The Incorporation of Waste Sludge into the Production of High-Temperature-Resistant Adhesive Ceramic Materials
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Samples
2.2. Thermal Cycling Test of the Prepared Ceramic Joint Specimens
2.3. The Characterization of Raw and Sintered Samples
2.3.1. The Chemical Composition of Waste Sludge and Raw Samples
2.3.2. The Phase Composition (XRD) of the Raw and Sintered Samples
2.3.3. Leaching Tests of the Raw and Sintered Samples
2.3.4. Dilatometry Analysis of Raw Samples
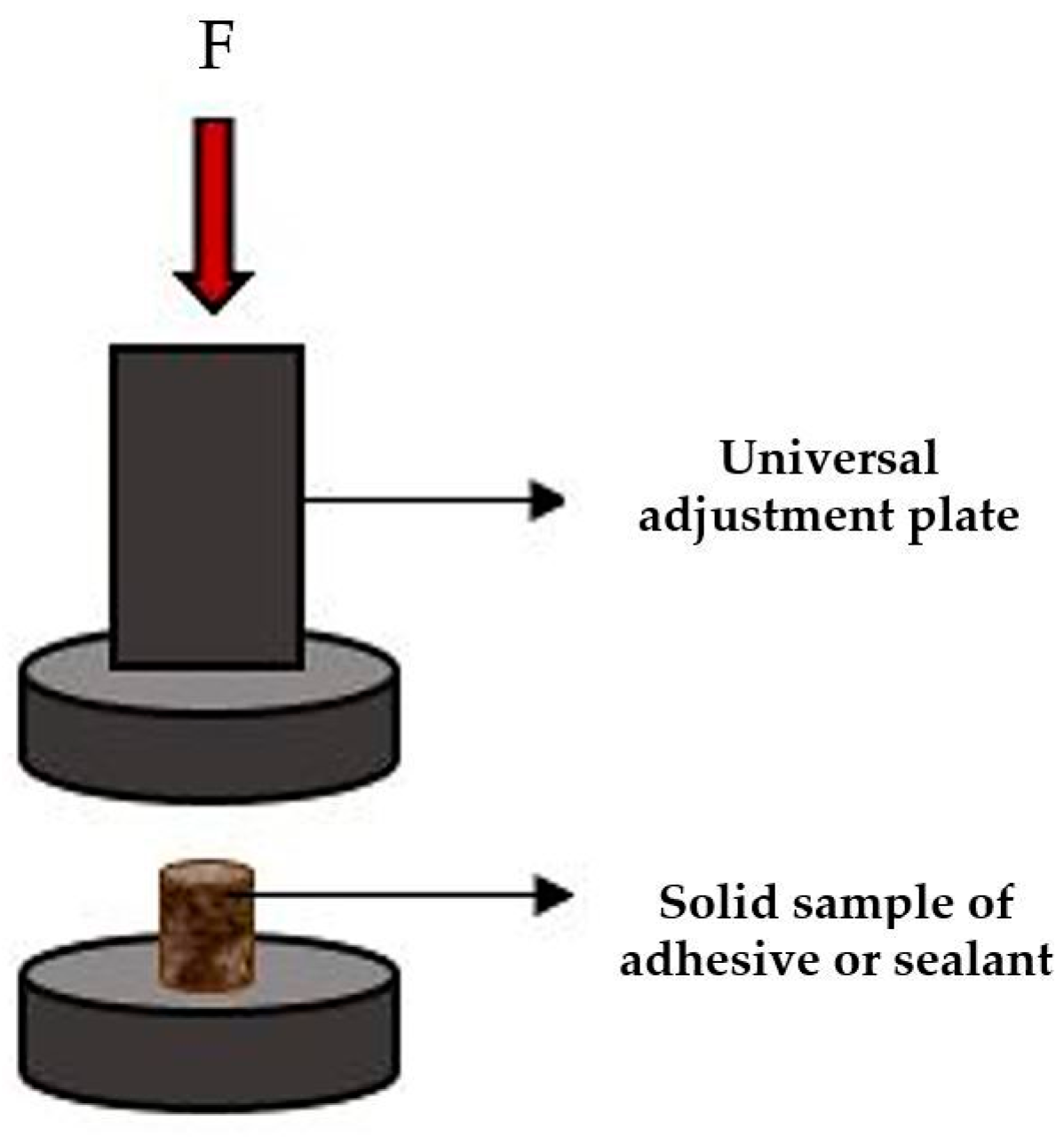
2.3.5. The Mechanical Properties of the Sintered Samples
2.4. The Performance of Materials for Potential Application as IT-SOFC Sealants
2.4.1. The Phase Composition (XRD) and Morphology (FESEM) of the Raw and Sintered Samples
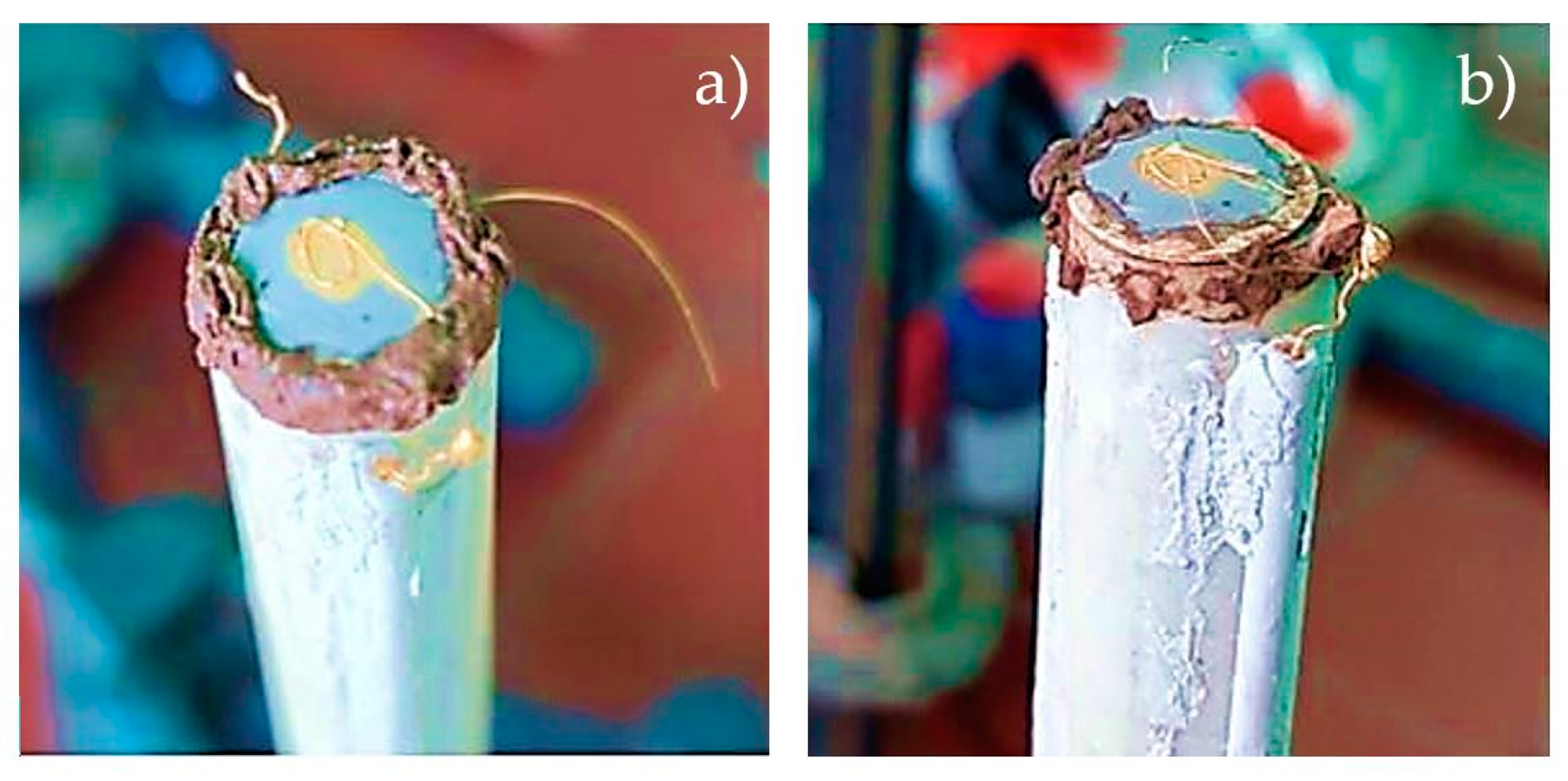
2.4.2. Open Circuit Voltage (OCV) Test—Leakage Detection of a Single Laboratory Fuel Cell
3. Results and Discussion
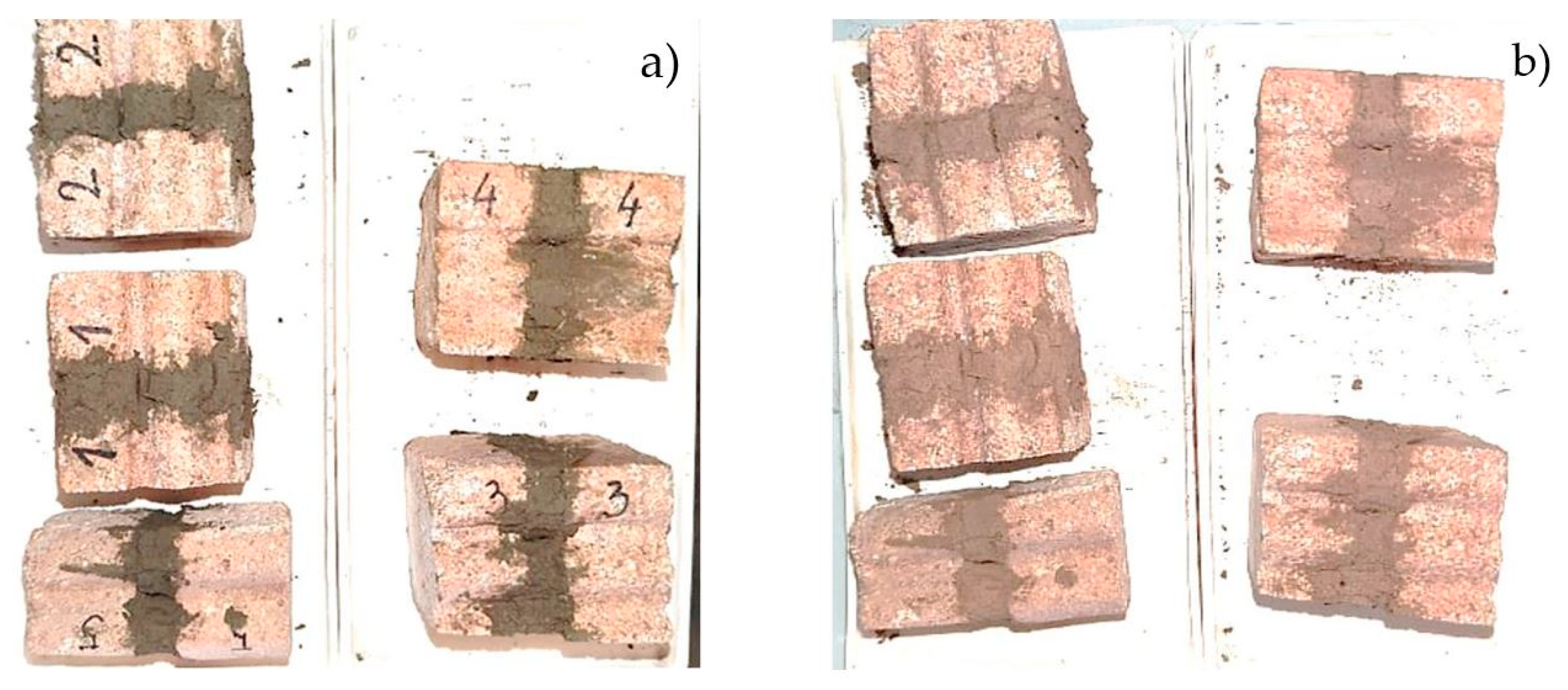
3.1. The Results of the Thermal Cycling Test of Ceramic Joint Specimens
3.2. Results of the Characterization of Raw and Sintered Samples
3.2.1. Results of the Chemical Composition of the Waste Sludge and Raw Samples
