Abstract
The stabilisation of soft soils using the traditional binders cement and quicklime are known to emit large amounts of carbon dioxide. To reduce this carbon footprint, substitutes such as industrial by-products have been thoroughly tested as viable alternatives for soil stabilisation. However, recent research has also shown that biochar from biomass pyrolysis can in some instances have a positive stabilisation effect and even result in a carbon-negative footprint. This paper presents a laboratory study to investigate the stabilisation effect of five industrial by-products and four types of biochar on three natural Norwegian soils: two clays with low and high water contents and one peat with a very high water content. The soils and binders were characterised by their mineralogical and chemical compositions. The biochars had varying stabilisation effects on the clays when combined with cement, with some negative stabilisation effects, whilst the effect was very beneficial in the peat, with a strength increase of up to 80%. The industrial by-products showed opposite results, with beneficial effects in the clays and a strength increase of up to 150%, but negative stabilisation effects in the peat. Correlating the mineralogical and chemical compositions to stabilisation effects was found to be challenging.
Keywords:
soil stabilisation; clay; peat; industrial by-products; biochar; shear strength; stiffness 1. Introduction
Soil stabilisation is used extensively throughout the world to improve soil characteristics. One application of soil stabilisation involves the improvement of engineering properties of thick soft soil deposits, typically referred to as deep soil stabilisation or deep mixing, to enable the development and construction of buildings, roads, railways and other land use. Deep soil stabilisation is performed by mixing a binder into the soil, whereupon chemical reactions strengthen the soil matrix. Traditionally, cement (CEM) and quicklime (QL) have been the preferred binders. However, their production is well known to emit considerable amounts of carbon dioxide (). Globally, cement production alone contributes to approximately 8% of global anthropogenic emissions [1,2]. To the best of the authors’ knowledge, there is no estimation of the total emissions generated from the deep soil stabilisation of soft soil deposits. However, it is safe to assume that it is considerable.
There are major research efforts being made across the world to decrease emissions in the soil stabilisation industry as well as in the cement and concrete industries. One way of achieving this is to substitute CEM and QL with alternative binder types and, since society is creating large amounts of unused industrial by-products (IBPs), these could be a potential substitute. IBPs often come in the form of ashes from the combustion of the original waste product. In the soil stabilisation industry, research and testing on a laboratory scale have been conducted using a wide variety of IBPs, e.g., cement kiln dust [3,4,5], lime kiln dust [6,7], fly ash [8,9,10], bottom ash [11], gypsum [12,13], ground-granulated blast-furnace slag (GGBS) [14,15,16,17,18] and ladle slag [12]. In addition, other binders or additives have been studied, such as biopolymers [19,20], geopolymers [21], fibres [22], reactive magnesia [5], colloidal silica [23] and natural or synthetic zeolites [24,25]. These studies have illustrated the potential to reduce emissions when using IBPs. However, despite all the research efforts, it is difficult to establish a common framework for their stabilisation effect. This might be attributed to the fact that IBPs can differ considerably in their composition and to the high variability of soils in terms of, e.g., grain size distribution, mineralogical composition, water content, organic content, etc. It is, therefore, vital that studies on the stabilisation effect of different IBPs in different countries and regions continue to be performed.
Recently, there has also been increased interest and research on the use of biochar as an alternative binder. Biochar is manufactured by the pyrolysis of biomass, i.e., combustion in an inert atmosphere (i.e., the absence of oxygen), resulting in a non-biodegradable carbonaceous material with typical carbon contents exceeding 75%. It is characterised by a high porosity and large surface area and, hence, a high water-absorption capacity [26,27], and has several potential benefits, including soil fertility improvement and contaminant immobilisation [28]. Recent studies have also shown that biochar in mixtures with cement can have a positive stabilisation effect in soils [27,29,30,31,32,33,34,35,36,37,38,39], meaning that it can be used as an alternative binder along with its carbon sequestration potential. Since 1.0 kg of biochar contains a minimum of approximately 0.75 kg of carbon (C), it can sequester over ~2.7 kg of as no decomposition or erosion takes place in low permeable thick clay deposits. This amount of sequestered carbon is substantial, considering that corresponding emissions for CEM and QL, as examples, are approximately 0.65 kg and 1.0 kg , respectively, per kg binder [6]. To make a CEM-biochar-based binder climate neutral, i.e., resulting in net zero emissions, one thus needs only to replace approximately 20–30% of the CEM with biochar.
This paper presents results from a laboratory study where five different IBPs and four different biochars were used to stabilise three soft Norwegian soils: a soft, low-sensitive clay from Onsøy [40], located in southern Norway; a quick clay; and a peat, both from Tiller-Flotten [41,42], located in mid-Norway. All binders and soils were characterised by their composition and mineralogy, and the potential for soil stabilisation was investigated by strength and stiffness testing.
The purpose of this study was to investigate if and how the stabilisation effects of the IBPs and biochars differed for the different soil types and whether the stabilisation effect could be correlated to the binder and soil compositions. As few studies compare several types of IBPs and biochars in both clay and peat, the results presented herein give new insights into how their effectiveness can vary. This study shows that the differences can be considerable and highlights the importance of taking both the type of binder and the type of soil into consideration. It is believed that the results can also provide valuable guidance for further testing.
2. Materials and Methods
2.1. Natural Soils
The soft, low-sensitive Onsøy clay was sampled from the Norwegian GeoTest Site (NGTS) located outside of the city of Fredrikstad in southern Norway [40]. The sample depth was approximately 5–8 m. The clay had a bulk density () of 1.5–1.6 t/m3, natural water content () of 60–80%, sensitivity of 5–9, organic content of 3–4%, plasticity index () of 40–50%, and liquidity index () of approximately 0.9–1.0. The clay content was approximately 60–70%, the remaining being predominantly quartz silt grains.
The Tiller-Flotten quick clay was sampled from a depth of 9–13 m at the NGTS test site outside of Trondheim, mid-Norway [41]. The quick clay had a of 1.75–1.85 t/m3, of 40–45%, sensitivity of 150–250, organic content of <1%, of approximately 10–15%, and of approximately 1.5–2.0. The clay content was similar to that of Onsøy, with the remaining being mostly quartz silt grains.
The peat was sampled from a depth of 1–1.5 m at the NGTS site at Tiller-Flotten [41,42]. The peat had a of approximately 800–1000% and a close to 1.0 t/m3. The degree of humification according to the von Post classification [43] was determined at H2–H3, i.e., a very low degree of humification with insignificant or very slight decomposition with an identifiable plant structure. The sampling depth was approximately 1.5 m.
2.2. Binders
A Portland cement of type CEM I 52.5 R (EN 197-1 [44]), produced by Norcem at Brevik, near Porsgrunn, Norway, was used. The CEM I cement was chosen to avoid any content of fly ash or slag that could interact with the biochars and IBPs.
Four different types of biochars (BCs) were used, all produced in a full-scale microwave-assisted pyrolysis (MAP) unit with a residence time of ~20 min. The reactor temperature was approximately 470–600 °C. The four biochars were as follows:
- BC1: originated from demolition wood, i.e., wood panels, furniture and composite wood materials, which hence contained some metals and glue remains.
- BC2: originated from municipal sewage which had been sedimented to a bottom sludge and thereafter left to decay for some time. Approximately 39 wt.% of limestone (CaCO3) was added for hygenisation and workability before the sludge was used for biochar production.
- BC3: originated from sewage and food waste. To speed up the sedimentation, iron chloride (FeCl3) was added for flocculation. The bottom sludge was used to produce the biochar.
- BC4: originated from garden waste, i.e., branches, leaves and grass, but also contained some soil and sand.
The five types of IBPs included two types of bioashes, one paper-sludge ash and two ladle slags:
- The bioashes were one fly ash (FA) and one bottom ash (BA) obtained from the Bergene Holm’s combustion plant at Brandval, near Kongsvinger, Norway. This plant is a grate-fire combustion plant where the boiler temperature is 1000–1200 °C. The resulting fly and bottom ash account for approximately 10% and 90%, respectively, of the total ash generated. The biomass consisted of a mixture of ~35–40% dry wood chips and ~60–65% bark. Bioash is mostly used for agricultural purposes [45]; however, a few studies have also been made on soil stabilisation [46,47,48,49,50,51].
