Application of Immobilized Biocatalysts in the Biotransformation of Non-Steroidal Anti-Inflammatory Drugs
Abstract
Featured Application
Abstract
1. Introduction
2. Materials and Methods
2.1. Immobilization of Bacterial Strains
2.2. NSAIDs Degradation Experiments
2.3. Biochemical Analysis
2.4. Determination of NSAIDs Concentration
2.4.1. HPLC Analysis
2.4.2. Gas Chromatography–Mass Spectrometry (GC/MS) Analysis
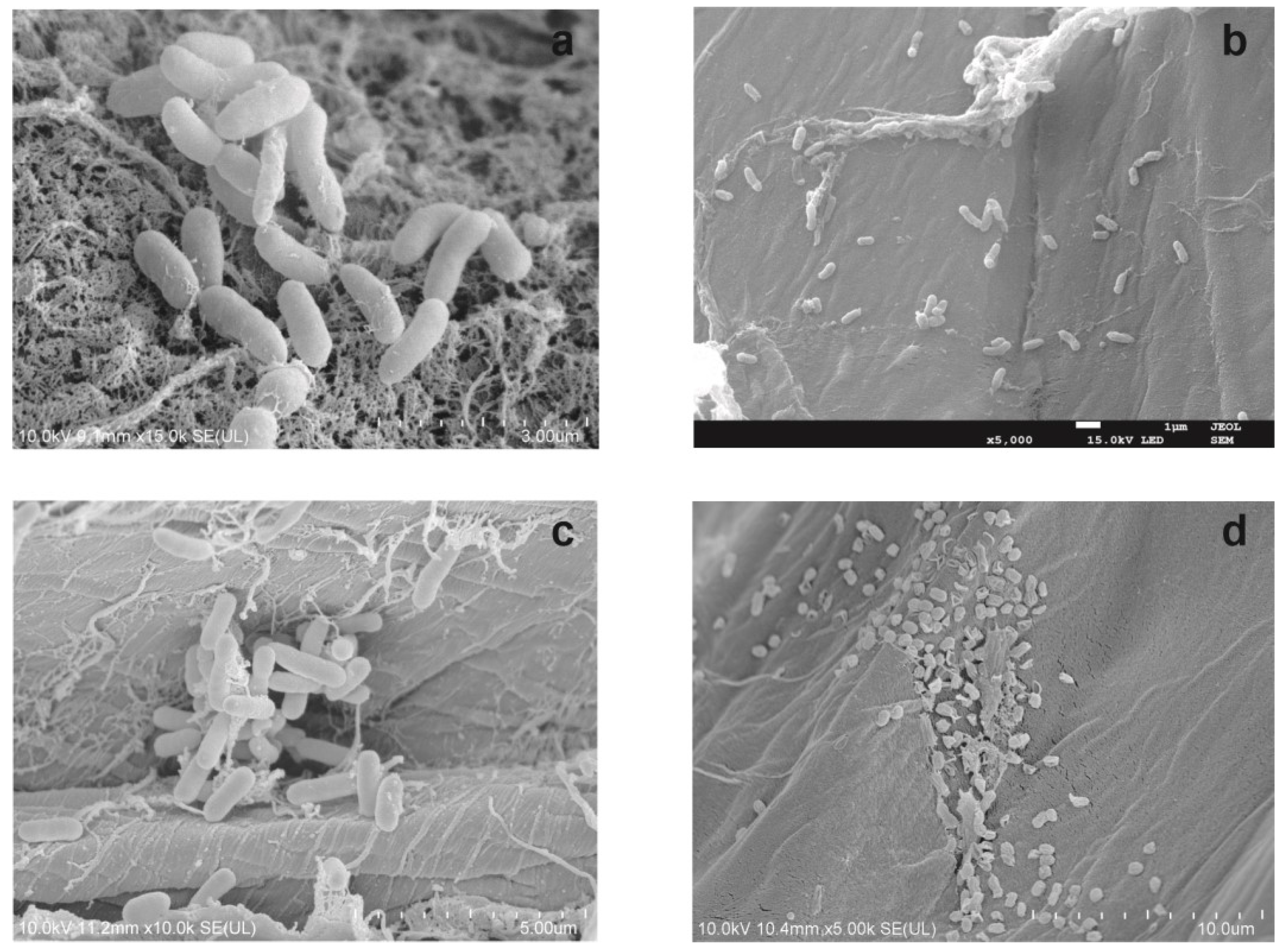
