Possibility of Using Vitreous Enamel Waste in the Construction Industry as the Concept of Cleaner Production
Abstract
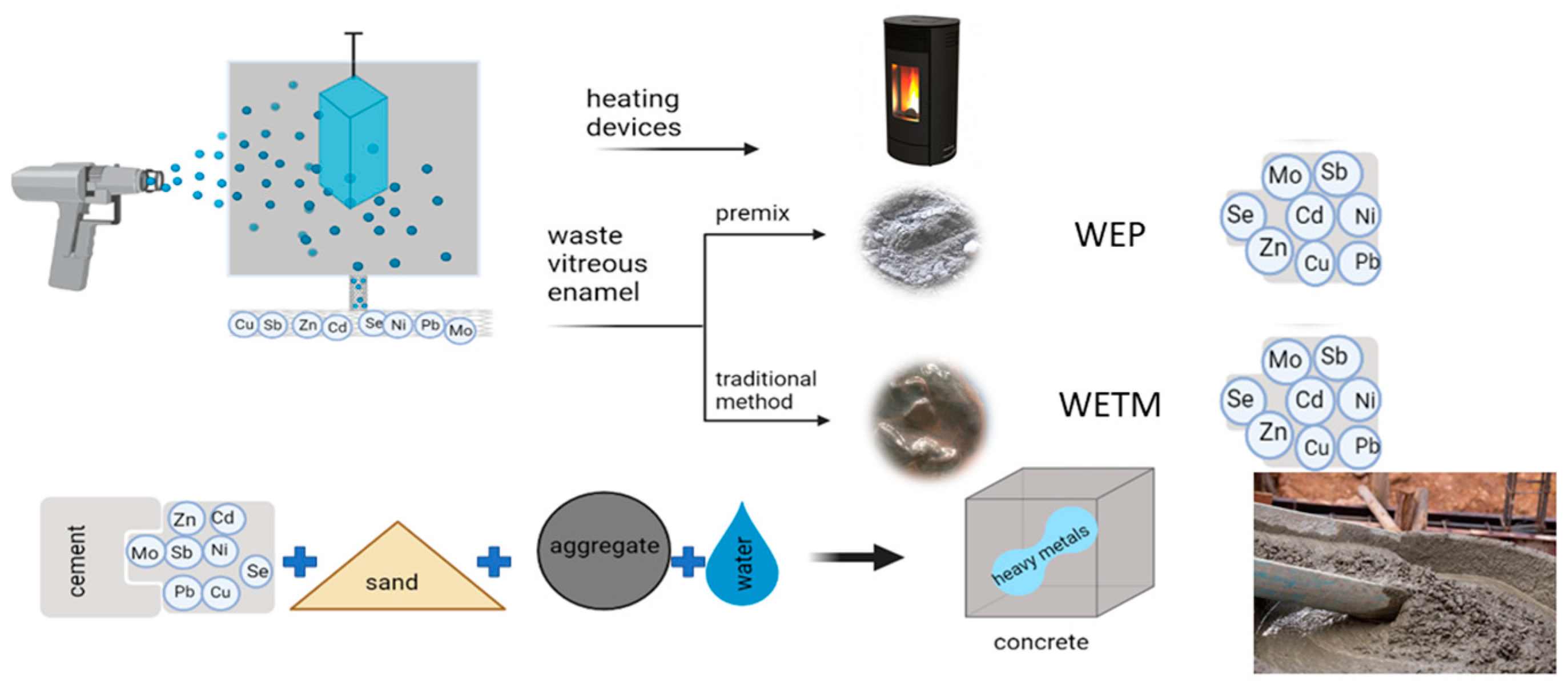
1. Introduction
2. Materials and Methods
2.1. Sampling
2.2. Physical-Chemical Analysis of Waste Vitreous Enamels
2.2.1. Chemical Analysis and Determination of the Content of Heavy Metals
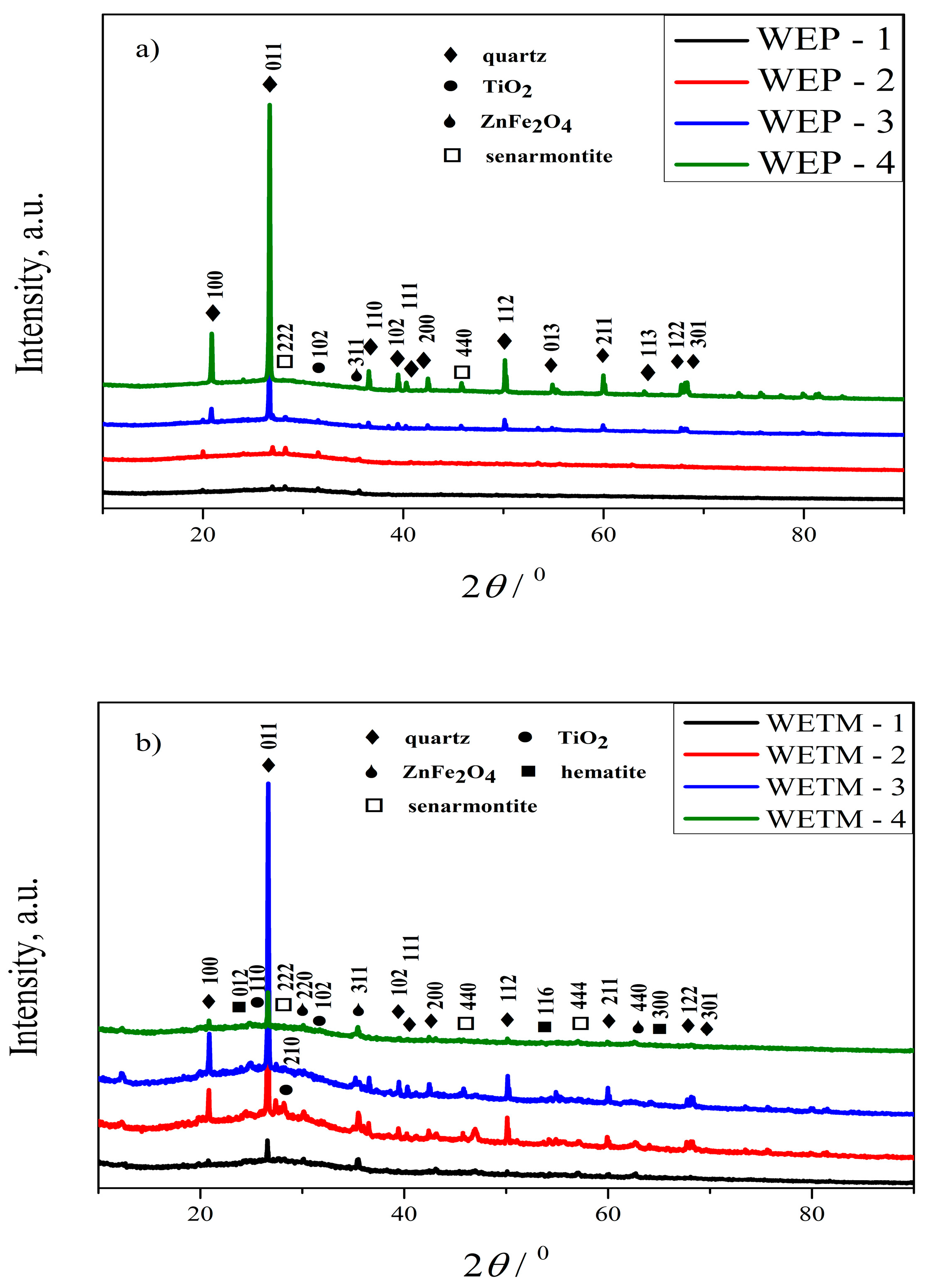
2.2.2. X-ray Structural Analysis (XRPD)
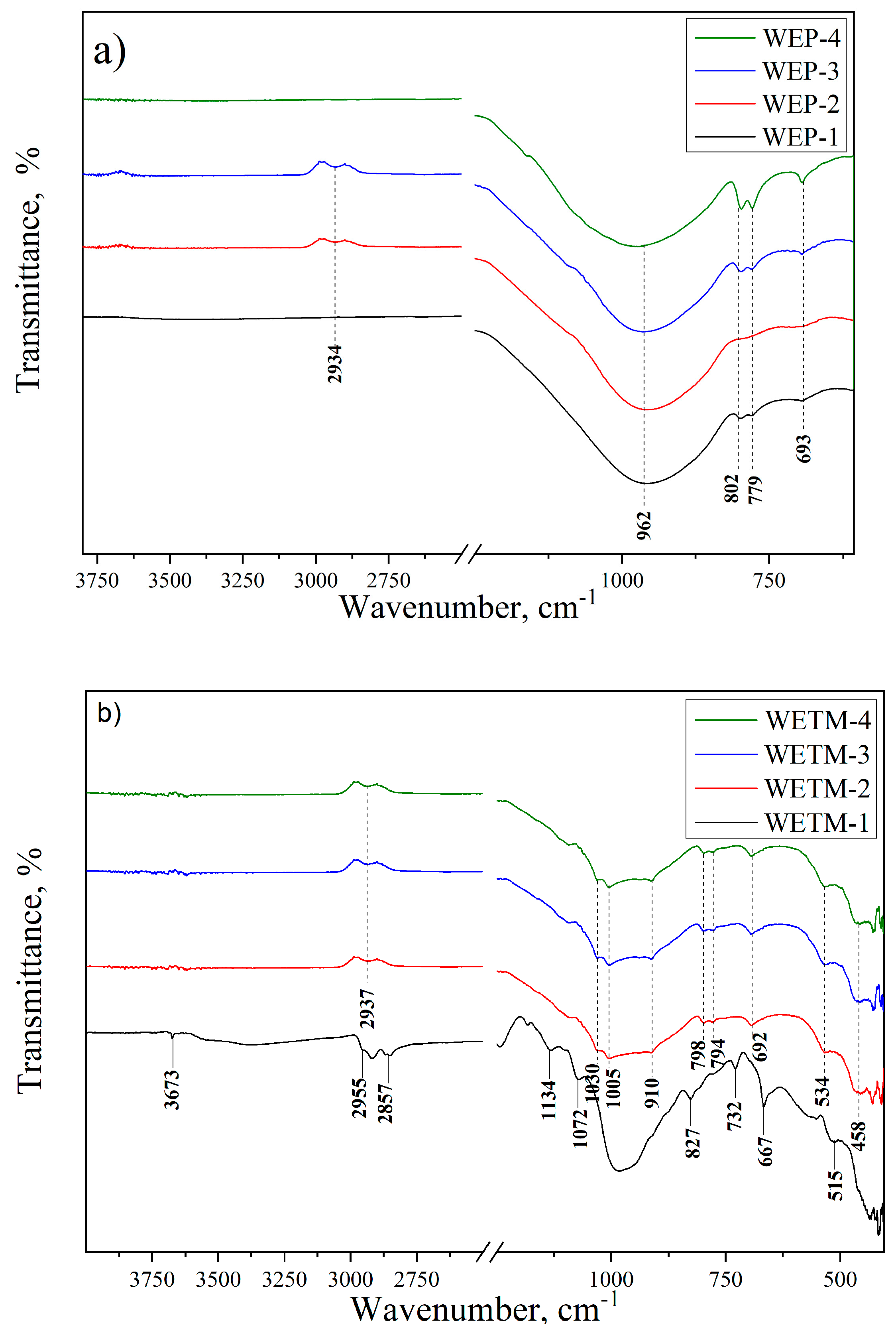
2.2.3. Fourier-Transform Infrared Spectroscopy (FTIR) with Attenuated Total Reflection (ATR)
2.2.4. Determination of the Specific Surface by the BET Method
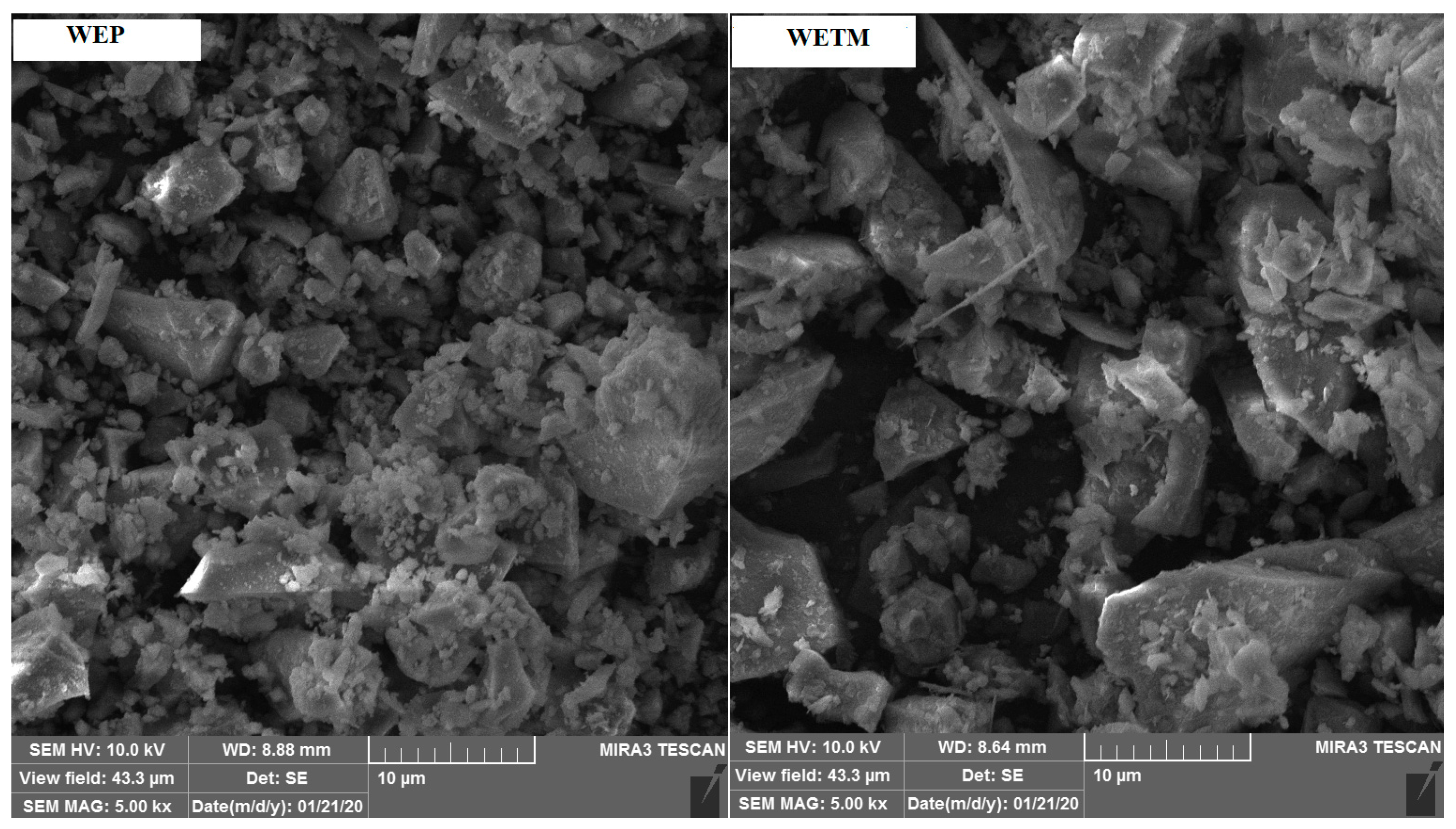
2.2.5. Field Emission Scanning Electron Microscopy (FE-SEM)
2.3. Pozzolanic Activity of Waste Vitreous Enamels
2.4. Preparation and Testing of Mortar with Waste Enamels
2.5. Preparation and Testing of Concrete with Waste Enamels
2.6. Methods of Investigations of Properties of Mortar and Concrete
3. Results and Discussion
3.1. Physical-Chemical Characterization of the Waste Vitreous Enamels
3.1.1. Chemical Analysis and Determination of the Content of Heavy Metals
3.1.2. Results of X-ray Structural Analysis (XRPD)
3.1.3. Results of Fourier-Transform Infrared Spectroscopy (FTIR)
3.1.4. Results of Determination of the Specific Surface by the BET Method
3.1.5. Results of Field Emission Scanning Electron Microscopy (FESEM)
3.2. Results of Determination of the Pozzolanic Activity, Parameters of Cement Paste, Mortar and Concrete Produced with the Addition of Waste Enamels
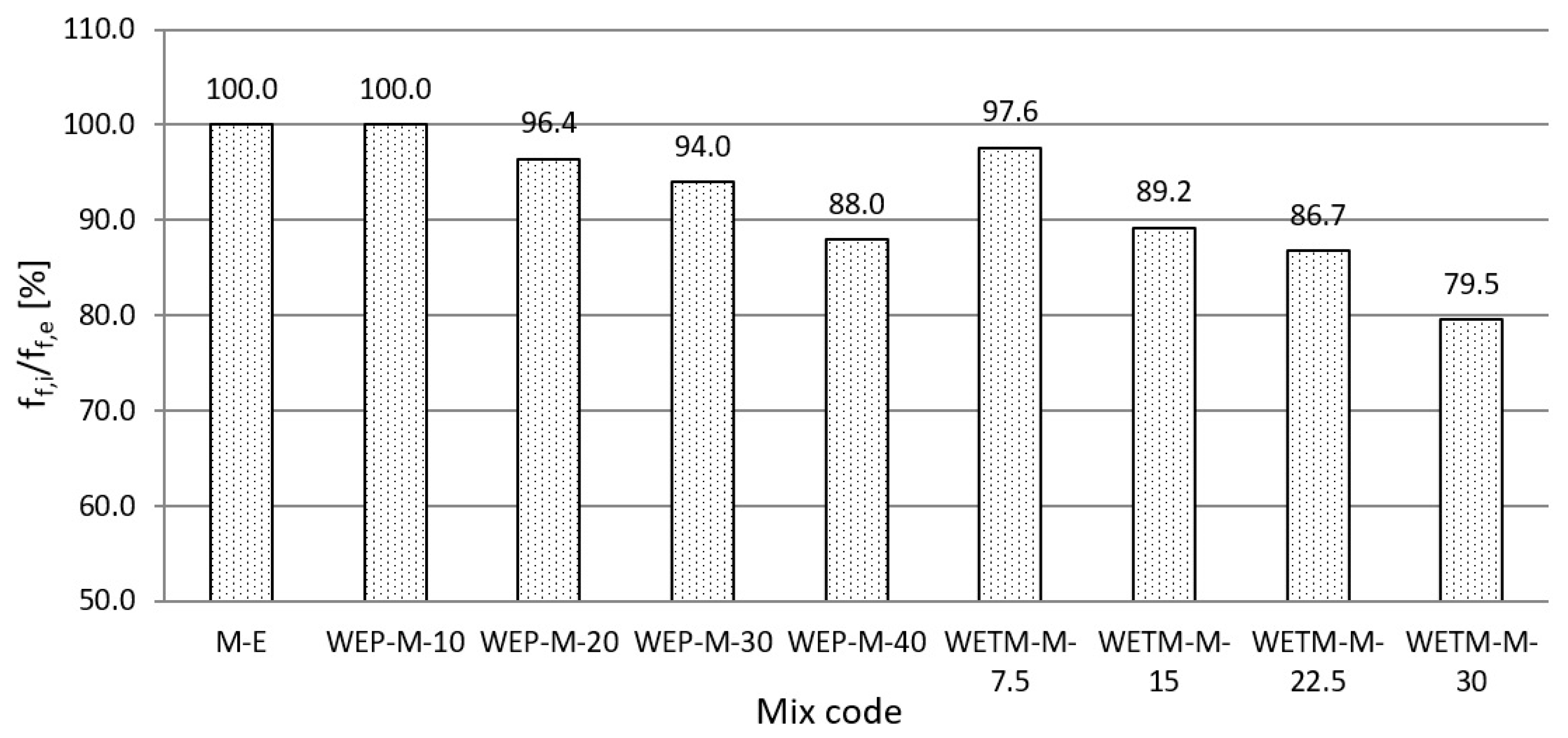
3.2.1. Determination of the Pozzolanic Activity of Waste Enamels
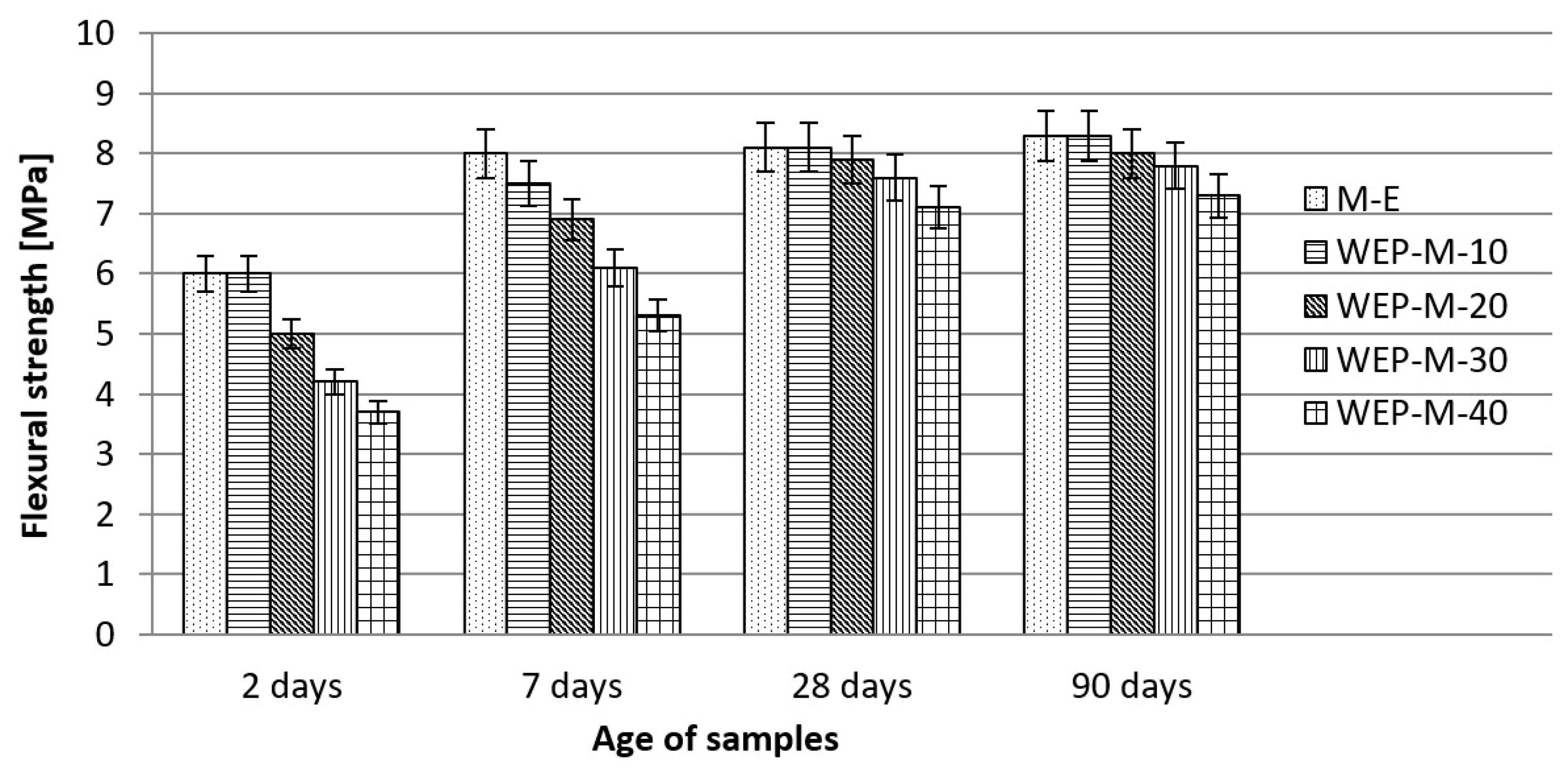
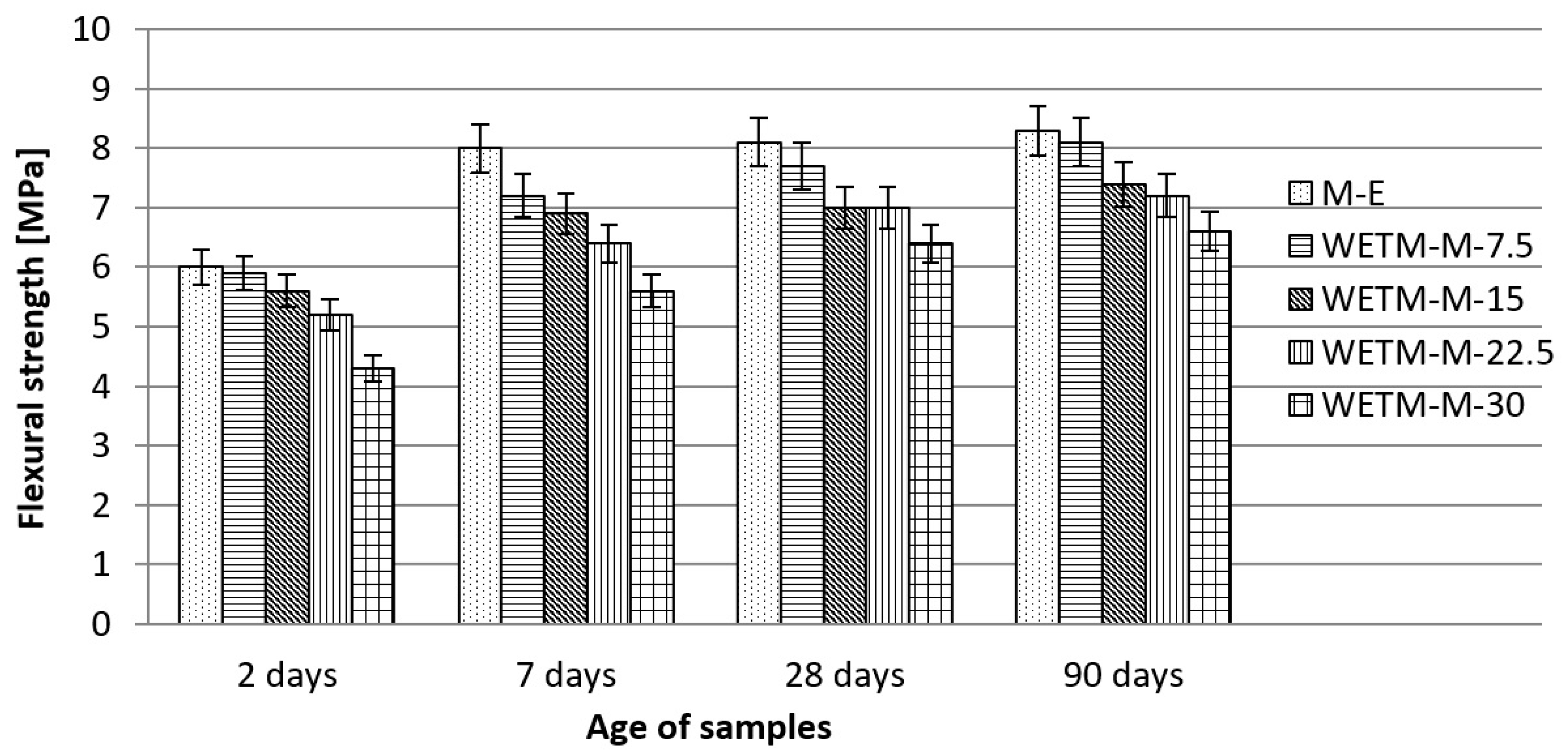
3.2.2. Effects of Waste Enamels on Mortar Properties
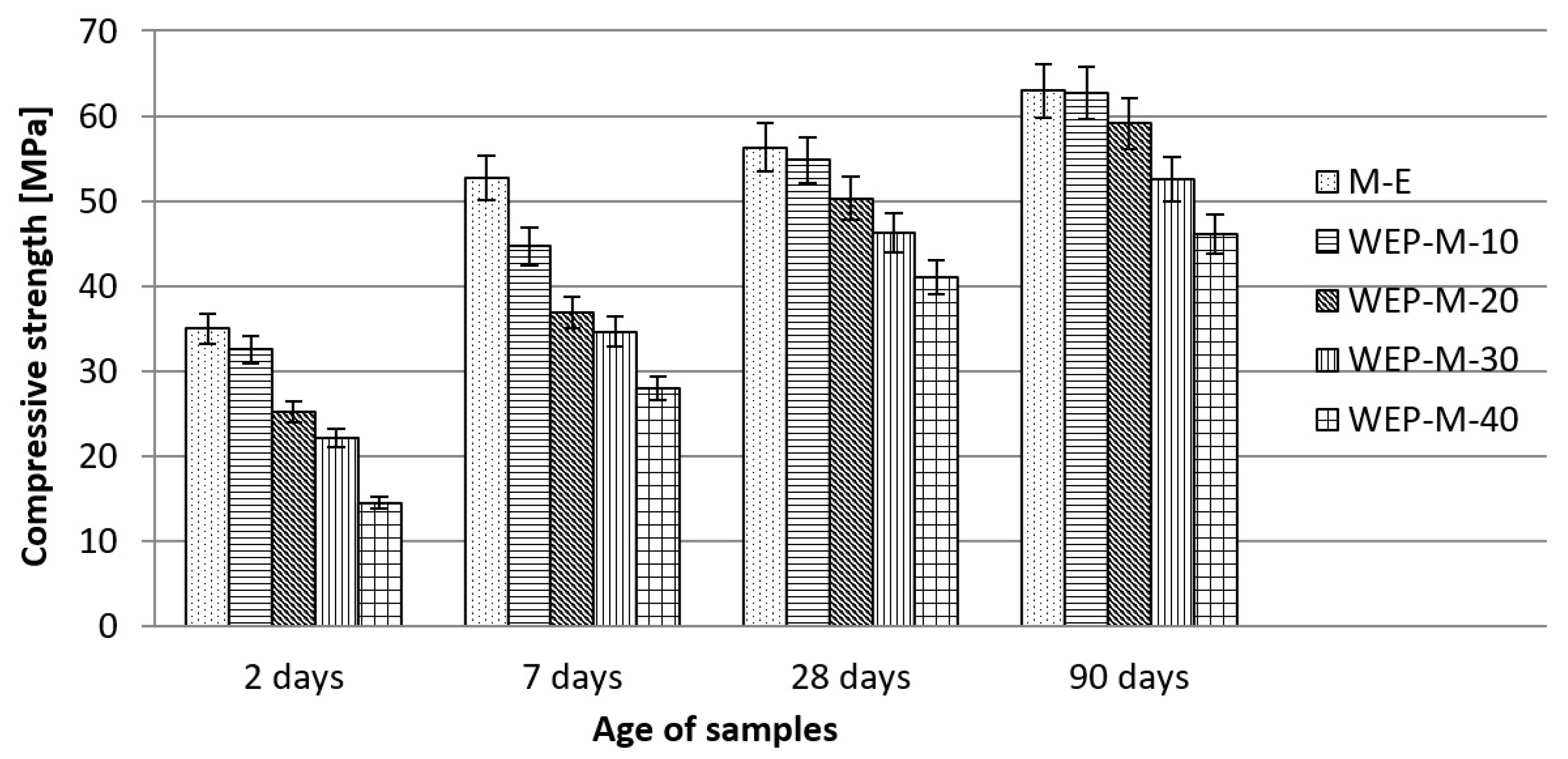
3.2.3. Effects of Waste Enamels on Concrete Properties
3.2.4. The Leaching Test
4. Conclusions
- Used waste enamels possess pozzolanic activity and belong to class 15 (WEP), that is 5 (WETM) of pozzolanic materials. Additionally, both waste enamels meet the criteria for the activity index according to EN 450-1, which refers to their possible use as a type II admixture for the production of concrete in accordance with EN 206. Waste enamels WEP and WETM in terms of water requirement, initial setting time and soundness meet the criteria prescribed by the Standard.
- Examinations of the physical and mechanical properties of mortars with waste enamels have shown that these materials can be used as a replacement for cement in the production of mortar, where is recommended a maximum cement replacement in the amount of 30% for WEP, and 20% for WETM. These replacements of cement with waste enamels in mortar do not significantly affect its physical and mechanical characteristics in comparison with the characteristics of the reference mortar prepared with 100% cement.
