First Study of a HEXITEC Detector for Secondary Particle Characterisation during Proton Beam Therapy
Abstract
:1. Introduction
2. Methods
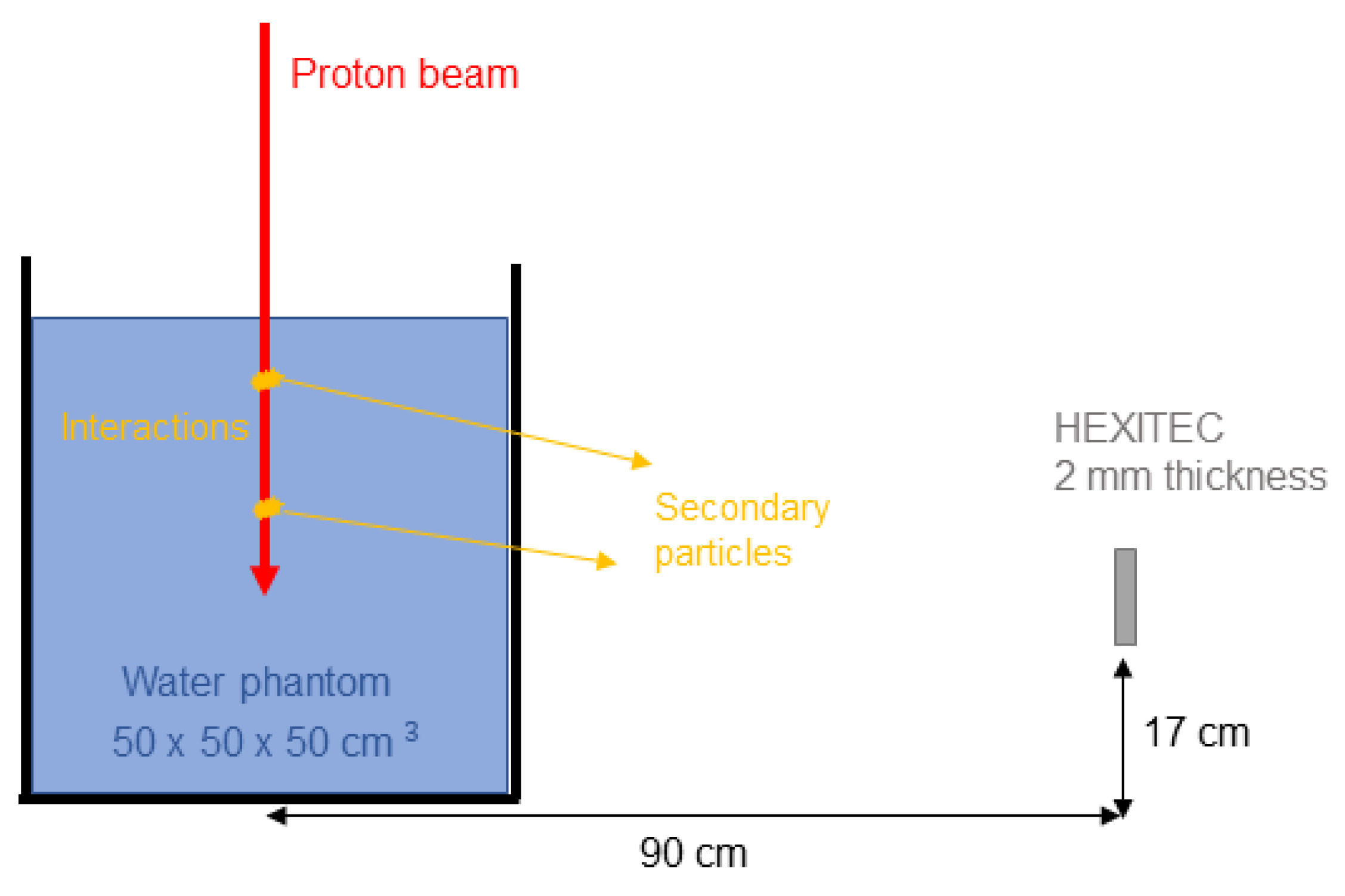
2.1. Experimental Scenario
2.2. Simulation Scenario
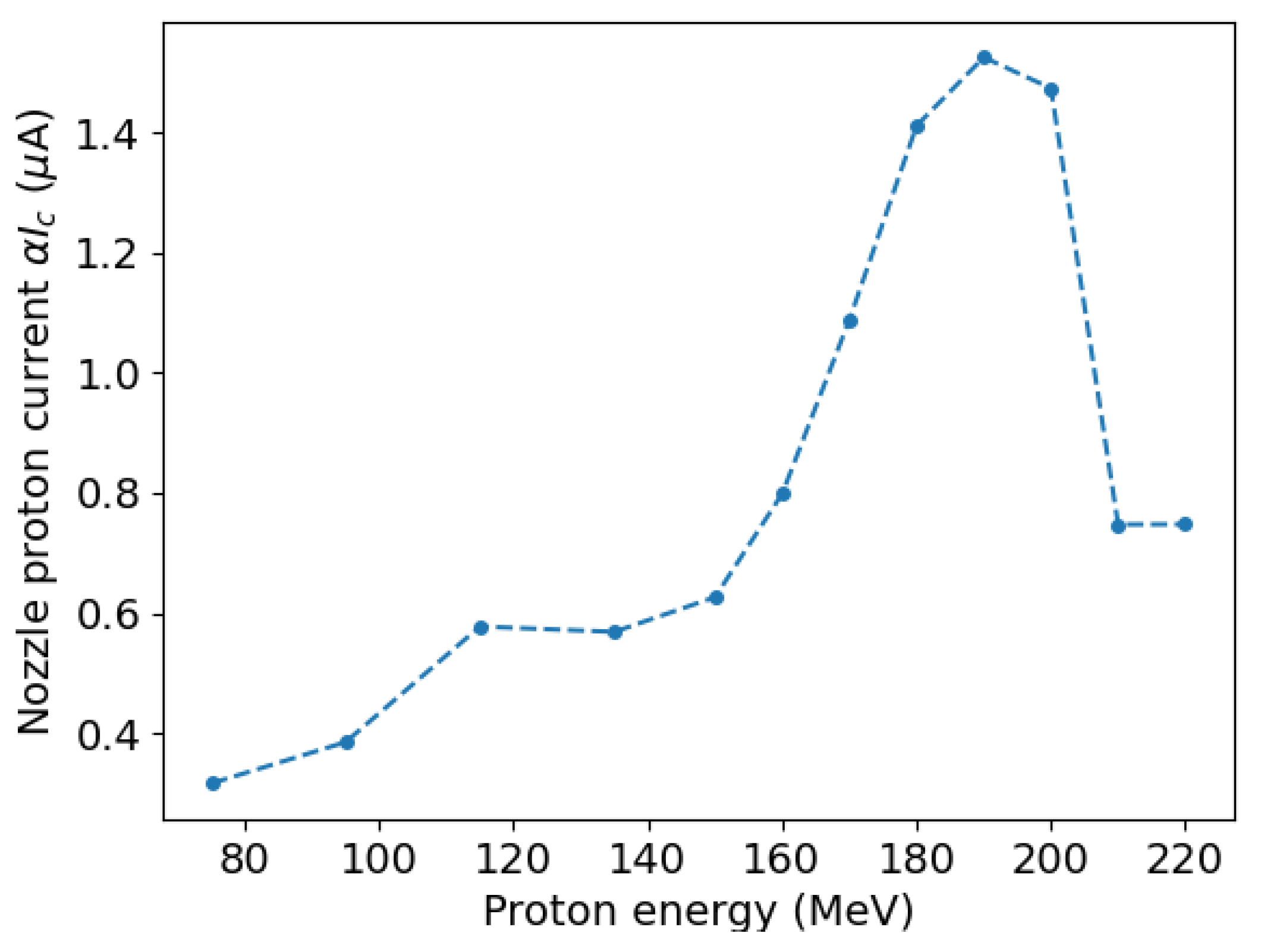
2.3. Equivalence between Scenarios
3. Results
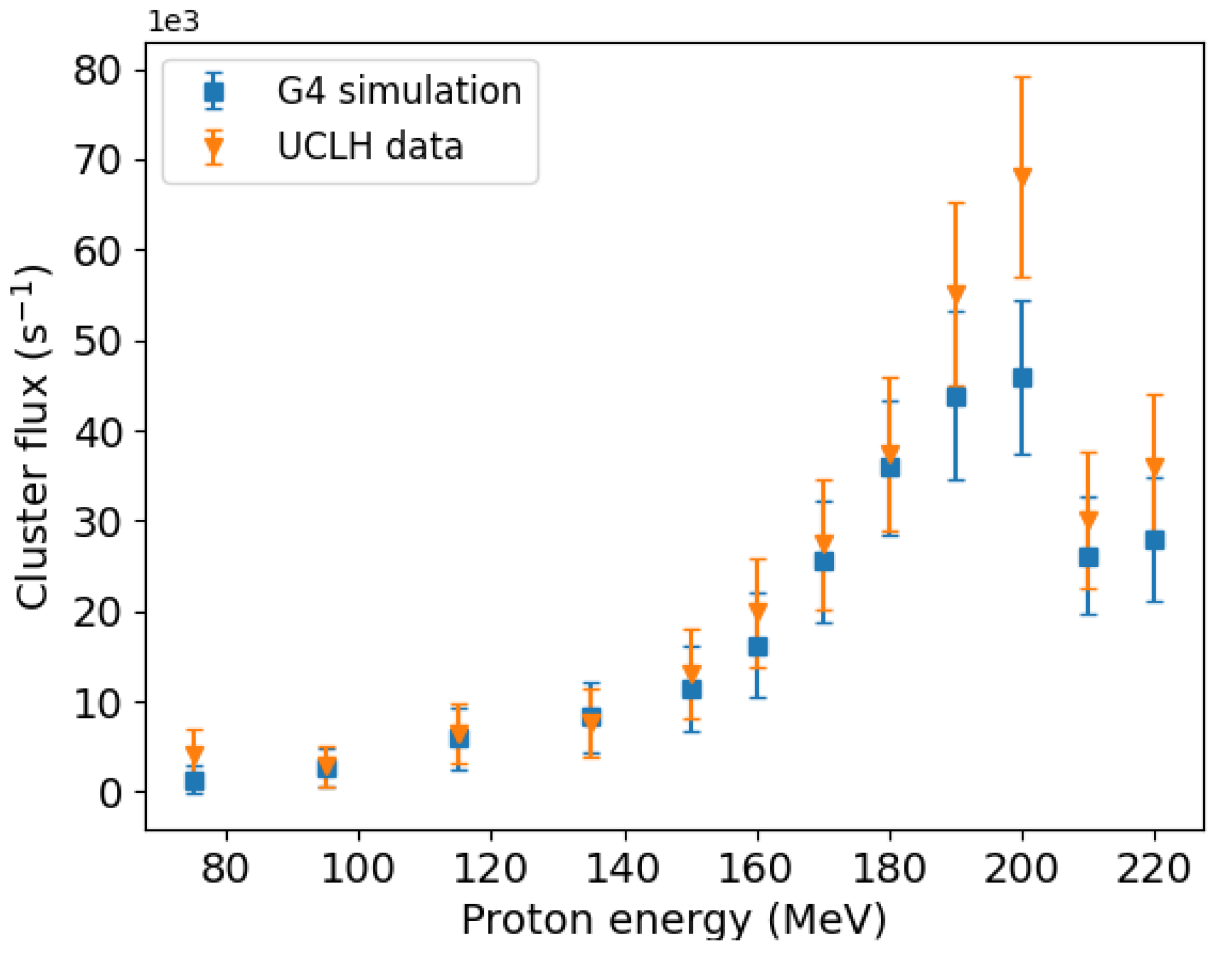
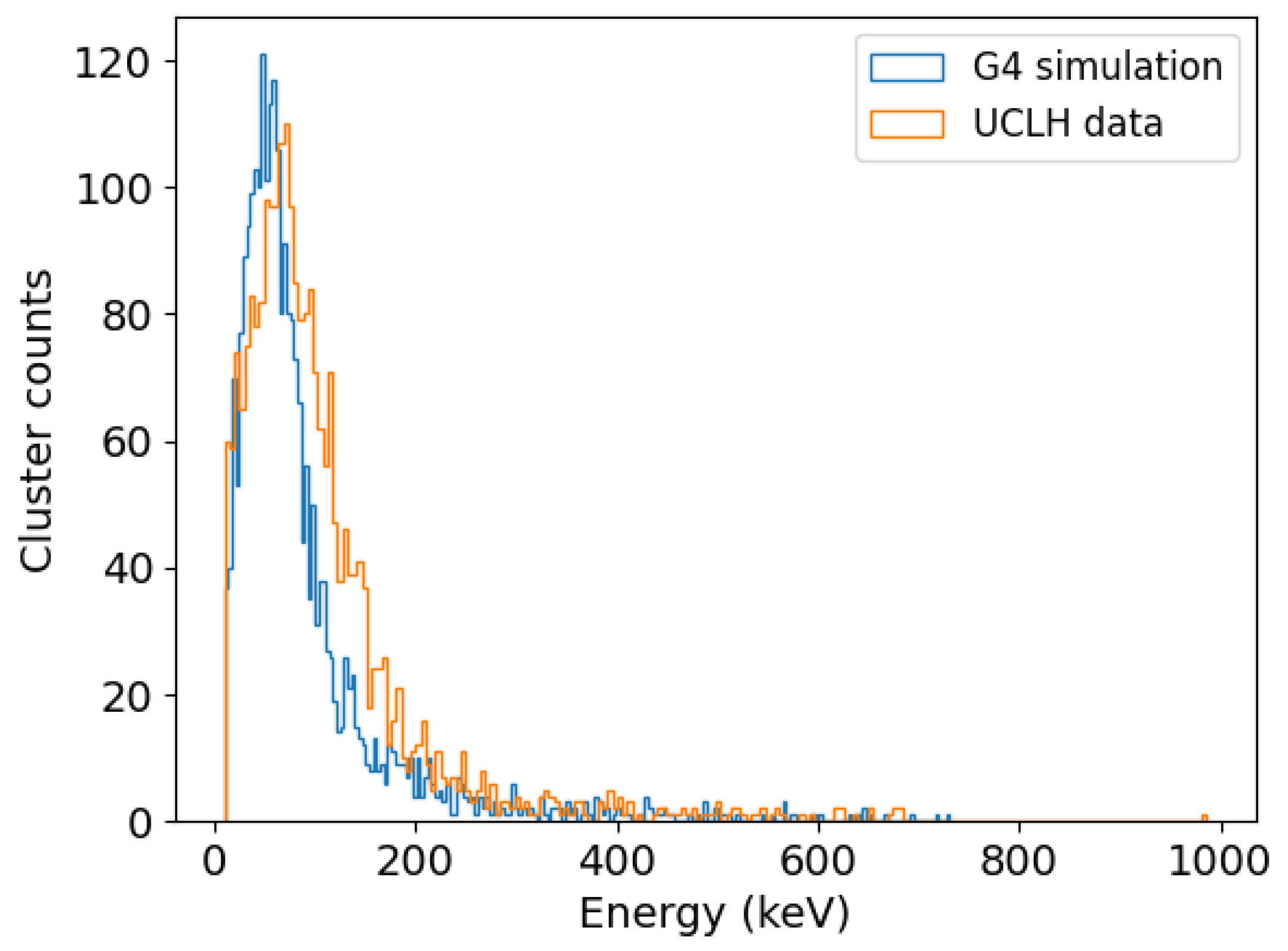
3.1. Comparison between Simulation and Experiment
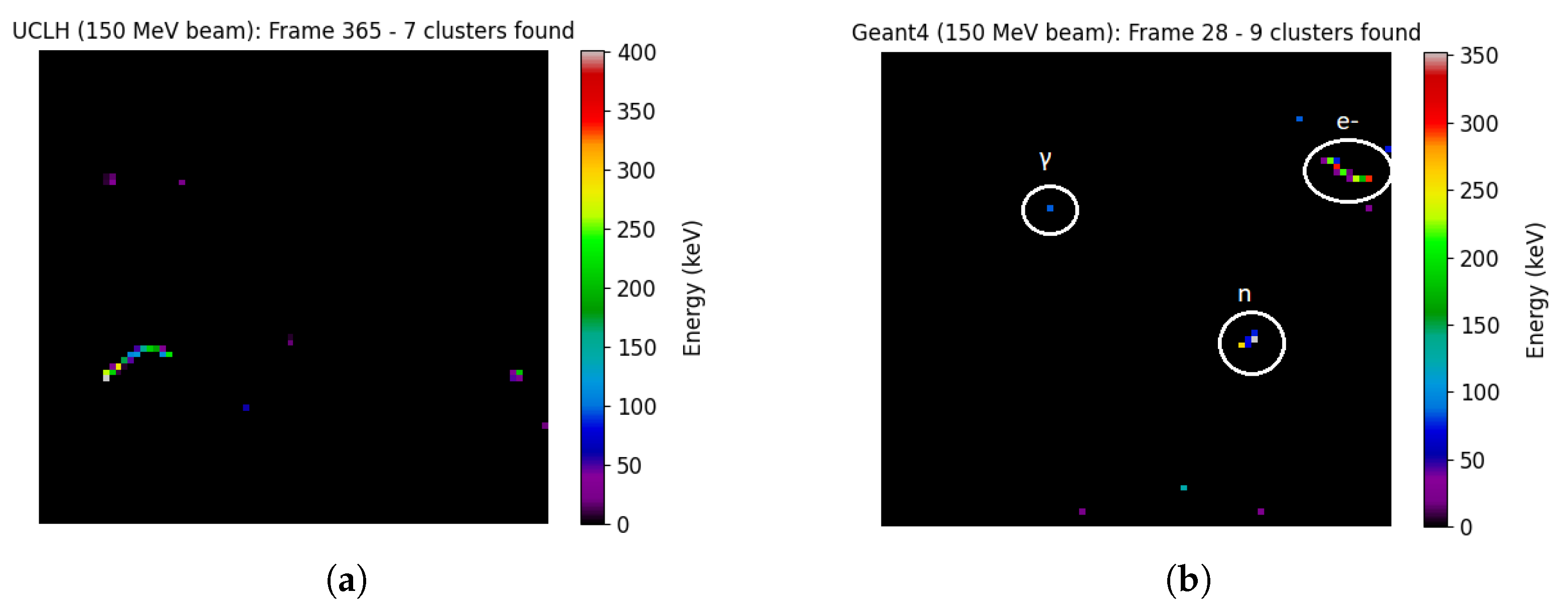
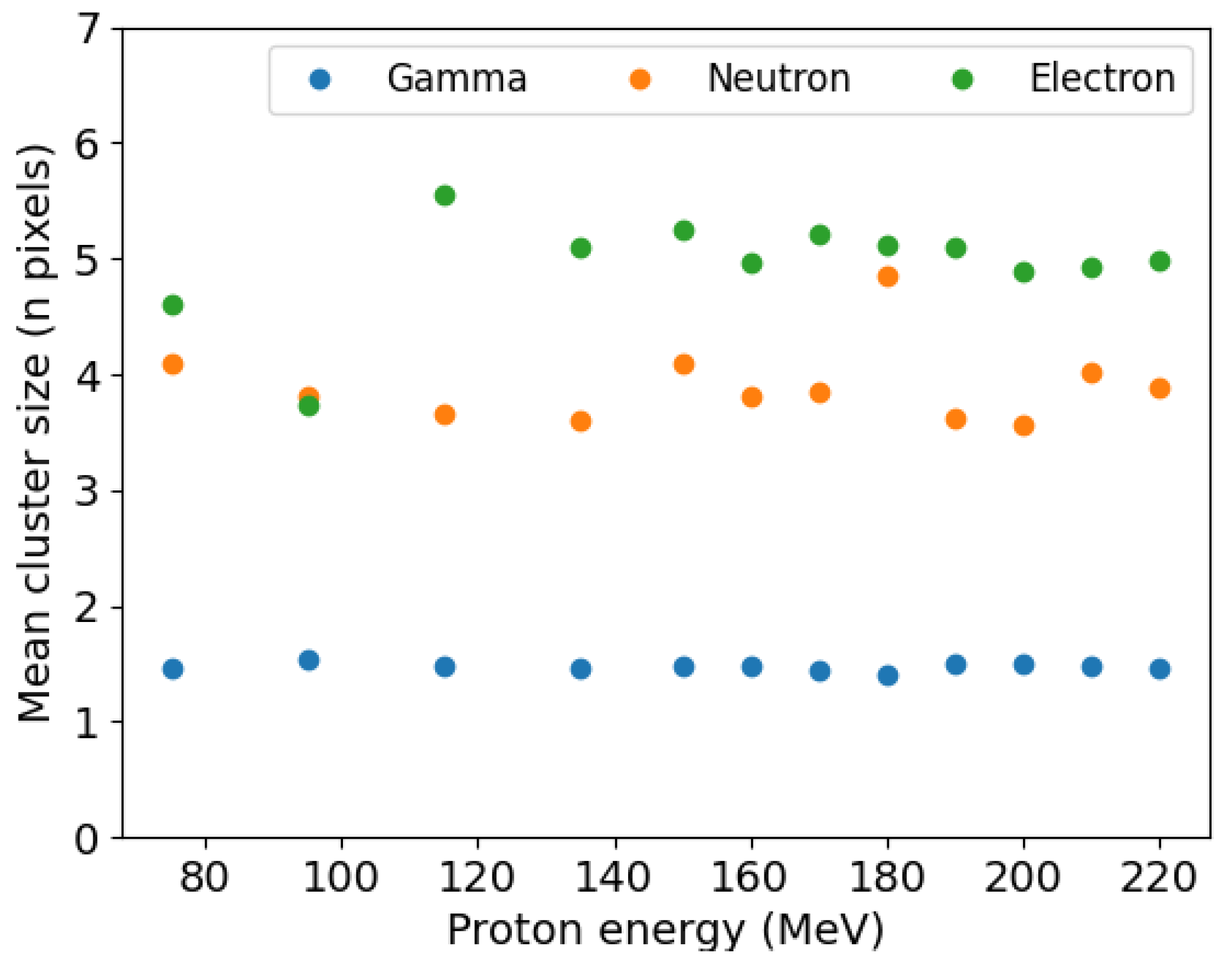
3.2. Secondary Particle Characterisation
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wilson, R.R. Radiological use of fast protons. Radiology 1946, 47, 487–491. [Google Scholar] [CrossRef]
- Newhauser, W.D.; Zhang, R. The physics of proton therapy. Phys. Med. Biol. 2015, 60, R155. [Google Scholar] [CrossRef]
- Kim, P.J.; Shih, H.A. The place of ion beams in clinical applications. In Ion Beam Therapy; Springer: Berlin/Heidelberg, Germany, 2012; pp. 17–29. [Google Scholar]
- Paganetti, H. Range uncertainties in proton therapy and the role of Monte Carlo simulations. Phys. Med. Biol. 2012, 57, R99. [Google Scholar] [CrossRef]
