Abstract
Agro-industrial by-products and by-products from the wine industry (pomace, peels, leaves, stems, and seeds) represent a potential economic interest because they are usually relevant natural sources of bioactive compounds, which may present significant biological activities related to human health and well-being. This article aims to review wine and winery industry by-products as potential natural sources of antioxidant, antimicrobial, anti-inflammatory, antiaging, and anticancer compounds, as well as briefly highlighting the extraction methods used to obtain these bioactive compounds and explore their potential applications in the food, cosmetic, and packaging industries. Although there are some studies of wine industry by-products with different origins, this revision will be mainly focused on the Portuguese vineyard industry since it represents an import industrial sector as proof of the diversity of the bioactive compounds identified. Therefore, the recovery of these bioactive molecules that act as antioxidants and health-promoting agents may promote a variety of industries at the same time as the circular economy.
1. Introduction
The agro-industrial sector generates approximately 1.3 billion tons per year of food by-products, according to the Food and Agriculture Organization of the United Nations (FAO) [1]. Currently, these by-products are used as animal feed or are disposed of in the field, and most are discarded without treatment, causing environmental concerns [2,3,4,5]. However, agro-industrial by-products can be highly valuable due to their abundant sources of bioactive compounds, which have a wide range of applications and can add value to the cosmetic, pharmaceutical, and food industries. Among these bioactive compounds, polyphenols are well recognized compounds with committed biological activities that contribute to preventing oxidative reactions and, consequently, related processes and diseases, such as inflammatory processes and cardiovascular diseases, among others [6,7]. Therefore, studies on the development of strategies and processes towards the use and valorisation of plant agro-industrial by-products have been increasing in recent years, arousing the interest of food scientists and the food industry [8,9]. Among the various agro-industrial by-products produced, the by-products of viticulture stand out because these by-products (grape pomace, skins, and seeds) are rich sources of phenolic compounds that represent high potential economic interest. The wine sector is one of most productive and important agro-industries in the world. Every year, 259.9 million hl of wine are produced around the world, leading to the generation of 20 million tons of biological by-products [10,11].
For millennia, grapes have been a crucial fruit crop, revered by ancient civilizations for their role in the art of winemaking. They hold a special place in history as one of the oldest and most significant crops [12]. There exist about 2000 different grape varieties grown around the world. According to the International Organization of Vine and Wine (OIV), grape production in 2018 was 77.8 million tons globally. Of this production, 7% was converted to dried grapes, 36% were destined to be table grapes, and 57% of cultivated grapes have been used for winemaking processes [13,14]. Wine is one of the most widely produced beverages in the world, and its global production in 2018 was approximately 294 million hectolitres (MhL), while in 2020, 2021, and 2022, approximately 262, 260 [13] and 259.9 MhL were produced for each year, respectively [15]. Specifically for Europe, in 2021, the top five wine producers were Italy (44.5 MhL), Spain (35.0 MhL), France (34.2 MhL), Germany (8.8 MhL), and Portugal (6.5 MhL) [13]. In 2021, the weather conditions did not favour grape growers in the European Union (EU), and wine production was estimated at 145 MhL (excluding juices and musts). Portugal, Germany, Romania, and Hungary were the only EU countries that recorded harvests greater than 2020 [13]. On average, about 30% of the grapes used for wine production become by-products, making this sector a good source of natural bioactive compounds [3,16,17,18,19]. These by-products are composed of vitamins, phenolic compounds (tannins, phenolic acids, anthocyanins, and resveratrol), water, proteins, lipids, carbohydrates, minerals, and compounds such as fiber [20]. Their concentration depends on the type of cultivars and climatic conditions of cultivation [21,22,23,24]. Some of these compounds present biological activities, such as antioxidant properties, and can be used in the food, pharmaceutical, and cosmetics industries [25,26,27]. In addition, several scientific studies are demonstrating the influence of bioactive compounds extracted from wine processing by-products on various diseases. Sahpazidou et al. [28] reported that the bioactive compounds from grape stem had an anti-carcinogenic effect against colon, breast, and kidney cancers. Researchers have discovered valuable compounds in grape stems that possess strong antioxidant properties and antimicrobial abilities [29]. Haas [30] analyzed grape pomace, which was of interest because the use of trans-resveratrol is associated with its anti-inflammatory and anticancer activities and cardioprotective, neuroprotective, and antioxidant effects. Various traditional and modern techniques are commonly employed to extract the essential bioactive compounds [31]. Emergent techniques are usually more advantageous, as they are less time-consuming and more environmentally friendly than conventional extraction methods, which have higher solvent volume consumption (usually with higher toxicity), lower extraction efficiency, and environmental disposal concerns [32,33]. The use of these technologies holds great promise in extracting high value-added compounds, particularly from winery by-products [34,35]. Ultrasound-assisted extraction (UAE), microwave-assisted extraction (MAE), or pressurized solvent extraction (PSE) are examples of some technologies that have been studied to improve extractions that also take into account the increasing application of green chemistry and so-called eco-friendly methods/techniques [36].
The recycling and reuse of winery by-products into high value-added compounds are imperative to promote the circular bioeconomy of the sector. The main goal of this review is to provide updated information on the bioactive compounds that can be extracted from by-products generated by the wine sector industries, through conventional and non-conventional technologies. In the following sections, we review the importance of bioactive compounds and some conventional and emerging extraction technologies that can be used to obtain them. Finally, the literature on bioactive compounds present in by-products of the wine industry of the last five years were reviewed in detail and with a focus on the work carried out in Portugal. The practical application of bioactive compounds derived from wine industry by-products was also explored, as well as a future perspective the circular bioeconomy context.
