Abstract
Japanese Encephalitis (JE) is caused by infection with the Japanese encephalitis virus (JEV). This vector-borne disease has been listed as a nationally notifiable public health risk in various countries. Predominantly found in Southeast Asia, JE can result in long-term neurological and psychiatric sequelae in both adults and children and is the leading cause of viral encephalitis in this region. Globally, there are an estimated 100,000 cases of JE and 25,000 associated deaths per year. Despite the significant effort to stop JE transmission through implementing vaccination programmes, JEV infection continues to be a global problem, with recent outbreaks in several countries, including outside of Asia. This raises a public health alert and establishes a need for future surveillance. Here, we reviewed the recent JE epidemic in Australia, focusing on its trends, impact and intervention. We recommend that a collaborative research effort together with a globally unified disease control strategy is urgently required to improve our understanding of JEV transmission and surveillance and to be better prepared for future outbreaks.
Keywords:
Australia; endemic; epidemiology; Japanese encephalitis; JE; JEV; vaccination; virus outbreak 1. Epidemiology of Japanese Encephalitis and the Outbreak in Australia
Globally, there are an estimated 68,000–100,000 clinical JE cases annually, causing approximately 15,000–25,000 fatalities [1,2]. Although historically most common in countries such as Japan and China, large-scale vaccination programmes have significantly reduced infection rates in these populations [2]. On 4 March 2022, an identified outbreak of Japanese Encephalitis (JE) in Australia was declared a “communicable disease incident of national significance” by Australia’s Acting Chief Medical Officer, Dr. Sonya Bennett [3,4]. At this time, Dr. Bennett called for a national approach to the “coordination of health policy, interventions and public messaging” surrounding this new outbreak [5]. Cases of Japanese encephalitis virus (JEV) infection have been found in piggeries across New South Wales and Victoria since January of that year, with more identified in Queensland and South Australia in the following months [6]. Australia’s Chief Veterinary Officer, Dr. Mark Schipp, noted that disease symptoms were most likely to be seen in piglets, with increased rates of stillbirth, impaired neurological functioning and hindlimb paralysis observed in the affected animal populations [5].
After identifying these animal cases in temperate regions of Australia not typically associated with the disease, it was hypothesised that the first human fatality from JE documented in the country since 1998, which occurred in February 2021, was actually the sentinel human case for the 2022 epidemic [3]. As such, this single case heralded a new infectious disease outbreak that would cause 40 further infections and 5 deaths by August 2022 [6]. Linked genetically to this sentinel case, the JEV genotype responsible for the recent outbreak has been identified as genotype IV, a previously rare variant found in China, Indonesia, Malaysia and Papua New Guinea [7]. The most recent media release from the Australian government in June 2023 reported a total of 45 human cases (35 with laboratory confirmation and 10 probable cases linked epidemiologically) and 7 deaths associated with the outbreak [8,9]. As no new cases have been detected in the Australian population since December 2022, the communication also announced the standing down of the JEV emergency response.
While this was not the first outbreak of JE in Australia, the situation was unique, as it occurred against the backdrop of an ongoing global pandemic of another communicable disease (COVID-19). The genotype involved in the outbreak (genotype IV) is also different from the two previous JEV outbreaks (I in 1995 and II in 1998) [10]. Although JEV was known to circulate in Queensland in the ten years following the 1995 outbreak, there are numerous theories as to why it is suddenly following a different pattern of expansion now. Jakob et al. noted that 2021 and 2022 were La Niña years with high levels of rainfall typically associated with outbreaks of mosquito-borne zoonotic infections [10]. Unprecedented flooding events have also likely contributed to JEV’s emergence in Australia’s mainland [11]. There is some evidence that endemicity in feral pig populations has already been established, and while JEV is a notifiable disease in both animals and humans in Australia, the contribution of these animals to the overall zoonotic infection rate is unknown [8,11].
Climate change plays a major role in vector-borne disease transmission, with rain and flood water providing the environmental conditions for mosquito population growth due to an “abundance of larval habitats,” and other weather conditions promoting different migration patterns for birds and other animal populations who are potential reservoirs of infection [12,13,14]. Van den Hurk et al. noted that the incursion of JEV into southeastern Australia represents a shift “at least 2500 km below its previous southern geographical limit” (Figure 1) [12]. As weather conditions continue to support survival and dispersal of disease-carrying mosquitoes, these authors suggest that JEV is unlikely to be eliminated in Australia [12]. As an immunologically naïve population, all Australians are at risk of JE if the virus spreads to densely populated areas in future outbreaks [1].
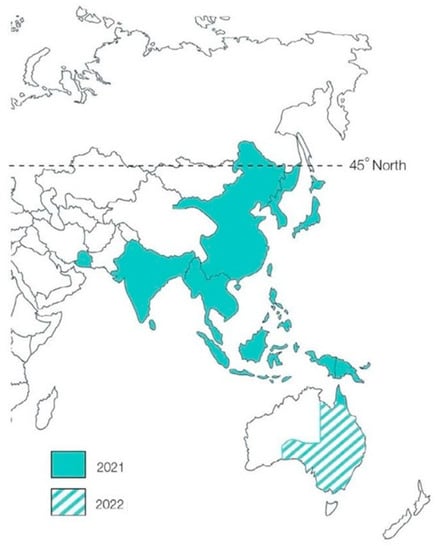
Figure 1.
Distribution of JEV beyond far north Queensland. This figure was adapted from the WHO under a creative commons licence and is taken, with permission, from the QIMR Berghofer website.
2. Host Invasion by JEV, Pathogenesis and Clinical Course
The aetiological factor causing JE is an infection with a flavivirus that is transmitted from a mosquito belonging to the genus Culex to a mammalian host [15]. While up to 14 species of mosquitoes have been identified as key transmission vectors, animals such as pigs and water birds, have been named amplifying and maintenance hosts of the virus, respectively [16,17]. Therefore, viral transmission can often occur in agricultural locations, including rice or pig farms, and can be seasonal according to environmental conditions that favour the breeding of mosquitoes (e.g., tropical climates and floods). However, there is no evidence that the virus is transmitted from human-to-human or by eating the flesh from an infected animal [15]. In many cases, infected animals show only mild clinical disease, although some may experience an elevated temperature, jaundice, lethargy, weight loss, oedema, neurological problems (e.g., depression, dysphagia and impaired vision), or develop infertility [18].
