Proximate Analysis and Antioxidant Properties of Young Plants of Sinapis alba L. Depend on the Time of Harvest and Variety
Abstract
:1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Field Experiment
2.3. Proximate Analysis
2.4. Extract Preparation, Antioxidant Capacity and Total Polyphenol Content
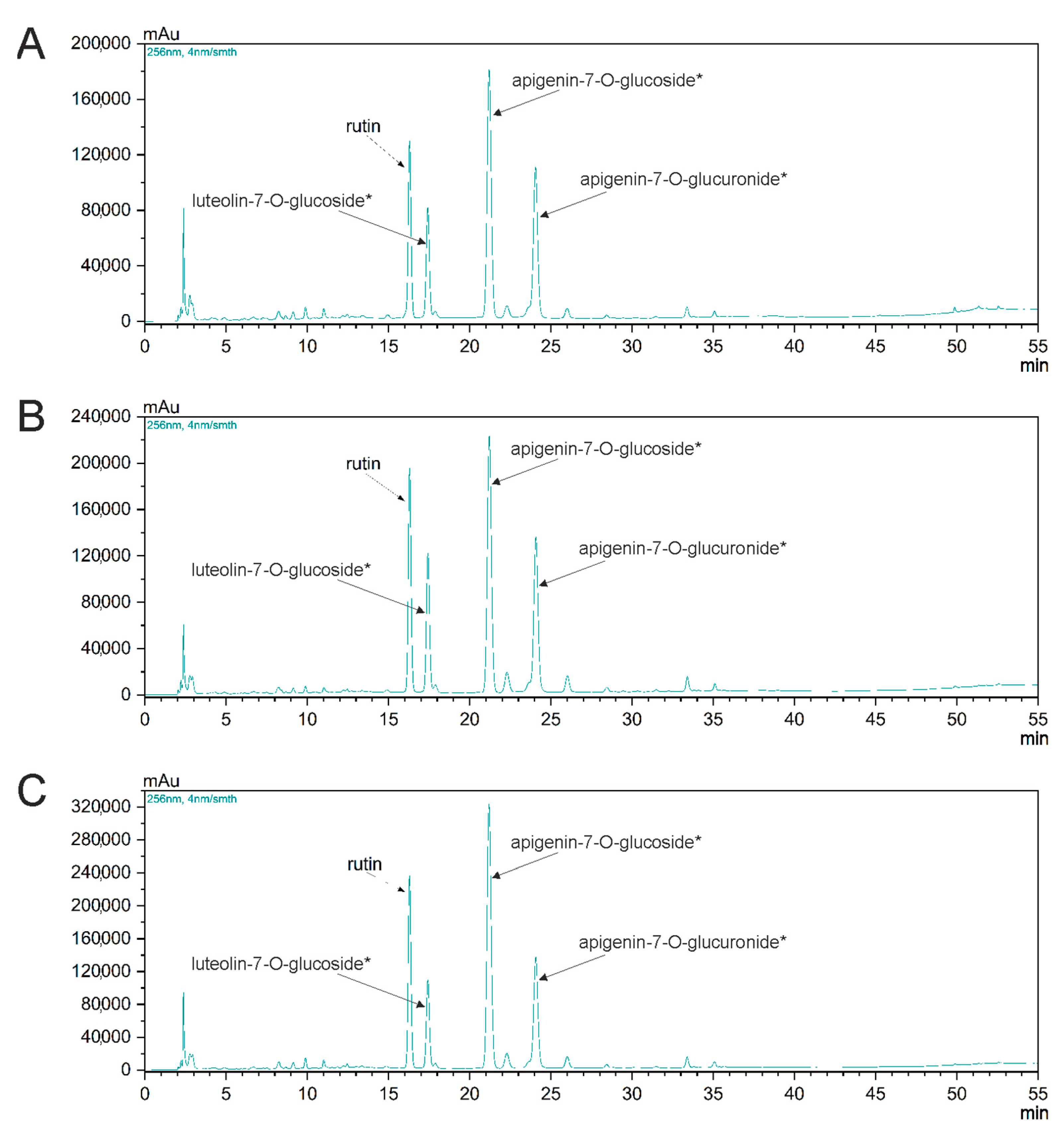
2.5. HPLC Analysis of Mustard Plant
2.6. Identification of Some Phenolic Compounds
2.7. Statistical Analysis
3. Results
4. Discussion
4.1. Proximate Composition
4.2. Antioxidant Activity and Total Polyphenolic Compound Content
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Avato, P.; Argentieri, M.P. Brassicaceae: A rich source of health improving phytochemicals. Phytochem. Rev. 2015, 14, 1019–1033. [Google Scholar] [CrossRef]
- Torrijos, R.; Righetti, L.; Cirlini, M.; Calani, L.; Mañes, J.; Meca, G.; Dall’Asta, C. Phytochemical profiling of volatile and bioactive compounds in yellow mustard (Sinapis alba) and oriental mustard (Brassica juncea) seed flour and bran. LWT 2023, 173, 114221. [Google Scholar] [CrossRef]
- Rahman, M.; Khatun, A.; Liu, L.; Barkla, B.J. Brassicaceae mustards: Traditional and agronomic uses in Australia and New Zealand. Molecules 2018, 23, 231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sawicka, B.; Kotiuk, E. Gorczyce jako rośliny wielofunkcyjne. Mustard species as multi-functional plants. Acta Sci. Pol. Agric. 2007, 6, 17–27. Available online: https://docplayer.pl/16221829-Acta-sci-pol-agricultura-6-2-2007-17-27.html (accessed on 14 April 2023). (In Polish).
