Acid-Catalyzed Organosolv Treatment of Potato Peels to Boost Release of Polyphenolic Compounds Using 1- and 2-Propanol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Reagents and Chemicals
2.2. Collection and Handling of Potato Peels (PP)
2.3. Solvent Screening and Organosolv Treatment
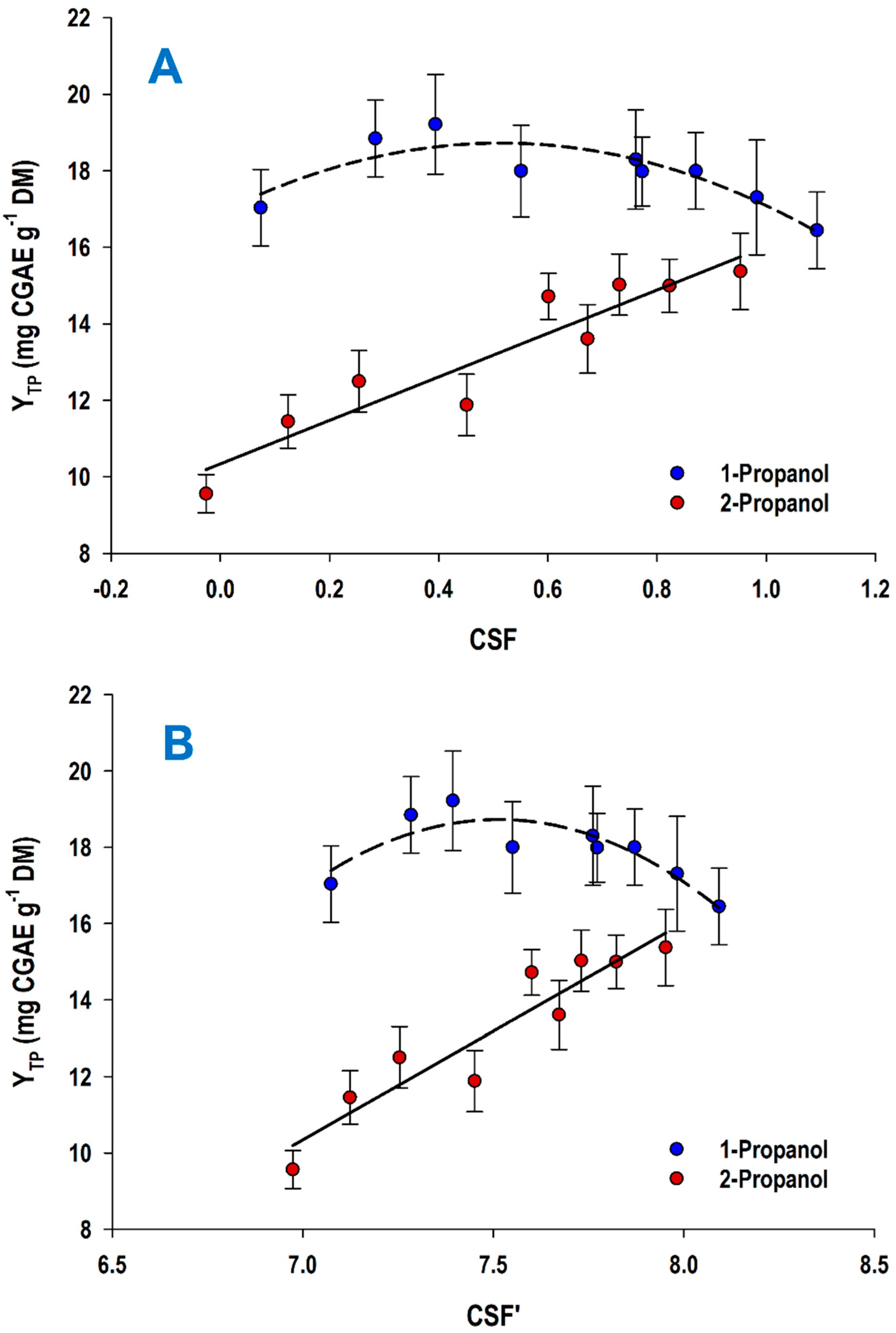
2.4. Determination of Severity Factor
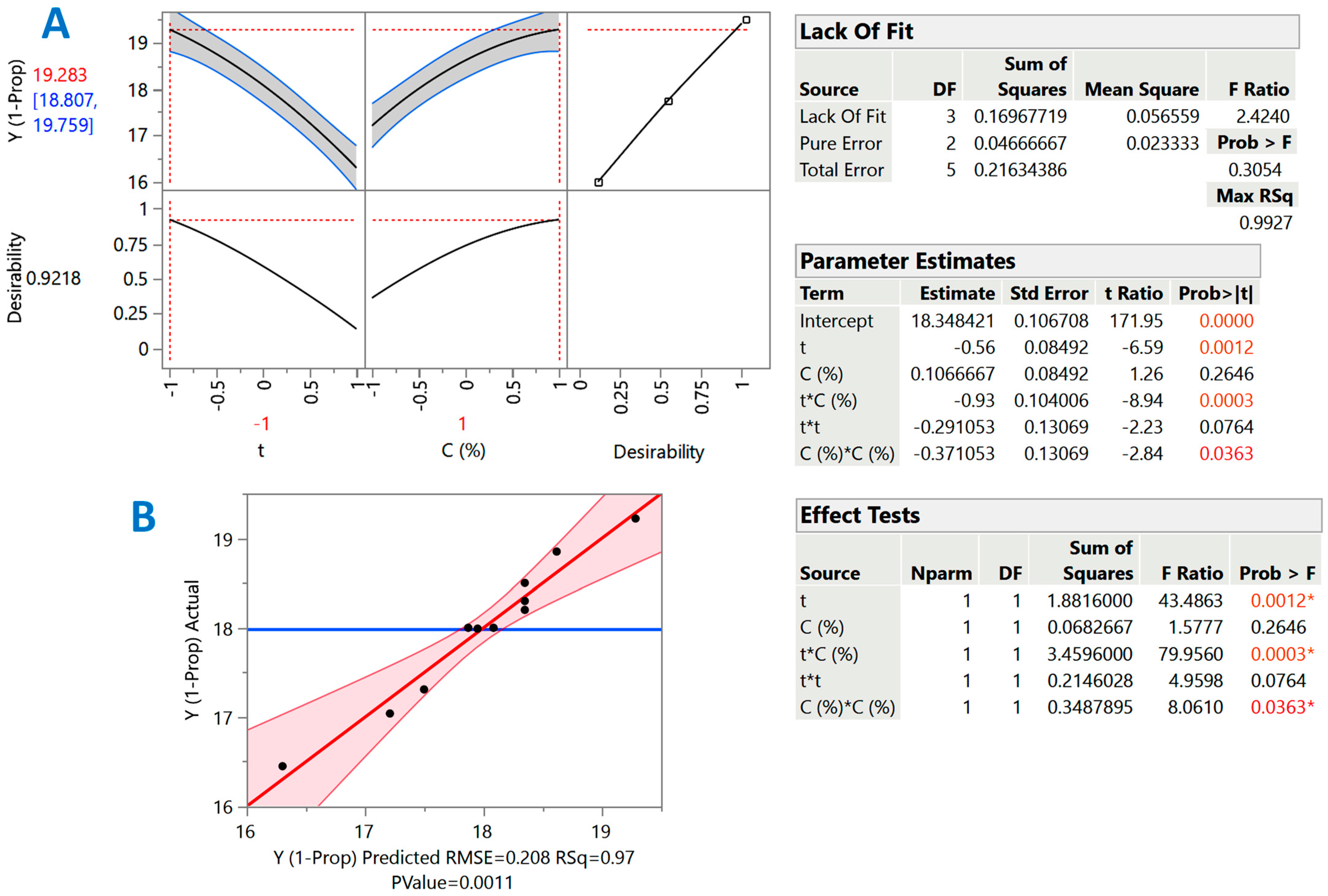
2.5. Design of Experiment and Response Surface Optimization
2.6. Determinations
2.7. Liquid Chromatography—Tandem Mass Spectrometry (LC-MS/MS)
2.8. Statistical Analyses
3. Results and Discussion
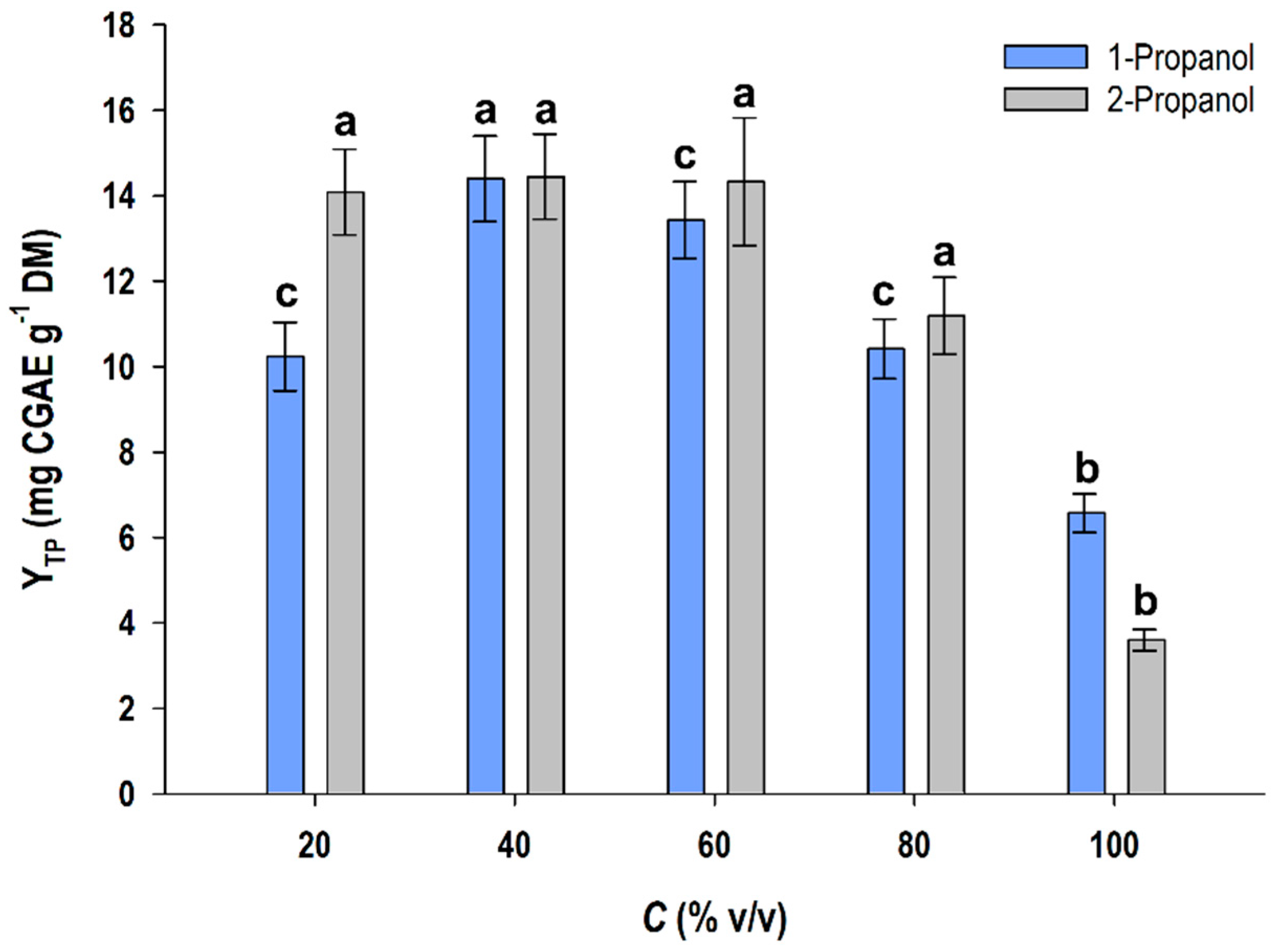
3.1. Solvent Assay
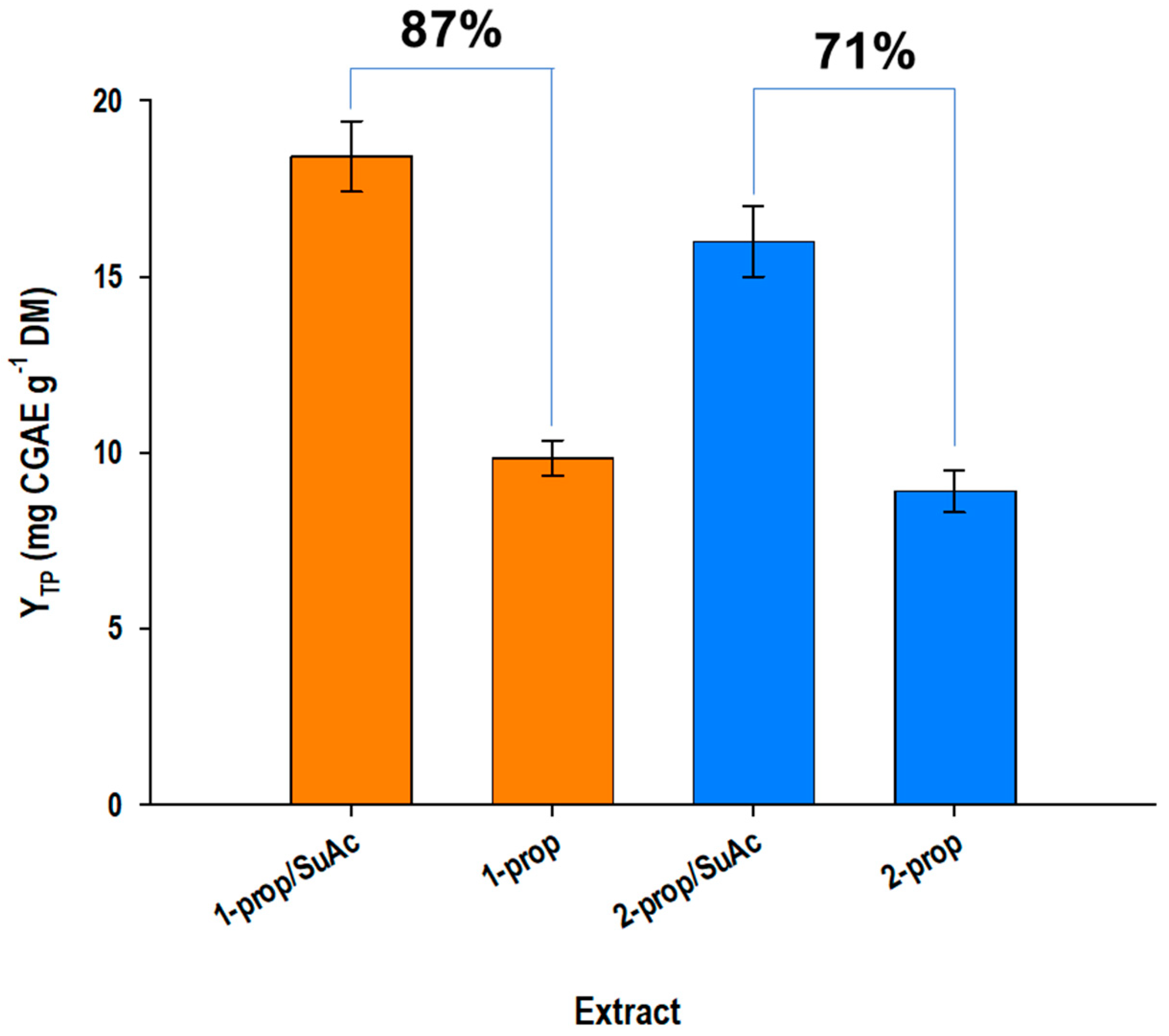
3.2. Acid-Catalyzed Treatment and Solvents Effects
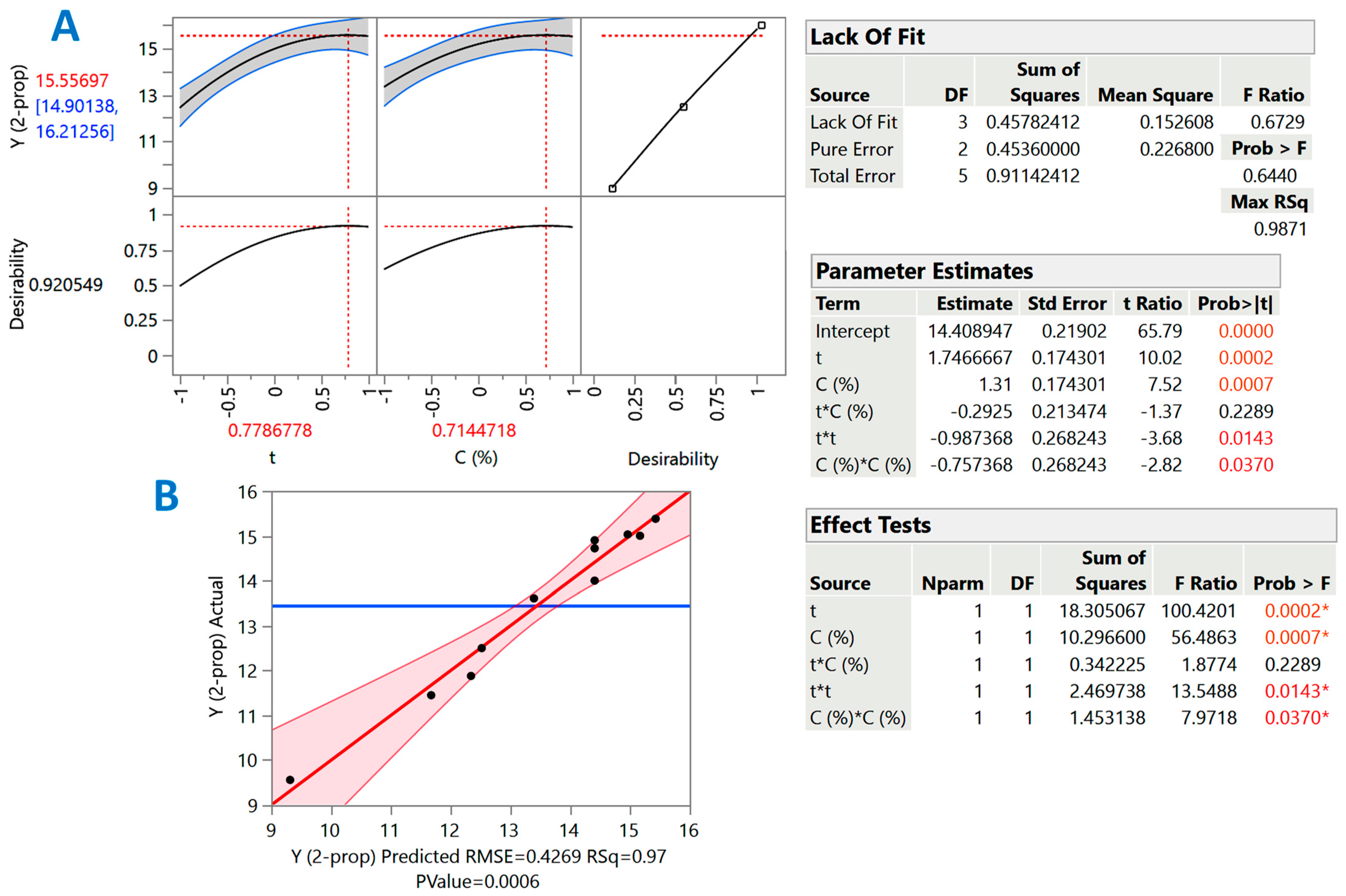
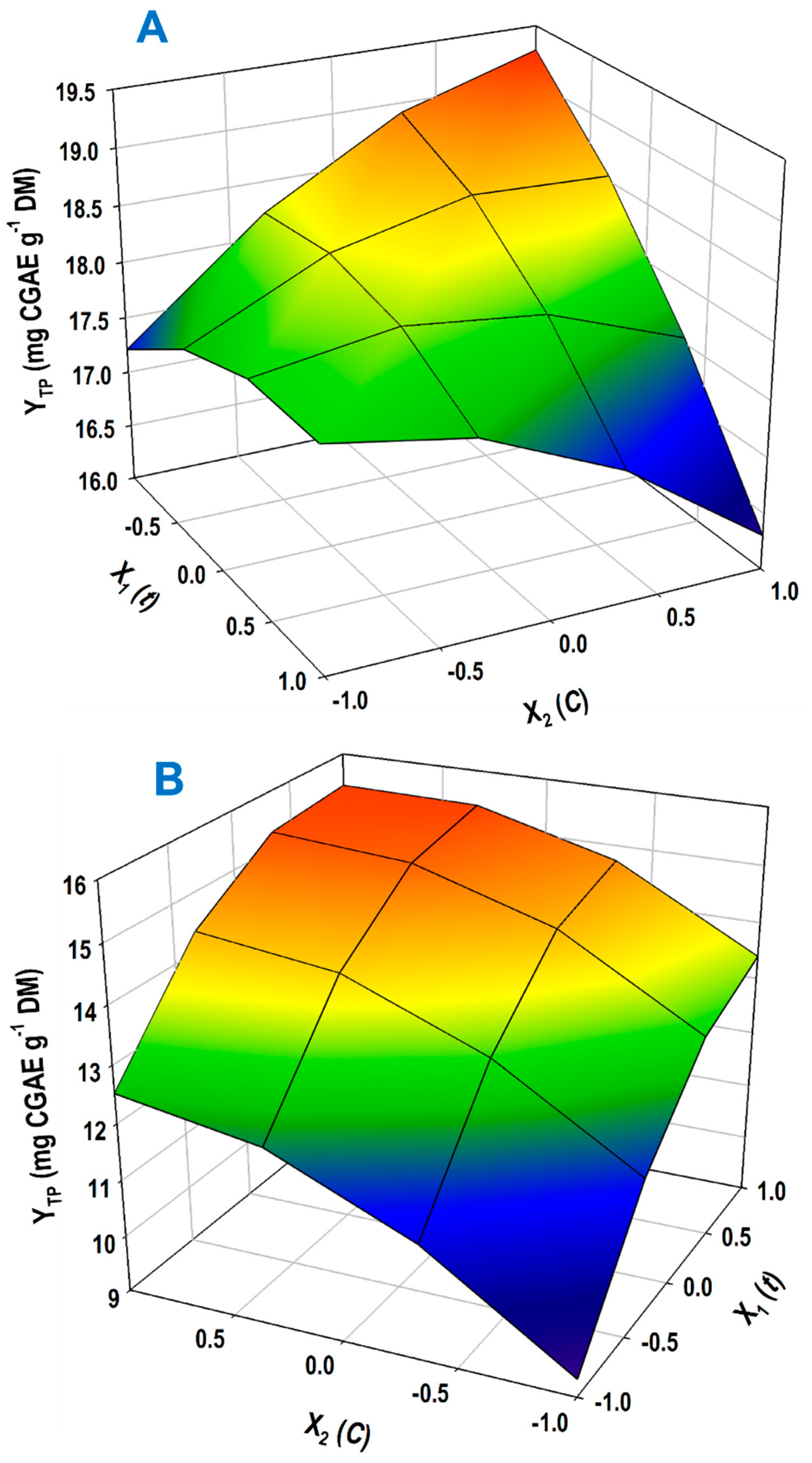
3.3. Response Surface Process Optimization
3.4. Polyphenolic Composition and Antioxidant Activity
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bakan, B.; Bernet, N.; Bouchez, T.; Boutrou, R.; Choubert, J.-M.; Dabert, P.; Duquennoi, C.; Ferraro, V.; Garcia-Bernet, D.; Gillot, S. Circular economy applied to organic residues and wastewater: Research challenges. Waste Biomass Valoriz. 2022, 13, 1267–1276. [Google Scholar]
- Yaashikaa, P.; Kumar, P.S.; Varjani, S. Valorization of agro-industrial wastes for biorefinery process and circular bioeconomy: A critical review. Bioresour. Technol. 2022, 343, 126126. [Google Scholar]
- Amran, M.A.; Palaniveloo, K.; Fauzi, R.; Satar, N.M.; Mohidin, T.B.M.; Mohan, G.; Razak, S.A.; Arunasalam, M.; Nagappan, T.; Sathiya Seelan, J.S. Value-added metabolites from agricultural waste and application of green extraction techniques. Sustainability 2021, 13, 11432. [Google Scholar] [CrossRef]