- Knopf, A.C.; Lomax, A. In vivo proton range verification: A review. Phys. Med. Biol. 2013, 58, R131. [Google Scholar] [CrossRef]
- Meißner, H.; Fuchs, H.; Hirtl, A.; Reschl, C.; Stock, M. Towards offline PET monitoring of proton therapy at MedAustron. Z. Für Med. Phys. 2019, 29, 59–65. [Google Scholar] [CrossRef]
- Knopf, A.; Parodi, K.; Bortfeld, T.; Shih, H.A.; Paganetti, H. Systematic analysis of biological and physical limitations of proton beam range verification with offline PET/CT scans. Phys. Med. Biol. 2009, 54, 4477. [Google Scholar] [CrossRef]
- Nischwitz, S.P.; Bauer, J.; Welzel, T.; Rief, H.; Jäkel, O.; Haberer, T.; Frey, K.; Debus, J.; Parodi, K.; Combs, S.E.; et al. Clinical implementation and range evaluation of in vivo PET dosimetry for particle irradiation in patients with primary glioma. Radiother. Oncol. 2015, 115, 179–185. [Google Scholar] [CrossRef]
- Yoshida, E.; Tashima, H.; Shinaji, T.; Shimizu, K.; Wakizaka, H.; Mohammadi, A.; Nishikido, F.; Yamaya, T. Development of a Whole-Body Dual Ring OpenPET for in-Beam PET. IEEE Trans. Radiat. Plasma Med. Sci. 2017, 1, 293–300. [Google Scholar] [CrossRef]
