Comparison of the Three-Dimensional Accuracy of Guided Apicoectomy Performed with a Drill or a Trephine: An In Vitro Study
Abstract
:1. Introduction
2. Materials and Methods
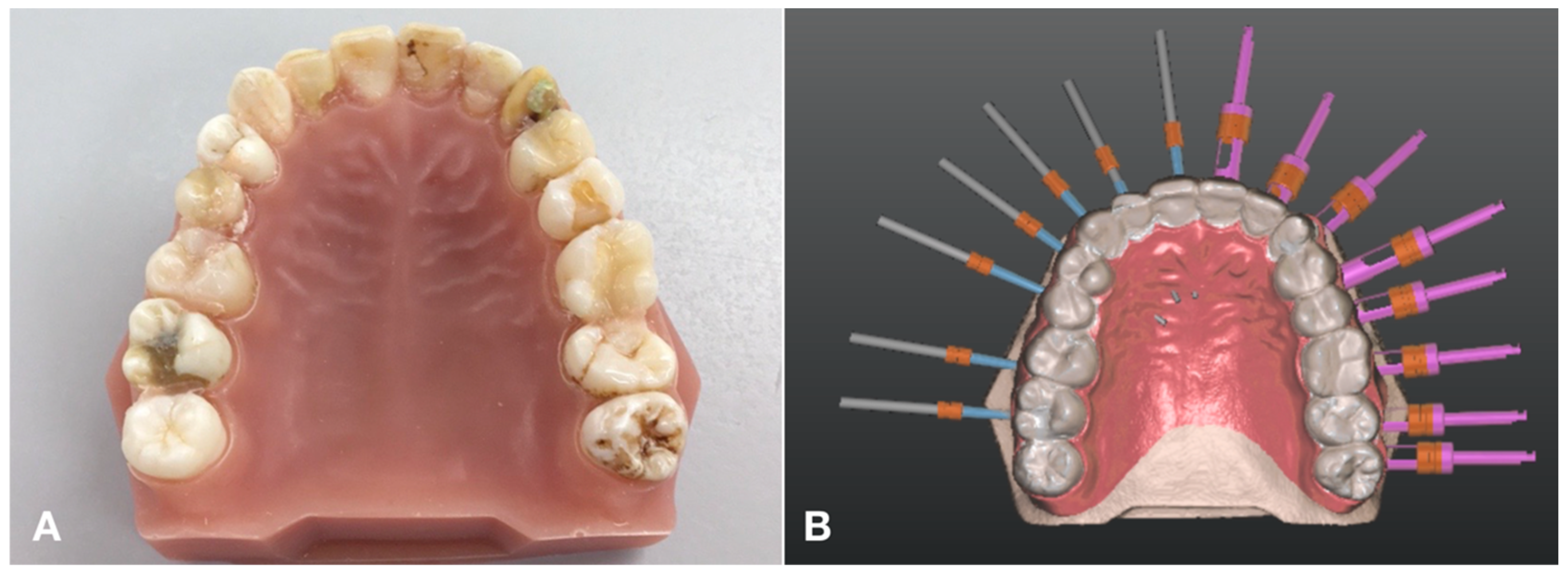
2.1. Model Preparation and Imaging
2.2. Planning
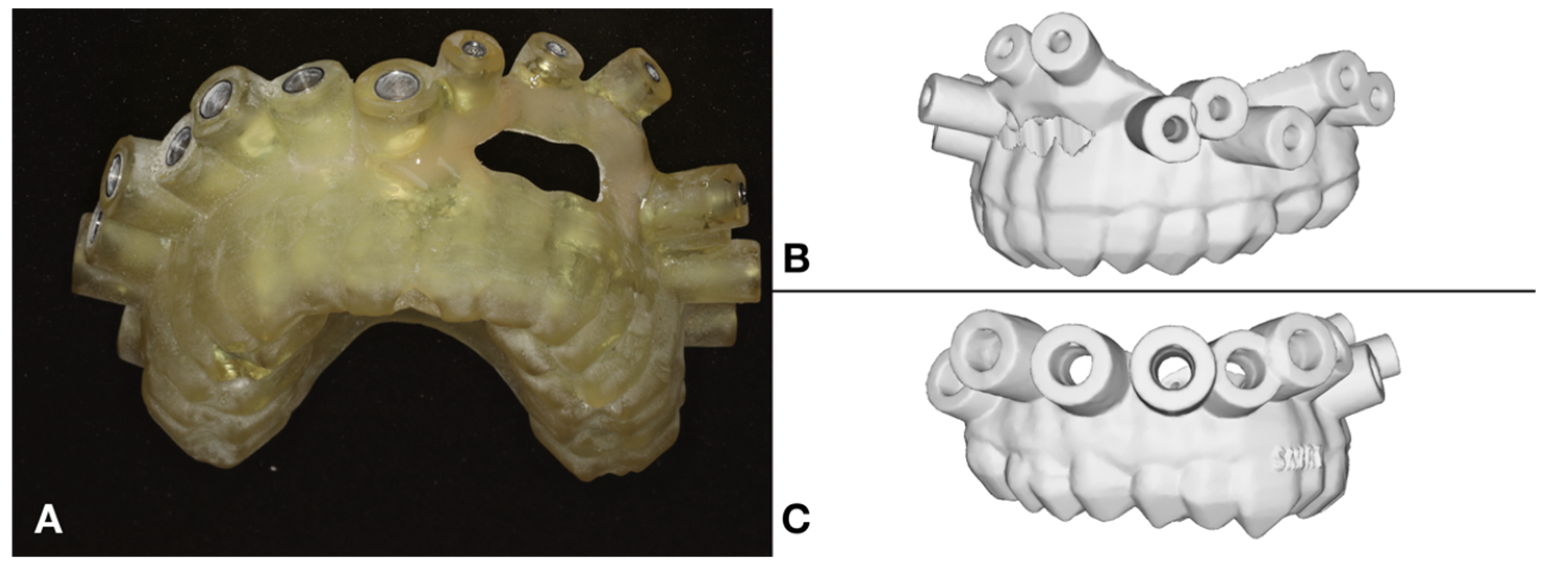
2.3. The Surgical Guide and the Applied Instruments
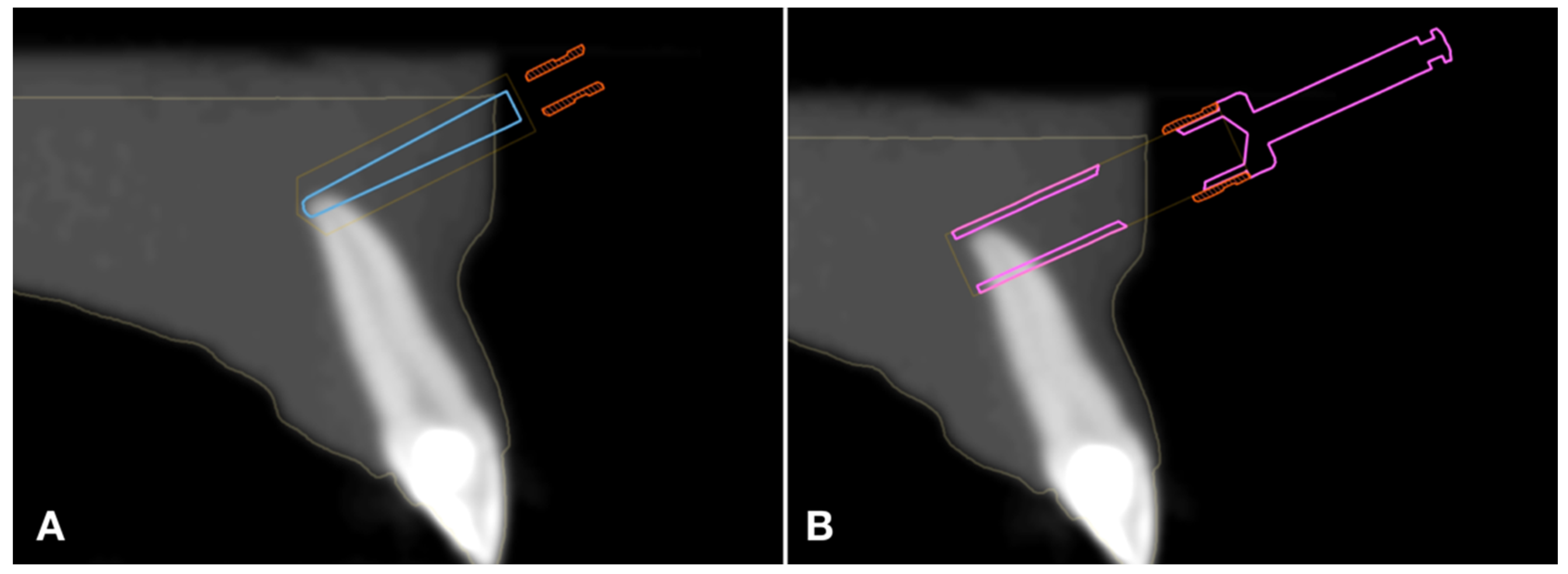
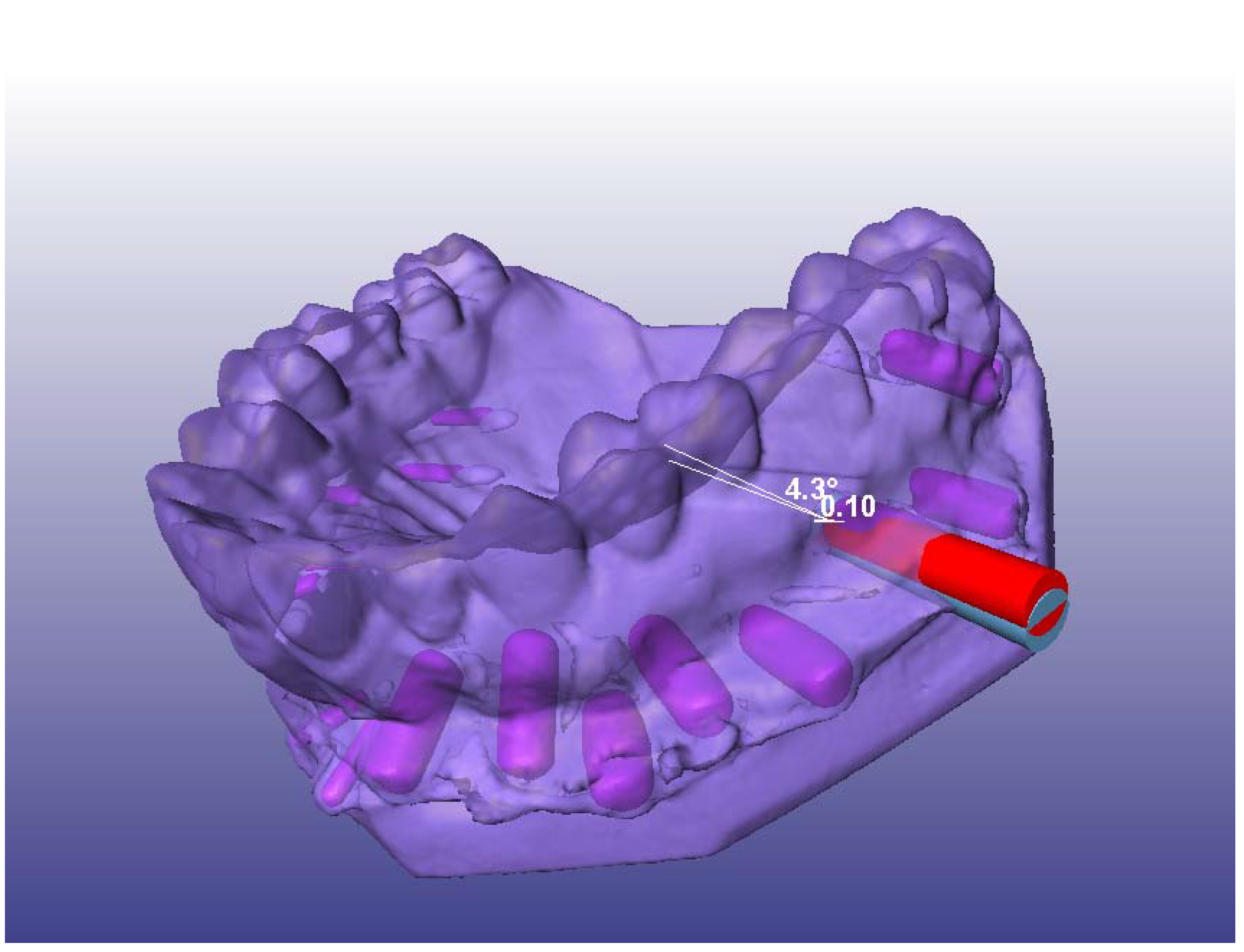
2.4. Simulated Surgery and Postoperative Analysis
2.5. Statistical Analyses
3. Results
3.1. Distal Global Deviation
3.2. Angular Deviation
4. Discussion
5. Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Karabucak, B.; Setzer, F. Criteria for the ideal treatment option for failed endodontics: Surgical or nonsurgical? Compend. Contin. Educ. Dent. 2007, 28, 391–397, quiz 398, 407. [Google Scholar]
- Tavares, W.L.F.; Fonseca, F.O.; Maia, L.M.; de Carvalho Machado, V.; Franca Alves Silva, N.R.; Junior, G.M.; Ribeiro Sobrinho, A.P. 3D Apicoectomy Guidance: Optimizing Access for Apicoectomies. J. Oral Maxillofac. Surg. 2020, 78, 357.e1. [Google Scholar] [CrossRef] [PubMed]
- von Arx, T. Apical surgery: A review of current techniques and outcome. Saudi Dent. J. 2011, 23, 9–15. [Google Scholar] [CrossRef]
- Kim, S.; Kratchman, S. Modern endodontic surgery concepts and practice: A review. J. Endod. 2006, 32, 601–623. [Google Scholar] [CrossRef] [PubMed]
- Niemczyk, S.P. Essentials of endodontic microsurgery. Dent. Clin. N. Am. 2010, 54, 375–399. [Google Scholar] [CrossRef]