- One type of paper-sludge ash (PSA) was used, originating from the Norske Skog factory at Skogn, mid-Norway, where paper production and recycling is performed. A mixture of ~58% biofuel (demolition wood), ~25% deinked pulp sludge, ~14% bio sludge and ~3% plastic/juice cartons, etc., was combusted at a temperature of approximately 850 °C. In this study, fly ash from the combustion was used. PSA has been used as an alternative binder in both mortars and concrete [52,53,54] and in soil stabilisation [55,56,57,58,59,60,61].
- The two ladle slags originated from Celsa Steel Services, where the recycling of steel is carried out to produce reinforcement steel bars. Both electric arc furnace slag and steelmaking slag are generated from different stages of the melting process. The ladle slags used herein were a mixture of these slags and were extracted from two different locations: at the melt shop (LS1), i.e., a fresh ladle slag, and from an intermediate repository (LS2). Since LS is cooled rather slowly, it develops a high crystallinity and thus possesses relatively low hydraulic reactivity compared to GGBS [62]. LS can be alkali-activated using, e.g., sodium hydroxide, sodium silicate (‘waterglass’), QL, CEM or reactive magnesia [63,64,65], albeit somewhat less effectively than GGBS [65,66]. LS has also been used for research purposes in soil stabilisation [12,67,68,69,70,71].
All biochars and IBPs were ground and sieved to a <1 mm fraction and dried before being used for soil stabilisation. Figure 1 shows the IBPs and an example of one biochar.

Figure 1.
Photographs of selected biochars and IBPs: (a) biochar (BC3), (b) ladle slag (LS1), (c) ladle slag (LS2), (d) paper sludge ash (PSA), (e) bioash (BA) and (f) bioash (FA).
2.3. Microstructural and Compositional Analyses
Water contents () and particle densities () were determined according to ISO 17892-1 and -3, respectively. The was determined using the fluid pycnometer with moist specimens. Two separate determinations were made and an average value was calculated if the difference was <0.03 t/m3. These analyses were performed at the geotechnical laboratory at the Norwegian Geotechnical Institute (NGI).
Specific surface area (SFA) determinations were made by the adsorption of gas and calculated according to the Brunauer–Emmett–Teller (BET) theory [72]. The SFA analyses were performed by Nemko Norlab in Norway.
Thermogravimetric analyses (TGA) were performed on samples dried at 105 °C prior to analyses which were then, from room temperature, exposed to a temperature increase rate of 10 °C/min in a nitrogen atmosphere [73,74]. The TGA was performed at SINTEF in Norway.
X-ray fluorescence (XRF) analyses of major elements were performed using a PANalytical Axios 4 kW equipped with a rhodium X-ray tube on fused glass beads. The material was mixed with a lithium borate flux and heated to 1000 °C. X-ray diffraction (XRD) analyses were made on whole rock, i.e., not sieved, and were hand-mortared prior to the analyses. The analyses were made with a BRUKER D8 Advance using CuKα radiation (40 kV/40 mA). Scans were acquired on a rotating disk using 2.5° Soller slits and a fixed divergence slit (0.6 mm) in the range of 3 to 75° 2Ө with a step size of 0.02° 2Ө and a 1 s count time. Mineral identifications were performed using the BRUKER Diffrac.EVA ver. 5.2 software, Crystallographic Open Database (COD) and International Centre for Diffraction Data (ICDD) databases. Mineral quantification was conducted using Rietveld modelling with the TOPAS 5.0 software. The XRF and XRD analyses were performed by X-ray Mineral Services, UK.
2.4. Geotechnical Testing
The preparation of stabilised soil specimens was performed by first remoulding the natural clays and peat until they were visually considered homogeneous, whereupon the dry binder mixture was added and homogenised for ~3–5 min. The soil–binder mixtures were then moulded in plastic cylinders (diameter 54 mm; height 100 mm) using a rodding technique, i.e., tamping or pushing a rod onto the material to minimise the air content [75,76]. Three replicate samples from each mixture were produced and cured for 28 days in the sealed cylinders at a room temperature of approximately 20 °C [77]. Unconfined compression (UC) tests were then performed with a strain rate of ~3.7%/min. From the UC tests, the strength was interpreted as the maximum shear stress (), which is typically used in Nordic practice instead of the unconfined compressive strength (). Stiffness was interpreted as secant Young’s modulus () up to 50% of the peak stress.
Table 1 shows the complete laboratory strength testing programme. Based on the results from previous research [27,29,46], different binder quantities () and combinations were used for the clays and peat and for the IBPs and biochars. For the clays stabilised with IBPs, mixtures of 50 wt.% CEM and 50 wt.% IBP were used, with a total of 60 kg/m3. For comparison, specimens with 100% CEM were also prepared with of 30 and 60 kg/m3. For clays and biochar (BC), mixtures of 33 wt.% CEM and 67 wt.% BC were prepared with of 150 kg/m3, i.e., 50 kg/m3 of CEM and 100 kg/m3 of BC. For comparison, specimens with 100% CEM with of 50 kg/m3 were prepared.

Table 1.
Laboratory strength testing programme. Mixtures were designated by binder quantity of CEM and binder quantity of biochar or IBP. For example, CEM-30/BC4-30 denotes a mixture of 30 kg of CEM and 30 kg of BC4 per m3 of natural soil, i.e., a total binder quantity of 60 kg/m3. For all mixtures, three replicate specimens were prepared.
For the peat specimens, all mixtures were prepared with 33 wt.% CEM and 67 wt.% IBP or BC with a total of 300 kg/m3. Again, for comparison, specimens with 100% CEM with of 100 kg/m3 were prepared.
To further investigate the stabilisation effect of the IBPs and biochars, testing according to the ‘strength activity index’ (SAI) [78] was performed, although not using the same recipe and geometry as specified in the ASTM standard due to available laboratory equipment. Specimens with mixtures of 12.5 wt.% water, 25 wt.% dry binder and 62.5 wt.% clean quartz sand were manufactured. One reference specimen with 100% CEM was prepared, followed by mixtures where 20% of the CEM was replaced with the respective IBP and biochar. Thus, in total, 10 mixtures were prepared, all moulded in cylinders (diameter 54 mm; height 100 mm) and cured for 14 days before being subjected to UC tests.
3. Results
3.1. The Characterisation of Natural Soils and Binders
Results from the , and SFA determinations are shown in Table 2. The of the Tiller-Flotten quick clay (42%) was lower than that of the Onsøy clay (73%); however, the SFA was similar for both clays. The IBPs and biochars exhibited a large variation in , although all were dried prior to being mixed with the natural soils. The PSA, BA, LS1 and BC2 had a (Table 2). The biochars had varying , and SFA. The high SFA of the biochars BC1, BC2 and BC3 was expected [26,27].

Table 2.
Results of water content, particle density and specific surface area (SFA) determinations. Note that the water contents for the IBPs and biochars were determined after receiving the binders in the laboratory. Before mixing with the soil, all IBPs and biochars were dried.
The results of the compositional (XRF) and phase (XRD) analyses are shown in Table 3, Table 4 and Table 5. In general, the compositions were similar to those of previously published results. For example, the two ladle slags had high contents of and [65,79]. The PSA also showed similar compositions as those reported in the literature, albeit with somewhat lower contents [53]. The BC2 was particularly high in because of the addition of limestone to the sludge prior to pyrolysis. Similarly, the BC3 contained a high amount of because of the addition of iron chloride in the sedimentation process. This also resulted in higher particle densities compared to BC1 and BC4 (Table 2).

Table 3.
Results of major element X-ray fluorescence analyses (XRF). All values in per cent. ND = not determined as abundance was below the detection limit.

Table 4.