- Monteiro, A.R.; Battisti, A.P.; Valencia, G.A.; de Andrade, C.J. The Production of High-Added-Value Bioproducts from Non-Conventional Biomasses: An Overview. Biomass 2023, 3, 123–137. [Google Scholar] [CrossRef]
- De Camargo, A.C.; Schwember, A.R.; Parada, R.; Garcia, S.; Maróstica Júnior, M.R.; Franchin, M.; Regitano-d’Arce, M.A.B.; Shahidi, F. Opinion on the hurdles and potential health benefits in value-added use of plant food processing by-products as sources of phenolic compounds. Int. J. Mol. Sci. 2018, 19, 3498. [Google Scholar] [CrossRef] [PubMed]
- Chemat, F.; Vian, M.A.; Ravi, H.K. Toward petroleum-free with plant-based chemistry. Curr. Opin. Green Sustain. Chem. 2021, 28, 100450. [Google Scholar] [CrossRef]
- Carpentieri, S.; Soltanipour, F.; Ferrari, G.; Pataro, G.; Donsì, F. Emerging green techniques for the extraction of antioxidants from agri-food by-products as promising ingredients for the food industry. Antioxidants 2021, 10, 1417. [Google Scholar] [CrossRef] [PubMed]
- Ravindran, R.; Desmond, C.; Jaiswal, S.; Jaiswal, A.K. Optimisation of organosolv pretreatment for the extraction of polyphenols from spent coffee waste and subsequent recovery of fermentable sugars. Bioresour. Technol. Rep. 2018, 3, 7–14. [Google Scholar] [CrossRef]
- Wei Kit Chin, D.; Lim, S.; Pang, Y.L.; Lam, M.K. Fundamental review of organosolv pretreatment and its challenges in emerging consolidated bioprocessing. Biofuels Bioprod. Biorefin. 2020, 14, 808–829. [Google Scholar] [CrossRef]
