Investigation of the Thermodynamics for the Removal of As(III) and As(V) from Water Using Synthesized ZnO Nanoparticles and the Effects of pH, Temperature, and Time
Abstract
:1. Introduction
2. Methods
2.1. Materials
2.2. Synthesis of ZnO Nanomaterials
2.3. Adsorbent Characterization
2.4. ICP-OES Analysis
2.5. pH Studies
2.6. Adsorption Isotherms
2.7. Time Dependency Studies
3. Results and Discussion
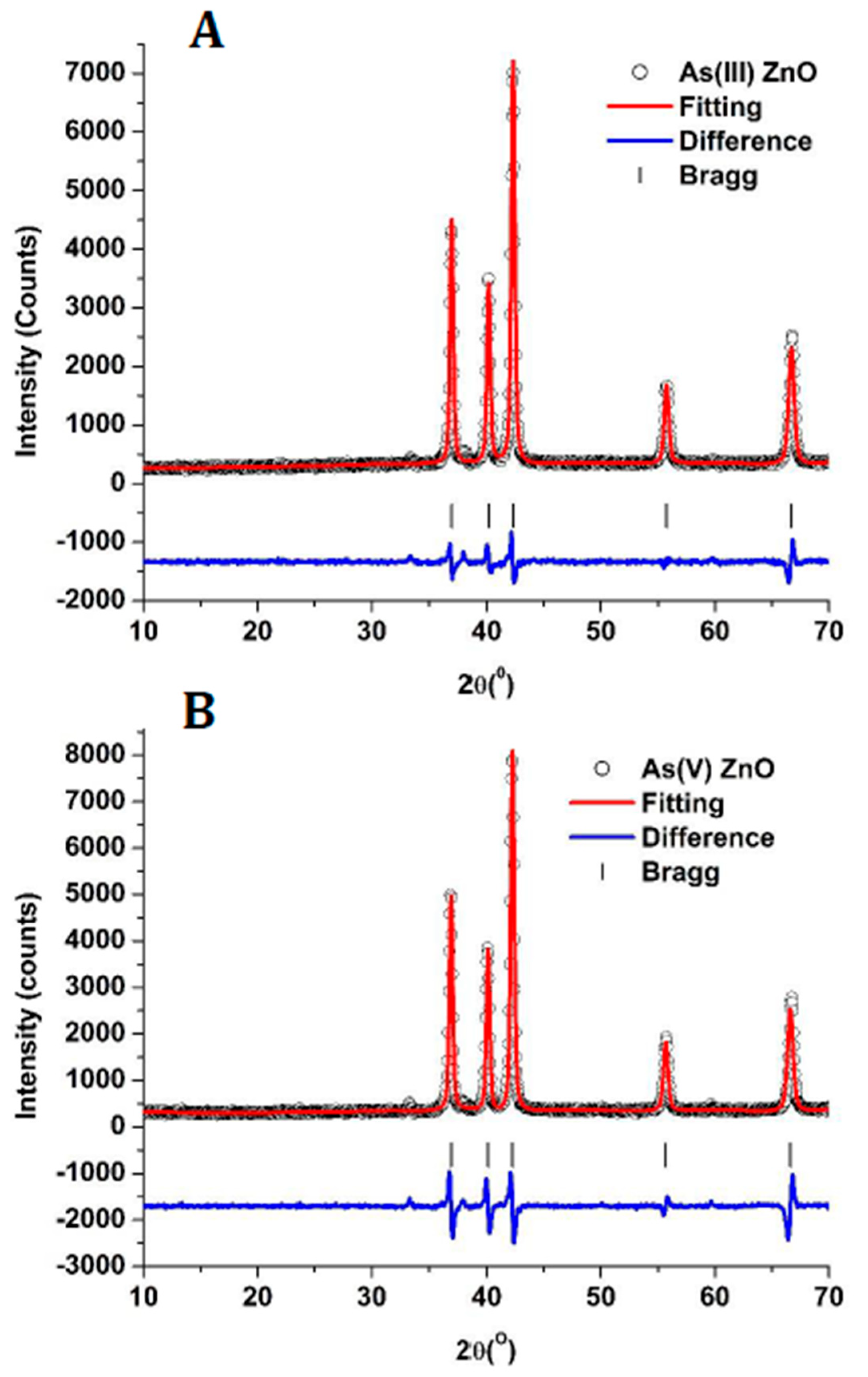
3.1. X-ray Diffraction
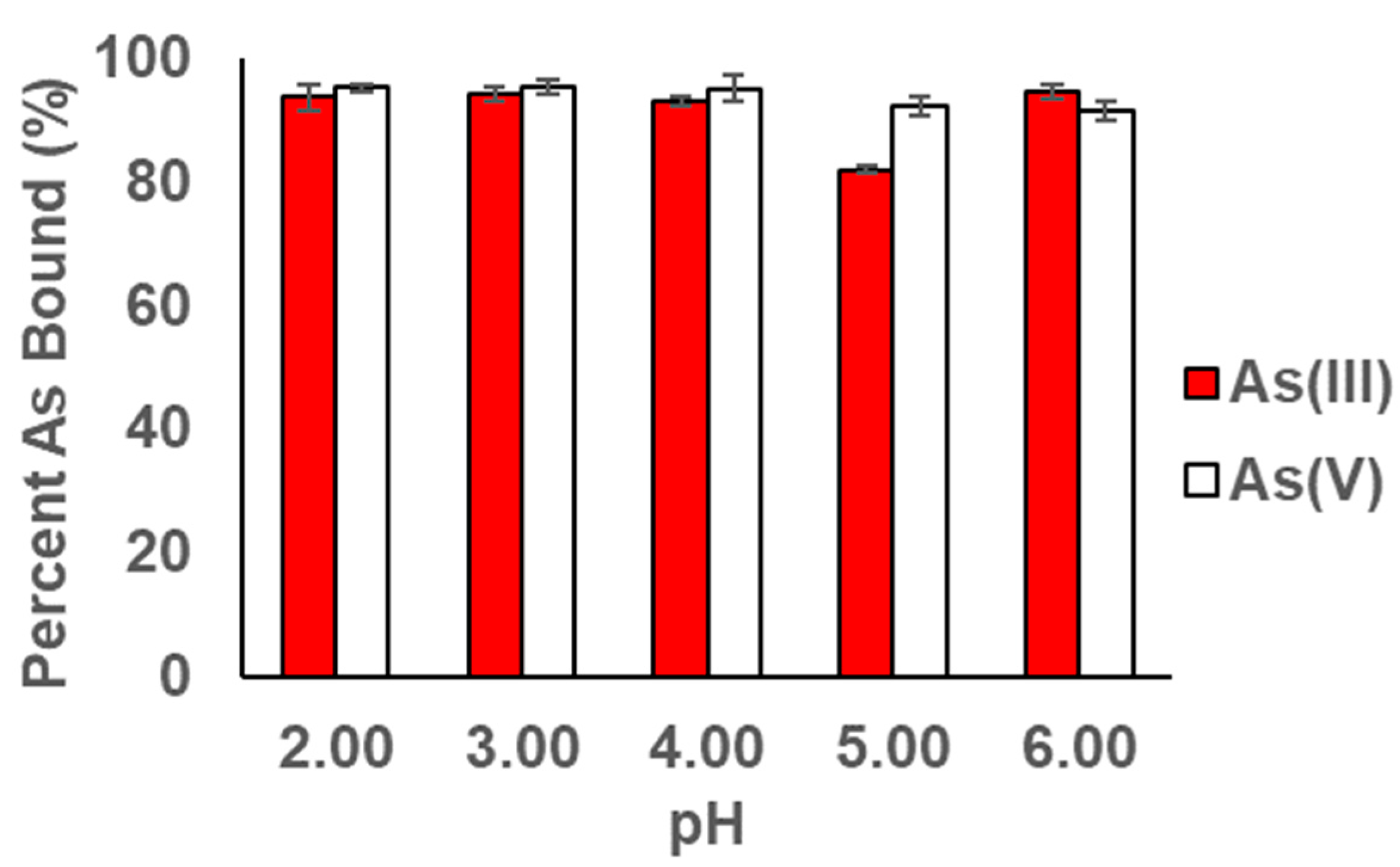
3.2. pH Profile Studies
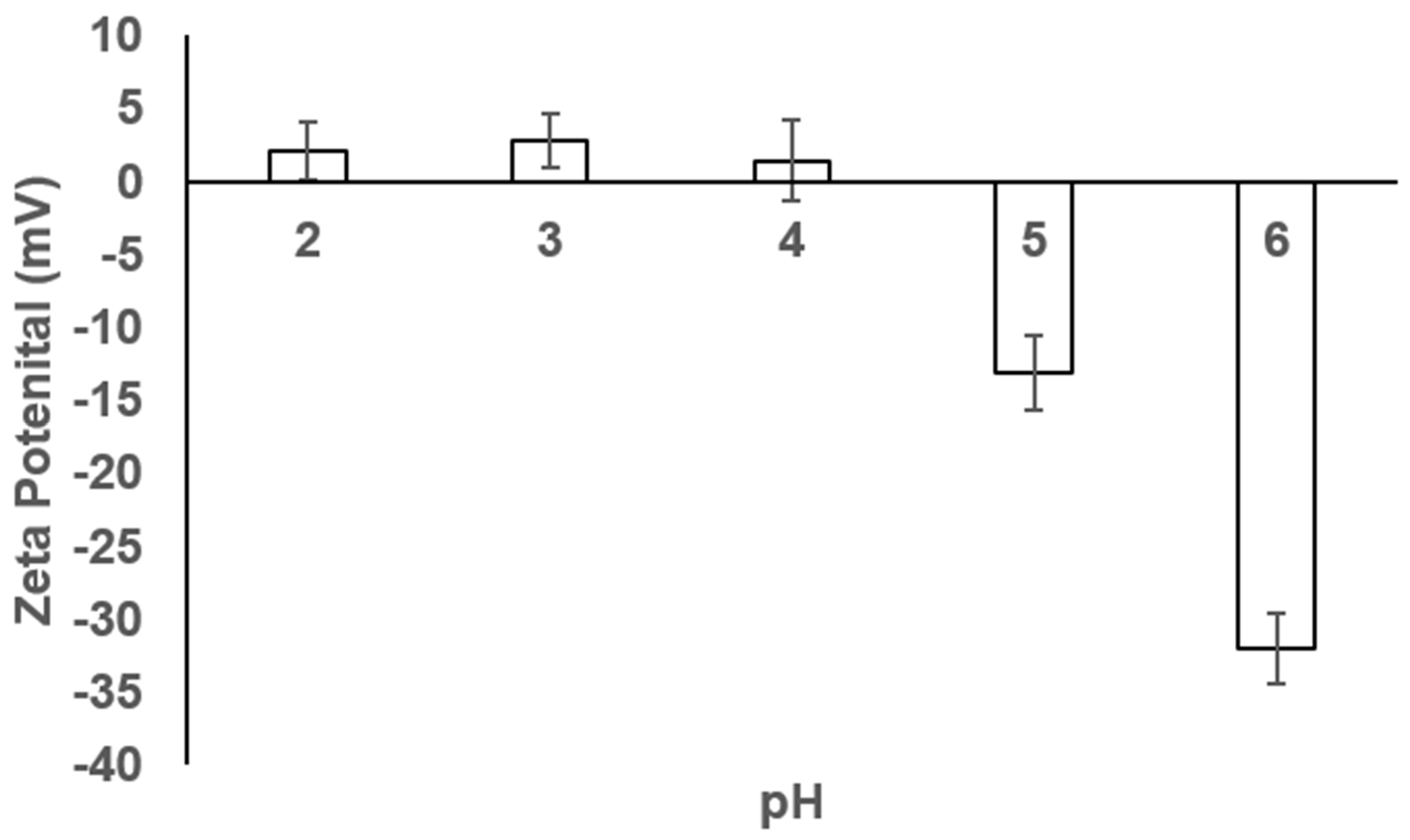
3.3. Zeta Potential of the ZnO Nanoparticles
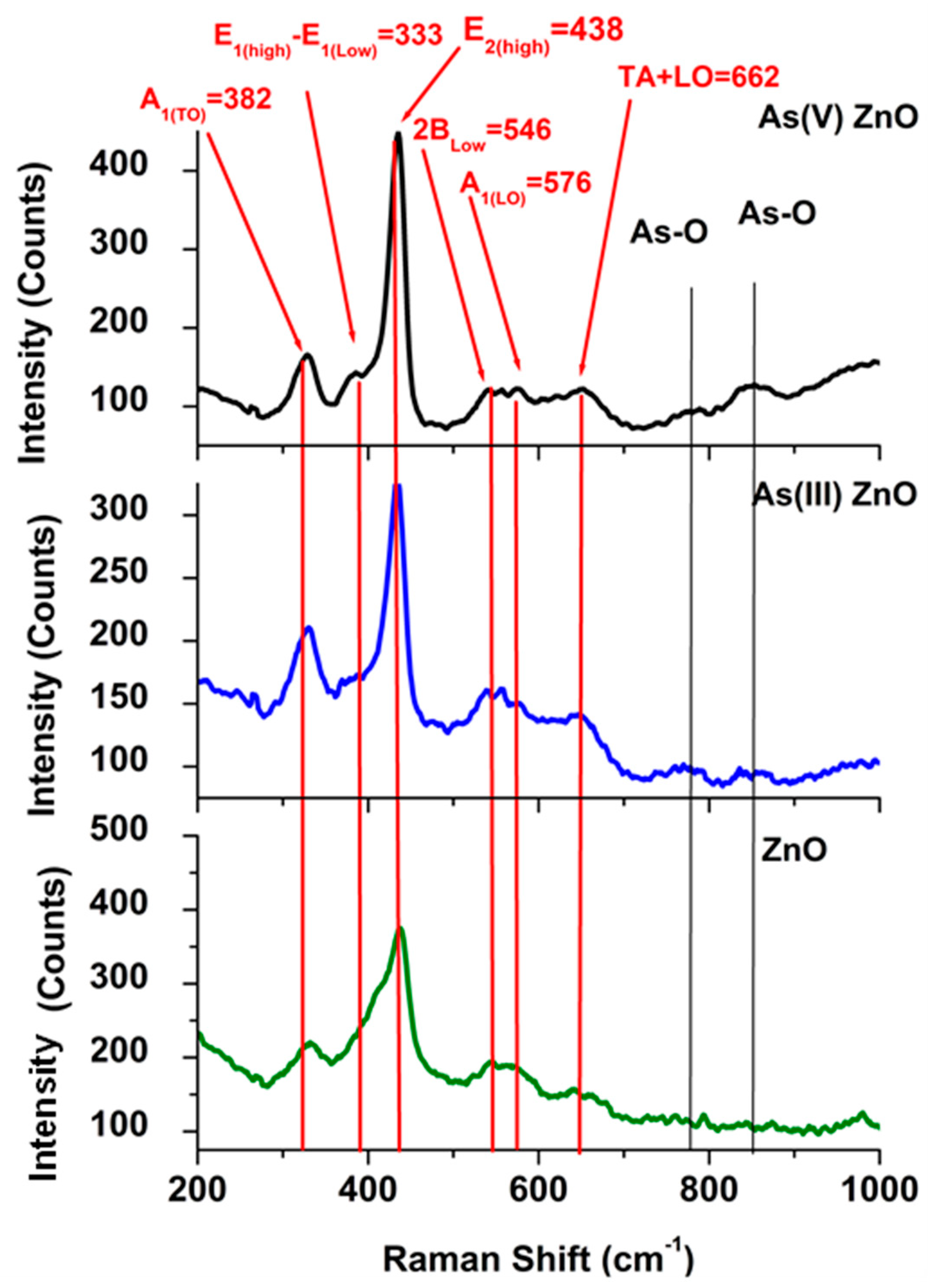
3.4. Particle Characterization after Reaction
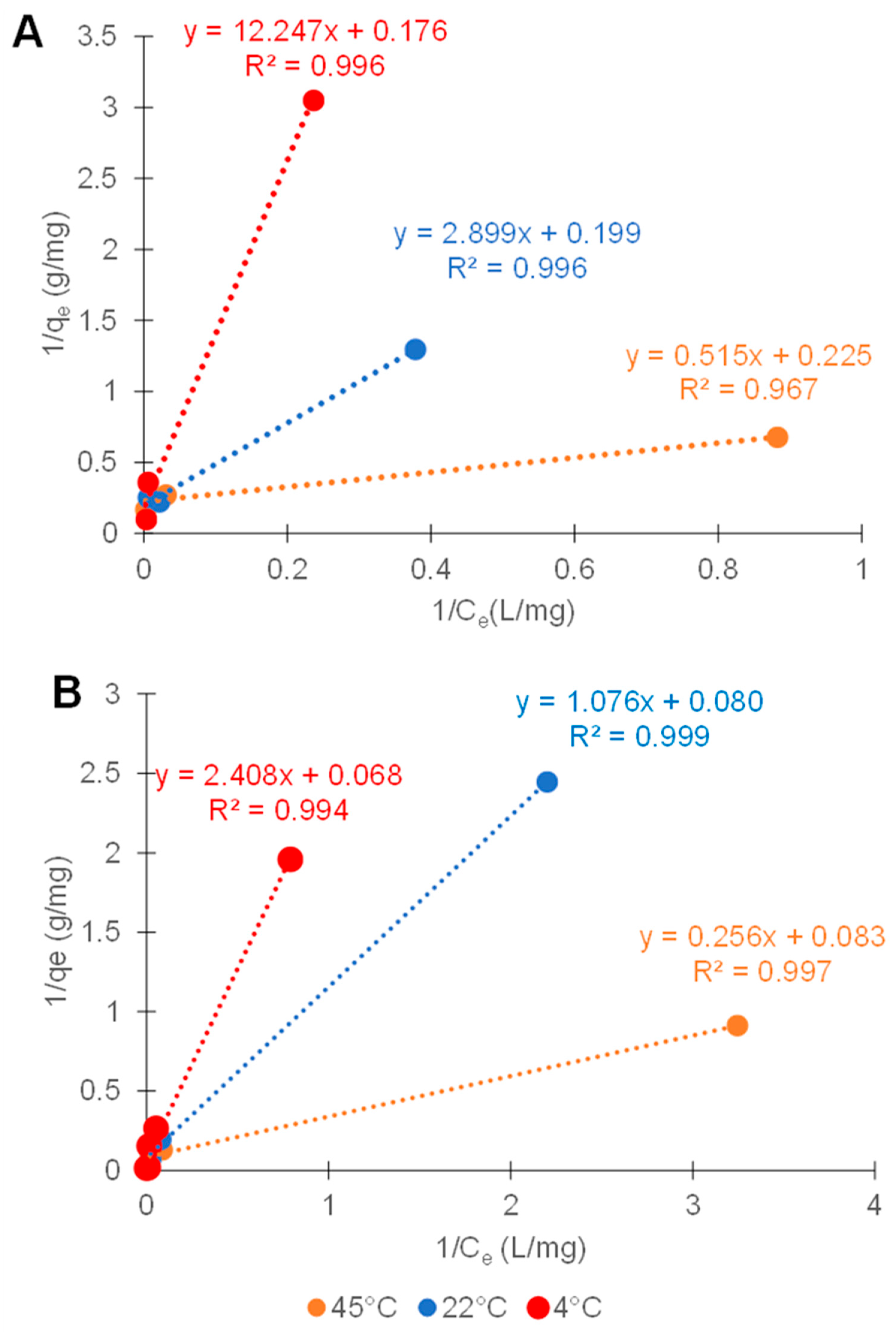
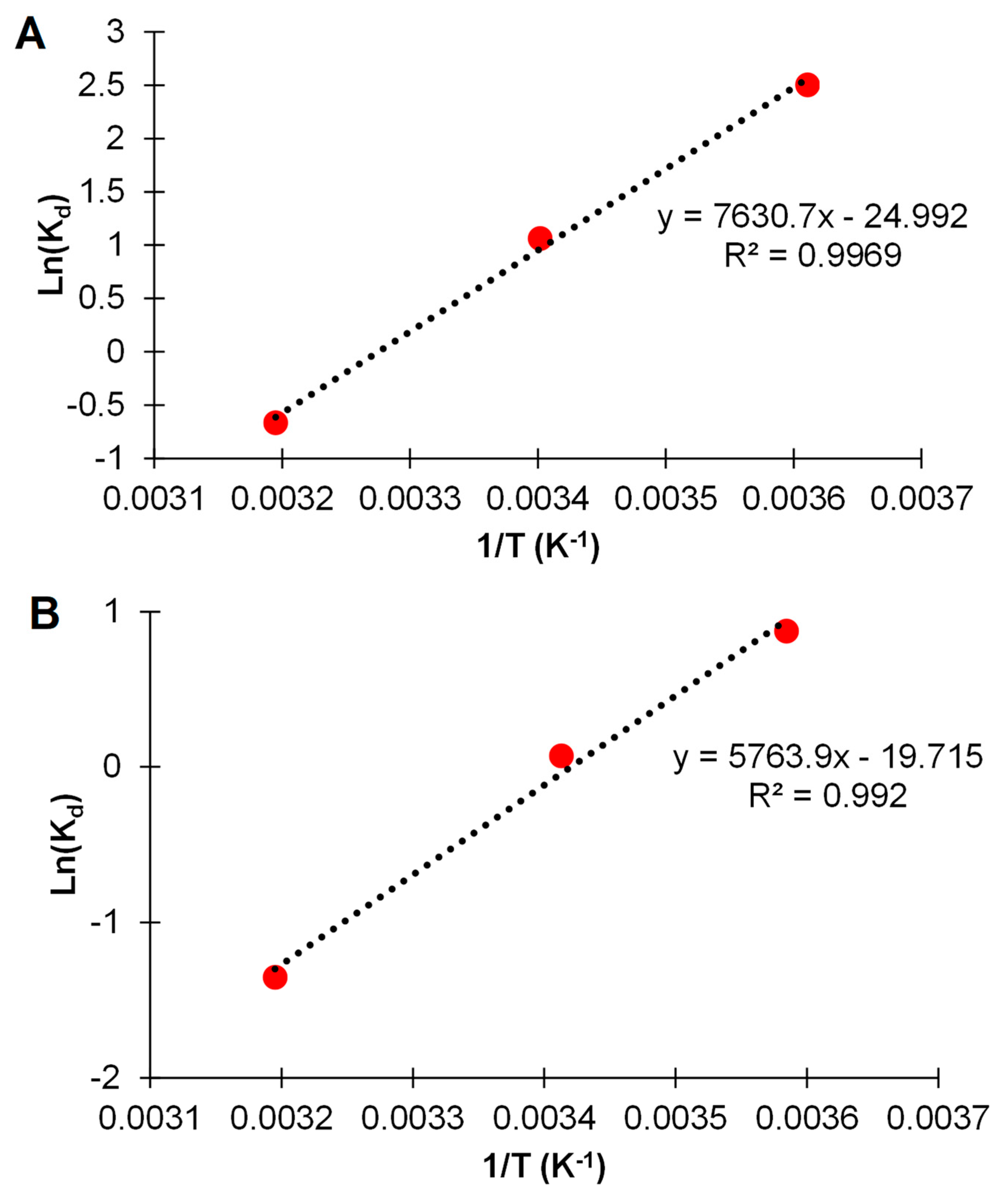
3.5. Adsorption Isotherms
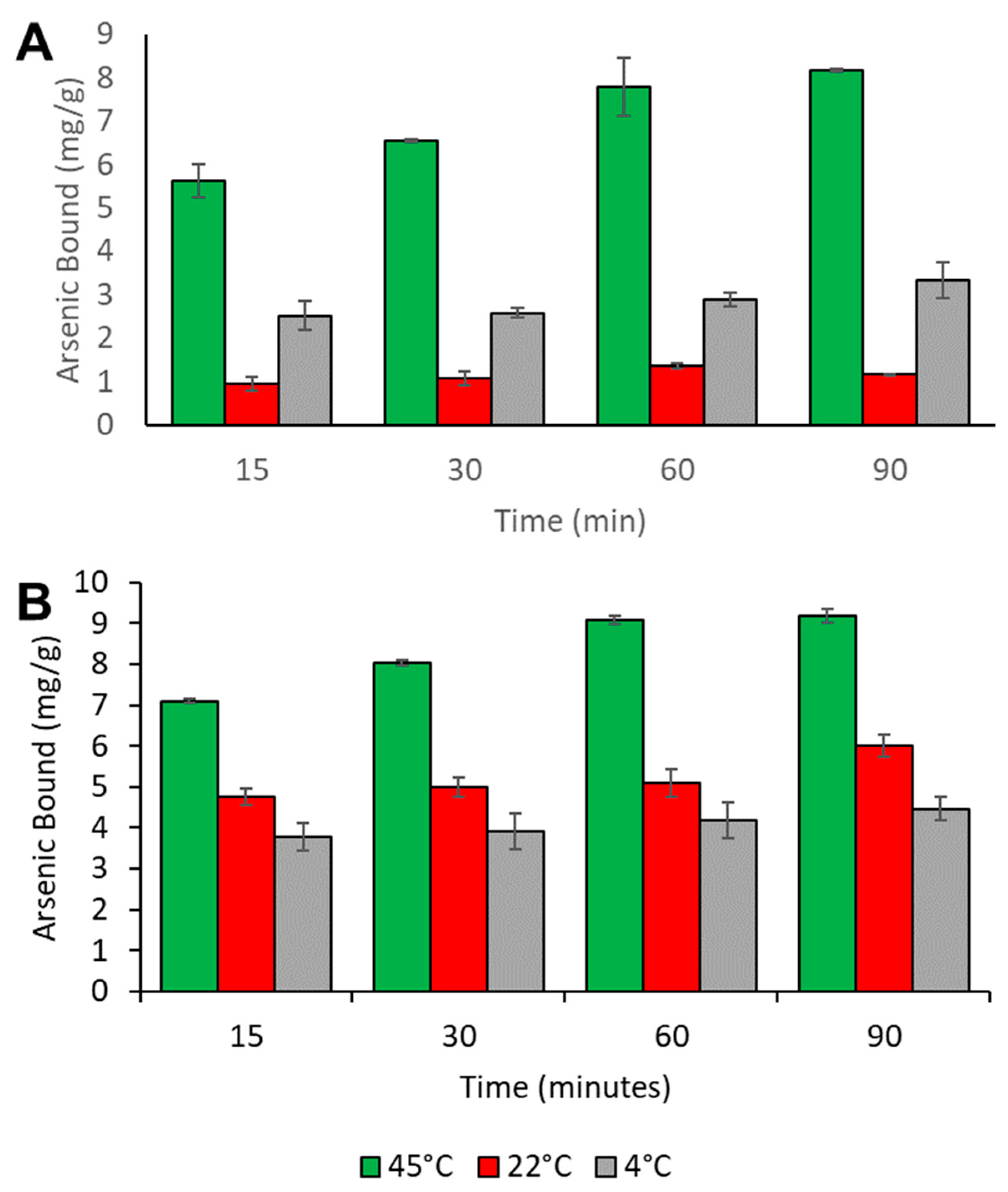
3.6. Time Dependency Studies
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bissen, M.; Frimmel, F.H. Arsenic—A review. Part I: Occurrence, toxicity, speciation, mobility. Acta Hydrochim. Hydrobiol. 2003, 31, 9–18. [Google Scholar] [CrossRef]
- Choong, T.S.; Chuah, T.G.; Robiah, Y.; Gregory Koay, F.L.; Azni, I. Arsenic toxicity, health hazards and removal techniques from water: An overview. Desalination 2007, 217, 139–166. [Google Scholar] [CrossRef]
- Gorby, M. Arsenic Poisoning. West. J. Med. 1988, 149, 308–315. [Google Scholar] [PubMed]
- Siddique, T.A.; Dutta, N.K.; Choudhury, N.R. Nanofiltration for arsenic removal: Challenges, recent developments, and perspectives. Nanomaterials 2020, 10, 1323. [Google Scholar] [CrossRef] [PubMed]
- Raju, N.J. Arsenic in the geo-environment: A review of sources, geochemical processes, toxicity and removal technologies. Environ. Res. 2022, 203, 111782. [Google Scholar] [CrossRef] [PubMed]
- Tchounwou, P.B.; Udensi, U.K.; Isokpehi, R.D.; Yedjou, C.G.; Kumar, S. 23—Arsenic and cancer. In Handbook of Arsenic Toxicology, 2nd ed.; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2023; pp. 607–630. [Google Scholar]
- Amen, R.; Bashir, H.; Bibi, I.; Shaheen, S.M.; Niazi, N.K.; Shahid, M.; Hussain, M.M.; Antoniadis, V.; Shakoor, M.B.; Al-Solaimani, S.G.; et al. A critical review on arsenic removal from water using biochar-based sorbents: The significance of modification and redox reactions. Chem. Eng. J. 2020, 396, 125195. [Google Scholar] [CrossRef]
- Sharma, V.K.; Sohn, M. Aquatic arsenic: Toxicity, speciation, transformations, and remediation. Environ. Int. 2009, 35, 743–759. [Google Scholar] [CrossRef]
- Jain, C.K.; Ali, I. Arsenic: Occurrence, toxicity and speciation techniques. Water Res. 2000, 34, 4304–4312. [Google Scholar] [CrossRef]
