From Seeing to Knowing with Artificial Intelligence: A Scoping Review of Point-of-Care Ultrasound in Low-Resource Settings
Abstract
1. Introduction
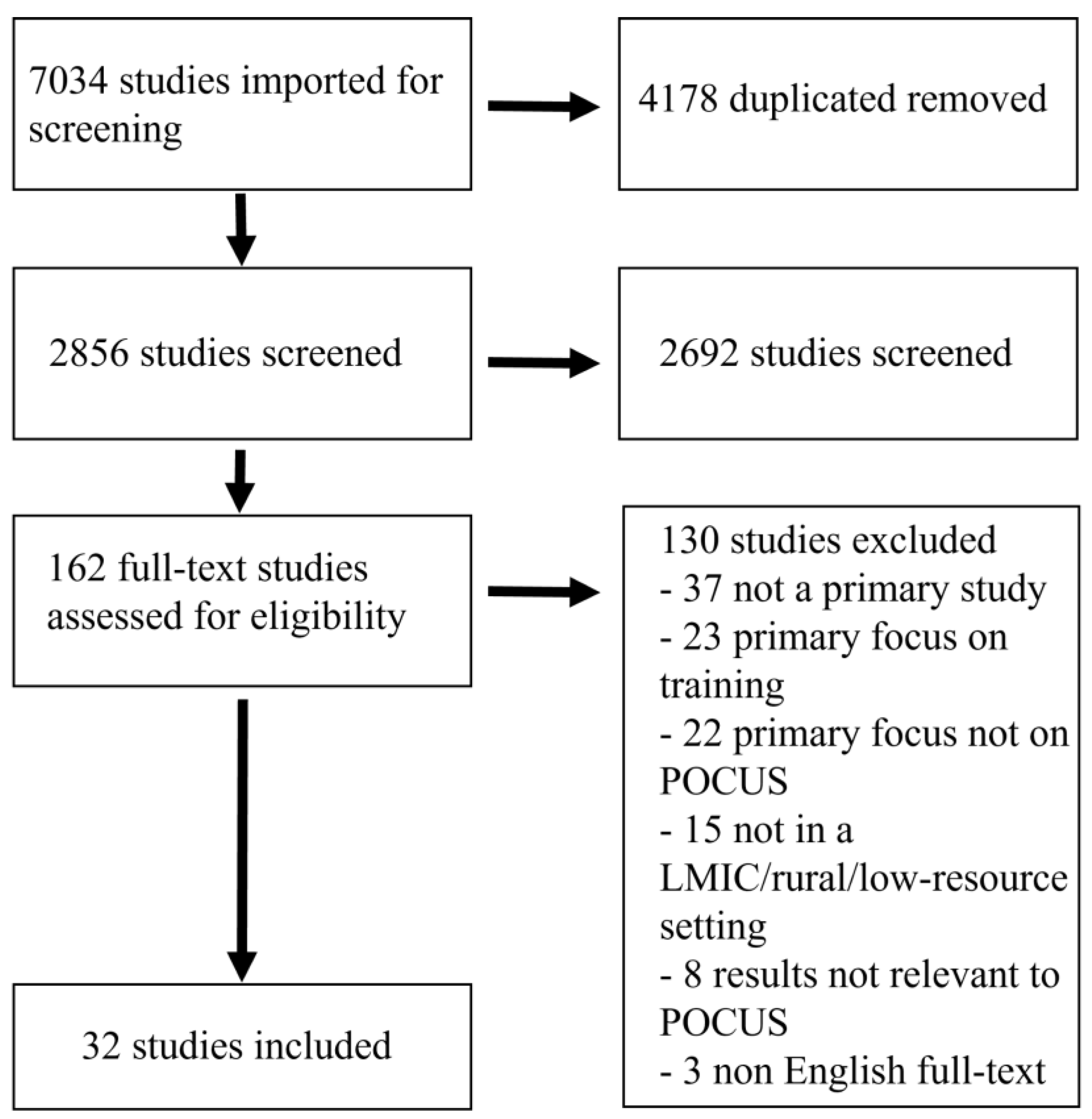
2. Materials and Methods
3. Results
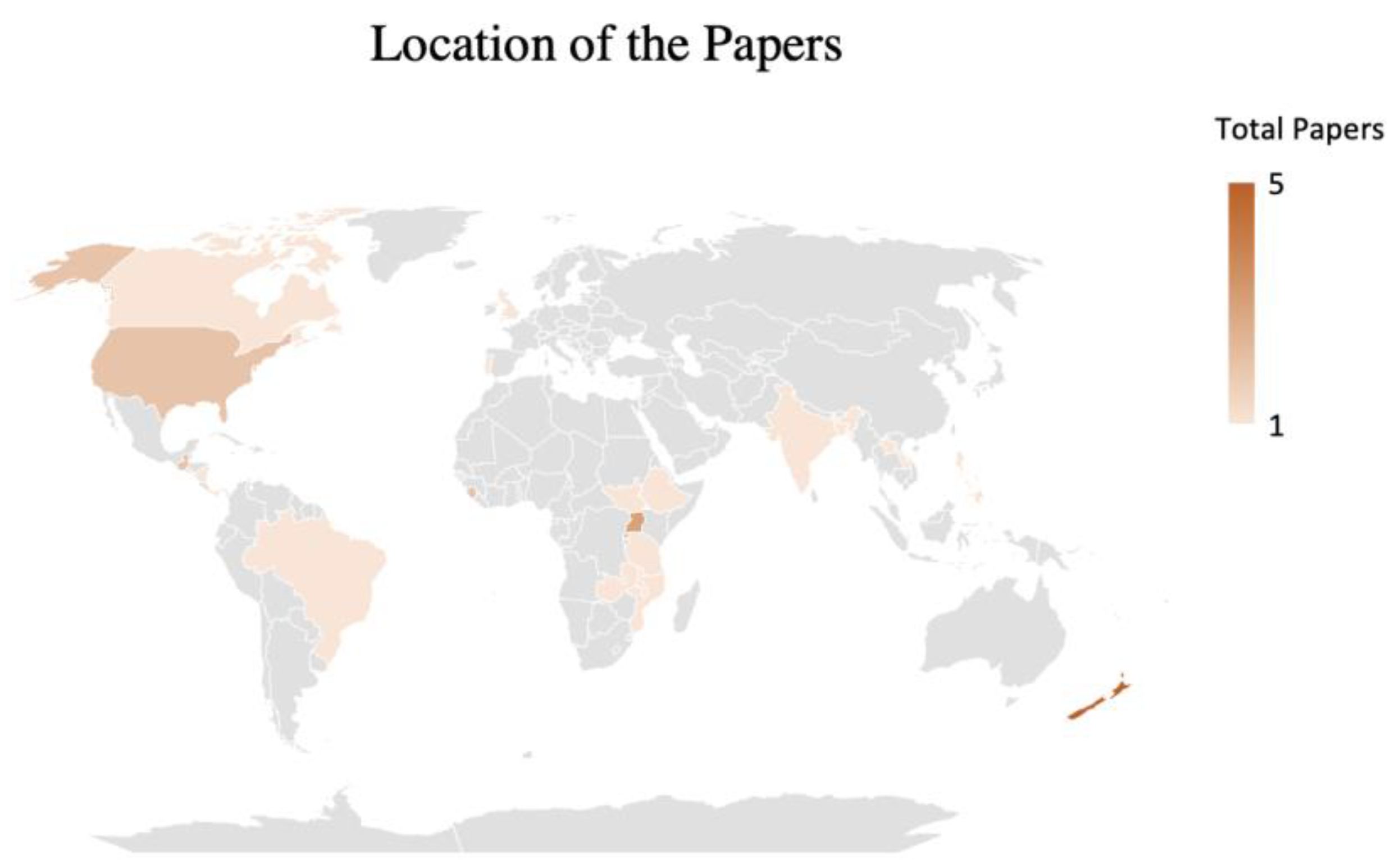
4. Setting and Context
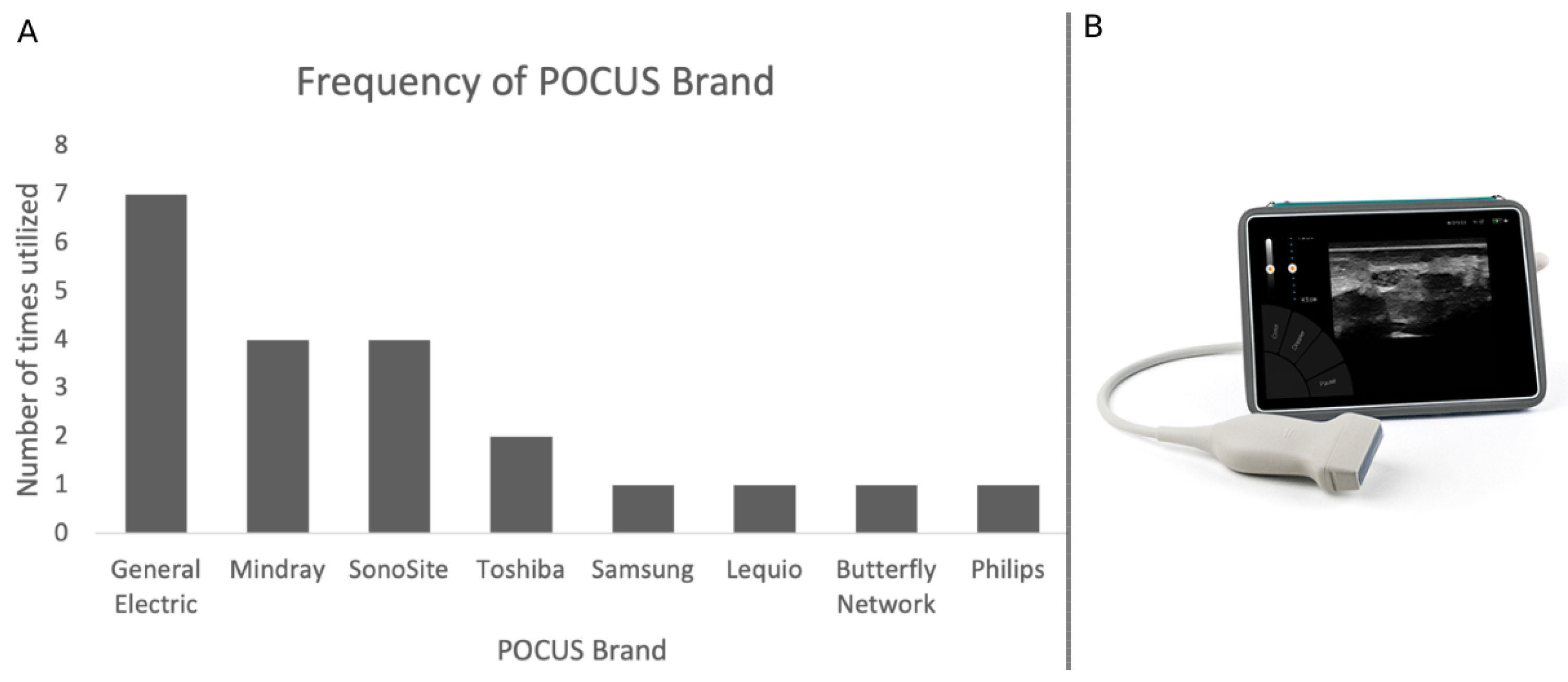
5. Types of Devices
6. Clinical Applications of POCUS
7. Outcomes Measured
8. Discussion
8.1. Clinical Applications of POCUS in LRS
8.2. Implementation of POCUS in LRS and Barriers
8.3. Potentials and the Integration of AI-Enhanced POCUS in LRS
8.4. Future Perspectives and Research Opportunities
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Newman, P.G.; Rozycki, G.S. The History of Ultrasound. Surg. Clin. N. Am. 1998, 78, 179–195. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-M.; Park, S.-K.; Park, S.-G. A Study on the Performance Evaluation Criteria and Methods of Abdominal Ultrasound Devices Based on International Standards. Safety 2021, 7, 31. [Google Scholar] [CrossRef]
- Carovac, A.; Smajlovic, F.; Junuzovic, D. Application of Ultrasound in Medicine. Acta Inform. Med. 2011, 19, 168. [Google Scholar] [CrossRef] [PubMed]
- Hertzberg, B.S.; Kliewer, M.A.; Bowie, J.D.; Carroll, B.A.; DeLong, D.H.; Gray, L.; Nelson, R.C. Physician Training Requirements in Sonography: How Many Cases Are Needed for Competence? Am. J. Roentgenol. 2000, 174, 1221–1227. [Google Scholar] [CrossRef]
- Gurara, D.; Klyuev, V.; Mwase, N.; Presbitero, A.; Xu, X.C.; Bannister, M.G.J. Trends and Challenges in Infrastructure Investment in Low-Income Developing Countries. IMF Work. Pap. 2017, 17, 1. [Google Scholar] [CrossRef]
- Le, M.-P.T.; Voigt, L.; Nathanson, R.; Maw, A.M.; Johnson, G.; Dancel, R.; Mathews, B.; Moreira, A.; Sauthoff, H.; Gelabert, C.; et al. Comparison of four handheld point-of-care ultrasound devices by expert users. Ultrasound J. 2022, 14, 27. [Google Scholar] [CrossRef]
- Marik, P.E.; Linde-Zwirble, W.T.; Bittner, E.A.; Sahatjian, J.; Hansell, D. Fluid administration in severe sepsis and septic shock, patterns and outcomes: An analysis of a large national database. Intensive Care Med. 2017, 43, 625–632. [Google Scholar] [CrossRef]
- Atkinson, P.; Bowra, J.; Milne, J.; Lewis, D.; Lambert, M.; Jarman, B.; Noble, V.E.; Lamprecht, H.; Harris, T.; Connolly, J.; et al. International Federation for Emergency Medicine Consensus Statement: Sonography in hypotension and cardiac arrest (SHoC): An international consensus on the use of point of care ultrasound for undifferentiated hypotension and during cardiac arrest. Can. J. Emerg. Med. 2017, 19, 459–470. [Google Scholar] [CrossRef]
- Long, B.; Alerhand, S.; Maliel, K.; Koyfman, A. Echocardiography in cardiac arrest: An emergency medicine review. Am. J. Emerg. Med. 2018, 36, 488–493. [Google Scholar] [CrossRef]
- Smallwood, N.; Dachsel, M. Point-of-care ultrasound (POCUS): Unnecessary gadgetry or evidence-based medicine? Clin. Med. 2018, 18, 219–224. [Google Scholar] [CrossRef]
- Seif, D.; Perera, P.; Mailhot, T.; Riley, D.; Mandavia, D. Bedside Ultrasound in Resuscitation and the Rapid Ultrasound in Shock Protocol. Crit. Care Res. Pract. 2012, 2012, 503254. [Google Scholar] [CrossRef] [PubMed]
- Bidner, A.; Bezak, E.; Parange, N. Evaluation of antenatal Point-of-Care Ultrasound (PoCUS) training: A systematic review. Med. Educ. Online 2022, 27, 2041366. [Google Scholar] [CrossRef] [PubMed]
- Meinen, R.D.; Bauer, A.S.; Devous, K.; Cowan, E. Point-of-care ultrasound use in umbilical line placement: A review. J. Perinatol. 2020, 40, 560–566. [Google Scholar] [CrossRef]
- Shaddock, L.; Smith, T. Potential for Use of Portable Ultrasound Devices in Rural and Remote Settings in Australia and Other Developed Countries: A Systematic Review. J. Multidiscip. Healthc. 2022, 15, 605–625. [Google Scholar] [CrossRef] [PubMed]
- Blanco, P.; Buendía, C.M. Point-of-care ultrasound in cardiopulmonary resuscitation: A concise review. J. Ultrasound 2017, 20, 193–198. [Google Scholar] [CrossRef] [PubMed]
- Andersen, C.A.; Holden, S.; Vela, J.; Rathleff, M.S.; Jensen, M.B. Point-of-Care Ultrasound in General Practice: A Systematic Review. Ann. Fam. Med. 2019, 17, 61–69. [Google Scholar] [CrossRef]
- Kok, B.; Wolthuis, D.; Bosch, F.; van der Hoeven, H.; Blans, M. POCUS in dyspnea, nontraumatic hypotension, and shock; a systematic review of existing evidence. Eur. J. Intern. Med. 2022, 106, 9–38. [Google Scholar] [CrossRef]
- World Health Organization. Increasing Access to Health Workers in Remote and Rural Areas through Improved Retention: Global Policy Recommendations; World Health Organization: Geneva, Switzerland, 2010; Volume 71. [Google Scholar]