- Pennazio, F.; Battistoni, G.; Bisogni, M.G.; Camarlinghi, N.; Ferrari, A.; Ferrero, V.; Fiorina, E.; Morrocchi, M.; Sala, P.; Sportelli, G.; et al. Carbon ions beam therapy monitoring with the INSIDE in-beam PET. Phys. Med. Biol. 2018, 63, 145018. [Google Scholar] [CrossRef]
- Parodi, K. Latest developments in in-vivo imaging for proton therapy. Br. J. Radiol. 2020, 93, 20190787. [Google Scholar] [CrossRef]
- Lang, K. Towards high sensitivity and high-resolution PET scanners: Imaging-guided proton therapy and total body imaging. Bio-Algorithms Med-Syst. 2022, 18, 96–106. [Google Scholar] [CrossRef]
- Ozoemelam, I.; Van der Graaf, E.; Van Goethem, M.J.; Kapusta, M.; Zhang, N.; Brandenburg, S.; Dendooven, P. Feasibility of quasi-prompt PET-based range verification in proton therapy. Phys. Med. Biol. 2020, 65, 245013. [Google Scholar] [CrossRef]
- Min, C.H.; Kim, C.H.; Youn, M.Y.; Kim, J.W. Prompt gamma measurements for locating the dose falloff region in the proton therapy. Appl. Phys. Lett. 2006, 89, 183517. [Google Scholar] [CrossRef]
- Singh, R. Nuclear Reactions; New Age International: Delhi, India, 1996. [Google Scholar]
- Krimmer, J.; Dauvergne, D.; Letang, J.; Testa, E. Prompt-gamma monitoring in hadrontherapy: A review. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 878, 58–73. [Google Scholar] [CrossRef]
