The Influence of Circular Physical Human–Machine Interfaces of Three Shoulder Exoskeletons on Tissue Oxygenation
Abstract
1. Introduction
- (i)
- pHMIs of shoulder exoskeletons restrict the blood supply underneath the pHMI and result in a decreased oxygen saturation in the soft tissue in resting posture.
- (ii)
- Dynamic arm movements with the shoulder exoskeletons have a neutralizing effect on the tissue oxygen saturation underneath the interface compared to resting conditions.
- (iii)
- The design of the pHMI influences the circumferential pressure and the tissue oxygenation underneath the pHMI.
2. Materials and Methods
2.1. Participants
2.2. Physical Characteristics of the Participants
2.3. Equipment
2.4. Physical Human–Machine Interfaces
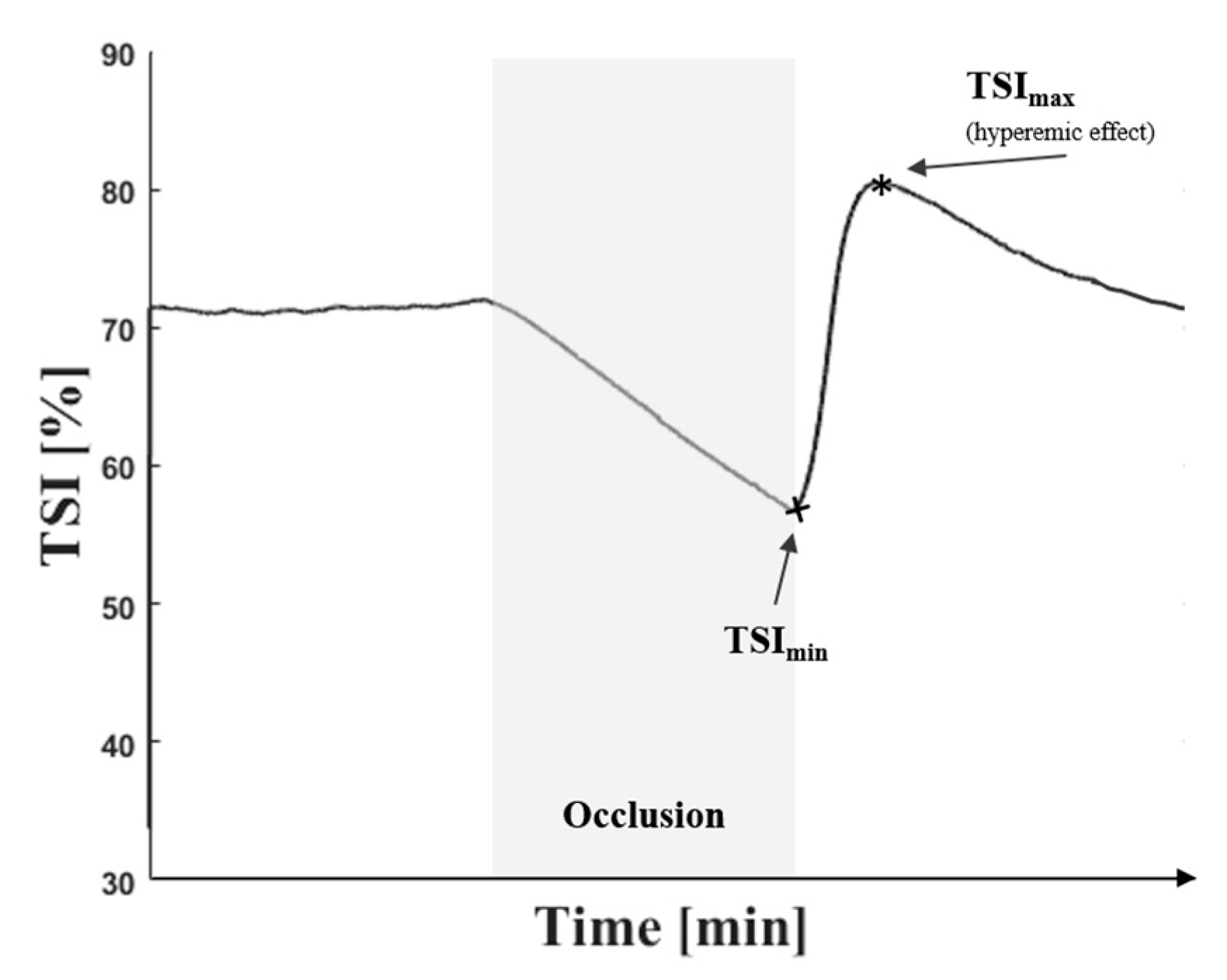
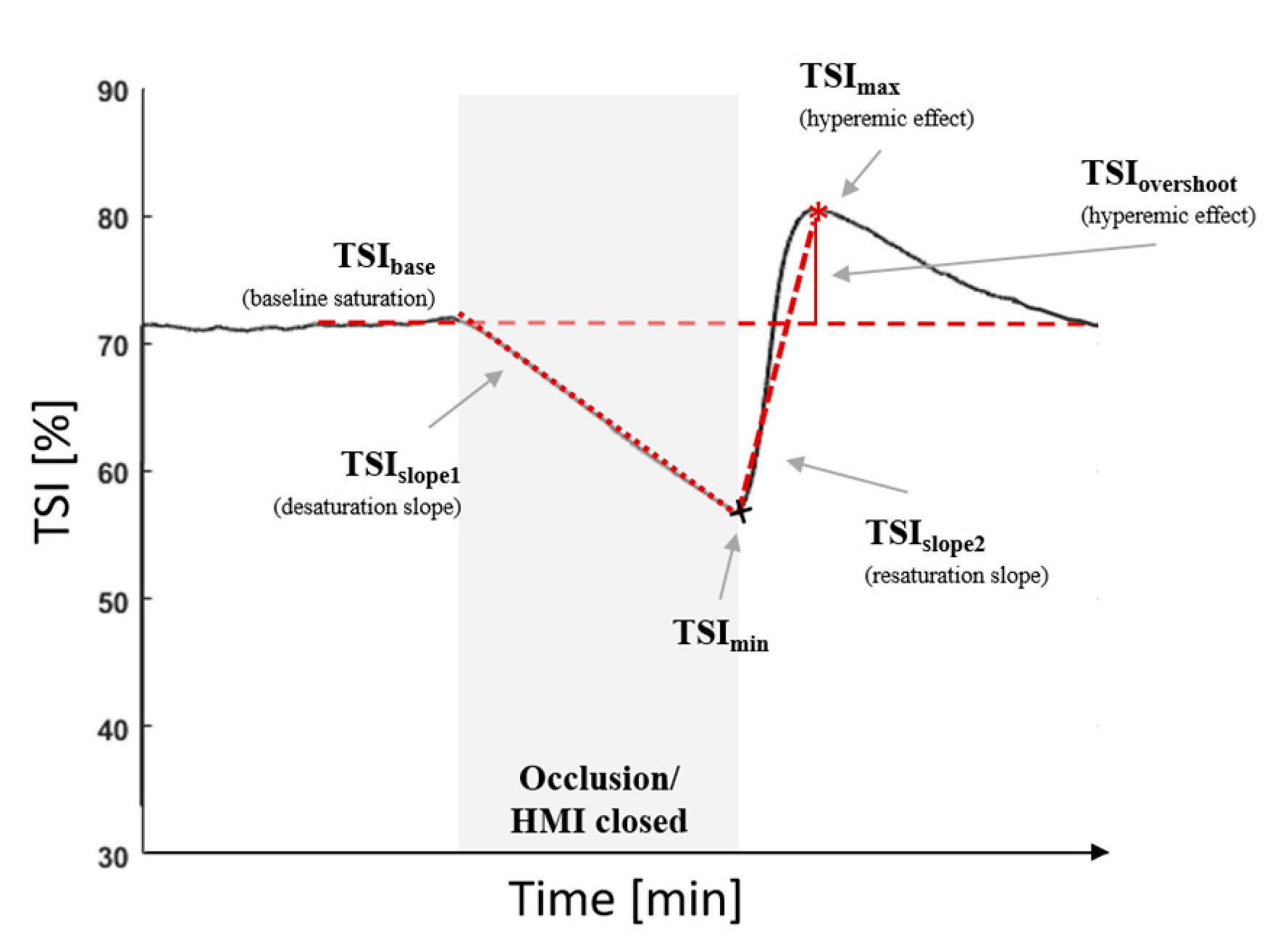
2.5. Vascular Occlusion Method
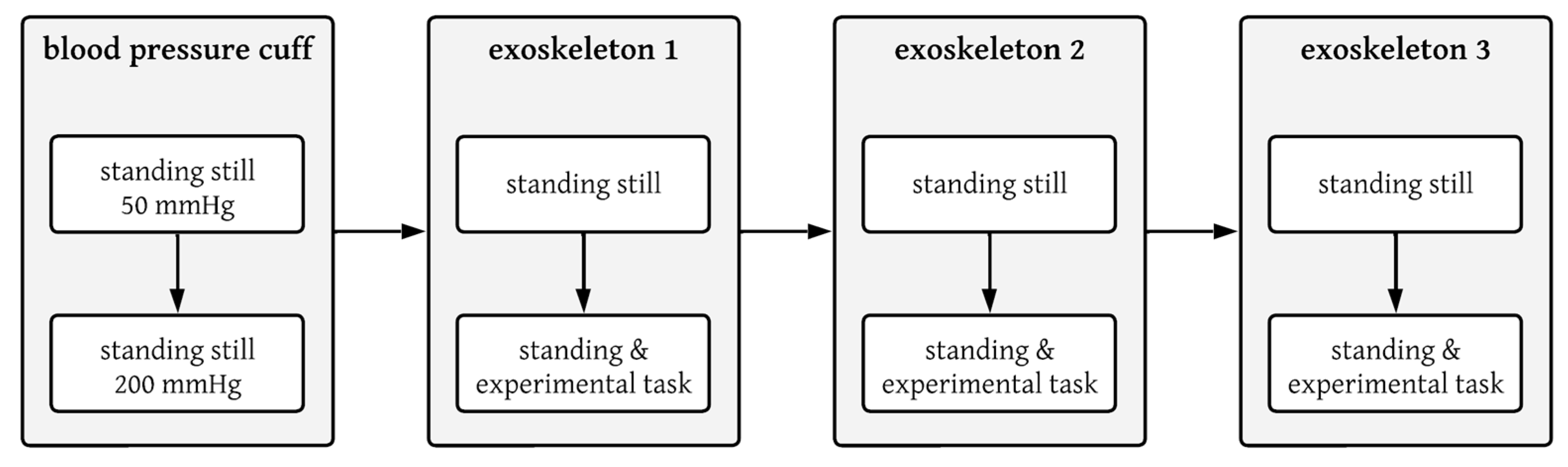
2.6. Testing Protocol
2.7. Dynamic Movement
2.8. Data and Statistical Analyses
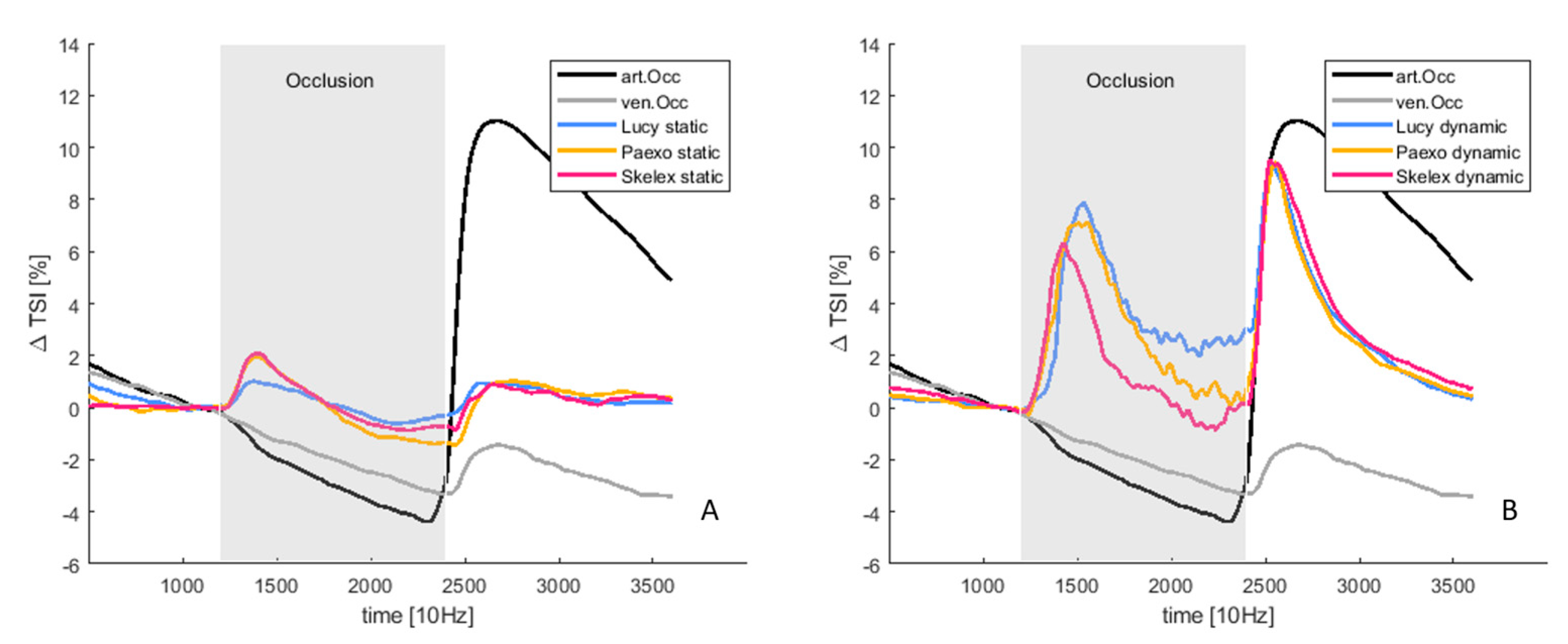
3. Results
3.1. Pressure
3.2. Tissue Oxygenation
| Outcome Parameter | Overall (p-Value (F-Value, η2)) | Sample 1 | Sample 2 | Distance | p-Value | CI-95% |
|---|---|---|---|---|---|---|
| Mean pressure [mmHg] | <0.001 (767.32, 0.991) * | Art.Occ. | Ven.Occ. | 147.550 | <0.001 * | [144.5–150.5] |
| Lucy | 184.499 | <0.001 * | [179.7–179.7] | |||
| Paexo | 160.019 | <0.001 * | [140.2–179.7] | |||
| Skelex | 176.848 | <0.001 * | [160.1–193.5] | |||
| Ven.Occ. | Lucy | 36.949 | <0.001 * | [27.2–46.6] | ||
| Paexo | 12.469 | 0.437 | [−7.9–32.9] | |||
| Skelex | 29.298 | <0.001 * | [14.3–44.2] | |||
| Lucy | Paexo | 24.480 | 0.006 * | [−41.2–−7.7] | ||
| Skelex | −7.6 | 0.427 | [−20.1–4.8] | |||
| Paexo | Skelex | 16.829 | 0.204 | [−5.8–39.5] | ||
| TSISlope1 | 0.124 (1.984, 0.221) | |||||
| TSISlope2 | 0.002 (13.194, 0.653) * | Art.Occ. | Ven.Occ. | 27.985 | 0.022 * | [3.9–52.0] |
| Lucy | 29.679 | 0.084 | [−3.9–62.6] | |||
| Paexo | 28.838 | 0.042 * | [1.0–56.6] | |||
| Skelex | 26.134 | 0.054 | [−0.3–52.6] | |||
| Ven.Occ. | Lucy | 1.694 | 1.000 | [−11.6–14.9] | ||
| Paexo | 0.853 | 1.000 | [−9.7–11.4] | |||
| Skelex | −1.851 | 1.000 | [−15.8–12.1] | |||
| Lucy | Paexo | −0.841 | 1.000 | [−10.5–8.8] | ||
| Skelex | −3.545 | 1.000 | [−16.6–9.5] | |||
| Paexo | Skelex | −2.704 | 1.000 | [−10.4–4.6] | ||
| TSIOvershoot | <0.001(17.710, 0.717) * | Art.Occ. | Ven.Occ. | 11.541 | 0.004 * | [4.1–18.8] |
| Lucy | 9.328 | 0.011 * | [2.2–16.4] | |||
| Paexo | 8.545 | 0.032 * | [0.7–16.3] | |||
| Skelex | 8.9 | 0.014 * | [1.8–16.1] | |||
| Ven.Occ. | Lucy | −2.2 | 1.000 | [−8.4–4.5] | ||
| Paexo | −2.9 | 0.114 | [−6.5–0.5] | |||
| Skelex | −2.5 | 1.000 | [−8.3–3.0] | |||
| Lucy | Paexo | −0.782 | 1.000 | [−5.8–4.3] | ||
| Skelex | −0.436 | 1.000 | [−4.4–3.7] | |||
