.; Gowthambabu, V.; Halimabi Alias Shakila Banu, S.; Devabharathi, V.; Balamurugan, A. Enhancement of Corrosion Inhibition of Mild Steel in Acidic Media by Green-Synthesized Nano-Manganese Oxide. Mater. Today Proc. 2021, 47, 889–893. [Google Scholar] [CrossRef]
- Ikumapayi, O.M.; Akinlabi, E.T. A Comparative Assessment of Tensile Strength and Corrosion Protection in Friction Stir Processed Aa7075-T651 Matrix Composites Using Fly Ashes Nanoparticles as Reinforcement Inhibitors in 3.5% NaCl. Int. J. Mech. Prod. Eng. Res. Dev. 2019, 9, 839–854. [Google Scholar] [CrossRef]
- González, E.A.; Leiva, N.; Vejar, N.; Sancy, M.; Gulppi, M.; Azócar, M.I.; Gomez, G.; Tamayo, L.; Zhou, X.; Thompson, G.E.; et al. Sol-Gel Coatings Doped with Encapsulated Silver Nanoparticles: Inhibition of Biocorrosion on 2024-T3 Aluminum Alloy Promoted by Pseudomonas Aeruginosa. J. Mater. Res. Technol. 2019, 8, 1809–1818. [Google Scholar] [CrossRef]
- Karekar, S.E.; Bagale, U.D.; Sonawane, S.H.; Bhanvase, B.A.; Pinjari, D.V. A Smart Coating Established with Encapsulation of Zinc Molybdate Centred Nanocontainer for Active Corrosion Protection of Mild Steel: Release Kinetics of Corrosion Inhibitor. Compos. Interfaces 2018, 25, 785–808. [Google Scholar] [CrossRef]
- Liu, X.; Zhang, T.C.; He, H.; Ouyang, L.; Yuan, S. A Stearic Acid/CeO2 Bilayer Coating on AZ31B Magnesium Alloy with Superhydrophobic and Self-Cleaning Properties for Corrosion Inhibition. J. Alloys Compd. 2020, 834, 155210. [Google Scholar] [CrossRef]
- Fu, J.; Chen, T.; Wang, M.; Yang, N.; Li, S.; Wang, Y.; Liu, X. Acid and Alkaline Dual Stimuli-Responsive Mechanized Hollow Mesoporous Silica Nanoparticles as Smart Nanocontainers for Intelligent Anticorrosion Coatings. ACS Nano 2013, 7, 11397–11408. [Google Scholar] [CrossRef]
- Gaballah, S.; Shehata, N.; Shaaban, M.; Nosier, S.; Hefnawy, A.; Hamed, A.; Samir, E. Corrosion Inhibition of Aluminum in Hydrochloric Acid Solution Using Ceria Doped Polyvinyl Chloride Nanofiber. Int. J. Electrochem. Sci. 2017, 12, 1094–1105. [Google Scholar] [CrossRef]
- Toorani, M.; Aliofkhazraei, M.; Sabour Rouhaghdam, A. Microstructural, Protective, Inhibitory and Semiconducting Properties of PEO Coatings Containing CeO2 Nanoparticles Formed on AZ31 Mg Alloy. Surf. Coat. Technol. 2018, 352, 561–580. [Google Scholar] [CrossRef]
- Mirzayi, B.; Basharnavaz, H.; Babapoor, A.; Kamali, H.; Khodayari, A.; Sohrabnezhad, S. Effects of Aluminum Terephthalate Metal-Organic Framework and Its Nanocomposites on the Corrosion of AM60B Magnesium Alloy in Ethylene Glycol Solution Containing Chloride Ions. Mater. Chem. Phys. 2021, 272, 125056. [Google Scholar] [CrossRef]
- Keshmiri, N.; Najmi, P.; Ramezanzadeh, M.; Ramezanzadeh, B. Designing an Eco-Friendly Lanthanide-Based Metal Organic Framework (MOF) Assembled Graphene-Oxide with Superior Active Anti-Corrosion Performance in Epoxy Composite. J. Clean. Prod. 2021, 319, 128732. [Google Scholar] [CrossRef]
- Farooq, S.A.; Raina, A.; Mohan, S.; Singh, R.A.; Jayalakshmi, S.; Haq, M.I.U. Nanostructured Coatings: Review on Processing Techniques, Corrosion Behaviour and Tribological Performance. Nanomaterials 2022, 12, 1323. [Google Scholar] [CrossRef]
