The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) Larvae
Abstract
:1. Introduction
2. Materials and Methods
2.1. Material
2.2. Pulsed Electric Field Treatment (PEF)
2.3. Freeze Drying (FD)
2.4. Physical Properties
2.4.1. Water Activity
2.4.2. Rehydration Properties
2.4.3. Hygroscopic Properties
2.4.4. Color
2.5. Chemical Composition
2.5.1. Moisture Content and Dry Matter Content
2.5.2. Protein Content
2.5.3. Fat Content
2.5.4. Ash Content
2.5.5. FTIR Analysis
2.6. Structure with µTomography
2.7. Microbiological Assessment
2.8. Statistical Analysis
3. Results and Discussion
3.1. Freeze-Drying Process of Edible Insects Larvae
3.2. Physical Properties of Freeze-Dried Edible Insects Larvae
3.2.1. Dry Matter Content and Water Activity of Freeze-Dried Edible Insects Larvae
3.2.2. Rehydration Rate and Hygroscopic Properties of Freeze-Dried Edible Insects Larvae
3.2.3. Color of Freeze-Dried Edible Insects Larvae
3.3. Structure of Freeze-Dried Edible Insects Larvae
3.4. Chemical Composition of Freeze-Dried Edible Insect Larvae
3.5. Microbiological Quality of Freeze-Dried Edible Insects Larvae
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Duda, A.; Adamczak, J.; Chełmińska, P.; Juszkiewicz, J.; Kowalczewski, P. Quality and Nutritional/Textural Properties of Durum Wheat Pasta Enriched with Cricket Powder. Foods 2019, 8, 46. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Pankiewicz, U. Nutritional, Physiochemical, and Antioxidative Characteristics of Shortcake Biscuits Enriched with Tenebrio Molitor Flour. Molecules 2020, 25, 5629. [Google Scholar] [CrossRef] [PubMed]
- Zielińska, E.; Karaś, M.; Baraniak, B. Comparison of Functional Properties of Edible Insects and Protein Preparations Thereof. LWT 2018, 91, 168–174. [Google Scholar] [CrossRef]
- Montowska, M.; Kowalczewski, P.Ł.; Rybicka, I.; Fornal, E. Nutritional Value, Protein and Peptide Composition of Edible Cricket Powders. Food Chem. 2019, 289, 130–138. [Google Scholar] [CrossRef]
- Boukid, F.; Riudavets, J.; del Arco, L.; Castellari, M. Impact of Diets Including Agro-Industrial By-Products on the Fatty Acid and Sterol Profiles of Larvae Biomass from Ephestia Kuehniella, Tenebrio Molitor and Hermetia Illucens. Insects 2021, 12, 672. [Google Scholar] [CrossRef]
- Krzyżaniak, M.; Aljewicz, M.; Bordiean, A.; Stolarski, M.J. Yellow Mealworm Composition after Convective and Freeze Drying—Preliminary Results. Agriculture 2022, 12, 149. [Google Scholar] [CrossRef]
- Lemke, B.; Siekmann, L.; Grabowski, N.T.; Plötz, M.; Krischek, C. Impact of the Addition of Tenebrio Molitor and Hermetia Illucens on the Physicochemical and Sensory Quality of Cooked Meat Products. Insects 2023, 14, 487. [Google Scholar] [CrossRef]
- Franco, A.; Salvia, R.; Scieuzo, C.; Schmitt, E.; Russo, A.; Falabella, P. Lipids from Insects in Cosmetics and for Personal Care Products. Insects 2021, 13, 41. [Google Scholar] [CrossRef]
- Kröncke, N.; Böschen, V.; Woyzichovski, J.; Demtröder, S.; Benning, R. Comparison of Suitable Drying Processes for Mealworms (Tenebrio Molitor). Innov. Food Sci. Emerg. Technol. 2018, 50, 20–25. [Google Scholar] [CrossRef]