2. Characterization of the Grape Cluster and Vinification Processes
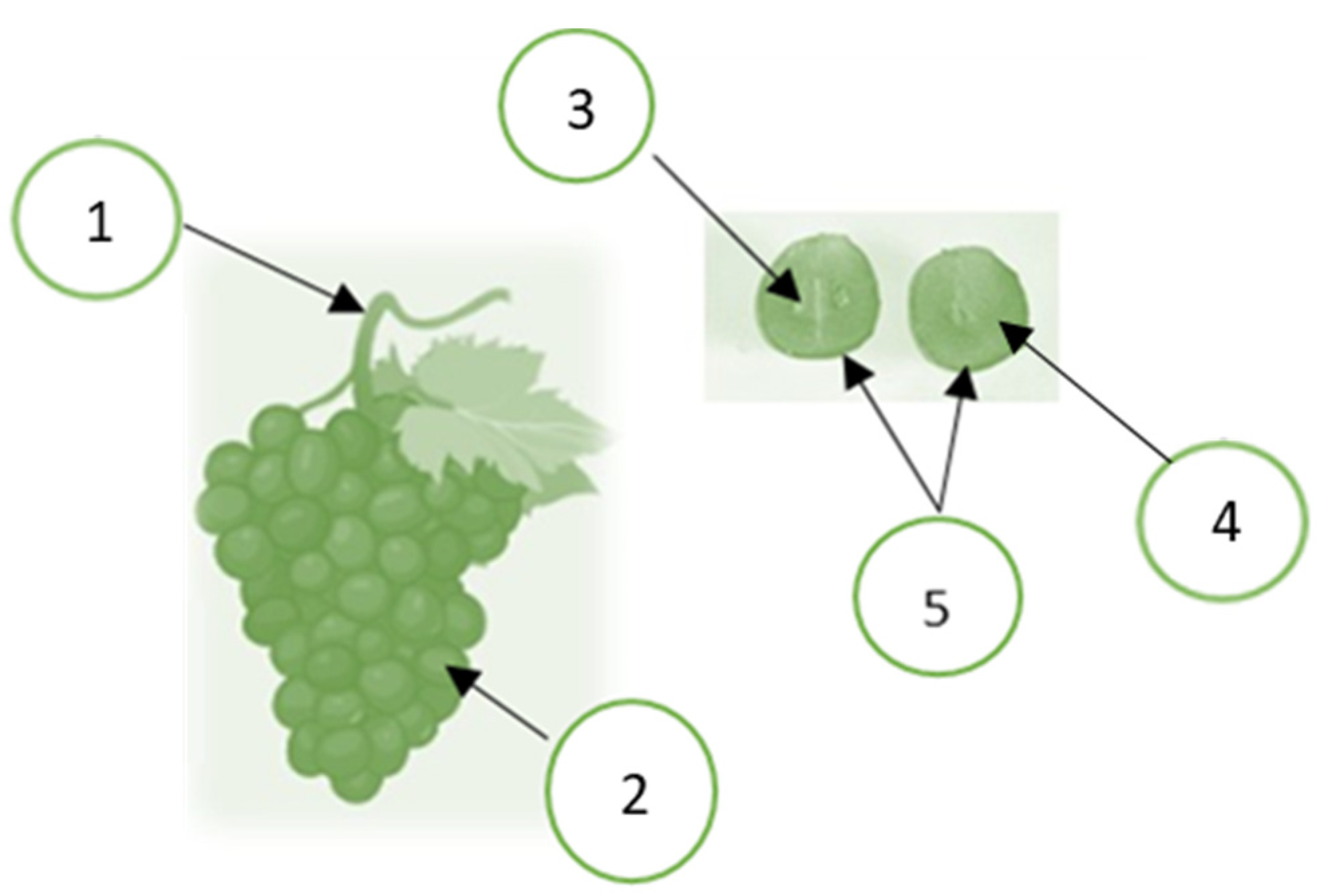
In grapevines, berries are attached to the stem to form a grape cluster, as shown in Figure 1. The berries consist of 85 to 92% of pulp, 6 to 12% of skin, and 2 to 5% of seed. The pulp consists of 65 to 85% of water, 12 to 25% of reducing sugars, 0.6 to 1.4% of organic acids, 0.25 to 0.5% of mineral substances, 0.05 to 0.1% of nitrogen compounds, and various water-soluble and fat-soluble vitamins [37]. Grape seeds are approximately 40% fiber, 16% lipids (oil), 11% protein, 7% phenolic compounds (mainly tannins), sugars, mineral salts, etc. In addition, grape seeds have a large amount of monomeric phenolic compounds, such as (+)-catechin, (-)-epicatechin, and (-)-epicatechin-3-O-gallate, and dimeric, trimeric, and tetrameric procyanidins [7,38]. Grape skin is a source of anthocyanidins and anthocyanidin natural dyes and has antioxidant properties and antimutagenic activities [39], as cited by the author of [40]. Anthocyanins are mainly found in grape skin [40]. Anthocyanins can react with the flavonols to produce more stable pigments, either directly or through different aldehydes. The color intensity depends on the anthocyanins’ type, concentration, pH, degree of polymerization, etc. [41]. Flavan-3-ol is a significant group of phenolic compounds that contribute to the darkening process of grapes and wine. They also interact with anthocyanins to stabilize the color of red wine [42,43].
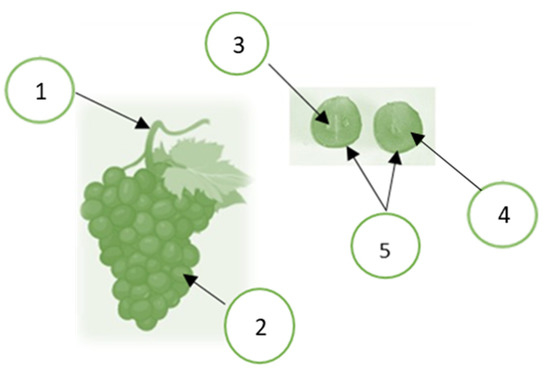
Figure 1.
The grape cluster and berry morphology: 1. stem; 2. berries; 3. seed; 4. pulp; 5. skin. Created by the authors using BioRender (https://biorender.com).
The stem is formed by the frame of the grape cluster that supports the fruit. The stem represents 3 to 7% of the grape cluster total [44]. It is a main residue produced during the winemaking process, and also has a high content of lignocellulosic material [45]. It contains a high concentration of tannins, which, if chewed, have a rough and astringent taste. Conveniently, the tannins present in the stem are not incorporated into the wine, and the grape should be destemmed before being pumped into the fermentation tanks.