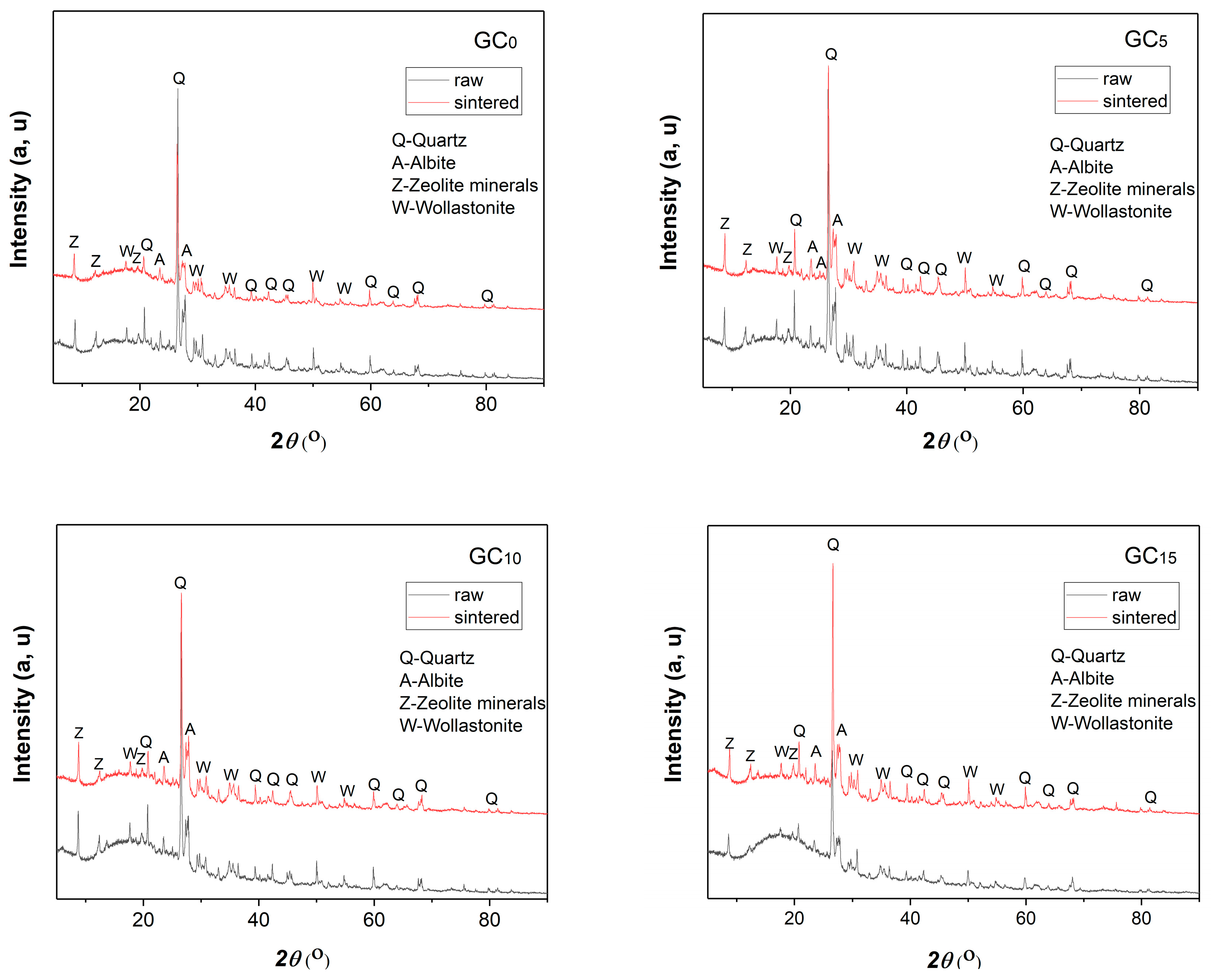
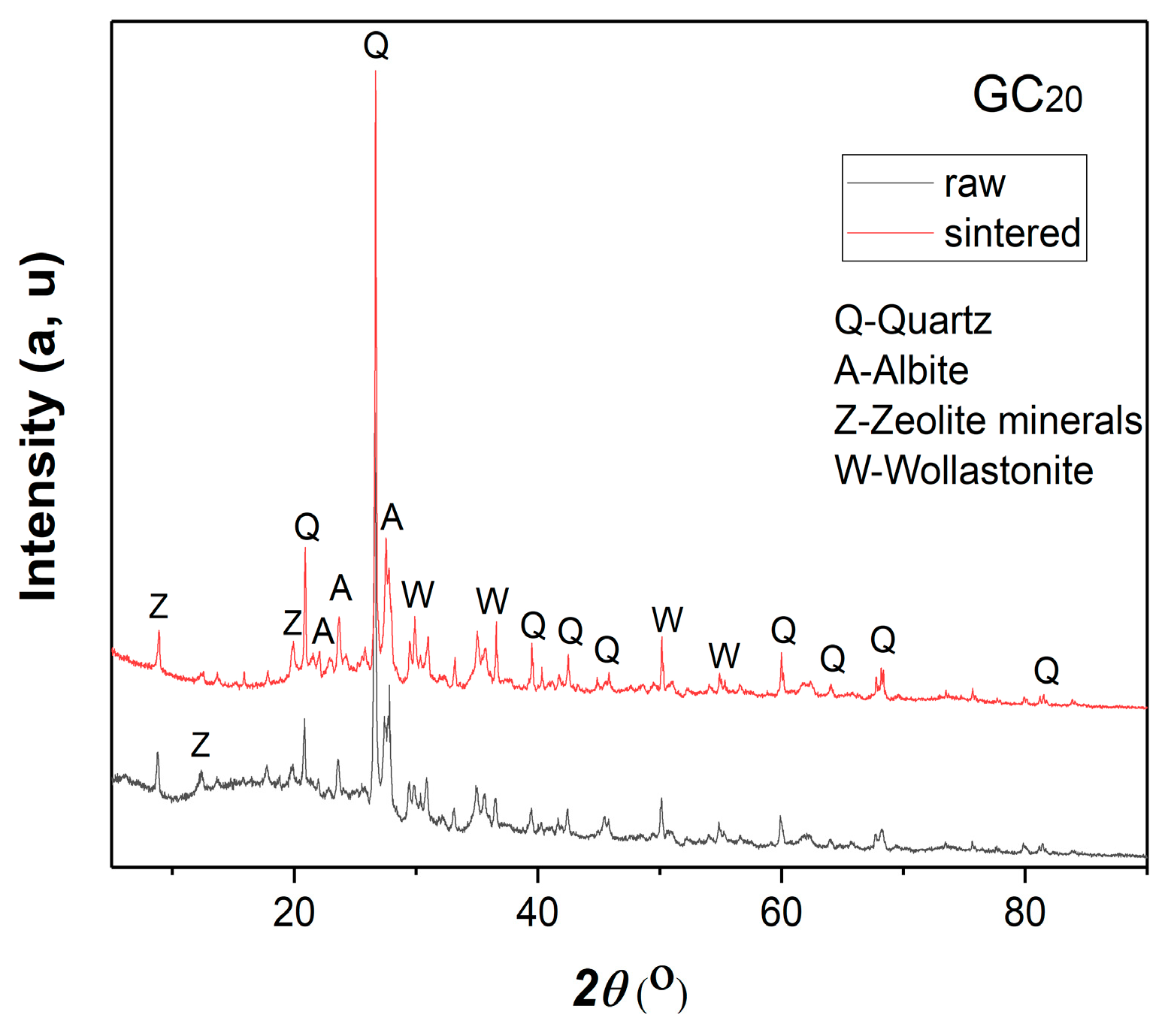
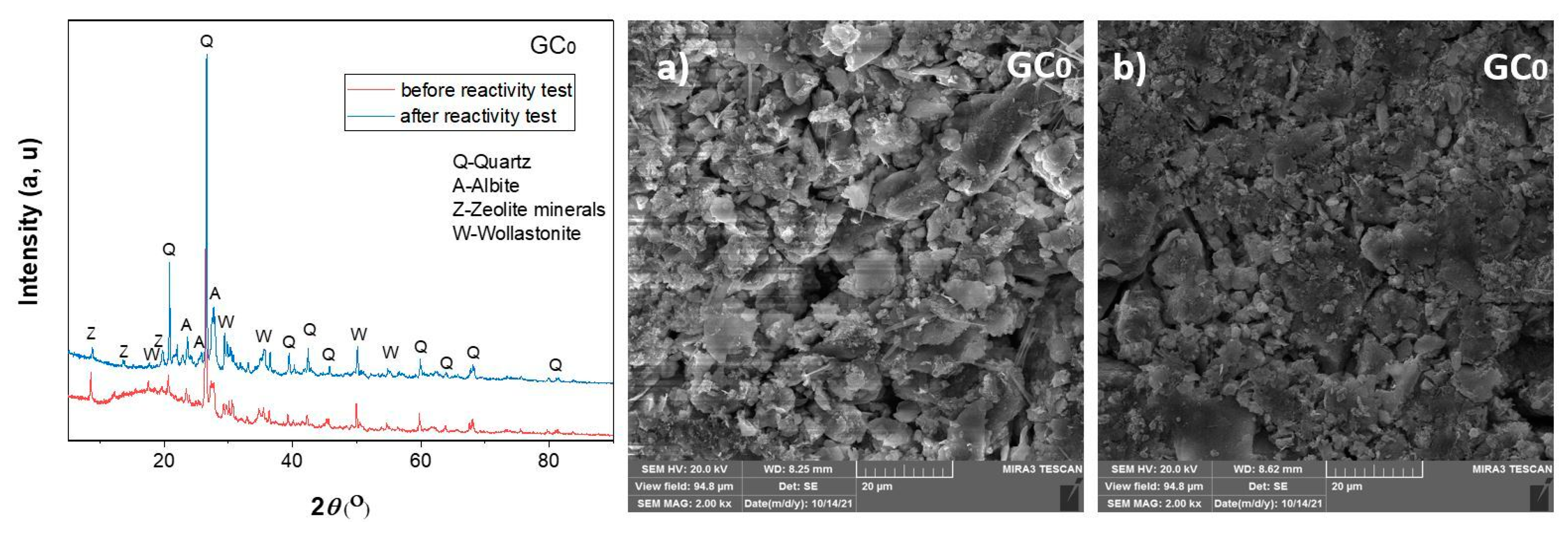
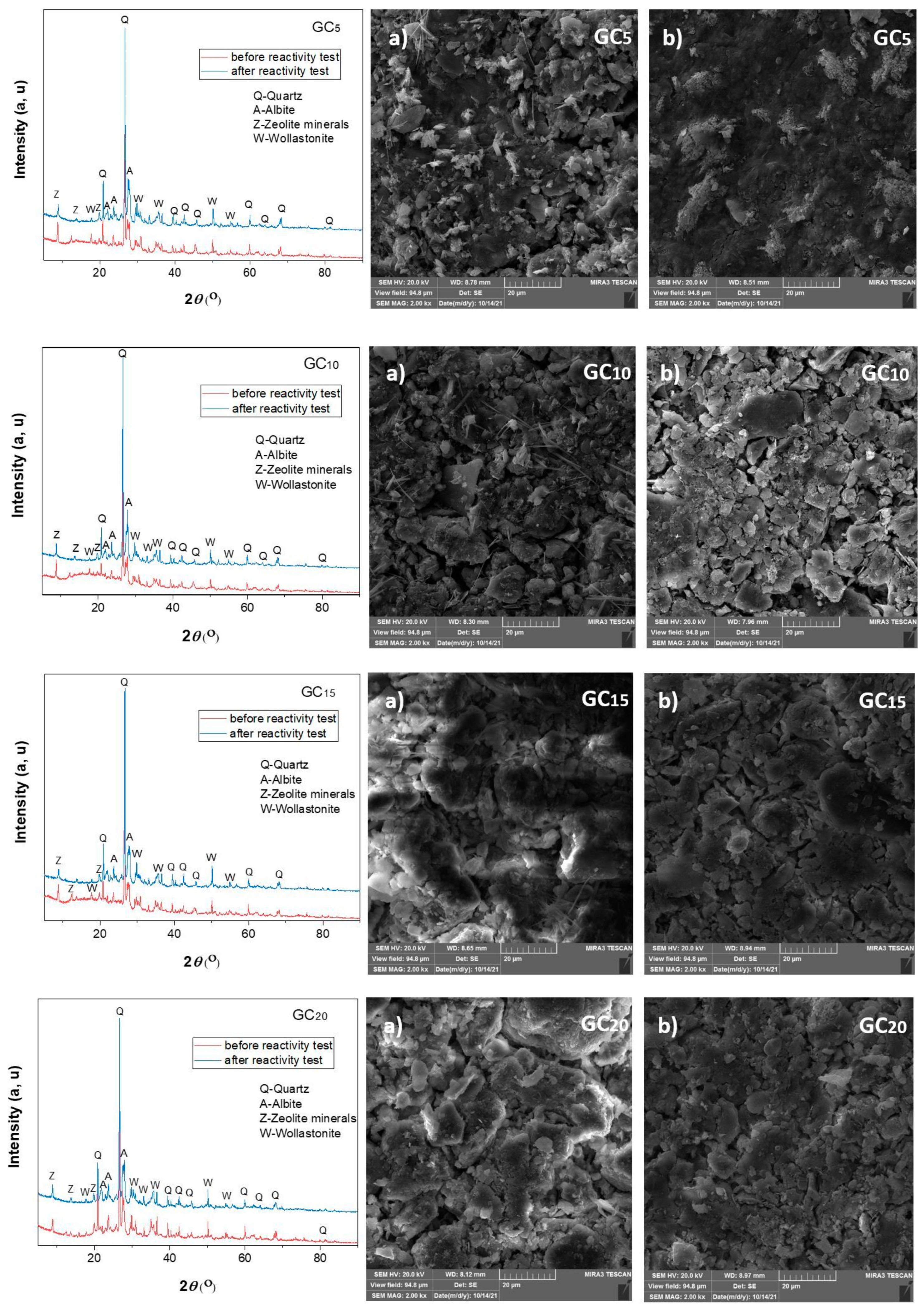
3.2.2. Results of the XRD Analysis of the Raw and Sintered Samples
3.2.3. Results of the Leaching Tests of the Raw and Sintered Samples
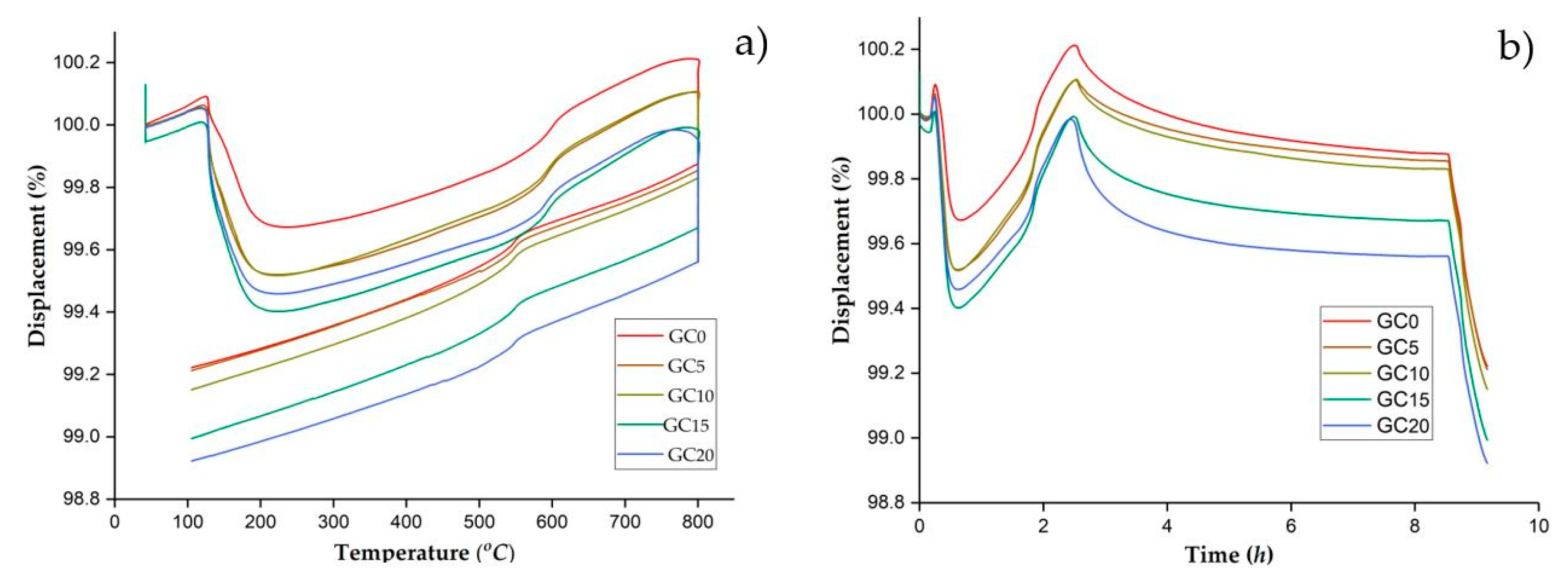
3.2.4. Results of the Dilatometry Analysis of the Raw Samples
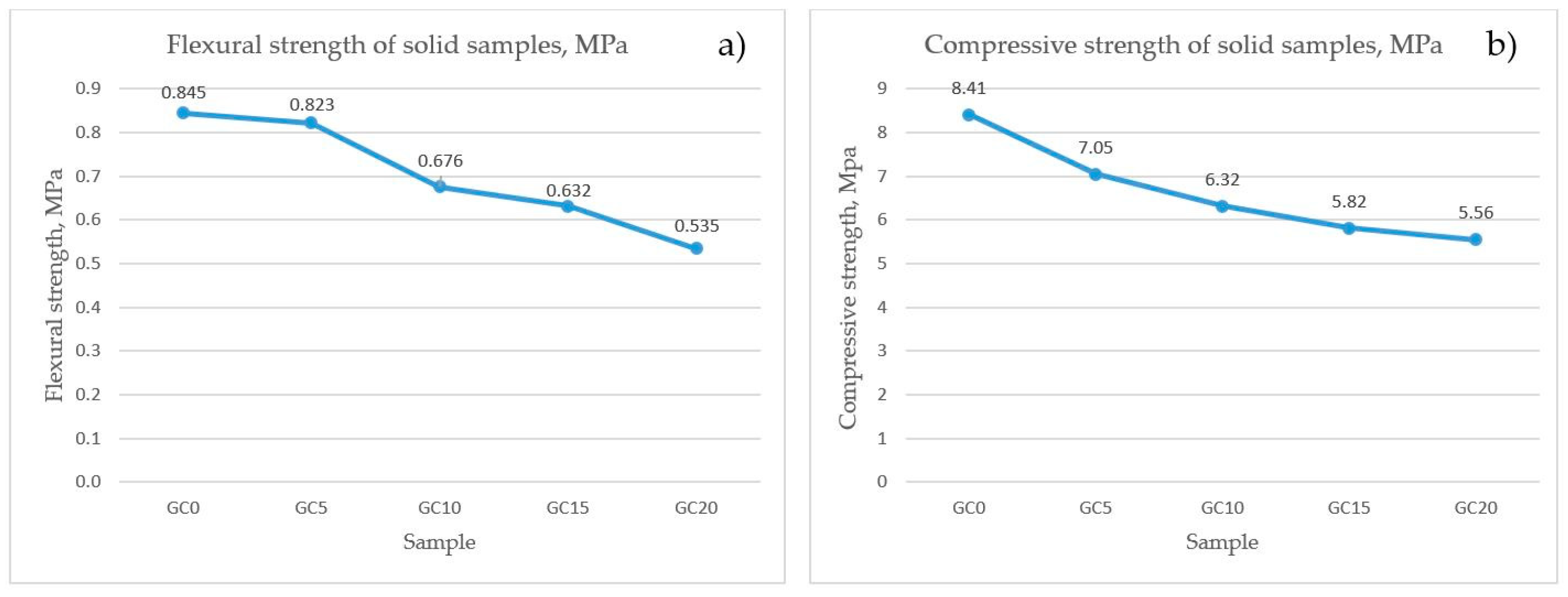
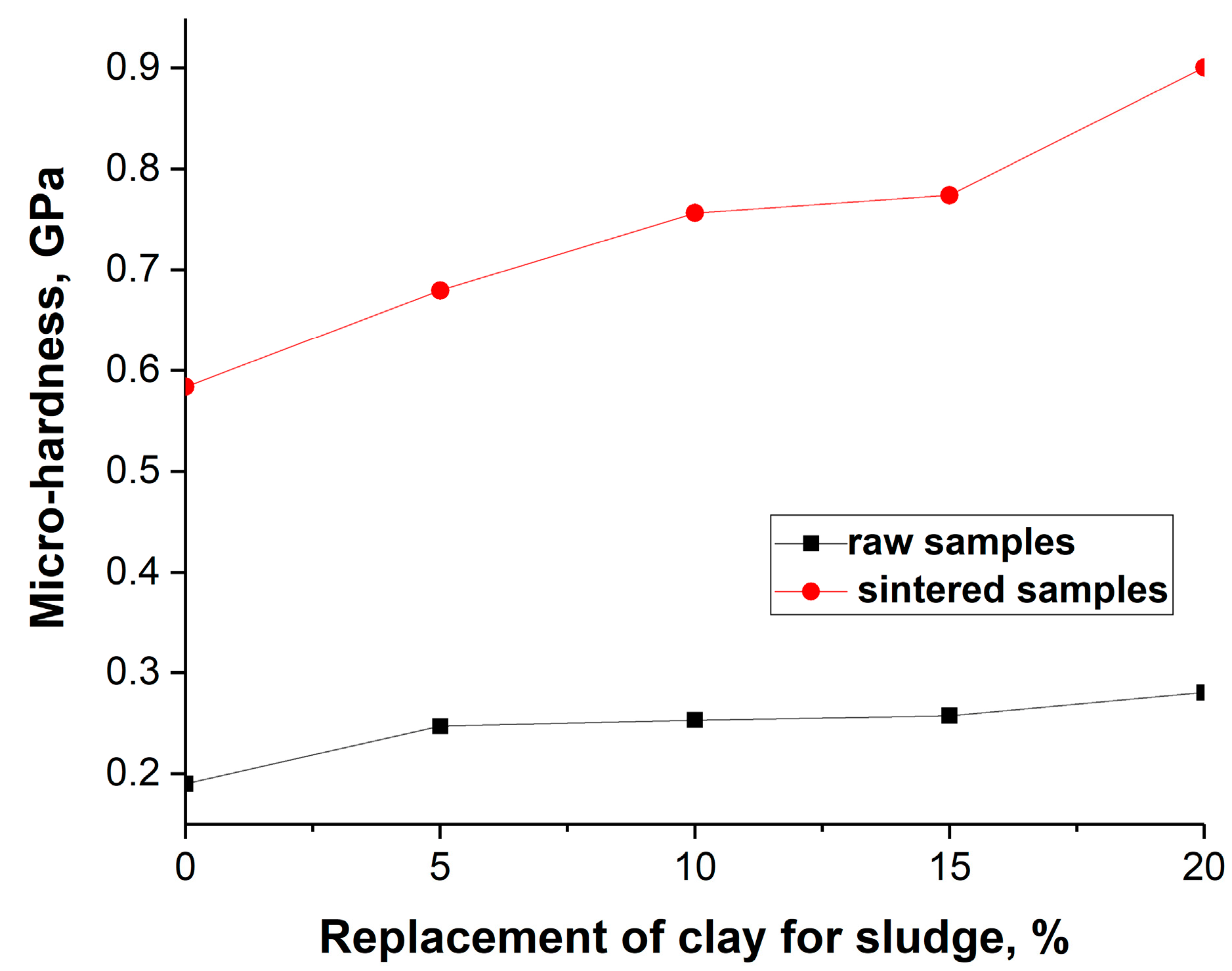
3.2.5. Results of the Mechanical Properties of the Sintered Samples
3.3. Results of Material Performance for Potential Application as IT-SOFC Sealants
3.3.1. Results of the XRD and FESEM Analyses of the Raw and Sintered Samples
3.3.2. Results of the Open Circuit Voltage (OCV) Test—Leakage Detection of a Single Laboratory Fuel Cell
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nasrollahi, Z.; Hashemi, M.S.; Bameri, S.; Mohamad Taghvaee, V. Environmental pollution, economic growth, population, industrialization, and technology in weak and strong sustainability: Using STIRPAT model. Environ. Dev. Sustain. 2020, 22, 1105–1122. [Google Scholar] [CrossRef]