- Xie, Y.; Bentefour, E.H.; Janssens, G.; Smeets, J.; Vander Stappen, F.; Hotoiu, L.; Yin, L.; Dolney, D.; Avery, S.; O’Grady, F.; et al. Prompt Gamma Imaging for In Vivo Range Verification of Pencil Beam Scanning Proton Therapy. Int. J. Radiat. Oncol. 2017, 99, 210–218. [Google Scholar] [CrossRef]
- Pausch, G.; Berthold, J.; Enghardt, W.; Römer, K.; Straessner, A.; Wagner, A.; Werner, T.; Kögler, T. Detection systems for range monitoring in proton therapy: Needs and challenges. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2020, 954, 161227. [Google Scholar] [CrossRef]
- Terzioglu, F.; Kuchment, P.; Kunyansky, L. Compton camera imaging and the cone transform: A brief overview. Inverse Probl. 2018, 34, 054002. [Google Scholar] [CrossRef]
- Gutierrez, A.; Baker, C.; Boston, H.; Chung, S.; Judson, D.; Kacperek, A.; Le Crom, B.; Moss, R.; Royle, G.; Speller, R.; et al. Progress towards a semiconductor Compton camera for prompt gamma imaging during proton beam therapy for range and dose verification. J. Instrum. 2018, 13, C01036. [Google Scholar] [CrossRef] [Green Version]