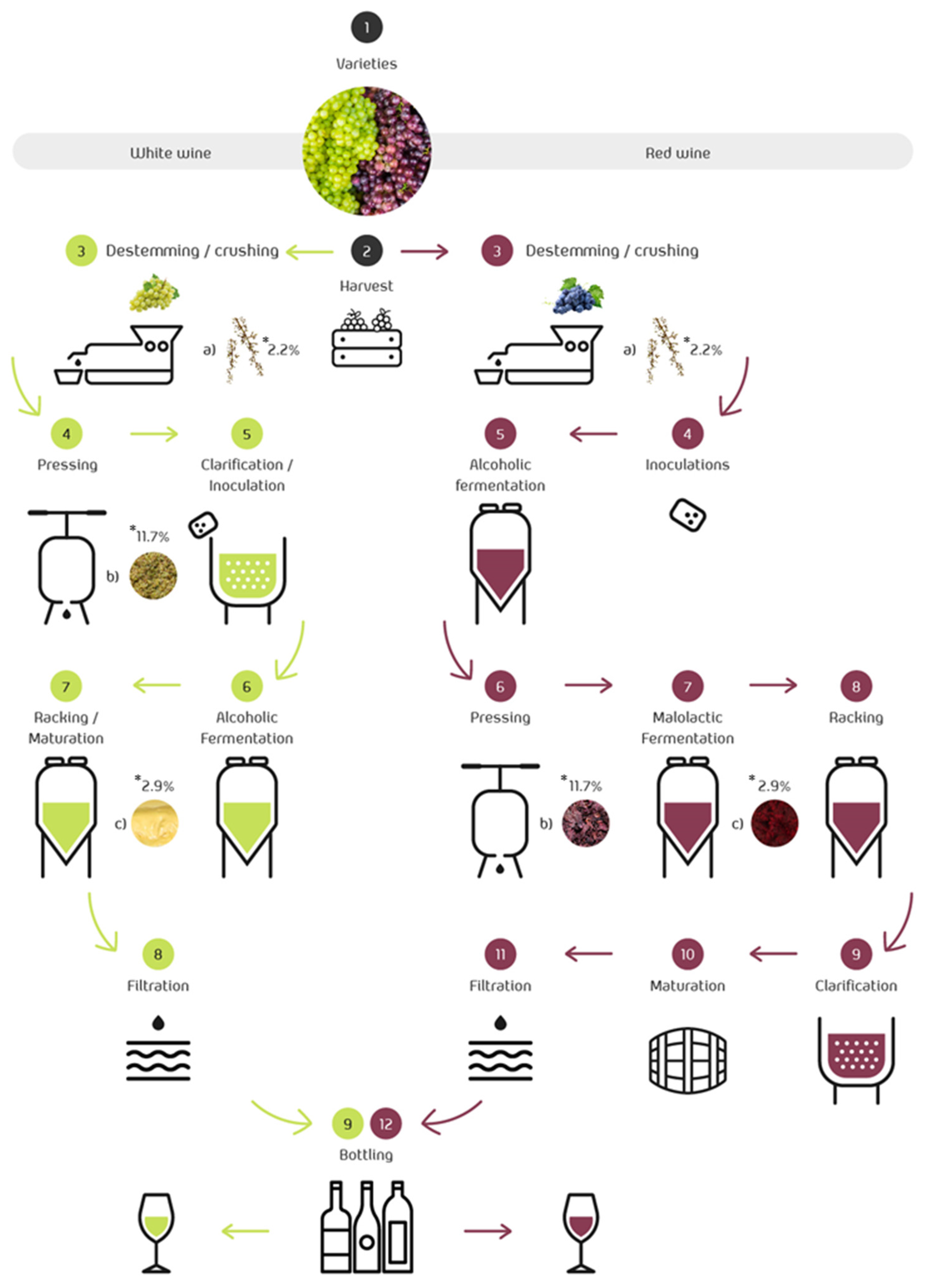
The main difference between white and red wine is that white wine is generally made from white grapes, but it can also be made from pink and red grapes. In this case, it is necessary to separate the liquid immediately from the solid phase, which contains the pigments responsible for the color. On the other hand, red wine is made only from red grapes. Figure 2 shows the winemaking process for white and red wine. Grapes are harvested, destemmed, and crushed. After that, the proper wine elaboration starts and 17% of the total weight of grapes used in the wine-making process are discarded (skins, seeds, stem, and lees, represented in step 3; see Figure 2) [46].
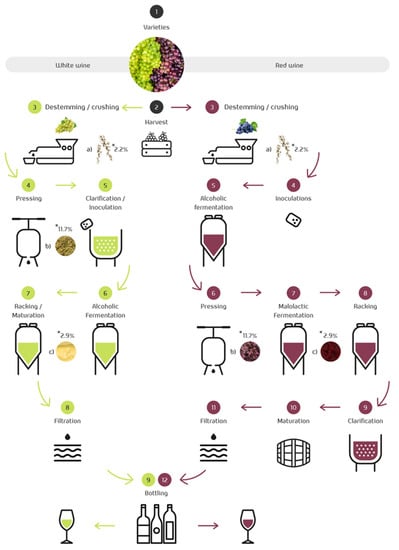
Figure 2.
Vinification process. By-products obtained during wine processing: (a) stem, (b) grape pomace, and (c) lees. * By-products’ percentages are indicated in reference to the mass of grapes used in the manufacture of wine.
During maceration, the skin of red grapes is the source of extraction for anthocyanins and other phenolic compounds [47]. These phenolic compounds give the wine color, structure, body, and originality, as well as the claimed health properties [48]. In the production of red wine, maceration is followed by the inoculation of yeasts and alcoholic fermentation before the material is pressed and bottled. The inoculum consists of commercial yeasts produced in the laboratory that are selected for their fermentative behavior and the metabolites they produce. The inoculum helps the yeast perform more evenly and correctly, overcoming the deficiencies of the must and the needs of the yeast. The so-called malolactic fermentation follows the second fermentation. After that, there is an aging period before the final stages.