- Baiano, A. Edible Insects: An Overview on Nutritional Characteristics, Safety, Farming, Production Technologies, Regulatory Framework, and Socio-Economic and Ethical Implications. Trends Food Sci. Technol. 2020, 100, 35–50. [Google Scholar] [CrossRef]
- Garofalo, C.; Milanović, V.; Cardinali, F.; Aquilanti, L.; Clementi, F.; Osimani, A. Current Knowledge on the Microbiota of Edible Insects Intended for Human Consumption: A State-of-the-Art Review. Food Res. Int. 2019, 125, 108527. [Google Scholar] [CrossRef]
- Ng’ang’a, J.; Imathiu, S.; Fombong, F.; Ayieko, M.; Vanden Broeck, J.; Kinyuru, J. Microbial Quality of Edible Grasshoppers Ruspolia Differens (Orthoptera: Tettigoniidae): From Wild Harvesting to Fork in the Kagera Region, Tanzania. J. Food Saf. 2019, 39, 1–6. [Google Scholar] [CrossRef]
- Messina, C.M.; Gaglio, R.; Morghese, M.; Tolone, M.; Arena, R.; Moschetti, G.; Santulli, A.; Francesca, N.; Settanni, L. Microbiological Profile and Bioactive Properties of Insect Powders Used in Food and Feed Formulations. Foods 2019, 8, 400. [Google Scholar] [CrossRef]
- Yan, X.; Laurent, S.; Hue, I.; Cabon, S.; Grua-Priol, J.; Jury, V.; Federighi, M.; Boué, G. Quality of Tenebrio Molitor Powders: Effects of Four Processes on Microbiological Quality and Physicochemical Factors. Foods 2023, 12, 572. [Google Scholar] [CrossRef] [PubMed]
- Ssepuuya, G.; Wynants, E.; Verreth, C.; Crauwels, S.; Lievens, B.; Claes, J.; Nakimbugwe, D.; Van Campenhout, L. Microbial Characterisation of the Edible Grasshopper Ruspolia Differens in Raw Condition after Wild-Harvesting in Uganda. Food Microbiol. 2019, 77, 106–117. [Google Scholar] [CrossRef] [PubMed]
- Garofalo, C.; Osimani, A.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Riolo, P.; Ruschioni, S.; Isidoro, N.; Clementi, F. The Microbiota of Marketed Processed Edible Insects as Revealed by High-Throughput Sequencing. Food Microbiol. 2017, 62, 15–22. [Google Scholar] [CrossRef]
- Osimani, A.; Garofalo, C.; Milanović, V.; Taccari, M.; Cardinali, F.; Aquilanti, L.; Pasquini, M.; Mozzon, M.; Raffaelli, N.; Ruschioni, S.; et al. Insight into the Proximate Composition and Microbial Diversity of Edible Insects Marketed in the European Union. Eur. Food Res. Technol. 2017, 243, 1157–1171. [Google Scholar] [CrossRef]
- Nyangena, D.N.; Mutungi, C.; Imathiu, S.; Kinyuru, J.; Affognon, H.; Ekesi, S.; Nakimbugwe, D.; Fiaboe, K.K.M. Effects of Traditional Processing Techniques on the Nutritional and Microbiological Quality of Four Edible Insect Species Used for Food and Feed in East Africa. Foods 2020, 9, 574. [Google Scholar] [CrossRef]
- Saucier, L.; M’ballou, C.; Ratti, C.; Deschamps, M.-H.; Lebeuf, Y.; Vandenberg, G.W. Comparison of Black Soldier Fly Larvae Pre-Treatments and Drying Techniques on the Microbial Load and Physico-Chemical Characteristics. J. Insects Food Feed. 2022, 8, 45–64. [Google Scholar] [CrossRef]