Results of whole rock X-ray diffraction analyses (XRD) of natural soils, showing clay minerals in crystalline phase and amorphous phase separately. All values in per cent.

Table 5.
Results of X-ray diffraction analyses (XRD) of cement and industrial by-products, showing minerals in crystalline phase and amorphous separately. All values in per cent.
Bassanite () was identified in the CEM I sample at a concentration of 7.2% (Table 5). Bassanite was unexpected, but the chemical composition favoured the phase with sulphur and calcium at reasonable levels (Table 3). It was expected to find (), a common constituent in cement, but no compelling evidence was present in the diffractograms. The high amorphous content (Table 5) made it difficult to argue for an aluminium-containing phase () instead of a calcium–sulphate phase.
Results from the TGA for the natural soils are plotted in Figure 2, together with their respective derivative differential thermogravimetry (DTG) values. The clays displayed DTG peaks at approximately 480 °C for the Onsøy clay and 590 °C and 670 °C for the Tiller-Flotten clay, corresponding to the dehydroxylation of clay minerals such as illite and chlorite. The Tiller-Flotten clay also contained 2.1% calcite (Table 4), which was most likely reflected by the peak at approximately 590 °C. The TGA on peat naturally exhibited a high weight loss due to the high organic content, as could also be observed concerning the high LOI and amorphous contents in the XRF and XRD analyses, respectively. Overall, the results agree with both XRF and XRD as well as previous studies [40,41].
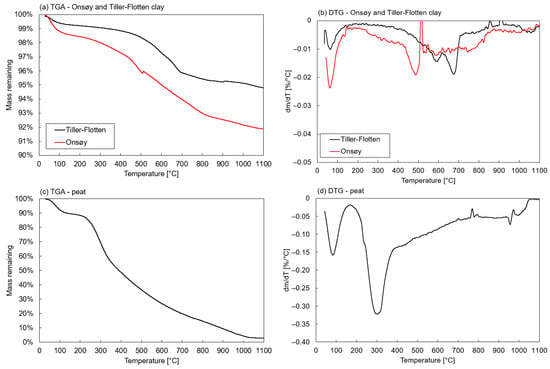
Figure 2.
Results from thermogravimetric analyses on natural soils: (a,b) Onsøy and Tiller-Flotten clay, (c,d) peat.
For the Tiller-Flotten peat, a DTG peak was observed between temperatures of 220–350 °C, caused by the dehydration of the organic content in the peat. A high weight loss in this temperature range indicated that the peat had a low degree of humification, e.g., a large number of intact fibres and leaves [73], which was coherent with the von Post classification of H2-H3. The TGA further showed a continuous weight loss at higher temperatures, which is typical for peat due to its rather complex composition [80].
TGA and DTG results for the CEM, IBPs and biochars are shown in Figure 3. The FA and LS2, in particular, had a high weight loss in the temperature range of approximately 40–120 °C as the free and adsorbed water evaporated, in line with the high water content determinations. The BA and LS2 showed additional weight loss at temperatures of 380–480 °C, i.e., dehydration of . These also had the highest content of portlandite, as shown by the XRD analyses. At temperatures of 700–800 °C, the FA and PSA exhibited weight loss due to the decomposition of . Again, this was observed in the XRD analyses with high calcite contents. Two outlying results were identified: a weight increase for the PSA at temperatures of 380–630 °C and for LS1 at temperatures of 900–1050 °C. A possible reason for this might be baseline drift during the TGA tests; however, despite this, it should be of no particular relevance to the stabilisation effect of the binders. In general, the TGA and DTG analyses of the IBPs were similar to those found in the literature [54,61,65,69,81].
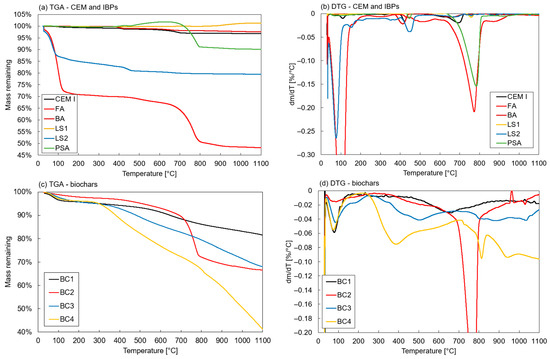
Figure 3.
Results from thermogravimetric analyses of binders: (a,b) CEM and IBPs, (c,d) biochars.
The TGA and DTG results for the biochar showed similar characteristics as those for the peat, i.e., a gradual weight loss over a large temperature range. This was expected since they had relatively similar compositions, as observed in the XRF analyses. One exception was the BC2, which exhibited a DTG peak at temperatures of 700–800 °C, i.e., decomposition of the due to the high calcite content, as was observed in the XRD analysis.
3.2. Stabilisation Effect
Figure 4 shows results from the UC tests, i.e., strength and stiffness for the stabilised specimens cured at 28 days. The stabilised Onsøy clay (see Figure 4a) obtained values ranging between 50 and 100 kPa, except for CEM as a single binder with of 60 kg/m3, i.e., CEM-60, which reached almost 200 kPa. Adding 100 kg/m3 of biochar had varying stabilisation effects. Biochars BC2 and BC3 had negative stabilisation effects on the strength development, whilst BC1 and BC4 had positive stabilisation effects. The same differences were also observed for (see Figure 4b). The stabilisation effect of adding the calcium-based IBPs also varied. However, all IBPs had positive stabilisation effects with increased strength. The PSA and BA in particular seemed to give a relatively high strength and stiffness contribution.
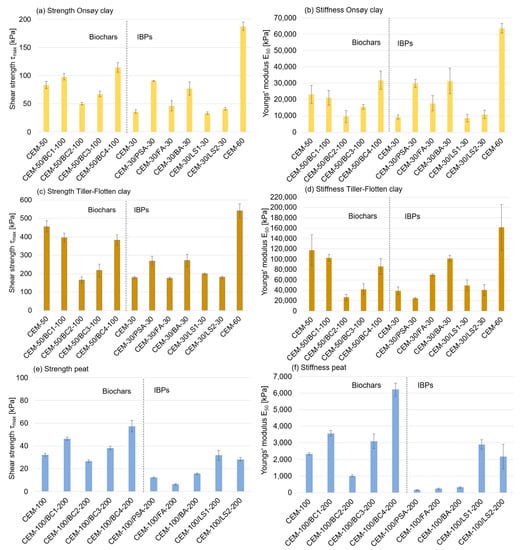
Figure 4.
Results of shear strength () and stiffness Youngs’ modulus ( ) from UC tests for the (a,b) Onsøy clay, (c,d) Tiller-Flotten clay and (e,f) peat. Error bars show the standard deviation.
The Tiller-Flotten clay (see Figure 4c,d) showed a considerably greater strength development, where ranged from just below 200 kPa up to approximately 550 kPa. However, for the Tiller-Flotten clay, all biochars had a negative stabilisation effect, although to a lesser degree for BC1 and BC4. For the IBPs, the same pattern as that for the Onsøy clay was observed, i.e., the PSA and BA had the highest stabilisation effect.
The results for the stabilised peat are shown in Figure 4e,f. All biochars, especially BC1 and BC4, had a positive stabilisation effect with an increased compared to CEM as a single binder. The effect of adding IBPs, however, reduced the strength considerably, except for LS1 and LS2. The same pattern was obtained for stiffness .
The average relationships between stiffness and strength , often referred to as the rigidity index, are plotted in Figure 5. For the clays, the ratio was generally between 200 and 400, equivalent to between ~100 and ~200, which is in line with other studies [29,82,83]. The CEM and PSA stabilised specimens deviated from this (CEM-30/PSA-30), where the ratio was ~90, i.e., considerably lower than that for the other IBPs. It was further observed that specimens stabilised with biochar in general had a lower ratio compared to CEM as a single binder and CEM combined with IBPs. This implies that stabilisation with biochar improves stiffness to a lesser degree than strength. For the peat specimens, the ratio varied between ~40 and ~100, independent of IBP or biochar, in line with previous studies on biochar in peat [27]; however, there was also an exceptionally low ratio for the CEM-PSA-specimen, where was ~13.