- Opoku, E.E.O.; Aluko, O.A. Heterogeneous effects of industrialization on the environment: Evidence from panel quantile regression. Struct. Chang. Econ. Dyn. 2021, 59, 174–184. [Google Scholar] [CrossRef]
- Usman, M.; Balsalobre-Lorente, D. Environmental concern in the era of industrialization: Can financial development, renewable energy and natural resources alleviate some load? Energy Policy 2022, 162, 112780. [Google Scholar] [CrossRef]
- Das, S.; Lee, S.H.; Kumar, P.; Kim, K.H.; Lee, S.S.; Bhattacharya, S.S. Solid waste management: Scope and the challenge of sustainability. J. Clean. Prod. 2019, 228, 658–678. [Google Scholar] [CrossRef]
- Abouelela, A.R.; Mussa, A.A.; Talhami, M.; Das, P.; Hawari, A.H. Industrial sludge valorization and decontamination via lipid extraction and heavy metals removal using low-cost protic ionic liquid. Sci. Total Environ. 2022, 835, 155451. [Google Scholar] [CrossRef]
- Basu, D.; Pal, P.; Prakash, A. Utilization of waste sludge in cementitious matrix: A feasibility study. Mater. Today Proc. 2022, 65, 1375–1381. [Google Scholar] [CrossRef]