The spherical-shaped virus is approximately 50 nm in diameter and is encapsulated by a lipid membrane consisting of structural proteins, such as the Envelope ‘E’ protein, precursor to membrane ‘prM’ protein and the capsid ‘C’ protein [16]. When the mosquito carrying the virus bites the host, the virus is delivered into the tissue and lymph nodes proximal to the contact site before entering circulation. The virus attaches to the host cell membrane and is endocytosed into the cytoplasm. During the replication process, the virus multiplies its genomic material, which translates into three structural and seven non-structural viral proteins. These proteins are then assembled into immature virions in the Golgi complex of the host cell before they are released as mature viruses [19]. The structural proteins consist of the capsid (C), pre-membrane (prM) and envelope (E) proteins, while the non-structural proteins (NS 1, 2A, 2B, 3, 4A, 4B and 5) drive viral replication where NS5 protein is key in suppressing interferon-dependent immune responses in the host [19,20]. The route of virus invasion and replication mechanisms are detailed and illustrated in Sahu et al., Zhu et al. and Neufeldt et al. [19,21,22]. As replication actively occurs between 4 and 15 days, with an average incubation period of 7 days, the viral load becomes detectable in the blood during this time and the patient can either remain asymptomatic or may develop non-specific symptoms, such as diarrhoea, low-grade fever, nausea, headache, myalgia and malaise, which can persist for several days [15]. In most cases, the host immune response is potent enough to eradicate the JEV infection over time and symptoms resolve. More severe symptoms such as seizures and changes in consciousness or behaviour may reveal that the virus has been carried to the central nervous system (CNS) via the bloodstream and penetrates the blood brain barrier (BBB) [15,23]. Fatal consequences may also ensue due to the neurotoxic effects of the virus, often accompanied by an aggressive inflammatory response, neuronal cell death and glial scar formation [15,21].
These symptoms can be attributed to the capacity of the virus to activate the inflammasome, a protein complex made up of innate immune system receptors and sensors [24]. Inflammasome activation triggers transcription of genes encoding NLR family pyrin domain containing 3 (NFLRP3), caspase 1, and Pycard, thus exerting pro-inflammatory and apoptotic effects in response to various stimuli, such as infections with pathogens, in an attempt to keep the host organism alive [24,25]. Inflammasomes have been implicated in a series of autoinflammatory and autoimmune disorders, including neurodegenerative diseases [24]. Hence, it is no surprise that the inflammasome plays a role in driving the pathological events observed in the brains of patients diagnosed with JE. One of the most significant inflammasomes responsible for the neuroinflammatory effects seen in JE is the NLRP3 inflammasome [26]. This inflammasome consists of a sensor (NLRP3; NOD-, LRR- and pyrin domain-containing protein 3), an effector (caspase 1) and an adaptor (known as PYCARD).
The NLRP3 gene codes for an intracellular protein sensor that detects an extensive range of microbial antigens, endogenous damage-associated molecular patterns and environmental toxins, causing cellular stress [27]. The exact molecular mechanisms of how the NLRP3 protein senses cellular stress and terminates NLRP3 activation and inflammasome formation remain to be determined [27]. However, it is well established that when the NLRP3 protein becomes activated, it dissociates from the endoplasmic reticulum in the cytoplasm and interacts with the mitochondria and the mitochondrial membrane, thus escalating mitochondrial stress, which forms a positive feedback cycle generating toxic reactive oxygen species and inducing mitophagy. This process ultimately aims to eliminate any mitochondria that are damaged or dysfunctional [27]. Animal studies have shown that JEV infection of mouse brain tissues, microglia cells and astrocytes led to increased production of inflammatory cytokines via the activation of various inflammatory signalling pathways (e.g., ERK/MAPKp38/AP-1/NF-κB) that were positively correlated with the expression of the NLRP3 gene and the inflammasome [28]. These molecular observations align with findings from NLRP3 gene studies conducted on JE patients, confirming the importance of NLRP3 inflammasome-dependent processes in the neuroinflammation and death of brain tissues as patients progress in JE [25].
The CASPASE 1 gene encodes an evolutionarily conserved inflammatory mediated enzyme that plays a pivotal role in regulating the innate immune system primarily through acting as a protease that cleaves and activates pro-inflammatory cytokines, such as interleukin-1 β (IL-1β) and interleukin-18 (IL-18) [29]. The caspase 1 protein also regulates inflammatory-induced cell death through proteolytic activation of Gasdermin D, as well as metabolic regulations, such as homeostasis and lipid metabolism [30,31]. In the context of JE, caspase 1 regulates JEV-induced inflammatory responses through regulating the extent of pro-inflammatory cytokine production, a process that involves other JE-related inflammasomes. In a mouse model of JE, JEV infection induced the activation of caspase 1, resulting in the production of pro-inflammatory factors proIL-1β and proIL-18 [26]. It has been well established that the role of caspase 1 in innate immunity is interlinked with the NLRP3 inflammasome, which regulates neuroinflammation in JE [26]. Indeed, caspase 1 governs the production of the above pro-inflammatory cytokines in an NLRP3-dependent manner, as the depletion of NLRP3 not only reduced caspase-1 activity but also decreased the subsequent production of these cytokines [26]. Collectively, caspase 1 plays an integral role in the signalling pathways associated with the inflammatory response to JEV infection. These findings are also supported in patients with JE, in which the expression of both CASPASE 1 and NLRP3 genes appears to positively correlate with the progression of disease severity, with a marked elevation identified in severe cases but not among patients with mild symptoms [25]. Although preliminary, findings from this study provide new insights into our understanding of the role that caspase 1 plays in regulating JE progression and ultimately therapeutic development.