For white wine production, the grapes are first pressed and clarified, then alcoholic fermentation is allowed, avoiding the maceration step, followed by wine racking and aging in the tank [49]. Once malolactic fermentation begins, both wines are handled similarly. Indeed, they are clarified and stabilized by reducing the tartaric salts, then they are filtered and bottled (Figure 2).
The first residue generated during winemaking is the grape stem, which is accumulated during the destemming step. As described above, the pressing of the grape depends on the type of wine [49]. This step can occur before the alcoholic fermentation or after the yeast-driven fermentation for white and red wines [50]. Pressing gives rise to the grape pomace, also known as grape pomace. Wine lees are produced after the racking stages, i.e., twice during red wine production and only once during white winemaking. The final steps in winemaking involve the fining and clarification process, which typically leaves by-products known as fining sediments. Then, the wine is stabilized, i.e., the excessive tartaric salts are removed through cooling or other methods, leading to tartrate sediments. Finally, filtration of the product allows the collection of the filtrated by-products [51]. In every step mentioned, wastewaters are generated and may contain grape pulp leftovers, skin, and seeds, and various compounds used in the filtration, precipitation, and cleaning processes [52].
Grape pomace accounts for about 69% of the total residue produced, while stems account for 13% and lees for 17% [53]. Considering these values and the total Portuguese wine production in 2021, 124.0 Mkg of grape pomace, 23.4 Mkg of stems, and 30.6 Mkg of lees were generated [13]. Wine lees and pomace are considered by-products according to the European Council Regulation (EC) N° 479/2008 on the typical organization of the market in wine [54]. Therefore, they may be used as substrates in distilleries to obtain alcohol and tartrates. After distillation, vinasse and, once again, more winery wastewater remains as the liquid by-products. There is also a solid residue known as exhausted marc that is produced. The aerobic depuration of vinasse and wastewaters gives rise to another solid waste: the winery sludge [49]. The liquid and solid by-products generated in the wineries and distilleries typically have acidic pH and a high organic content consisting of polyphenols. Several relevant minerals are present, including sodium, potassium, phosphorous, and even heavy metals [55].
3. Bioactive Compounds in Wine By-Products
Bioactive compounds refer to a diverse range of phytochemicals that are produced through secondary metabolism in plants. Plants produce primary metabolites, such as carbohydrates, proteins, and fats, to sustain vital life processes, such as growth, development, respiration, storage, and reproduction. Additionally, they create secondary metabolites, including glucosinolates, carotenoids, or polyphenols, to protect themselves from external abiotic factors, such as light and water scarcity, as well as to ward off pathogens and communicate with other plants [56,57,58,59,60]. Polyphenols are the primary bioactive compounds found in grapes and their by-products. In recent decades, these compounds have gained significant interest from the scientific community and various industries, such as food, pharmaceutical, and cosmetics (Section 4.1, Section 4.2 and Section 4.3). Utilizing winemaking by-products as a source of polyphenols is a promising strategy to elevate the worth of these by-products [61].
The chemical compounds known as polyphenols are intricate structures made up of one or more aromatic rings. They can have different levels of hydroxylation, methylation, and glycosylation, which affect the fruit’s color, astringency, and bitterness [62,63,64]. There are two classes of grape polyphenols that are distinguished by the number of phenolic rings and the type of structural unit that binds them together. These classes are non-flavonoid and flavonoid [65,66]. Non-flavonoid includes stilbenes and phenolic acids subclasses, while the flavonoid class is subdivided into flavanols, flavonols, and anthocyanidins subclasses.
The profile of polyphenols in wine varies greatly depending on the grape variety, aging, ripening stages, climatic and geographical conditions, and cultivation practices [34,67,68,69]. Grape varieties are distinguished by their different properties, including their ability to form specific organic compounds and their more remarkable ability to produce particular amounts of these components [70,71].
Grape is one of the fruits with the highest antioxidant compounds present in large amounts in the skin, pulp, and seeds [40]. Therefore, solid by-products, such as grape skin, grape pomace (skins and seeds), and stems, among others, obtained from industrially processed grapes, are of great economic interest [72]. The grape skin, considered a solid residue from grape processing for juices and wine production, may contain different but significant concentrations of polyphenols and available antioxidants [70,71]. The grape pomace consists of the skins and seeds, considering that the stem is separated in the previous stage, called disengage [73]. Grape pomace is considered of high economic interest due to its alcoholic and tartaric richness but also because of its physical components. Grape pomace is also a rich source of phenolic acids, colored anthocyanins, flavonoids, and tannins. The bioactive compounds extracted from grape by-products tend to differ in composition based on several factors, including the grape variety, processing method, environmental conditions, and the proportions of bark, seeds, and cuttings utilized [74,75] (Table 1). Moreira et al. [21] extracted 33.7 mg GAE.g−1 of phenolic compounds from the stem residue of the TR variety from the Douro region, while Gouvinhas et al. [23] obtained a much higher concentration between 78.02 and 103.49 mg GAE.g−1 for ‘Moscatel’ grape stem samples of the lower altitude Douro regions. Gouvinhas et al. [24] extracted 42.04 to 96.29 mg GAE.g−1 and 29.46 to 76.20 mg CAT.g−1 of total phenolics and flavonoids, respectively, also from the stem (Table 1). Gouvinhas and Barros [76] compared the content of the phenolic compounds and flavonoids in wine lees from the Douro region of two different varieties. They found that the content of phenolics and flavonoids was higher in the ‘Tinta Barroca’ variety, 125.39 mg GAE.g−1 DW and 128.34 mg CAT.g−1 DW, respectively, compared to the ‘Sousão’ variety, which are traditionally cultivated in the Região Demarcada do Douro, in northern Portugal, at 15.44 mg GAE.g−1 and 18.50 mg CAT.g−1 DW, respectively. The same authors also determined the concentration of these compounds in the pomace and stems, and they found no significant difference between the two varieties in terms of the phenolic and flavonoid content. The stems of the ‘Tinta Barroca’ variety were the residue that presented the highest content of phenolic compounds and flavonoids, and this extract also presented greater antioxidant activity. Peixoto et al. [77] analyzed the extract from three different wine residue samples, pomace, skin, and seeds, and found that the skins have a higher concentration of anthocyanins and the seeds have a higher content of phenolic compounds, as well as higher antioxidant, cytotoxic, and antibacterial potentials.