- Sun, C.; Song, G.; Pan, Z.; Tu, M.; Kharaziha, M.; Zhang, X.; Show, P.-L.; Sun, F. Advances in organosolv modified components occurring during the organosolv pretreatment of lignocellulosic biomass. Bioresour. Technol. 2022, 368, 128356. [Google Scholar]
- Chemat, F.; Rombaut, N.; Sicaire, A.-G.; Meullemiestre, A.; Fabiano-Tixier, A.-S.; Abert-Vian, M. Ultrasound assisted extraction of food and natural products. Mechanisms, techniques, combinations, protocols and applications. A review. Ultrason. Sonochem. 2017, 34, 540–560. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Li, S.; Wu, R.; Liu, D. Organosolv fractionating pre-treatment of lignocellulosic biomass for efficient enzymatic saccharification: Chemistry, kinetics, and substrate structures. Biofuels Bioprod. Biorefin. 2017, 11, 567–590. [Google Scholar] [CrossRef]
- Zhu, S.; Guo, J.; Wang, X.; Wang, J.; Fan, W. Alcoholysis: A promising technology for conversion of lignocellulose and platform chemicals. ChemSusChem 2017, 10, 2547–2559. [Google Scholar] [CrossRef]
- Vaidya, A.A.; Murton, K.D.; Smith, D.A.; Dedual, G. A review on organosolv pretreatment of softwood with a focus on enzymatic hydrolysis of cellulose. Biomass Convers. Biorefin. 2022, 12, 5427–5442. [Google Scholar] [CrossRef]
- Rodríguez-Martínez, B.; Gullón, B.; Yáñez, R. Identification and recovery of valuable bioactive compounds from potato peels: A comprehensive review. Antioxidants 2021, 10, 1630. [Google Scholar] [CrossRef]
- Rasheed, H.; Ahmad, D.; Bao, J. Genetic diversity and health properties of polyphenols in potato. Antioxidants 2022, 11, 603. [Google Scholar] [CrossRef]
- Eliopoulos, C.; Markou, G.; Langousi, I.; Arapoglou, D. Reintegration of food industry by-products: Potential applications. Foods 2022, 11, 3743. [Google Scholar] [CrossRef]