- Hueso-González, F.; Pausch, G.; Petzoldt, J.; Römer, K.; Enghardt, W. Prompt gamma rays detected with a BGO block Compton camera reveal range deviations of therapeutic proton beams. IEEE Trans. Radiat. Plasma Med. Sci. 2016, 1, 76–86. [Google Scholar] [CrossRef]
- McCleskey, M.; Kaye, W.; Mackin, D.; Beddar, S.; He, Z.; Polf, J. Evaluation of a multistage CdZnTe Compton camera for prompt γ imaging for proton therapy. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2015, 785, 163–169. [Google Scholar] [CrossRef]
- Solevi, P.; Munoz, E.; Solaz, C.; Trovato, M.; Dendooven, P.; Gillam, J.E.; Lacasta, C.; Oliver, J.F.; Rafecas, M.; Torres-Espallardo, I.; et al. Performance of MACACO Compton telescope for ion-beam therapy monitoring: First test with proton beams. Phys. Med. Biol. 2016, 61, 5149. [Google Scholar] [CrossRef]
- Panthi, R.; Maggi, P.; Peterson, S.; Mackin, D.; Polf, J.; Beddar, S. Secondary particle interactions in a Compton camera designed for in vivo range verification of proton therapy. IEEE Trans. Radiat. Plasma Med. Sci. 2021, 5, 383–391. [Google Scholar] [CrossRef] [PubMed]
- Veale, M.; Seller, P.; Wilson, M.; Liotti, E. HEXITEC: A high-energy X-ray spectroscopic imaging detector for synchrotron applications. Synchrotron Radiat. News 2018, 31, 28–32. [Google Scholar] [CrossRef]