- Tegtmeier, D.; Hurka, S.; Klüber, P.; Brinkrolf, K.; Heise, P.; Vilcinskas, A. Cottonseed Press Cake as a Potential Diet for Industrially Farmed Black Soldier Fly Larvae Triggers Adaptations of Their Bacterial and Fungal Gut Microbiota. Front. Microbiol. 2021, 12, 563. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Crauwels, S.; Lievens, B.; Luca, S.; Claes, J.; Borremans, A.; Bruyninckx, L.; Van Campenhout, L. Effect of Post-Harvest Starvation and Rinsing on the Microbial Numbers and the Bacterial Community Composition of Mealworm Larvae (Tenebrio Molitor). Innov. Food Sci. Emerg. Technol. 2017, 42, 8–15. [Google Scholar] [CrossRef]
- Fasolato, L.; Cardazzo, B.; Carraro, L.; Fontana, F.; Novelli, E.; Balzan, S. Edible Processed Insects from E-Commerce: Food Safety with a Focus on the Bacillus Cereus Group. Food Microbiol. 2018, 76, 296–303. [Google Scholar] [CrossRef] [PubMed]
- Grabowski, N.T.; Klein, G. Microbiology of Processed Edible Insect Products—Results of a Preliminary Survey. Int. J. Food Microbiol. 2017, 243, 103–107. [Google Scholar] [CrossRef] [PubMed]
- Wynants, E.; Frooninckx, L.; Van Miert, S.; Geeraerd, A.; Claes, J.; Van Campenhout, L. Risks Related to the Presence of Salmonella Sp. during Rearing of Mealworms (Tenebrio Molitor) for Food or Feed: Survival in the Substrate and Transmission to the Larvae. Food Control 2019, 100, 227–234. [Google Scholar] [CrossRef]
- Stoops, J.; Crauwels, S.; Waud, M.; Claes, J.; Lievens, B.; Van Campenhout, L. Microbial Community Assessment of Mealworm Larvae (Tenebrio Molitor) and Grasshoppers (Locusta Migratoria Migratorioides) Sold for Human Consumption. Food Microbiol. 2016, 53, 122–127. [Google Scholar] [CrossRef]
- Milanović, V.; Osimani, A.; Roncolini, A.; Garofalo, C.; Aquilanti, L.; Pasquini, M.; Tavoletti, S.; Vignaroli, C.; Canonico, L.; Ciani, M.; et al. Investigation of the Dominant Microbiota in Ready-to-Eat Grasshoppers and Mealworms and Quantification of Carbapenem Resistance Genes by QPCR. Front. Microbiol. 2018, 9, 424106. [Google Scholar] [CrossRef]
- Wendin, K.; Martensson, L.; Djerf, H.; Langton, M. Product Quality during the Storage of Foods with Insects as an Ingredient: Impact of Particle Size, Antioxidant, Oil Content and Salt Content. Foods 2020, 9, 791. [Google Scholar] [CrossRef]
- Melgar-Lalanne, G.; Hernández-Álvarez, A.J.; Salinas-Castro, A. Edible Insects Processing: Traditional and Innovative Technologies. Compr. Rev. Food Sci. Food Saf. 2019, 18, 1166–1191. [Google Scholar] [CrossRef]
- Ciurzyńska, A.; Falacińska, J.; Kowalska, H.; Kowalska, J.; Galus, S.; Marzec, A.; Domian, E. The Effect of Pre-Treatment (Blanching, Ultrasound and Freezing) on Quality of Freeze-Dried Red Beets. Foods 2021, 10, 132. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. The Freeze-Drying of Foods—The Characteristic of the Process Course and the Effect of Its Parameters on the Physical Properties of Food Materials. Foods 2020, 9, 1488. [Google Scholar] [CrossRef]