3.1. Extraction Methods of Bioactive Compounds
The extraction is a unit operation that aims to separate the compounds from different matrices, solid or liquid, by chemical, physical, and/or mechanical methods [78]. The most critical factors in an extraction process are the type of solvent, the process conditions (temperature, pH, and the ratio between the solvent and the plant matrix), and the properties of the plant material (composition and particle size) [79]. The solvents used and their polarity can affect the transfer of electrons and hydrogen atoms, which is a critical aspect in the extraction of polyphenols and may affect the extraction yield and their antioxidant capacity [80].
3.1.1. Conventional Extraction Methods
Conventional extraction techniques can be used to extract bioactive compounds from by-products of the wine industry. The effectiveness of the extraction process depends on commonly used traditional extraction techniques of solid–liquid extractions, such as pressing, Soxhlet extraction, maceration, decoction or infusion, and steam-dragging extraction/hydro-distillation [77,81,82,83]. These methodologies represent an essential part of many industrial processes and involve a series of analogous and successive phases between the plant particle containing the solute and the solvent. The process of extracting compounds from solid material involves several steps, including the introduction of a solvent into the matrix, the dissolution of the compounds, the movement of the dissolved substance from inside to outside the matrix through diffusion, the transfer of the compounds from the particle’s external surface to the solvent via convection, and, finally, the separation of the extract from the solvent through evaporation, if the solvent has a lower boiling point than the extracted compound [84,85,86].
Although these conventional techniques have been used to extract bioactive compounds from wine by-products [77,81,82,83], they have some associated problems, such as high energy consumption for the heating process, which can also result in the degradation of thermolabile compounds [34], or the use of hazardous solvents [87].
3.1.2. Emerging Extraction Methods
Promising new ‘green’ and advanced extraction techniques are being developed and applied to bioactive extraction from wine by-products (Table 1) to overcome conventional extraction limitations by increasing the overall yield and the selectivity of bioactive components from plant materials without fragmenting the treated tissue, reducing the subsequent purification steps and achieving a cleaner and safer extraction [88,89,90]. Some of the most promising new methods are microwave-assisted extraction (MAE), high-pressure extraction (HPE), pulsed electric fields extraction (PEFE), ohmic heating (OH), pressurized hot water extraction (PHWE), supercritical fluid extraction (SFE), ultrasound extraction (UAE), and the use of natural deep eutectic solvents (NADES) [34,87,91,92,93,94,95]. Recent studies have proven the success of using these methods for the extraction of bioactive compounds from wine by-products [87,91,96,97].
Microwave-assisted extraction (MAE): This is a process that utilizes temperature to extract polar compounds. Essentially, electromagnetic energy within the frequency range of 300 MHz and 300 GHz is converted into heat through ionic conduction and dipole rotation [34,98]. The advantage of this method is heating for the extraction of the compounds. However, this may also be a disadvantage for the extraction of thermosensitive compounds. Other advantages include reduced thermal gradients, reduced equipment size, and increased extract yield [99,100]. Rocha and Noroña [94] used an acidic aqueous solution with 2% citric acid as a solvent to separate the phenolic compounds from grape pomace, using both UAE and MAE. The results show that for both extraction methods, the content of the total phenolic compounds and the antioxidant activity measured by ABTS and DPPH increased with time, highlighting that MAE provided the best condition for extraction. Pezzini et al. [97] produced bioactive-enriched extracts from grape juice by-products using three different methods (UAE, MAE, and liquid–liquid). Among the methods studied, the MAE of grape juice by-products using polar solvents, such as ethanol and water, provided the best yield and chemical composition, obtaining flavonoid-rich extracts.
High-pressure extraction (HPE): This method is based on two principles: (1) the isostatic principle, which states that, regardless of the size and geometry of the material, the pressure acts uniformly, instantaneously, and homogeneously in all directions and (2) the principle of Le Chatelier, in which, for each phenomenon, when the volume decreases, the pressure is increased/decreased, bringing the system to a new state of equilibrium [101,102]. In HPE, heat is not used in order to avoid its deleterious effects on bioactive compounds and, consequently, preserve their biological activities [103]. When subjected to high pressures ranging from 100 to 800 MPa or even up to 1000 MPa, the matrices may undergo structural changes. These changes include the destruction of plant tissues, protein denaturation, cellular deformation, damage to cellular membranes and organelles, and an increase in solvent transfer into the materials and the soluble components into the solvents [103,104,105,106]. Putnik et al. [107] performed the extraction of anthocyanins from grape skin pomace using HPE. They tested the use of different concentrations of water/ethanol and water/methanol solutions but concluded that the type of solvent used did not have a significant influence on the extraction yield. However, increasing the percentage of the solvent in the water favored this process and increased the temperature from 22 to 30 °C. On the other hand, increasing the pressure up to 500 MPa had no significant effect on the process yield. These authors verified that 55.7% of the anthocyanins present in the extracts were malvidin and the optimal conditions for their extraction were 268 MPa at 30 °C for 3.3 min.
Ohmic heating (OH): This is based on alternating current used for internal heat generation [93]. Moreover, this technique is associated with less aggressive heat treatments than conventional methods, as it can heat materials uniformly and rapidly, avoiding the denaturation of thermosensitive substances, including phenolic compounds [108,109]. For example, El Darra et al. [110] applied OH pre-treatment (50 °C and 400 or 800 V.cm−1) to extract polyphenols from red grape pomace and found that under the same extraction conditions (30% ethanol at 50 °C for 60 min), the polyphenols yield increased from 440 to 620 mg GAE.100 g−1 DW with the pre-treatment.