- Gherghel, A.; Teodosiu, C.; De Gisi, S. A review on wastewater sludge valorisation and its challenges in the context of circular economy. J. Clean. Prod. 2019, 10, 244–263. [Google Scholar] [CrossRef]
- Patil, R.A.; Ramakrishna, S. A comprehensive analysis of e-waste legislation worldwide. Environ. Sci. Pollut. Res. 2020, 27, 14412–14431. [Google Scholar] [CrossRef] [PubMed]
- Bekezhanov, D.; Omirali, A.; Aitimov, B.; Nurmukhankyzy, D.; Zhumagulov, T. Environmental and legal framework for regulating consumer and industrial waste management. J. Environ. Manag. Tour. 2020, 11, 186–193. [Google Scholar] [CrossRef]
- Neves, S.A.; Marques, A.C. Drivers and barriers in the transition from a linear economy to a circular economy. J. Clean. Prod. 2022, 341, 130865. [Google Scholar] [CrossRef]
- Meath, C.; Karlovšek, J.; Navarrete, C.; Eales, M.; Hastings, P. Co-designing a multi-level platform for industry level transition to circular economy principles: A case study of the infrastructure CoLab. J. Clean. Prod. 2022, 347, 131080. [Google Scholar] [CrossRef]
- Charef, R.; Lu, W.; Hall, D. The transition to the circular economy of the construction industry: Insights into sustainable approaches to improve the understanding. J. Clean. Prod. 2022, 364, 132421. [Google Scholar] [CrossRef]