- The use of waste enamels in concrete, as a partial replacement of cement, contributes to a slight decrease in physical and mechanical properties, while on the other hand, it does not compromise the durability of the concrete. Test results indicate that the replacement of cement with WEP up to 30%, or WETM up to 20% does not significantly affect the quality of concrete compared to the quality of the reference concrete prepared with 100% cement.
- Bearing in mind that vitreous waste enamels can be used for the production of cement composites, the problem of their disposal is solved, which is a great contribution to preserving a healthy environment.
- Further research should be focused on studying the behavior of reinforcement in concrete produced with the addition of waste enamels, as well as the possibility of the production of concrete paving flags and blocks.
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Inocente, J.M.; Nandi, V.S.; Rosso, F.; Oliveira, A.; Zaccaron, A. Study for Vitreous Waste Recovery in the Formulation of Heavy Clay Ceramics. Mater. Sci. Eng. Int. J. 2017, 1, 56–60. [Google Scholar] [CrossRef]
- Taha, Y.; Benzaazoua, M.; Hakkou, R.; Mansori, M. Coal mine wastes recycling for coal recovry and eco-friendly bricks production. Miner. Eng. 2017, 107, 123–138. [Google Scholar] [CrossRef]
- Li, X.; Zhu, Y.; Zhang, Z. An LCA-based environmental impact assessment model for construction processes. Build. Environ. 2010, 45, 766–775. [Google Scholar] [CrossRef]
- Shen, L.-Y.; Lu, W.-S.; Yao, H.; Wu, D.-H. A computer-based scoring method for measuring the environmental performance of construction activities. Autom. Constr. 2005, 14, 297–309. [Google Scholar] [CrossRef]
- Morledge, R.; Jackson, F. Reducing environmental pollution caused by construction plant. Environ. Manag. Health 2001, 12, 191–206. [Google Scholar] [CrossRef]
- Ball, J. Can ISO 14000 and eco-labelling turn the construction industry green? Build. Environ. 2002, 37, 421–428. [Google Scholar] [CrossRef]
- Lam, P.T.; Chan, E.H.; Chau, C.; Poon, C.; Chun, K. Environmental management system vs green specifications: How do they complement each other in the construction industry? J. Environ. Manag. 2011, 92, 788–795. [Google Scholar] [CrossRef]
- Francis, A.J. The Cement Industry 1796–1914: A History; David and Charles: Newton Abbot, UK, 1977. [Google Scholar]
- Mangat, P.S.; Khatib, J.M. Influence of Fly Ash, Silica Fume, and Slag on Sulfate Resistance of Concrete. ACI Mater. J. 1993, 92, 542–552. [Google Scholar] [CrossRef]
- Al-Amoudi, O.; Maslehuddin, M.; Shameem, M.; Ibrahim, M. Shrinkage of plain and silica fume cement concrete under hot weather. Cem. Concr. Compos. 2007, 29, 690–699. [Google Scholar] [CrossRef]
- Barbuta, M. Effect of different types of superplasticizer on the properties of high strength concrete incorporating large amounts of silica fume. Bull. Polytech. Inst. Iasi 2005, 51, 69–74. [Google Scholar]
- Sun, H.; Qian, J.; Yang, Y.; Fan, C.; Yue, Y. Optimization of gypsum and slag contents in blended cement containing slag. Cem. Concr. Compos. 2020, 112, 103674. [Google Scholar] [CrossRef]
- HYoon, H.N.; Seo, J.; Kim, S.; Lee, H.K.; Park, S. Characterization of blast furnace slag-blended Portland cement for immobilization of Co. Cem. Concr. Res. 2020, 134, 106089. [Google Scholar] [CrossRef]