- Yigit, A. Baharatlar. In Gıda Coğrafyası; No: 3274; Açıköğretim Fakültesi Yayını; TC Anadolu Üniversitesi Yayını: Eskişehir, Turkey, 2016; p. 2137. ISBN 978-975-06-1896-3. (In Turkish) [Google Scholar]
- Dogan, Y.; Baslar, S.; Ay, G.; Mert, H.H. The use of wild edible plants in western and central Anatolia (Turkey). Econ. Bot. 2004, 58, 684–690. [Google Scholar] [CrossRef]
- Ertug, F. Wild edible plants of the Bodrum area (Mugla, Turkey). Turk. J. Bot. 2004, 28, 161–174. Available online: https://journals.tubitak.gov.tr/botany/vol28/iss1/16 (accessed on 23 February 2023).
- Kargioglu, M.; Cenkci, S.; Serteser, A.; Konuk, M.; Vural, G. Traditional uses of wild plants in the middle Aegean region of Turkey. Hum. Ecol. 2010, 38, 429–450. [Google Scholar] [CrossRef]
- Bridges, M.; Jones, A.M.; Bones, A.M.; Hodgson, C.; Cole, R.; Bartlet, E.; Wallsgrove, R.; Karapapa, V.K.; Watts, N.; Rossite, J.T. Spatial organization of the glucosinolate–myrosinase system in Brassica specialist aphids is similar to that of the host plant. Proceedings of the Royal Society of London. Ser. B Biol. Sci. 2002, 269, 187–191. [Google Scholar] [CrossRef]
- Bartnik, M.; Facey, P.C. Glycosides. In Pharmacognosy; Badals, S., Delgoda, R., Eds.; Academic Press: Cambridge, MA, USA, 2017; pp. 101–161. [Google Scholar] [CrossRef]
- Piętka, T.; Krzymanski, J.; Krótka, K. Pierwsza podwójnie ulepszona odmiana gorczycy białej (Sinapis alba L.) [The first double low variety of white mustard (Sinapis alba L.)]. Rośl. Oleiste-Oilseed Crops 2010, 31, 177–200. [Google Scholar]
- Piętka, T.; Krzymański, J.; Krótka, K.; Bartkowiak-Broda, I. Double low white mustard (Sinapis alba L. syn. Brassica hirta) is a source of protein and oil. Rośl. Oleiste-Oilseed Crops 2014, 35, 21–35. (In Polish) [Google Scholar]
- Capuano, E.; Dekker, M.; Verkerk, R.; Oliviero, T. Food as pharma? The case of glucosinolates. Curr. Pharm. Design. 2017, 23, 2697–2721. [Google Scholar] [CrossRef]
- Yokozawa, T.; Kim, H.Y.; Cho, E.J.; Yamabe, N.; Choi, J.S. Protective effects of mustard leaf (Brassica juncea) against diabetic oxidative stress. J. Nutr. Sci. Vitaminol. 2003, 49, 87–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Björkman, M.; Klingen, I.; Birch, A.N.E.; Bones, A.M.; Bruce, T.J.-A.; Johansen, T.J.; Meadow, R.; Mølmann, J.; Seljåsen, R.; Smart, L.E.; et al. Phytochemicals of Brassicaceae in plant protection and human health—Influences of climate, environment and agronomic practice. Phytochemistry 2011, 72, 538–556. [Google Scholar] [CrossRef] [PubMed]
- Ares, A.M.; Nozal, M.J.; Bernal, J. Extraction, chemical characterization and biological activity determination of broccoli health promoting compounds. J. Chromatogr. A 2013, 1313, 78–95. [Google Scholar] [CrossRef] [PubMed]
