Effect of Adjunctive Use of Probiotics in the Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Inclusion Criteria
2.2. Data Extraction (Selection and Coding)
2.3. Extracted Data
2.4. Quality Assessment and Risk of Bias
2.5. Statistical Analysis
3. Results
3.1. Literature Search
3.2. Characteristics of Included Studies
3.3. Definition of Peri-Implant Mucositis (PiM)
3.4. Clinical Parameters
3.5. Submucosal Instrumentation and Administration of Probiotics
3.6. Administration of Probiotics
3.7. Probiotic Strains
3.8. Clinical Parameter Results
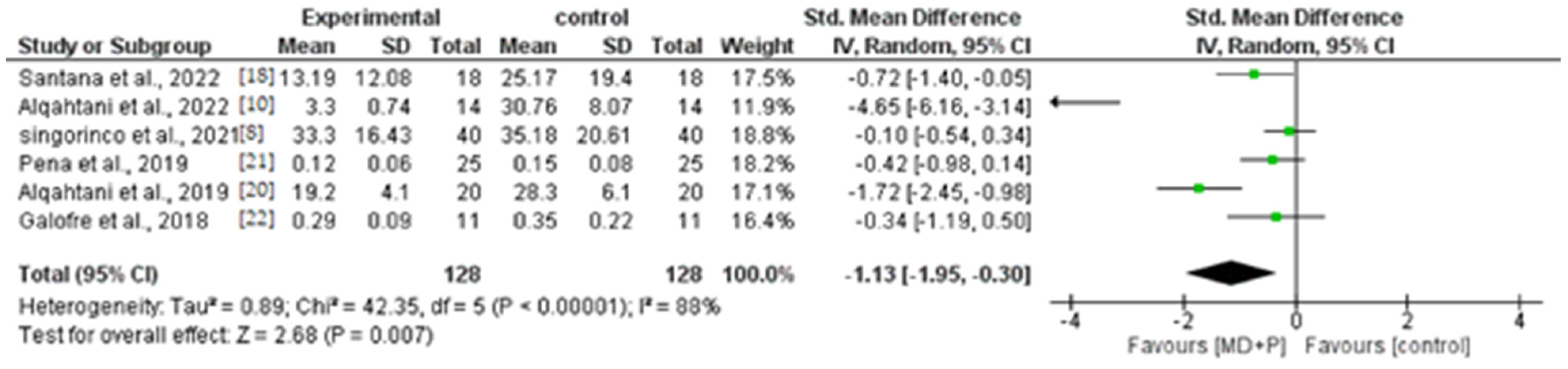
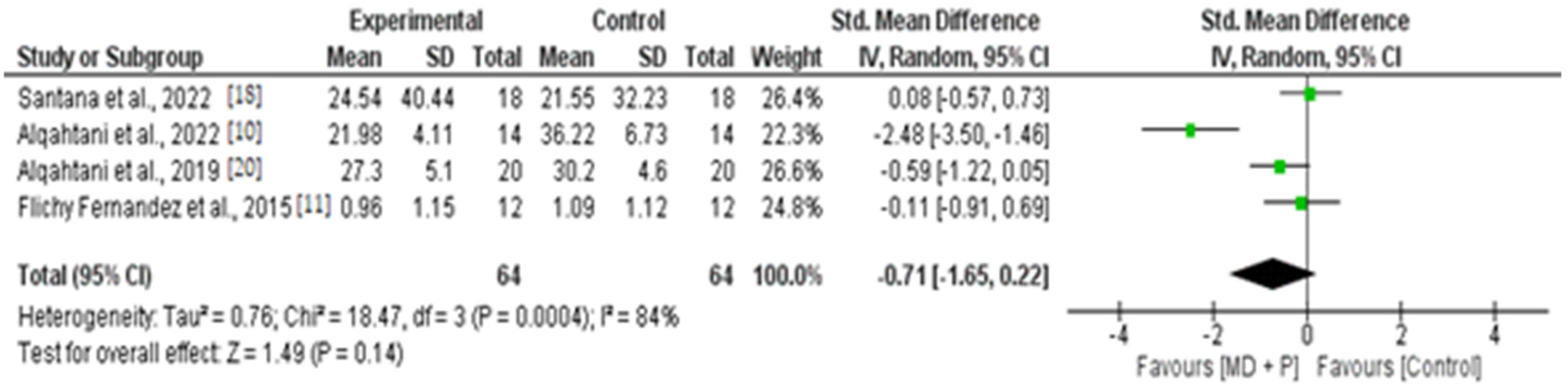
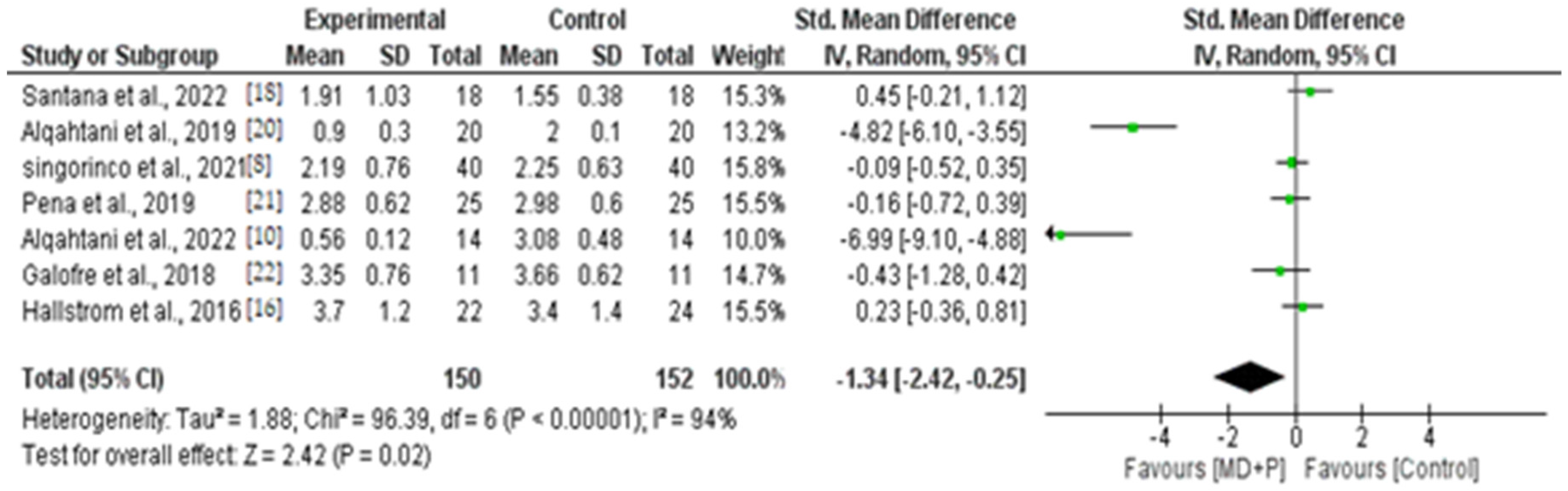
3.9. Quantitative Results of BOP, PI, and PPD
3.10. Microbiological Parameters
3.11. Microbiological Techniques
3.12. Results of Microbiological Parameters
3.13. Adverse Effects
3.14. Quality Evaluation
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Figuero, E.; Graziani, F.; Sanz, I.; Herrera, D.; Sanz, M. Management of peri-implant mucositis and peri-implantitis. Periodontology 2000 2014, 66, 255–273. [Google Scholar] [CrossRef] [PubMed]
- Berglundh, T.; Armitage, G.; Araujo, M.G.; Avila-Ortiz, G.; Blanco, J.; Camargo, P.M.; Chen, S.; Cochran, D.; Derks, J.; Figuero, E.; et al. Peri-implant diseases and conditions: Consensus report of workgroup 4 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89 (Suppl. S1), S313–S318. [Google Scholar] [CrossRef]
- Verket, A.; Koldsland, O.C.; Bunaes, D.; Lie, S.A.; Romandini, M. Non-surgical therapy of peri-implant mucositis-Mechanical/physical approaches: A systematic review. J. Clin. Periodontol. 2023, 50, 135–145. [Google Scholar] [CrossRef] [PubMed]
- Ramanauskaite, A.; Fretwurst, T.; Schwarz, F. Efficacy of alternative or adjunctive measures to conventional non-surgical and surgical treatment of peri-implant mucositis and peri-implantitis: A systematic review and meta-analysis. Int. J. Implant Dent. 2021, 7, 112. [Google Scholar] [CrossRef] [PubMed]
- Saiz, P.; Taveira, N.; Alves, R. Probiotics in Oral Health and Disease: A Systematic Review. Appl. Sci. 2021, 11, 8070. [Google Scholar] [CrossRef]
- Reid, G. Probiotics: Definition, scope and mechanisms of action. Best practice & research. Clin. Gastroenterol. 2016, 30, 17–25. [Google Scholar] [CrossRef]