- Manoj, A.; Ramachandran, R.; Menezes, P.L. Self-Healing and Superhydrophobic Coatings for Corrosion Inhibition and Protection. Int. J. Adv. Manuf. Technol. 2020, 106, 2119–2131. [Google Scholar] [CrossRef]
- Ahirwar, H.; Zhou, Y.; Mahapatra, C.; Ramakrishna, S.; Kumar, P.; Nanda, H.S. Materials for Orthopedic Bioimplants: Modulating Degradation and Surface Modification Using Integrated Nanomaterials. Coatings 2020, 10, 264. [Google Scholar] [CrossRef]
- Ouyang, Y.; Li, L.X.; Xie, Z.H.; Tang, L.; Wang, F.; Zhong, C.J. A Self-Healing Coating Based on Facile PH-Responsive Nanocontainers for Corrosion Protection of Magnesium Alloy. J. Magnes. Alloys 2022, 10, 836–849. [Google Scholar] [CrossRef]
- Chen, L.; Lu, D.; Zhang, Y. Organic Compounds as Corrosion Inhibitors for Carbon Steel in HCl Solution: A Comprehensive Review. Materials 2022, 15, 2023. [Google Scholar] [CrossRef]
- Olivieri, F.; Castaldo, R.; Cocca, M.; Gentile, G.; Lavorgna, M. Mesoporous Silica Nanoparticles as Carriers of Active Agents for Smart Anticorrosive Organic Coatings: A Critical Review. Nanoscale 2021, 13, 9091–9111. [Google Scholar] [CrossRef] [PubMed]
- Faísca, F.; Filipe, L.; Petrovski, Z.; Santos, M.M.; Gago, S.; Branco, L.C. Ionic Systems and Nanomaterials as Antiseptic and Disinfectant Agents for Surface Applications: A Review. Surfaces 2021, 4, 169–190. [Google Scholar] [CrossRef]
- Tiringer, U.; Durán, A.; Castro, Y.; Milošev, I. Self-Healing Effect of Hybrid Sol-Gel Coatings Based on GPTMS, TEOS, SiO2 Nanoparticles and Ce(NO3)3 Applied on Aluminum Alloy 7075-T6. J. Electrochem. Soc. 2018, 165, C213–C225. [Google Scholar] [CrossRef]
- Sudha, M.; Surendhiran, S.; Gowthambabu, V.; Balamurugan, A.; Anandarasu, R.; Syed Khadar, Y.A.; Vasudevan, D. Enhancement of Corrosive-Resistant Behavior of Zn and Mg Metal Plates Using Biosynthesized Nickel Oxide Nanoparticles. J. Bio-Tribo-Corrosion 2021, 7, 60. [Google Scholar] [CrossRef]
- Yuan, H.; Qi, F.; Zhao, N.; Wan, P.; Zhang, B.; Xiong, H.; Liao, B.; Ouyang, X. Graphene Oxide Decorated with Titanium Nanoparticles to Reinforce the Anti-Corrosion Performance of Epoxy Coating. Coatings 2020, 10, 129. [Google Scholar] [CrossRef]
- Ress, J.; Martin, U.; Bastidas, D.M. Improved Corrosion Protection of Acrylic Waterborne Coating by Doping with Microencapsulated Corrosion Inhibitors. Coatings 2021, 11, 1134. [Google Scholar] [CrossRef]
- Gouda, M.; Abd El-Lateef, H.M. Novel Cellulose Derivatives Containing Metal (Cu, Fe, Ni) Oxide Nanoparticles as Eco-Friendly Corrosion Inhibitors for c-Steel in Acidic Chloride Solutions. Molecules 2021, 26, 7006. [Google Scholar] [CrossRef]
- González, E.; Stuhr, R.; Vega, J.M.; García-lecina, E.; Grande, H.J.; Leiza, J.R.; Paulis, M. Assessing the Effect of CeO2 Nanoparticles as Corrosion Inhibitor in Hybrid Biobased Waterborne Acrylic Direct to Metal Coating Binders. Polymers 2021, 13, 848. [Google Scholar] [CrossRef]
- Kaseem, M.; Ko, Y.G. A Novel Hybrid Composite Composed of Albumin, WO3, and LDHs Film for Smart Corrosion Protection of Mg Alloy. Compos. Part B Eng. 2021, 204, 108490. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Du, X.; Du, Z.; Cheng, X.; Wang, H. A Self-Healing Polyurethane-Based Composite Coating with High Strength and Anti-Corrosion Properties for Metal Protection. Compos. Part B Eng. 2021, 225, 109273. [Google Scholar] [CrossRef]
