Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Methods
2.2.1. Formulation of the Hydrogel
2.2.2. Visual and Sensory Inspection of Prepared Formulations
2.2.3. pH Determination
2.2.4. Spreadability Test
2.2.5. Rheology Measurements
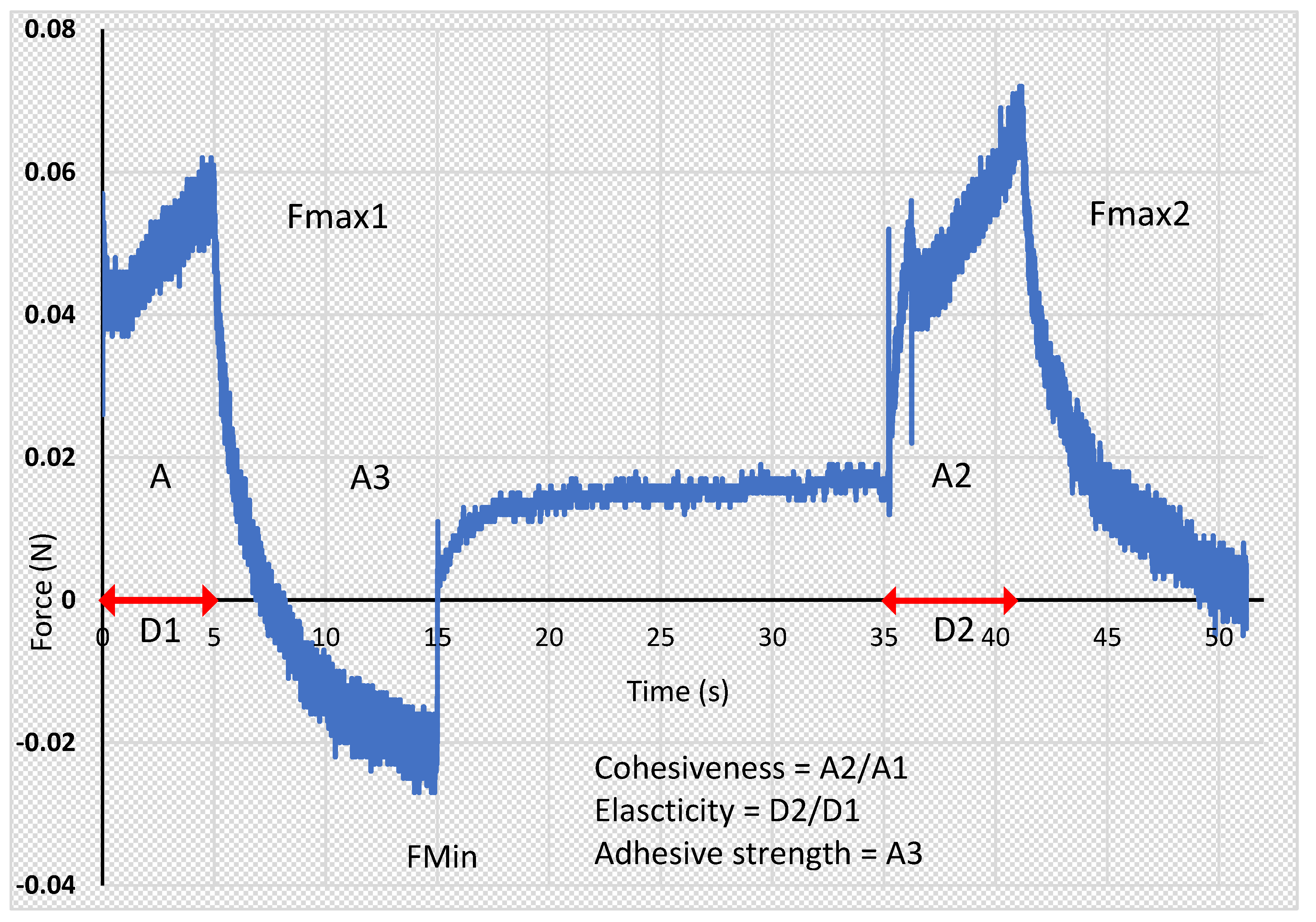
2.2.6. Texture Analysis
2.2.7. Stability Test
2.2.8. Ex Vivo Skin Permeation Experiments
2.2.9. Statistical Analysis
3. Results
3.1. Visual and Sensory Inspection of Prepared Formulations
3.2. pH Determination
3.3. Spreadability Test
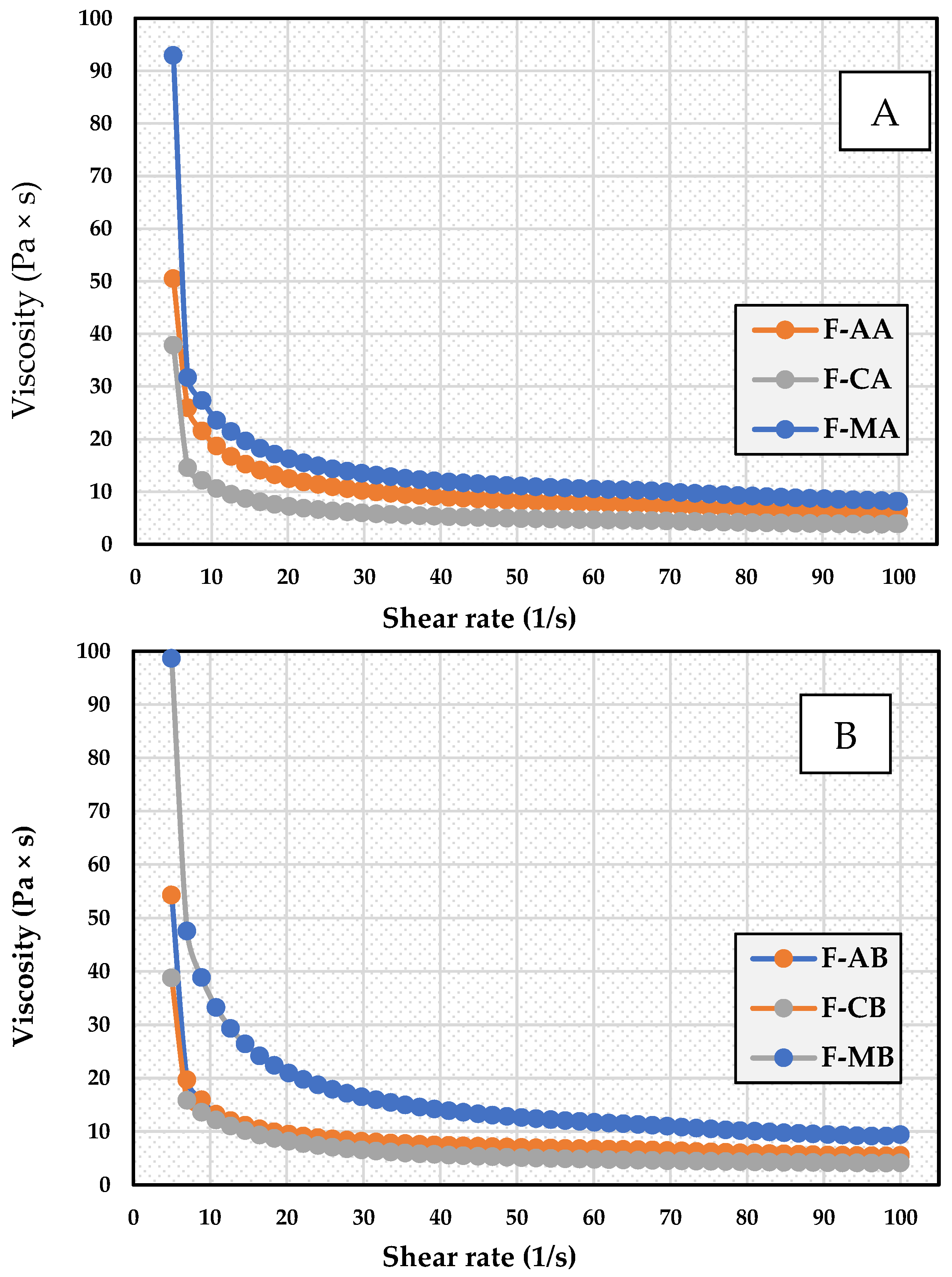
3.4. Apparent Viscosity
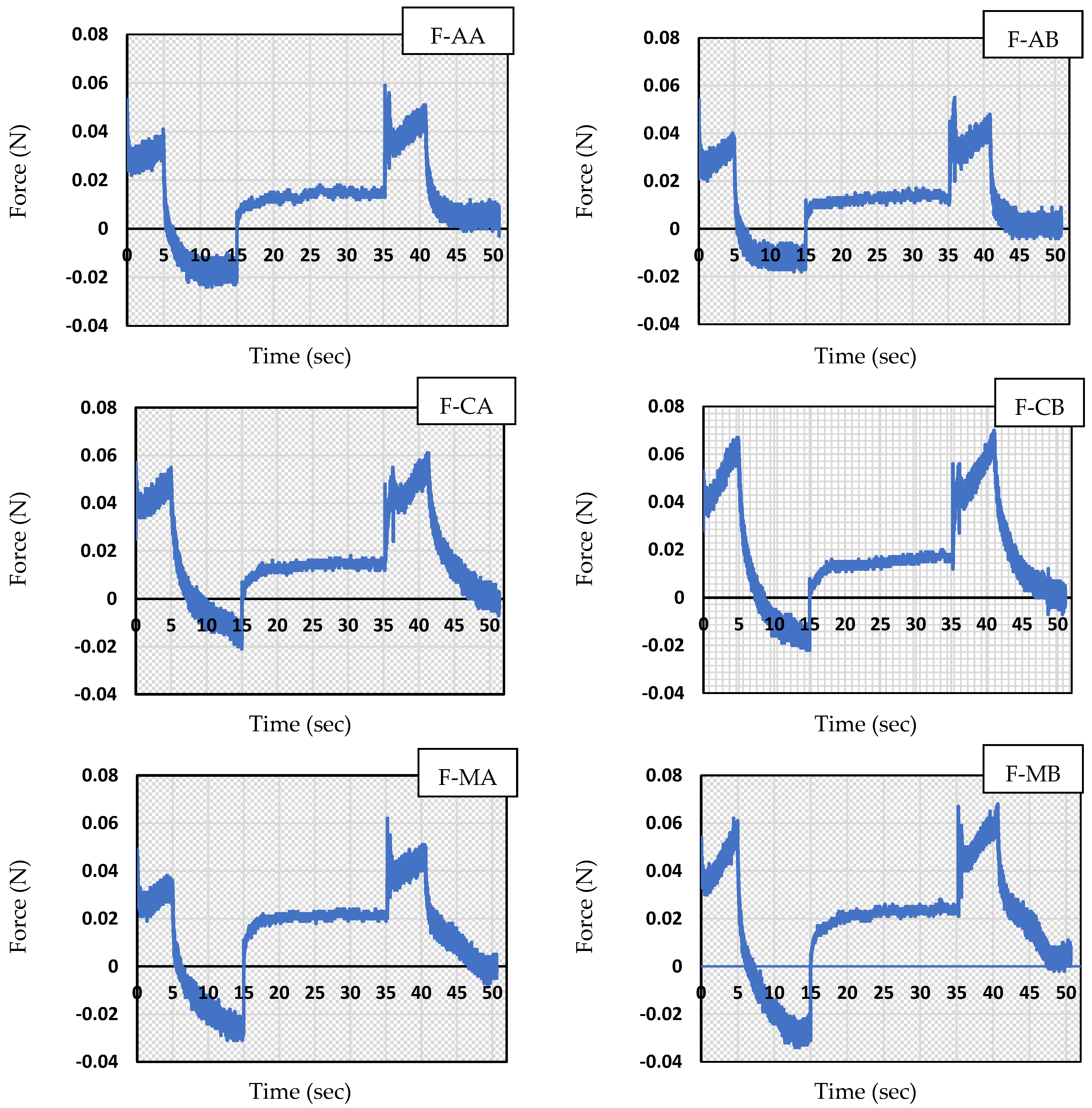
3.5. Texture Analysis Parameters
3.6. Stability Studies
3.7. Ex Vivo Skin Permeation Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Anand David, A.V.; Arulmoli, R.; Parasuraman, S. Overviews of biological importance of quercetin: A bioactive flavonoid. Pharmacogn. Rev. 2016, 10, 84–89. [Google Scholar] [PubMed]
- Seo, M.J.; Lee, Y.J.; Hwang, J.H.; Kim, K.J.; Lee, B.Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef]
- Li, D.; Jiang, C.; Mei, G.; Zhao, Y.; Chen, L.; Liu, J.; Tang, Y.; Gao, C.; Yao, P. Quercetin alleviates ferroptosis of pancreatic β cells in type 2 diabetes. Nutrients 2020, 12, 2954. [Google Scholar] [CrossRef] [PubMed]
- Ghafouri-Fard, S.; Shabestari, F.A.; Vaezi, S.; Abak, A.; Shoorei, H.; Karimi, A.; Taheri, M.; Basiri, A. Emerging impact of quercetin in the treatment of prostate cancer. Biomed. Pharmacother. 2021, 138, 111548. [Google Scholar] [CrossRef]
- Ebrahimpour, S.; Zakeri, M.; Esmaeili, A. Crosstalk between obesity, diabetes, and alzheimer’s disease: Introducing quercetin as an effective triple herbal medicine. Ageing Res. Rev. 2020, 62, 101095. [Google Scholar] [CrossRef] [PubMed]
- Guan, T.; Cao, C.; Hou, Y.; Li, Y.; Wei, X.; Li, S.; Jia, S.; Zhao, X. Effects of quercetin on the alterations of serum elements in chronic unpredictable mild stress-induced depressed rats. Biometals 2021, 34, 589–602. [Google Scholar] [CrossRef]
- Wu, W.; Li, R.; Li, X.; He, J.; Jiang, S.; Liu, S.; Yang, J. Quercetin as an antiviral agent inhibits influenza A virus (IAV) entry. Viruses 2015, 8, 6. [Google Scholar] [CrossRef]
- Khan, K.; Najmi, A.K.; Akhtar, M. A natural phenolic compound quercetin showed the usefulness by targeting inflammatory, oxidative stress markers and augment 5-ht levels in one of the animal models of depression in mice. Drug Res. 2019, 69, 392–400. [Google Scholar] [CrossRef]
- Winnica, D.E.; Monzon, A.; Ye, S.; Vladar, E.K.; Saal, M.; Cooney, R.; Liu, C.; Sharma, S.; Holguin, F. Airway epithelial Paraoxonase-2 in obese asthma. PLoS ONE 2022, 17, e0261504. [Google Scholar] [CrossRef]
- Cesarone, M.R.; Belcaro, G.; Hu, S.; Dugall, M.; Hosoi, M.; Ledda, A.; Feragalli, B.; Maione, C.; Cotellese, R. Supplementary prevention and management of asthma with quercetin phytosome: A pilot registry. Minerva Med. 2019, 110, 524–529. [Google Scholar] [CrossRef]
- Lesjak, M.; Beara, I.; Simin, N.; Pintać, D.; Majkić, T.; Bekvalac, K.; Orčić, D.; Mimica-Dukić, N. Antioxidant and anti-inflammatory activities of quercetin and its derivatives. J. Funct. Foods 2018, 40, 68–75. [Google Scholar] [CrossRef]