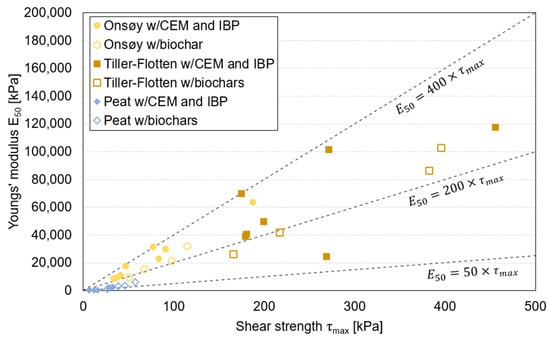
Figure 5.
Relationship between shear strength () and stiffness Youngs’ modulus ( ). Average values for the three replicate specimens for each mixture. Points denoted ‘w/CEM and IBP’ show mixtures with both CEM as a single binder and CEM and IBP in combination.
The results from the strength activity index (SAI) are shown in Figure 6. All biochars and IBPs reduced the strength but to various degrees, ranging from ~70% reduced strength for BC4, BA and FA to ~90% reduced strength for BC3. On average, the strength reduction was 81% for the biochars compared to 73% for the IBPs. This difference was very much affected by the low reduction for BC3 (93%). It should, however, be noted that the variation for each mixture was relatively large, as shown by the error bars.
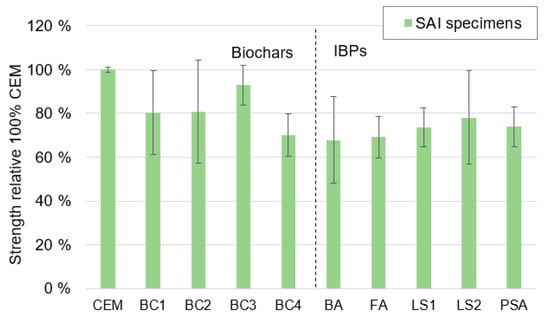
Figure 6.
Results of the strength activity index (SAI) for 100% CEM and mixtures where 20% of the CEM was replaced with biochar or IBPs. Error bars show coefficients of variation.
4. Discussion
4.1. Stabilisation Effect for Different Binders in Clays
To further analyse the comparative stabilisation effects of biochars and IBPs, the relative strengths for both the Onsøy and the Tiller-Flotten clays when the biochars and IBPs were added to CEM are plotted in Figure 7. It was observed that most of the biochars had a negative effect on strength development (see Figure 7a), apart from BC1 and BC4 in the Onsøy clay, where the strength increase was ~20–40%. Notably, BC1 and BC4 also had the least negative effect in the Tiller-Flotten clay, where the strength only decreased ~15%. The largest negative effect was observed for BC2, where the strength in the Tiller-Flotten clay decreased by a considerable ~65%.
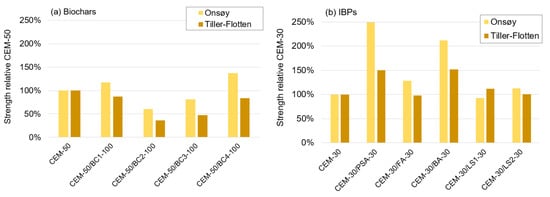
Figure 7.
Average strength of mixtures of biochars and IBPs relative to mixtures with 100% CEM as a binder for the Onsøy and Tiller-Flotten clays, (a) biochars, and (b) IBPs.
These results indicate that the use of biochar in the stabilisation of clays results in relatively few benefits, which can be expected since it does not contain any calcium-based cementitious properties. However, previous tests have revealed positive stabilisation effects on the Tiller-Flotten clay [29], although with a different type of biochar. Notably, the stabilisation effect of biochar in soils has been observed to be highly dependent on the type of biochar and its properties such as SFA, water content and grain size distribution, and the resulting stabilisation effect thus varies greatly [37,84].
Research on mortars, concrete and asphalt has also shown that biochar can be beneficial as a substitution or additive, although negative effects also have been reported [85,86,87,88,89]. In those cases where the biochar has shown positive effects, this has mostly been attributed to the water retention properties of biochar. This reduces water evaporation during curing, reduces microcracking and provides a continuous source of water for the cement hydration occurring over time [85,86]. In addition, the micropores of the biochar can provide space for the cementitious compounds resulting from pozzolanic reactions [90,91]. Whether a positive or negative effect is obtained depends on the biochar content in relation to the cement content, the water saturation of biochar prior to mixing and the grain size distribution. Generally, finer-grained and pre-soaked biochar is preferred in cement mortar mixtures [38]. Typically, positive effects have been reported for biochar contents up to ~2–3%, beyond which, a negative effect is often observed, especially if the content is over ~10% [88,92].
How these findings translate to soil stabilisation has thus far not been properly investigated. As clays typically have a large amount of water readily available for cement hydration, the potential benefits from water retention properties might be lower compared to those from mortars and cement. On the other hand, biochar could enable densification in clays with high porosity and, thus, both the initial porosity and amount of biochar per unit volume of clay would affect the stabilisation effect. Previous tests on soils have shown that an optimal amount of biochar can be found [29,38], which should be studied further with the soils used herein.
Another important parameter that highly affects the stabilisation effect is the binder uniformity, i.e., the distribution of binder in the soil that is obtained after mixing. The higher binder uniformity of calcium-based binders such as CEM and QL was observed to be beneficial [93]. How these results apply to biochar is not known and, although it could be assumed to have the same effect, this effect must be studied further.
Based on simple linear regression analyses, there appeared to be no correlations between the stabilisation effect and the mineralogical or chemical oxide compositions of the biochars as shown by the XRD and XRF analyses. For example, BC2 contained a considerable amount of in a calcite mineral structure, originating from the addition of limestone to the sludge prior to pyrolysis. However, this had no stabilisation effect since the was not available for pozzolanic reactions with the CEM. This was also observed previously when QL and calcite () were used for the stabilisation of Tiller-Flotten clay [6].
The stabilisation effects of the calcium-based IBPs are shown in Figure 7b. Most of the IBPs had a positive stabilisation effect. However, there are a few exceptions where no effect was observed, specifically for FA, LS1 and LS2. Ladle slags in general are known for their relatively low effectiveness and often need to be alkali-activated [65,94], which appears not to have been achieved with the added quantity of CEM. The BA and PSA performed well, with an increased strength of 50% to 150%. This indicates that the in these binders was available for chemical reactions with pozzolans in the binder or soil. In fact, in free-form in the BA and PSA at 16% and 4.8%, respectively, was also noted by the XRD analyses (Table 5). However, these contents were not directly proportional to the relative strength increase.
Typically, for calcium-based products such as the ashes and ladle slags used herein, the -content is correlated to the amount of cementitious products obtained in the soil–binder mixture. This, however, depends on the amount of available for chemical reactions with the soil particles, also referred to as the active or free content. This should be considered in further work.
4.2. The Stabilisation Effect for Different Binders in Peat
Figure 8 shows the stabilisation effect of the biochars and IBPs in the peat. Here, biochar was observed to be far more effective compared to the IBPs, with a strength increase of up to 80% for BC4. These results are similar to previous findings on peat stabilisation [27,31,95]. The stabilisation effect can possibly be attributed to the water retention (high absorption capacity) of the biochar and densification [32,96]. This could be a larger effect in peat with a very high water content and high porosity compared to clays with considerably lower porosity, as indicated by the water content determinations (Table 2).
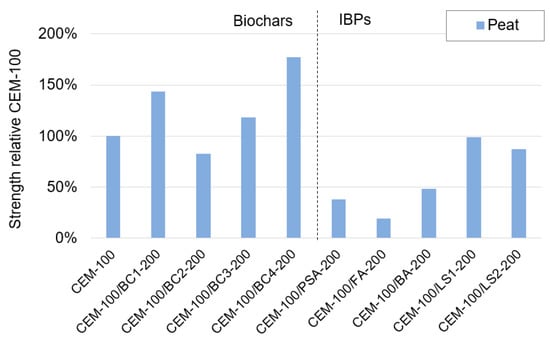
Figure 8.