| Paexo | Skelex | 0.436 | 1.000 | [−3.3–4.1] |
| Outcome Parameter | pHMI Design | Movement | pHMI Design × Movement |
|---|---|---|---|
| Mean pressure | 0.002 (22.488, 0.882) * | <0.001 (35.522, 0.835) * | 0.918 (0.086, 0.028) |
| TSISlope1 | 0.058 (3.408, 0.299) | <0.001 (28.561, 0.781) * | 0.421 (0.914, 0.102) |
| TSISlope2 | 0.637 (0.464, 0.055) | 0.001 (23.916, 0.749) * | 0.818 (0.203, 0.025) |
| TSIOvershoot | 0.988 (0.012, 0.002) | <0.001 (25.951, 0.764) * | 0.901 (0.100, 0.012) |
4. Discussion
4.1. Pressure
4.2. Tissue Oxygenation in Resting Position
4.3. Tissue Oxygenation in Dynamic Arm Movement
4.4. Considerations on the Design of pHMIs
4.5. Limitations
4.6. Future Work
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- da Costa, B.R.; Vieira, E.R. Risk Factors for Work-Related Musculoskeletal Disorders: A Systematic Review of Recent Longitudinal Studies. Am. J. Ind. Med. 2010, 53, 285–323. [Google Scholar] [CrossRef] [PubMed]
- Bosch, T.; van Eck, J.; Knitel, K.; de Looze, M. The Effects of a Passive Exoskeleton on Muscle Activity, Discomfort and Endurance Time in Forward Bending Work. Appl. Ergon. 2016, 54, 212–217. [Google Scholar] [CrossRef] [PubMed]
- Otten, B.; Weidner, R.; Linnenberg, C. Leichtgewichtige Und Inhärent Biomechanisch Kompatible Unterstützungssysteme Für Tätigkeiten in Und Über Kopfhöhe. In Proceedings of the 2. Transdisziplinäre Konferenz “Technische Unterstützungssysteme, Die Die Menschen Wirklich Wollen”, Hamburg, Germany, 12–13 December 2016; pp. 495–505. [Google Scholar]
- Ersoysal, S.; Hoffmann, N.; Ralfs, L.; Weidner, R. Towards a Modular Elbow Exoskeleton: Concepts for Design and System Control. In Annals of Scientific Society for Assembly, Handling and Industrial Robotics 2021; Springer: Berlin/Heidelberg, Germany, 2022; pp. 141–152. [Google Scholar] [CrossRef]
- Yao, Z.; Linnenberg, C.; Argubi-Wollesen, A.; Weidner, R.; Wulfsberg, J.P. Biomimetic Design of an Ultra-Compact and Light-Weight Soft Muscle Glove. Prod. Eng. 2017, 11, 731–743. [Google Scholar] [CrossRef]
- Maurice, P.; Ivaldi, S.; Babic, J.; Camernik, J.; Gorjan, D.; Schirrmeister, B.; Bornmann, J.; Tagliapietra, L.; Latella, C.; Pucci, D.; et al. Objective and Subjective Effects of a Passive Exoskeleton on Overhead Work. IEEE Trans. Neural Syst. Rehabil. Eng. 2020, 28, 152–164. [Google Scholar] [CrossRef] [PubMed]
- Otten, B.; Weidner, R.; Argubi-Wollesen, A. Evaluation of a Novel Active Exoskeleton for Tasks at or above Head Level. IEEE Robot. Autom. Lett. 2018, 3, 2408–2415. [Google Scholar] [CrossRef]
- Pacifico, I.; Aprigliano, F.; Parri, A.; Cannillo, G.; Melandri, I.; Sabatini, A.M.; Violante, F.S.; Molteni, F.; Giovacchini, F.; Vitiello, N.; et al. Evaluation of a Spring-Loaded Upper-Limb Exoskeleton in Cleaning Activities. Appl. Ergon. 2023, 106, 103877. [Google Scholar] [CrossRef]