The PYCARD gene encodes for an adaptor protein consisting of the PYRIN-PAAD-DAPIN domain (PYD) and the caspase-recruitment domain (CARD) at the N and C terminals, respectively [32]. These domains are members of the six-helix bundle death domain superfamily, which, via the activation of Caspase, mediates the assembly of signalling complexes involved in inflammatory and apoptotic pathways [32]. Additionally known as Apoptosis-associated speck-like protein containing a CARD (ASC), the Pycard protein is typically localised in the nuclei of macrophages and monocytes; however, following infection, it can move to the cytoplasm, endoplasmic reticulum and other sites where it performs its function in activating the inflammasome [33]. The function of ASC in assembling the NLRP3 inflammasome is believed to be a response to inflammatory cytokine stimulation [34]. In 2011, Gupta and Rao suggested that JEV pathogenesis involved the activation of Pycard and various other pattern recognition receptors, including Toll-like receptors, C-type lectins, Cathepsin, Il1β, and Caspases 1 and 4, in addition to the possible generation of inflammasomes when JEV infects the host [35]. By 2020, Chowdhury and Khan demonstrated significant elevation of Pycard gene expression among patients with severe JE compared to those with milder symptoms. This observation is consistent with the upregulation of NLRP3 and Caspase 1 in severe cases, as mentioned above, thus suggesting a possible protective role of the NLRP3 inflammasome in the early stages of JEV infection. Similar pathways involving gene expression of Pycard and NLRP3 and inflammasome activation leading to neurological symptoms are also seen in West Nile Virus infections and, more recently, those caused by the SARS-CoV-2 (COVID-19) virus [36].
Taken together, viral infections caused by JEV activates molecular mechanisms driven by NLRP3 inflammasome activation, with Chowdhury and Khan concluding that “the variation in gene expression of inflammasomes may be associated with magnitude of JE pathogenesis and could be used as a probable marker for JE severity” [25]. The cellular changes that ensue include the efflux of potassium or chloride ions and influx of calcium ions across the plasma membrane, lysosomal disruption, mitochondrial dysfunction, abnormal metabolic changes and trans-Golgi disassembly [27]. The combined effects of these processes lead to a rapid and inflammation-dependent kind of lytic cell death (i.e., pyroptosis) for the prompt removal of infected cells from the host, thereby triggering some of the common symptoms described below [27].
The clinical course of JE can take place in four stages. During the prodromal phase, the patient suffers from a high-grade fever, malaise and anorexia. The next stage, known as the “acute stage”, results from increased intracranial pressure, caused by the build-up of inflammatory exudate in affected tissues. This stage is marked by more severe neurological symptoms consisting of loss of concentration or mental clarity, convulsion and impairment of sight, speech and/or hearing [16]. Up to 30% of patients succumb to death during this stage [16,37]. The third stage, known as the “defervescence phase”, occurs as fever subsides, but transient extrapyramidal features become prominent in patients exhibiting weakness of facial muscles, involuntary muscle contractions, rigidity and tremors [16]. The “sequelae phase” presents permanent neurological damage and approximately half of surviving patients experience one or more sequela: pyramidal weakness, convulsions, cognitive/language impairment, mental retardation and paralysis [16]. This final stage of JE affects children more than adults and those affected are unable to escape from at least some neurological symptoms and learning disabilities [16].
3. The Long-Term Impact of JEV Infection on Health and Socio-Economic Burden
The WHO report shows that JEV is a major cause of neurological disability in Asia and causes thousands of severe encephalitis cases and deaths each year. While the vast majority of JEV infections are asymptomatic, the risk of fatality amongst those who become symptomatic is approximately 30% [38]. Furthermore, about 30–50% of encephalitis cases experience significant acute or long-term neurological and psychiatric sequelae [39,40,41] such as behaviour impairments, intellectual disability [42], Parkinsonism and amnesia [43], and paralysis [41]. Movement disorders, neurologic deficits, and seizures are particularly common in children [15]. These neurological and psychiatric sequelae are largely due to viral infections in the CNS. Unfortunately, JEV is neuro-invasive, and the BBB is not equipped to block JEV from entering the CNS. Infiltration of the JEV into neurons and glial cells not only damages these cells but importantly induces neuroinflammation and disrupts brain homeostasis [28,44]. As a result, these JEV-induced CNS infections damage the structural and functional integrity of the brain, ultimately leading to neurological and psychiatric sequelae and long-term consequences.
JEV-induced CNS damage, sequalae and medical complications cause significant medical and social-economic burdens due to direct and indirect medical costs and long-term support needs from families and communities [45,46]. A study from Indonesia showed that 50% of children with clinical JE either died or were left with serious sequalae that significantly impaired their abilities and would require lifelong support [47]. In adults, there are significant medical costs associated with JE diagnosis, with the subsequent-year costs being even higher due to medical complications, such as comorbid diabetes and epilepsy events [46]. Despite this, a robust analysis of the corresponding health and economic burden is limited. One study from China analysed the medical cost of JE in Zhejiang province between 2013–2018 and estimated a total cost of US $12.01 million for 149 cases (over US $80,000 per patient) during hospitalisation [48]. A study estimated that over 1500 million people live in areas susceptible to endemic JEV transmission [11,37]. Considering this large volume of patient cases, the resultant economic burden is likely beyond the calculated estimates. As cases and deaths associated with JE are significantly underreported, the true burden of the disease is not well understood in most endemic countries and remains largely underestimated.
4. Management Strategies for Curb JEV Infection
Despite being one of the leading causes of encephalitic disorders, particularly in the Asia-Pacific region, there is still no cure for JE due to the lack of effective anti-viral therapy [17]. Current treatments do not reverse the permanent neurological damage caused by the infection; rather, they only provide patients with relief from some of the symptoms. These are usually limited to intravenous fluids (i.e., saline) and drugs (e.g., immunoglobulins, interferon alpha-2 and dexamethasone) that reduce inflammation, seizures, behavioural changes and/or psychotic episodes [15,19]. A high level of care provided by medical staff or personal carers may be required by patients who are either bedridden or otherwise incapacitated and unable to perform routine daily activities independently [16].