- Micks, T.; Sue, K.; Rogers, P. Barriers to point-of-care ultrasound use in rural emergency departments. Can. J. Emerg. Med. 2016, 18, 475–479. [Google Scholar] [CrossRef]
- Hershman, M.; Hershman, M.; Blain, Y.; Moise, M.E.; Monchil, N.; Schmit, B.P. Experience with an ultrasound donation program in a low-income country. J. Glob. Radiol. 2021, 7, 1101. [Google Scholar] [CrossRef]
- Ienghong, K.; Cheung, L.W.; Tiamkao, S.; Bhudhisawasdi, V.; Apiratwarakul, K. Development and Remodeling of Point-of-Care Ultrasound Education for Emergency Medicine Residents in Resource Limited Countries during the COVID-19 Pandemic. Tomography 2021, 7, 721–733. [Google Scholar] [CrossRef]
- Sepulveda-Ortiz, V.; Warkentine, F.; Starr-Seal, R.; Rominger, A. The effectiveness of a longitudinal ultrasound curriculum for general pediatricians working in a Puerto Rican emergency department: A pilot study. Ultrasound J. 2020, 12, 20. [Google Scholar] [CrossRef] [PubMed]
- Self, A.; Chen, Q.; Desiraju, B.K.; Dhariwal, S.; Gleed, A.D.; Mishra, D.; Thiruvengadam, R.; Chandramohan, V.; Craik, R.; Wilden, E.; et al. Developing Clinical Artificial Intelligence for Obstetric Ultrasound to Improve Access in Underserved Regions: Protocol for a Computer-Assisted Low-Cost Point-of-Care UltraSound (CALOPUS) Study. JMIR Res. Protoc. 2022, 11, e37374. [Google Scholar] [CrossRef] [PubMed]
- Shokoohi, H.; LeSaux, M.A.; Roohani, Y.H.; Liteplo, A.; Huang, C.; Blaivas, M. Enhanced Point-of-Care Ultrasound Applications by Integrating Automated Feature-Learning Systems Using Deep Learning. J. Ultrasound Med. 2019, 38, 1887–1897. [Google Scholar] [CrossRef]
- Recker, F.; Höhne, E.; Damjanovic, D.; Schäfer, V.S. Ultrasound in Telemedicine: A Brief Overview. Appl. Sci. 2022, 12, 958. [Google Scholar] [CrossRef]
- Huang, Q.; Zhang, F.; Li, X. Machine Learning in Ultrasound Computer-Aided Diagnostic Systems: A Survey. BioMed Res. Int. 2018, 2018, 5137904. [Google Scholar] [CrossRef] [PubMed]
- Brattain, L.J.; Telfer, B.A.; Dhyani, M.; Grajo, J.R.; Samir, A.E. Machine learning for medical ultrasound: Status, methods, and future opportunities. Abdom. Radiol. 2018, 43, 786–799. [Google Scholar] [CrossRef]
- Doig, M.; Dizon, J.; Guerrero, K.; Parange, N. Exploring the availability and impact of antenatal point-of-care ultrasound services in rural and remote communities: A scoping review. Australas. J. Ultrasound Med. 2019, 22, 174–185. [Google Scholar] [CrossRef]
- Buonsenso, D.; De Rose, C. Implementation of lung ultrasound in low- to middle-income countries: A new challenge global health? Eur. J. Pediatr. 2022, 181, 447–452. [Google Scholar] [CrossRef]
- Baloescu, C.; Parhar, A.; Liu, R.; Wanjiku, G.W. Effect of Point-of-Care Ultrasound on Clinical Outcomes in Low-Resource Settings: A Systematic Review. Ultrasound Med. Biol. 2022, 48, 1711–1719. [Google Scholar] [CrossRef]
- Cherniak, W.; Anguyo, G.; Meaney, C.; Kong, L.Y.; Malhame, I.; Pace, R.; Sodhi, S.; Silverman, M. Effectiveness of advertising availability of prenatal ultrasound on uptake of antenatal care in rural Uganda: A cluster randomized trial. PLoS ONE 2017, 12, e0175440. [Google Scholar] [CrossRef]
- Baker, D.E.; Nolting, L.; Brown, H.A. Impact of point-of-care ultrasound on the diagnosis and treatment of patients in rural Uganda. Trop. Dr. 2021, 51, 291–296. [Google Scholar] [CrossRef] [PubMed]
- Barron, K.R.; Lai, J.C.; MenkinSmith, L.P.; Lee, J.S.; Humphrey, M.E.; Hall, J.W. Point-of-Care Ultrasound as Part of a Short-Term Medical Mission to Rural Nicaragua. South. Med. J. 2018, 111, 434–438. [Google Scholar] [CrossRef]
- Bobbio, F.; Di Gennaro, F.; Marotta, C.; Kok, J.; Akec, G.; Norbis, L.; Monno, L.; Saracino, A.; Mazzucco, W.; Lunardi, M. Focused ultrasound to diagnose HIV-associated tuberculosis (FASH) in the extremely resource-limited setting of South Sudan: A cross-sectional study. BMJ Open 2019, 9, e027179. [Google Scholar] [CrossRef] [PubMed]
- Milart, P.H.C.; Molina, C.A.D.; Prieto-Egido, I.; Martínez-Fernández, A. Use of a portable system with ultrasound and blood tests to improve prenatal controls in rural Guatemala. Reprod. Health 2016, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Dalmacion, G.V.; Reyles, R.T.; Habana, A.E.; Cruz, L.M.V.; Chua, M.C.; Ngo, A.T.; Tia-Jocson, M.J.; Baja, E.S. Handheld ultrasound to avert maternal and neonatal deaths in 2 regions of the Philippines: An iBuntis® intervention study. BMC Pregnancy Childbirth 2018, 18, 32. [Google Scholar] [CrossRef]