- Gómez, B.; Munekata, P.E.S.; Gavahian, M.; Barba, F.J.; Martí-Quijal, F.J.; Bolumar, T.; Campagnol, P.C.B.; Tomasevic, I.; Lorenzo, J.M. Application of Pulsed Electric Fields in Meat and Fish Processing Industries: An Overview. Food Res. Int. 2019, 123, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Telfser, A.; Gómez Galindo, F. Effect of Reversible Permeabilization in Combination with Different Drying Methods on the Structure and Sensorial Quality of Dried Basil (Ocimum Basilicum L.) Leaves. LWT 2019, 99, 148–155. [Google Scholar] [CrossRef]
- Wu, Y.; Zhang, D. Pulsed Electric Field Enhanced Freeze-Drying of Apple Tissue. Czech J. Food Sci. 2019, 37, 432–438. [Google Scholar] [CrossRef]
- Nowak, D.; Jakubczyk, E. Effect of Pulsed Electric Field Pre-Treatment and the Freezing Methods on the Kinetics of the Freeze-Drying Process of Apple and Its Selected Physical Properties. Foods 2022, 11, 2407. [Google Scholar] [CrossRef] [PubMed]
- Wu, Y.; Zhang, D. Effect of Pulsed Electric Field on Freeze-Drying of Potato Tissue. Int. J. Food Eng. 2014, 10, 857–862. [Google Scholar] [CrossRef]
- Alles, M.C.; Smetana, S.; Parniakov, O.; Shorstkii, I.; Toepfl, S.; Aganovic, K.; Heinz, V. Bio-Refinery of Insects with Pulsed Electric Field Pre-Treatment. Innov. Food Sci. Emerg. Technol. 2020, 64, 102403. [Google Scholar] [CrossRef]
- Shorstkii, I.; Comiotto Alles, M.; Parniakov, O.; Smetana, S.; Aganovic, K.; Sosnin, M.; Toepfl, S.; Heinz, V. Optimization of Pulsed Electric Field Assisted Drying Process of Black Soldier Fly (Hermetia Illucens) Larvae. Dry. Technol. 2020, 40, 595–603. [Google Scholar] [CrossRef]
- Bogusz, R.; Smetana, S.; Wiktor, A.; Parniakov, O.; Pobiega, K.; Rybak, K.; Nowacka, M. The Selected Quality Aspects of Infrared-Dried Black Soldier Fly (Hermetia Illucens) and Yellow Mealworm (Tenebrio Molitor) Larvae Pre-Treated by Pulsed Electric Field. Innov. Food Sci. Emerg. Technol. 2022, 80, 103085. [Google Scholar] [CrossRef]
- Fauster, T.; Giancaterino, M.; Pittia, P.; Jaeger, H. Effect of Pulsed Electric Field Pretreatment on Shrinkage, Rehydration Capacity and Texture of Freeze-Dried Plant Materials. LWT 2020, 121, 108937. [Google Scholar] [CrossRef]
- Santos, N.C.; Almeida, R.L.J.; de Oliveira Brito, A.C.; de Alcântara Silva, V.M.; Albuquerque, J.C.; Saraiva, M.M.T.; Santos, R.M.S.; de Sousa, F.M.; de Alcântara Ribeiro, V.H.; de Oliveira Carvalho, R.; et al. Effect of Pulse Electric Field (PEF) Intensity Combined with Drying Temperature on Mass Transfer, Functional Properties, and in Vitro Digestibility of Dehydrated Mango Peels. J. Food Meas. Charact. 2023, in press. [Google Scholar] [CrossRef]
- Lammerskitten, A.; Wiktor, A.; Mykhailyk, V.; Samborska, K.; Gondek, E.; Witrowa-Rajchert, D.; Toepfl, S.; Parniakov, O. Pulsed Electric Field Pre-Treatment Improves Microstructure and Crunchiness of Freeze-Dried Plant Materials: Case of Strawberry. LWT 2020, 134, 110266. [Google Scholar] [CrossRef]
- Fijałkowska, A.; Nowacka, M.; Witrowa-Rajchert, D. The Physical, Optical and Reconstitution Properties of Apples Subjected to Ultrasound before Drying. Ital. J. Food Sci. 2017, 29, 344–356. [Google Scholar]