- Nguyen, M.D.; Thomas, M.; Surapaneni, A.; Moon, E.M.; Milne, N.A. Beneficial reuse of water treatment sludge in the context of circular economy. Environ. Technol. Innov. 2022, 13, 102651. [Google Scholar] [CrossRef]
- Gomes, S.D.C.; Zhou, J.L.; Li, W.; Long, G. Progress in manufacture and properties of construction materials incorporating water treatment sludge: A review. Resour. Conserv. Recycl. 2019, 145, 148–159. [Google Scholar] [CrossRef]
- Limami, H.; Manssouri, I.; Cherkaoui, K.; Khaldoun, A. Recycled wastewater treatment plant sludge as a construction material additive to ecological lightweight earth bricks. Clean. Eng. Technol. 2021, 2, 100050. [Google Scholar] [CrossRef]
- Heniegal, A.M.; Ramadan, M.A.; Naguib, A.; Agwa, I.S. Study on properties of clay brick incorporating sludge of water treatment plant and agriculture waste. Case Stud. Constr. Mater. 2020, 13, e00397. [Google Scholar] [CrossRef]
- Meda, S.R.; Sharma, S.K.; Tyagi, G.D. Utilization of waste sludge as a construction material—A review. Mater. Today Proc. 2021, 46, 4195–4202. [Google Scholar] [CrossRef]