- Setzer, F.C.; Kohli, M.R.; Shah, S.B.; Karabucak, B.; Kim, S. Outcome of endodontic surgery: A meta-analysis of the literature—Part 2: Comparison of endodontic microsurgical techniques with and without the use of higher magnification. J. Endod. 2012, 38, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Kohli, M.R.; Berenji, H.; Setzer, F.C.; Lee, S.M.; Karabucak, B. Outcome of Endodontic Surgery: A Meta-analysis of the Literature—Part 3: Comparison of Endodontic Microsurgical Techniques with 2 Different Root-end Filling Materials. J. Endod. 2018, 44, 923–931. [Google Scholar] [CrossRef] [PubMed]
- Torabinejad, M.; Parirokh, M.; Dummer, P.M.H. Mineral trioxide aggregate and other bioactive endodontic cements: An updated overview—Part II: Other clinical applications and complications. Int. Endod. J. 2018, 51, 284–317. [Google Scholar] [CrossRef] [PubMed]
- Tsesis, I.; Rosen, E.; Taschieri, S.; Telishevsky Strauss, Y.; Ceresoli, V.; Del Fabbro, M. Outcomes of surgical endodontic treatment performed by a modern technique: An updated meta-analysis of the literature. J. Endod. 2013, 39, 332–339. [Google Scholar] [CrossRef]
- Wang, Z.H.; Zhang, M.M.; Wang, J.; Jiang, L.; Liang, Y.H. Outcomes of Endodontic Microsurgery Using a Microscope and Mineral Trioxide Aggregate: A Prospective Cohort Study. J. Endod. 2017, 43, 694–698. [Google Scholar] [CrossRef]
- Pinsky, H.M.; Champleboux, G.; Sarment, D.P. Periapical surgery using CAD/CAM guidance: Preclinical results. J. Endod. 2007, 33, 148–151. [Google Scholar] [CrossRef] [PubMed]