- Elsayes, A.; Rohren, S.; Islam, N.; Blair, K.; Silvestre, A.; Segura, D.; Dubinsky, T. Role of portable ultrasound during a short-term medical service trip to rural Guatemala: A collaborative mission of trainees and physicians. Rural. Remote Health 2021, 21, 6056. [Google Scholar] [CrossRef]
- Epstein, D.; Petersiel, N.; Klein, E.; Marcusohn, E.; Aviran, E.; Harel, R.; Azzam, Z.S.; Neuberger, A.; Fuchs, L. Pocket-size point-of-care ultrasound in rural Uganda—A unique opportunity “to see”, where no imaging facilities are available. Travel Med. Infect. Dis. 2018, 23, 87–93. [Google Scholar] [CrossRef]
- Gomes, D.J.; Kaufman, B.; Aluisio, A.R.; Kendall, S.; Thomas, V.; Bloem, C. Assessment of Acute Obstetrical Needs and the Potential Utility of Point-Of-Care Ultrasound in the North East Region of Haiti: A Cross-Sectional Study. Ann. Glob. Health 2020, 86, 72. [Google Scholar] [CrossRef]
- Haldeman, M.S.; Kunka, E.; Makasa, M.; Birkland, B. Resident perception on the impact of point-of-care ultrasound in clinical care at a family medicine training program in Zambia. Ultrasound J. 2022, 14, 18. [Google Scholar] [CrossRef]
- Huson, M.A.M.; Kling, K.; Chankongsin, S.; Phongluxa, K.; Keoluangkhot, V.; Newton, P.N.; Dance, D.; Heller, T.; Neumayr, A. Point-of-Care Ultrasound in the Diagnosis of Melioidosis in Laos. Am. J. Trop. Med. Hyg. 2020, 103, 675–678. [Google Scholar] [CrossRef]
- Kodaira, Y.; Pisani, L.; Boyle, S.; Olumide, S.; Orsi, M.; Adeniji, A.O.; Pisani, E.; Zanette, M.; Putoto, G.; Koroma, M.M. Reliability of ultrasound findings acquired with handheld apparatuses to inform urgent obstetric diagnosis in a high-volume resource-limited setting. Int. J. Gynecol. Obstet. 2021, 153, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Lamorte, A.; Boero, E.; Crida, P.; Conteh, A.R.; Foletti, M.; Narcisi, P. The Sierra Leone Ultrasound Rainbow4Africa Project (SLURP): An observational study of ultrasound effectiveness in developing countries. Crit. Ultrasound J. 2016, 8, 14. [Google Scholar] [CrossRef]
- Leopold, S.J.; Ghose, A.; Plewes, K.A.; Mazumder, S.; Pisani, L.; Kingston, H.W.F.; Paul, S.; Barua, A.; Sattar, M.A.; Huson, M.A.M.; et al. Point-of-care lung ultrasound for the detection of pulmonary manifestations of malaria and sepsis: An observational study. PLoS ONE 2018, 13, e0204832. [Google Scholar] [CrossRef] [PubMed]
- Limani, F.; Dula, D.; Keeley, A.J.; Joekes, E.; Phiri, T.; Tembo, E.; Gadama, L.; Nnensa, V.; Jordan, S.; Mallewa, J.; et al. Diagnostic point-of-care ultrasound in medical inpatients at Queen Elizabeth Central Hospital, Malawi: An observational study of practice and evaluation of implementation. Trans. R. Soc. Trop. Med. Hyg. 2021, 115, 863–869. [Google Scholar] [CrossRef] [PubMed]
- Lobo, M.J.C.D.; Tavares, S.C.C.N.M.; de Almeida, R.P.P. Point of care prehospital ultrasound in Basic Emergency Services in Portugal. Health Sci. Rep. 2022, 5, e847. [Google Scholar] [CrossRef]
- Mazza, G.; Romo, C.M.; Torres, M.; Duffens, A.; Vyas, A.; Moran, K.; Livingston, J.; Gonzales, S.; Lahham, S.; Shniter, I.; et al. Assessment of clinical dehydration using point of care ultrasound for pediatric patients in rural Panama. World J. Emerg. Med. 2019, 10, 46. [Google Scholar] [CrossRef]
- Mengarelli, M.; Nepusz, A.; Kondrashova, T. A Comparison of Point-of-Care Ultrasonography Use in Rural Versus Urban Emergency Departments Throughout Missouri. Mo. Med. 2018, 115, 56–60. [Google Scholar]
- Nacarapa, E.; Munyangaju, I.; Osório, D.; Zindoga, P.; Mutaquiha, C.; Jose, B.; Macuacua, A.; Chongo, B.; de-Almeida, M.; Verdu, M.-E.; et al. Extrapulmonary tuberculosis mortality according to clinical and point of care ultrasound features in Mozambique. Sci. Rep. 2022, 12, 16675. [Google Scholar] [CrossRef]
- Nixon, G.; Blattner, K.; Finnie, W.; Lawrenson, R.; Kerse, N. Use of point-of-care ultrasound for the assessment of intravascular volume in five rural New Zealand hospitals. Can. J. Rural Med. 2019, 24, 109. [Google Scholar] [CrossRef]
- Nixon, G.; Blattner, K.; Koroheke-Rogers, M.; Muirhead, J.; Finnie, W.L.; Lawrenson, R.; Kerse, N. Point-of-care ultrasound in rural New Zealand: Safety, quality and impact on patient management. Aust. J. Rural Health 2018, 26, 342–349. [Google Scholar] [CrossRef]
- Nixon, G.; Blattner, K.; Muirhead, J.; Finnie, W.; Lawrenson, R.; Kerse, N. Scope of point-of-care ultrasound practice in rural New Zealand. J. Prim. Health Care 2018, 10, 224–236. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.; Blattner, K.; Muirhead, J.; Kerse, N. Rural point-of-care ultrasound of the kidney and bladder: Quality and effect on patient management. J. Prim. Health Care 2018, 10, 324–330. [Google Scholar] [CrossRef] [PubMed]