- Lenaerts, S.; Van Der Borght, M.; Callens, A.; Van Campenhout, L. Suitability of Microwave Drying for Mealworms (Tenebrio Molitor) as Alternative to Freeze Drying: Impact on Nutritional Quality and Colour. Food Chem. 2018, 254, 129–136. [Google Scholar] [CrossRef] [PubMed]
- Janssen, R.H.; Vincken, J.P.; Van Den Broek, L.A.M.; Fogliano, V.; Lakemond, C.M.M. Nitrogen-to-Protein Conversion Factors for Three Edible: Tenebrio Molitor, Alphitobius Diaperinus, and Hermetia. J. Agric. Food Chem. 2017, 65, 2275. [Google Scholar] [CrossRef]
- García-Gutiérrez, N.; Mellado-Carretero, J.; Bengoa, C.; Salvador, A.; Sanz, T.; Wang, J.; Ferrando, M.; Güell, C.; Lamo-Castellví, S. de ATR-FTIR Spectroscopy Combined with Multivariate Analysis Successfully Discriminates Raw Doughs and Baked 3D-Printed Snacks Enriched with Edible Insect Powder. Foods 2021, 10, 1806. [Google Scholar] [CrossRef]
- Wynants, E.; Crauwels, S.; Verreth, C.; Gianotten, N.; Lievens, B.; Claes, J.; Van Campenhout, L. Microbial Dynamics during Production of Lesser Mealworms (Alphitobius Diaperinus) for Human Consumption at Industrial Scale. Food Microbiol. 2018, 70, 181–191. [Google Scholar] [CrossRef]
- Abbaspour-Gilandeh, Y.; Kaveh, M.; Fatemi, H.; Khalife, E.; Witrowa-Rajchert, D.; Nowacka, M. Effect of Pretreatments on Convective and Infrared Drying Kinetics, Energy Consumption and Quality of Terebinth. Appl. Sci. 2021, 11, 7672. [Google Scholar] [CrossRef]
- Chauhan, O.P.; Shayanfar, S.; Topefl, S. Cell Permeabilisation, Microstructure and Quality of Dehydrated Apple Slices Treated with Pulsed Electric Field During Blanching. Def. Life Sci. J. 2018, 4, 38–44. [Google Scholar] [CrossRef]
- Dadan, M.; Nowacka, M. The Assessment of the Possibility of Using Ethanol and Ultrasound to Design the Properties of Dried Carrot Tissue. Appl. Sci. 2021, 11, 689. [Google Scholar] [CrossRef]
- Barba, F.J.; Mariutti, L.R.B.; Bragagnolo, N.; Mercadante, A.Z.; Barbosa-Cánovas, G.V.; Orlien, V. Bioaccessibility of Bioactive Compounds from Fruits and Vegetables after Thermal and Nonthermal Processing. Trends Food Sci. Technol. 2017, 67, 195–206. [Google Scholar] [CrossRef]
- Janiszewska-Turak, E.; Kołakowska, W.; Pobiega, K.; Gramza-Michałowska, A. Influence of Drying Type of Selected Fermented Vegetables Pomace on the Natural Colorants and Concentration of Lactic Acid Bacteria. Appl. Sci. 2021, 11, 7864. [Google Scholar] [CrossRef]
- Kaveh, M.; Abbaspour-Gilandeh, Y.; Taghinezhad, E.; Witrowa-Rajchert, D.; Nowacka, M. The Quality of Infrared Rotary Dried Terebinth (Pistacia Atlantica L.)-Optimization and Prediction Approach Using Response Surface Methodology. Molecules 2021, 26, 1999. [Google Scholar] [CrossRef] [PubMed]
- Psarianos, M.; Dimopoulos, G.; Ojha, S.; Cavini, A.C.M.; Bußler, S.; Taoukis, P.; Schlüter, O.K. Effect of Pulsed Electric Fields on Cricket (Acheta Domesticus) Flour: Extraction Yield (Protein, Fat and Chitin) and Techno-Functional Properties. Innov. Food Sci. Emerg. Technol. 2022, 76, 102908. [Google Scholar] [CrossRef]