For an incurable condition, such as JE, preventative measures are of critical importance to halt the spread of this potentially life-threatening zoonotic disease. Chemical control of mosquitoes using larvicides containing bacterial toxins or insect growth regulators, adulticides such as fogging or surface application of a synthetic pyrethroid, and surface films or oils may be considered to reduce mosquito populations in high-risk areas [49]. However, these chemicals are also toxic to other land and marine wildlife and their efficacy declines within a week. Therefore, the use of these substances should be minimised where possible, and instead, regular surveillance of mosquito breeding grounds must be carried out. In 2022, the rapid JEV spread in eastern Australia across Queensland, NSW, Victoria, South Australia, and the Northern Territory strongly suggests that the natural transmission of the virus had been present for several months or years prior to being detected by the current surveillance systems. Therefore, additional animal surveillance protocols such as testing backyard pigs and their wastewater for the presence of viruses and newer mosquito sampling should be put in place for early detection systems in the future [50]. Future surveillance should not only incorporate the use of sentinel chicken flocks but also increase mosquito collections for virus isolation. It should also be targeted at wetlands where Ardeid bird feed and in areas of feral pig abundance [51]. Health campaigns, with the support of local councils, can also be developed to raise public awareness of JE and educate individuals and agricultural communities located in high-risk zones on the significance of taking precautions to avoid mosquito bites. These measures include reducing time spent outdoors, especially between dusk and dawn, wearing protective clothing, applying repellents containing permethrin or N,N-Diethyl-meta-toluamide, and sleeping within mosquito nets or in air-conditioned spaces [15].
Vaccination programmes are also available for people living in endemic areas, travellers who are likely to live in these areas during the transmission season for a month or more, or those who plan to spend significant time outdoors in rural or agricultural locations [15]. Residents from certain countries (e.g., United States of America) who plan to live in Asia for 6–12 months are also offered a JE vaccine as they may travel outside of urban areas for work or leisure [52]. Inactivated vaccines (e.g., JEspect, also known as Ixiaro®) have been developed by infecting Vero cells with the SA14-14-2 viral strain. The virus pool is collected, purified with protamine sulphate, and then inactivated with formaldehyde [53]. For this vaccine, two intramuscular doses must be administered 28 days apart [16]. Booster doses can be supplied a year after the second dose and are recommended for people at risk of infection with JEV. Live attenuated vaccines, such as Imojev®, require only a single subcutaneous dose, but are not appropriate for people who are immunocompromised, pregnant or younger than nine months of age [52]. This recombinant chimeric vaccine was created by swapping the prM and E coding regions of the yellow fever live attenuated 17D virus with that of the SA14-14–2 live attenuated JE virus [54]. Individual access to vaccines may vary depending on availability, health care advice and vaccination eligibility criteria across the different countries [55].
Australia’s emergency response to the 2022 JE epidemic demonstrates the importance of a coordinated national approach to disease outbreaks, with 125,000 vaccine doses delivered to at-risk individuals between February 2022 and the conclusion of the response in June 2023 [9]. A targeted education campaign also formed part of this public health intervention, with health professionals and the public providing vital information regarding how to protect themselves from infection. Another key insight from this recent outbreak focuses on the importance of One Health, with Australia’s national science agency, the CSIRO, reiterating the need to consider the interconnectedness of human, animal and environmental health [56].
5. Future Implications and Concluding Remarks
As mentioned above, although the risk of acquiring JE is low, the disease can be severe. Laboratory testing for JEV is complex due to the diversity of viral strains. The whole genomic sequences have identified five genotypes (GI, GII, GIII, GIV and GV) of JEV and the genetic diversity of JEV has remained high since 2000 [57]. Therefore, most cases are diagnosed by serology [11,19], demonstrating that securing timely diagnosis and treatment is often challenging. Thus, prevention through increased JE vaccination coverage is key to reducing disease spread and represents a cost-effective health strategy expected to have a large impact on reducing the burden of JE on public and environmental health. High vaccination coverage in countries such as Japan, Taiwan and South Korea has demonstrated a successful reduction of JE burden to almost zero [58,59], suggesting that while vaccination does not eliminate JEV, it can effectively reduce endemic JE transmission to humans. From 2010 to 2019, there was an overall decline in the mean number of JE deaths [37]. It is estimated that effective vaccination programmes have prevented approximately 315,000 cases and 115,000 deaths in the last ten years [37].
Despite these successes, there is still low vaccination coverage in many countries with a persistently high JE burden, including Bangladesh, Indonesia, Pakistan and the Philippines, due to the absence of a national vaccination programme [37]. Therefore, the extent of JE vaccination coverage can be seen to vary a great deal in many Asian countries with high JEV transmission [37]. Moreover, there is often an age-specific difference in vaccination coverage within the same country [37]. As such, JE remains a major cause of morbidity and mortality throughout Asia, particularly South and East Asia. Considering the size of current at-risk JE populations [37], increased JE vaccination coverage is needed. Increased funding to support large-scale vaccine programmes is required for low-income countries with high JE burden to achieve long-term elimination. Indeed, increased funding for JEV vaccination in recent years, including through support from Gavi, the Vaccine Alliance beginning in 2013, provides an opportunity to extend the successes experienced in higher-income countries to other countries that still experience a significant JE disease burden.
While vaccination is a key indicator, ongoing JEV surveillance remains a challenge. Travellers living outside of JE high-risk countries are more vulnerable to acquiring JE and the severe symptoms of the disease due to a lack of understanding of the need for vaccination in these regions [41]. Hence, travellers from low-risk countries should be informed of the potential risks of acquiring JE when visiting regions where the virus is endemic and should be offered vaccinations. Differences in risk profiles according to the itinerary and season of travel should also be disclosed.