- Lee, K.; Lee, H.; Lee, S.; Kim, G. Autogeneous shrinkage of concrete containing granulated blast furnace slag. Cem. Concr. Res. 2006, 36, 1279–1285. [Google Scholar] [CrossRef]
- Bilim, C.; Atiş, C.D.; Tanyildizi, H.; Karahan, O. Predicting the compressive strength of ground granulated blast furnace slag concrete using artificial neural network. Adv. Eng. Softw. 2009, 40, 334–340. [Google Scholar] [CrossRef]
- Nassar, R.-U.; Soroushian, P. Strength and durability of recycled aggregate concrete containing milled glass as partial replacement for cement. Constr. Build. Mater. 2012, 29, 368–377. [Google Scholar] [CrossRef]
- Islam, G.S.; Rahman, M.; Kazi, N. Waste glass powder as partial replacement of cement for sustainable concrete practice. Int. J. Sustain. Built Environ. 2017, 6, 37–44. [Google Scholar] [CrossRef]
- Du, H.; Tan, K.H. Use of waste glass as sand in mortar: Part II-Alkali-silica reaction and mitigation methods. Cem. Concr. Compos. 2013, 35, 118–126. [Google Scholar] [CrossRef]
- Topcu, I.B.; Canbaz, M. Properties of concrete containing waste glass. Cem. Concr. Res. 2004, 34, 267–274. [Google Scholar] [CrossRef]
- Dai, S.; Zhao, L.; Peng, S.; Chou, C.-L.; Wang, X.; Zhang, Y.; Li, D.; Sun, Y. Abundances and distribution of minerals and elements in high-alumina coal fly ash from the Jungar Power Plant, Inner Mongolia, China. Int. J. Coal Geol. 2010, 81, 320–332. [Google Scholar] [CrossRef]
- Wongsa, A.; Boonserm, K.; Waisurasingha, C.; Sata, V.; Chindaprasirt, P. Use of municipal solid waste incinerator (MSWI) bottom ash in high calcium fly ash geopolymer matrix. J. Clean. Prod. 2017, 148, 49–59. [Google Scholar] [CrossRef]
- Kou, S.-C.; Poon, C.-S. A novel polymer concrete made with recycled glass aggregate, fly ash and metakaolin. Constr. Build. Mater. 2013, 41, 146–151. [Google Scholar] [CrossRef]
- Ren, J.; Zhang, L.; Nicolas, R.S. San Nicolas, Degradation process of alkali-activated slag/fly ash and Portland cement-based pastes exposed to phosphoric acid. Constr. Build. Mater. 2019, 232, 117209. [Google Scholar] [CrossRef]
- Mehta, A.; Siddique, R. Sulfuric acid resistance of fly ash based geopolymer concrete. Constr. Build. Mater. 2017, 146, 136–143. [Google Scholar] [CrossRef]
- Sang, L.; Idowu, T.; Okumu, V. Evaluation of the performance of waste marble dust as a mineral filler in hot-mix asphalt concrete. J. Civ. Eng. Sci. Technol. 2021, 12, 1–14. [Google Scholar] [CrossRef]
- Gonfa, L.G.; Quezon, E.T.Q.T.; Geremew, A. Experimental study on application of marble waste as conventional aggregate for base course materials. Int. J. Civ. Eng. Technol. 2020, 11, 144–163. [Google Scholar] [CrossRef]
- Chin, W.Q.; Lee, Y.H.; Amran, M.; Fediuk, R.; Vatin, N.; Kueh, A.B.H.; Lee, Y.Y. A Sustainable Reuse of Agro-Industrial Wastes into Green Cement Bricks. Materials 2022, 15, 1713. [Google Scholar] [CrossRef]
- Kueh, A.; Razali, A.; Lee, Y.; Hamdan, S.; Yakub, I.; Suhaili, N. Acoustical and mechanical characteristics of mortars with pineapple leaf fiber and silica aerogel infills—Measurement and modeling. Mater. Today Commun. 2023, 35, 105540. [Google Scholar] [CrossRef]