- Nixon, G.; Blattner, K.; Muirhead, J.; Kiuru, S.; Kerse, N. Point-of-care ultrasound for FAST and AAA in rural New Zealand: Quality and impact on patient care. Rural Remote Health 2019, 19, 5027. [Google Scholar] [CrossRef] [PubMed]
- Pellegrini, J.A.S.; Cordioli, R.L.; Grumann, A.C.B.; Ziegelmann, P.K.; Taniguchi, L.U. Point-of-care ultrasonography in Brazilian intensive care units: A national survey. Ann. Intensive Care 2018, 8, 50. [Google Scholar] [CrossRef] [PubMed]
- Peterman, N.J.; Yeo, E.; Kaptur, B.; Smith, E.J.; Christensen, A.; Huang, E.; Rasheed, M. Analysis of Rural Disparities in Ultrasound Access. Cureus 2022, 14, e25425. [Google Scholar] [CrossRef] [PubMed]
- Reynolds, T.A.; Amato, S.; Kulola, I.; Chen, C.-J.J.; Mfinanga, J.; Sawe, H.R. Impact of point-of-care ultrasound on clinical decision-making at an urban emergency department in Tanzania. PLoS ONE 2018, 13, e0194774. [Google Scholar] [CrossRef]
- Sheppard, G.; Devasahayam, A.; Campbell, C.; Najafizada, M.; Yi, Y.; Power, A. The prevalence and patterns of use of point-of-care ultrasound in Newfoundland and Labrador. Can. J. Rural Med. 2021, 26, 160. [Google Scholar] [CrossRef]
- Shumbusho, J.P.; Duanmu, Y.; Kim, S.H.; Bassett, I.V.; Boyer, E.W.; Ruutiainen, A.T.; Riviello, R.; Ntirenganya, F.; Henwood, P.C. Accuracy of Resident-Performed Point-of-Care Lung Ultrasound Examinations Versus Chest Radiography in Pneumothorax Follow-up after Tube Thoracostomy in Rwanda. J. Ultrasound Med. 2020, 39, 499–506. [Google Scholar] [CrossRef]
- Stachura, M.; Landes, M.; Aklilu, F.; Venugopal, R.; Hunchak, C.; Berman, S.; Maskalyk, J.; Sarrazin, J.; Kebede, T.; Azazh, A. Evaluation of a point-of-care ultrasound scan list in a resource-limited emergency centre in Addis Ababa Ethiopia. Afr. J. Emerg. Med. 2017, 7, 118–123. [Google Scholar] [CrossRef]
- Umuhire, O.F.; Henry, M.B.; Levine, A.C.; Cattermole, G.N.; Henwood, P. Impact of ultrasound on management for dyspnea presentations in a Rwandan emergency department. Ultrasound J. 2019, 11, 18. [Google Scholar] [CrossRef]
- Strasser, R. Rural health around the world: Challenges and solutions. Fam. Pract. 2003, 20, 457–463. [Google Scholar] [CrossRef] [PubMed]
- Kovell, L.C.; Ali, M.T.; Hays, A.G.; Metkus, T.S.; Madrazo, J.A.; Corretti, M.C.; Mayer, S.A.; Abraham, T.P.; Shapiro, E.P.; Mukherjee, M. Defining the Role of Point-of-Care Ultrasound in Cardiovascular Disease. Am. J. Cardiol. 2018, 122, 1443–1450. [Google Scholar] [CrossRef]
- Moulson, N.; Jaff, Z.; Wiltshire, V.; Taylor, T.; O’Connor, H.M.; Hopman, W.M.; Johri, A.M. Feasibility and Reliability of Nonexpert POCUS for Cardiovascular Preparticipation Screening of Varsity Athletes: The SHARP Protocol. Can. J. Cardiol. 2019, 35, 35–41. [Google Scholar] [CrossRef] [PubMed]
- Panebianco, N.; Baston, C.M.; Mehta, M.; Ferrari, V.A.; Jagasia, D.; Scherrer-Crosbie, M.; Adusumalli, S. Collaboration during Crisis: A Novel Point-of-Care Ultrasound Alliance among Emergency Medicine, Internal Medicine, and Cardiology in the COVID-19 Era. J. Am. Soc. Echocardiogr. 2021, 34, 325–326. [Google Scholar] [CrossRef]
- Bhagat, G.; Scheels, W. POCUS evaluation of symptomatic aortic stenosis. Vis. J. Emerg. Med. 2022, 29, 101569. [Google Scholar] [CrossRef]
- Zeller, L.; Fuchs, L.; Maman, T.; Fainguelernt, T.S.; Fainguelernt, I.; Barski, L.; Liel-Cohen, N.; Kobal, S.L. Pocket-sized Ultrasound versus Cardiac Auscultation in Diagnosing Cardiac Valve Pathologies: A Prospective Cohort Study. Int. J. Med. Stud. 2021, 9, 294–299. [Google Scholar] [CrossRef]
- Drake, A.; Dreyer, N.; Hoffer, M.; Boniface, K. Point-of-care Ultrasound for the Evaluation of Acute Arterial Pathology in the Emergency Department: A Case Series. Clin. Pract. Cases Emerg. Med. 2022, 6, 13–19. [Google Scholar] [CrossRef]
- Gupta, A.; Kindarara, D.M.; Chun, K.C.; Datta, S.; Anderson, R.C.; Irwin, Z.T.; Newton, E.A.; Lee, E.S. Accuracy of Point-of-Care Ultrasound in Follow Up Abdominal Aortic Aneurysm Imaging. Vasc. Endovasc. Surg. 2022, 56, 649–654. [Google Scholar] [CrossRef]
- Evans, P.T.; Zhang, R.S.; Cao, Y.; Breslin, S.; Panebianco, N.; Baston, C.M.; DiBardino, D.M. The Use of Thoracic Ultrasound to Predict Transudative and Exudative Pleural Effusion. Pocus J. 2021, 6, 97–102. [Google Scholar] [CrossRef]
- Soni, N.J.; Franco, R.; Velez, M.I.; Schnobrich, D.; Dancel, R.; Restrepo, M.I.; Mayo, P.H. Ultrasound in the diagnosis and management of pleural effusions: Ultrasound and Pleural Effusions. J. Hosp. Med. 2015, 10, 811–816. [Google Scholar] [CrossRef]
- Pourmand, A.; Dimbil, U.; Drake, A.; Shokoohi, H. The Accuracy of Point-of-Care Ultrasound in Detecting Small Bowel Obstruction in Emergency Department. Emerg. Med. Int. 2018, 2018, 3684081. [Google Scholar] [CrossRef] [PubMed]