- Yu, S.C.; Zhang, Y.C.; Zhu, C.H.; Zhou, H.; Liu, J.; Sun, J.Y.; Li, A.; Pei, D.D. Adjunctive Diode Laser Therapy and Probiotic Lactobacillus Therapy in the Treatment of Periodontitis and Peri-Implant Disease. Jove-J. Vis. Exp. 2022, 183, e63893. [Google Scholar] [CrossRef]
- Signorinco, F.; Zouri, N.N.; Allocca, G.; Maiorana, C.; Poli, P.P. Effectiveness of orally administered probiotic Lactobacillus reuteri in patients with peri-implant mucositis: A prospective clinical study. J. Osseointegration 2021, 13, 144–149. [Google Scholar] [CrossRef]
- Sargolzaei, N.; Arab, H.; Gerayeli, M.; Ivani, F. Evaluation of the Topical Effect of Probiotic Mouthwash in the Treatment of Patients with Peri-Implant Mucositis. J. Long Term Eff. Med. Implant 2022, 32, 85–91. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alshaikh, M.; Mehmood, A.; Alqhtani, N.; Alkhtani, F.; Alenazi, A. Role of Probiotics for the Treatment of Peri-Implant Mucositis in Patients with and without Type 2 Diabetes Mellitus. J. Oral Implantol. 2022, 48, 37–42. [Google Scholar] [CrossRef]
- Flichy-Fernandez, A.J.; Ata-Ali, J.; Alegre-Domingo, T.; Candel-Marti, E.; Ata-Ali, F.; Palacio, J.R.; Penarrocha-Diago, M. The effect of orally administered probiotic Lactobacillus reuteri-containing tablets in peri-implant mucositis: A double-blind randomized controlled trial. J. Periodontal Res. 2015, 50, 775–785. [Google Scholar] [CrossRef]
- Cereda, E.; Caraccia, M.; Caccialanza, R. Probiotics and mucositis. Curr. Opin. Clin. Nutr. Metab. Care 2018, 21, 399–404. [Google Scholar] [CrossRef] [PubMed]
- Barootchi, S.; Ravidà, A.; Tavelli, L.; Wang, H.L. Nonsurgical treatment for peri-implant mucositis: A systematic review and meta-analysis. Int. J. Oral Implantol. 2020, 13, 123–139. [Google Scholar]
- Arbildo-Vega, H.I.; Panda, S.; Bal, A.; Mohanty, R.; Rendón-Alvarado, A.; Das, A.C.; Cruzado-Oliva, F.H.; Infantes-Ruíz, E.D.; Manfredi, B.; Vásquez-Rodrigo, H.; et al. Clinical effectiveness of Lactobacillus reuteri in the treatment of peri-implant diseases: A systematic review and meta-analysis. J. Biol. Regul. Homeost. Agents 2021, 35, 79–88. [Google Scholar] [CrossRef] [PubMed]
- Sayardoust, S.; Johansson, A.; Jönsson, D. Do Probiotics Cause a Shift in the Microbiota of Dental Implants—A Systematic Review and Meta-Analysis. Front. Cell. Infect. Microbiol. 2022, 12, 823985. [Google Scholar] [CrossRef]