- Andrew, A.I. Porcelain Enamels; Garrad Press: Champaign, IL, USA, 1961. [Google Scholar]
- Kuchinski, F.A. Corrosion resistant thick films by enamelling. In Ceramic Films and Coatings; Wachtman, J.B., Haber, R.A., Eds.; Noyes Publications: Park Ridge, NJ, USA, 1993; pp. 77–130. [Google Scholar]
- Scrinzi, E.; Rossi, S. The aesthetic and functional properties of enamel coatingson steel. J. Mater. Des. 2010, 31, 4138–4146. [Google Scholar] [CrossRef]
- Bazayants, G.V.; Svetlichnyi, V.A.; Demchuk, V.V.; Ryzhikov, V.A. Abrasive wear of glass enamels and slag sitall used in heat energetics. Glass Ceram. 1984, 40, 295–296. [Google Scholar] [CrossRef]
- Rossi, S.; Russo, F.; Calovi, M. Durability of vitreous enamel coatings and their resistance to abrasion, chemicals and corrosion: A review. J. Coat. Technol. Res. 2021, 18, 39–52. [Google Scholar] [CrossRef]
- Schäfer, S.; Annamalai, V. Degradation of Glass Linings and Coatings. In Reference Module in Materials Science and Materials Engineering; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Johnston, N.R.; Strobel, S.A. Principles of fluoride toxicity and the cellular response: A review. Arch. Toxicol. 2020, 94, 1051–1069. [Google Scholar] [CrossRef] [PubMed]
- Dietzel, A.H. Emaillierung-Wissenschaftliche Grundlagen und Grundstaze der Technologie, 1st ed.; Springer: Berlin/Heidelberg, Germany, 1981. [Google Scholar] [CrossRef]
- Rodtsevich, S.P.; Eliseev, S.Y.; Tavgen, V.V. Low-melting Chemically Resistant Enamel for Steel Kitchenware. Glass Ceram. 2003, 60, 23–25. [Google Scholar] [CrossRef]
- Nedeljković, A.; Stojmenović, M.; Gulicovski, J.; Ristić, N.; Milićević, S.; Krstić, J.; Kragović, M. Waste Slag from Heating Plants as a Partial Replacement for Cement in Mortar and Concrete Production. Part I—Physical–Chemical and Physical–Mechanical Characterization of Slag. Minerals 2020, 10, 992. [Google Scholar] [CrossRef]
- Kraus, W.; Nolze, G. POWDER CELL—A program for the representation and manipulation of crystal structures and calculation of the resulting X-ray powder patterns. J. Appl. Crystallogr. 1996, 29, 301–303. [Google Scholar] [CrossRef]
- PowderCell for Windows 2.4. Available online: http://powdercell-for-windows.software.informer.com/2.4/ (accessed on 15 February 2019).
- American Mineralogist Crystal Data Structure Base (AMCDSB). Available online: http://rruff.geo.arizona.edu/AMS/amcsd.php (accessed on 31 July 2018).
- Gregg, S.J.; Sing, K.S.W. Adsorption, Surface Area and Porosity; Academic Press: London, UK, 1982; pp. 35–120. [Google Scholar]
- Barrett, E.P.; Joyner, L.G.; Halenda, P.P. The determination of pore volume and area distributions in porous substances. I. Computations from nitrogen isotherms. J. Am. Chem. Soc. 1951, 73, 373–380. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishii, C.; Ruike, M.; Kuwabara, H. Origin of superhigh surface area and microcrystalline graphitic structures of activated carbons. Carbon 1992, 30, 1075–1088. [Google Scholar] [CrossRef]