- Pontius, F.W.; Brown, K.G.; Chen, C.J. Health implications of arsenic in drinking water. J. Am. Water Work. Assn. 1994, 86, 52–63. [Google Scholar] [CrossRef]
- Machado-Neves, M.; Souza, A.C.F. 17—The Effect of Arsenical Compounds on Mitochondrial Metabolism; de Oliveira, M.R., Ed.; Mitochondrial Intoxication; Academic Press: Oxford, UK, 2023; pp. 379–407. [Google Scholar]
- Wen, Z.; Li, S.; Zhang, G.; Chen, R.; Zhang, Y.; Liao, X.; Cheng, G.; Chen, R. Effective roxarsone (ROX) and arsenate (As(V)) sequestration from water with magnetic hollow Fe3O4-based Mg/Al layered double hydroxide: Performance and mechanism. J. Clean. Prod. 2023, 389, 136085. [Google Scholar] [CrossRef]
- Zhao, F.; Liu, Y.; Zhang, F.; Dong, R.; Yu, W.; Zhang, H.; Han, X.; Gong, P.; Zhang, X.; Liu, Y.; et al. Simultaneous determination and distribution analysis of eleven arsenic species in vegetables. Microchem. J. 2023, 193, 109168. [Google Scholar] [CrossRef]
- Chen, Q.Y.; Costa, M. Arsenic: A Global Environmental Challenge. Annu. Rev. Pharmacol. Toxicol. 2021, 61, 47–63. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.B.; Athar, M. 11—Advances in cutaneous toxicology of arsenic. In Handbook of Arsenic Toxicology, 2nd ed.; Flora, S.J.S., Ed.; Academic Press: Oxford, UK, 2023; pp. 327–354. [Google Scholar]
- Dos Santos, L.M.G.; Barata-Silva, C.; Neto, S.A.V.; Magalhaes, C.D.; Moreira, J.C.; Jacob, S.C. Analysis and risk assessment of arsenic in rice from different regions of Brazil. J. Food Compos. Anal. 2021, 99, 103853. [Google Scholar] [CrossRef]
- Figoli, A.; Fuoco, I.; Apollaro, C.; Chabane, M.; Mancuso, R.; Gabriele, B.; De Rosa, R.; Vespasiano, G.; Barca, D.; Criscuoli, A. Arsenic-contaminated groundwaters remediation by nanofiltration. Sep. Purif. Technol. 2020, 238, 116461. [Google Scholar] [CrossRef]
- Khan, N.U.; Shahid, M.; Khalid, S.; Natasha, N.; Alothman, Z.A.; Al-Kahtani, A.A.; Imran, M.; Murtaza, B. Arsenic level in groundwater and biological samples in Khanewal, Pakistan. Environ. Geochem. Health 2023. epub ahead of print. [Google Scholar] [CrossRef] [PubMed]
- Teixeira, M.C.; Santos, A.C.; Fernandes, C.S.; Ng, J.C. Arsenic contamination assessment in Brazil–Past, present and future concerns: A historical and critical review. Sci. Total Environ. 2020, 730, 138217. [Google Scholar] [CrossRef]
- Alka, S.; Shahir, S.; Ibrahim, N.; Ndejiko, M.J.; Vo, D.V.N.; Manan, F.A. Arsenic removal technologies and future trends: A mini review. J. Clean. Prod. 2021, 278, 123805. [Google Scholar] [CrossRef]
- Kabir, F.; Chowdhury, S. Arsenic removal methods for drinking water in the developing countries: Technological developments and research needs. Environ. Sci. Pollut. Res. 2017, 24, 24102–24120. [Google Scholar] [CrossRef]