- Li, H.; Chen, F.J.; Yang, W.L.; Qiao, H.Z.; Zhang, S.J. Quercetin improves cognitive disorder in aging mice by inhibiting Nlrp3 inflammasome activation. Food Funct. 2021, 12, 717–725. [Google Scholar] [CrossRef] [PubMed]
- Alkushi, A.G.R.; Elsawy, N.A.M. Quercetin attenuates, indomethacin-induced acute gastric ulcer in rats. Folia Morphol. 2017, 76, 252–261. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Tawab, M.S.; Mostafa Tork, O.; Mostafa-Hedeab, G.; Ewaiss Hassan, M.; Azmy Elberry, D. Protective effects of quercetin and melatonin on indomethacin induced gastric ulcers in rats. Rep. Biochem. Mol. Biol. 2020, 9, 278–290. [Google Scholar] [CrossRef]
- Ma, Z.X.; Zhang, R.Y.; Rui, W.J.; Wang, Z.Q.; Feng, X. Quercetin alleviates chronic unpredictable mild stress-induced depressive-like behaviors by promoting adult hippocampal neurogenesis via FoxG1/CREB/ BDNF signaling pathway. Behav. Brain Res. 2021, 406, 113245. [Google Scholar] [CrossRef]
- Grewal, A.K.; Singh, T.G.; Sharma, D.; Sharma, V.; Singh, M.; Rahman, M.H.; Najda, A.; Walasek-Janusz, M.; Kamel, M.; Albadrani, G.M.; et al. Mechanistic insights and perspectives involved in neuroprotective action of quercetin. Biomed. Pharmacother. 2021, 140, 111729. [Google Scholar] [CrossRef]
- Jiang, Y.H.; Jiang, L.Y.; Wang, Y.C.; Ma, D.F.; Li, X. Quercetin attenuates atherosclerosis via modulating oxidized LDL-induced endothelial cellular senescence. Front. Pharmacol. 2020, 11, 512. [Google Scholar] [CrossRef]
- Li, H.; Xiao, L.; He, H.; Zeng, H.; Liu, J.; Jiang, C.; Mei, G.; Yu, J.; Chen, H.; Yao, P.; et al. Quercetin attenuates atherosclerotic inflammation by inhibiting galectin-3-NLRP3 signaling pathway. Mol. Nutr. Food Res. 2021, 65, e2000746. [Google Scholar] [CrossRef]
- Luo, X.; Weng, X.; Bao, X.; Bai, X.; Lv, Y.; Zhang, S.; Chen, Y.; Zhao, C.; Zeng, M.; Huang, J.; et al. A novel anti-atherosclerotic mechanism of quercetin: Competitive binding to KEAP1 via Arg483 to inhibit macrophage pyroptosis. Redox Biol. 2022, 57, 102511. [Google Scholar] [CrossRef]
- Dehghani, F.; Sezavar Seyedi Jandaghi, S.H.; Janani, L.; Sarebanhassanabadi, M.; Emamat, H.; Vafa, M. Effects of quercetin supplementation on inflammatory factors and quality of life in post-myocardial infarction patients: A double blind, placebo-controlled, randomized clinical trial. Phytother. Res. 2021, 35, 2085–2098. [Google Scholar] [CrossRef]
- Majewska, M.; Czeczot, H. Flavonoids in prevention and therapy of diseases. Farm. Pol. 2009, 65, 369–377. [Google Scholar]
- Batiha, G.E.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef]
- Salehi, B.; Machin, L.; Monzote, L.; Sharifi-Rad, J.; Ezzat, S.M.; Salem, M.A.; Merghany, R.M.; El Mahdy, N.M.; Kılıç, C.S.; Sytar, O.; et al. Therapeutic potential of quercetin: New insights and perspectives for human health. ACS Omega 2020, 5, 11849–11872. [Google Scholar] [CrossRef] [PubMed]
- Woźnicka, E.; Kuźniar, A.; Nowak, D.; Nykiel, E.; Kopacz, M.; Gruszecka, J.; Golec, K. Comparative study on the antibacterial activity of some flavonoids and their sulfonic derivatives. Acta Pol. Pharm. 2013, 70, 567–571. [Google Scholar] [PubMed]