2.5. Carrier Analysis by Scanning Electron Microscopy (SEM)
2.6. Statistical Analysis
3. Results and Discussion
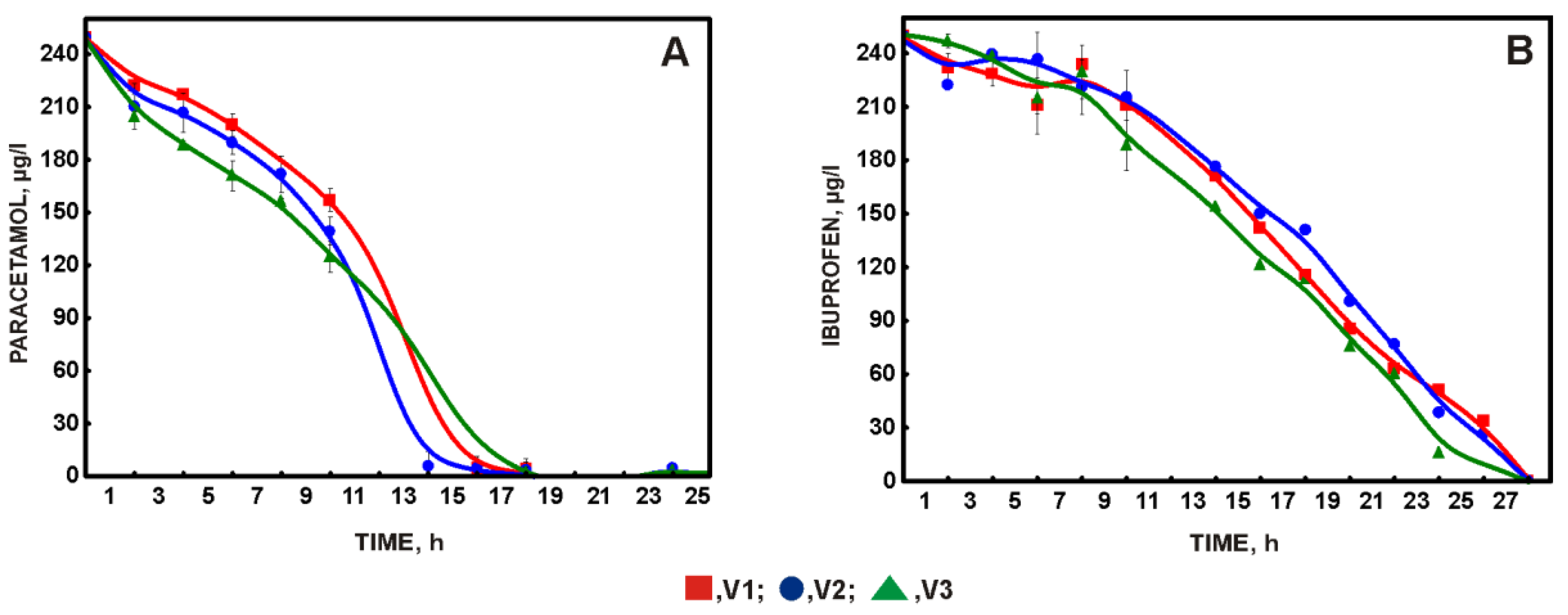
3.1. Development of the Qualitative Composition of the Biopreparation
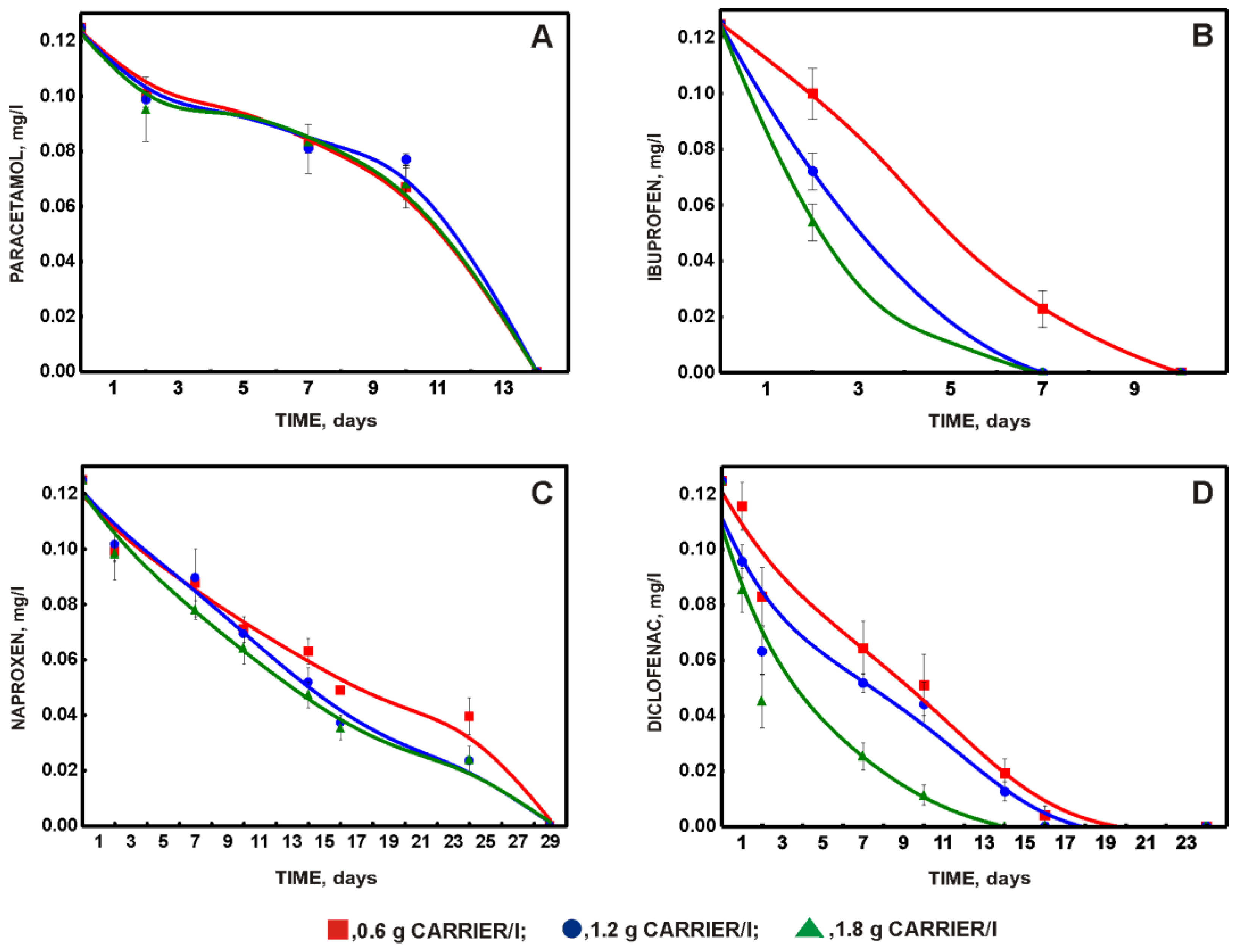
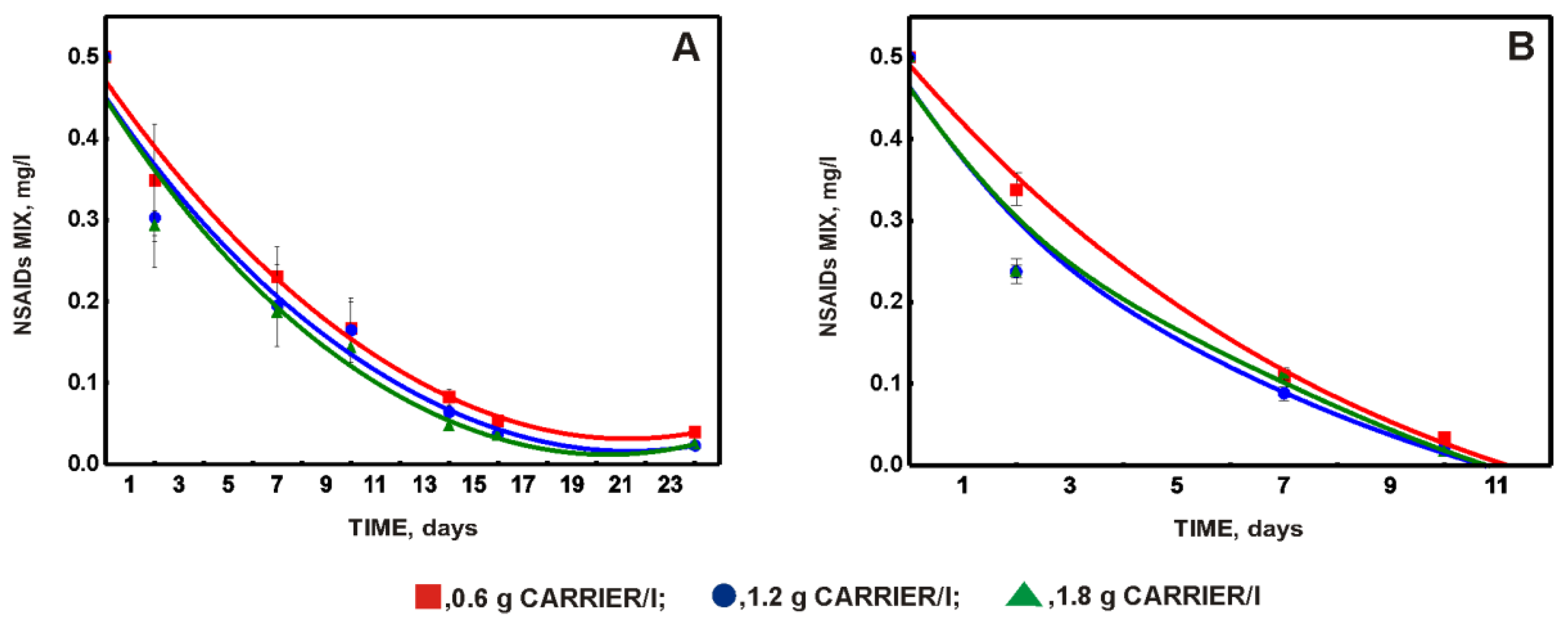
3.2. Choosing the Optimal Amount of Carrier with Immobilized Biomass and Effect of Phenol on the Degradation of a Mixture of NSAIDs and Paracetamol
4. Conclusions
- Due to slight differences in the degradation of diclofenac, which is challenging to decompose and is currently recommended for monitoring in wastewater treatment plants, the authors commend a system with 1.2 g carrier/L as the optimal system;
- This system is more economical and less burdensome for the functioning of the sewage treatment plant infrastructure due to the limited amount of the carrier itself;
- The system is based on four strains: Stenotrophomonas maltophilia KB2, Planococcus sp. S5, Bacillus thuringiensis B1(2015b), and Pseudomonas moorei KB4 immobilized on a plant sponge;
- The co-pollution, which is phenol, not only does not inhibit the functioning of the system but is a good stimulator of the system;
- This system ensures stable operation and effective decomposition with a variable load of pollutants.