- Giacomino, C.M.; Ray, J.J.; Wealleans, J.A. Targeted Endodontic Microsurgery: A Novel Approach to Anatomically Challenging Scenarios Using 3-dimensional-printed Guides and Trephine Burs—A Report of 3 Cases. J. Endod. 2018, 44, 671–677. [Google Scholar] [CrossRef] [PubMed]
- Hawkins, T.K.; Wealleans, J.A.; Pratt, A.M.; Ray, J.J. Targeted endodontic microsurgery and endodontic microsurgery: A surgical simulation comparison. Int. Endod. J. 2020, 53, 715–722. [Google Scholar] [CrossRef]
- Buniag, A.G.; Pratt, A.M.; Ray, J.J. Targeted Endodontic Microsurgery: A Retrospective Outcomes Assessment of 24 Cases. J. Endod. 2021, 47, 762–769. [Google Scholar] [CrossRef]
- Lin, S.; Platner, O.; Metzger, Z.; Tsesis, I. Residual bacteria in root apices removed by a diagonal root-end resection: A histopathological evaluation. Int. Endod. J. 2008, 41, 469–475. [Google Scholar] [CrossRef]
- Benjamin, G.; Ather, A.; Bueno, M.R.; Estrela, C.; Diogenes, A. Preserving the Neurovascular Bundle in Targeted Endodontic Microsurgery: A Case Series. J. Endod. 2021, 47, 509–519. [Google Scholar] [CrossRef] [PubMed]
- Patel, S.; Brown, J.; Pimentel, T.; Kelly, R.D.; Abella, F.; Durack, C. Cone beam computed tomography in Endodontics—A review of the literature. Int. Endod. J. 2019, 52, 1138–1152. [Google Scholar] [CrossRef]
- Reda, R.; Zanza, A.; Bhandi, S.; Biase, A.; Testarelli, L.; Miccoli, G. Surgical-anatomical evaluation of mandibular premolars by CBCT among the Italian population. Dent. Med. Probl. 2022, 59, 209–216. [Google Scholar] [CrossRef]