- Cartea, M.E.; Francisco, M.; Soengas, P.; Velasco, P. Phenolic compounds in Brassica vegetables. Molecules 2011, 16, 251–280. [Google Scholar] [CrossRef] [PubMed]
- Dufresne, C.J.; Farnworth, E.R. A review of latest research findings on the health promotion properties of tea. J. Nutr. Biochem. 2001, 12, 404–421. [Google Scholar] [CrossRef]
- Martínez-Sánchez, A.; Gil-Izquierdo, A.; Gil, M.I.; Ferreres, F. A comparative study of flavonoid compounds, vitamin C, and antioxidant properties of baby leaf Brassicaceae species. J. Agric. Food Chem. 2008, 56, 2330–2340. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef]
- Wang, S.; Melnyk, J.P.; Tsao, R.; Marcone, M.F. How natural dietary antioxidants in fruits, vegetables and legumes promote vascular health. Food Res. Int. 2011, 44, 14–22. [Google Scholar] [CrossRef]
- Jo, S.H.; Cho, C.Y.; Ha, K.S.; Lee, J.Y.; Choi, H.Y.; Kwon, Y.I.; Apostolidis, E. In vitro and in vivo anti-hyperglycemic effects of green and red mustard leaves (Brassica juncea var. integrifolia). J. Food Biochem. 2018, 42, 12583. [Google Scholar] [CrossRef]
- Yao, X.H.; Zhang, Z.B.; Song, P.; Hao, J.Y.; Zhang, D.Y.; Zhang, Y.F. Different harvest seasons modify bioactive compounds and antioxidant activities of Pyrola incarnata. Ind. Crops Prod. 2016, 94, 405–412. [Google Scholar] [CrossRef]
- Ribeiro, D.A.; Camilo, C.J.; Nonato, C.D.F.A.; Rodrigues, F.F.G.; Menezes, I.R.A.; Ribeiro-Filho, J.; da Costa, J.G.M. Influence of seasonal variation on phenolic content and in vitro antioxidant activity of Secondatia floribunda A. DC. (Apocynaceae). Food Chem. 2020, 315, 126277. [Google Scholar] [CrossRef] [PubMed]
- Soengas, P.; Sotelo, T.; Velasco, P.; Cartea, M.E. Antioxidants properties of Brassica vegetables. In Functional Plant Science and Biotechnology; Teixeira da Silva, J., Ed.; Volume 5 (Special Iusse 2); Global Science Books: London, UK, 2011; pp. 43–55. [Google Scholar]
- Paszkiewicz-Jasinska, A. The effect od selected agrotechnical factors on development, yielding and quality of white mustard. II. The effect of nitrogen fertilization and sowing density on chemical composition of white mustard [Sinapis alba L.]. Rośl. Oleiste-Oilseed Crops 2005, 26, 467–478. (In Polish) [Google Scholar]
- Kwon, H.Y.; Choi, S.I.; Park, H.I.; Choi, S.H.; Sim, W.S.; Yeo, J.H.; Lee, O.H. Comparative analysis of the nutritional components and antioxidant activities of different Brassica juncea cultivars. Foods 2020, 9, 840. [Google Scholar] [CrossRef]
- Singh, P.; Sinhal, V.K. Effect of aphid infestation on the biochemical constituents of mustard (Brassica juncea) plant. J. Phytol. 2011, 3, 28–33. [Google Scholar]
- The Polish National List. Research Centre For Cultivar Testing. Available online: https://coboru.gov.pl/index_en (accessed on 25 April 2023).