- Pradel, M.; Aissani, L. Environmental impacts of phosphorus recovery from a “product” Life Cycle Assessment perspective: Allocating burdens of wastewater treatment in the production of sludge-based phosphate fertilizers. Sci. Total Environ. 2019, 656, 55–69. [Google Scholar] [CrossRef]
- Chen, Y.H.; Ngo, T.N.L.T.; Chiang, K.Y. Enhanced hydrogen production in co-gasification of sewage sludge and industrial wastewater sludge by a pilot-scale fluidized bed gasifier. Int. J. Hydrog. Energy 2021, 46, 14083–14095. [Google Scholar] [CrossRef]
- Kamyab, H.; Yuzir, A.; Ashokkumar, V.; Hosseini, S.E.; Balasubramanian, B.; Kirpichnikova, I. Review of the application of gasification and combustion technology and waste-to-energy technologies in sewage sludge treatment. Fuel 2022, 316, 123199. [Google Scholar] [CrossRef]
- Nkuna, S.G.; Olwal, T.O.; Chowdhury, S.D. Assessment of thermochemical technologies for wastewater sludge-to-energy: An advance MCDM model. Clean. Eng. Technol. 2022, 9, 100519. [Google Scholar] [CrossRef]
- Available online: https://www.industryarc.com/Research/High-Temperature-Adhesives-Sealants-Market-Research-502948 (accessed on 25 May 2023).