- Wilson, M.; Dummott, L.; Duarte, D.D.; Green, F.; Pani, S.; Schneider, A.; Scuffham, J.; Seller, P.; Veale, M. A 10 cm × 10 cm CdTe Spectroscopic Imaging Detector based on the HEXITEC ASIC. J. Instrum. 2015, 10, P10011. [Google Scholar] [CrossRef]
- Jowitt, L.; Wilson, M.; Seller, P.; Angelsen, C.; Wheater, R.; Cline, B.; Schöne, D.; Lauba, F.; Goede, M.; Ball, R.; et al. HEXITEC 2 × 2 tiled hard X-ray spectroscopic imaging detector system. J. Instrum. 2022, 17, P01012. [Google Scholar] [CrossRef]
- Scuffham, J.W.; Wilson, M.D.; Pani, S.; Duarte, D.D.; Veale, M.C.; Bell, S.; Seller, P.; Sellin, P.J.; Cernik, R.J. Evaluation of a new small-pixel CdTe spectroscopic detector in dual-tracer SPECT brain imaging. In Proceedings of the 2012 IEEE Nuclear Science Symposium and Medical Imaging Conference Record (NSS/MIC), Anaheim, CA, USA, 29 October–3 November 2012; pp. 3115–3118. [Google Scholar] [CrossRef]
- Zhang, J.; Zannoni, E.M.; Du, Y.; Frey, E.; Meng, L.J. Alpha-SPECT: Hyperspectral single photon imaging of targeted α-emission therapy. J. Nucl. Med. 2019, 60, 311. [Google Scholar]