- AOAC. Official Methods of Analysis of the AOAC, 21st ed.; AOAC: Gaithersburg, MD, USA, 2019. [Google Scholar]
- Metzger, L.E.; Nielsen, S.S. Nutrition labeling. In Food Analysis, 5th ed.; Nielsen, S.S., Ed.; Springer: Cham, Switzerland, 2017; pp. 35–43. [Google Scholar] [CrossRef]
- Swain, P.; Hillis, W.E. The phenolic constituents of Prunus domestica (L.). The quantity of analisys of phenolic constituents. J. Sci. Food Agric. 1959, 10, 63–68. [Google Scholar] [CrossRef]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant activity applying an improved ABTS radical cation decolorization assay. Free. Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Benzie, I.F.; Strain, J.J. The ferric reducing ability of plasma (FRAP) as a measure of “antioxidant power”: The FRAP assay. Anal. Biochem. 1996, 239, 70–76. [Google Scholar] [CrossRef] [Green Version]
- Vo Van, L.; Pham, E.C.; Nguyen, C.V.; Duong, N.T.N.; Vi Le Thi, T.; Truong, T.N. In vitro and in vivo antidiabetic activity, isolation of flavonoids, and in silico molecular docking of stem extract of Merremia tridentata (L.). Biomed Pharmacother. 2022, 146, 112611. [Google Scholar] [CrossRef]
- Cipollini, D.; Stevenson, R.; Enright, S.; Eyles, A.; Bonello, P. Phenolic metabolites in leaves of the invasive shrub, Lonicera maackii, and their potential phytotoxic and anti-herbivore effects. J. Chem. Ecol. 2008, 34, 144–152. [Google Scholar] [CrossRef]
- Zhu, X.; Zhang, H.; Lo, R. Phenolic compounds from the leaf extract of artichoke (Cynara scolymus L.) and their antimicrobial activities. J. Agric. Food Chem. 2004, 52, 7272–7278. [Google Scholar] [CrossRef]
- Cardoso Reis, A.C.; Valente, G.M.; Silva, B.M.; de Brito Magalhães, C.L.; Kohlhoff, M.; Brandão, G.C. Anti-arboviral activity and chemical characterization of hispidulin and ethanolic extracts from Millingtonia hortensis L. f. and Oroxylum indicum (L.) Kurz (Bignoniaceae). Nat. Prod. Res. 2023, 37, 613–617. [Google Scholar] [CrossRef] [PubMed]
- da Cruz, A.F.G.; Reis, A.C.C.; Sousa, J.A.C.; Vaz, L.B.A.; de Mello Silva, B.; de Brito Magalhães, C.L.; Kohlhoff, M.; de Oliveira, A.B.; Brandão, G.C. High-Resolution Mass Spectrometry Identification and Characterization of Flavonoids from Fridericia chica Leaves Extract with Anti-Arbovirus Activity. Molecules 2022, 27, 6043. [Google Scholar] [CrossRef]
- Available online: www.reaxys.com (accessed on 1 July 2023).
- Kisielewska, W.; Harasimowicz-Hermann, G. Impact of sowing time on accumulation of mineral elements by white mustard cultivated as intercrop. Rośl. Oleiste-Oilseed Crop. 2008, 29, 209–216. (In Polish) [Google Scholar]
- Nowakowski, M.; Szymczak-Nowak, J. Yields of fresh and dry matter and antinematode effect of white mustard and oil radish depending on cultivar and nitrogen fertilization. Rośl. Oleiste-Oilseed Crop. 2003, 24, 501–508. (In Polish) [Google Scholar]
- Sawicka, B.; Kotiuk, E.; Bienia, B.; Wójcik, S. The importance of mustard (Sinapis alba) indian mustard (Brassica juncea var. sareptana) and black mustard (Brassica nigra) in nutrition and phytotherapy. In Proceedings of the I Międzynarodowa Konferencja “Ziołolecznictwo, Biokosmetyki i Żywność Funkcjonalna”, Krosno, Poland, 18–19 April 2013; pp. 18–19. (In Polish). [Google Scholar]
- Kapusta-Duch, J.; Borczak, B.; Kopeć, A.; Filipiak-Florkiewicz, A.; Leszczyńska, T. The influence of packaging type and time of frozen storage on antioxidative properties of Brussels sprouts. J. Food Process. Pres. 2014, 38, 1089–1096. [Google Scholar] [CrossRef]
- Rożek, E.; Nurzyńska-Wierdak, R.; Dzida, K. Factors modifying yield quantity and quality, as well as the chemical composition of the leaves of leaf celery Apium graveolens L. var. secalinum Alef. grown from seedlings. Acta Sci. Pol. Hortoru 2012, 11, 201–210. (In Polish) [Google Scholar]