From a public health perspective, managing JE in the context of the COVID-19 pandemic in Australia in 2022 represented a communication challenge, with mosquito monitoring and animal diagnostic surveillance forming the bulk of the health system response to JE [11], as opposed to the mass vaccination campaigns and targeted use of anti-viral therapies used in the nation’s COVID-19 response [60]. The two available vaccines for JEV retained strict eligibility criteria throughout most of Australia during the recent outbreak, due to limited supply, with those most at risk being prioritised for vaccination, including those living in flood-affected areas, people employed on pig farms, and individuals working to remove stagnant waters following floods [61]. However, these vaccines have not become available to children as young as 2 months old, even if the people they live with meet one of these criteria [34]. Despite the restrictive eligibility criteria, Australian residents are currently able to access these vaccinations not only at hospitals or their local councils, but also from community pharmacists who have been provided online training via the JE eLearning module, a government education initiative aimed at expanding the health workforce to assist in vaccine rollout programmes [34].
To ultimately eliminate JE in humans, research that focuses on protection and treatment is required. While significant efforts have been focused on developing the JE vaccine as the primary approach for protection, there are currently no treatments for JE [38] primarily due to poor understanding of the virus per se and its precise infection mechanism. Pathogenesis of viral infections of the CNS is poorly understood and there is a paucity of evidence as to how JE infects the CNS and causes subsequent neural damage. Understanding the detailed mechanisms by which JE invades and damages the CNS specifically will ultimately aid the development of effective treatment strategies. In light of the increasing public health threat of JE around the world, emerging evidence has increased our understanding of the modes of JEV infection and the delivery of the virus to various organs [28,62] as well as new insights into the development of novel JE models for research [63]. Using human-derived stem cells, brain organoids are being developed and used as an experimental model to study viral infections, such as JEV and the Zika virus [63]. Such pre-clinical research could shed light on new therapeutic developments targeting JEV-induced CNS infections, hence preventing or ameliorating neurological and psychiatric sequelae and their long-term functional consequences. The global spread of JEV infection is still not under control. Alarmingly, while JE remains most prevalent in Asia, there is a clear trend towards global expansion, including throughout Australia [2,12,38], Europe [64,65], America [66] and Africa [44], as evidenced by increasing case numbers. This raises a public health alert and has implications for future surveillance. JE affects not only individuals but also families and communities.
Nanotechnology-based vector control systems are the focus of current research efforts to intervene in the different stages of the life cycles of mosquito vectors. The use of nanotechnology to generate repellents and formulations that target the larvae stage are deemed to be less toxic to other wildlife due to increased water solubility. Nevertheless, they are deemed to be more efficacious with wider coverage and sustained release of the pesticide or mosquito repellent [12,52]. Nanotechnology has also enabled the development of biometallic nano-sensors that can rapidly detect mosquito-borne diseases [12]. The use of nanotechnology brings new hope to the prevention and treatment of JE, and this research area has progressed extensively in 2022 [12]. Following the recent advancement in the understanding of JEV replication and immune evasion mechanisms, chemotherapeutic targets are emerging, representing a new therapeutic strategy that could directly and effectively combat viral infection [67].
In conclusion, the recent JE outbreak in Australia demonstrates the need for a large collaborative research effort to improve our understanding of the epidemiology and pathogenesis regarding JEV infection, surveillance, diagnosis and treatment to ultimately eliminate JE globally. Furthermore, new and improved environmental, agricultural, animal husbandry and health practices and interventions must be considered holistically to form a unified disease control strategy that will effectively minimise viral transmission and curb the growth of present JE viral endemics into an epidemic and beyond [19]. Adopting a One Health approach that recognises the important role of viral reservoirs and environmental hazards in disease spread is essential for reducing JEV-related morbidity and mortality.
Author Contributions
Conceptualization, F.A.A.K., E.K. and J.X.; Investigation, F.A.A.K., E.K. and J.X.; Resources, F.A.A.K., E.K. and J.X.; Writing—Original Draft Preparation, F.A.A.K., E.K. and J.X. Writing—Final Review & Editing, F.A.A.K.; Project Administration, F.A.A.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The authors would like to acknowledge the School of Health Sciences, Swinburne University of Technology, for their support during the preparation of this review article.
Conflicts of Interest
The authors have no conflict of interest to declare.
References
- Furuya-Kanamori, L.; Gyawali, N.; Mills, D.J.; Hugo, L.E.; Devine, G.J.; Lau, C.L. The Emergence of Japanese Encephalitis in Australia and the Implications for a Vaccination Strategy. Trop. Med. Infect. Dis. 2022, 7, 85. [Google Scholar] [CrossRef] [PubMed]
- Williams, C.R.; Webb, C.E.; Higgs, S.; van den Hurk, A.F. Japanese Encephalitis Virus Emergence in Australia: Public Health Importance and Implications for Future Surveillance. Vector Borne Zoonotic Dis. 2022, 22, 529–534. [Google Scholar] [CrossRef] [PubMed]
- Waller, C.; Tiemensma, M.; Currie, B.J.; Williams, D.T.; Baird, R.W.; Krause, V.L. Japanese Encephalitis in Australia—A Sentinel Case. N. Engl. J. Med. 2022, 387, 661–662. [Google Scholar] [CrossRef] [PubMed]
- McGuinness, S.L.; Lau, C.L.; Leder, K. The evolving Japanese encephalitis situation in Australia and implications for travel medicine. J. Travel. Med. 2023, 30, taad029. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. Japanese Encephalitis Virus Situation Declared a Communicable Disease Incident of National Significance. Available online: https://www.health.gov.au/news/japanese-encephalitis-virus-situation-declared-a-communicable-disease-incident-of-national-significance#:~:text=%E2%80%9CI%20have%20declared%20the%20JEV,messaging%2C%E2%80%9D%20Dr%20Bennett%20said (accessed on 5 January 2023).
- Hutchinson, D.; Kunasekaran, M.; Chen, X.; Moa, A. Japanese Encephalitis Outbreak in South-Eastern Australia. Global Biosecurity Watching Brief. 2022. Available online: https://jglobalbiosecurity.com/articles/10.31646/gbio.159 (accessed on 10 February 2023).