- Bozinou, E.; Palaiogiannis, D.; Athanasiadis, V.; Chatzilazarou, A.; Lalas, S.I.; Makris, D.P. Glycerol-based deep eutectic solvents for simultaneous organosolv treatment/extraction: High-performance recovery of antioxidant polyphenols from onion solid wastes. Sustainability 2022, 14, 15715. [Google Scholar] [CrossRef]
- Shaheen, S.; Grigorakis, S.; Halahlah, A.; Loupassaki, S.; Makris, D.P. Extractor dimensions affect optimization of laboratory-scale batch solid-liquid extraction of polyphenols from plant material: Potato peels as a case study. Chem. Eng. Commun. 2021, 208, 1618–1629. [Google Scholar] [CrossRef]
- Morsli, F.; Grigorakis, S.; Halahlah, A.; Poulianiti, K.P.; Makris, D.P. Appraisal of the combined effect of time and temperature on the total polyphenol yield in batch stirred-tank extraction of medicinal and aromatic plants: The extraction efficiency factor. J. Appl. Res. Med. Arom. Plants 2021, 25, 100340. [Google Scholar] [CrossRef]
- Papadaki, E.S.; Palaiogiannis, D.; Lalas, S.I.; Mitlianga, P.; Makris, D.P. Polyphenol release from wheat bran using ethanol-based organosolv treatment and acid/alkaline catalysis: Process modeling based on severity and response surface optimization. Antioxidants 2022, 11, 2457. [Google Scholar] [CrossRef]
- Overend, R.P.; Chornet, E. Fractionation of lignocellulosics by steam-aqueous pretreatments. Phil. Trans. R. Soc. Lond. Ser. A Math. Phys. Sci. 1987, 321, 523–536. [Google Scholar]
- Sidiras, D.; Politi, D.; Giakoumakis, G.; Salapa, I. Simulation and optimization of organosolv based lignocellulosic biomass refinery: A review. Bioresour. Technol. 2022, 343, 126158. [Google Scholar] [CrossRef]