- Kumar, R.; Patel, M.; Singh, P.; Bundschuh, J.; Pittman, C.U.; Trakal, L.; Mohan, D. Emerging technologies for arsenic removal from drinking water in rural and peri-urban areas: Methods, experience from, and options for Latin America. Sci. Total Environ. 2019, 694, 133427. [Google Scholar] [CrossRef]
- Mohanty, D. Conventional as well as Emerging Arsenic Removal Technologies—A Critical Review. Water Air Soil Pollut. 2017, 228, 381. [Google Scholar] [CrossRef]
- Nicomel, N.R.; Leus, K.; Folens, K.; Van Der Voort, P.; Du Laing, G. Technologies for arsenic removal from water: Current status and future perspectives. Int. J. Environ. Res. Public Health 2015, 13, 62. [Google Scholar] [CrossRef] [PubMed]
- Ng, K.S.; Ujang, Z.; Le-Clech, P. Arsenic removal technologies for drinking water treatment. Rev. Environ. Sci. Biotechnol. 2004, 3, 43–53. [Google Scholar] [CrossRef]
- Nidheesh, P.V.; Singh, T.S. Arsenic removal by electrocoagulation process: Recent trends and removal mechanism. Chemosphere 2017, 181, 418–432. [Google Scholar] [CrossRef] [PubMed]
- Siddiqui, S.I.; Chaudhry, S.A. Iron oxide and its modified forms as an adsorbent for arsenic removal: A comprehensive recent advancement. Process Saf. Environ. Prot. 2017, 111, 592–626. [Google Scholar] [CrossRef]
- Wang, S.; Lu, Y.; Ouyang, X.-K.; Liang, X.X.; Yu, D.; Yang, L.-Y.; Huang, F. Fabrication of chitosan-based MCS/ZnO@Alg gel microspheres for efficient adsorption of As(v). Int. J. Biol. Macromol. 2019, 139, 886–895. [Google Scholar] [CrossRef]
- Zakhar, R.; Derco, J.; Cacho, F. An overview of main arsenic removal technologies. Acta Chim. Slovaca 2018, 11, 107–113. [Google Scholar]
- Parsons, J.; Lopez, M.; Peralta-Videa, J.; GardeaTorresdey, J. Determination of arsenic(iii) and arsenic(v) binding to microwave assisted hydrothermal synthetically prepared Fe3O4, Mn3O4, and MnFe2O4 nanoadsorbents. Microchem. J. 2019, 91, 100–106. [Google Scholar] [CrossRef]
- Babaee, Y.; Mulligan, C.N.; Rahaman, M.S. Removal of arsenic (III) and arsenic (V) from aqueous solutions through adsorption by Fe/Cu nanoparticles. J. Chem. Technol. Biotechnol. 2018, 93, 63–71. [Google Scholar] [CrossRef]
- Asere, T.G.; Stevens, C.V.; Du Laing, G. Use of (modified) natural adsorbents for arsenic remediation: A review. Sci. Total Environ. 2019, 676, 706–720. [Google Scholar] [CrossRef]
- Carneiro, M.A.; Pintor, A.M.A.; Boaventura, R.A.R.; Botelho, C.M.S. Current trends of arsenic adsorption in continuous mode: Literature review and future perspectives. Sustainability 2021, 13, 1186. [Google Scholar] [CrossRef]