- Corbella, S.; Walter, C.; Tsesis, I. Effectiveness of root resection techniques compared with root canal retreatment or apical surgery for the treatment of apical periodontitis and tooth survival: A systematic review. Int. Endod. J. 2022; online ahead of print. [Google Scholar] [CrossRef]
- Kim, J.E.; Shim, J.S.; Shin, Y. A new minimally invasive guided endodontic microsurgery by cone beam computed tomography and 3-dimensional printing technology. Restor. Dent. Endod. 2019, 44, e29. [Google Scholar] [CrossRef]
- Anderson, J.; Wealleans, J.; Ray, J. Endodontic applications of 3D printing. Int. Endod. J. 2018, 51, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Ye, S.; Zhao, S.; Wang, W.; Jiang, Q.; Yang, X. A novel method for periapical microsurgery with the aid of 3D technology: A case report. BMC Oral Health 2018, 18, 85. [Google Scholar] [CrossRef] [PubMed]
- Antal, M.; Nagy, E.; Sanyo, L.; Braunitzer, G. Digitally Planned Root End Surgery with Static Guide and Custom Trephine Burs: A Case Report. Int. J. Med. Robot. Comput. Assist. Surg. MRCAS 2020, 16, e2115. [Google Scholar] [CrossRef] [PubMed]
- Popowicz, W.; Palatynska-Ulatowska, A.; Kohli, M.R. Targeted Endodontic Microsurgery: Computed Tomography-based Guided Stent Approach with Platelet-rich Fibrin Graft: A Report of 2 Cases. J. Endod. 2019, 45, 1535–1542. [Google Scholar] [CrossRef]
- Patel, S.; Aldowaisan, A.; Dawood, A. A novel method for soft tissue retraction during periapical surgery using 3D technology: A case report. Int. Endod. J. 2017, 50, 813–822. [Google Scholar] [CrossRef] [PubMed]