- Pedersen, M.; Meyer, A.S. Lignocellulose pretreatment severity–relating pH to biomatrix opening. New Biotechnol. 2010, 27, 739–750. [Google Scholar] [CrossRef] [PubMed]
- Athanasiadis, V.; Palaiogiannis, D.; Bozinou, E.; Lalas, S.I.; Makris, D.P. β-Cyclodextrin-aided aqueous extraction of antioxidant polyphenols from peppermint (Mentha × piperita L.). Oxygen 2022, 2, 424–436. [Google Scholar] [CrossRef]
- Lakka, A.; Lalas, S.; Makris, D.P. Development of a low-temperature and high-performance green extraction process for the recovery of polyphenolic phytochemicals from waste potato peels using hydroxypropyl β-cyclodextrin. Appl. Sci. 2020, 10, 3611. [Google Scholar] [CrossRef]
- Wang, S.; Lin, A.H.-M.; Han, Q.; Xu, Q. Evaluation of direct ultrasound-assisted extraction of phenolic compounds from potato peels. Processes 2020, 8, 1665. [Google Scholar] [CrossRef]
- Wu, T.; Yan, J.; Liu, R.; Marcone, M.F.; Aisa, H.A.; Tsao, R. Optimization of microwave-assisted extraction of phenolics from potato and its downstream waste using orthogonal array design. Food Chem. 2012, 133, 1292–1298. [Google Scholar] [CrossRef]
- Riciputi, Y.; Diaz-de-Cerio, E.; Akyol, H.; Capanoglu, E.; Cerretani, L.; Caboni, M.F.; Verardo, V. Establishment of ultrasound-assisted extraction of phenolic compounds from industrial potato by-products using response surface methodology. Food Chem. 2018, 269, 258–263. [Google Scholar] [CrossRef]
- Alvarez, V.H.; Cahyadi, J.; Xu, D.; Saldaña, M.D. Optimization of phytochemicals production from potato peel using subcritical water: Experimental and dynamic modeling. J. Supercrit. Fluids 2014, 90, 8–17. [Google Scholar] [CrossRef]