- De Bock, S.; Ampe, T.; Rossini, M.; Tassignon, B.; Lefeber, D.; Rodriguez-Guerrero, C.; Roelands, B.; Geeroms, J.; Meeusen, R.; De Pauw, K. Passive Shoulder Exoskeleton Support Partially Mitigates Fatigue-Induced Effects in Overhead Work. Appl. Ergon. 2023, 106, 103903. [Google Scholar] [CrossRef]
- Schmalz, T.; Schändlinger, J.; Schuler, M.; Bornmann, J.; Schirrmeister, B.; Kannenberg, A.; Ernst, M. Biomechanical and Metabolic Effectiveness of an Industrial Exoskeleton for Overhead Work. Int. J. Environ. Res. Public Health 2019, 16, 4792. [Google Scholar] [CrossRef]
- Schröter, F.; Weidner, R.; Dehmel, P.; Jakobsen, T.; Wulfsberg, J. Der Beflügelte Mensch—Gesteigerte Konzentration Durch Unterstützungssysteme in Der Produktion. In Proceedings of the 51. DGPs-Kongress “Psychologie gestaltet”, Frankfurt am Main, Germany, 15–20 September 2018. [Google Scholar]
- Weidner, R.; Linnenberg, C.; Hoffmann, N.; Prokop, G.; Edwards, V. Exoskelette Für Den Industriellen Kontext: Systematisches Review Und Klassifikation. In Dokumentation des 66. Arbeitswissenschaftlichen Kongresses TU Berlin—Digitaler Wandel, Digitale Arbeit, Digitaler Mensch; GfA-Press: Dortmund, Germany, 2020. [Google Scholar]
- Nguiadem, C.; Raison, M.; Achiche, S. Motion Planning of Upper-Limb Exoskeleton Robots: A Review. Appl. Sci. 2020, 10, 7626. [Google Scholar] [CrossRef]
- Kuber, P.M.; Rashedi, E. Product Ergonomics in Industrial Exoskeletons: Potential Enhancements for Workforce Efficiency and Safety. Theor. Issues Ergon. Sci. 2020, 22, 729–752. [Google Scholar] [CrossRef]
- Yandell, M.B.; Quinlivan, B.T.; Popov, D.; Walsh, C.; Zelik, K.E. Physical Interface Dynamics Alter How Robotic Exosuits Augment Human Movement: Implications for Optimizing Wearable Assistive Devices. J. Neuroeng. Rehabil. 2017, 14, 40. [Google Scholar] [CrossRef] [PubMed]
- Linnenberg, C.; Weidner, R.; Wulfsberg, J.P.; Klabunde, J. Entwicklungsansatz Für Physische Mensch-Technik-Schnittstellen von Exoskeletten. In Proceedings of the Band zur Dritten Transdisziplinäre Konferenz “Technische Unterstützungssysteme, Die Die Menschen Wirklich Wollen, 11–12 December 2018”; Weidner, R., Karafillidis, A., Eds.; Helmut-Schmidt-Universität: Hamburg, Germany, 2018; pp. 417–424. [Google Scholar]
- Xiloyannis, M.; Alicea, R.; Georgarakis, A.M.; Haufe, F.L.; Wolf, P.; Masia, L.; Riener, R. Soft Robotic Suits: State of the Art, Core Technologies, and Open Challenges. IEEE Trans. Robot. 2022, 38, 1343–1362. [Google Scholar] [CrossRef]
- Francisco, A.; Yoe, J.; Morales, R.; Torres, G.O.; De Jes, F.; Sorcia, V.; Rojas, A.C.; Aurelio, J.; Mendoza, B. Soft Exoskeletons: Development, Requirements, and Challenges of the Last Decade. Actuators 2021, 10, 166. [Google Scholar] [CrossRef]