5. Patents
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ivshina, I.B.; Tyumina, E.A.; Kuzmina, M.V.; Vikhareva, E.V. Features of diclofenac biodegradation by Rhodococcus ruber IEGM 346. Sci. Rep. 2019, 9, 9159. [Google Scholar] [CrossRef] [PubMed]
- Moreira, I.S.; Bessa, V.S.; Murgolo, S.; Piccirillo, C.; Mascolo, G.; Castro, P.M.L. Biodegradation of diclofenac by the bacterial strain Labrys portucalensis F11. Ecotoxicol. Environ. Saf. 2018, 152, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszyńska, D.; Guzik, U. Naproxen in the environment: Its occurrence, toxicity to nontarget organisms and biodegradation. Appl. Microbiol. Biotechnol. 2020, 104, 1849–1856. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszyńska, D.; Łagoda, K.; Guzik, U. Diclofenac biodegradation by microorganisms and with immobilized systems—A review. Catalysts 2023, 13, 412. [Google Scholar] [CrossRef]
- Parolini, M. Toxicity of the non-steroidal anti-inflammatory drugs (NSAIDs) acetylsalicylic acid, paracetamol, diclofenac, ibuprofen and naproxen towards freshwater invertebrates: A review. Sci. Total Environ. 2020, 740, 140043. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Guzik, U.; Wojcieszyńska, D. Over-the counter monocyclic non-steroidal anti-inflammatory drugs in environment—Sources, risks, biodegradation. Water Air Soil Pollut. 2015, 226, 355. [Google Scholar] [CrossRef]
- Jimenez-Silva, V.A.; Santoyo-Tepole, F.; Ruiz-Ordaz, N.; Galindez-Mayer, J. Study of the ibuprofen impact on wastewater treatment mini-plants with bioaugmented sludge. Process Saf. Environ. Prot. 2019, 123, 140. [Google Scholar] [CrossRef]
- Yu, Q.; Geng, J.; Huo, H.; Xu, K.; Huang, H.; Hu, H.; Ren, H. Bioaugmentated activated sludge degradation of progesterone: Kinetics and mechanism. Chem. Eng. J. 2018, 352, 214–224. [Google Scholar] [CrossRef]
- Aguilar-Romero, I.; Torre-Zuniga, J.; Quesada, J.M.; Haidour, A.; O’Connel, G.; McAmmond, B.M.; Van Hamme, J.D.; Romero, E.; Wittich, R.M.; Dillewijn, P. Effluent decontamination by the ibuprofen-mineralizing strain, Sphingopyxis granuli RW412: Metabolic processes. Environ. Pollut. 2021, 274, 116536. [Google Scholar] [CrossRef]
- Żur, J.; Michalska, J.; Piński, A.; Mrozik, A.; Nowak, A. Effects of low concentration of selected analgesics and successive bioaugmentation of the activated sludge on its activity and metabolic diversity. Water 2020, 12, 1133. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Evaluation of the defined bacterial consortium efficacy in the biodegradation of NSAIDs. Molecules 2023, 28, 2185. [Google Scholar] [CrossRef] [PubMed]
- Mehrotra, T.; Dev, S.; Banerjee, A.; Chatterjee, A.; Singh, R.; Aggarwal, S. Use of immobilized bacteria for environmental bioremediation: A review. J. Environ. Chem. Eng. 2021, 9, 105920. [Google Scholar] [CrossRef]
- Datta, S.; Veena, R.; Samuel, M.S.; Selvarajan, E. Immobilization of laccases and application for the detection and remediation of pollutants: A review. Environ. Chem. Lett. 2021, 19, 521–538. [Google Scholar] [CrossRef]