- Hao, L.; Wang, N.; Wang, C.; Li, G. Arsenic removal from water and river water by the combined adsorption—UF membrane process. Chemosphere 2018, 202, 768–776. [Google Scholar] [CrossRef] [PubMed]
- Lata, S.; Samadder, S.R. Removal of arsenic from water using nano adsorbents and challenges: A review. J. Environ. Manag. 2016, 166, 387–406. [Google Scholar] [CrossRef] [PubMed]
- Massoudinejad, M.; Keramati, H.; Ghaderpoori, M. Investigation of photo-catalytic removal of arsenic from aqueous solutions using UV/H2O2 in the presence of ZnO nanoparticles. Chem. Eng. Commun. 2020, 207, 1605–1615. [Google Scholar] [CrossRef]
- Singh, N.; Singh, S.; Gupta, V.; Yadav, H.K.; Ahuja, T.; Tripathy, S.S. A process for the selective removal of arsenic from contaminated water using acetate functionalized zinc oxide nanomaterials. Environ. Prog. Sustain. Energy. 2013, 32, 1023–1029. [Google Scholar] [CrossRef]
- Kataria, N.; Garg, V.K. Optimization of Pb (II) and Cd (II) adsorption onto ZnO nanoflowers using central composite design: Isotherms and kinetics modelling. J. Mol. Liq. 2018, 271, 228–239. [Google Scholar] [CrossRef]
- Gu, M.; Hao, L.; Wang, Y.; Li, X.; Chen, Y.; Li, W.; Jiang, L. The selective heavy metal ions adsorption of zinc oxide nanoparticles from dental wastewater. Chem. Phys. 2020, 534, 110750. [Google Scholar] [CrossRef]
- Cantu, J.; Gonzalez, L.E.; Goodship, J.; Contreras, M.; Joseph, M.; Garza, C.; Eubanks, T.M.; Parsons, J.G. Removal of arsenic from water using synthetic Fe7S8 nanoparticles. Chem. Eng. J. 2016, 290, 428–437. [Google Scholar] [CrossRef]
- Luther, S.; Borgfeld, N.; Kim, J.; Parsons, J.G. Removal of arsenic from aqueous solution: A study of the effects of pH and interfering ions using iron oxide nanomaterials. Microchem. J. 2012, 101, 30–36. [Google Scholar] [CrossRef]
- Albertsson, J.; Abrahams, S.C.; Kvick, A. Atomic displacement, anharmonic thermal vibration, expansivity and pyroelectric coefficient thermal dependences in ZnO. Acta Crystallogr. Sect. B 1989, 45, 34–40. [Google Scholar] [CrossRef]
- Rodriguez-Carvajal, J. Recent advances in magnetic structure determination by neutron powder diffraction. Physica B 1993, 192, 55–69. [Google Scholar] [CrossRef]
- Zhou, J.; Zhao, F.; Wang, Y.; Zhang, Y.; Yang, L. Size-controlled synthesis of ZnO nanoparticles and their photoluminescence properties. J. Lumin. 2007, 122–123, 195–197. [Google Scholar] [CrossRef]
- Jegadeesan, G.; Al-Abed, S.R.; Sundaram, V.; Choi, H.; Scheckel, K.G.; Dionysiou, D.D. Arsenic sorption on TiO2 nanoparticles: Size and crystallinity effects. Water Res. 2010, 44, 965–973. [Google Scholar] [CrossRef] [PubMed]