- Baltrusch, S.J.; van Dieën, J.H.; van Bennekom, C.A.M.; Houdijk, H. The Effect of a Passive Trunk Exoskeleton on Functional Performance in Healthy Individuals. Appl. Ergon. 2018, 72, 94–106. [Google Scholar] [CrossRef]
- de Looze, M.P.; Bosch, T.; Krause, F.; Stadler, K.S.; O’Sullivan, L.W. Exoskeletons for Industrial Application and Their Potential Effects on Physical Work Load. Ergonomics 2015, 59, 671–681. [Google Scholar] [CrossRef]
- Tamez-Duque, J.; Cobian-Ugalde, R.; Kilicarslan, A.; Venkatakrishnan, A.; Soto, R.; Contreras-Vidal, J.L. Real-Time Strap Pressure Sensor System for Powered Exoskeletons. Sensors 2015, 15, 4550–4563. [Google Scholar] [CrossRef]
- Quinlivan, B.; Asbeck, A.; Wagner, D.; Ranzani, T.; Russo, S.; Walsh, C. Force Transfer Characterization of a Soft Exosuit for Gait Assistance. In Proceedings of the ASME 2015 International Design Engineering Technical Conferences & Computers and Information in Engineering Conference (IDETC/CIE 2015), Boston, MA, USA, 2–5 August 2015; 5A-2015; pp. 1–8. [Google Scholar] [CrossRef]
- Agam, L.; Gefen, A. Pressure Ulcers and Deep Tissue Injury: A Bioengineering Perspective. J. Wound Care 2007, 16, 336–342. [Google Scholar] [CrossRef]
- Bouten, C.V.; Oomens, C.W.; Baaijens, F.P.; Bader, D.L. The Etiology of Pressure Ulcers: Skin Deep or Muscle Bound? Arch. Phys. Med. Rehabil. 2003, 84, 616–619. [Google Scholar] [CrossRef]
- Järvholm, U.; Palmerud, G.; Karlsson, D.; Herberts, P.; Kadefors, R. Intramuscular Pressure and Electromyography in Four Shoulder Muscles. J. Orthop. Res. 1991, 9, 609–619. [Google Scholar] [CrossRef]
- Charles, L.E.; Ma, C.C.; Burchfiel, C.M.; Dong, R.G. Vibration and Ergonomic Exposures Associated with Musculoskeletal Disorders of the Shoulder and Neck. Saf. Health Work 2018, 9, 125–132. [Google Scholar] [CrossRef]
- Garg, A.; Hegmann, K.; Kapellusch, J. Short-Cycle Overhead Work and Shoulder Girdle Muscle Fatigue. Int. J. Ind. Ergon. 2006, 36, 581–597. [Google Scholar] [CrossRef]
- Meier, T.O.; Tschirch, F.F.; Weisheupt, D.; Hug, U.; Giovanoli, P.; Schiller, A. Thoracic-Outlet-Syndrom (TOS). gefaessmedizin.net 2008, 4, 18–50. [Google Scholar] [CrossRef]
- Buckle, P.; Devereux, J. The Nature of Work-Related Neck and Upper Limb Musculoskeletal Disorders. Appl. Ergon. 2002, 33, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Armstrong, T.; Buckle, P.; Fine, L.J.; Hagberg, M.; Jonsson, B.; Kilborn, A.; Kuorinka, I.A.A.; Silverstein, B.A.; Sjogaard, G.; Viikari-juntura, E.R.A. A Conceptual Model for Work-Related Musculoskeletal Disorders Neck and Upper-Limb. Scand. J. Work. Environ. Health 1993, 19, 73–84. [Google Scholar] [CrossRef] [PubMed]
- Oomens, C.W.J.; Loerakker, S.; Bader, D.L. The Importance of Internal Strain as Opposed to Interface Pressure in the Prevention of Pressure Related Deep Tissue Injury. J. Tissue Viability 2010, 19, 35–42. [Google Scholar] [CrossRef]