- Kruk, M.; Jaroniec, M.; Gadkaree, K.P. Nitrogen adsorption studies of novel synthetic active carbons. J. Colloid Interface Sci. 1997, 192, 250–256. [Google Scholar] [CrossRef]
- Kaneko, K.; Ishii, C.; Kanoh, H.; Hanzawa, Y.; Setoyama, N.; Suzuki, T. Characterization of porous carbons with high resolution as-analysis and low temperature magnetic susceptibility. Adv. Colloid Interface Sci. 1998, 76–77, 295–320. [Google Scholar] [CrossRef]
- Reis, S.T.; Koenigstein, M.; Fan, L.; Chen, G.; Pavić, L.; Moguš-Milanković, A. The Effects of Silica on the Properties of Vitreous Enamels. Materials 2019, 12, 248. [Google Scholar] [CrossRef] [PubMed]
- Chi, M.; Sun, X.; Lozano-Blanco, G.; Tatarchuk, B.J. XPS and FTIR investigations of the transient photocatalytic decomposition of surface carbon contaminants from anatase TiO2 in UHV starved water/oxygen environments. J. Appl. Surf. Sci. 2021, 570, 151147. [Google Scholar] [CrossRef]
- Najam, H.M.; Yaseen, H.M. Synthesis of Sm3+ doped with TiO2 nanoparticles powder as mid-ir optical filter. MINAR Int. J. Appl. Sci. Technol. 2021, 3, 1–7. [Google Scholar] [CrossRef]
- Bullen, J.C.; Lapinee, C.; Miller, L.A.; Bullough, F.; Berry, A.J.; Najorka, J.; Cibin, G.; Vilar, R.; Weiss, D.J. Spectroscopic (XAS, FTIR) investigations into arsenic adsorption onto TiO2/Fe2O3 composites: Evaluation of the surface complexes, speciation and precipitation predicted by modelling. Res. Surf. Interfaces 2022, 9, 100084. [Google Scholar] [CrossRef]
- Azimifar, M.; Ghorbani, M.; Peyravi, M. Fabrication and evaluation of a photocatalytic membrane based on Sb2O3/CBO composite for improvement of dye removal efficiency. J. Mol. Struct. 2022, 1270, 133957. [Google Scholar] [CrossRef]
- Rosaline, D.R.; Suganthi, A.; Vinodhkumar, G.; Inbanathan, S.; Umar, A.; Ameen, S.; Akhtar, M.S.; Foletto, L.E. Enhanced sunlight-driven photocatalytic activity of SnO2-Sb2O3 composite towards emerging contaminant degradation in water. J. Alloys Compd. 2022, 897, 162935. [Google Scholar] [CrossRef]
- Cai, W.; Elveny, M.; Akhtar, M.N. Enhanced X-band wave dissipation performance in bilayer absorber composed of bare epoxy resin and epoxy resin filled with [CaTiO3/ZnFe2O4]@C nanocomposite. J. Magn. Magn. Mater. 2021, 539, 168385. [Google Scholar] [CrossRef]
- Torkzaban, S.; Feyzi, M.; Norouzi, L. A novel robust CaO/ZnFe2O4 hollow magnetic microspheres heterogenous catalyst for synthesis biodiesel from waste frying sunflower oil. Renew. Energy 2022, 200, 996–1007. [Google Scholar] [CrossRef]
- Devi, N.K.; Wareppam, B.; Singh, L.H. Effect of sintering temperature on the magnetic properties of ZnFe2O4 composite with cobaltic oxide synthesized by chemical co precipitation method. Mater. Today Proc. 2022, 68, 196–199. [Google Scholar] [CrossRef]
- Shokri, B.; Firouzjah, M.A.; Hosseini, S.I. FTIR Analysis of Silicon Dioxide Thin Film Deposited by Metal Organic-Based PECVD. In Proceedings of the 19th International Symposium on Plasma Chemistry, Bochum, Germany, 26–31 July 2009; Available online: https://www.ispc-conference.org/ispcproc/papers/791.pdf (accessed on 20 June 2023).