Pulsed electric fields (PEFE): Electric fields are applied to a material placed between two electrodes. The pulse amplitude ranges from 100–300 V.cm−1 to 20–80 kV.cm−1 [111]. Typically, PEFE is performed at ambient temperature or slightly higher than the ambient temperature for a treatment time less than 1 s (ms or µs) [112,113]. The exposure of plant cells to a specific electric field can result in damage to the cell membranes, causing temporary (reversible) or permanent (irreversible) pore formation. This process is referred to as ‘electroporation’. PEFE was used to study the extraction efficiency of polyphenols from two grape varieties (white—Muscat Ottonel and red—Pinot Noir). The authors showed that the application of PEFE significantly increased the content of the total polyphenols and flavonoids and the antioxidant capacity. In addition, PEFE in combination with a homogenization step leads to a significant increase in bioactive compounds and antioxidant capacity compared to PEFE-treated samples of crushed grapes [114].
Supercritical fluid extraction (SFE): This method uses the solvation of the compounds of interest in a solvent maintained above its critical pressure and temperature. The solvent is immediately recovered when the system returns to atmospheric conditions. One of the most commonly used supercritical fluids is CO2. It is a green solvent that is cheap, environmentally friendly, easy-to-reach critical point (31 °C and 7.4 MPa), non-flammable, non-toxic, and non-carcinogenic [115]. The advantages of this method are a short extraction time, a low amount of solvent required, that it is environmentally friendly, and provides rapid solvent recovery. The main disadvantages are related to the high cost of the equipment and operation and complex optimization [116,117,118].
Pressurized hot water extraction (PHWE): This is a non-conventional method based on the extraction of molecules using hot liquid water as a solvent. To use this method, one must have both high temperature and pressure, exceeding 100 °C and 0.1 MPa, respectively (which is the boiling point of water at atmospheric pressure), but remaining below the critical point values (374 °C, 22.1 MPa). If water is used as a solvent, the PHWE method can be referred to by several names, including subcritical water extraction, superheated liquid extraction, pressurized liquid extraction, or accelerated solvent extraction [34,119]. Otero-Pareja et al. [120] compared two extraction processes for the recovery of antioxidants from grape pomace. They compared PHWE (mixture of 50% ethanol–water, 9 MPa, 120 °C for 90 min) and supercritical CO2 extraction (55 °C, 10 MPa, and 20% ethanol). It was found that the total yield and the yield of anthocyanins and phenolic compounds extracted were higher when the pressurized ethanolic solution was used. This process was more efficient in terms of the phenolic concentration and antioxidant activity. Pedras et al. [121] optimized the temperature of the extraction process of carbohydrates and phenolic compounds of grape pomace with PHWE. The experiment, which led to a higher concentration of the phenolic compounds (2.6 g.100 g−1 of grape pomace) and a more significant carbohydrate recovery (85%), was carried out at 210 °C and 10 MPa.
Ultrasound (UAE): An ultrasound can be utilized to enhance heat and mass transfer in plants by breaking down their cell walls through the cavitation effect [122]. UAE promotes greater solvent penetration into the cell material and shorter processing and residence times, resulting in better extraction yields and reproducibility. It also minimizes the use of solvents and emulsifier consumption, provides high extraction throughput, extraction of heat-labile components, significant savings in maintenance, lower energy requirements for extraction, and promotes a more eco-friendly and cost-effective processing method [111,122,123]. UAE can be easily combined with existing equipment as part of the technological plant or, if necessary, set up as a new production line [34,124]. This extractive method was used by some authors in recent studies to recover phenolic compounds from winery residues [125,126,127]. Frum et al. [128] appointed Nicolai et al. [95] to use the UAE method to determine the total phenolic compounds that constitute different wine by-products from the Alentejo and Ribatejo regions. They found that the concentration ranged between 0.16 and 1.93 mmol GAE.g−1 of the extract. González-Centeno et al. [96] extracted phenolic compounds and antioxidants from grape pomace and compared the efficiency of UAE with a conventional method (water was used as an extraction solvent with mechanical stirring). It was found that the properties of the extracts obtained with both methods were similar. However, at 50 °C, the UAE method was 8 times faster than the method with mechanical stirring (200 rpm). To increase the extraction yields, in the study developed by Drevelegka and Goula [129], enzymatic pre-treatment of the residue was carried out, followed by UAE in combination with MAE.
Natural deep eutectic solvents (NADES): These can be created by blending a hydrogen bond acceptor component (quaternary ammonium salts, such as choline chloride), natural hydrogen bond donors (such as sugars, alcohols, and amines), and up to 50% (v/v) water; it is possible to customize their physicochemical properties [91,92]. The advantages of using these solvents are the versatility of their physicochemical properties, the relatively easy precess [92], and a wide range of polarity and high solubilization strength. Thus, they can be used as an alternative greener solvent [91,92,130]. However, there are also some disadvantages, namely, a high viscosity and very low vapor pressure. The latter parameter makes it difficult to evaporate the solvent to isolate the target compound, commonly performed by distillation. However, anti-solvents can be added, and liquid–liquid or solid–liquid extraction can be performed [92]. Bioactive compounds can be extracted and preserved with the use of NADES, making them suitable for various applications in the food, pharmaceutical, and cosmetic industries [87]. Recent studies have proven the success of using NADES for the extraction of polyphenols from wine by-products [87,91,92,130,131,132]. The use of NADES containing choline chloride, tartaric acid, and water led to a 112% increase in the extraction efficiency of anthocyanins from grape pomace, when compared to the extraction efficiency achieved with acidified ethanol under the same conditions [87].
All the emergent extraction methods listed above can enhance the efficiency of extracting polyphenols, flavonoids, flavonols, sugars, minerals, and carotenoids from plant materials and by-products [34]. These methods are crucial for the food industry, among others. They aid in product innovation, leading to a wider range of food industry products and more efficient processes, making the industry more competitive [133,134].

Table 1.
Bioactive compounds (concentration and some biological properties) extracted from different types of by-products resulting from different Portuguese grape cultivars and origins.