- Ismaeel, S.M.; Day, N.J.; Earnshaw, D.; Lorains, J.W. Training requirements for point of care ultrasound in acute medicine. Acute Med. 2010, 9, 87–90. [Google Scholar] [CrossRef] [PubMed]
- Guttman, J.; Stone, M.B.; Kimberly, H.H.; Rempell, J.S. Point-of-care ultrasonography for the diagnosis of small bowel obstruction in the emergency department. Can. J. Emerg. Med. 2015, 17, 206–209. [Google Scholar] [CrossRef]
- Zhang, Y.N.; Fowler, K.J.; Ozturk, A.; Potu, C.K.; Louie, A.L.; Montes, V.; Henderson, W.C.; Wang, K.; Andre, M.P.; Samir, A.E.; et al. Liver fibrosis imaging: A clinical review of ultrasound and magnetic resonance elastography. J. Magn. Reson. Imaging 2020, 51, 25–42. [Google Scholar] [CrossRef]
- Bélard, S.; Joekes, E.; Tamarozzi, F.; Heller, T.; Bustinduy, A.L.; Kuhn, W.; Brunetti, E.; Wallrauch, C.; Grobusch, M.P. Point-of-Care Ultrasound Assessment of Tropical Infectious Diseases—A Review of Applications and Perspectives. Am. J. Trop. Med. Hyg. 2016, 94, 8–21. [Google Scholar] [CrossRef] [PubMed]
- Abbinante, G.; Vitiello, L.; Coppola, A.; Salerno, G.; Gagliardi, V.; Pellegrino, A. Optic Nerve Ultrasound Evaluation in Children: A Review. Diagnostics 2023, 13, 535. [Google Scholar] [CrossRef]
- Tessaro, M.O.; Friedman, N.; Al-Sani, F.; Gauthey, M.; Maguire, B.; Davis, A. Pediatric point-of-care ultrasound of optic disc elevation for increased intracranial pressure: A pilot study. Am. J. Emerg. Med. 2021, 49, 18–23. [Google Scholar] [CrossRef]
- Vinayak, S.; Brownie, S. Collaborative task-sharing to enhance the Point-Of-Care Ultrasound (POCUS) access among expectant women in Kenya: The role of midwife sonographers. J. Interprofessional Care 2018, 32, 641–644. [Google Scholar] [CrossRef]
- Kwon, A.S.; Lahham, S.; Fox, J.C. Can an 8th grade student learn point of care ultrasound? World J. Emerg. Med. 2019, 10, 109. [Google Scholar] [CrossRef]
- Wang, S.; Parsons, M.; Stone-McLean, J.; Rogers, P.; Boyd, S.; Hoover, K.; Meruvia-Pastor, O.; Gong, M.; Smith, A. Augmented Reality as a Telemedicine Platform for Remote Procedural Training. Sensors 2017, 17, 2294. [Google Scholar] [CrossRef]
- Kolbe, N.; Killu, K.; Coba, V.; Neri, L.; Garcia, K.M.; McCulloch, M.; Spreafico, A.; Dulchavsky, S. Point of care ultrasound (POCUS) telemedicine project in rural Nicaragua and its impact on patient management. J. Ultrasound 2015, 18, 179–185. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.-C.; Sarkar, N.; Hsia, R.Y. Structural Inequities for Historically Underserved Communities in the Adoption of Stroke Certification in the United States. JAMA Neurol. 2022, 79, 777. [Google Scholar] [CrossRef] [PubMed]
- Übeylı, E.D.; Güler, İ. Feature extraction from Doppler ultrasound signals for automated diagnostic systems. Comput. Biol. Med. 2005, 35, 735–764. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.K.; Verma, K.; Thoke, A.S. Fuzzy cluster based neural network classifier for classifying breast tumors in ultrasound images. Expert Syst. Appl. 2016, 66, 114–123. [Google Scholar] [CrossRef]
- Prabusankarlal, K.M.; Thirumoorthy, P.; Manavalan, R. Assessment of combined textural and morphological features for diagnosis of breast masses in ultrasound. Hum.-Centric Comput. Inf. Sci. 2015, 5, 12. [Google Scholar] [CrossRef]
- Virmani, J.; Kumar, V.; Kalra, N.; Khandelwal, N. SVM-Based Characterization of Liver Ultrasound Images Using Wavelet Packet Texture Descriptors. J. Digit. Imaging 2013, 26, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wu, F.; Jiang, T.; Zhu, J.; Kong, D. Cascade convolutional neural networks for automatic detection of thyroid nodules in ultrasound images. Med. Phys. 2017, 44, 1678–1691. [Google Scholar] [CrossRef]
- Gao, X.; Li, W.; Loomes, M.; Wang, L. A fused deep learning architecture for viewpoint classification of echocardiography. Inf. Fusion 2017, 36, 103–113. [Google Scholar] [CrossRef]
- Brattain, L.J.; Telfer, B.A.; Liteplo, A.S.; Noble, V.E. Automated B-Line Scoring on Thoracic Sonography. J. Ultrasound Med. 2013, 32, 2185–2190. [Google Scholar] [CrossRef]
- Veeramani, S.K.; Muthusamy, E. Detection of abnormalities in ultrasound lung image using multi-level RVM classification. J. Matern.-Fetal Neonatal Med. 2015, 29, 1844–1852. [Google Scholar] [CrossRef]
- Cheema, B.S.; Walter, J.; Narang, A.; Thomas, J.D. Artificial Intelligence–Enabled POCUS in the COVID-19 ICU. JACC Case Rep. 2021, 3, 258–263. [Google Scholar] [CrossRef] [PubMed]