- Ray, J.J.; Giacomino, C.M.; Wealleans, J.A.; Sheridan, R.R. Targeted Endodontic Microsurgery: Digital Workflow Options. J. Endod. 2020, 46, 863–871. [Google Scholar] [CrossRef]
- Gilheany, P.A.; Figdor, D.; Tyas, M.J. Apical dentin permeability and microleakage associated with root end resection and retrograde filling. J. Endod. 1994, 20, 22–26. [Google Scholar] [CrossRef]
- Le, S.H.; Tonami, K.; Umemori, S.; Nguyen, L.B.; Ngo, L.Q.; Araki, K.; Nitta, H. Relationship between preoperative dental anxiety and short-term inflammatory response following oral surgery. Aust. Dent. J. 2021, 66, 13–19. [Google Scholar] [CrossRef]
- von Arx, T.; Peñarrocha, M.; Jensen, S. Prognostic factors in apical surgery with root-end filling: A meta-analysis. J. Endod. 2010, 36, 957–973. [Google Scholar] [CrossRef]
- Nagy, E.; Braunitzer, G.; Gryschka, D.G.; Barrak, I.; Antal, M.A. Accuracy of digitally planned, guided apicoectomy with a conventional trephine and a custom-made endodontic trephine: An in vitro comparative study. J. Stomatol. Oral Maxillofac. Surg. 2021, 123, 388–394. [Google Scholar] [CrossRef]
- Antal, M.; Nagy, E.; Braunitzer, G.; Fráter, M.; Piffkó, J. Accuracy and clinical safety of guided root end resection with a trephine: A case series. Head Face Med. 2019, 15, 30. [Google Scholar] [CrossRef] [PubMed]
- Strbac, G.D.; Schnappauf, A.; Giannis, K.; Moritz, A.; Ulm, C. Guided Modern Endodontic Surgery: A Novel Approach for Guided Osteotomy and Root Resection. J. Endod. 2017, 43, 496–501. [Google Scholar] [CrossRef] [PubMed]