- Khatun, H.; Claes, J.; Smets, R.; De Winne, A.; Akhtaruzzaman, M.; Van Der Borght, M. Characterization of Freeze-Dried, Oven-Dried and Blanched House Crickets (Acheta Domesticus) and Jamaican Field Crickets (Gryllus Assimilis) by Means of Their Physicochemical Properties and Volatile Compounds. Eur. Food Res. Technol. 2021, 247, 1291–1305. [Google Scholar] [CrossRef]
- Lee, H.; Kim, J.; Ji, D.; Lee, C. Effects of Heating Time and Temperature on Functional Properties of Proteins of Yellow Mealworm Larvae (Tenebrio Molitor L.). Food Sci. Anim. Resour. 2019, 39, 296–308. [Google Scholar] [CrossRef]
- Nowacka, M.; Wedzik, M. Effect of Ultrasound Treatment on Microstructure, Colour and Carotenoid Content in Fresh and Dried Carrot Tissue. Appl. Acoust. 2016, 103, 163–171. [Google Scholar] [CrossRef]
- Tobolková, B.; Takáč, P.; Mangová, B.; Kozánek, M. A Comparative Study of Colour Characteristics of Thermally/Non-Thermally Treated Mealworm Larvae (Tenebrio Molitor) by Means of UV/Vis Spectroscopy and Multivariate Analysis. J. Food Meas. Charact. 2021, 15, 3791–3799. [Google Scholar] [CrossRef]
- Azzollini, D.; Derossi, A.; Severini, C. Understanding the Drying Kinetic and Hygroscopic Behaviour of Larvae of Yellow Mealworm (Tenebrio Molitor) and the Effects on Their Quality. J. Insects Food Feed. 2016, 2, 233–243. [Google Scholar] [CrossRef]
- Sánchez, M.; Gómez, C.; Avendaño, C.; Harmsen, I.; Ortiz, D.; Ceballos, R.; Villamizar-Sarmiento, M.G.; Oyarzun-Ampuero, F.; Wacyk, J.; Valenzuela, C. House Fly (Musca Domestica) Larvae Meal as an Ingredient with High Nutritional Value: Microencapsulation and Improvement of Organoleptic Characteristics. Food Res. Int. 2021, 145, 110423. [Google Scholar] [CrossRef]
- Queiroz, L.S.; Regnard, M.; Jessen, F.; Mohammadifar, M.A.; Sloth, J.J.; Petersen, H.O.; Ajalloueian, F.; Brouzes, C.M.C.; Fraihi, W.; Fallquist, H.; et al. Physico-Chemical and Colloidal Properties of Protein Extracted from Black Soldier Fly (Hermetia Illucens) Larvae. Int. J. Biol. Macromol. 2021, 186, 714–723. [Google Scholar] [CrossRef]
- Jóźwiak, T.; Filipkowska, U.; Bakuła, T.; Bralewska-Piotrowicz, B.; Karczmarczyk, K.; Gierszewska, M.; Olewnik-Kruszkowska, E.; Szyryńska, N.; Lewczuk, B. The Use of Chitin from the Molts of Mealworm (Tenebrio Molitor) for the Removal of Anionic and Cationic Dyes from Aqueous Solutions. Materials 2023, 16, 545. [Google Scholar] [CrossRef] [PubMed]
- Baigts-Allende, D.; Doost, A.S.; Ramírez-Rodrigues, M.; Dewettinck, K.; Van der Meeren, P.; de Meulenaer, B.; Tzompa-Sosa, D. Insect Protein Concentrates from Mexican Edible Insects: Structural and Functional Characterization. LWT 2021, 152, 112267. [Google Scholar] [CrossRef]
- Bolat, B.; Ugur, A.E.; Oztop, M.H.; Alpas, H. Effects of High Hydrostatic Pressure Assisted Degreasing on the Technological Properties of Insect Powders Obtained from Acheta Domesticus & Tenebrio Molitor. J. Food Eng. 2021, 292, 110359. [Google Scholar] [CrossRef]