- Li, S.M.; Ji, Y.Z.; Wu, S.S.; Zhan, S.Y.; Wang, B.; Liu, L.R.; Li, S.Y.; Wang, N.L.; Wang, J.J. Multifocal versus single vision lenses intervention to slow progression of myopia in school-age children: A meta-analysis. Surv. Ophthalmol. 2011, 56, 451–460. [Google Scholar] [CrossRef] [PubMed]
- Higgins, J.P.; Altman, D.G.; Gotzsche, P.C.; Juni, P.; Moher, D.; Oxman, A.D.; Savovic, J.; Schulz, K.F.; Weeks, L.; Sterne, J.A. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ Clin. Res. Ed. 2011, 343, d5928. [Google Scholar] [CrossRef]
- Santana, S.I.; Silva, P.H.F.; Salvador, S.L.; Casarin, R.C.V.; Furlaneto, F.A.C.; Messora, M.R. Adjuvant use of multispecies probiotic in the treatment of peri-implant mucositis: A randomized controlled trial. J. Clin. Periodontol. 2022, 49, 828–839. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alshaikh, M.; Mehmood, A.; Alqhtani, N.; Alkhtani, F.; Alenazi, A. Efficacy of Antibiotic Versus Probiotics as Adjuncts to Mechanical Debridement for the Treatment of Peri-Implant Mucositis. J. Oral Implantol. 2022, 48, 99–104. [Google Scholar] [CrossRef]
- Alqahtani, F.; Alqahtani, M.; Shafqat, S.S.; Akram, Z.; Al-Kheraif, A.A.; Javed, F. Efficacy of mechanical debridement with adjunctive probiotic therapy in the treatment of peri-implant mucositis in cigarette-smokers and never-smokers. Clin. Implant Dent. Relat. Res. 2019, 21, 734–740. [Google Scholar] [CrossRef]
- Pena, M.; Barallat, L.; Vilarrasa, J.; Vicario, M.; Violant, D.; Nart, J. Evaluation of the effect of probiotics in the treatment of peri-implant mucositis: A triple-blind randomized clinical trial. Clin. Oral Investig. 2019, 23, 1673–1683. [Google Scholar] [CrossRef] [PubMed]
- Galofre, M.; Palao, D.; Vicario, M.; Nart, J.; Violant, D. Clinical and microbiological evaluation of the effect of Lactobacillus reuteri in the treatment of mucositis and peri-implantitis: A triple-blind randomized clinical trial. J. Periodontal Res. 2018, 53, 378–390. [Google Scholar] [CrossRef] [PubMed]
- Hallstrom, H.; Lindgren, S.; Widen, C.; Renvert, S.; Twetman, S. Probiotic supplements and debridement of peri-implant mucositis: A randomized controlled trial. Acta Odontol. Scand. 2016, 74, 60–66. [Google Scholar] [CrossRef] [PubMed]
- Ahmedbeyli, D.R.; Seyidbekov, O.S.; Dirikan, I.S.; Mamedov, F.Y.; Ahmedbeyli, R.M. Efficacy of probiotic application in the treatment and prevention of peri-implant mucositis. Stomatologiia 2019, 98, 20–24. [Google Scholar] [CrossRef]