- Bhardwaj, P.; Kaur, N.; Selvaraj, M.; Ghramh, H.A.; Al-Shehri, B.M.; Sinhg, G.; Arya, S.K.; Bhatt, K.; Ghotekar, S.; Mani, R.; et al. Laccase-assisted degradation of emerging recalcitrant compounds—A review. Biores. Technol. 2022, 364, 128031. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Gu, L.; Ge, S.; Liu, X.; Zhang, X.; Chen, X. Remediation potential of immobilized bacterial consortium with biochar as carrier in pyrene-Cr(VI) co-contaminated soil. Environ. Technol. 2019, 40, 2345–2353. [Google Scholar] [CrossRef]
- Fu, X.; Qiao, Y.; Xue, J.; Cheng, D.; Chen, C.; Bai, Y.; Jiang, Q. Analyses of community structure and role of immobilized bacteria system in the bioremediation process of diesel pollution seawater. Sci. Total Environ. 2021, 799, 149439. [Google Scholar] [CrossRef]
- Górny, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. Naproxen ecotoxicity and biodegradation by Bacillus thuringiensis B1(2015b) strain. Ecotoxicol. Environ. Saf. 2019, 167, 505–512. [Google Scholar] [CrossRef]
- Shu, W.; Price, G.W.; Jamieson, R.; Lake, C. Biodegradation kinetics of individual and mixture non-steroidal anti-inflammatory drugs in an agricultural soil receiving alkaline treated biosolids. Sci. Total Environ. 2021, 755, 132520. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Domaradzka, D.; Guzik, U.; Wojcieszyńska, D. Bacillus thuringiensis B1(2015b) is a gram-positive bacteria able to degrade naproxen and ibuprofen. Water Air Soil Pollut. 2016, 227, 197. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Hupert-Kocurek, K.; Adamczyk-Habrajska, M.; Guzik, U. Immobilization of Planococcus sp. S5 strain on the loofah sponge and its application in naproxen removal. Catalysts 2018, 8, 176. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Habrajska, M.; Guzik, U. Enhanced degradation of naproxen by immobilization of Bacillus thuringiensis B1(2015b) on loofah sponge. Molecules 2020, 25, 872. [Google Scholar] [CrossRef] [PubMed]
- Surma, R.; Wojcieszyńska, D.; Karcz, J.; Guzik, U. Effect of Pseudomonas moorei KB4 cells’ immobilization on their degradation potential and tolerance towards paracetamol. Molecules 2021, 26, 820. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszyńska, D.; Klamka, J.; Marchlewicz, A.; Potocka, I.; Żur-Pińska, J.; Guzik, U. Immobilized Stenotrophomonas maltophilia KB2 in naproxen degradation. Molecules 2022, 27, 5795. [Google Scholar] [CrossRef]
- Żur, J.; Wojcieszyńska, D.; Hupert-Kocurek, K.; Marchlewicz, A.; Guzik, U. Paracetamol-toxicity and microbial utilization. Pseudomonas moorei KB4 as a case study for exploring degradation pathway. Chemosphere 2018, 206, 192–202. [Google Scholar] [CrossRef] [PubMed]
- Guzik, U.; Greń, I.; Wojcieszyńska, D.; Łabużek, S. Isolation and characterization of a novel strain of Stenotrophomonas maltophilia possessing various dioxygenases for monocyclic hydrocarbon degradation. Braz. J. Microbiol. 2009, 40, 285–291. [Google Scholar] [CrossRef]
- Greń, I.; Wojcieszyńska, D.; Guzik, U.; Perkosz, M.; Hupert-Kocurek, K. Enhanced biotransformation of mononitrophenols by Stenotrophomonas maltophilia KB2 in the presence of aromatic compounds of plant origin. World J. Microbiol. Biotechnol. 2010, 26, 289–295. [Google Scholar] [CrossRef]