- Weng, Z.Y.; Zhang, B.D.; Asadi, S.; Sismanopoulos, N.; Butcher, A.; Fu, X.; Katsarou-Katsari, A.; Antoniou, C.; Theoharides, T.C. Quercetin is more effective than cromolyn in blocking human mast cell cytokine release and inhibits contact dermatitis and photosensitivity in humans. PLoS ONE 2012, 7, e33805. [Google Scholar] [CrossRef]
- Park, E.J.; Kim, J.Y.; Jeong, M.S.; Park, K.Y.; Park, K.H.; Lee, M.W.; Joo, S.S.; Seo, S.J. Effect of topical application of quercetin-3-O-(2″-gallate)-alpha-l-rhamnopyranoside on atopic dermatitis in NC/Nga mice. J. Dermatol. Sci. 2015, 77, 166–172. [Google Scholar] [CrossRef]
- Beken, B.; Serttas, R.; Yazicioglu, M.; Turkekul, K.; Erdogan, S. Quercetin improves inflammation, oxidative stress, and impaired wound healing in atopic dermatitis model of human keratinocytes. Pediatr. Allergy Immunol. Pulmonol. 2020, 33, 69–79. [Google Scholar] [CrossRef]
- Bagde, A.; Patel, K.; Mondal, A.; Kutlehria, S.; Chowdhury, N.; Gebeyehu, A.; Patel, N.; Kumar, N.; Singh, M. Combination of UVB absorbing titanium dioxide and quercetin nanogel for skin cancer chemoprevention. AAPS PharmSciTech 2019, 20, 240. [Google Scholar] [CrossRef]
- Nan, W.; Ding, L.; Shi, X.; Sui, X.-B. Topical use of quercetin-loaded chitosan nanoparticles against ultraviolet B radiation. Front. Pharmacol. 2018, 9, 826. [Google Scholar] [CrossRef]
- Rajnochová Svobodová, A.; Ryšavá, A.; Čížková, K.; Roubalová, L.; Ulrichová, J.; Vrba, J.; Zálešák, B.; Vostálová, J. Effect of the flavonoids quercetin and taxifolin on UVA-induced damage to human primary skin keratinocytes and fibroblasts. Photochem. Photobiol. Sci. 2022, 21, 59–75. [Google Scholar] [CrossRef]
- Zhu, X.; Li, N.; Wang, Y.; Ding, L.; Chen, H.; Yu, Y.; Shi, X. Protective effects of quercetin on UVB irradiation-induced cytotoxicity through ROS clearance in keratinocyte cells. Oncol. Rep. 2017, 37, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Chitkara, A.; Mangla, B.; Kumar, P.; Javed, S.; Ahsan, W.; Popli, H. Design-of-experiments (doe)-assisted fabrication of quercetin-loaded nanoemulgel and its evaluation against human skin cancer cell lines. Pharmaceutics 2022, 14, 2517. [Google Scholar] [CrossRef] [PubMed]
- Mansi, K.; Kumar, R.; Narula, D.; Pandey, S.K.; Kumar, V.; Singh, K. Microwave-Induced CuO Nanorods: A comparative approach between curcumin, quercetin, and rutin to study their antioxidant, antimicrobial, and anticancer effects against normal skin cells and human breast cancer cell lines MCF-7 and T-47D. ACS Appl. Bio Mater. 2022, 5, 5762–5778. [Google Scholar] [CrossRef]
- Caddeo, C.; Nacher, A.; Vassallo, A.; Armentano, M.F.; Pons, R.; Fernàndez-Busquets, X.; Carbone, C.; Valenti, D.; Fadda, A.M.; Manconi, M. Effect of quercetin and resveratrol co-incorporated in liposomes against inflammatory/oxidative response associated with skin cancer. Int. J. Pharm. 2016, 513, 153–163. [Google Scholar] [CrossRef] [PubMed]
- Ali, H.; Dixit, S. Quercetin attenuates the development of 7, 12-dimethyl benz (a) anthracene (DMBA) and croton oil-induced skin cancer in mice. J. Biomed. Res. 2015, 29, 139–144. [Google Scholar] [PubMed]