Strength of mixtures of biochars and IBPS relative to mixtures with 100% CEM as a binder for the peat.
In contrast to their positive stabilisation effect on clay, most of the IBPs had a negative effect on the strength of the peat. Adding PSA, FA and BA to CEM yielded strengths that were only ~20–50% of that for CEM as a single binder. The reason for this is not known. LS1 and LS2 had a lesser negative effect, with equivalent values of ~88–98%. Here, a possible explanation is that the ladle slags were activated by the higher amount of CEM than that in the clays and hence gave a cementitious contribution by pozzolanic reactions, similar to the effects of GGBS [13,97].
4.3. The Strength Difference between the Onsøy and Tiller-Flotten Clays
The absolute strength difference between the Onsøy and Tiller-Flotten clays was noticeable. The strength values for the Tiller-Flotten clay were ~3–6 times higher than those for the Onsøy clay and ~3.7 times higher on average. For CEM as a single binder, the strength was ~2.9 times higher. It was noted that the deviations were similar between the two clays, which might reflect the fact that the specimen quality was similar for both clays. The strength difference is, therefore, believed not to be caused by, for example, differences in specimen quality. Further, based on previous research on similar types of clays, these differences seem too large to be explained by differences in, for example, the organic content alone, which was 3–4% for the Onsøy clay and <1% for the Tiller-Flotten clay [13,83]. Previous research has also shown that a lower liquidity index could imply a higher strength [98], but the opposite effect was observed herein as the liquidity indices were approximately 0.9–1.0 and 1.5–2.0 for the Onsøy and Tiller-Flotten clays, respectively.
Moreover, the difference cannot be explained by the differences in the water content relative to the binder quantity. This is typically analysed by using the water–binder ratio, i.e., the weight ratio between the water in the soil to the total added during stabilisation [6,99,100]. With a of ~42% and ~73% for the Tiller-Flotten and Onsøy clays, respectively, this corresponds to a content of water of approximately 530 l/m3 and 660 l/m3, respectively, which results in water–binder ratios of ~9 and ~11, respectively. Previous research on water–binder ratios vs. strength testing on Norwegian soils [6,82] indicates that the strength difference should only be approximately 20%, i.e., this does not explain the large strength differences between the two clays studied herein.
The strength differences between the two clays were also difficult to assess based on the compositional analyses. In addition, since previous studies have shown large strength differences between stabilised clays from different geographical regions without a satisfactory explanation [6,82,83,101], there is a need for further studies on this topic.
5. Conclusions
Four biochars (BCs) and five industrial by-products (IBPs) were characterised and tested for stabilisation effects on three natural Norwegian soils: the Onsøy and Tiller-Flotten clays and peat. The following conclusions were made:
- Two of the biochars (BC1 and BC4) had beneficial stabilisation effects (i.e., strength and stiffness) on the Onsøy clay with a relatively high water content (~73%). All four biochars had negative stabilisation effects on the Tiller-Flotten clay with a relatively low water content (~42%); however, BC1 and BC4 had the least negative effect.
- Almost all of the IBPs had positive stabilisation effects on the two clays. The greatest effect was observed with the paper sludge ash (PSA) and bottom ash (BA). The ladle slags (LS1 and LS2) had a negligible effect on strength and stiffness development.
- Three biochars (BC1, BC3 and BC4) had a positive stabilisation effect on the peat. Three IBPs had negative stabilisation effects, whilst the ladle slags LS1 and LS2 again had a negligible effect.
- The stiffness-to-strength ratios () of most of the mixtures ranged between 200 and 400, except for all peat samples, which had values of approximately 50. The low values also applied to some mixtures with biochar and all PSA-stabilised specimens.
The varying results in strength for the biochars are believed to depend on the biochar characteristics such as grain size and water absorption capacity in relation to the porosity and water content of the soil. The results indicate that the stabilisation effect of the biochars increases with increasing water content of the soil; however, this has not been investigated in detail. Further research on biochar in soft soil stabilisation is needed as the climate mitigation potential is considerable.
No apparent correlation between stabilisation effect and the chemical composition of the biochars or IBPs was observed. This highlights the difficulty in predicting strength development based on chemical and mineralogical compositions alone and stresses the importance of performing laboratory testing.
The need for further studies is noted. In particular, further research should investigate the observed large strength and stiffness differences when stabilising different clays. In addition, further studies are needed to explain the effects of, for example, the grain size distribution of IBPs and biochars and the effects of the binder uniformity of biochar on the stabilisation effect.
Author Contributions
Conceptualization, S.R. and P.P.; methodology, S.R., C.S. and P.P.; software, S.H.; validation, S.H., P.P. and M.L.; formal analysis, S.H. and C.S.; investigation, S.H.; resources, S.R., P.P., G.C. and M.L.; data curation, S.H.; writing—original draft preparation, S.H.; writing—review and editing, S.H., P.P., S.R., G.C., C.S. and M.L.; visualization, S.H.; supervision, P.P.; project administration, S.R.; funding acquisition, S.R. and G.C. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Research Council of Norway, grant number 328767 ‘GOAL—Green Soil Stabilisation’.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
All data are available from the corresponding author upon reasonable request.
Acknowledgments
The authors are grateful to Ingrid Peikli and Geir Wold Åsli at the NGI geotechnical laboratory in Oslo for performing many of the laboratory tests presented herein. The authors are also grateful to the staff at Nemko Norlab, SINTEF and X-ray Mineral Services for performing several of the characterisation investigations. The authors are especially grateful to Jonathan Wilkins at X-ray Mineral Services for contributing to the interpretation of the XRF and XRD analyses. The contributions from the industrial partners Norske Skog Skogn, Bergene Holm, Lindum and Celsa Steel Services are appreciated. The authors appreciate the valuable comments from the anonymous reviewers, which improved the quality of the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andrew, R.M. Global CO2 emissions from cement production, 1928–2018. In Earth System Science Data; Copernicus GmbH: Göttingen, Germany, 2019; Volume 11, pp. 1675–1710. [Google Scholar] [CrossRef]
- Gao, T.; Shen, L.; Shen, M.; Chen, F.; Liu, L.; Gao, L. Analysis on differences of carbon dioxide emission from cement production and their major determinants. J. Clean. Prod. 2015, 103, 160–170. [Google Scholar] [CrossRef]
- Miller, G.A.; Azad, S. Influence of soil type on stabilization with cement kiln dust. Constr. Build. Mater. 2000, 14, 89–97. [Google Scholar] [CrossRef]
- Yoobanpot, N.; Jamsawang, P.; Horpibulsuk, S. Strength behavior and microstructural characteristics of soft clay stabilized with cement kiln dust and fly ash residue. Appl. Clay Sci. 2017, 141, 146–156. [Google Scholar] [CrossRef]
- Jegandan, S.; Liska, M.; Osman, A.A.M.; Al-Tabbaa, A. Sustainable binders for soil stabilisation. Proc. Inst. Civ. Eng. Ground Improv. 2010, 163, 53–61. [Google Scholar] [CrossRef]