- Weston, E.B.; Alizadeh, M.; Hani, H.; Knapik, G.G.; Souchereau, R.A.; Marras, W.S. A Physiological and Biomechanical Investigation of Three Passive Upper-Extremity Exoskeletons during Simulated Overhead Work. Ergonomics 2022, 65, 105–117. [Google Scholar] [CrossRef]
- Muramatsu, Y.; Kobayashi, H. Assessment of Local Muscle Fatigue by NIRS—Development and Evaluation of Muscle Suit. Robomech J. 2014, 1, 19. [Google Scholar] [CrossRef]
- Hefferle, M.; Lechner, M.; Kluth, K.; Christian, M. Development of a Standardized Ergonomic Assessment Methodology for Exoskeletons Using Both Subjective and Objective Measurement Techniques. In Advances in Human Factors in Robots and Unmanned Systems, Proceedings of the AHFE 2016 International Conference on Human Factors in Robots and Unmanned Systems, Orlando, FL, USA, 27–31 July 2016; Savage-Knepshield, P., Chen, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2016; pp. 49–59. ISBN 978-3-319-41959-6. [Google Scholar]
- Zhu, Y.; Weston, E.B.; Mehta, R.K.; Marras, W.S. Neural and Biomechanical Tradeoffs Associated with Human-Exoskeleton Interactions. Appl. Ergon. 2021, 96, 103494. [Google Scholar] [CrossRef]
- Caliandro, P.; Molteni, F.; Simbolotti, C.; Guanziroli, E.; Iacovelli, C.; Reale, G.; Giovannini, S.; Padua, L. Exoskeleton-Assisted Gait in Chronic Stroke: An EMG and Functional near-Infrared Spectroscopy Study of Muscle Activation Patterns and Prefrontal Cortex Activity. Clin. Neurophysiol. 2020, 131, 1775–1781. [Google Scholar] [CrossRef]
- Kermavnar, T.; O’Sullivan, K.J.; de Eyto, A.; O’Sullivan, L.W. Discomfort/Pain and Tissue Oxygenation at the Lower Limb During Circumferential Compression: Application to Soft Exoskeleton Design. Hum. Factors 2020, 62, 475–488. [Google Scholar] [CrossRef]
- Kermavnar, T.; O’Sullivan, K.J.; Casey, V.; de Eyto, A.; O’Sullivan, L.W. Circumferential Tissue Compression at the Lower Limb during Walking, and Its Effect on Discomfort, Pain and Tissue Oxygenation: Application to Soft Exoskeleton Design. Appl. Ergon. 2020, 86, 103093. [Google Scholar] [CrossRef]
- Schiele, A.; van der Helm, F.C.T. Influence of Attachment Pressure and Kinematic Configuration on PHRI with Wearable Robots. Appl. Bionics Biomech. 2009, 6, 157–173. [Google Scholar] [CrossRef]
- Linnenberg, C.; Weidner, R. Industrial Exoskeletons for Overhead Work: Circumferential Pressures on the Upper Arm Caused by the Physical Human-Machine-Interface. Appl. Ergon. 2022, 101, 103706. [Google Scholar] [CrossRef]
- van Beekvelt, M.C.P.; Borghuis, M.S.; van Engelen, B.G.M.; Wevers, R.A.; Colier, W.N.J.M. Adipose Tissue Thickness Affects in Vivo Quantitative Near-IR Spectroscopy in Human Skeletal Muscle. Clin. Sci. 2001, 101, 21–28. [Google Scholar] [CrossRef]