- Pendrey, C.; Martin, G. Japanese encephalitis clinical update: Changing diseases under a changing climate. Aust. J. Gen. Pract. 2023, 52, 275–280. [Google Scholar] [CrossRef]
- Australian Government Department of Health and Aged Care. Japanese Encephalitis Virus. Available online: https://www.health.gov.au/diseases/japanese-encephalitis (accessed on 10 February 2023).
- Australian Government Department of Health and Aged Care. Statement on the End of Japanese Encephalitis Virus Emergency Response. 3 August 2023. Available online: https://www.health.gov.au/news/statement-on-the-end-of-japanese-encephalitis-virus-emergency-response#:~:text=Since%201%20January%202021%2C%2045,in%20Australia%20since%20December%202022 (accessed on 4 August 2023).
- Yakob, L.; Hu, W.; Frentiu, F.D.; Gyawali, N.; Hugo, L.E.; Johnson, B.; Lau, C.; Furuya-Kanamori, L.; Magalhaes, R.S.; Devine, G. Japanese Encephalitis Emergence in Australia: The Potential Population at Risk. Clin. Infect. Dis. 2023, 76, 335–337. [Google Scholar] [CrossRef]
- Pham, D.; Howard-Jones, A.R.; Hueston, L.; Jeoffreys, N.; Doggett, S.; Rockett, R.J.; Eden, J.S.; Sintchenko, V.; Chen, S.C.A.; O’Sullivan, M.V.; et al. Emergence of Japanese encephalitis in Australia: A diagnostic perspective. Pathology 2022, 54, 669–677. [Google Scholar] [CrossRef]
- van den Hurk, A.F.; Skinner, E.; Ritchie, S.A.; Mackenzie, J.S. The Emergence of Japanese Encephalitis Virus in Australia in 2022: Existing Knowledge of Mosquito Vectors. Viruses 2022, 14, 1208. [Google Scholar] [CrossRef]
- Hsu, S.M.; Yen, A.M.; Chen, T.H. The impact of climate on Japanese encephalitis. Epidemiol. Infect. 2008, 136, 980–987. [Google Scholar] [CrossRef]
- Furlong, M.; Adamu, A.M.; Hoskins, A.; Russell, T.L.; Gummow, B.; Golchin, M.; Hickson, R.I.; Horwood, P.F. Japanese Encephalitis Enzootic and Epidemic Risks across Australia. Viruses 2023, 15, 450. [Google Scholar] [CrossRef]
- Simon, L.V.; Sandhu, D.S.; Goyal, A.; Kruse, B. Japanese Encephalitis. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2022. Available online: https://www.ncbi.nlm.nih.gov/books/NBK470423/ (accessed on 4 August 2023).
- Mehta, A.; Singh, R.; Mani, V.E.; Poddar, B. Japanese B Encephalitis. Indian J. Crit. Care Med. 2022, 25 (Suppl. S2), S171–S174. [Google Scholar]
- Auerswald, H.; Maquart, P.O.; Chevalier, V.; Boyer, S. Mosquito Vector Competence for Japanese Encephalitis Virus. Viruses 2021, 13, 1154. [Google Scholar] [CrossRef] [PubMed]
- Animal Health Australia. Japanese Encephalitis. Available online: https://animalhealthaustralia.com.au/japanese-encephalitis/ (accessed on 4 August 2023).
- Sahu, R.C.; Suthar, T.; Pathak, A.; Jain, K. Interventions for the Prevention and Treatment of Japanese Encephalitis. Curr. Infect. Dis. Rep. 2022, 24, 189–204. [Google Scholar] [CrossRef] [PubMed]
- Ye, J.; Chen, Z.; Li, Y.; Zhao, Z.; He, W.; Zohaib, A.; Song, Y.; Deng, C.; Zhang, B.; Chen, H.; et al. Japanese Encephalitis Virus NS5 Inhibits Type I Interferon (IFN) Production by Blocking the Nuclear Translocation of IFN Regulatory Factor 3 and NF-κB. J. Virol. 2017, 91, e00039-17. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, Z.; Qi, Z. Virus-host Interactions in Early Japanese Encephalitis Virus Infection. Virus Res. 2023, 331, 199120. [Google Scholar] [CrossRef]
- Neufeldt, C.J.; Cortese, M.; Acosta, E.G.; Bartenschlager, R. Rewiring cellular networks by members of the Flaviviridae family. Nat. Rev. Microbiol. 2018, 16, 125–142. [Google Scholar] [CrossRef]
- Allen, S.; Cooper, C.M.; Taranath, A.; Cheng, A.C.; Britton, P.N. Japanese encephalitis virus: Changing the clinical landscape of encephalitis in Australia. Med. J. Aust. 2023, 218, 344–347. [Google Scholar] [CrossRef]
- Guo, H.; Callaway, J.B.; Ting, J.P.Y. Inflammasomes: Mechanism of action, role in disease, and therapeutics. Nat. Med. 2015, 21, 677–687. [Google Scholar] [CrossRef] [PubMed]
- Chowdhury, P.; Khan, S.A. Differential expression pattern of host inflammasome genes in Japanese encephalitis virus infected patients. Int. J. Infect. Dis. 2020, 101, 6–7. [Google Scholar] [CrossRef]
- Kaushik, D.K.; Gupta, M.; Kumawat, K.L.; Basu, A. NLRP3 inflammasome: Key mediator of neuroinflammation in murine Japanese encephalitis. PLoS ONE 2012, 7, e32270. [Google Scholar] [CrossRef]
- Swanson, K.V.; Deng, M.; Ting, J.P.Y. The NLRP3 inflammasome: Molecular activation and regulation to therapeutics. Nat. Rev. Immunol. 2019, 19, 477–489. [Google Scholar] [CrossRef] [PubMed]