- Edelman, M.; Colt, M. Nutrient value of leaf vs. seed. Front. Chem. 2016, 4, 32. [Google Scholar] [CrossRef] [Green Version]
- Cho, Y.S.; Park, S.K.; Chun, S.S.; Moon, J.S.; Ha, B.S. Proximate, sugar and amino acid compositions of Dolsan leaf mustard (Brassica juncea). J. Korean Soc. Food Sci. Nutr. 1993, 22, 48–52. [Google Scholar]
- Sharma, A.; Verma, A.K.; Gupta, R.K.; Neelabh; Dwivedi, P.D. A comprehensive review on mustard-induced allergy and implications for human health. Clin. Rev. Allerg. Immu. 2019, 57, 39–54. [Google Scholar] [CrossRef]
- Abul-Fadl, M.M.; El-Badry, N.; Ammar, M.S. Nutritional and chemical evaluation for two different varieties of mustard seeds. WASJ 2011, 15, 1225–1233. [Google Scholar]
- Sawicka, B.; Kotiuk, E.; Kiełtyka-Dadasiewicz, A.; Krochmal-Marczak, B. Fatty acids composition of mustard oil from two cultivars and physico-chemical characteristics of the seeds. J. Oleo Sci. 2020, 69, 207–217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piętka, T.; Krótka, K.; Krzymanski, J. Double low white mustard (Sinapis alba L.)—Alternative spring oilseed crop. Rośl. Oleiste-Oilseed Crops 2004, 25, 403–413. (In Polish) [Google Scholar]
- Piętka, T.; Krzymański, J. Bamberka-zero erucic white mustard. Rośl. Oleiste-Oilseed Crops 2007, 28, 119–124. (In Polish) [Google Scholar]
- Karydogianni, S.; Roussis, I.; Mavroeidis, A.; Kakabouki, I.; Tigka, E.; Beslemes, D.; Bilalis, D. The Influence of Fertilization and Plant Density on the Dry Matter Yield and Quality of Black Mustard [Brassica nigra (L.) Koch]: An Alternative Forage Crop. Plants 2022, 11, 2683. [Google Scholar] [CrossRef] [PubMed]
- Mitra, S.; Begum, A. Osmotic stress induced alterations in fatty acid composition and other metabolic responses in seedlings of Sinapis alba. Environ. Exp. Biol. 2023, 21, 11–19. [Google Scholar] [CrossRef]
- Hagen, S.F.; Borge, G.I.A.; Solhaug, K.A.; Bengtsson, G.B. Effect of cold storage and harvest date on bioactive compounds in curly kale (Brassica oleracea L. var. acephala). Postharvest Biol. Tec. 2009, 51, 36–42. [Google Scholar] [CrossRef]
- Cabrera-De la Fuente, M.; González-Morales, S.; Juárez-Maldonado, A.; Leija-Martínez, P.; Benavides-Mendoza, A. Plant nutrition and agronomic management to obtain crops with better nutritional and nutraceutical quality. In Therapeutic Foods; Academic Press: London, UK, 2018; pp. 99–140. [Google Scholar] [CrossRef]
- Piątkowska, E.; Witkowicz, R.; Janeczko, Z.; Kopeć, A.; Leszczyńska, T.; Pisulewska, E.; Suchecki, S. Basic chemical composition and antioxidant activity leaves of selected buckwheat’s varietes and tartary buckwheat. Fragm. Agron. 2015, 32, 92–100. [Google Scholar]
- Oh, S.; Kim, K.; Choi, M. Antioxidant activity of different parts of Dolsan Leaf Mustard. Food Sci. Biotechnol. 2016, 25, 1463–1467. [Google Scholar] [CrossRef]
- Drozdowska, M.; Leszczyńska, T.; Koronowicz, A.; Piasna-Słupecka, E.; Dziadek, K. Comparative study of young shoots and the mature red headed cabbage as antioxidant food resources with antiproliferative effect on prostate cancer cells. RSC Adv. 2020, 10, 43021–43034. [Google Scholar] [CrossRef]
- Choe, U.; Yu, L.L.; Wang, T.T. The science behind microgreens as an exciting new food for the 21st century. J. Agric. Food Chem. 2018, 66, 11519–11530. [Google Scholar] [CrossRef]
- Szajdek, A.; Borowska, J. Antioxidant properties of plant-based food products. Żywn. Nauka Technol. Jakość 2004, 11, 5–28. Available online: http://agro.icm.edu.pl/agro/element/bwmeta1.element.dl-catalog-7da5b096-f6d5-4ca8-9d86-2eb50b13e35b/c/01_Szajdek.pdf (accessed on 28 March 2023). (In Polish).