- Martinez, M.A.; Abenojar, J.; Bahrami, M.; Velasco, F. One-Step Enameling and Sintering of Low-Carbon Steels. Metals 2021, 11, 1007. [Google Scholar] [CrossRef]
- Grdić, D.Z.; Topličić-Ćurčić, G.A.; Grdić, Z.J.; Ristić, N.S. Durability Properties of Concrete Supplemented with Recycled CRT Glass as Cementitious Material. Materials 2021, 14, 4421. [Google Scholar] [CrossRef]
- Kragović, M.; Stojmenović, M.; Ristić, N.; Milićević, S.; Živković, S.; Liu, S.; Gulicovski, J. Application of the hazardous waste vitreous enamel generated in heating devices production process as partial replacement for cement. Buildings 2022, 12, 1287. [Google Scholar] [CrossRef]
- Neville, A.; Brooks, J.J. Concrete Technology, 2nd ed.; England Prentice Hall: Harlow, UK, 2010. [Google Scholar]



| Chemical Composition | ||||||||
|---|---|---|---|---|---|---|---|---|
| SiO2 | Fe2O3 | Al2O3 | CaO | MgO | SO3 | Na2O | K2O | LOI |
| 21.62 | 2.60 | 7.00 | 60.16 | 2.34 | 2.55 | 0.33 | 0.66 | 2.68 |
| Mineral properties | ||||||||
| brownmillerite; calcium-silicate oxide; calcite; larnite; magnesium-silicate; calcium-hydroxide | ||||||||
| Designat. of Mortar | Cement [g] | WEP [g] | WETM [g] | River Sand 0/2 mm [g] | Water [g] | Superplasticizer [g] |
|---|---|---|---|---|---|---|
| M-E | 450.00 | 0 | - | 1350.00 | 225.00 | 1.00 |
| WEP-M-10 | 405.00 | 45.00 | - | 1350.00 | 225.00 | 1.00 |
| WEP-M-20 | 360.00 | 90.00 | - | 1350.00 | 225.00 | 1.00 |
| WEP-M-30 | 315.00 | 135.00 | - | 1350.00 | 225.00 | 1.00 |
| WEP-M-40 | 270.00 | 180.00 | - | 1350.00 | 225.00 | 1.00 |
| WETM-M-7.5 | 416.25 | - | 33.75 | 1350.00 | 225.00 | 1.00 |
| WETM-M-15 | 382.50 | - | 67.50 | 1350.00 | 225.00 | 1.00 |
| WETM-M-22.5 | 348.75 | - | 101.25 | 1350.00 | 225.00 | 1.00 |
| WETM-M-30 | 315.00 | - | 135.00 | 1350.00 | 225.00 | 1.00 |
| Designat. of Concrete | Cement [kg] | WEP [kg] | WETM [kg] | River Sand 0/4 mm [kg] | Crushed Agg. 4/8 mm [kg] | Crushed Agg. 8/16 mm [kg] | Water [kg] | Superplasticizer [kg] |
|---|---|---|---|---|---|---|---|---|
| M-E | 380.0 | - | - | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WEP-C-10 | 342.0 | 38.0 | - | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WEP-C-20 | 304.0 | 76.0 | - | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WEP-C-30 | 266.0 | 114.0 | - | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WEP-C-40 | 228.0 | 152.0 | - | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WETM-C-10 | 342.0 | - | 38.0 | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WETM-C-20 | 304.0 | - | 76.0 | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| WETM-C-30 | 266.0 | - | 114.0 | 808.0 | 376.0 | 696.0 | 180.0 | 3.04 |
| Batch | Content, mg/kg | ||||||||
|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | Maximum Values for Disposal | |||||
| Parameter | WEP | WETM | WEP | WETM | WEP | WETM | WEP | WETM | |
| Mo | 449.5 | 4.5 | 439.5 | 4.5 | 399.5 | 3.5 | 387.5 | 5.5 | 10 * 30 ** |
| Hg | <0.15 | <0.15 | <0.15 | <0.15 | <0.15 | <0.15 | <0.15 | <0.15 | 0.2 * 2.0 ** |
| Sb | 38.0 | <0.5 | 35.0 | <0.5 | 39.0 | <0.5 | 42.0 | <0.5 | 0.7 * 5.0 ** |
| Se | 4.5 | 1.5 | 3.5 | 1.5 | 3.5 | 2.5 | 3.5 | 1.5 | 0.5 * 7.0 ** |
| Sr | 100.5 | 25.0 | 101.5 | 23.0 | 112.5 | 27.0 | 112.5 | 26.0 | / */ ** |
| Ba | int | 2.5 | 54.5 | 2.5 | 64.3 | 2.5 | 32.5 | 1.5 | 100 * 300 ** |
| Ca | 13,700.0 | 1550.0 | 13,720.0 | 1560.0 | 13,722.0 | 1549.0 | 13,765.0 | 1548.0 | / */ ** |
| Mg | 1315.0 | 5245.0 | 1313.0 | 5235.0 | 1333.0 | 5248.0 | 1315.0 | 5244.0 | / */ ** |
| Ti | 1200 | 71.0 | 1220 | 75.0 | 1235 | 78.0 | 1228 | 78.0 | / */ ** |
| V | 18.0 | 2.5 | 19.0 | 3.5 | 18.0 | 2.5 | 18.0 | 2.0 | / */ ** |
| Mn | 6850.0 | 8750.0 | 6840.0 | 8758.0 | 6843.0 | 8758.0 | 6885.0 | 8722.0 | / */ ** |
| Fe | 7300.0 | 26,000.0 | 7320.0 | 26,080.0 | 7322.0 | 260,256.0 | 7301.0 | 26,033.0 | / */ ** |
| Co | 4245.0 | 45.0 | 4225.0 | 46.0 | 4244.0 | 42.0 | 4248.0 | 44.0 | / */ ** |
| Cu | 3350 | 26.0 | 3251 | 20.0 | 3368 | 28.0 | 3368 | 27.0 | 50 * 100 ** |
| Zn | 127.5 | 6.5 | 128.5 | 12.5 | 123.5 | 409.5 | 122.0 | 422.5 | 50 * 200 ** |
| Ni | 150.0 | 2.0 | 151.0 | 2.0 | 155.0 | 61.0 | 151.0 | 62.0 | 10 * 40 ** |
| Cd | 2.0 | 1.5 | 2.5 | 0.5 | 3.0 | 6.5 | 2.0 | 5.5 | 1 * 5.0 ** |
| Al | 2280.0 | 1220.0 | 2281.0 | 1225.0 | 2200.0 | 1225.0 | 2266.0 | 1248.0 | / */ ** |
| Pb | 26.5 | 10.0 | 23.5 | 9.0 | 22.5 | 10.0 | 25.0 | 10.0 | 10 * 50 ** |
| As | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | <0.5 | 2 * 25 ** |
| Be | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | <0.05 | / */ ** |
| Cr | 3.5 | 11.2 | 3.5 | 13.5 | 3.5 | 12.2 | 5.5 | 10.2 | 10 * 70 ** |
| Tl | 54.5 | 73.5 | 52.0 | 75.5 | 50.5 | 70.5 | 49.5 | 68.5 | / */ ** |
| Sn | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | <1.0 | / */ ** |