- Granja, C.; Jakubek, J.; Polansky, S.; Zach, V.; Krist, P.; Chvatil, D.; Stursa, J.; Sommer, M.; Ploc, O.; Kodaira, S.; et al. Resolving power of pixel detector Timepix for wide-range electron, proton and ion detection. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2018, 908, 60–71. [Google Scholar] [CrossRef]
- Agostinelli, S.; Allison, J.; Amako, K.A.; Apostolakis, J.; Araujo, H.; Arce, P.; Asai, M.; Axen, D.; Banerjee, S.; Barrand, G.; et al. GEANT4—A simulation toolkit. Nucl. Instrum. Methods Phys. Res. Sect. A Accel. Spectrometers Detect. Assoc. Equip. 2003, 506, 250–303. [Google Scholar] [CrossRef] [Green Version]
- Chen, Z.; Yang, P.; Lei, Q.; Wen, Y.; He, D.; Wu, Z.; Gou, C. Comparison of BNCT dosimetry calculations using different GEANT4 physics lists. Radiat. Prot. Dosim. 2019, 187, 88–97. [Google Scholar] [CrossRef]
- Wrońska, A.; Kasper, J.; Ahmed, A.A.; Andres, A.; Bednarczyk, P.; Gazdowicz, G.; Herweg, K.; Hetzel, R.; Konefał, A.; Kulessa, P.; et al. Prompt-gamma emission in GEANT4 revisited and confronted with experiment. Phys. Med. 2021, 88, 250–261. [Google Scholar] [CrossRef]
- Kramer, O. Scikit-learn. In Machine Learning for Evolution Strategies; Springer: Berlin/Heidelberg, Germany, 2016; pp. 45–53. [Google Scholar]