- Singh, A.P.; Kishore, P.S.; Kar, S.; Dewanjee, S. Secondary Metabolites of Brassica juncea (L.) czern and Coss: Occurence, Variations and Importance. Brassica–Variations and Importance; IntechOpen: London, UK, 2022. [Google Scholar] [CrossRef]
- Rochfort, S.J.; Imsic, M.; Jones, R.; Trenerry, V.C.; Tomkins, B. Characterization of flavonol conjugates in immature leaves of pak choi [Brassica rapa L. Ssp. Chinensis L. (Hanelt.)] by HPLC-DAD and LC-MS/MS. J. Agric. Food Chem. 2006, 54, 4855–4860. [Google Scholar] [CrossRef] [PubMed]
- Romani, A.; Vignolini, P.; Isolani, L.; Ieri, F.; Heimler, D. HPLC-DAD/MS characterization of flavonoids and hydroxycinnamic derivatives in turnip tops (Brassica rapa L. Subsp. sylvestris L.). J. Agric. Food Chem. 2006, 54, 1342–1346. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, B.; Tian, Y.X.; Jiang, M.; Yuan, Q.; Chen, Q.; Zhang, Y.; Luo, Y.; Zhang, F.; Tang, H.R. Variation in the main health-promoting compounds and antioxidant activity of whole and individual edible parts of baby mustard (Brassica juncea var. gemmifera). RSC Adv. 2018, 8, 33845–33854. [Google Scholar] [CrossRef] [PubMed]


| Treatment | Cultivar | Harvest Date the Number of Days after Sowing | Means for Cultivar | ||
|---|---|---|---|---|---|
| 31 | 38 | 45 | |||
| Dry mass [g/100 g FM *] | Warta | 9.99 ± 0.51 a | 11.22 ± 0.62 a | 17.40 ± 2.39 a | 12.87 ± 3.60 A |
| Bamberka | 10.33 ± 1.05 a | 11.13 ± 2.00 a | 17.95 ± 0.85 a | 13.14 ± 7.37 A | |
| Borowska | 10.80 ± 1.43 a | 10.36 ± 0.48 a | 17.80 ± 0.71 a | 12.99 ± 3.62 A | |
| Means for harvest time | 10.38 ± 1.06 A | 10.90 ± 1.23 A | 17.72 ± 1.45 B | ||
| Warta | 36.28 ± 1.07 f | 34.80 ± 1.13 ef | 29.53 ± 0.76 c | 33.53 ± 3.16 C | |
| Protein | Bamberka | 22.96 ± 0.47 a | 33.27 ± 1.00 e | 24.39 ± 0.88 a | 26.87 ± 4.82 A |
| Borowska | 33.82 ± 1.58 e | 26.12 ± 1.53 b | 31.40 ± 1.68 d | 30.44 ± 3.65 B | |
| Means for harvest time | 31.82 ± 6.13 B | 31.40 ± 4.11 B | 28.44 ± 3.27 A | ||
| Crude fat | Warta | 0.83 ± 0.41 a | 0.82 ± 0.44 a | 1.37 ± 0.56 b | 1.01 ± 0.50 A |
| Bamberka | 1.35 ± 0.09 b | 1.19 ± 0.24 ab | 2.77 ± 0.44 c | 1.77 ± 0.79 B | |
| Borowska | 1.56 ± 0.09 b | 1.37 ± 0.05 b | 2.76 ± 0.17 c | 1.90 ± 0.65 B | |
| Means for harvest time | 1.25 ± 0.39 A | 1.13 ± 0.35 A | 2.30 ± 0.79 B | ||
| Total carbohydrates | Warta | 46.69 ± 1.37 a | 49.50 ± 1.24 b | 57.34 ± 0.79 ef | 51.18 ± 4.82 A |
| Bamberka | 59.06 ± 0.54 fg | 51.87 ± 1.43 c | 60.12 ± 0.89 g | 57.02 ± 3.94 C | |
| Borowska | 46.66 ± 1.57 a | 56.82 ± 1.68 e | 53.90 ± 1.94 d | 52.46 ± 4.73 B | |
| Means for harvest time | 50.81 ± 6.20 A | 52.73 ± 3.45 B | 57.12 ± 2.91 C | ||
| Ash | Warta | 16.20 ± 0.24 ef | 14.87 ± 0.32 d | 11.76 ± 0.51 a | 14.28 ± 1.97 A |
| Bamberka | 16.63 ± 0.06 f | 13.66 ± 0.68 c | 12.72 ± 0.40 b | 14.34 ± 1.79 A | |
| Borowska | 17.96 ± 0.53 g | 15.69 ± 0.18 e | 11.95 ± 0.41 a | 15.20 ± 2.62 B | |