- Shin, E.J.; Lee, J.S.; Hong, S.; Lim, T.G.; Byun, S. Quercetin Directly Targets JAK2 and PKCδ and Prevents UV-Induced Photoaging in Human Skin. Int. J. Mol. Sci. 2019, 20, 5262. [Google Scholar] [CrossRef] [PubMed]
- Aghamohammadi, M.; Zolghadr, L.; Nezhad, N.S.; Ahmadpour Yazdi, H.; Esfahani, A.J.; Gheibi, N. Investigating the effects of quercetin fatty acid esters on apoptosis, mechanical properties, and expression of ERK in melanoma cell line (A375). Life Sci. 2022, 310, 121007. [Google Scholar] [CrossRef]
- Fu, J.; Huang, J.; Lin, M.; Xie, T.; You, T. Quercetin promotes diabetic wound healing via switching macrophages from M1 to M2 polarization. J. Surg. Res. 2019, 246, 213–223. [Google Scholar] [CrossRef]
- Mi, Y.; Zhong, L.; Lu, S.; Hu, P.; Pan, Y.; Ma, X.; Yan, B.; Wei, Z.; Yang, G. Quercetin promotes cutaneous wound healing in mice through Wnt/β-catenin signaling pathway. J. Ethnopharmacol. 2022, 290, 115066. [Google Scholar] [CrossRef]
- Wang, J.; Song, M.; Pan, J.; Shen, X.; Liu, W.; Zhang, X.; Li, H.; Deng, X. Quercetin impairs Streptococcus pneumoniae biofilm formation by inhibiting sortase A activity. J. Cell. Mol. Med. 2018, 22, 6228–6237. [Google Scholar] [CrossRef]
- Sadeghi-Ghadi, Z.; Vaezi, A.; Ahangarkani, F.; Ilkit, M.; Ebrahimnejad, P.; Badali, H. Potent in vitro activity of curcumin and quercetin co-encapsulated in nanovesicles without hyaluronan against Aspergillus and Candida isolates. J. Mycol. Med. 2020, 30, 101014. [Google Scholar] [CrossRef] [PubMed]
- Hanif, H.; Abdollahi, V.; Javani Jouni, F.; Nikoukar, M.; Rahimi Esboei, B.; Shams, E.; Vazini, H. Quercetin nano phytosome: As a novel anti-leishmania and anti-malarial natural product. J. Parasit. Dis. 2023, 12, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Bidone, J.; Argenta, D.F.; Kratz, J.; Pettenuzzo, L.F.; Horn, A.P.; Koester, L.S.; Bassani, V.L.; Simões, C.M.; Teixeira, H.F. Antiherpes activity and skin/mucosa distribution of flavonoids from achyrocline satureioides extract incorporated into topical nanoemulsions. Biomed. Res. Int. 2015, 2015, 238010. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yang, X.; Wang, Z.; Liu, Y.; Guo, J.; Zhu, Y.; Shao, J.; Li, J.; Wang, L.; Wang, K. Antibacterial. antioxidant and biocompatible nanosized quercetin-PVA xerogel films for wound dressing. Colloids Surf. B Biointerfaces 2022, 209, 12175. [Google Scholar] [CrossRef]
- Cikrikci, S.; Mert, B.; Oztop, M.H. Development of ph sensitive alginate/gum tragacanth based hydrogels for oral insulin delivery. J. Agric. Food Chem. 2018, 66, 11784–11796. [Google Scholar] [CrossRef] [PubMed]
- Rioux, Y.; Fradette, J.; Maciel, Y.; Bégin-Drolet, A.; Ruel, J. Biofabrication of sodium alginate hydrogel scaffolds for heart valve tissue engineering. Int. J. Mol. Sci. 2022, 23, 8567. [Google Scholar] [CrossRef]
- Liu, C.; Qin, W.; Wang, Y.; Ma, J.; Liu, J.; Wu, S.; Zhao, H. 3D printed gelatin/sodium alginate hydrogel scaffolds doped with nano-attapulgite for bone tissue repair. Int. J. Nanomed. 2021, 16, 8417–8432. [Google Scholar] [CrossRef]
- Yao, Z.; Qian, Y.; Jin, Y.; Wang, S.; Li, J.; Yuan, W.E.; Fan, C. Biomimetic multilayer polycaprolactone/sodium alginate hydrogel scaffolds loaded with melatonin facilitate tendon regeneration. Carbohydr. Polym. 2022, 277, 118865. [Google Scholar] [CrossRef]