- Hov, S.; Paniagua, P.; Sætre, C.; Rueslåtten, H.; Størdal, I.; Mengede, M.; Mevik, C. Lime-cement stabilisation of Trondheim clays and its impact on carbon dioxide emissions. Soils Found. 2022, 1, 62. [Google Scholar] [CrossRef]
- Ramírez, A.L.; Korkiala-Tanttu, L. Stabilisation of Malmi soft clay with traditional and low-CO2 binders. Transp. Geotech. 2023, 38, 100920. [Google Scholar] [CrossRef]
- Mypati, V.N.K.; Saride, S. Feasibility of Alkali-Activated Low-Calcium Fly Ash as a Binder for Deep Soil Mixing. J. Mater. Civil. Eng. 2022, 34, 04021410. [Google Scholar] [CrossRef]
- Sharma, A.K.; Sivapullaiah, P.V. Strength development in fly ash and slag mixtures with lime. Proc. Inst. Civ. Eng. Ground Improv. 2016, 169, 194–205. [Google Scholar] [CrossRef]
- Sukmak, P.; Horpibulsuk, S.; Shen, S.L. Strength development in clay-fly ash geopolymer. Constr. Build. Mater. 2013, 40, 566–574. [Google Scholar] [CrossRef]
- Wu, J.; Deng, Y.; Zhang, G.; Zhou, A.; Tan, Y.; Xiao, H. A Generic Framework of Unifying Industrial By-products for Soil Stabilization. J. Clean. Prod. 2021, 25, 321. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Soft Clay Stabilization Using Three Industry Byproducts. J. Mater. Civil. Eng. 2021, 33, 06021002. [Google Scholar] [CrossRef]
- Åhnberg, H.; Johansson, S.-E.; Pihl, H.; Carlsson, T. Stabilising effects of different binders in some Swedish soils. Proc. Inst. Civ. Eng. Ground Improv. 2003, 7, 9–23. [Google Scholar] [CrossRef]
- James, R.; Kamruzzaman, A.H.M.; Haque, A.; Wilkinson, A. Behaviour of lime—Slag-treated clay. Proc. Inst. Civ. Eng. Ground Improv. 2008, 161, 207–216. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Z.; Song, Z.; Li, J. Engineering properties of marine soft clay stabilized by alkali residue and steel slag: An experimental study and ANN model. Acta Geotech. 2022, 17, 5089–5112. [Google Scholar] [CrossRef]
- Wilkinson, A.; Haque, A.; Kodikara, J. Stabilisation of clayey soils with industrial by-products: Part, A. Proc. Inst. Civ. Eng. Ground Improv. 2010, 163, 149–163. [Google Scholar] [CrossRef]
- Wilkinson, A.; Haque, A.; Kodikara, J. Stabilisation of clayey soils with industrial by-products: Part B. Proc. Inst. Civ. Eng. Ground Improv. 2010, 163, 165–172. [Google Scholar] [CrossRef]
- Yi, Y.; Gu, L.; Liu, S.; Puppala, A.J. Carbide slag-activated ground granulated blastfurnace slag for soft clay stabilization. Can. Geotech. J. 2015, 52, 656–663. [Google Scholar] [CrossRef]
- Armistead, S.J.; Smith, C.C.; Staniland, S.S. Sustainable biopolymer soil stabilisation: The effect of microscale chemical characteristics on macroscale mechanical properties. Acta Geotech. 2022, 18, 3213–3227. [Google Scholar] [CrossRef]
- Soldo, A.; Miletić, M.; Auad, M.L. Biopolymers as a sustainable solution for the enhancement of soil mechanical properties. Sci. Rep. 2020, 1, 10. [Google Scholar] [CrossRef]
- Voottipruex, P.; Teerawattanasuk, C.; Sramoon, W.; Meepon, I. Stabilization of Soft Clay Using Perlite Geopolymer Activated by Sodium Hydroxide. Int. J. Geosynth. Ground Eng. 2022, 8, 5. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Venda Oliveira, P.J.; Custódio, D.G. Effect of polypropylene fibres on the compressive and tensile strength of a soft soil, artificially stabilised with binders. Geotext. Geomembr. 2015, 43, 97–106. [Google Scholar] [CrossRef]
- Zhao, M.; Liu, G.; Zhang, C.; Guo, W.; Luo, Q. State-of-the-art of colloidal silica-based soil liquefaction mitigation: An emerging technique for ground improvement. Appl. Sci. 2020, 10, 15. [Google Scholar] [CrossRef]
- Ahmadi Chenarboni, H.; Hamid Lajevardi, S.; MolaAbasi, H.; Zeighami, E. The effect of zeolite and cement stabilization on the mechanical behavior of expansive soils. Constr. Build. Mater. 2021, 272, 121630. [Google Scholar] [CrossRef]
- Eyo, E.U.; Ngambi, S.; Abbey, S.J. Performance of clay stabilized by cementitious materials and inclusion of zeolite/alkaline metals-based additive. Transp. Geotech. 2020, 23, 100330. [Google Scholar] [CrossRef]
- Sørmo, E.; Silvani, L.; Thune, G.; Gerber, H.; Schmidt, H.P.; Smebye, A.B. Waste timber pyrolysis in a medium-scale unit: Emission budgets and biochar quality. Sci. Total Environ. 2020, 718, 137335. [Google Scholar] [CrossRef]
- Ritter, S.; Paniagua, P.; Hansen, C.B.; Cornelissen, G. Biochar amendment for improved and more sustainable peat stabilisation. Proc. Inst. Civil. Eng. Ground Improv. 2022; ahead of print. [Google Scholar] [CrossRef]
- Jeffery, S.; Bezemer, T.M.; Cornelissen, G.; Kuyper, T.W.; Lehmann, J.; Mommer, L. The way forward in biochar research: Targeting trade-offs between the potential wins. GCB Bioenergy 2015, 1, 1–13. [Google Scholar] [CrossRef]
- Ritter, S.; Paniagua, P.; Cornelissen, G. Biochar in Quick Clay Stabilization: Reducing Carbon Footprint and Improving Shear Strength. Proc. Geo-Congr. 2023, 15–24. [Google Scholar] [CrossRef]
- GuhaRay, A.; Guoxiong, M.; Sarkar, A.; Bordoloi, S.; Garg, A.; Pattanayak, S. Geotechnical and chemical characterization of expansive clayey soil amended by biochar derived from invasive weed species Prosopis juliflora. Innov. Infrastruct. Solut. 2019, 4, 44. [Google Scholar] [CrossRef]
- Lau, J. Static and Dynamic Performance of Biochar Enhanced Cement Stabilised Peat. Ph.D. Thesis, University of Cambridge, Cambridge, UK, 2018. [Google Scholar]
- Lau, J.; Biscontin, G.; Berti, D. Effects of biochar on cement-stabilised peat soil. Proc. Inst. Civ. Eng. Ground Improv. 2021, 176, 76–87. [Google Scholar] [CrossRef]
- Pardo, G.S.; Sarmah, A.K.; Orense, R.P. Mechanism of improvement of biochar on shear strength and liquefaction resistance of sand. Geotechnique 2019, 69, 471–480. [Google Scholar] [CrossRef]
- Pardo, G.S.; Orense, R.P.; Sarmah, A.K. Cyclic strength of sand mixed with biochar: Some preliminary results. Soils Found. 2018, 58, 241–247. [Google Scholar] [CrossRef]
- Vincevica-gaile, Z.; Teppand, T.; Kriipsalu, M.; Krievans, M.; Jani, Y.; Klavins, M. Towards sustainable soil stabilization in peatlands: Secondary raw materials as an alternative. Sustainability 2021, 13, 6726. [Google Scholar] [CrossRef]
- Lu, S.G.; Sun, F.F.; Zong, Y.T. Effect of rice husk biochar and coal fly ash on some physical properties of expansive clayey soil (Vertisol). Catena 2014, 114, 37–44. [Google Scholar] [CrossRef]
- Zong, Y.; Chen, D.; Lu, S. Impact of biochars on swell–shrinkage behavior, mechanical strength, and surface cracking of clayey soil. J. Plant Nutr. Soil. Sci. 2014, 177, 920–926. [Google Scholar] [CrossRef]
- William, J.; Latifi, N.; Vahedifard, F. Effects of Biochar Amendment on Mechanical Properties of Buckshot Clay. In Proceedings of the IFCEE 2018: Innovations in Ground Improvement for Soils, Pavements, and Subgrades, Orlando, FL, USA, 5–10 March 2018; pp. 125–134. [Google Scholar]
- Sadasivam, Y.; Reddy, K. Shear strength of waste-wood biochar and biochar-amended soil used for sustainable landfill cover systems. In Proceedings of the 15th Pan-American Conference on Soil. Mechanics and Geotechnical Engineering, Buenos Aires, Argentina, 15–18 November 2015; pp. 745–752. [Google Scholar]
- Gundersen, A.S.; Hansen, R.C.; Lunne, T.; L’Heureux, J.S.; Strandvik, S.O. Characterization and engineering properties of the NGTS Onsøy soft clay site. AIMS Geosci. 2019, 5, 665–703. [Google Scholar] [CrossRef]
- L’Heureux, J.S.; Lindgård, A.; Emdal, A. The Tiller-Flotten research site: Geotechnical characterization of a very sensitive clay deposit. AIMS Geosci. 2019, 5, 831–867. [Google Scholar] [CrossRef]
- Long, M.; Paniagua, P.; Grimstad, G.; Trafford, A.; Degago, S.; L’Heureux, J.S. Engineering properties of Norwegian peat for calculation of settlements. Eng. Geol. 2022, 308, 106799. [Google Scholar] [CrossRef]
- von Post, L.; Granlund, E. Peat Resources in Southern Sweden; Swedish Geological Survey: Uppsala, Sweden, 1926. (In Swedish) [Google Scholar]
- EN 197-1:2011; Cement Part 1: Composition, Specifications and Conformity for Common Cements. European Committe for Standardization: Brussels, Belgium, 2011.