| Means for harvest time | 16.93 ± 0.84 C | 14.74 ± 0.96 B | 12.14 ± 0.59 A | ||
| Compound | Cultivar | Number of Days after Sowing | Means for Cultivar | ||
|---|---|---|---|---|---|
| 31 | 38 | 45 | |||
| Warta | 217 ± 4 e | 1755 ± 3 b | 163 ± 1 a | 1855 ± 25 A | |
| ABTS | Bamberka | 225 ± 3 f | 255 ± 1 g | 193 ± 3 c | 225 ± 27 C |
| Borowska | 173 ± 7 b | 273 ± 2 h | 200 ± 1 d | 216 ± 45 B | |
| Means for harvest time | 205 ± 24 B | 234 ± 45 C | 185 ± 17 A | ||
| FRAP | Warta | 1511 ± 98 c | 1549.39 ± 5 c | 1399.37 ± 6 b | 1486.54 ± 84 B |
| Bamberka | 1368 ± 12 b | 1890.06 ± 8 d | 922.57 ± 7 a | 1393.63 ± 419 A | |
| Borowska | 1539 ± 10 c | 1963.47 ± 15 e | 1523.29 ± 10 c | 1675.25 ± 217 C | |
| Means for harvest time | 1477 ± 94 B | 180 ± 192 C | 1282 ± 275 A | ||
| Total polyphenols | Warta | 827 ± 24 f | 7582 ± 14 c | 6048 ± 14 a | 7301 ± 986 A |
| Bamberka | 796 ± 14 d | 8194 ± 21 e | 8249 ± 21 f | 8135 ± 133 B | |
| Borowska | 689 ± 15 b | 8964 ± 29 g | 6036 ± 8 a | 7297 ± 1304 A | |
| Means for harvest time | 7709 ± 628 B | 824 ± 600 C | 6778 ± 1104 A | ||
| Compound | Borowska | Bamberka | Warta | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Number of Days after Sowing | |||||||||
| 31 | 38 | 45 | 31 | 38 | 45 | 31 | 38 | 45 | |
| Acacetin | 6.22 ± 0.29 e | 3.59 ± 0.09 b | 3.23 ± 0.02 a | 4.93 ± 0.19 d | 4.03 ± 0.16 c | 4.95 ± 0.03 d | 4.25 ± 0.09 c | 4.98 ± 0.02 d | 4.73 ± 0.20 d |
| Catechin | 40.06 ± 2.39 b | 33.42 ± 1.13 a | 44.08 ± 0.92 bc | 69.05 ± 0.40 e | 76.01 ± 4.92 f | 58.00 ± 2.60 d | 58.89 ± 0.09 d | 48.01 ± 0.25 c | 40.07 ± 0.37 b |
| Epicatechin | 95.79 ± 0.36 bc | 58.43 ± 1.75 a | 98.83 ± 7.47 bc | 92.56 ± 0.02 b | 98.61 ± 5.71 bc | 139.17 ± 2.94 d | 100.88 ± 0.56 c | 177.75 ± 0.94 e | 175.51 ± 0.30 e |
| Hispidulin | 2.02 ± 0.05 a | 2.25 ± 0.00 b | 2.01 ± 0.06 a | 8.78 ± 0.00 g | 2.08 ± 0.05 a | 2.37 ± 0.00 c | 2.53 ± 0.01 d | 3.41 ± 0.00 f | 3.04 ± 0.00 e |
| Kaempferol | 5.63 ± 0.07 a | 6.58 ± 0.02 d | 6.61 ± 0.00 d | 5.81 ± 0.05 ab | 6.10 ± 0.03 bc | 8.83 ± 0.53 f | 6.37 ± 0.07 cd | 10.10 ± 0.05 g | 8.04 ± 0.03 e |
| Luteolin | 3.88 ± 0.02 a | 7.07 ± 0.05 c | 4.38 ± 0.02 ab | 4.53 ± 2.97 ab | 6.59 ± 0.03 bc | 4.47 ± 0.35 ab | 3.00 ± 0.04 a | 5.08 ± 0.02 abc | 3.44 ± 0.02 a |
| Myricetin | 10.52 ± 0.03 e | 7.85 ± 0.05 c | 12.40 ± 0.15 f | 10.12 ± 0.03 d | 6.78 ± 0.08 a | 13.26 ± 0.05 g | 7.25 ± 0.03 b | 10.05 ± 0.21 d | 6.97 ± 0.07 a |
| Naringin | 2.62 ± 0.07 b | 2.88 ± 0.02 bc | 6.39 ± 0.27 e | 2.88 ± 0.02 bc | 1.89 ± 0.03 a | 4.57 ± 0.31 d | 3.11 ± 0.03 c | 17.92 ± 0.28 g | 11.15 ± 0.1 f |
| Quercetin | 5.65 ± 0.42 a | 8.17 ± 0.02 d | 7.14 ± 0.02 b | 7.90 ± 0.03 cd | 7.53 ± 0.02 bc | 5.51 ± 0.20 a | 8.16 ± 0.04 d | 10.52 ± 0.31 e | 8.24 ± 0.02 d |
| Rutin | 1365.07 ± 13.8 d | 2013.88 ± 1.61 f | 2286.00 ± 1.24 h | 2103.88 ± 4.86 g | 1760.55 ± 13.3 e | 1084.55 ± 50.9 c | 837.22 ± 0.53 b | 1115.81 ± 0.78 c | 583.12 ± 0.13 a |