- Najjar, R.; Katourani, S.A.; Hosseini, M.G. Self-Healing and Corrosion Protection Performance of Organic Polysulfide@urea-Formaldehyde Resin Core-Shell Nanoparticles in Epoxy/PANI/ZnO Nanocomposite Coatings on Anodized Aluminum Alloy. Prog. Org. Coat. 2018, 124, 110–121. [Google Scholar] [CrossRef]
- Taghavikish, M.; Dutta, N.K.; Choudhury, N.R. Emerging Corrosion Inhibitors for Interfacial Coating. Coatings 2017, 7, 217. [Google Scholar] [CrossRef]
- Dakhil, R.M.; Gaaz, T.S.; Al-Amiery, A.A.; Kadhum, A.A.H. Inhibitive Impacts Extract of Citrus Aurantium Leaves of Carbon Steel in Corrosive Media. Green Chem. Lett. Rev. 2018, 11, 559–566. [Google Scholar] [CrossRef]
- Berdimurodov, E.; Kholikov, A.; Akbarov, K.; Obot, I.B.; Guo, L. Thioglycoluril Derivative as a New and Effective Corrosion Inhibitor for Low Carbon Steel in a 1 M HCl Medium: Experimental and Theoretical Investigation. J. Mol. Struct. 2021, 1234, 130165. [Google Scholar] [CrossRef]
- Asaad, M.A.; Ismail, M.; Tahir, M.M.; Huseien, G.F.; Raja, P.B.; Asmara, Y.P. Enhanced Corrosion Resistance of Reinforced Concrete: Role of Emerging Eco-Friendly Elaeis Guineensis/Silver Nanoparticles Inhibitor. Constr. Build. Mater. 2018, 188, 555–568. [Google Scholar] [CrossRef]
- Asaad, M.A.; Huseien, G.F.; Baghban, M.H.; Raja, P.B.; Fediuk, R.; Faridmehr, I.; Alrshoudi, F. Gum Arabic Nanoparticles as Green Corrosion Inhibitor for Reinforced Concrete Exposed to Carbon Dioxide Environment. Materials 2021, 14, 7867. [Google Scholar] [CrossRef] [PubMed]
- Rylski, A.; Siczek, K. The Effect of Addition of Nanoparticles, Especially ZrO2-Based, on Tribological Behavior of Lubricants. Lubricants 2020, 8, 23. [Google Scholar] [CrossRef]
- Coating, S.N.E.N.; Dhakal, D.R.; Kshetri, Y.K.; Chaudhary, B.; Kim, T.; Lee, S.W.; Kim, B.S.; Song, Y.; Kim, H.S.; Kim, H.H. Particle-Size-Dependent Anticorrosion Performance of the Si3N4-Nanoparticle-Incorporated. Coatings 2022, 12, 9. [Google Scholar]
- Birdeanu, M.; Epuran, C.; Fratilescu, I.; Fagadar-cosma, E. Structured Thin Films Based on Synergistic Effects of MnTa2O6 Oxide and Bis-Carboxy-Phenyl-Substituted Porphyrins, Capable to Inhibit Steel Corrosion. Processes 2021, 9, 1890. [Google Scholar] [CrossRef]
- Bahgat Radwan, A.; Mannah, C.A.; Sliem, M.H.; Al-Qahtani, N.H.S.; Okonkwo, P.C.; Berdimurodov, E.; Mohamed, A.M.; Abdullah, A.M. Electrospun Highly Corrosion-Resistant Polystyrene–Nickel Oxide Superhydrophobic Nanocomposite Coating. J. Appl. Electrochem. 2021, 51, 1605–1618. [Google Scholar] [CrossRef]
- Preethi, P.S.; Suganya, M.; Narenkumar, J.; AlSalhi, M.S.; Devanesan, S.; Nanthini, A.U.R.; Kamalakannan, S.; Rajasekar, A. M Acrolepiota-Mediated Synthesized Silver Nanoparticles as a Green Corrosive Inhibitor for Mild Steel in Re-Circulating Cooling Water System. Bioprocess Biosyst. Eng. 2022, 45, 493–501. [Google Scholar] [CrossRef] [PubMed]
- Samadianfard, R.; Seifzadeh, D.; Habibi-Yangjeh, A. Sol-Gel Coating Filled with SDS-Stabilized Fullerene Nanoparticles for Active Corrosion Protection of the Magnesium Alloy. Surf. Coat. Technol. 2021, 419, 127292. [Google Scholar] [CrossRef]