- Nagy, E.; Fráter, M.; Antal, M. Guided modern endodontic microsurgery by use of a trephine bur. Orv. Hetil. 2020, 161, 1260–1265. [Google Scholar] [CrossRef] [PubMed]
- Ahn, S.Y.; Kim, N.H.; Kim, S.; Karabucak, B.; Kim, E. Computer-aided Design/Computer-aided Manufacturing-guided Endodontic Surgery: Guided Osteotomy and Apex Localization in a Mandibular Molar with a Thick Buccal Bone Plate. J. Endod. 2018, 44, 665–670. [Google Scholar] [CrossRef]
- Varga, E., Jr.; Antal, M.; Major, L.; Kiscsatári, R.; Braunitzer, G.; Piffkó, J. Guidance means accuracy: A randomized clinical trial on freehand versus guided dental implantation. Clin. Oral Implants Res. 2020, 31, 417–430. [Google Scholar] [CrossRef] [PubMed]
- Möhlhenrich, S.C.; Modabber, A.; Steiner, T.; Mitchell, D.A.; Hölzle, F. Heat generation and drill wear during dental implant site preparation: Systematic review. Br. J. Oral Maxillofac. Surg. 2015, 53, 679–689. [Google Scholar] [CrossRef]
- Atkinson, P.J.; Powell, K.; Woodhead, C. Cortical structure of the pig mandible after the insertion of metallic implants into alveolar bone. Arch. Oral Biol. 1977, 22, 383–391. [Google Scholar] [CrossRef]
- Powell, K.; Atkinson, P.J.; Woodhead, C. Cortical bone structure of the pig mandible. Arch. Oral Biol. 1973, 18, 171–180. [Google Scholar] [CrossRef]
- Štembírek, J.; Kyllar, M.; Putnová, I.; Stehlík, L.; Buchtová, M. The pig as an experimental model for clinical craniofacial research. Lab. Anim. 2012, 46, 269–279. [Google Scholar] [CrossRef]