| Caffeic acid | 7.24 ± 0.07 d | 6.25 ± 0.03 c | 9.30 ± 0.03 f | nd | nd | nd | 5.52 ± 0.19 b | 7.79 ± 0.02 e | 5.27 ± 0.02 a |
| Chlorogenic acid | 1.90 ± 0.02 b | 6.10 ± 0.00 g | 1.97 ± 0.06 b | 7.90 ± 0.03 h | 5.38 ± 0.10 f | 3.31 ± 0.02 d | 1.76 ± 0.01 a | 2.53 ± 0.08 c | 3.48 ± 0.02 e |
| Ferulic acid | 12.72 ± 0.66 b | 9.59 ± 0.05 a | 19.60 ± 0.02 e | 12.61 ± 0.19 b | 9.90 ± 0.77 a | 22.45 ± 0.56 f | 13.67 ± 0.19 c | 29.04 ± 0.03 g | 14.99 ± 0.08 d |
| Gallic acid | 8.23 ± 0.19 b | 9.41 ± 0.21 c | 11.22 ± 0.71 d | 13.64 ± 0.32 f | 12.31 ± 0.26 e | 7.18 ± 0.43 a | Nd | nd | nd |
| p-Coumaric acid | 4.98 ± 0.02 b | 4.40 ± 0.09 ab | 8.08 ± 0.18 c | 4.62 ± 0.05 ab | 4.06 ± 0.08 a | 10.44 ± 0.84 d | 8.32 ± 0.10 c | 16.45 ± 0.02 e | 7.88 ± 0.12 c |
| Sinapinic acid | 23.15 ± 0.67 b | 10.48 ± 0.31 a | 132.58 ± 0.02 c | 27.29 ± 1.99 b | 20.58 ± 1.32 b | 126.66 ± 7.59 c | 183.87 ± 7.63 e | 356.53 ± 0.17 f | 159.41 ± 0.57 d |
| Syringic acid | 2.32 ± 0.07 b | 2.33 ± 0.07 b | 5.76 ± 0.23 d | 1.85 ± 0.05 a | 8.17 ± 0.16 f | 24.86 ± 0.30 h | 7.71 ± 0.16 e | 4.24 ± 0.02 c | 20.69 ± 0.17 g |
| Vanillic acid | 3.03 ± 0.21 a | 3.27 ± 0.05 a | 7.66 ± 0.03 d | 5.96 ± 0.13 c | 4.82 ± 0.07 b | 22.48 ± 0.18 f | 9.83 ± 0.92 e | 8.37 ± 0.21 d | 3.39 ± 0.08 a |
| Carnosol | 47.84 ± 0.55 e | 17.18 ± 0.26 d | 16.01 ± 0.05 bc | 17.05 ± 0.05 d | 16.62 ± 0.10 cd | 16.14 ± 0.51 bc | 13.32 ± 0.07 a | 15.52 ± 0.30 b | 15.59 ± 0.15 b |
| Carnosolic acid | 44.13 ± 4.13 f | 23.42 ± 0.14 e | 19.89 ± 0.94 d | 5.70 ± 0.10 b | 9.18 ± 0.28 c | 48.71 ± 1.36 g | 1.50 ± 0.11 a | 5.38 ± 0.05 b | 3.75 ± 0.22 ab |
| TOTAL | 1692.99 ± 10.13 c | 2236.53 ± 0.88 f | 2703.14 ± 10.36 h | 2407.05 ± 0.05 g | 2061.18 ± 26.48 e | 1607.90 ± 51.06 c | 1277.15 ± 6.51 b | 1849.47 ± 1.70 d | 1078.75 ± 1.26 a |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska, U.; Jewiarz, K.; Kopak, M.; Dziadek, K.; Francik, R.; Kopeć, A. Proximate Analysis and Antioxidant Properties of Young Plants of Sinapis alba L. Depend on the Time of Harvest and Variety. Appl. Sci. 2023, 13, 7980. https://doi.org/10.3390/app13137980
Sadowska U, Jewiarz K, Kopak M, Dziadek K, Francik R, Kopeć A. Proximate Analysis and Antioxidant Properties of Young Plants of Sinapis alba L. Depend on the Time of Harvest and Variety. Applied Sciences. 2023; 13(13):7980. https://doi.org/10.3390/app13137980
Chicago/Turabian StyleSadowska, Urszula, Klaudia Jewiarz, Magdalena Kopak, Kinga Dziadek, Renata Francik, and Aneta Kopeć. 2023. "Proximate Analysis and Antioxidant Properties of Young Plants of Sinapis alba L. Depend on the Time of Harvest and Variety" Applied Sciences 13, no. 13: 7980. https://doi.org/10.3390/app13137980
APA StyleSadowska, U., Jewiarz, K., Kopak, M., Dziadek, K., Francik, R., & Kopeć, A. (2023). Proximate Analysis and Antioxidant Properties of Young Plants of Sinapis alba L. Depend on the Time of Harvest and Variety. Applied Sciences, 13(13), 7980. https://doi.org/10.3390/app13137980