- Dzionek, A.; Dzik, J.; Wojcieszyńska, D.; Guzik, U. Fluorescein diacetate hydrolysis using the whole biofilm as a sensitive tool to evaluate the physiological state of immobilized bacterial cells. Catalysts 2018, 8, 434. [Google Scholar] [CrossRef]
- Dzionek, A.; Wojcieszyńska, D.; Adamczyk-Habrajska, M.; Karczewski, J.; Potocka, I.; Guzik, U. Xanthan gum as a carrier for bacterial cell entrapment: Developing a novel immobilized biocatalyst. Mater. Sci. Eng. C Mater. Biol. Appl. 2021, 118, 111474. [Google Scholar] [CrossRef]
- Kosjek, T.; Heath, E.; Kompare, B. Removal of pharmaceutical residues in a pilot wastewater treatment plant. Anal. Bioanal. Chem. 2007, 387, 1379–1387. [Google Scholar] [CrossRef]
- Dalecka, B.; Strods, M.; Cacivkins, P.; Ziverte, E.; Rajarao, G.K.; Juhna, T. Removal of pharmaceutical compounds from municipal wastewater by bioaugmentation with fungi: An emerging strategy using fluidized bed pelleted bioreactor. Environ. Adv. 2021, 5, 100086. [Google Scholar] [CrossRef]
- Wu, S.; Zhang, L.; Chen, J. Paracetamol in the environment and its degradation by microorganisms. Appl. Microbiol. Biotechnol. 2012, 96, 875–884. [Google Scholar] [CrossRef] [PubMed]
- Rios-Miguel, A.B.; Smith, G.J.; Cremers, G.; Alen, T.; Jetten, M.S.M.; Op den Camp, H.J.M.; Welte, C.U. Microbial paracetamol degradation involves a high diversity of novel amidase enzyme candidates. Water Res. X 2022, 16, 100152. [Google Scholar] [CrossRef] [PubMed]
- Chopra, S.; Kumar, D. Ibuprofen as an emerging organic contaminant in environment, distribution and remediation. Heliyon 2020, 6, e04087. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. Genetic and chemical characterization of ibuprofen degradation by Sphingomonas Ibu-2. Microbiology 2013, 159, 621–632. [Google Scholar] [CrossRef]
- Murdoch, R.W.; Hay, A.G. The biotransformation of ibuprofen to trihydroxyibuprofen in activated sludge and by Variovorax Ibu-1. Biodegradation 2015, 26, 105–113. [Google Scholar] [CrossRef]
- Aracagök, Y.D.; Göker, H.; Cihangir, N. Biodegradation of micropollutant naproxen with a selected fungal strain and identification of metabolites. Z. Naturforsch. 2017, 72, 173–179. [Google Scholar] [CrossRef]
- Żur, J.; Piński, A.; Wojcieszyńska, D.; Smułek, W.; Guzik, U. Diclofenac degradation—Enzymes, genetic background and cellular alteration triggered in diclofenac-metabolizing strain Pseudomonas moorei KB4. Int. J. Mol. Sci. 2020, 21, 6786. [Google Scholar] [CrossRef]
- Marchlewicz, A.; Guzik, U.; Smułek, W.; Wojcieszyńska, D. Exploring the degradation of ibuprofen by Bacillus thuringiensis B1(2015b): The new pathway and factors affecting degradation. Molecules 2017, 22, 1676. [Google Scholar] [CrossRef] [PubMed]
- Górny, D.; Guzik, U.; Hupert-Kocurek, K.; Wojcieszyńska, D. A new pathway for naproxen utilization by Bacillus thuringiensis B1(2015b) and its decomposition in the presence of organic and inorganic contaminants. J. Environ. Manag. 2019, 239, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Wojcieszyńska, D.; Domaradzka, D.; Hupert-Kocurek, K.; Guzik, U. Bacterial degradation of naproxen—Undisclosed pollutant in the environment. J. Environ. Manag. 2014, 145, 157–161. [Google Scholar] [CrossRef]