- Martinez-Fernandez, J.S.; Seker, A.; Davaritouchaee, M.; Gu, X.; Chen, S. Recovering valuable bioactive compounds from potato peels with sequential hydrothermal extraction. Waste Biomass Valoriz. 2021, 12, 1465–1481. [Google Scholar]
- Zhu, X.; Cheng, Y.; Chen, P.; Peng, P.; Liu, S.; Li, D.; Ruan, R. Effect of alkaline and high-pressure homogenization on the extraction of phenolic acids from potato peels. Innov. Food Sci. Emerg. Technol. 2016, 37, 91–97. [Google Scholar] [CrossRef]
- Kumari, B.; Tiwari, B.K.; Hossain, M.B.; Rai, D.K.; Brunton, N.P. Ultrasound-assisted extraction of polyphenols from potato peels: Profiling and kinetic modelling. Int. J. Food Sci. Technol. 2017, 52, 1432–1439. [Google Scholar] [CrossRef]
- López-Cobo, A.; Gómez-Caravaca, A.M.; Cerretani, L.; Segura-Carretero, A.; Fernández-Gutiérrez, A. Distribution of phenolic compounds and other polar compounds in the tuber of Solanum tuberosum L. by HPLC-DAD-q-TOF and study of their antioxidant activity. J. Food Compos. Anal. 2014, 36, 1–11. [Google Scholar] [CrossRef]
- Jimenez-Champi, D.; Romero-Orejon, F.L.; Moran-Reyes, A.; Muñoz, A.M.; Ramos-Escudero, F. Bioactive compounds in potato peels, extraction methods, and their applications in the food industry: A review. CyTA J. Food 2023, 21, 418–432. [Google Scholar] [CrossRef]
- Heo, H.J.; Kim, Y.J.; Chung, D.; Kim, D.-O. Antioxidant capacities of individual and combined phenolics in a model system. Food Chem. 2007, 104, 87–92. [Google Scholar] [CrossRef]
- Abou Samra, M.; Chedea, V.S.; Economou, A.; Calokerinos, A.; Kefalas, P. Antioxidant/prooxidant properties of model phenolic compounds: Part I. Studies on equimolar mixtures by chemiluminescence and cyclic voltammetry. Food Chem. 2011, 125, 622–629. [Google Scholar] [CrossRef]