- Barstow, T.J. Understanding near Infrared Spectroscopy and Its Application to Skeletal Muscle Research. J. Appl. Physiol. 2019, 126, 1360–1376. [Google Scholar] [CrossRef]
- Patterson, M.S.; Chance, B.; Wilson, B.C. Time Resolved Reflectance and Transmittance for the Non-Invasive Measurement of Tissue Optical Properties. Appl. Opt. 1989, 28, 2331–2336. [Google Scholar] [CrossRef]
- van der Zee, P.; Cope, M.; Arridge, S.R.; Essenpreis, M.; Potter, L.A.; Edwards, A.D.; Wyatt, J.S.; Mccormick, D.C.; Roth, S.; Reynolds, E.O.R. Experimentally Measured Optical Pathlengths for the Adult Head, Calf and Forearm and the Head of the Newborn Infant as a Function of Inter Optode Spacing. Adv. Exp. Med. Biol. 1992, 316, 143–153. [Google Scholar]
- Ferrari, M.; Wei, Q.; Carraresi, L.; De Blasi, R.A.; Zaccanti, G. Time-Resolved Spectroscopy of the Human Forearm. J. Photochem. Photobiol. B 1992, 16, 141–153. [Google Scholar] [CrossRef]
- Bezemer, R.; Lima, A.; Myers, D.; Klijn, E.; Heger, M.; Goedhart, P.T.; Bakker, J.; Ince, C. Assessment of Tissue Oxygen Saturation during a Vascular Occlusion Test Using Near-Infrared Spectroscopy: The Role of Probe Spacing and Measurement Site Studied in Healthy Volunteers. Crit. Care 2009, 13 (Suppl. S5), 244. [Google Scholar] [CrossRef]
- Futier, E.; Christophe, S.; Robin, E.; Petit, A.; Pereira, B.; Desbordes, J.; Bazin, J.E.; Vallet, B. Use of Near-Infrared Spectroscopy during a Vascular Occlusion Test to Assess the Microcirculatory Response during Fluid Challenge. Crit. Care 2011, 15, R214. [Google Scholar] [CrossRef]
- Payen, D.; Luengo, C.; Heyer, L.; Resche-Rigon, M.; Kerever, S.; Damoisel, C.; Losser, M.R. Is Thenar Tissue Hemoglobin Oxygen Saturation in Septic Shock Related to Macrohemodynamic Variables and Outcome? Crit. Care 2009, 13 (Suppl. S5), S6. [Google Scholar] [CrossRef]
- Jones, S.; Tillin, T.; Williams, S.; Rapala, A.; Chaturvedi, N.; Hughes, A.D. Skeletal Muscle Tissue Saturation Changes Measured Using Near Infrared Spectroscopy During Exercise Are Associated With Post-Occlusive Reactive Hyperaemia. Front. Physiol. 2022, 13, 919754. [Google Scholar] [CrossRef]
- Massardi, S.; Rodriguez-Cianca, D.; Pinto-Fernandez, D.; Moreno, J.C.; Lancini, M.; Torricelli, D. Characterization and Evaluation of Human–Exoskeleton Interaction Dynamics: A Review. Sensors 2022, 22, 3993. [Google Scholar] [CrossRef]
- De Blasi, R.A.; Ferrari, M.; Natali, A.; Conti, G.; Mega, A.; Gasparetto, A. Noninvasive Measurement of Forearm Blood Flow and Oxygen Consumption by Near-Infrared Spectroscopy. J. Appl. Physiol. 1994, 76, 1388–1393. [Google Scholar] [CrossRef]
- Hach, W. VenenChirurgie: Operative, Interventionelle und Konservative Aspekte, 3rd ed.; Schattauer: Stuttgart, Germany, 2013; ISBN 9783794528424. [Google Scholar]