- Uncu, O.; Napiórkowska, A.; Szajna, T.K.; Ozen, B. Evaluation of Three Spectroscopic Techniques in Determination of Adulteration of Cold Pressed Pomegranate Seed Oils. Microchem. J. 2020, 158, 105128. [Google Scholar] [CrossRef]
- Nowosad, K.; Sujka, M.; Pankiewicz, U.; Kowalski, R. The Application of PEF Technology in Food Processing and Human Nutrition. J. Food Sci. Technol. 2021, 58, 397–411. [Google Scholar] [CrossRef]
- Li, L.; Yang, R.; Zhao, W. The Effect of Pulsed Electric Fields (PEF) Combined with Temperature and Natural Preservatives on the Quality and Microbiological Shelf-Life of Cantaloupe Juice. Foods 2021, 10, 2606. [Google Scholar] [CrossRef] [PubMed]
- Muszewska, A.; Piłsyk, S.; Perlińska-Lenart, U.; Kruszewska, J. Diversity of Cell Wall Related Proteins in Human Pathogenic Fungi. J. Fungi 2017, 4, 6. [Google Scholar] [CrossRef]
- Martínez, J.M.; Delso, C.; Álvarez, I.; Raso, J. Pulsed Electric Field-assisted Extraction of Valuable Compounds from Microorganisms. Compr. Rev. Food Sci. Food Saf. 2020, 19, 530–552. [Google Scholar] [CrossRef]
- Chandrapala, J.; Oliver, C.; Kentish, S.; Ashokkumar, M. Ultrasonics in Food Processing. Ultrason. Sonochem. 2012, 19, 975–983. [Google Scholar] [CrossRef]
- Bodzen, A.; Jossier, A.; Dupont, S.; Mousset, P.-Y.; Beney, L.; Lafay, S.; Gervais, P. Design of a New Lyoprotectant Increasing Freeze-Dried Lactobacillus Strain Survival to Long-Term Storage. BMC Biotechnol. 2021, 21, 66. [Google Scholar] [CrossRef]
- Polo, L.; Mañes-Lázaro, R.; Olmeda, I.; Cruz-Pio, L.E.; Medina, Á.; Ferrer, S.; Pardo, I. Influence of Freezing Temperatures Prior to Freeze-Drying on Viability of Yeasts and Lactic Acid Bacteria Isolated from Wine. J. Appl. Microbiol. 2017, 122, 1603–1614. [Google Scholar] [CrossRef] [PubMed]
- Ostermeier, R.; Hill, K.; Dingis, A.; Töpfl, S.; Jäger, H. Influence of Pulsed Electric Field (PEF) and Ultrasound Treatment on the Frying Behavior and Quality of Potato Chips. Innov. Food Sci. Emerg. Technol. 2021, 67, 102553. [Google Scholar] [CrossRef]
- Niu, D.; Zeng, X.-A.; Ren, E.-F.; Xu, F.-Y.; Li, J.; Wang, M.-S.; Wang, R. Review of the Application of Pulsed Electric Fields (PEF) Technology for Food Processing in China. Food Res. Int. 2020, 137, 109715. [Google Scholar] [CrossRef] [PubMed]
- Chatterjee, A.; Abraham, J. Microbial Contamination, Prevention, and Early Detection in Food Industry. In Microbial Contamination and Food Degradation; Elsevier: Amsterdam, The Netherlands, 2018; pp. 21–47. [Google Scholar]
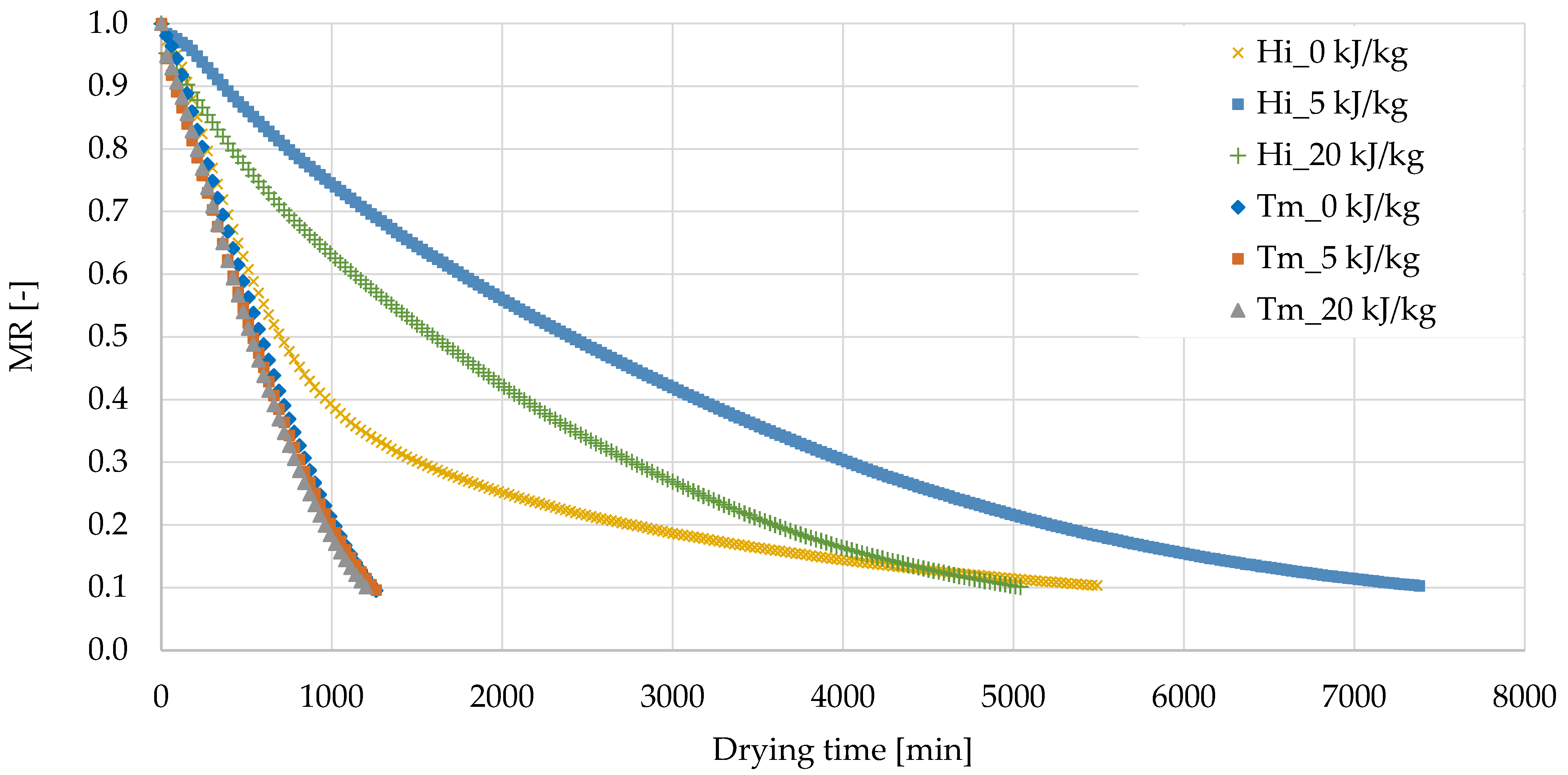

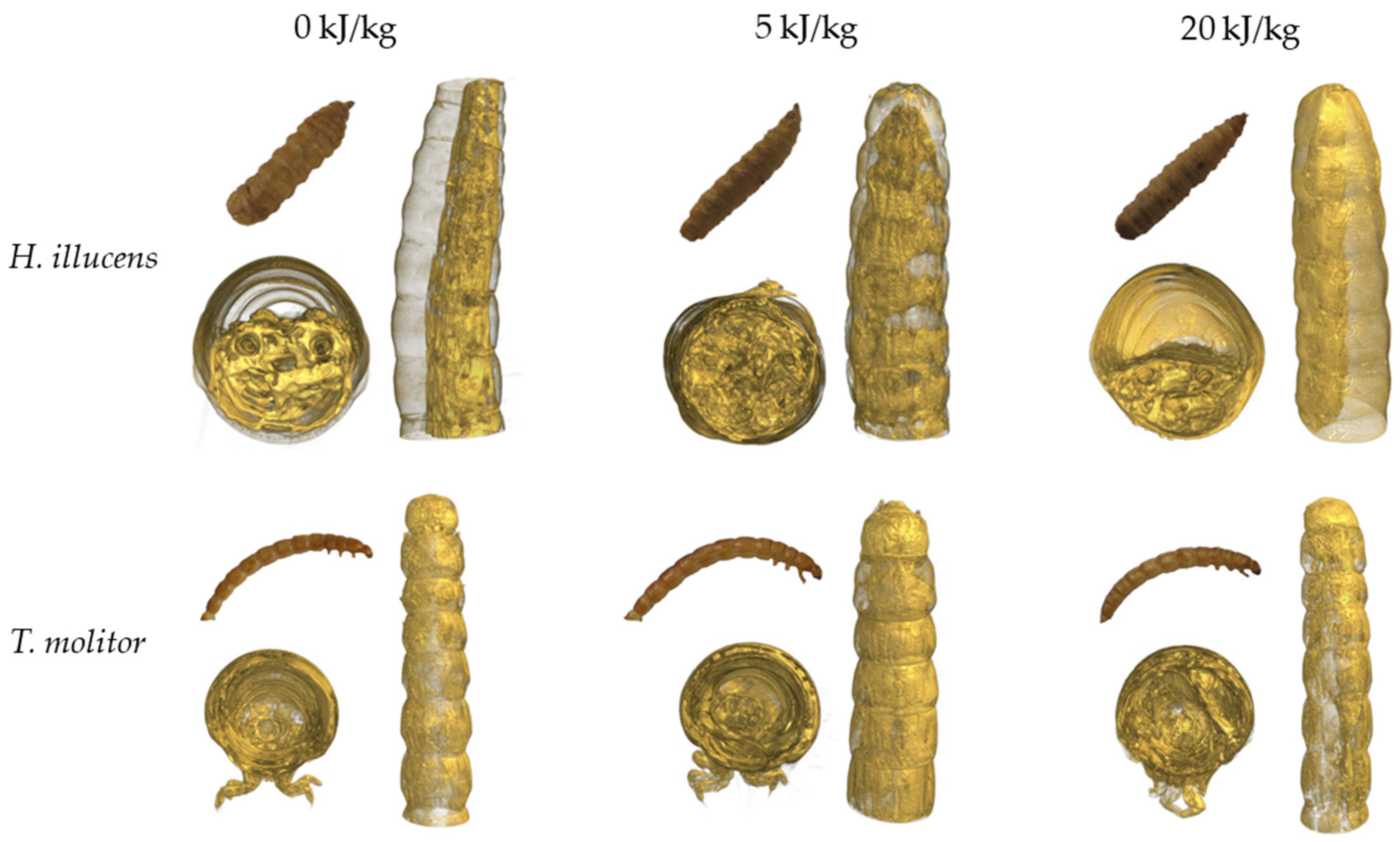
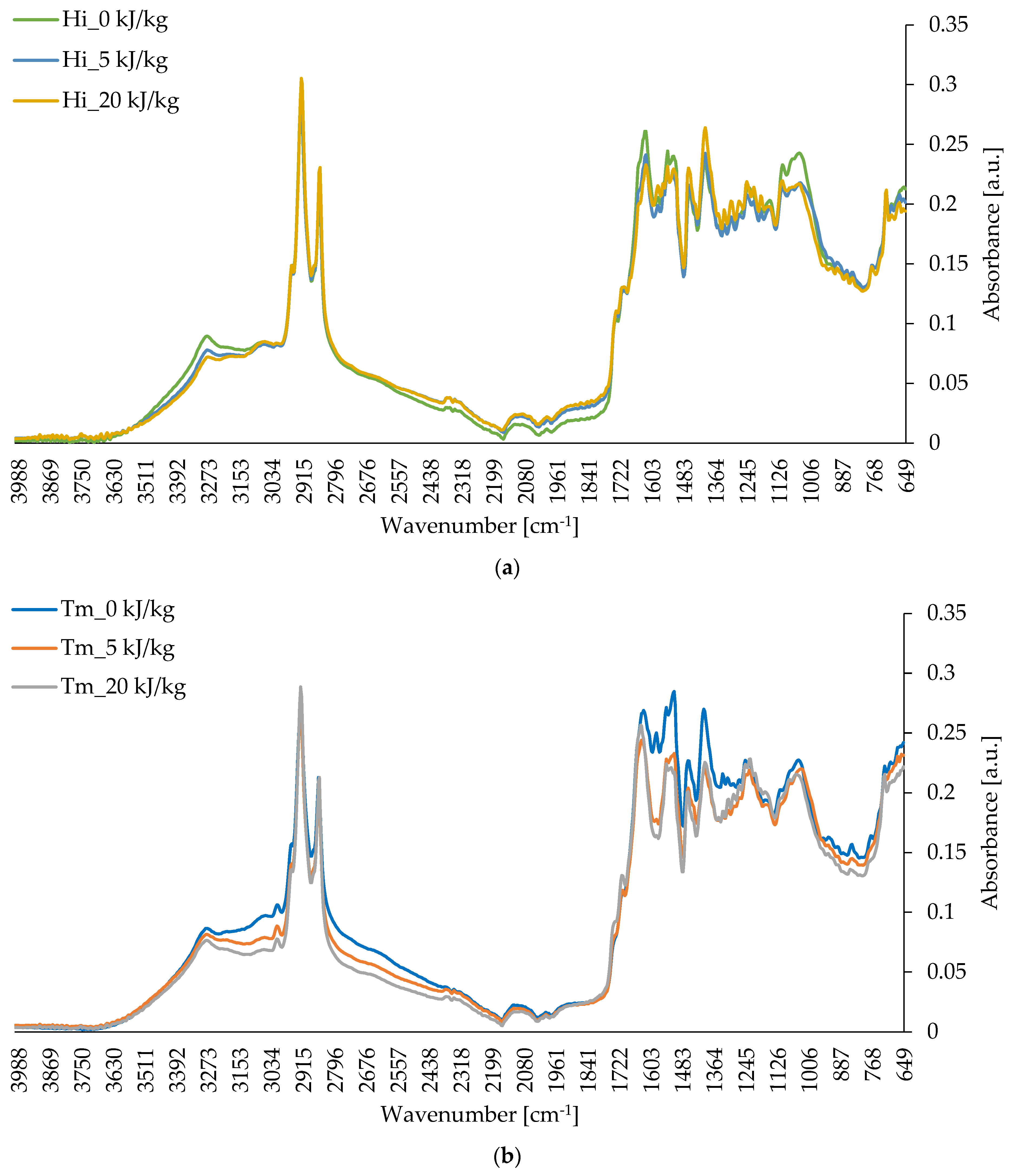
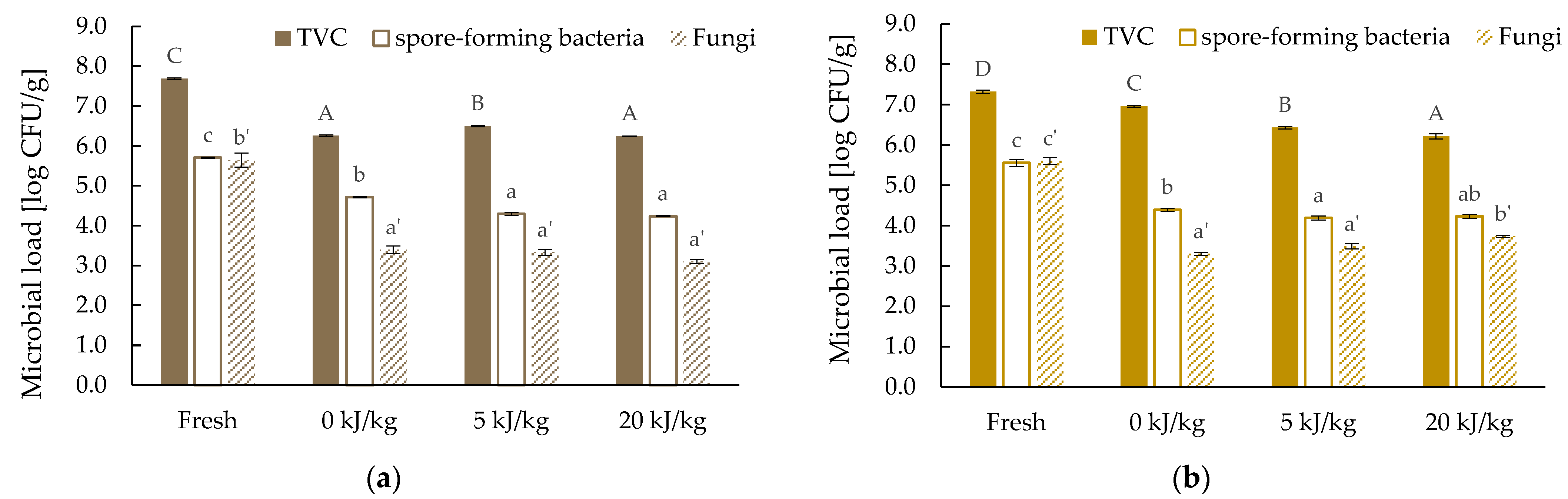
| Sample | Drying Time to MR = 0.1 (min) | Dry Matter Content (%) | Water Activity (−) | |
|---|---|---|---|---|
| H. illucens | 0 kJ/kg | 5160 ± 467 a,* | 83.2 ± 2.3 a | 0.165 ± 0.008 a |
| 5 kJ/kg | 7320 ± 85 b | 87.5 ± 0.5 b | 0.232 ± 0.008 b | |
| 20 kJ/kg | 5175 ± 191 a | 83.2 ± 2.0 a | 0.405 ± 0.001 c | |