- Jézéquel, H.; Chu, K.H. Removal of Arsenate from Aqueous Solution by Adsorption onto Titanium Dioxide Nanoparticles. J. Environ. Sci. Health Part A 2006, 41, 1519–1528. [Google Scholar] [CrossRef]
- Feng, L.; Cao, M.; Ma, X.; Zhu, Y.; Hu, C. Superparamagnetic high-surface-area Fe3O4 nanoparticles as adsorbents for arsenic removal. J. Hazard. Mater. 2012, 217–218, 439–446. [Google Scholar] [CrossRef]
- Rehman, H.; Ali, Z.; Hussain, M.; Gilani, S.R.; Shahzady, T.G.; Zahra, A.; Hussin, S.; Hussain, H.; Hussain, I.; Farooq, M.U.H. Synthesis and Characterization of ZnO Nanoparticles and their use as an Adsorbent for the Arsenic Removal from Drinking Water. Dig. J. Nanomater. Biostruct. 2019, 14, 1033–1040. [Google Scholar]
- Bulut, G.; Yenial, U.; Emirolu, E.; Sirkeci, A.A. Arsenic removal from aqueous solution using pyrite. J. Clean. Prod. 2014, 84, 526–532. [Google Scholar] [CrossRef]
- Garcia, S.; Sardar, S.; Maldonado, S.; Garcia, V.; Tamez, C.; Parsons, J.G. Study of As(III) and As(V) Oxoanion Adsorption onto Single and Mixed Ferrite and Hausmannite Nanomaterials. Microchem. J. 2012, 117, 52–60. [Google Scholar] [CrossRef]
- Goswami, A.; Raul, P.K.; Purkait, M.K. Arsenic adsorption using copper (II) oxide nanoparticles. Chem. Eng. Res. Des. 2012, 90, 1387–1396. [Google Scholar] [CrossRef]
- Guan, X.; Du, J.; Meng, X.; Sun, Y.; Sun, B.; Hu, Q. Application of titanium dioxide in arsenic removal from water: A review. J. Hazard. Mater. 2012, 215–216, 1–16. [Google Scholar] [CrossRef]
- Kong, S.; Wang, Y.; Zhan, H.; Yuan, S.; Yu, M.; Liu, M. Adsorption/Oxidation of arsenic in groundwater by nanoscale FeMn binary oxides loaded on zeolite. Water Environ. Res. 2014, 86, 147–155. [Google Scholar] [CrossRef]
- Lunge, S.; Singh, S.; Sinha, A. Magnetic iron oxide (Fe3O4) nanoparticles from tea waste for arsenic removal. J. Magn. Magn. Mater. 2014, 356, 21–31. [Google Scholar] [CrossRef]
- Mamindy-Pajany, Y.; Hurel, C.; Marmier, N.; Romeo, M. Arsenic (V) adsorption from aqueous solution onto goethite, hematite, magnetite and zero-valent iron: Effects of pH, concentration and reversibility. Desalination 2011, 281, 93–99. [Google Scholar] [CrossRef]
- Wen, Z.; Zhang, Y.; Dai, C.; Chen, B.; Guo, S.; Yu, H.; Wu, D. Synthesis of ordered mesoporous iron manganese bimetal oxides for arsenic removal from aqueous solutions. Microporous Mesoporous Mater. 2014, 200, 235–244. [Google Scholar] [CrossRef]
- Fatehah, M.O.; Aziz, H.A.; Stoll, S. Stability of ZnO Nanoparticles in Solution. Influence of pH, Dissolution, Aggregation and Disaggregation Effects. J. Colloid Sci. Biotechnol. 2014, 3, 75–84. [Google Scholar] [CrossRef]