- Silva, F.C.; Cruz, N.C.; Tarelho, L.A.C.; Rodrigues, S.M. Use of biomass ash-based materials as soil fertilisers: Critical review of the existing regulatory framework. J. Clean. Prod. 2019, 214, 112–124. [Google Scholar] [CrossRef]
- Paniagua, P.; Ritter, S.; Moseid, M.; Okkenhaug, G. Bioashes and steel slag as alternative binders in ground improvement of quick clays. In Proceedings of the Geo-Congress 2023, Los Angeles, CA, USA, 26–29 March 2023. [Google Scholar] [CrossRef]
- Jafarbiglookarami, A. Alternative Binders for Improvement of Soft Soils—A Geoenvironmental Approach. Master’s Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2021. [Google Scholar]
- Liu, Y.; Chang, C.W.; Namdar, A.; She, Y.; Lin, C.H.; Yuan, X. Stabilization of expansive soil using cementing material from rice husk ash and calcium carbide residue. Constr. Build. Mater. 2019, 221, 1–11. [Google Scholar] [CrossRef]
- Okagbue, C.O. Stabilization of Clay Using Woodash. J. Mater. Civ. Eng. 2007, 19, 14–18. [Google Scholar] [CrossRef]
- Nath, B.D.; Sarkar, G.; Siddiqua, S.; Rokunuzzaman, M.; Islam, M.R. Geotechnical Properties of Wood Ash-Based Composite Fine-Grained Soil. Adv. Civil. Eng. 2018, 2018, 9456019. [Google Scholar] [CrossRef]
- Ayobami, A.B. Performance of wood bottom ash in cement-based applications and comparison with other selected ashes: Overview. Resour. Conserv. Recycl. 2021, 166, 105351. [Google Scholar] [CrossRef]
- Azrizal, M.F.; Noorsuhada, M.N.; Latif, M.F.; Arshad, M.F.; Sulaiman, H. The properties of wastepaper sludge ash and its generic applications. J. Phys. Conf. Ser. 2019, 1349, 012087. [Google Scholar] [CrossRef]
- Zmamou, H.; Leblanc, N.; Levacher, D.; Kubiak, J. Recycling of high quantities of wastepaper sludge ash for production of blended cements and alternative materials. Environ. Technol. Innov. 2021, 23, 101524. [Google Scholar] [CrossRef]
- Bai, J.; Chaipanich, A.; Kinuthia, J.M.; O’Farrell, M.; Sabir, B.B.; Wild, S. Compressive strength and hydration of wastepaper sludge ash–ground granulated blastfurnace slag blended pastes. Cem. Concr. Res. 2003, 33, 1189–1202. [Google Scholar] [CrossRef]
- Baloochi, H.; Aponte, D.; Barra, M. Soil Stabilization Using Waste Paper Fly Ash: Precautions for Its Correct Use. Appl. Sci. 2020, 10, 8750. [Google Scholar] [CrossRef]
- Mavrouhdou, M.; Ziniatis, A.; Gray, C.; Ebad, Z.; del Rosario, J.; Kanak, S. Alternative calcium-based chemical stabilisers for ground improvement: Paper Sludge Ash treatment of London Clay. In Proceedings of the 15th International Conference on Environmental Science and Technology, Rhodes, Greece, 31 August–2 September 2017. [Google Scholar]
- Mavroulidou, M. Use of waste paper sludge ash as a calcium-based stabiliser for clay soils. Waste Manag. Res. J. A Sustain. Circ. Econ. 2018, 36, 1066–1072. [Google Scholar] [CrossRef] [PubMed]
- Bujulu, P.M.S.; Sorta, A.R.; Priol, A.; Emdal, A. Potential of wastepaper sludge ash to replace cement in deep stabilization of quick clay. In Proceedings of the “Characterization and Improvement of Soils and Materials” Session of the 2007 Annual Conference of the Transportation Association of Canada, Saskatoon, SK, Canada, 14–17 October 2007; pp. 1–16. [Google Scholar]
- Bujulu, P.M.S. Deep-Mix Stabilization of Quick Clay: A Potential Area for Utilization of Wastepaper Sludge Ash. Ph.D. Thesis, Norwegian University of Science and Technology, Trondheim, Norway, 2008. [Google Scholar]
- Rahmat, M.N.; Kinuthia, J.M. Effects of mellowing sulfate-bearing clay soil stabilized with wastepaper sludge ash for road construction. Eng. Geol. 2011, 117, 170–179. [Google Scholar] [CrossRef]
- Rahmat, N. Soil Stabilization Utilising Wastepaper Sludge Ash. Ph.D. Thesis, University of Glamorgan, Pontypridd, UK, 2004. [Google Scholar]
- Pinheiro, C.; Rios, S.; Viana da Fonseca, A.; Fernández-Jiménez, A.; Cristelo, N. Application of the response surface method to optimize alkali activated cements based on low-reactivity ladle furnace slag. Constr. Build. Mater. 2020, 264, 120271. [Google Scholar] [CrossRef]
- Yi, Y.; Li, C.; Liu, S. Alkali-Activated Ground-Granulated Blast Furnace Slag for Stabilization of Marine Soft Clay. J. Mater. Civil. Eng. 2015, 27, 04014146. [Google Scholar] [CrossRef]
- Yi, Y.; Liska, M.; Jin, F.; Al-Tabbaa, A. Mechanism of reactive magnesia—Ground granulated blastfurnace slag (GGBS) soil stabilization. Can. Geotech. J. 2016, 53, 773–782. [Google Scholar] [CrossRef]
- Najm, O.; El-Hassan, H.; El-Dieb, A. Ladle slag characteristics and use in mortar and concrete: A comprehensive review. J. Clean. Prod. 2021, 288, 125584. [Google Scholar] [CrossRef]
- Manso, J.M.; Losañez, M.; Polanco, J.A.; Gonzalez, J.J. Ladle Furnace Slag in Construction. J. Mater. Civ. Eng. 2005, 17, 5. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Stabilisation/solidification of lead-contaminated soil by using ladle furnace slag and carbon dioxide. Soils Found. 2022, 62, 101205. [Google Scholar] [CrossRef]
- Xu, B.; Yi, Y. Soft clay stabilization using ladle slag-ground granulated blastfurnace slag blend. Appl. Clay Sci. 2019, 178, 105136. [Google Scholar] [CrossRef]
- Brand, A.S.; Singhvi, P.; Fanijo, E.O.; Tutumluer, E. Stabilization of a clayey soil with ladle metallurgy furnace slag fines. Materials 2020, 13, 4251. [Google Scholar] [CrossRef]
- Manso, J.M.; Ortega-López, V.; Polanco, J.A.; Setién, J. The use of ladle furnace slag in soil stabilization. Constr. Build. Mater. 2013, 40, 126–134. [Google Scholar] [CrossRef]
- Espinosa, A.; Revilla-Cuesta, V.; Lopez-Ausin, V.; Serrano-Lopez, V.; Fiol, F. Study of clayey soils stabilized with ladle furnace slag as alternative binder for use in road works. Key Eng. Mater. 2022, 929, 187–192. [Google Scholar] [CrossRef]
- Brunauer, S.; Emmet, P.H.; Teller, E. Adsorption of Gases in Multimolecular Layers. J. Am. Chem. Soc. 1938, 60, 309–319. [Google Scholar] [CrossRef]
- Plante, A.F.; Fernández, J.M.; Leifeld, J. Application of thermal analysis techniques in soil science. Geoderma 2009, 153, 1–10. [Google Scholar] [CrossRef]
- Mitchell, J.; Soga, K. Fundamentals of Soil Behavior, 3rd ed.; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2005. [Google Scholar]
- Kitazume, M.; Grisolia, M.; Leder, E.; Marzano, I.P.; Correia, A.A.S.; Venda Oliveira, P.J.; Åhnberg, H.; Andersson, M. Applicability of molding procedures in laboratory mix tests for quality control and assurance of the deep mixing method. Soils Found. 2015, 55, 761–777. [Google Scholar] [CrossRef]
- Hov, S.; Falle, F.; Paniagua, P. Optimization of Laboratory Molding Techniques for Nordic Dry Deep Mixing. ASTM Geotech. Test. J. 2022, 1, 45. [Google Scholar] [CrossRef]
- Bache, B.K.; Wiersholm, P.; Paniagua, P.; Emdal, A. Effect of Temperature on the Strength of Lime–Cement Stabilized Norwegian Clays. J. Geotech. Geoenvironmental. Eng. 2022, 148, 04021198. [Google Scholar] [CrossRef]
- ASTM C311-07; Standard Test Methods for Sampling and Testing Fly Ash or Natural Pozzolans for Use in Portland-Cement Concrete. ASTM International: West Conshohocken, PA, USA, 2022.