- Choueiri, L.; Chedea, V.S.; Calokerinos, A.; Kefalas, P. Antioxidant/pro-oxidant properties of model phenolic compounds. Part II: Studies on mixtures of polyphenols at different molar ratios by chemiluminescence and LC–MS. Food Chem. 2012, 133, 1039–1044. [Google Scholar]
| Process Variables | Codes | Coded Variable Level | ||
|---|---|---|---|---|
| −1 | 0 | 1 | ||
| t (min) | X1 | 60 | 180 | 300 |
| CSuAc (% w/v) | X2 | 0.5 | 5.0 | 1.5 |
| CSuAc (% w/v) | t (min) | CSF | CSF′ | YTP (mg CGAE g−1 DM) | |||
|---|---|---|---|---|---|---|---|
| 1-Pr | 2-Pr | 1-Pr | 2-Pr | 1-Pr | 2-Pr | ||
| 0.5 | 60 | 0.07 a | −0.03 a | 7.07 a | 6.97 a | 17.04 a | 9.56 a |
| 180 | 0.55 c | 0.45 c | 7.55 c | 7.45 c | 18.00 c | 11.88 c | |
| 300 | 0.77 c | 0.67 c | 7.77 c | 7.67 c | 17.99 c | 13.61 c | |
| 1.0 | 60 | 0.28 a | 0.12 a | 7.28 a | 7.12 a | 18.85 b | 11.45 a |
| 180 | 0.76 c | 0.60 c | 7.76 c | 7.60 c | 18.30 c | 14.72 c | |
| 300 | 0.98 b | 0.82 b | 7.98 b | 7.82 b | 17.31 c | 15.00 b | |
| 1.5 | 60 | 0.39 c | 0.25 a | 7.39 c | 7.25 c | 19.22 b | 12.50 c |
| 180 | 0.87 c | 0.73 c | 7.87 c | 7.73 c | 18.00 c | 15.03 b | |
| 300 | 1.09 b | 0.95 b | 8.09 b | 7.95 b | 16.45 a | 15.38 b | |
| Design Point | Independent Variables | Response (YTP, mg CGAE g−1 DM) | ||||
|---|---|---|---|---|---|---|
| X1 (t) | X2 (CSuAc) | 1-Propanol | 2-Propanol | |||
| Measured | Predicted | Measured | Predicted | |||
| 1 | −1 | −1 | 17.04 | 17.21 | 9.56 | 9.32 |
| 2 | −1 | 1 | 19.22 | 19.28 | 12.5 | 12.52 |
| 3 | 1 | −1 | 17.99 | 17.95 | 13.61 | 13.39 |
| 4 | 1 | 1 | 16.45 | 16.30 | 15.38 | 15.43 |
| 5 | −1 | 0 | 18.85 | 18.62 | 11.45 | 11.67 |
| 6 | 1 | 0 | 17.31 | 17.50 | 15.00 | 15.17 |