- Gaffuri, S.; Audino, E.; Salvadori, M.; Garo, M.L.; Salgarello, S. Accuracy of a minimally invasive surgical guide in microsurgical endodontics: A human cadaver study. G. Ital. Endod. 2021, 35. [Google Scholar] [CrossRef]
- Kim, S. Color Atlas of Microsurgery in Endodontics; Saunders: Hong Kong, China, 2001. [Google Scholar]
- Younes, F.; Cosyn, J.; De Bruyckere, T.; Cleymaet, R.; Bouckaert, E.; Eghbali, A. A randomized controlled study on the accuracy of free-handed, pilot-drill guided and fully guided implant surgery in partially edentulous patients. J. Clin. Periodontol. 2018, 45, 721–732. [Google Scholar] [CrossRef] [PubMed]
- Di Giacomo, G.A.; Cury, P.R.; de Araujo, N.S.; Sendyk, W.R.; Sendyk, C.L. Clinical application of stereolithographic surgical guides for implant placement: Preliminary results. J. Periodontol. 2005, 76, 503–507. [Google Scholar] [CrossRef] [PubMed]
- Nickenig, H.J.; Wichmann, M.; Hamel, J.; Schlegel, K.A.; Eitner, S. Evaluation of the difference in accuracy between implant placement by virtual planning data and surgical guide templates versus the conventional free-hand method—A combined in vivo—In vitro technique using cone-beam CT (Part II). J. Craniomaxillofac. Surg. 2010, 38, 488–493. [Google Scholar] [CrossRef]
- Arisan, V.; Karabuda, Z.C.; Ozdemir, T. Accuracy of two stereolithographic guide systems for computer-aided implant placement: A computed tomography-based clinical comparative study. J. Periodontol. 2010, 81, 43–51. [Google Scholar] [CrossRef] [PubMed]


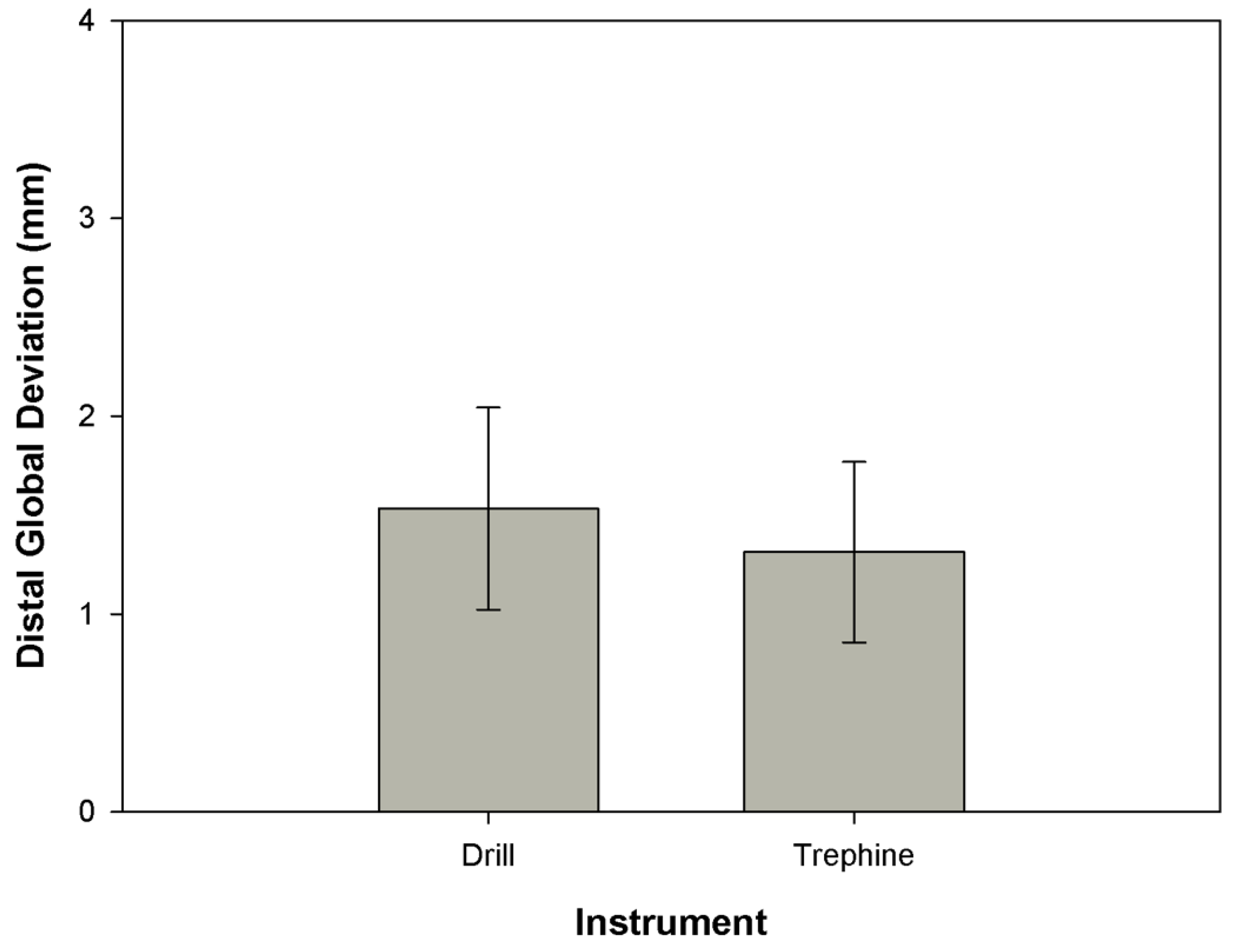
| Drill | Trephine | ||