- Ashraf, U.; Ding, Z.; Deng, S.; Ye, J.; Cao, S.; Chen, Z. Pathogenicity and virulence of Japanese encephalitis virus: Neuroinflammation and neuronal cell damage. Virulence 2021, 12, 968–980. [Google Scholar] [CrossRef] [PubMed]
- Sollberger, G.; Strittmatter, G.E.; Garstkiewicz, M.; Sand, J.; Beer, H.D. Caspase-1: The inflammasome and beyond. Innate Immun. 2014, 20, 115–125. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Gao, W.; Shao, F. Pyroptosis: Gasdermin-Mediated Programmed Necrotic Cell Death. Trends Biochem. Sci. 2017, 42, 245–254. [Google Scholar] [CrossRef]
- Molla, M.D.; Ayelign, B.; Dessie, G.; Geto, Z.; Admasu, T.D. Caspase-1 as a regulatory molecule of lipid metabolism. Lipids Health Dis. 2020, 19, 34. [Google Scholar] [CrossRef]
- Lu, A.; Magupalli, V.G.; Ruan, J.; Yin, Q.; Atianand, M.K.; Vos, M.R.; Schröder, G.F.; Fitzgerald, K.A.; Wu, H.; Egelman, E.H. Unified polymerization mechanism for the assembly of ASC-dependent inflammasomes. Cell 2014, 156, 1193–1206. [Google Scholar] [CrossRef]
- Sharma, M.; de Alba, E. Structure, Activation and Regulation of NLRP3 and AIM2 Inflammasomes. Int. J. Mol. Sci. 2021, 22, 872. [Google Scholar] [CrossRef]
- Srinivasula, S.M.; Poyet, J.L.; Razmara, M.; Datta, P.; Zhang, Z.; Alnemri, E.S. The PYRIN-CARD protein ASC is an activating adaptor for caspase-1. J. Biol. Chem. 2002, 277, 21119–21122. [Google Scholar] [CrossRef]
- Gupta, N.; Rao, P.V.L. Transcriptomic profile of host response in Japanese encephalitis virus infection. Virol. J. 2011, 8, 92. [Google Scholar] [CrossRef]
- Albornoz, E.A.; Amarilla, A.A.; Modhiran, N.; Parker, S.; Li, X.X.; Wijesundara, D.K.; Aguado, J.; Zamora, A.P.; McMillan, C.L.D.; Liang, B.; et al. SARS-CoV-2 Drives NLRP3 Inflammasome Activation in human Microglia through Spike Protein. Mol. Psychiatry 2022. [Google Scholar] [CrossRef]
- Moore, S.M. The current burden of Japanese encephalitis and the estimated impacts of vaccination: Combining estimates of the spatial distribution and transmission intensity of a zoonotic pathogen. PLoS Negl. Trop. Dis. 2021, 15, e0009385. [Google Scholar] [CrossRef]
- World Health Organization. Japanese Encephalitis—Australia. Available online: https://www.who.int/emergencies/disease-outbreak-news/item/2022-DON365 (accessed on 29 January 2023).
- Gao, X.; Li, X.; Li, M.; Fu, S.; Wang, H.; Lu, Z.; Cao, Y.; He, Y.; Zhu, W.; Zhang, T.; et al. Vaccine strategies for the control and prevention of Japanese encephalitis in Mainland China, 1951–2011. PLoS Negl. Trop. Dis. 2014, 8, e3015. [Google Scholar] [CrossRef] [PubMed]
- Quan, T.M.; Thao, T.T.N.; Duy, N.M.; Nhat, T.M.; Clapham, H. Estimates of the global burden of Japanese encephalitis and the impact of vaccination from 2000–2015. Elife 2020, 9, e51027. [Google Scholar] [CrossRef]
- Turtle, L.; Easton, A.; Defres, S.; Ellul, M.; Bovill, B.; Hoyle, J.; Jung, A.; Lewthwaite, P.; Solomon, T. ‘More than devastating’-patient experiences and neurological sequelae of Japanese encephalitis§. J. Travel. Med. 2019, 26, taz064. [Google Scholar] [CrossRef] [PubMed]
- Yin, Z.; Wang, X.; Li, L.; Li, H.; Zhang, X.; Li, J.; Ning, G.; Li, F.; Liang, X.; Gao, L.; et al. Neurological sequelae of hospitalized Japanese encephalitis cases in Gansu province, China. Am. J. Trop. Med. Hyg. 2015, 92, 1125–1129. [Google Scholar] [CrossRef]
- Monnet, F.P. Behavioural disturbances following Japanese B encephalitis. Eur. Psychiatry 2003, 18, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Filgueira, L.; Lannes, N. Review of Emerging Japanese Encephalitis Virus: New Aspects and Concepts about Entry into the Brain and Inter-Cellular Spreading. Pathogens 2019, 8, 111. [Google Scholar] [CrossRef]
- Griffiths, M.J.; Lemon, J.V.; Rayamajhi, A.; Poudel, P.; Shrestha, P.; Srivastav, V.; Kneen, R.; Medina-Lara, A.; Singh, R.R.; Solomon, T. The functional, social and economic impact of acute encephalitis syndrome in Nepal—A longitudinal follow-up study. PLoS Negl. Trop. Dis. 2013, 7, e2383. [Google Scholar] [CrossRef]
- Chen, H.Y.; Yang, C.Y.; Hsieh, C.Y.; Yeh, C.Y.; Chen, C.C.; Chen, Y.C.; Lai, C.C.; Harris, R.C.; Ou, H.T.; Ko, N.Y.; et al. Long-term neurological and healthcare burden of adults with Japanese encephalitis: A nationwide study 2000–2015. PLoS Negl. Trop. Dis. 2021, 15, e0009703. [Google Scholar] [CrossRef]
- Maha, M.S.; Moniaga, V.A.; Hills, S.L.; Widjaya, A.; Sasmito, A.; Hariati, R.; Kupertino, Y.; Artastra, I.K.; Arifin, M.Z.; Supraptono, B.; et al. Outcome and extent of disability following Japanese encephalitis in Indonesian children. Int. J. Infect. Dis. 2009, 13, e389–e393. [Google Scholar] [CrossRef]