- Chai, M.H.H.; Amir, N.; Yahya, N.; Saaid, I.M. Characterization and Colloidal Stability of Surface Modified Zinc Oxide Nanoparticle. J. Phys. Conf. Ser. 2018, 1123, 01200. [Google Scholar]
- Kim, K.M.; Choi, M.H.; Lee, J.K.; Jeong, J.; Kim, Y.R.; Kim, M.K.; Paek, S.M.; Oh, J.M. Physicochemical properties of surface charge-modified ZnO nanoparticles with different particle sizes. Int. J. Nanomed. 2014, 9 (Suppl. S2), 41–56. [Google Scholar] [CrossRef]
- Cusco, R.; Alarcon-Llado, E.; Ibanez, J.; Artus, L.; Jimenez, J.; Wang, B.; Callahan, M.J. Temperature dependence of RAMAN scattering in ZnO. Phys. Rev. B Condens. Mater. 2007, 75, 165202. [Google Scholar] [CrossRef]
- Jaramillo, A.; Baez-Cruz, R.; Montoya, L.F.; Medinam, C.; Pérez-Tijerina, E.; Salazar, F.; Rojas, D.; Melendrez, M. Estimation of the surface interaction mechanism of zno nanoparticles modified with organosilane groups by raman spectroscopy. Ceram. Int. 2017, 43, 11838–11847. [Google Scholar] [CrossRef]
- Song, Y.; Zhang, S.; Zhang, C.; Yang, Y.; Lv, K. Raman spectra and microstructure of zinc oxide irradiated with swift heavy ion. Crystals 2019, 9, 395. [Google Scholar] [CrossRef]
- Frost, R.L.; Martens, W.; Williams, P.A.; Kloprogge, J.T. Raman spectroscopic study of the vivianite arsenate minerals. Raman Spectrosc. 2003, 34, 751–759. [Google Scholar] [CrossRef]
- Frost, R.L.; Čejka, J.; Sejkora, J.; Plášil, J.; Reddy, B.J.; Keeffe, E.C. Raman spectroscopic study of a hydroxy-arsenate mineral containing bismuth–atelestite Bi2O(OH)(AsO4). Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2011, 78, 494–496. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-H.; Chuang, Y.-H.; Chen, T.-Y.; Tian, Y.; Li, H.; Wang, M.-K.; Zhang, W. Mechanism of Arsenic Adsorption on Magnetite Nanoparticles from Water: Thermodynamic and Spectroscopic Studies. Environ. Sci. Technol. 2015, 49, 7726–7734. [Google Scholar] [CrossRef] [PubMed]
- Mulvihill, M.; Tao, A.; Benjauthrit, K.; Arnold, J.; Yang, P. Surface-Enhanced Raman Spectroscopy for Trace Arsenic Detection in Contaminated Water. Angew. Chem. Int. Ed. Engl. 2008, 7, 6456–6460. [Google Scholar] [CrossRef] [PubMed]
- Burton, E.D.; Bush, R.T.; Johnston, S.G.; Watling, K.M.; Hocking, R.K.; Sullivan, L.A.; Parker, G.K. Sorption of Arsenic(V) and Arsenic(III) to Schwertmannite. Environ. Sci. Technol. 2009, 43, 9202–9207. [Google Scholar] [CrossRef]
- Xu, Z.; Hao, J.; Lib, F.; Meng, X. Surface-enhanced Raman spectroscopy of arsenate and arsenite using Ag nanofilm prepared by modified mirror reaction. J. Colloid Interface Sci. 2010, 347, 90–95. [Google Scholar] [CrossRef]