- Cui, M.; Yu, Y.; Zheng, Y. Effective Corrosion Inhibition of Carbon Steel in Hydrochloric Acid by Dopamine-Produced Carbon Dots. Polymers 2021, 13, 1923. [Google Scholar] [CrossRef]
- Yan, D.; Liu, J.; Zhang, Z.; Wang, Y.; Zhang, M.; Song, D.; Zhang, T.; Liu, J.; He, F.; Wang, J. Dual-Functional Graphene Oxide-Based Nanomaterial for Enhancing the Passive and Active Corrosion Protection of Epoxy Coating. Compos. Part B Eng. 2021, 222, 109075. [Google Scholar] [CrossRef]
- Zhang, Y.; Tan, B.; Zhang, X.; Guo, L.; Zhang, S. Synthesized Carbon Dots with High N and S Content as Excellent Corrosion Inhibitors for Copper in Sulfuric Acid Solution. J. Mol. Liq. 2021, 338, 116702. [Google Scholar] [CrossRef]
- Umoren, S.A.; Madhankumar, A. Effect of Addition of CeO2 Nanoparticles to Pectin as Inhibitor of X60 Steel Corrosion in HCl Medium. J. Mol. Liq. 2016, 224, 72–82. [Google Scholar] [CrossRef]
- Habib, S.; Fayyad, E.; Nawaz, M.; Khan, A.; Shakoor, R.A.; Kahraman, R.; Abdullah, A. Cerium Dioxide Nanoparticles as Smart Carriers for Self-Healing Coatings. Nanomaterials 2020, 10, 791. [Google Scholar] [CrossRef]
- Nnaji, N.; Nwaji, N.; Mack, J.; Nyokong, T. Ball-Type Phthalocyanines and Reduced Graphene Oxide Nanoparticles as Separate and Combined Corrosion Inhibitors of Aluminium in HCl. J. Mol. Struct. 2021, 1236, 130279. [Google Scholar] [CrossRef]
- Dippong, T.; Levei, E.A.; Cadar, O. Recent Advances in Synthesis and Applications of MFe2O4 (M = Co, Cu, Mn, Ni, Zn) Nanoparticles. Nanomaterials 2021, 11, 1560. [Google Scholar] [CrossRef] [PubMed]
- Kartsonakis, I.A.; Dragatogiannis, D.A.; Koumoulos, E.P.; Karantonis, A.; Charitidis, C.A. Corrosion Behaviour of Dissimilar Friction Stir Welded Aluminium Alloys Reinforced with Nanoadditives. Mater. Des. 2016, 102, 56–67. [Google Scholar] [CrossRef]
- Lekbach, Y.; Bennouna, F.; El Abed, S.; Balouiri, M.; El Azzouzi, M.; Aouniti, A.; Ibnsouda Koraichi, S. Green Corrosion Inhibition and Adsorption Behaviour of Cistus Ladanifer Extract on 304L Stainless Steel in Hydrochloric Acid Solution. Arab. J. Sci. Eng. 2021, 46, 103–113. [Google Scholar] [CrossRef]
- Jmiai, A.; Tara, A.; El Issami, S.; Hilali, M.; Jbara, O.; Bazzi, L. A New Trend in Corrosion Protection of Copper in Acidic Medium by Using Jujube Shell Extract as an Effective Green and Environmentally Safe Corrosion Inhibitor: Experimental, Quantum Chemistry Approach and Monte Carlo Simulation Study. J. Mol. Liq. 2021, 322, 114509. [Google Scholar] [CrossRef]
- Solomon, M.M.; Gerengi, H.; Umoren, S.A. Carboxymethyl Cellulose/Silver Nanoparticles Composite: Synthesis, Characterization and Application as a Benign Corrosion Inhibitor for St37 Steel in 15% H2SO4 Medium. ACS Appl. Mater. Interfaces 2017, 9, 6376–6389. [Google Scholar] [CrossRef]
- Sazou, D.; Deshpande, P.P. Conducting Polyaniline Nanocomposite-Based Paints for Corrosion Protection of Steel. Chem. Pap. 2017, 71, 459–487. [Google Scholar] [CrossRef]
- Shafaamri, A.; Cheng, C.H.; Wonnie Ma, I.A.; Baig, S.B.; Kasi, R.; Subramaniam, R.; Balakrishnan, V. Effects of TiO2 Nanoparticles on the Overall Performance and Corrosion Protection Ability of Neat Epoxy and PDMS Modified Epoxy Coating Systems. Front. Mater. 2020, 6, 336. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).