- Deng, X.; Yan, R.; Li, Z.Q.; Tang, X.W.; Zhou, Y.; He, H. Economic and disease burden of Japanese encephalitis in Zhejiang Province, 2013–2018. PLoS Negl. Trop. Dis. 2021, 15, e0009505. [Google Scholar] [CrossRef]
- Hribar, L.J.; Boehmler, M.B.; Murray, H.L.; Pruszynski, C.A.; Leal, A.L. Mosquito Surveillance and Insecticide Resistance Monitoring Conducted by the Florida Keys Mosquito Control District, Monroe County, Florida, USA. Insects 2022, 13, 927. [Google Scholar] [CrossRef] [PubMed]
- Mackenzie, J.S.; Smith, D.W.; Speers, D.J. Japanese encephalitis disease: Overview of the virus, its risk to Australia and the need for better surveillance. Intern. Med. J. 2022, 52, 2029–2033. [Google Scholar] [CrossRef] [PubMed]
- Spencer, J.D.; Azoulas, J.; Broom, A.K.; Buick, T.D.; Currie, B.; Daniels, P.W.; Doggett, S.L.; Hapgood, G.D.; Jarrett, P.J.; Lindsay, M.D.; et al. Murray Valley encephalitis virus surveillance and control initiatives in Australia. National Arbovirus Advisory Committee of the Communicable Diseases Network Australia. Commun. Dis. Intell. Q. Rep. 2001, 25, 33–47. [Google Scholar]
- Mileno, M.D. Japanese Encephalitis Vaccine. RI Med. J. (2013) 2020, 103, 49–50. [Google Scholar]
- Lutmer, H.; Beall, M.; Bolt, B.; Felton, C.; Germann, L.; Gildersleeve, N.; Goode, A.L.; Hall, A.; Kryal, S.; Lu, K.; et al. Ixiaro Japanese Encephalitis Vaccine; Precision Vaccinations: Houston, TX, USA, 2022; Volume 2022. [Google Scholar]
- Appaiahgari, M.B.; Vrati, S. Clinical development of IMOJEV®—A recombinant Japanese encephalitis chimeric vaccine (JE-CV). Expert Opin. Biol. Ther. 2012, 12, 1251–1263. [Google Scholar] [CrossRef]
- World Health Organization. Japanese Encephalitis Vaccines: WHO position paper, February 2015 Recommendations. Vaccine 2016, 34, 302–303. [Google Scholar] [CrossRef]
- CSIRO. Our One Health Approach to Japanese Encephalitis. Available online: https://www.csiro.au/en/research/animals/pests/mosquito-borne-diseases_japaneseencephalitis (accessed on 4 August 2023).
- Gao, X.; Liu, H.; Li, M.; Fu, S.; Liang, G. Insights into the evolutionary history of Japanese encephalitis virus (JEV) based on whole-genome sequences comprising the five genotypes. Virol. J. 2015, 12, 43. [Google Scholar] [CrossRef]
- Erlanger, T.E.; Weiss, S.; Keiser, J.; Utzinger, J.; Wiedenmayer, K. Past, present, and future of Japanese encephalitis. Emerg. Infect. Dis. 2009, 15, 1–7. [Google Scholar] [CrossRef]
- Wang, H.; Liang, G. Epidemiology of Japanese encephalitis: Past, present, and future prospects. Ther. Clin. Risk Manag. 2015, 11, 435–448. [Google Scholar]
- Australian Government Department of Health and Aged Care. Coronavirus (COVID-19) Pandemic. Available online: https://www.health.gov.au/health-alerts/covid-19 (accessed on 25 November 2022).
- Victorian Government Department of Health. Japanese Encephalitis Virus. Available online: https://www.health.vic.gov.au/infectious-diseases/japanese-encephalitis-virus (accessed on 27 January 2023).
- Yadav, P.; Chakraborty, P.; Jha, N.K.; Dewanjee, S.; Jha, A.K.; Panda, S.P.; Mishra, P.C.; Dey, A.; Jha, S.K. Molecular Mechanism and Role of Japanese Encephalitis Virus Infection in Central Nervous System-Mediated Diseases. Viruses 2022, 14, 2686. [Google Scholar] [CrossRef]
- Depla, J.A.; Mulder, L.A.; de Sá, R.V.; Wartel, M.; Sridhar, A.; Evers, M.M.; Wolthers, K.C.; Pajkrt, D. Human Brain Organoids as Models for Central Nervous System Viral Infection. Viruses 2022, 14, 634. [Google Scholar] [CrossRef] [PubMed]
- Cleton, N.; Koopmans, M.; Braks, M.; Van Maanen, K.; Reusken, C. Japanese encephalitis in Southern Europe. Tijdschr Diergeneeskd 2014, 139, 20–25. [Google Scholar] [PubMed]
- Hernández-Triana, L.M.; Folly, A.J.; Sewgobind, S.; Lean, F.Z.X.; Ackroyd, S.; Nuñez, A.; Delacour, S.; Drago, A.; Visentin, P.; Mansfield, K.L.; et al. Susceptibility of Aedes albopictus and Culex quinquefasciatus to Japanese encephalitis virus. Parasit Vectors 2022, 15, 210. [Google Scholar] [CrossRef] [PubMed]
- Nemeth, N.M.; Bosco-Lauth, A.M.; Sciulli, R.H.; Gose, R.B.; Nagata, M.T.; Bowen, R.A. Serosurveillance for Japanese encephalitis and West Nile viruses in resident birds in Hawai’i. J. Wildl. Dis. 2010, 46, 659–664. [Google Scholar] [CrossRef]
- Nemeth, N.M.; Bosco-Lauth, A.M.; Sciulli, R.H.; Gose, R.B.; Nagata, M.T.; Bowen, R.A. Chemotherapy in Japanese encephalitis: Are we there yet? Infect. Disord. Drug Targets 2011, 11, 300–314. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).