Table 1.
Bioactive compounds (concentration and some biological properties) extracted from different types of by-products resulting from different Portuguese grape cultivars and origins.
| By-Product | Variety | Origin | Compounds Determined | Extraction Methods and Detection Method | Concentrations | References |
|---|---|---|---|---|---|---|
| Wine lees | ‘Sousão’ and ‘Tinta Barroca’ | Douro region (Northern Portugal) | Total phenols, ortho-diphenols and flavonoids | Extraction by ultrasound with MeOH (70%, v/v) at 70 °C for 40 min. | ‘Sousão’: Total phenols: 15.44 mg GAE.g−1 DW Ortho-diphenols: 118.91 mg GAE.g−1 DW Flavonoids: 18.50 mg CAT.g−1 DW ABTS: 1.71 mmol Trolox.g−1 DW DPPH: 1.24 mmol Trolox.g−1 DW FRAP: 1.54 mmol Trolox.g−1 DW ‘Tinta Barroca’: Total phenols: 125.39 mg GAE.g−1 DW Ortho-diphenols: 136.03 mg GAE.g−1 DW Flavonoids: 128.34 mg CAT.g−1 DW ABTS: 3.28 mmol Trolox.g−1 DW DPPH: 1.58 mmol Trolox.g−1 DW FRAP: 1.96 mmol Trolox.g−1 DW | [76] |
| Pomace | ‘Sousão’ and ‘Tinta Barroca’ | Douro region (Northern Portugal) | Total phenols, ortho-diphenols and flavonoids | Extraction by ultrasound with MeOH (70%, v/v) at 70 °C for 40 min. | ‘Sousão’: Total phenols: 153.70 mg GAE.g−1 DW Ortho-diphenols: 151.78 mg GAE.g−1 DW Flavonoids: 144.81 mg CAT.g−1 DW ABTS: 5.54 mmol Trolox.g−1 DW DPPH: 1.64 mmol Trolox.g−1 DW FRAP: 1.75 mmol Trolox.g−1 DW ‘Tinta Barroca’: Total phenols: 135.32 mg GAE.g−1 DW Ortho-diphenols: 138.70 mg GAE.g−1 DW Flavonoids: 129.93 mg CAT.g−1 DW ABTS: 4.01 mmol Trolox.g−1 DW DPPH: 1.59 mmol Trolox.g−1 DW FRAP: 1.61 mmol Trolox.g−1 DW | [76] |
| Stems | ‘Sousão’ and ‘Tinta Barroca’ | Douro region (Northern Portugal) | Total phenols, ortho-diphenols and flavonoids | Extraction by ultrasound with MeOH (70%, v/v) at 70 °C for 40 min. | ‘Sousão’: Total phenols: 156.81 mg GAE.g−1 DW Ortho-diphenols: 162.53 mg GAE.g−1 DW Flavonoids: 143.90 mg CAT.g−1 DW ABTS: 5.62 mmol Trolox.g−1 DW DPPH: 1.49 mmol Trolox.g−1 DW FRAP: 1.69 mmol Trolox.g−1 DW ‘Tinta Barroca’: Total phenols: 180.68 mg GAE.g−1 DW Ortho-diphenols: 170.24 mg GAE.g−1 DW Flavonoids: 160.71 mg CAT.g−1 DW ABTS: 8.02 mmol Trolox.g−1 DW DPPH: 1.85 mmol Trolox.g−1 DW FRAP: 2.02 mmol Trolox.g−1 DW | [76] |
| Stems | ‘Tinta Roriz’, Touriga | Quinta do Pinto, Alenquer (Lisbon). | Catechins | Conventional extraction with MeOH (70%, v/v) at RT for 30 min. | Catechin: 0.44 ± 0.02–2.03 ± 0.08 mg.g−1 DW Extracts with antioxidant activity: ABTS: 0.84 ± 0.06. DPPH: 0.64 ± 0.05. FRAP: 1.03 ± 0.06 mmol Trolox.g−1 DW. | [22] |
| Stems | ‘Alvarinho’, ‘Loureiro’ ‘Touriga’ ‘National’ and ‘Tinta Roriz’ (TR) | Sogrape Wines, S. A. (Porto, Portugal) and collected at Quinta dos Carvalhais (from the Dão region), Quinta do Seixo (from the Douro region) and Quinta de Azevedo (from the Minho region). | Phenolic compounds | Subcritical water extraction at 150 °C and 4 MPa for 40 min. | TR from the Douro region (33.7 ± 1.9 mg GAE.g−1 DW). IC50 for the Loureiro variable (56.68 ± 2.60 µg.mL−1). There were no adverse effects on the dermis cells HaCaT and HFF-1 in concentrations below 100 and 1000 µg.mL−1. | [21] |
| Stems | ‘Rabigato’, ‘Malvasia Fina’, ‘Fernão Pires’, Viosinho and ‘Moscatel’ | In the Baixo Corgo and Douro Region (Portugal). | Phenolic compounds | Conventional extraction with MeOH (70%, v/v) at RT for 30 min. Qualitative and quantitative analysis of phenolic composite (HPLC). | Total phenolic compounds: (94.71–123.09 mg−1 GAE). Individual phenolics: 0.02–73.79 mg.g−1. Antioxidant: 0.37–1.17 mmol Trolox.g−1 | [135] |
| Stems | ‘Rabigato’, ‘Malvasia Fina’, ‘Fernão Pires’, ‘Viosinho’ and ‘Moscatel’ | Douro Region (Portugal). | Phenolics | Conventional extraction with MeOH (70%, v/v) at RT for 30 min. Phenolic profile: evaluated by reverse-phase—high-performance liquid chromatography—diode ray detector (RP-HPLC-DAD). | PC: higher in regions of lower altitude (varying from 78.02 ± 0.70–103.49 ± 4.36 mg GAE.g−1 in contrast with concentrations between 32.35 ± 3.35 and 88.32 ± 1.75 mg GAE.g−1), Grape stem samples: Higher antioxidant activities (0.73 ± 0.00–0.85 ± 0.04 versus 0.24 ± 0.06–0.75 ± 0.01 mmol Trolox.g−1). | [23] |
| Stems | ‘Tinta Barroca’, ‘Sousão’ and ‘Syrah’ | Douro demarcated region in the north of Portugal; Quinta do Bonfim, vine located in the sub-region of Cima Corgo. | Total phenolic compounds and flavonoids | Conventional extraction (solvents) with MeOH (70%, v/v) at RT for 30 min. | TP: 42.04–96.29 mg GAE.g−1; Ortho-diphenols: 45.52–81.11 mg GAE.g−1; Flavonoids: 29.46–76.20 mg CAT.g−1. ABTS: 4.28–8.56; DPPH: 0.46–1.00 mmol Trolox.g−1. | [24] |