| 7 | 0 | −1 | 18.02 | 17.87 | 11.88 | 12.34 |
| 8 | 0 | 1 | 18.07 | 18.08 | 15.03 | 14.96 |
| 9 | 0 | 0 | 18.30 | 18.35 | 14.72 | 14.41 |
| 10 | 0 | 0 | 18.22 | 18.35 | 14.01 | 14.41 |
| 11 | 0 | 0 | 18.54 | 18.35 | 14.91 | 14.41 |
| Sample | Phenolic Acid Content (μg g−1 DM) | Total | AAR (μmol DPPH g−1 DM) | PR (μmol AAE g−1 DM) | ||
|---|---|---|---|---|---|---|
| CA | CGA | p-CouA | ||||
| 40% 1-prop/1.5% SuAc | 772.25 ± 54.30 | 1771.84 ± 98.52 | 17.00 ± 2.34 | 2561.00 | 148.66 ± 10.32 | 29.94 ± 1.85 |
| 40% 2-prop/1.36% SuAc | 764.20 ± 63.02 | 1181.80 ± 84.61 | 24.04 ± 1.87 | 1970.00 | 192.03 ± 14.20 | 24.88 ± 2.02 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casasni, S.; Guenaoui, A.; Grigorakis, S.; Makris, D.P. Acid-Catalyzed Organosolv Treatment of Potato Peels to Boost Release of Polyphenolic Compounds Using 1- and 2-Propanol. Appl. Sci. 2023, 13, 9484. https://doi.org/10.3390/app13169484
Casasni S, Guenaoui A, Grigorakis S, Makris DP. Acid-Catalyzed Organosolv Treatment of Potato Peels to Boost Release of Polyphenolic Compounds Using 1- and 2-Propanol. Applied Sciences. 2023; 13(16):9484. https://doi.org/10.3390/app13169484
Chicago/Turabian StyleCasasni, Selma, Akram Guenaoui, Spyros Grigorakis, and Dimitris P. Makris. 2023. "Acid-Catalyzed Organosolv Treatment of Potato Peels to Boost Release of Polyphenolic Compounds Using 1- and 2-Propanol" Applied Sciences 13, no. 16: 9484. https://doi.org/10.3390/app13169484
APA StyleCasasni, S., Guenaoui, A., Grigorakis, S., & Makris, D. P. (2023). Acid-Catalyzed Organosolv Treatment of Potato Peels to Boost Release of Polyphenolic Compounds Using 1- and 2-Propanol. Applied Sciences, 13(16), 9484. https://doi.org/10.3390/app13169484