- Anghel, N.; Dinu, M.V.; Zaltariov, M.; Pamfil, D.; Spiridon, I. New cellulose-collagen-alginate materials incorporated with quercetin, anthocyanins and lipoic acid. Int. J. Biol. Macromol. 2021, 181, 30–40. [Google Scholar] [CrossRef]
- Shafei, S.; Khanmohammadi, M.; Heidari, R.; Ghanbari, H.; Taghdiri Nooshabadi, V.; Farzamfar, S.; Akbariqomi, M.; Sanikhani, N.S.; Absalan, M.; Tavoosidana, G. Exosome loaded alginate hydrogel promotes tissue regeneration in full-thickness skin wounds: An in vivo study. J. Biomed. Mater. Res. A 2020, 108, 545–556. [Google Scholar] [CrossRef]
- Wang, T.; Yi, W.; Zhang, Y.; Wu, H.; Fan, H.; Zhao, J.; Wang, S. Sodium alginate hydrogel containing platelet-rich plasma for wound healing. Colloids Surf. B Biointerfaces 2023, 222, 113096. [Google Scholar] [CrossRef] [PubMed]
- Naeem, A.; Yu, C.; Zhu, W.; Chen, X.; Wu, X.; Chen, L.; Zang, Z.; Guan, Y. Gallic acid-loaded sodium alginate-based (polyvinyl alcohol-co-acrylic acid) hydrogel membranes for cutaneous wound healing: Synthesis and characterization. Molecules 2022, 27, 8397. [Google Scholar] [CrossRef] [PubMed]
- Garg, D.; Matai, I.; Agrawal, S.; Sachdev, A. Hybrid gum tragacanth/sodium alginate hydrogel reinforced with silver nanotriangles for bacterial biofilm inhibition. Biofouling 2022, 38, 965–983. [Google Scholar] [CrossRef]
- Mali, K.K.; Dhawale, S.C.; Dias, R.J.; Dhane, N.S.; Ghorpade, V.S. Citric acid crosslinked carboxymethyl cellulose-based composite hydrogel films for drug delivery. Indian J. Pharm. Sci. 2018, 80, 657–667. [Google Scholar] [CrossRef]
- Niemczyk-Soczynska, B.; Gradys, A.; Kolbuk, D.; Krzton-Maziopa, A.; Rogujski, P.; Stanaszek, L.; Lukomska, B.; Sajkiewicz, P. A methylcellulose/agarose hydrogel as an innovative scaffold for tissue engineering. RSC Adv. 2022, 2, 26882–26894. [Google Scholar] [CrossRef] [PubMed]
- Bonetti, L.; De Nardo, L.; Farè, S. Thermo-responsive methylcellulose hydrogels: From design to applications as smart biomaterials. Tissue Eng. Part B Rev. 2021, 27, 486–513. [Google Scholar] [CrossRef]
- Lim, S.J.; Lee, J.H.; Piao, M.G.; Lee, M.K.; Oh, D.H.; Hwangdu, H.; Quan, Q.Z.; Yong, C.S.; Choi, H.G. Effect of sodium carboxymethylcellulose and fucidic acid on the gel characterization of polyvinylalcohol-based wound dressing. Arch. Pharm. Res. 2010, 33, 1073–1081. [Google Scholar] [CrossRef] [PubMed]
- Kanikireddy, V.; Varaprasad, K.; Jayaramudu, T.; Karthikeyan, C.; Sadiku, R. Carboxymethyl cellulose-based materials for infection control and wound healing: A review. Int. J. Biol. Macromol. 2020, 164, 963–975. [Google Scholar] [CrossRef]
- Lee, I.S.; Yang, H.M.; Kim, J.W.; Maeng, Y.J.; Lee, C.W.; Kang, Y.S.; Rang, M.J.; Kim, H.Y. Terminology development and panel training for sensory evaluation of skin care products including aqua cream. J. Sens. Stud. 2005, 20, 421–433. [Google Scholar] [CrossRef]
- Siemiradzka, W.; Franczyk, A.; Bułaś, L.; Dolińska, B. Somatotropin penetration testing from formulations applied topically to the skin. Appl. Sci. 2023, 13, 2588. [Google Scholar] [CrossRef]