- Setién, J.; Hernández, D.; González, J.J. Characterization of ladle furnace basic slag for use as a construction material. Constr. Build. Mater. 2009, 23, 1788–1794. [Google Scholar] [CrossRef]
- Zhang, W.; Henschel, T.; Söderlind, U.; Tran, K.Q.; Han, X. Thermogravimetric and Online Gas Analysis on various Biomass Fuels. In Energy Procedia; Elsevier: Amsterdam, The Netherlands, 2017; pp. 162–167. [Google Scholar] [CrossRef]
- Ashraf, M.S.; Ghouleh, Z.; Shao, Y. Production of eco-cement exclusively from municipal solid waste incineration residues. Resour. Conserv. Recycl. 2019, 149, 332–342. [Google Scholar] [CrossRef]
- Paniagua, P.; Bache, B.K.; Karlsrud, K.; Lund, A.K. Strength and stiffness of laboratory-mixed specimens of stabilised Norwegian clays. Proc. Inst. Civ. Eng. Ground Improv. 2022, 175, 150–163. [Google Scholar] [CrossRef]
- Hov, S.; Larsson, S. Strength and Stiffness Properties of Laboratory-Improved Soft Swedish Clays. Int. J. Geosynth. Ground Eng. 2023, 9, 11. [Google Scholar] [CrossRef]
- Sadasivam, B.Y.; Reddy, K.R. Engineering properties of waste wood-derived biochars and biochar-amended soils. Int. J. Geotech. Eng. 2015, 9, 521–535. [Google Scholar] [CrossRef]
- Zhang, Y.; He, M.; Wang, L.; Yan, J.; Ma, B.; Zhu, X. Biochar as construction materials for achieving carbon neutrality. Biochar 2022, 4, 59. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W. Effect of water entrainment by pre-soaked biochar particles on strength and permeability of cement mortar. Constr. Build. Mater. 2018, 159, 107–125. [Google Scholar] [CrossRef]
- Chen, L.; Zhang, Y.; Labianca, C.; Wang, L.; Ruan, S.; Poon, C.S. Carbon-negative cement-bonded biochar particleboards. Biochar 2022, 4, 58. [Google Scholar] [CrossRef]
- Danish, A.; Ali Mosaberpanah, M.; Usama Salim, M.; Ahmad, N.; Ahmad, F.; Ahmad, A. Reusing biochar as a filler or cement replacement material in cementitious composites: A review. Constr. Build. Mater. 2021, 300, 124295. [Google Scholar] [CrossRef]
- Dixit, A.; Gupta, S.; Pang, S.D.; Kua, H.W. Cement Replacement and Improved Hydration in Ultra-High Performance Concrete Using Biochar. In Proceedings of the 3rd International Conference on the Application of Superabsorbent Polymers (SAP) and Other New Admixtures Towards Smart Concrete, Skukuza, South Africa, 25–27 November 2019; pp. 222–229. [Google Scholar] [CrossRef]
- Yang, X.; Wang, X.Y. Hydration-strength-durability-workability of biochar-cement binary blends. J. Build. Eng. 2021, 42, 103064. [Google Scholar] [CrossRef]
- Wang, Y.S.; Wang, X.Y. Multi-characterizations of the hydration, microstructure, and mechanical properties of a biochar–limestone calcined clay cement (LC3) mixture. J. Mater. Res. Technol. 2023, 24, 3691–3703. [Google Scholar] [CrossRef]
- Gupta, S.; Kua, H.W.; Pang, S.D. Biochar-mortar composite: Manufacturing, evaluation of physical properties and economic viability. Constr. Build. Mater. 2018, 167, 874–889. [Google Scholar] [CrossRef]
- Larsson, S. State of Practice Report—Execution, monitoring and quality control. In Proceedings of the International Conference on Deep Mixing: Best Practice and Recent Advances, Stockholm, Sweden, 23–25 May 2005; pp. 732–785. [Google Scholar]
- Marinho, B.; Santos, M.; Franco De Carvalho, M.; Mendes, J.C.; Brigolini, J.; Peixoto, F. Ladle Furnace Slag as Binder for Cement-Based Composites. J. Mater. Civ. Eng. 2017, 29, 04017207. [Google Scholar] [CrossRef]
- Hernandez-Martinez, F.G.; Al-Tabbaa, A.; Medina-Cetina, Z.; Yousefpour, N. Stiffness and Strength of Stabilized Organic Soils—Part I/II: Experimental Database and Statistical Description for Machine Learning Modelling. Geosciences 2021, 11, 243. [Google Scholar] [CrossRef]
- Berti, D.; Biscontin, G.; Asce, A.M.; Lau, J. Effect of Biochar Filler on the Hydration Products and Microstructure in Portland Cement–Stabilized Peat. J. Mater. Civ. Eng. 2021, 33, 04021263. [Google Scholar] [CrossRef]
- Timoney, M.J.; Mccabe, B.A.; Bell, A.L. Experiences of dry soil mixing in highly organic soils. Proc. Inst. Civ. Eng. Ground Improv. 2012, 165, 3–14. [Google Scholar] [CrossRef]
- Correia, A.A.S.; Venda Oliveira, P.J.; Lemos, L.J.L. Strength assessment of chemically stabilised soft soils. Proc. Inst. Civ. Eng. Geotech. Eng. 2019, 172, 218–227. [Google Scholar] [CrossRef]
- Kitazume, M.; Terashi, M. The Deep Mixing Method; Taylor & Francis Group: London, UK, 2013. [Google Scholar]
- Horpibulsuk, S.; Rachan, R.; Suddeepong, A. State of the art in strength development of soil-cement columns. Proc. Inst. Civ. Eng. Ground Improv. 2012, 165, 201–215. [Google Scholar] [CrossRef]
- Åhnberg, H.; Pihl, H. Type of Lime and Its Effect on Stabilisation Effect; Swedish Deep Stabilization Research Centre: Linköping, Sweden, 1997. (In Swedish) [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).