- Pokaprakarn, T.; Prieto, J.C.; Price, J.T.; Kasaro, M.P.; Sindano, N.; Shah, H.R.; Peterson, M.; Akapelwa, M.M.; Kapilya, F.M.; Sebastião, Y.V.; et al. AI Estimation of Gestational Age from Blind Ultrasound Sweeps in Low-Resource Settings. NEJM Evid. 2022, 1, EVIDoa2100058. [Google Scholar] [CrossRef] [PubMed]
- Kuroda, Y.; Kaneko, T.; Yoshikawa, H.; Uchiyama, S.; Nagata, Y.; Matsushita, Y.; Hiki, M.; Minamino, T.; Takahashi, K.; Daida, H.; et al. Artificial intelligence-based point-of-care lung ultrasound for screening COVID-19 pneumoniae: Comparison with CT scans. PLoS ONE 2023, 18, e0281127. [Google Scholar] [CrossRef] [PubMed]
- Blaivas, M.; Blaivas, L. Are All Deep Learning Architectures Alike for Point-of-Care Ultrasound?: Evidence From a Cardiac Image Classification Model Suggests Otherwise. J. Ultrasound Med. 2020, 39, 1187–1194. [Google Scholar] [CrossRef]
- Karlsson, J.; Arvidsson, I.; Sahlin, F.; Åström, K.; Overgaard, N.C.; Lång, K.; Heyden, A. Classification of point-of-care ultrasound in breast imaging using deep learning. In Proceedings of the Medical Imaging 2023: Computer-Aided Diagnosis, San Diego, CA, USA, 19–23 February 2023; Iftekharuddin, K.M., Chen, W., Eds.; SPIE: San Diego, CA, USA, 2023; p. 51. [Google Scholar]
- Baribeau, Y.; Sharkey, A.; Chaudhary, O.; Krumm, S.; Fatima, H.; Mahmood, F.; Matyal, R. Handheld Point-of-Care Ultrasound Probes: The New Generation of POCUS. J. Cardiothorac. Vasc. Anesth. 2020, 34, 3139–3145. [Google Scholar] [CrossRef]
- Gohar, E.; Herling, A.; Mazuz, M.; Tsaban, G.; Gat, T.; Kobal, S.; Fuchs, L. Artificial Intelligence (AI) versus POCUS Expert: A Validation Study of Three Automatic AI-Based, Real-Time, Hemodynamic Echocardiographic Assessment Tools. J. Clin. Med. 2023, 12, 1352. [Google Scholar] [CrossRef]
- Jafari, M.H.; Girgis, H.; Van Woudenberg, N.; Moulson, N.; Luong, C.; Fung, A.; Balthazaar, S.; Jue, J.; Tsang, M.; Nair, P.; et al. Cardiac point-of-care to cart-based ultrasound translation using constrained CycleGAN. Int. J. Comput. Assist. Radiol. Surg. 2020, 15, 877–886. [Google Scholar] [CrossRef]
| Inclusion Criteria | Exclusion Criteria |
|---|---|
|
|
| Author (s); Journal; Year | Population in Study (Criteria) | Study Type and Purpose | Geographical Context | Results | Device(s) Utilized |
|---|---|---|---|---|---|
| Baker et al., Tropical Doctor, 2021 [32] | 144 patients | Cross-sectional study
| Uganda, LMIC, 20 mobile clinics from local churches and schools in 6 separate rural locations, Masindi region in Western Uganda |
| 3 Philips Lumify ultrasound probes attached to a Samsung Galaxy tablet with preloaded Lumify software; Phased: −4 to 1 MHz Linear: −12 to 4 MHz Curvilinear: −5 to 2 MHz |
| Barron et al., Southern Medical Journal, 2018 [33] | All patients who received POCUS in standard clinical practice during an Short Term Medical Mission (STMM) | Observational prospective study How POCUS would change medical management | Nicaragua (Sébaco), LMIC, rural Nicaraguan clinic |
| 2 General Electric Vscans. Single low frequency probe: (1.7–3.8 MHz) Dual low and high frequency probe: (Phased array––1.7–3.8 MHz; Linear array––3.3–8.0 MHz) |
| Bobbio et al., BMJ Open, 2018 [34] | 100 HIV-positive patients registered for antiretroviral treatment | Cross-sectional study
| South Sudan, LMIC, Voluntary Counselling and Testing Centre (VCT) of Yirol Hospital |
| 2 Mindray portable black US scanners Convex probe: 3.5 MHz Linear probe: 7 MHz |
| Cherniak et al., PLoS ONE, 2017 [31] | All women who were currently aware of being pregnant and presented to ANC | Non-blinded cluster-randomized controlled trial
| Uganda, LMIC, rural communities in southwestern Uganda, Kabale District |
| Device not specified |
| Milart et al., Reproductive Health, 2016 [35] | Pregnant women intervention group decided by the Health Directorate | Observational study
| Guatemala, LMIC, Rural areas of the districts of Senahu, Campur, and Carcha, in Alta Verapaz Department |
| Device not specified |
| Dalmacion et al., BMC Pregnancy and Childbirth, 2018 [36] | Pregnant women who were not allergic to gel, did not have concurrent medical or surgical conditions, and provided informed consents | Cross-sectional study
| Philippines, LMIC, Parañaque city, the urban study site, and Tagum city, the rural study site |
| General electric Vscan with dual probe: (Phased array––1.7–3.8 MHz; Linear array––3.3–8.0 MHz); Utilized General Electric Logic 5 Premium as reference standard |
| Elsayes et al., Rural Remore Health, 2021 [37] | All adult patients over the age of 18 at the mobile clinic in Antigua, Guatemala | Retrospective observational study
| Guatemala, LMIC, a mobile clinic in Antigua, Guatemala |
| Device not specified |
| Epstein et al., Travel Medicine and infectious disease, 2018 [38] | 101 patients at Kiboga hospital | Medical management