- Mongardini, C.; Pilloni, A.; Farina, R.; Di Tanna, G.; Zeza, B. Adjunctive efficacy of probiotics in the treatment of experimental peri-implant mucositis with mechanical and photodynamic therapy: A randomized, cross-over clinical trial. J. Clin. Periodontol. 2017, 44, 410–417. [Google Scholar] [CrossRef]
- Lauritano, D.; Carinci, F.; Palmieri, A.; Cura, F.; Caruso, S.; Candotto, V. Reuterinos((R)) as adjuvant for peri-implant treatment: A pilot study. Int. J. Immunopathol. Pharmacol. 2019, 33, 2058738419827745. [Google Scholar] [CrossRef]
- Mombelli, A.; van Oosten, M.A.; Schurch, E., Jr.; Land, N.P. The microbiota associated with successful or failing osseointegrated titanium implants. Oral Microbiol. Immunol. 1987, 2, 145–151. [Google Scholar] [CrossRef]
- Zhou, K.; Xie, J.; Su, Y.; Fang, J. Lactobacillus reuteri for chronic periodontitis: Focus on underlying mechanisms and future perspectives. Biotechnol. Genet. Eng. Rev. 2023, 39, 1–28. [Google Scholar] [CrossRef]
- Staab, B.; Eick, S.; Knöfler, G.; Jentsch, H. The influence of a probiotic milk drink on the development of gingivitis: A pilot study. J. Clin. Periodontol. 2009, 36, 850–856. [Google Scholar] [CrossRef]
- Oliveira, L.F.; Salvador, S.L.; Silva, P.H.; Furlaneto, F.A.; Figueiredo, L.; Casarin, R.; Ervolino, E.; Palioto, D.B.; Souza, S.L.; Taba, M., Jr.; et al. Benefits of Bifidobacterium animalis subsp. lactis Probiotic in Experimental Periodontitis. J. Periodontol. 2017, 88, 197–208. [Google Scholar] [CrossRef]
- Cardoso, R.S.; Messora, M.R.; Silva, P.H.F.; Oliveira, L.F.; Leite-Panissi, C.; Salvador, S.; Casarin, R.; Novaes, A.B., Jr.; Palioto, D.B.; Furlaneto, F.A.C. Effects of Bifidobacterium animalis subsp. lactis HN019 on ligature-induced periodontitis in rats with experimental rheumatoid arthritis. Benef. Microbes 2020, 11, 33–46. [Google Scholar] [CrossRef] [PubMed]
- Ali, M.S.; Lee, E.B.; Lim, S.K.; Suk, K.; Park, S.C. Isolation and Identification of Limosilactobacillus reuteri PSC102 and Evaluation of Its Potential Probiotic, Antioxidant, and Antibacterial Properties. Antioxidants 2023, 12, 238. [Google Scholar] [CrossRef] [PubMed]
- Villafuerte, K.R.V.; Martinez, C.J.H.; Nobre, A.V.V.; Maia, L.P.; Tirapelli, C. What are microbiological effects of the adjunctive use of probiotics in the treatment of periodontal diseases? A systematic review. Benef. Microbes 2021, 12, 307–319. [Google Scholar] [CrossRef]