| Sample | WEP | WEMT | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Batch | Sp, m2/g | Vtotal, cm3/g | Vmeso, cm3/g | Vmicro, cm3/g | Dsr, nm | Dmax *, nm | Sp, m2/g | Vtotal, cm3/g | Vmeso, cm3/g | Vmicro, cm3/g | Dsr, nm | Dmax *, nm |
| 1 | 8.75 | 0.0818 | 0.0633 | 0.0185 | 10.87 | 2.48 | 6.08 | 0.0040 | 0.0002 | 0.0038 | 6.49 | 2.32 |
| 2 | 7.8 | 0.0816 | 0.0562 | 0.0254 | 9.92 | 2.53 | 7.06 | 0.0057 | 0.0003 | 0.0054 | 7.03 | 2.36 |
| 3 | 22.3 | 0.0708 | 0.0682 | 0.0026 | 9.80 | 2.25 | 20.08 | 0.0689 | 0.0622 | 0.0067 | 9.33 | 2.36 |
| 4 | 8.92 | 0.0816 | 0.0632 | 0.0184 | 10.95 | 2.59 | 6.23 | 0.0060 | 0.0002 | 0.0058 | 6.57 | 2.15 |
| Property | Parameters/Results | Requirement | |||
|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | ||
| Class of pozzolanic materials | Flexural strength: 4.5 mPa Comp. strength: 17.2 mPa | Flexural strength: 4.1 mPa Comp. strength: 16.8 mPa | Flexural strength: 2.1 mPa Comp. strength: 7.0 mPa | Flexural strength: 2.1 mPa Comp. strength: 7.4 mPa | >2.0/4.0 mPa (class 5/15) >5.0/15.0 mPa (class 5/15) |
| Activity index | After 28 days: 80.80% After 90 days—87.48% | After 28 days: 77.62% After 90 days—87.49% | After 28 days: 75.24% After 90 days—85.74% | After 28 days: 76.24% After 90 days—86.28% | >75% >85% |
| Water requirement | 93% | 94% | 93% | 94% | <95% |
| Standard consistency | 29.5% | 29.5% | 30% | 29.5% | not prescribed |
| Initial setting time Final setting time | 135 min 160 min | 145 min 180 min | 150 min 180 min | 140 min 170 min | <230 min not prescribed |
| Roundness | 1.0 mm | 1.0 mm | 1.0 mm | 1.0 mm | <10 mm |
| Property | Parameters/Results | Requirement | |||
|---|---|---|---|---|---|
| Batch 1 | Batch 2 | Batch 3 | Batch 4 | ||
| Class of pozzolanic materials | Flexural strength: 2.8 mPa Comp. strength: 8.4 mPa | Flexural strength: 2.1 mPa Comp. strength: 7.0 mPa | Flexural strength: 2.1 mPa Comp. strength: 6.9 mPa | Flexural strength: 2.2 mPa Comp. strength: 7.2 mPa | >2.0/4.0 mPa (class 5/15) >5.0/15.0 mPa (class 5/15) |
| Activity index | After 28 days: 73.45% After 90 days—85.10% | After 28 days: 78.62% After 90 days—89.29% | After 28 days: 75.86% After 90 days—86.13% | After 28 days: 76.78% After 90 days—87.08% | >75% >85% |
| Water requirement | 98% | 97% | 100% | 98% | <95% |
| Standard consistence | 31.0% | 30.5% | 31.5% | 31.0% | not prescribed |
| Initial setting time Final setting time | 175 min 200 min | 165 min 190 min | 185 min 210 min | 170 min 200 min | <230 min not prescribed |
| Soundness | 0.5 mm | 0.5 mm | 0.5 mm | 0.5 mm | <10 mm |
| Property | Unit | M-E | WEP-M-10 | WEP-M-20 | WEP-M-30 | WEP-M-40 |
|---|---|---|---|---|---|---|
| Consistency—by flow table | mm | 135 ± 2.0 | 141 ± 3.0 | 137 ± 2.5 | 130 ± 2.0 | 125 ± 3.0 |
| Bulk density of fresh mortar | kg/m3 | 2299 ± 8 | 2295 ± 7 | 2288 ± 6 | 2279 ± 8 | 2270 ± 9 |
| Bulk density of hardened mortar | kg/m3 | 2294 ± 7 | 2290 ± 9 | 2283 ± 7 | 2276 ± 6 | 2265 ± 8 |
| Water abs. at atm. pressure | % | 7.54 ± 0.12 | 7.70 ± 0.10 | 7.81 ± 0.08 | 7.91 ± 0.11 | 7.99 ± 0.10 |
| Property | Unit | M-E | WETM-M-7.5 | WETM-M-15 | WETM-M-22.5 | WETM-M-30 |
|---|---|---|---|---|---|---|
| Consistency—by flow table | mm | 135 ± 2.0 | 137 ± 2.5 | 143 ± 3.5 | 134 ± 3.0 | 125 ± 2.5 |
| Bulk density of fresh mortar | kg/m3 | 2299 ± 8 | 2301 ± 6 | 2285 ± 9 | 2272 ± 7 | 2265 ± 8 |
| Bulk density of hardened mortar | kg/m3 | 2294 ± 7 | 2297 ± 7 | 2281 ± 8 | 2268 ± 9 | 2260 ± 7 |
| Water abs. at atm. pressure | % | 7.54 ± 0.12 | 7.66 ± 0.11 | 7.75 ± 0.10 | 7.84 ± 0.12 | 7.94 ± 0.09 |
| Designation of Mortar | M-E | WEP-M-10 | WEP-M-20 | WEP-M-30 | WEP-M-40 |
|---|---|---|---|---|---|
| Age [Days] | |||||
| 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 0.25 ± 0.02 | 0.25 ± 0.02 | 0.24 ± 0.02 | 0.23 ± 0.02 | 0.23 ± 0.02 |
| 7 | 0.38 ± 0.03 | 0.36 ± 0.03 | 0.34 ± 0.01 | 0.31 ± 0.02 | 0.30 ± 0.03 |
| 14 | 0.59 ± 0.01 | 0.59 ± 0.03 | 0.60 ± 0.03 | 0.60 ± 0.03 | 0.61 ± 0.01 |
| 21 | 0.69 ± 0.03 | 0.73 ± 0.02 | 0.78 ± 0.02 | 0.82 ± 0.02 | 0.85 ± 0.02 |
| 28 | 0.91 ± 0.02 | 0.93 ± 0.02 | 0.97 ± 0.03 | 0.99 ± 0.03 | 1.02 ± 0.03 |
| 56 | 0.93 ± 0.02 | 0.94 ± 0.01 | 0.99 ± 0.01 | 1.01 ± 0.02 | 1.04 ± 0.02 |
| 90 | 0.94 ± 0.03 | 0.95 ± 0.03 | 1.01 ± 0.02 | 1.03 ± 0.01 | 1.06 ± 0.02 |
| Designation of Mortar | M-E | WETM-M-7.5 | WETM-M-15 | WETM-M-22.5 | WETM-M-30 |
|---|---|---|---|---|---|
| Age [Days] | |||||
| 3 | 0.00 | 0.00 | 0.00 | 0.00 | 0.00 |
| 4 | 0.25 ± 0.02 | 0.23 ± 0.02 | 0.20 ± 0.03 | 0.18 ± 0.02 | 0.17 ± 0.03 |
| 7 | 0.38 ± 0.03 | 0.37 ± 0.03 | 0.34 ± 0.02 | 0.32 ± 0.01 | 0.30 ± 0.01 |
| 14 | 0.59 ± 0.01 | 0.58 ± 0.03 | 0.58 ± 0.01 | 0.56 ± 0.03 | 0.55 ± 0.03 |
| 21 | 0.69 ± 0.03 | 0.71 ± 0.02 | 0.73 ± 0.02 | 0.74 ± 0.02 | 0.76 ± 0.02 |