- Schwingel, L.C.; Bianchi, S.E.; Zorzi, G.K.; Gonçalves, P.; Teixeira, H.F.; Bassani, V.L. Quercetin and 3-O-methylquercetin in vitro skin layers permeation/retention from hydrogels: Why only a methoxy group difference determines different behaviors? J. Pharm. Pharmacol. 2019, 71, 733–745. [Google Scholar] [CrossRef] [PubMed]
- Schürer, N. pH and Acne. Curr. Probl. Dermatol. 2018, 54, 115–122. [Google Scholar] [CrossRef] [PubMed]
- Rippke, F.; Berardesca, E.; Weber, T.M. pH and Microbial Infections. Curr. Probl. Dermatol. 2018, 54, 87–94. [Google Scholar]
- Lynde, C.; Tan, J.; Skotnicki, S.; Beecker, J.; Claveau, J.; Li, M.K.; Rao, J.; Salsberg, J.; Sauder, M.B.; Zip, C. Skin Surface pH. J. Drugs Dermatol. 2019, 18, 214. [Google Scholar]
- Sreedharan Nair, R.; Rahman, H.; Kong, M.X.; Tan, X.Y.; Chen, K.Y.; Shanmugham, S. Development and rheological evaluation of DEET (N,N-diethyL-3-methylbenzamide) microparticles loaded hydrogel for topical application. Turk. J. Pharm. Sci. 2021, 18, 352–359. [Google Scholar] [CrossRef]
- Osmałek, T.; Milanowski, B.; Froelich, A.; Górska, S.; Białas, W.; Szybowicz, M.; Kapela, M. Novel organogels for topical delivery of naproxen: Design, physicochemical characteristics and in vitro drug permeation. Pharm. Dev. Technol. 2017, 22, 521–536. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, M.T.; Karaman, S.; Dogan, M.; Yetim, H.; Kayacier, A. Characterization of O/W model system meat emulsions using shear creep and creep recovery tests based on mechanical simulation models and their correlation with texture profile analysis (TPA) parameters. J. Food Eng. 2012, 108, 327–336. [Google Scholar] [CrossRef]
- Franzén, H.M.; Draget, K.I.; Langebäck, J.; Nilsen-Nygaard, J. Characterization and properties of hydrogels made from neutral soluble chitosans. Polymers 2015, 7, 373–389. [Google Scholar] [CrossRef]
- U.S. Pharmacopeial Convention. United States Pharmacopeia, 32; U.S. Pharmacopeial Convention: Rockville, MD, USA, 2009; Volume 2, pp. 2282–2284. [Google Scholar]
- Yang, Y.; Xu, L.; Wang, J.; Meng, Q.; Zhong, S.; Gao, Y.; Cui, X. Recent advances in polysaccharide-based self-healing hydrogels for biomedical applications. Carbohydr. Polym. 2022, 283, 119161. [Google Scholar] [CrossRef]
- de Barros, D.P.C.; Santos, R.; Reed, P.; Fonseca, L.P.; Oliva, A. Design of quercetin-loaded natural oil-based nanostructured lipid carriers for the treatment of bacterial skin infections. Molecules 2022, 27, 8818. [Google Scholar] [CrossRef]
- Sapino, S.; Ugazio, E.; Gastaldi, L.; Miletto, I.; Berlier, G.; Zonari, D.; Oliaro-Bosso, S. Mesoporous silica as topical nanocarriers for quercetin: Characterization and in vitro studies. Eur. J. Pharm. Biopharm. 2015, 89, 116–125. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, H.; Tang, L.; Meng, F. Penetration enhancement of menthol on quercetin through skin: Insights from atomistic simulation. J. Mol. Model. 2019, 25, 235. [Google Scholar] [CrossRef] [PubMed]
- Scalia, S.; Franceschinis, E.; Bertelli, D.; Iannuccelli, V. Comparative evaluation of the effect of permeation enhancers, lipid nanoparticles and colloidal silica on in vivo human skin penetration of quercetin. Skin Pharmacol. Physiol. 2013, 26, 57–67. [Google Scholar] [CrossRef] [PubMed]