| Uganda, LMIC, Kiboga hospital (general governmental hospital), Kampala, Uganda, Central Uganda |
| General Electrical Vscan with a single low-frequency probe (1.7–3.8 MHz) |
| Gomes et al., Annals of Global Health, 2020 [39] | Women presenting to the obstetrical departments of Fort Liberte Hospital and Centre Medicosocial de Ouanaminthe | Cross-sectional study
| Haiti, LMIC, Obstetrical departments of two Ministry of Public Health and Population (MSPP)-affiliated public hospitals |
| Device not specified |
| Haldeman et al., The Ultrasound Journal, 2022 [40] | Residents enrolled in the UNZA Family Medicine program | Single-center, survey-based, prospective study
| Zambia, LMIC, Chilenje hospital |
| 3 Butterfly IQ handheld ultrasound devices (1–10 MHz) |
| Huson et al., The American Journal of Tropical Medicine and Hygiene, 2020 [41] | Patients with fever at Mahosot Hospital | Prospective observational study
| Laos, LMIC, Mahosot Hospital, Vientiane Laos |
| Mindray portable black-and-white ultrasound; Convex probe: 3.5 MHz Linear probe: 7.5 MHz |
| Kodaira et al., International Journal of Gynecology and Obstetrics, 2020 [42] | Pregnant women at Princess Christian Maternity Hospital | Single-center prospective observational study
| Sierra Leone, LMIC, high-volume, low-resource hospital, Western Area of Sierra Leone |
| Hand-held smartphone-based ultrasound devices; Lequio US-304 convex probe (3–5 MHz) Mindray SD-10 (3–5 MHz convex probe and 7–10 MHz trans-vaginal probe) |
| Lamorte et al., Crit Ultrasound Journal, 2016 [43] | 105 patients re- quested for ultrasound examination by the caring physician | Prospective, observational study
| Sierra Leone, LMIC, Holy Spirit Hospital, Makeni, Bombali district |
| Toshiba Memio 20 |
| Leopold et al., PLoS ONE, 2018 [44] | Patients enrolled in the general medical wards of Chittagong Medical College | Prospective, observational study
| Bangladesh, LMIC, Large tertiary government hospital (Chittagong Medical College), Chittagong, Bangladesh |
| General Healthcare Vivid-I portable ultrasound; Convex probe: 5 MHz |
| Limani et al., Transactions of The Royal Society of Tropical Medicine and Hygiene, 2020 [45] | Patients over 16 who had received an ultrasound examination | Prospective observational study
| Malawi, LMIC, Queen Elizabeth Central Hospital, Blantyre, Malawi, tertiary referral center for the southern region |
| POCUS: General Electrical V-scan, Mindray DP 30 Radiology department ultrasound: Mindray DC 30, General Electric vivid Q-i |
| Lobo et al., Health Science Reports, 2022 [46] | Voluson Ultrasound Equipment, Toshiba Némio XG ultrasound data across 2 emergency centers | Cross sectional, observational and longitudinal study
| Portugal, Developed Country, two remote locations in Portugal (SUB N, SUB S), basic emergency services (SUB) |
| General Electric Voluson SN: Convex and Linear probes; Toshiba Nemio XG ultrasound: Convex |
| Mazza et al., World J Emergency Medicine, 2019 [47] | Subjects from the age of 11 months to 13 years, with risk of dehydration | Prospective, observational study
| Panama, LMIC, Bocas del Toro region of rural Panama, Floating Doctors clinics |
| Mindray M7 portable ultrasound machine with single phased array probe |
| Mengarelli et al., Missouri Medicine, 2018 [48] | Hospitals that were in the records of the Missouri Department of Health and Senior Services | Survey
| United States, Developed Country, Missouri, hospitals with emergency departments, large, medium, and small hospitals |
| Device not specified |
| Nacarapa et al., Nature Scientific Reports, 2022 [49] | Patients over 15 years from the CHC dataset with ultrasound findings of extrapulmonary TB Manifestation | Prospective, observational study
| Mozambique, LMIC, rural Chókwè district, Mozambique, southern Gaza province, hospital |
| Samsung SonoAce R3 with a C2–4/20 convex probe (1–10 MHz frequency) |
| Nixon et al., Canadian Journal of Rural Medicine, 2019 [50] | 28 rural generalist physicians trained by the Royal NZ college of General Practitioners | Subgroup analysis
| New Zealand, Developed Country, Five New Zealand rural hospitals |
| Device not specified |
| Nixon et al., Australian Journal of Rural Health, 2018 [51] | All generalist doctors practicing ultrasound in study hospitals | Cross-sectional descriptive study
| New Zealand, Developed Country, Six rural small hospitals serving a range of communities in rural New Zealand |
| Device not specified |
| Nixon et al., Journal of Primary Health Care, 2018 [52] | All the generalist rural doctors practicing POCUS in the 6 rural hospitals | Mixed-methods descriptive study