- Huang, M.; Feng, W.; Xu, W.; Liu, P. An in situ gold-decorated 3D branched ZnO nanocomposite and its enhanced absorption and photo-oxidation performance for removing arsenic from water. RSC Adv. 2016, 6, 112877–112884. [Google Scholar] [CrossRef]
- Vaiano, V.; Iervolino, G.; Rizzo, L. Cu-doped ZnO as efficient photocatalyst for the oxidation of arsenite to arsenate under visible light. Appl. Catal. B Environ. 2018, 238, 471–479. [Google Scholar] [CrossRef]
- Cruz, G.J.; Mondal, D.; Rimaycuna, J.; Soukup, K.; Gomez, M.M.; Solis, J.L.; Lang, J. Agrowaste derived biochars impregnated with ZnO for removal of arsenic and lead in water. J. Environ. Chem. Eng. 2020, 8, 103800. [Google Scholar] [CrossRef]
- Mahato, B.N.; Krithiga, T.; Mary Thangam, M.A. Rapid adsorption of As(V) from aqueous solution by ZnO embedded in mesoporous aluminosilicate nanocomposite adsorbent: Parameter optimization, kinetic, and isotherms studies. Surf. Interfaces 2021, 23, 100636. [Google Scholar] [CrossRef]
- Yuvaraja, G.; Prasad, C.; Vijaya, Y.; Subbaiah, M.V. Application of ZnO nanorods as an adsorbent material for the removal of As(III) from aqueous solution: Kinetics, isotherms and thermodynamic studies. Int. J. Ind. Chem. 2018, 9, 17–25. [Google Scholar] [CrossRef]
- Altundogan, H.S.; Altundogan, S.; Tumen, F.; Bildik, M. Arsenic adsorption from aqueous solutions by activated red mud. Waste Manag. 2002, 22, 357–363. [Google Scholar] [CrossRef] [PubMed]
- Feng, Q.; Zhang, Z.; Ma, Y.; He, X.; Zhao, Y.; Chai, Z. Adsorption and desorption characteristics of arsenic onto ceria nanoparticles. Nanoscale Res. Lett. 2012, 7, 84. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.; Li, Q.; Gao, S.; Shang, J.K. High efficient As(III) removal by self-assembled zinc oxide micro-tubes synthesized by a simple precipitation process. J. Mater. Sci. 2011, 46, 5851–5858. [Google Scholar] [CrossRef]
- Yazdani, M.; Tuutijärvi, T.; Bhatnagar, A.; Vahala, R. Adsorptive removal of arsenic(V) from aqueous phase by feldspars: Kinetics, mechanism, and thermodynamic aspects of adsorption. J. Mol. Liq. 2016, 214, 149–156. [Google Scholar] [CrossRef]
- Navarathna, C.M.; Karunanayake, A.G.; Gunatilake, S.R.; Pittman, C.U.; Perez, F.; Mohan, D.; Mlsna, T. Removal of Arsenic(III) from water using magnetite precipitated onto Douglas fir biochar. J. Environ. Manag. 2019, 250, 109429. [Google Scholar] [CrossRef] [PubMed]
- Monarrez-Cordero, B.E.; Saenz-Trevizo, A.; Bautista-Carrillo, L.M.; Silva-Vidaurri, L.G.; Miki-Yoshida, M.; Amezaga-Madrid, P. Simultaneous and fast removal of As3+, As5+, Cd2+, Cu2+, Pb2+ and F− from water with composite Fe-Ti oxides nanoparticles. J. Alloys Compd. 2018, 757, 150–160. [Google Scholar] [CrossRef]


| Parameter | Setting |
|---|---|
| L | 193.696 nm |