- Li, B.; Xiao, T.; Guo, S.; Wu, Y.; Lai, R.; Liu, Z.; Luo, W.; Xu, Y. Oxymatrine-fatty acid deep eutectic solvents as novel penetration enhancers for transdermal drug delivery: Formation mechanism and enhancing effect. Int. J. Pharm. 2023, 637, 122880. [Google Scholar] [CrossRef] [PubMed]
- Dyja, R.; Jankowski, A. The effect of additives on release and in vitro skin retention of flavonoids from emulsion and gel semisolid formulations. Int. J. Cosmet. Sci. 2017, 39, 442–449. [Google Scholar] [CrossRef] [PubMed]
- Karim, M.; Boikess, R.S.; Schwartz, R.A.; Cohen, P.J. Dimethyl sulfoxide (DMSO): A solvent that may solve selected cutaneous clinical challenges. Arch. Dermatol. Res. 2022, 21, 588–600. [Google Scholar] [CrossRef]
- Kant, V.; Jangir, B.L.; Kumar, V.; Nigam, A.; Sharma, V. Quercetin accelerated cutaneous wound healing in rats by modulation of different cytokines and growth factors. Growth Factors 2020, 38, 105–119. [Google Scholar] [CrossRef]
- dal Belo, S.E.; Gaspar, L.R.; Maia Campos, P.M.; Marty, J.P. Skin penetration of epigallocatechin-3-gallate and quercetin from green tea and Ginkgo biloba extracts vehiculated in cosmetic formulations. Skin Pharmacol. Physiol. 2009, 22, 299–304. [Google Scholar] [CrossRef]
- Silva, V.C.; Silva, A.M.; Basílio, J.A.; Xavier, J.A.; do Nascimento, T.G.; Naal, R.M.; del Lama, M.P.; Leonelo, L.A.; Mergulhão, N.L.; Maranhão, F.C.; et al. New insights for red propolis of alagoas—Chemical constituents, topical membrane formulations and their physicochemical and biological properties. Molecules 2020, 25, 5811. [Google Scholar] [CrossRef]



| Ingredient (g) | Formulation Designation | |||||
|---|---|---|---|---|---|---|
| F-AA | F-CA | F-MA | F-AB | F-CB | F-MB | |
| Content (g) | ||||||
| Quercetin | 0.4 | 0.4 | 0.4 | 0.7 | 0.7 | 0.7 |
| Sodium alginate | 4.0 | 4.0 | ||||
| Methylcellulose | - | - | 4.0 | - | - | 4.0 |
| Sodium carboxymethyl cellulose | - | 4.0 | - | - | 4.0 | - |
| Glycol 86% | 10 | 10 | 10 | 10 | 10 | 10 |
| Dimethyl sulfoxide | 2 | 2 | 2 | 2 | 2 | 2 |
| Purified water | up to 100 | up to 100 | up to 100 | up to 100 | up to 100 | up to 100 |
| F-AA | F-CA | F-MA | F-AB | F-CB | F-MB |
|---|---|---|---|---|---|
| Color | |||||
| yellow | yellow | yellow | intensified yellow | intensified yellow | intensified yellow |
| Consistency | |||||
| very gentle semi-solid | very gentle semi-solid | very gentle semi-solid | very gentle semi-solid | soft semi-solid | soft semi-solid |
| Homogeneity | |||||
| homogenous | homogenous | homogenous | homogenous | homogenous | homogenous |
| Adhesion | |||||
| picks up easily, does not flow, forms a persistent typical cone on the fingertip | more difficult to pick up no characteristic cone | picks up easily, does not flow, forms a persistent typical cone on the fingertip | picks up easily, does not flow, forms a persistent typical cone on the fingertip | more difficult to pick up no characteristic cone | picks up easily, does not flow, forms a persistent typical cone on the fingertip |
| Stickiness (0–5) | |||||