- Hupert-Kocurek, K.; Guzik, U.; Wojcieszyńska, D. Characterization of catechol 2,3-dioxygenase from Planococcus sp. strain S5 induced by high initial phenol concentration. Acta Biochim. Pol. 2012, 59, 345–351. [Google Scholar] [CrossRef]
- Partovinia, A.; Khanpour-Alikelayeh, E.; Talebi, A.; Kermanian, H. Improving mass transfer rates in microbial cell immobilized system for environmental applications: Synergistic interaction of cells on crude oil biodegradation. J. Environ. Manag. 2023, 326, 116729. [Google Scholar] [CrossRef]
- Alanis-Sanchez, B.M.; Perez-Tapia, S.M.; Vazquez-Leyva, S.; Mejia-Calvo, I.; Macias-Palacios, Z.; Vallejo-Castillo, L.; Flores-Ortiz, C.M.; Guerrero-Barajas, C.; Cruz-Maya, J.A.; Jan-Roblero, J. Utilization of naproxen by Amycolatopsis sp. Poz 14 and detection of the enzymes involved in the degradation metabolic pathway. World J. Microbiol. Biotechnol. 2019, 35, 186. [Google Scholar] [CrossRef]
- Alvarado-Gómez, E.; Tapia, J.I.; Encinas, A. A sustainable hydrophobic luffa sponge for efficient removal of oils from water. Sustain. Mater. Technol. 2021, 28, e00273. [Google Scholar] [CrossRef]
- Corseuil, H.X.; Weber, W.J. Potential biomass limitation on rates of degradation of monoaromatic hydrocarbons by indigenous microbes in subsurface soils. Water Res. 1994, 28, 1415–1423. [Google Scholar] [CrossRef]
- Dhiman, N.; Jasrotia, T.; Sharma, P.; Negi, S.; Chaudhary, S.; Kumar, R.; Mahnashi, M.H.; Umar, A.; Kumar, R. Immobilization interaction between xenobiotic and Bjerkandera adusta for the biodegradation of atrazine. Chemosphere 2020, 257, 127060. [Google Scholar] [CrossRef] [PubMed]



| Carrier Mass | |||
|---|---|---|---|
| Strain | 0.6 g | 1.2 g | 1.8 g |
| KB4 | 10.2 mg | 20.4 mg | 30.6 mg |
| B1(2015b) | 9.0 mg | 18.0 mg | 27.0 mg |
| KB2 | 10.8 mg | 21.6 mg | 32.4 mg |
| S5 | 12.0 mg | 24.0 mg | 36.0 mg |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nowak, A.; Dzionek, A.; Wojcieszyńska, D.; Guzik, U. Application of Immobilized Biocatalysts in the Biotransformation of Non-Steroidal Anti-Inflammatory Drugs. Appl. Sci. 2023, 13, 7789. https://doi.org/10.3390/app13137789
Nowak A, Dzionek A, Wojcieszyńska D, Guzik U. Application of Immobilized Biocatalysts in the Biotransformation of Non-Steroidal Anti-Inflammatory Drugs. Applied Sciences. 2023; 13(13):7789. https://doi.org/10.3390/app13137789
Chicago/Turabian StyleNowak, Agnieszka, Anna Dzionek, Danuta Wojcieszyńska, and Urszula Guzik. 2023. "Application of Immobilized Biocatalysts in the Biotransformation of Non-Steroidal Anti-Inflammatory Drugs" Applied Sciences 13, no. 13: 7789. https://doi.org/10.3390/app13137789
APA StyleNowak, A., Dzionek, A., Wojcieszyńska, D., & Guzik, U. (2023). Application of Immobilized Biocatalysts in the Biotransformation of Non-Steroidal Anti-Inflammatory Drugs. Applied Sciences, 13(13), 7789. https://doi.org/10.3390/app13137789