- Yang, K.M.; Kim, J.S.; Kim, H.S.; Kim, Y.Y.; Oh, J.K.; Jung, H.W.; Park, D.S.; Bae, K.H. Lactobacillus reuteri AN417 cell-free culture supernatant as a novel antibacterial agent targeting oral pathogenic bacteria. Sci. Rep. 2021, 11, 1631. [Google Scholar] [CrossRef] [PubMed]
- Deandra, F.A.; Ketherin, K.; Rachmasari, R.; Sulijaya, B.; Takahashi, N. Probiotics and metabolites regulate the oral and gut microbiome composition as host modulation agents in periodontitis: A narrative review. Heliyon 2023, 9, e13475. [Google Scholar] [CrossRef] [PubMed]
- Barbagallo, G.; Santagati, M.; Guni, A.; Torrisi, P.; Spitale, A.; Stefani, S.; Ferlito, S.; Nibali, L. Microbiome differences in periodontal, peri-implant, and healthy sites: A cross-Sectional pilot study. Clin. Oral Investig. 2022, 26, 2771–2781. [Google Scholar] [CrossRef]
- Zhou, N.; Huang, H.; Liu, H.; Li, Q.; Yang, G.; Zhang, Y.; Ding, M.; Dong, H.; Mou, Y. Microbiota analysis of peri-implant mucositis in patients with periodontitis history. Clin. Oral Investig. 2022, 26, 6223–6233. [Google Scholar] [CrossRef]
- Zhang, H.; Duan, Y.; Cai, F.; Cao, D.; Wang, L.; Qiao, Z.; Hong, Q.; Li, N.; Zheng, Y.; Su, M.; et al. Next-Generation Probiotics: Microflora Intervention to Human Diseases. BioMed Res. Int. 2022, 2022, 5633403. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Villafuerte, K.R.V.; Martinez, C.d.J.H.; Santos, K.O. Effect of Adjunctive Use of Probiotics in the Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Appl. Sci. 2023, 13, 10940. https://doi.org/10.3390/app131910940
Villafuerte KRV, Martinez CdJH, Santos KO. Effect of Adjunctive Use of Probiotics in the Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Applied Sciences. 2023; 13(19):10940. https://doi.org/10.3390/app131910940
Chicago/Turabian StyleVillafuerte, Kelly Rocio Vargas, Cristhiam de Jesus Hernandez Martinez, and Karina Oliveira Santos. 2023. "Effect of Adjunctive Use of Probiotics in the Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis" Applied Sciences 13, no. 19: 10940. https://doi.org/10.3390/app131910940
APA StyleVillafuerte, K. R. V., Martinez, C. d. J. H., & Santos, K. O. (2023). Effect of Adjunctive Use of Probiotics in the Treatment of Peri-Implant Mucositis: A Systematic Review and Meta-Analysis. Applied Sciences, 13(19), 10940. https://doi.org/10.3390/app131910940