| medium sticky sticky (3/5) | moderately sticky (1/5) | medium sticky (3/5) | Medium sticky (3/5) | not sticky (0/5) | medium sticky (3/5) |
| Greasiness and greasing | |||||
| no oily film when applied | no oily film when applied | no oily film when applied | no oily film when applied | no oily film when applied | no oily film when applied |
| Pillow effect | |||||
| slight layer of formula felt on fingers | slight layer of formula felt on fingers | slight layer of formula felt on fingers | slight layer of formula felt on fingers | slight layer of formula felt on fingers | slight layer of formula felt on fingers |
| Formulation Code | Spreadability (mm2) | pH |
|---|---|---|
| F-AA | 304.70 ± 6.80 c | 5.62 ± 0.02 |
| F-CA | 327.30 ± 12.06 a | 6.40 ± 0.01 |
| F-MA | 273.76 ± 3.15 d | 4.62 ± 0.02 |
| F-AB | 269.96 ± 0.22 a,e | 5.60 ± 0.02 |
| F-CB | 295.50 ± 5.93 b | 6.39 ± 0.01 |
| F-MB | 256.03 ± 5.15 a,f | 4.51 ± 0.04 |
| Formulation Code | η (30 s−1) | η (60 s−1) | η (100 s−1) |
|---|---|---|---|
| F-AA | 11.68 ± 0.51 | 8.09 ± 0.23 | 8.06 ± 0.23 |
| F-CA | 7.04 ± 0.42 | 4.66 ± 0.21 | 3.86 ± 0.25 |
| F-MA | 13.45 ± 0.34 d | 10.43 ± 0.36 e | 8.47 ± 0.21 f |
| F-AB | 11.97 ± 0.49 | 9.35 ± 0.41 | 8.45 ± 0.41 |
| F-CB | 7.49 ± 0.41 | 5.35 ± 0.23 | 4.35 ± 0.22 |
| F-MB | 17.86 ± 0.65 a | 11.84 ± 0.20 b | 9.55 ± 0.37 c |
| Formulation Code | Hardness (N) | Cohesiveness | Adhesiveness (mJ) | Elasticity |
|---|---|---|---|---|
| F-AA | 0.0520 ± 0.001 | 2.316 ± 0.054 a | 0.150 ± 0.050 | 0.649 ± 0.008 a |
| F-CA | 0.0575 ± 0.001 a | 1.314 ± 0.116 a | 0.100 ± 0.000 a | 0.868 ± 0.065 a |
| F-MA | 0.0505 ± 0.002 | 2.698 ± 0.073 | 0.150 ± 0.050 | 0.583 ± 0.012 |
| F-AB | 0.0545 ± 0.001 b | 2.127 ± 0.199 | 0.150 ± 0.050 b | 0.665 ± 0.030 b |
| F-CB | 0.0645 ± 0.003 | 1.306 ± 0.025 b | 0.100 ± 0.000 b | 0.816 ± 0.034 b |
| F-MB | 0.0640 ± 0.003 | 2.157 ± 0.140 | 0.250 ± 0.050 | 0.580 ± 0.027 |
| Formulation Code | Cumulative Amount of Flavonoid Permeated (%) | Flavonoid Retention (μg/cm2) ± SD |
|---|---|---|
| F-AA | 0.10 ± 0.02 | 4.21 ± 0.62 c |
| F-CA | 0.07 ± 0.01 | 2.97 ± 0.26 c |
| F-MA | 0.13 ± 0.02 | 7.88 ± 0.91 a |
| F-AB | 0.12 ± 0.02 | 6.48 ± 0.73 d |
| F-CB | 0.08 ± 0.01 | 4.63 ± 0.73 d |
| F-MB | 0.16 ± 0.04 | 9.45 ± 0.89 b |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Szulc-Musioł, B.; Siemiradzka, W.; Dolińska, B. Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application. Appl. Sci. 2023, 13, 7826. https://doi.org/10.3390/app13137826
Szulc-Musioł B, Siemiradzka W, Dolińska B. Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application. Applied Sciences. 2023; 13(13):7826. https://doi.org/10.3390/app13137826
Chicago/Turabian StyleSzulc-Musioł, Beata, Wioletta Siemiradzka, and Barbara Dolińska. 2023. "Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application" Applied Sciences 13, no. 13: 7826. https://doi.org/10.3390/app13137826
APA StyleSzulc-Musioł, B., Siemiradzka, W., & Dolińska, B. (2023). Formulation and Evaluation of Hydrogels Based on Sodium Alginate and Cellulose Derivatives with Quercetin for Topical Application. Applied Sciences, 13(13), 7826. https://doi.org/10.3390/app13137826