| T. molitor | 0 kJ/kg | 1245 ± 21 B | 93.3 ± 0.2 A | 0.145 ± 0.001 B |
| 5 kJ/kg | 1245 ± 21 B | 95.8 ± 1.0 B | 0.023 ± 0.002 A | |
| 20 kJ/kg | 1200 ± 0 A | 95.8 ± 0.1 B | 0.023 ± 0.002 A | |
| Sample | RR [−] | SSL [−] | H [−] | |
|---|---|---|---|---|
| H. illucens | 0 kJ/kg | 1.26 ± 0.04 a,* | 1.13 ± 0.01 a | 1.31 ± 0.07 a |
| 5 kJ/kg | 1.25 ± 0.01 a | 1.10 ± 0.01 a | 1.44 ± 0.02 b | |
| 20 kJ/kg | 1.35 ± 0.15 a | 1.13 ± 0.01 a | 1.48 ± 0.06 b | |
| T. molitor | 0 kJ/kg | 1.52 ± 0.35 A | 1.06 ± 0.01 A | 1.73 ± 0.07 A |
| 5 kJ/kg | 1.46 ± 0.25 A | 1.04 ± 0.01 A | 1.73 ± 0.05 A | |
| 20 kJ/kg | 1.76 ± 0.18 A | 1.05 ± 0.02 A | 1.89 ± 0.14 A | |
| Sample | L* [−] | a* [−] | b* [−] | ΔE [−] | |
|---|---|---|---|---|---|
| H. illucens | Fresh | 39.4 ± 0.8 b,* | 7.9 ± 0.8 c | 24.0 ± 0.2 c | - |
| 0 kJ/kg | 42.1 ± 1.3 c | 5.6 ± 0.3 b | 17.8 ± 0.7 b | 7.3 | |
| 5 kJ/kg | 34.9 ± 2.2 a | 4.8 ± 0.4 a | 15.1 ± 1.1 a | 10.6 | |
| 20 kJ/kg | 34.7 ± 1.0 a | 4.5 ± 0.3 a | 14.9 ± 0.7 a | 10.8 | |
| T. molitor | Fresh | 39.9 ± 1.7 B | 3.1 ± 0.2 A | 17.1 ± 1.0 A | - |
| 0 kJ/kg | 33.8 ± 0.8 A | 10.8 ± 0.5 C | 20.7 ± 0.6 C | 10.5 | |
| 5 kJ/kg | 33.5 ± 0.6 A | 9.7 ± 0.3 B | 19.4 ± 0.5 B | 9.5 | |
| 20 kJ/kg | 34.3 ± 0.3 A | 9.4 ± 0.3 B | 18.9 ± 0.4 B | 8.6 | |
| Sample | Moisture (%) | Protein (%) | Fat (%) | Ash (%) | |
|---|---|---|---|---|---|
| H. illucens | 0 kJ/kg | 16.80 ± 2.33 b,* | 35.80 ± 1.04 a | 34.60 ± 1.96 a | 4.20 ± 0.01 a |
| 5 kJ/kg | 12.51 ± 0.51 a | 35.73 ± 1.00 a | 34.52 ± 1.83 a | 4.57 ± 0.02 c | |
| 20 kJ/kg | 16.77 ± 2.01 b | 37.38 ± 0.87 a | 33.33 ± 2.47 a | 4.30 ± 0.02 b | |
| T. molitor | 0 kJ/kg | 6.69 ± 0.25 B | 48.02 ± 0.54 B | 26.78 ± 0.21 A | 3.82 ± 0.02 A |
| 5 kJ/kg | 4.19 ± 1.05 A | 48.03 ± 0.49 B | 26.83 ± 0.80 A | 4.09 ± 0.02 C | |
| 20 kJ/kg | 4.15 ± 0.11 A | 45.40 ± 0.59 A | 26.90 ± 0.70 A | 3.93 ± 0.01 B | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bogusz, R.; Pobiega, K.; Rybak, K.; Wiktor, A.; Parniakov, O.; Smetana, S.; Nowacka, M. The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) Larvae. Appl. Sci. 2023, 13, 10251. https://doi.org/10.3390/app131810251
Bogusz R, Pobiega K, Rybak K, Wiktor A, Parniakov O, Smetana S, Nowacka M. The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) Larvae. Applied Sciences. 2023; 13(18):10251. https://doi.org/10.3390/app131810251
Chicago/Turabian StyleBogusz, Radosław, Katarzyna Pobiega, Katarzyna Rybak, Artur Wiktor, Oleksii Parniakov, Sergiy Smetana, and Małgorzata Nowacka. 2023. "The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) Larvae" Applied Sciences 13, no. 18: 10251. https://doi.org/10.3390/app131810251
APA StyleBogusz, R., Pobiega, K., Rybak, K., Wiktor, A., Parniakov, O., Smetana, S., & Nowacka, M. (2023). The Pulsed Electric Field Treatment Effect on Drying Kinetics and Chosen Quality Aspects of Freeze-Dried Black Soldier Fly (Hermetia illucens) and Yellow Mealworm (Tenebrio molitor) Larvae. Applied Sciences, 13(18), 10251. https://doi.org/10.3390/app131810251