- Wen, T.L.; Wang, D.; Chen, M.; Tu, H.; Lu, Z.; Zhang, Z.; Huang, W. Material research for planar SOFC stack. Solid State Ion. 2002, 148, 513–519. [Google Scholar] [CrossRef]
- Fergus, J.W. Sealants for solid oxide fuel cells. J. Power Sources 2005, 147, 46–57. [Google Scholar] [CrossRef]
- Zhang, J.; Luo, R.; Jiang, M.; Xiang, Q.; Li, J. The preparation and performance of a novel room-temperature-cured heat-resistant adhesive for ceramic bonding. Mater. Sci. Eng. A 2011, 528, 2952–2959. [Google Scholar] [CrossRef]
- Wang, M.; Song, Q.; Gu, Y.; Wu, C.; Liu, J.; Zhou, X.; Du, M. Multiple high-temperature resistant phases modified phosphate-based adhesive for engineering ceramic connection in extreme environment. Ceram. Int. 2019, 45, 516–521. [Google Scholar] [CrossRef]
- Tokyo Rigaku Corporation. PDXL Integrated X-ray Powder Diffraction Software; Rigaku Corporation: Tokyo, Japan, 2011. [Google Scholar]
- Qi, Y.; Szendrak, D.; Yuen, R.T.W.; Hoadley, A.F.; Mudd, G. Application of sludge dewatered products to soil and its effects on the leaching behaviour of heavy metals. J. Chem. Eng. 2011, 166, 586–595. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, X.; Yu, C.; Ding, J.; Deng, C.; Zhu, H. Properties of inorganic high-temperature adhesive for high-temperature furnace connection. Ceram. Int. 2019, 45, 8684–8689. [Google Scholar] [CrossRef]
- Siviour, C.R.; Gifford, M.J.; Walley, S.M.; Proud, W.G.; Field, J.E. Particle size effects on the mechanical properties of a polymer bonded explosive. J. Mater. Sci. 2004, 39, 1255–1258. [Google Scholar] [CrossRef]
- Rezaei, N.; Ghatee, M. Barium-calcium aluminosilicate glass/mica composite seals for intermediate solid oxide fuel cells. Ceram. Int. 2021, 47, 21679–21687. [Google Scholar] [CrossRef]
- Hasanabadi, M.F.; Malzbender, J.; Groß-Barsnick, S.M.; Abdoli, H.; Kokabi, A.H.; Faghihi-Sani, M.A. Micro-scale evolution of mechanical properties of glass-ceramic sealant for solid oxide fuel/electrolysis cells. Ceram. Int. 2021, 47, 3884–3891. [Google Scholar] [CrossRef]
- Latosińska, J.; Czapik, P. The ecological risk assessment and the chemical speciation of heavy metals in ash after the incineration of municipal sewage sludge. Sustainability 2020, 12, 6517. [Google Scholar] [CrossRef]
- Zhang, S.; Zhang, Y.; Wu, S.; Zhao, Z.; Wu, Y. Long-term leaching mechanism of chromium-containing slag after vitrification and heat treatment. Ceram. Int. 2022, 48, 13366–13378. [Google Scholar] [CrossRef]
- Available online: https://www.qsiquartz.com/thermal-properties-fused-quartz/ (accessed on 16 June 2023).
- Zilles, J.U. Wollastonites. In Fillers for Polymer Applications; Polymers and Polymeric Composites: A Reference Series; Rothon, R., Ed.; Springer: Berlin/Heidelberg, Germany, 2017; Volume 489, pp. 203–230. [Google Scholar] [CrossRef]
- Feng, D.; Provis, J.L.; Jannie, S.J.; van Deventer, J.S. Thermal activation of albite for the synthesis of one-part mix geopolymers. J. Am. Ceram. Soc. 2012, 95, 565–572. [Google Scholar] [CrossRef]
- Cruciani, G. Zeolites upon heating: Factors governing their thermal stability and structural changes. J. Phys. Chem. Solids 2006, 67, 1973–1994. [Google Scholar] [CrossRef]
- Rodríguez-López, S.; Wei, J.; Laurenti, K.C.; Mathias, I.; Justo, V.M.; Serbena, F.C.; Baudin, C.; Malzbenger, J.; Pascual, M.J. Mechanical properties of solid oxide fuel cell glass-ceramic sealants in the system BaO/SrO-MgO-B2O3-SiO2. Ceram. Soc. 2017, 37, 3579. [Google Scholar] [CrossRef]
- Heydari, F.; Maghsoudipour, A.; Hamnabard, Z.; Farhangdoust, S. Mechanical properties and microstructure characterization of zirconia nanoparticles glass composites for SOFC sealant. Mater. Sci. Eng. A 2012, 552, 119–124. [Google Scholar] [CrossRef]
- Lin, C.K.; Chen, J.Y.; Tian, J.W.; Chiang, L.K.; Wu, S.H. Joint strength of a solid oxide fuel cell glass-ceramic sealant with metallic interconnect. J. Power Sources 2012, 205, 307–317. [Google Scholar] [CrossRef]



| Concentrations, mg/kg, in Waste Sludge | ||||
|---|---|---|---|---|
| Parameter | LP Sludge | PE Sludge | LP+PE Sludge (1:1) | Ref. Values |
| Hg | <0.15 | <0.15 | <0.15 | 0.2 */2 ** |
| Sb | 0.85 | 85.5 | 43.175 | 0.7 */5 ** |
| Se | <0.2 | 2.5 | <1.35 | 0.5 */7 ** |
| Cu | 20.5 | 2050 | 1035.25 | 50 */100 ** |
| Zn | 37 | 175.5 | 106.25 | 50 */200 ** |
| Ni | 8 | 186 | 97 | 10 */40 ** |
| Cd | 2.5 | 6 | 4.25 | 1 */5 ** |
| Pb | 10 | 37 | 23.5 | 10 */50 ** |
| As | 2.5 | <0.5 | <1.5 | 2 */25 ** |
| Cr | 8 | 22 | 15 | 10 */70 ** |
| Mn | 600 | 4855 | 2727.5 | - |
| Tl | <0.05 | 2.5 | <1.275 | - |
| Fe | 18,750 | 13,250 | 16,000 | - |
| Co | 3.5 | 2330 | 1166.75 | - |