- Packer, C.F.; Bickel, S.; Dattilo, J.B. Vein Obstruction; StatPearls Publishing: Tampa, FL, USA, 2023. [Google Scholar]
- Casavola, C.; Paunescu, L.A.; Fantini, S.; Gratton, E. Blood Flow and Oxygen Consumption with Near-Infrared Spectroscopy and Venous Occlusion: Spatial Maps and the Effect of Time and Pressure of Inflation. J. Biomed. Opt. 2000, 5, 269. [Google Scholar] [CrossRef][Green Version]
- Linnenberg, C.; Weidner, R. Designing Physical Human-Machine-Interfaces for Exoskeletons Using 3D-Shape Analysis. In Proceedings of the 3DBODY.TECH 2019—10th International Conference and Exihbition on 3D Body Scanning and Processing Technologies, Lugano, Switzerland, 22–23 October 2019; pp. 85–95. [Google Scholar]
- Klinke, R. Physiologie; Georg Thieme Verlag: Stuttgart, Germany, 2005; ISBN 9783131924353. [Google Scholar]
- McManus, C.J.; Collison, J.; Cooper, C.E. Performance Comparison of the MOXY and PortaMon Near-Infrared Spectroscopy Muscle Oximeters at Rest and during Exercise. J. Biomed. Opt. 2018, 23, 1. [Google Scholar] [CrossRef]
- van Hooff, M.; Meijer, E.J.; Scheltinga, M.R.M.; Savelberg, H.H.C.M.; Schep, G. Test–Retest Reliability of Skeletal Muscle Oxygenation Measurement Using near-Infrared Spectroscopy during Exercise in Patients with Sport-Related Iliac Artery Flow Limitation. Clin. Physiol. Funct. Imaging 2022, 42, 114–126. [Google Scholar] [CrossRef]
- Baldassarre, A.; Lulli, L.G.; Cavallo, F.; Fiorini, L.; Mariniello, A.; Mucci, N.; Arcangeli, G. Industrial Exoskeletons from Bench to Field: Human-Machine Interface and User Experience in Occupational Settings and Tasks. Front. Public Health 2022, 10, 1039680. [Google Scholar] [CrossRef]
- de Looze, M.P.; Krause, F.; O’Sullivan, L.W. The Potential and Acceptance of Exoskeletons in Industry. In Wearable Robotics: Challenges and Trends; González-Vargas, J., Ibáñez, J., Contreras-Vidal, J., van der Kooij, H., Pons, J., Eds.; Springer: Berlin/Heidelberg, Germany, 2017; pp. 195–199. [Google Scholar]
- Perry, J.C.; Brower, J.R.; Carne, R.H.R.; Bogert, M.A. 3D Scanning of the Forearm for Orthosis and HMI Applications. Front. Robot. AI 2021, 8, 576783. [Google Scholar] [CrossRef]
- Baud, R.; Manzoori, A.R.; Ijspeert, A.; Bouri, M. Review of Control Strategies for Lower-Limb Exoskeletons to Assist Gait. J. Neuroeng. Rehabil. 2021, 18, 1–34. [Google Scholar] [CrossRef]
- Aguirre-Ollinger, G.; Colgate, J.E.; Peshkin, M.A.; Goswami, A. Active-Impedance Control of a Lower-Limb Assistive Exoskeleton. In Proceedings of the 2007 IEEE 10th International Conference on Rehabilitation Robotics, Noordwijk, The Netherlands, 13–15 June 2007; pp. 188–195. [Google Scholar] [CrossRef]