| RF power | 1500 W |
| Nebulizer | Gemcone (low flow) |
| Plasma flow | 15 L min−1 |
| Auxiliary flow | 0.2 L min−1 |
| Nebulizer flow | 0.55 L min−1 |
| Sample flow | 1.50 L min−1 |
| Injector | 2.0 mm Alumina |
| Spray chamber | Cyclonic |
| Integration time | 20 s |
| Replicates | 3 |
| Sample | a (°A) | b (°A) | c (°A) | α° | β° | γ° | χ2 |
|---|---|---|---|---|---|---|---|
| ZnOsyn | 3.245(8) | 3.245(8) | 5.199(8) | 90.0 | 90.0 | 120.0 | 3.08 |
| ZnOlit | 3.2417 | 3.2417 | 5.1876 | 90.0 | 90.0 | 120.0 |
| Sample | a (°A) | b (°A) | c (°A) | α° | β° | γ° | χ2 |
|---|---|---|---|---|---|---|---|
| As(III)ZnO | 3.245(8) | 3.245(8) | 5.214(8) | 90.0 | 90.0 | 120.0 | 3.08 |
| As(V)ZnO | 3.261(1) | 3.261(1) | 5.220(9) | 90.0 | 90.0 | 120.0 | 3.35 |
| Sample | Capacity (mg/g) |
|---|---|
| As(III) 4 °C | 5.83 |
| As(III) 22 °C | 5.03 |
| As(III) 45 °C | 4.44 |
| As(V) 4 °C | 14.68 |
| As(V) 22 °C | 12.56 |
| As(V) 45 °C | 12.09 |
| Sample | ∆G (kJmol−1) | ∆H (kJ mol−1) | ∆S (J mol−1K−1) |
|---|---|---|---|
| As(III) 4 °C | −5.76 | ||
| As(III) 22 °C | −2.60 | −63.4 | −233.6 |
| As(III) 45 °C | 1.72 | ||
| As(V) 4 °C | −2.04 | ||
| As(V) 22 °C | −0.18 | −47.29 | −164.25 |
| As(V) 45 °C | 3.52 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Morales, H.M.; Torreblanca, G.; Mar, A.; Alcoutlabi, M.; Eubanks, T.M.; Plata, E.; Parsons, J.G. Investigation of the Thermodynamics for the Removal of As(III) and As(V) from Water Using Synthesized ZnO Nanoparticles and the Effects of pH, Temperature, and Time. Appl. Sci. 2023, 13, 10525. https://doi.org/10.3390/app131810525
Morales HM, Torreblanca G, Mar A, Alcoutlabi M, Eubanks TM, Plata E, Parsons JG. Investigation of the Thermodynamics for the Removal of As(III) and As(V) from Water Using Synthesized ZnO Nanoparticles and the Effects of pH, Temperature, and Time. Applied Sciences. 2023; 13(18):10525. https://doi.org/10.3390/app131810525
Chicago/Turabian StyleMorales, Helia Magali, Grecia Torreblanca, Arnulfo Mar, Mataz Alcoutlabi, Thomas Mark Eubanks, Erik Plata, and Jason George Parsons. 2023. "Investigation of the Thermodynamics for the Removal of As(III) and As(V) from Water Using Synthesized ZnO Nanoparticles and the Effects of pH, Temperature, and Time" Applied Sciences 13, no. 18: 10525. https://doi.org/10.3390/app131810525
APA StyleMorales, H. M., Torreblanca, G., Mar, A., Alcoutlabi, M., Eubanks, T. M., Plata, E., & Parsons, J. G. (2023). Investigation of the Thermodynamics for the Removal of As(III) and As(V) from Water Using Synthesized ZnO Nanoparticles and the Effects of pH, Temperature, and Time. Applied Sciences, 13(18), 10525. https://doi.org/10.3390/app131810525