| 28 | 0.91 ± 0.02 | 0.92 ± 0.02 | 0.93 ± 0.03 | 0.93 ± 0.01 | 0.94 ± 0.03 |
| 56 | 0.93 ± 0.02 | 0.94 ± 0.01 | 0.96 ± 0.02 | 0.96 ± 0.03 | 0.97 ± 0.02 |
| 90 | 0.94 ± 0.03 | 0.96 ± 0.02 | 0.99 ± 0.01 | 0.99 ± 0.02 | 1.01 ± 0.02 |
| Property | Unit | C-E | WEP-C-10 | WEP-C-20 | WEP-C-30 | WEP-C-40 |
|---|---|---|---|---|---|---|
| Consistency—slump test | mm | 200 ± 10 | 210 ± 11 | 220 ± 10 | 180 ± 10 | 170 ± 12 |
| Density of fresh concrete | kg/m3 | 2466 ± 12 | 2452 ± 0 | 2490 ± 12 | 2488 ± 11 | 2492 ± 11 |
| Air content in fresh concrete | % | 2.6 ± 0.19 | 2.0 ± 0.15 | 1.8 ± 0.17 | 1.7 ± 0.16 | 1.5 ± 0.18 |
| Density of hardened concrete (water saturated) | kg/m3 | 2455 ± 10 | 2442 ± 11 | 2483 ± 10 | 2482 ± 12 | 2485 ± 11 |
| Determination of ultrasonic pulse velocity | km/s | 5.21 ± 0.022 | 5.15 ± 0.015 | 5.21 ± 0.017 | 5.19 ± 0.018 | 5.19 ± 0.016 |
| Property | Unit | WETM-C-E | WETM-M-10 | WETM-C-20 | WETM-C-30 |
|---|---|---|---|---|---|
| Consistency—slump test | mm | 200 ± 10 | 160 ± 11 | 110 ± 9 | 100 ± 10 |
| Density of fresh concrete | kg/m3 | 2466 ± 12 | 2488 ± 10 | 2479 ± 9 | 2455 ± 11 |
| Air content in fresh concrete | % | 2.6 ± 0.19 | 2.7 ± 0.17 | 2.5 ± 0.15 | 2.3 ± 0.16 |
| Density of hardened concrete (water saturated) | kg/m3 | 2455 ± 10 | 2484 ± 11 | 2471 ± 12 | 2447 ± 10 |
| Determination of ultrasonic pulse velocity | km/s | 5.21 ± 0.022 | 5.19 ± 0.021 | 5.16 ± 0.017 | 5.12 ± 0.016 |
| Property | Unit | C-E | WEP-C-10 | WEP-C-20 | WEP-C-30 | WEP-C-40 | |
|---|---|---|---|---|---|---|---|
| Flexural strength | 28 days | mPa | 7.0 ± 0.2 7.4 ± 0.3 | 5.1 ± 0.3 5.5 ± 0.3 | 4.9 ± 0.2 5.4 ± 0.1 | 4.7 ± 0.2 5.1 ± 0.3 | 4.6 ± 0.2 5.0 ± 0.2 |
| 90 days | |||||||
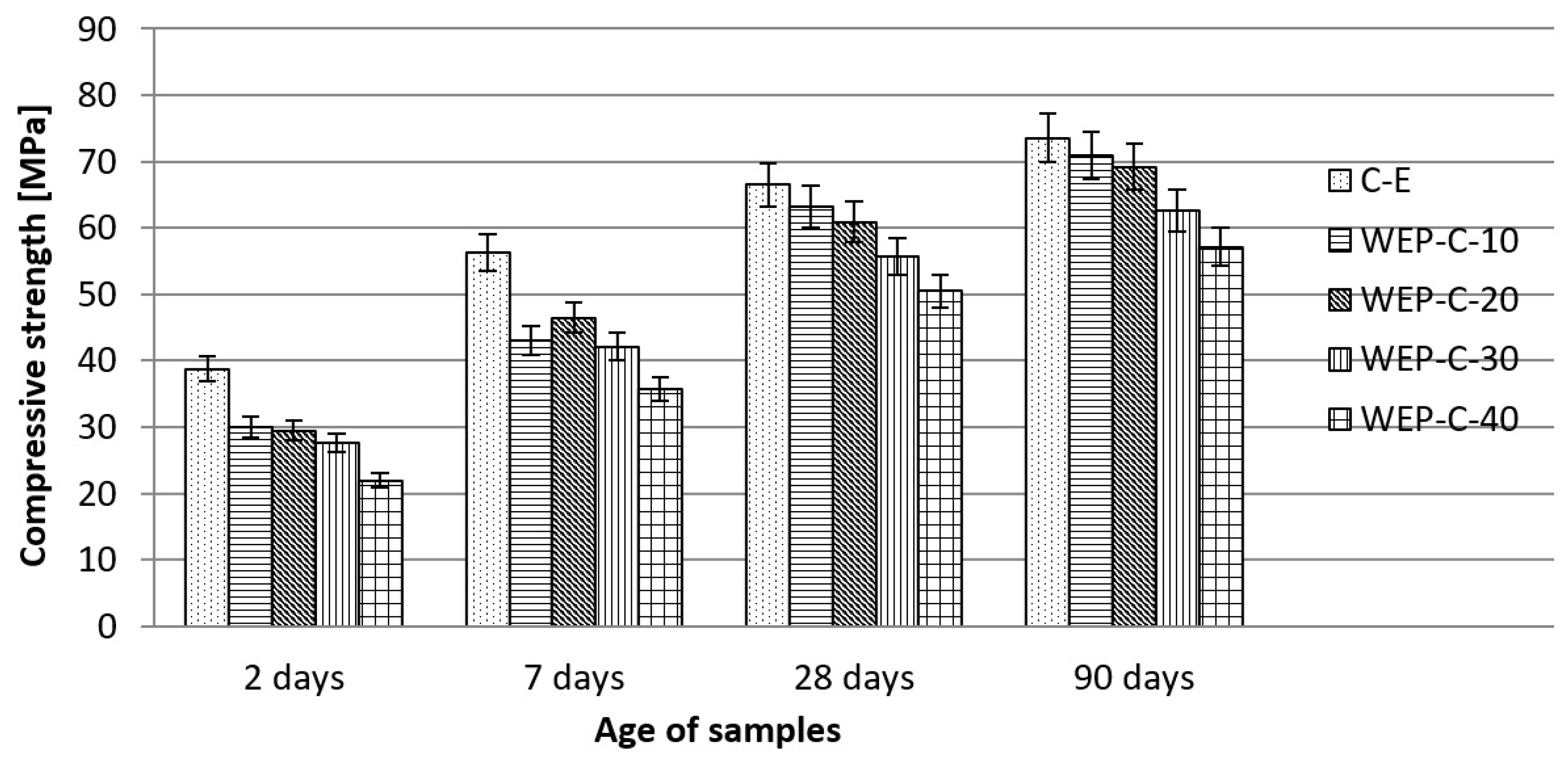
| Compressive strength | mPa | See Figure 11 | |||||
| Tensile splitting strength | 28 days | mPa | 3.9 ± 0.2 | 3.8 ± 0.3 | 3.7 ± 0.2 | 3.5 ± 0.3 | 3.2 ± 0.2 |
| Secant modulus of elasticity | 28 days | gPa | 33.0 ± 0.3 | 34.2 ± 0.3 | 34.9 ± 0.2 | 33.5 ± 0.2 | 32.7 ± 0.3 |
| Dept of penetration of water under pressure | mm | 12 ± 2 | 10 ± 2 | 14 ± 3 | 12 ± 2 | 13 ± 3 | |
| Freeze–thaw resistance with de-icing salts—Scaling | kg/m2 | 0.14 ± 0.02 | 0.16 ± 0.03 | 0.12 ± 0.03 | 0.15 ± 0.02 | 0.18 ± 0.03 | |
| Property | Unit | WETM-C-E | WETM-C-10 | WETM-C-20 | WETM-C-30 | |
|---|---|---|---|---|---|---|
| Flexural strength | 28 days | mPa | 7.0 ± 0.2 7.4 ± 0.3 | 6.3 ± 0.1 6.7 ± 0.2 | 5.9 ± 0.3 6.5 ± 0.223 | 5.7 ± 0.2 6.2 ± 0.3 |
| 90 days | ||||||
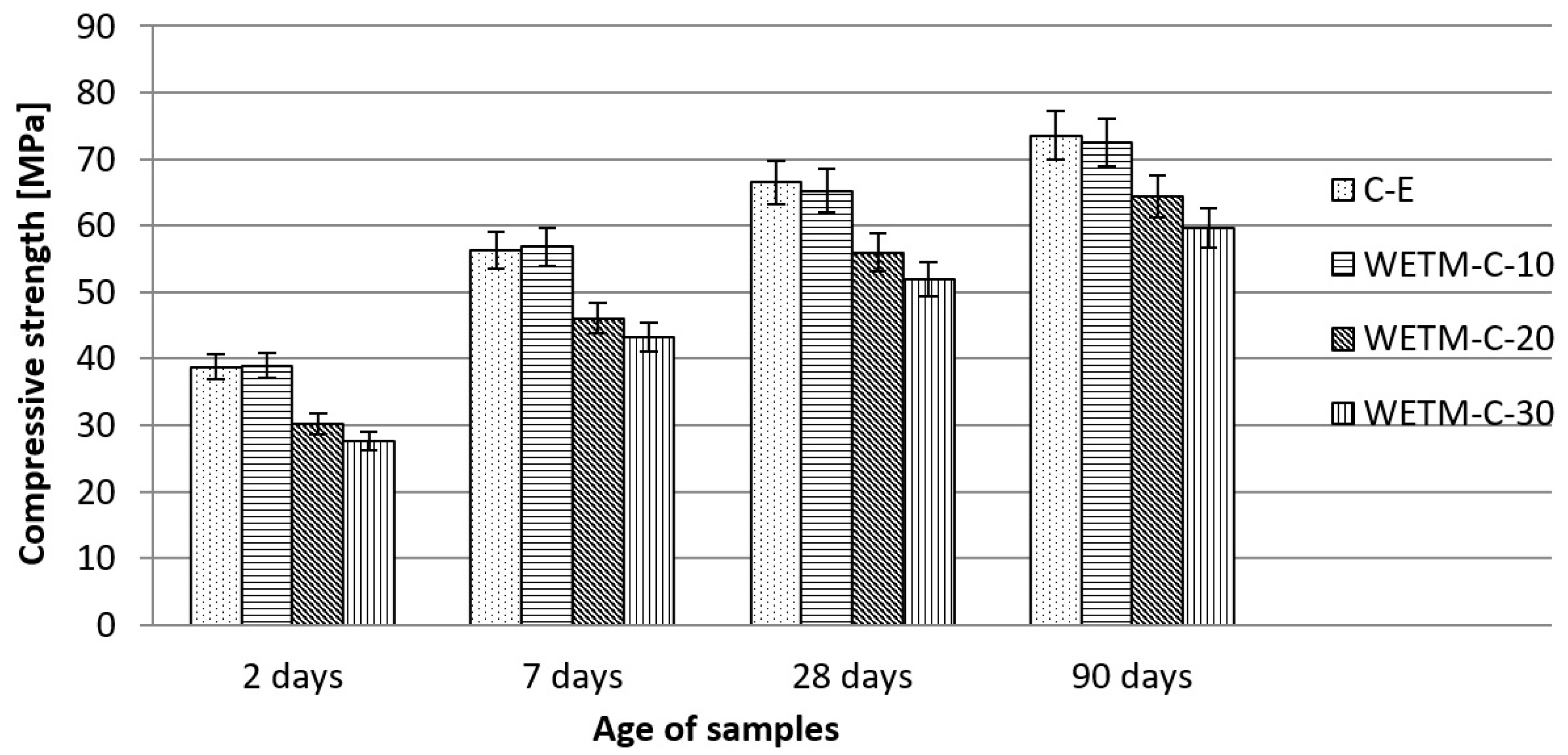
| Compressive strength | mPa | See Figure 12 | ||||
| Tensile splitting strength | 28 days | mPa | 3.9 ± 0.2 | 3.8 ± 0.3 | 3.6 ±0.2 | 3.5 ± 0.2 |
| Secant modulus of elasticity | 28 days | gPa | 33.0 ± 0.3 | 32.6 ± 0.3 | 32.3 ± 0.3 | 31.8 ± 0.2 |
| Dept of penetration of water under pressure | mm | 12 ± 2 | 14 ± 2 | 15 ± 3 | 17 ± 3 | |
| Freeze–thaw resistance with de-icing salts—Scaling | kg/m2 | 0.14 ± 0.02 | 0.14 ± 0.03 | 0.16 ± 0.02 | 0.19 ± 0.03 | |
| Released Element | Concentration, µg/dm3 | * Allowed Values, µg/dm3 | |
|---|---|---|---|
| WEP | WETM | ||
| Cu | 34 | 5 | <100 |
| Zn | 21 | 15 | <1000 |
| Ni | 10 | 20 | <100 |
| Cd | 1 | 1 | <10 |
| Pb | 20 | 30 | <100 |
| Cr | 7 | 2 | <500 |
| Hg | 48 | 4 | <1 |
| As | 22 | 31 | <50 |
| Mg | 8 | 5 | - |
| Fe | 1270 | 31,000 | - |
| Co | <10 | <10 | - |
| Al | 2280 | 544 | - |
| Sn | 698 | 5 | - |
| Si | 470 | 420 | - |
| Mo | 1 | <1 | - |
| Sr | 600 | 140 | - |
| Ca | 100 | 400 | - |
| Mn | <1 | <1 | - |
| V | 10 | <10 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gulicovski, J.; Kragović, M.; Nikolić, K.; Rosić, M.; Ristić, N.; Janković-Častvan, I.; Stojmenović, M. Possibility of Using Vitreous Enamel Waste in the Construction Industry as the Concept of Cleaner Production. Appl. Sci. 2023, 13, 8215. https://doi.org/10.3390/app13148215
Gulicovski J, Kragović M, Nikolić K, Rosić M, Ristić N, Janković-Častvan I, Stojmenović M. Possibility of Using Vitreous Enamel Waste in the Construction Industry as the Concept of Cleaner Production. Applied Sciences. 2023; 13(14):8215. https://doi.org/10.3390/app13148215
Chicago/Turabian StyleGulicovski, Jelena, Milan Kragović, Katarina Nikolić, Milena Rosić, Nenad Ristić, Ivona Janković-Častvan, and Marija Stojmenović. 2023. "Possibility of Using Vitreous Enamel Waste in the Construction Industry as the Concept of Cleaner Production" Applied Sciences 13, no. 14: 8215. https://doi.org/10.3390/app13148215
APA StyleGulicovski, J., Kragović, M., Nikolić, K., Rosić, M., Ristić, N., Janković-Častvan, I., & Stojmenović, M. (2023). Possibility of Using Vitreous Enamel Waste in the Construction Industry as the Concept of Cleaner Production. Applied Sciences, 13(14), 8215. https://doi.org/10.3390/app13148215