- Levesque, L.; Pardoel, S.; Doumit, M.; Lovrenovic, Z. Experimental Comfort Assessment of an Active Exoskeleton Interface. In Proceedings of the IEEE International Symposium on Robotics and Intelligent Sensors (IRIS), Ottawa, ON, Canada, 5–7 October 2017; pp. 5–7. [Google Scholar]



| Characteristics | Mean | SD | Unit |
|---|---|---|---|
| Age | 29 | 1 | yrs |
| Height | 1.82 | 0.02 | m |
| Weight | 79 | 2.7 | kg |
| Circumference of upper arm | 29.5 | 1 | cm |
| Skinfold thickness | 3.5 | 0.6 | mm |
| Systolic blood pressure | 118.3 | 3.3 | mmHg |
| Diastolic blood pressure | 68.7 | 4.9 | mmHg |
| Exoskeleton | Institution | Type | Technology | pHMI Strap | pHMI Fixation | Weight | Support Force | pHMI Width × Length |
|---|---|---|---|---|---|---|---|---|
| Skelex 360 | Skelex | Passive | Flex frame, spring | Elastic | Buckle | 2.8 kg | up to 4.9 kg | 13 × 22.5 cm |
| Lucy | HSU | Active | Air pressure | Elastic | Buckle | 6.6 kg | up to 8 Nm | 18 × 11.5 cm |
| Paexo Shoulder | Otto Bock | Passive | Mechanical pully | Elastic | Buckle | 1.9 kg | n/s * | 15 × 17 cm |
| Resting Posture | Dynamic Movement | |||||||
|---|---|---|---|---|---|---|---|---|
| Art. Occ. | Ven. Occ. | Lucy | Paexo | Skelex | Lucy | Paexo | Skelex | |
| Mean pressure [mmHg] | 200.1 (1.8) | 52.6 (0.4) | 16.1 (6.5) | 38.3 (14.3) | 23.8 (11.8) | 24.3 (12.9) | 55.7 (21.4) | 39.3 (10.7) |
| TSIBase [%] | 58.3 (3.1) | 60.7 (2.8) | 55.8 (8.0) | 57.3 (6.3) | 56.4 (5.8) | 56.4 (6.9) | 57.2 (5.3) | 56.4 (5.9) |
| TSISlope1 [%/min] | −2.1 (1.1) | −1.5 (0.5) | −1.6 (1.0) | −2.5 (2.2) | −2.7 (1.5) | −5.9 (3.6) | −9.0 (4.5) | −7.0 (4.3) |
| TSISlope2 [%/min] | 35.6 (18.4) | 6.5 (5.7) | 6.7 (5.9) | 7.7 (5.9) | 7.0 (5.5) | 46.1 (33.3) | 58.7 (25.4) | 51.6 (36.8) |
| TSIOvershoot [%] | 9.8 (4.2) | −1.2 (2.7) | 1.6 (2.7) | 1.4 (1.7.) | 1.4 (2.4) | 11.8 (6.4) | 9.6 (5.7) | 10.3 (5.8) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Linnenberg, C.; Reimeir, B.; Eberle, R.; Weidner, R. The Influence of Circular Physical Human–Machine Interfaces of Three Shoulder Exoskeletons on Tissue Oxygenation. Appl. Sci. 2023, 13, 10534. https://doi.org/10.3390/app131810534
Linnenberg C, Reimeir B, Eberle R, Weidner R. The Influence of Circular Physical Human–Machine Interfaces of Three Shoulder Exoskeletons on Tissue Oxygenation. Applied Sciences. 2023; 13(18):10534. https://doi.org/10.3390/app131810534
Chicago/Turabian StyleLinnenberg, Christine, Benjamin Reimeir, Robert Eberle, and Robert Weidner. 2023. "The Influence of Circular Physical Human–Machine Interfaces of Three Shoulder Exoskeletons on Tissue Oxygenation" Applied Sciences 13, no. 18: 10534. https://doi.org/10.3390/app131810534
APA StyleLinnenberg, C., Reimeir, B., Eberle, R., & Weidner, R. (2023). The Influence of Circular Physical Human–Machine Interfaces of Three Shoulder Exoskeletons on Tissue Oxygenation. Applied Sciences, 13(18), 10534. https://doi.org/10.3390/app131810534