| New Zealand, Developed Country, Rural New Zealand, six rural hospitals |
| Device not specified |
| Nixon et al., Journal of Primary Health Care, 2018 [53] | 28 doctors in 6 New Zealand rural hospitals | Subgroup analysis
| New Zealand, Developed Country, Rural New Zealand, 6 rural hospitals |
| Device not specified |
| Nixon et al., Rural and Remote Health, 2019 [54] | 28 physicians in 6 New Zealand rural hospitals | Subgroup analysis
| New Zealand, Developed Country, Rural New Zealand, 6 geographically dispersed rural hospitals |
| Device not specified |
| Pellegrini et al., Annals of Intensive Care, 2018 [55] | 1533 Brazilian intensivists | National survey
| Brazil, LMIC, Intensive Care Units (ICU), all regions of Brazil |
| Device not specified |
| Peterman et al., Cureus, 2022 [56] | 3011 public datasets on a county level | Geospatial Analysis
| United States, Developed Country, rural and metropolitan counties |
| Device not specified |
| Reynolds et al., PLoS ONE, 2018 [57] | Patients receiving POCUS at Muhimbili National Hospital’s Emergency Medical Department (MNH EMD) | Prospective descriptive cross-sectional study
| Tanzania, LMIC, Urban emergency department in Dar es Salaam, Tanzania, Muhimbili National Hospital |
| SonoSite mTurbo |
| Self et al., JMIR Research Protocols, 2022 [23] | Pregnant women in the 2 hospitals | Prospective study
| United Kingdom (Developed Country), India (LMIC), Group for Advanced Research on BirtH outcomes–Department of Biotechnology India Initiative (GARBH-Ini) cohort, John Radcliffe Hospital (Oxford) |
| General Electric Voluson E8 with curvilinear probes: C2–9 (3–9 MHz) and C1–5 (2–5 MHz) |
| Sheppard et al., Canadian Journal of Rural Medicine, 2021 [58] | 10 physicians (3 females, 5 rural) participated in the interviews | Mixed-methods cross-sectional study
| Canada (Developed Country), Newfoundland and Labrador, Urban (4) and rural geographic (19) locations |
| Device not specified |
| Shumbusho et al., Journal of ultrasound in medicine, 2019 [59] | Patients 5 years and older with PTX managed by chest tubes at CHUK | Prospective, observational study
| Rwanda, LMIC, Rwandan referral hospital (University Teaching Hospital of Kigali (CHUK)) |
| SonoSite M-Turbo |
| Stachura et al., African Journal of Emergency Medicine, 2017 [60] | 118 patients with clinical indications for POCUS | Prospective observational study
| Ethiopia, LMIC, Tikur Anbessa Specialized Hospital EC in Addis Ababa, urban, low-resource, academic EC in Ethiopia |
| SonoSite MicroMaxx and Full Digital Laptop Ultrasound Scanner (RUS-9000F) with 3.5 MHz curved array probe; Last two weeks of the study, there was a SeeMore USB ultrasound system with two probes: abdominal (GP3.5/5.0 MHz) and SP7.5/24.0 MHz high frequency probe |
| Umuhire et al., Ultrasound Journal, 2019 [61] | Adult participants presenting with dyspnea to an urban Rwandan emergency department | Prospective, observational study
| Rwanda, LMIC, Emergency Department at University Teaching Hospital of Kigali (UTH-K) in Rwanda |
| SonoSite M-Turbo |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Venkatayogi, N.; Gupta, M.; Gupta, A.; Nallaparaju, S.; Cheemalamarri, N.; Gilari, K.; Pathak, S.; Vishwanath, K.; Soney, C.; Bhattacharya, T.; et al. From Seeing to Knowing with Artificial Intelligence: A Scoping Review of Point-of-Care Ultrasound in Low-Resource Settings. Appl. Sci. 2023, 13, 8427. https://doi.org/10.3390/app13148427
Venkatayogi N, Gupta M, Gupta A, Nallaparaju S, Cheemalamarri N, Gilari K, Pathak S, Vishwanath K, Soney C, Bhattacharya T, et al. From Seeing to Knowing with Artificial Intelligence: A Scoping Review of Point-of-Care Ultrasound in Low-Resource Settings. Applied Sciences. 2023; 13(14):8427. https://doi.org/10.3390/app13148427
Chicago/Turabian StyleVenkatayogi, Nethra, Maanas Gupta, Alaukik Gupta, Shreya Nallaparaju, Nithya Cheemalamarri, Krithika Gilari, Shireen Pathak, Krithik Vishwanath, Carel Soney, Tanisha Bhattacharya, and et al. 2023. "From Seeing to Knowing with Artificial Intelligence: A Scoping Review of Point-of-Care Ultrasound in Low-Resource Settings" Applied Sciences 13, no. 14: 8427. https://doi.org/10.3390/app13148427
APA StyleVenkatayogi, N., Gupta, M., Gupta, A., Nallaparaju, S., Cheemalamarri, N., Gilari, K., Pathak, S., Vishwanath, K., Soney, C., Bhattacharya, T., Maleki, N., Purkayastha, S., & Gichoya, J. W. (2023). From Seeing to Knowing with Artificial Intelligence: A Scoping Review of Point-of-Care Ultrasound in Low-Resource Settings. Applied Sciences, 13(14), 8427. https://doi.org/10.3390/app13148427