| Content of Oxides (wt, %) in Waste Sludge | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Component | Al2O3 | SiO2 | Fe2O3 | Na2O | K2O | CaO | MgO | SO3 | P2O5 |
| LP sludge | 2.68 | 1.971 | 4.35 | 3.56 | 2.609 | 0.167 | 0.133 | 0.095 | 2.255 |
| PE sludge | 16.67 | 10.78 | 11.01 | 19.29 | 3.792 | 3.455 | 0.697 | 0.856 | 8.57 |
| LP+PE sludge (1:1) | 9.675 | 6.3755 | 7.68 | 11.425 | 3.201 | 1.811 | 0.415 | 0.475 | 5.413 |
| Concentration, mg/kg, in Adhesive and Sealant Sample Composition | |||||
|---|---|---|---|---|---|
| Parameter | GC5 | GC10 | GC15 | GC20 | Ref. Values |
| Hg | <0.15 | <0.15 | <0.15 | <0.15 | 0.2 */2 ** |
| Sb | 0.7038 | 1.4076 | 2.1114 | 2.8152 | 0.7 */5 ** |
| Se | <0.022 | <0.044 | <0.066 | <0.088 | 0.5 */7 ** |
| Cu | 16.8746 | 33.7492 | 50.6238 | 67.4984 | 50 */100 ** |
| Zn | 1.7319 | 3.4638 | 5.1957 | 6.9276 | 50 */200 ** |
| Ni | 1.5811 | 3.1622 | 4.7433 | 6.3244 | 10 */40 ** |
| Cd | 0.0693 | 0.1386 | 0.2079 | 0.2772 | 1 */5 ** |
| Pb | 0.383 | 0.7660 | 1.149 | 1.532 | 10 */50 ** |
| As | <0.0245 | <0.0490 | <0.0735 | <0.098 | 2 */25 ** |
| Cr | 0.2445 | 0.4890 | 0.7335 | 0.978 | 10 */70 ** |
| Mn | 44.4582 | 88.9164 | 133.3746 | 177.8328 | - |
| Tl | <0.0245 | <0.0490 | <0.0735 | <0.098 | - |
| Fe | 260.8 | 521.6000 | 782.4 | 1043.2 | - |
| Co | 19.0343 | 38.0686 | 57.1029 | 76.1372 | - |
| Concentration, mg/kg, in Adhesive and Sealant Samples’ Composition | |||||
|---|---|---|---|---|---|
| Parameter | GC15 Raw | GC20 Raw | GC15 Sint | GC20 Sint | Ref. Values |
| pH before test | 10.91 | 10.92 | 11.71 | 11.52 | - |
| pH after test | 10.67 | 10.67 | 10.9 | 10.39 | - |
| Al | 132.4 | 104.1 | 257.1 | 120.1 | - |
| As | 3.282 | 3.29 | 0.02452 | 0.2154 | 2 */25 ** |
| Ca | 64.96 | 58.45 | 769.1 | 616.1 | - |
| Cd | 0.008039 | 0.008372 | 1UDV | UDV | 1 */5 **- |
| Co | 0.1895 | 0.2401 | 0.008266 | 0.02789 | - |
| Cr | 0.09819 | 0.1081 | 10.11 | 10.25 | 10 */70 ** |
| Cu | 2.395 | 2.761 | 0.4003 | 0.7381 | 50 */100 ** |
| Fe | 12.18 | 13.63 | 0.3947 | 2.701 | - |
| Hg | 0.001594 | 0.000053 | 0.000661 | 0.000223 | 0.2 */2 ** |
| K | 187.3 | 198.2 | 62.92 | 92.2 | - |
| Mg | 17.66 | 16.44 | 34.25 | 35.56 | - |
| Mn | 0.4034 | 0.4925 | 0.009729 | 0.08828 | - |
| Mo | 1.89 | 2.659 | 2.86 | 3.452 | 10 */30 **- |
| Na | 25770 | 26420 | 693.1 | 2532 | - |
| Ni | 0.1875 | 0.1983 | UDV | 0.01233 | 10 */40 ** |
| P | 1514 | 1802 | 0.4047 | 52.39 | |
| Pb | 0.9608 | 1.265 | 0.07941 | 0.2532 | 10 */50 ** |
| S | 792.7 | 839.4 | 299.7 | 323.3 | |
| Se | 0.192 | 0.2617 | 0.009627 | 0.01374 | 0.5 */7 ** |
| V | 1.248 | 1.242 | 0.9937 | 1.106 | |
| Zn | 0.1989 | 0.3054 | UDV | 0.02292 | 50 */200 ** |
| Sample | No. | HV0.025, HV | [HV0.025]sr, HV | Sample | No. | HV0.05, HV | [HV0.05]sr, HV |
|---|---|---|---|---|---|---|---|
| GC0 raw | 1 | 19.5 | 19.4 | GC0 sintered | 1 | 56.8 | 59.57 |
| 2 | 19.6 | 2 | 59.7 | ||||
| 3 | 19.1 | 3 | 62.2 | ||||
| GC5 raw | 1 | 25.2 | 25.23 | GC5 sintered | 1 | 66.6 | 69.37 |
| 2 | 25.3 | 2 | 71.9 | ||||
| 3 | 25.2 | 3 | 69.6 | ||||
| GC10 raw | 1 | 26.2 | 25.83 | GC10 sintered | 1 | 77.0 | 77.2 |
| 2 | 25.8 | 2 | 79.3 | ||||
| 3 | 25.5 | 3 | 75.3 | ||||
| GC15 raw | 1 | 25.8 | 26.27 | GC15 sintered | 1 | 77 | 79.0 |
| 2 | 26.8 | 2 | 79.3 | ||||
| 3 | 26.2 | 3 | 80.7 | ||||
| GC20 raw | 1 | 28.4 | 28.63 | GC20 sintered | 1 | 91.7 | 91.9 |
| 2 | 28.8 | 2 | 90.0 | ||||
| 3 | 28.7 | 3 | 94.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nišić, N.; Kragović, M.; Gulicovski, J.; Žunić, M.; Basoli, F.; Gordić, M.; Stojmenović, M. The Incorporation of Waste Sludge into the Production of High-Temperature-Resistant Adhesive Ceramic Materials. Appl. Sci. 2023, 13, 9044. https://doi.org/10.3390/app13169044
Nišić N, Kragović M, Gulicovski J, Žunić M, Basoli F, Gordić M, Stojmenović M. The Incorporation of Waste Sludge into the Production of High-Temperature-Resistant Adhesive Ceramic Materials. Applied Sciences. 2023; 13(16):9044. https://doi.org/10.3390/app13169044
Chicago/Turabian StyleNišić, Neda, Milan Kragović, Jelena Gulicovski, Milan Žunić, Francesco Basoli, Milan Gordić, and Marija Stojmenović. 2023. "The Incorporation of Waste Sludge into the Production of High-Temperature-Resistant Adhesive Ceramic Materials" Applied Sciences 13, no. 16: 9044. https://doi.org/10.3390/app13169044
APA StyleNišić, N., Kragović, M., Gulicovski, J., Žunić, M., Basoli, F., Gordić, M., & Stojmenović, M. (2023). The Incorporation of Waste Sludge into the Production of High-Temperature-Resistant Adhesive Ceramic Materials. Applied Sciences, 13(16), 9044. https://doi.org/10.3390/app13169044