- Jones, L.; Bell, S.; Cline, B.; Gardiner, T.; Hart, M.; Prydderch, M.; Seller, P.; Veale, M.; Wilson, M. Spectroscopic X-ray imaging at MHz frame rates—The HEXITECMHz ASIC. J. Instrum. 2022, 17, C10012. [Google Scholar] [CrossRef]
- Pinto, M.; Bajard, M.; Brons, S.; Chevallier, M.; Dauvergne, D.; Dedes, G.; De Rydt, M.; Freud, N.; Krimmer, J.; La Tessa, C.; et al. Absolute prompt-gamma yield measurements for ion beam therapy monitoring. Phys. Med. Biol. 2014, 60, 565. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Perez-Lara, M.L.; Khong, J.C.; Wilson, M.D.; Cline, B.D.; Moss, R.M. First Study of a HEXITEC Detector for Secondary Particle Characterisation during Proton Beam Therapy. Appl. Sci. 2023, 13, 7735. https://doi.org/10.3390/app13137735
Perez-Lara ML, Khong JC, Wilson MD, Cline BD, Moss RM. First Study of a HEXITEC Detector for Secondary Particle Characterisation during Proton Beam Therapy. Applied Sciences. 2023; 13(13):7735. https://doi.org/10.3390/app13137735
Chicago/Turabian StylePerez-Lara, Maria L., Jia C. Khong, Matthew D. Wilson, Ben D. Cline, and Robert M. Moss. 2023. "First Study of a HEXITEC Detector for Secondary Particle Characterisation during Proton Beam Therapy" Applied Sciences 13, no. 13: 7735. https://doi.org/10.3390/app13137735