|---|---|---|---|
| DGD | Mean | 1.5318 | 1.3119 |
| SD | 0.51074 | 0.45690 | |
| Median | 1.4500 | 1.3300 | |
| Minimum | 0.60 | 0.46 | |
| Maximum | 3.45 | 2.58 | |
| AD | Mean | 3.323 | 3.500 |
| SD | 1.4199 | 1.6722 | |
| Median | 3.200 | 3.200 | |
| Minimum | 0.9 | 0.6 | |
| Maximum | 6.5 | 8.8 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kiscsatári, R.; Nagy, E.; Szabó, M.; Braunitzer, G.; Piffkó, J.; Fráter, M.; Antal, M.Á. Comparison of the Three-Dimensional Accuracy of Guided Apicoectomy Performed with a Drill or a Trephine: An In Vitro Study. Appl. Sci. 2023, 13, 9642. https://doi.org/10.3390/app13179642
Kiscsatári R, Nagy E, Szabó M, Braunitzer G, Piffkó J, Fráter M, Antal MÁ. Comparison of the Three-Dimensional Accuracy of Guided Apicoectomy Performed with a Drill or a Trephine: An In Vitro Study. Applied Sciences. 2023; 13(17):9642. https://doi.org/10.3390/app13179642
Chicago/Turabian StyleKiscsatári, Ramóna, Eszter Nagy, Máté Szabó, Gábor Braunitzer, József Piffkó, Márk Fráter, and Márk Ádám Antal. 2023. "Comparison of the Three-Dimensional Accuracy of Guided Apicoectomy Performed with a Drill or a Trephine: An In Vitro Study" Applied Sciences 13, no. 17: 9642. https://doi.org/10.3390/app13179642
APA StyleKiscsatári, R., Nagy, E., Szabó, M., Braunitzer, G., Piffkó, J., Fráter, M., & Antal, M. Á. (2023). Comparison of the Three-Dimensional Accuracy of Guided Apicoectomy Performed with a Drill or a Trephine: An In Vitro Study. Applied Sciences, 13(17), 9642. https://doi.org/10.3390/app13179642