| Pomace | ‘Arinto’ or Pederna, ‘Aragonesa’ and ‘Tália’ | Herdade da Malhadinha Nova, Alentejo; Herdade da Bombeira, Alentejo and Herdade de Vila Chã, Ribatejo. | Total phenolic compounds | Extraction by ultrasound with EtOH (96%, v/v) at RT for 30 min. | Total phenols: 0.16 and 1.93 mmol GAE.g−1 of extract. | [95] |
| Stems | ‘Touriga Nacional’, ‘Franca’ and ‘Tinta Roriz’ | Douro Demarcated Region (Cima Corgo and Douro upper Sub-regions), Portugal. | Polyphenols | Conventional extraction with Acetone, EtOH, and water (1:1:1) at RT for 180 min. | TPC: 0.40 g.L−1 | [65] |
| Pomace made from fermented grapes (skin, seeds, and mixtures) | - | Three different samples: skin, seeds, and a mixture | Phenolic compounds (anthocyanin) | Conventional extraction with MeOH/water (80%, v/v) at 25 °C for 60 min and 0.5% trifluoroacetic acid. LC-DAD-ESI/MSn determined the phenolic profile. -Anthocyanin. -Evaluation of bioactive properties (antioxidant activity, cytotoxicity, antibacterial). | Seeds: high antioxidant activity: DPPH, reduction potential, β—carotene (23, 110, 208 and 49.6 µg.mL−1). -Skins: higher values of anthocyanins (7.9 µg.g−1 extracts). | [77] |
| Seed | ‘Touriga Nacional’, Castelão, Trincadeira, Alfrocheiro, ‘Tinta Roriz’, ‘Trincadeira das Pratas’, ‘Síria’, ‘Terrantez’, ‘Galego’ ‘Dourado’, ‘Vital’, ‘Cerceal Branco’, ‘Avesso’, ‘Arinto’, ‘Malvasia Colares’, ‘Gouveio’, ‘Azal’, ‘Antão Vaz’, ‘Rabigato’, ‘Rabo de Ovelha’, ‘Cercial’, ‘Fernão Pires’, ‘Verdelho’ and ‘Tália’ | - | Fatty acids from the extracted oil | Extraction was made on a semi-continuous process with the organic solvent (petroleum ether) at 60 °C for 8 h. | A maximum oil level (18 + 0.62% DW) was observed in the Azal variety and a minimum in Malvasia Rei (5 + 0.15% DW). The profile of the fatty acids was similar in all the samples. The most recurrent fatty acid was the linoleic acid, which varied between 69 + 0.75% (‘Arinto’ (white grape) and ‘Syrah’(red grape)) to 75 + 0.3% (Malvasia Rei (white grape) and ‘Aragonês’ (red grape)), followed by the oleic acid, which ranged between 13 + 0.07% (Terrantez (white grape) and ‘Aragonês’) and 19 + 0.6% (‘Syrah’). | [136] |
| Pomace | ‘Tinta Roriz’ | Herdade do Esporão, Portugal | Polyphenols | Used a Soxhlet unit to determine the most efficient hydroethanolic composition to maximize the extraction of phenolic compounds by varying the environmental composition. (A total of 20, 40, 60, or 80% w/w ethanol in water at 83.1 °C for 8 h). | Total phenolic compounds: 1042.1 mg GAE.L−1. | [137] |
| Pomace | Portugal | Lipids, carbohydrates, protein, lignin. | Lyophilized pomace. Protein characterization, ashes, and lipids; HPLC method for the analysis of carbohydrates. Colorimetric method for the analysis of carbohydrates. | Characterization (g.100 g−1) of waste in the reactor after treatment with subcritical water (240 °C and 7 MPa): Lipids = 18.5; Carbohydrates = 6.2; Protein = 7.6; Lignin = 25.6. | [138] | |
| Pomace | - | Portugal (Esporão, Alentejo region) | Carbohydrates and phenolic compounds | Pomace from white wine processed with subcritical water in a semi-continuous reactor at 170, 190, and 210 °C, and 10 MPa. | At 210 °C and 10 MPa, the trial resulted in the highest recovery of carbohydrates and phenolic compounds. (2.6 g.100 g−1) The extracts showed higher antimicrobic activity for Gram-positive than for Gram-negative. The lignin content of the remaining residue after SBW (subcritical water hydrolyzed) treatment at 210 °C (8.6 ± 1.5 g/100 g WGP (white grape pomace)) indicates that around half of the lignin of WGP was effectively hydrolyzed by SBW. | [121] |
4. Potential of Bioactive Compounds Obtained from By-Products of the Wine Industry
4.1. Health and Cosmetics
The discovery that the by-products of wineries can generate compounds that are beneficial for human health has resulted in a growing market for various items, such as functional foods, nutraceuticals, food additives, and cosmetics [22]. Polyphenols, which have antioxidant, anti-inflammatory, anticarcinogenic, and antibacterial properties, have been linked to the bioactive ability of grapes, wine, and winery by-products [139] (Table 2).

Table 2.
Bioactive compounds reported in grape by-products with health and cosmetics interests.
Polyphenols can prevent health-related diseases contributing to pandemics, such as COVID-19 [145,146]. At the molecular level, these inhibitors have shown great promise in preventing viral proteases from replicating, with minimal risk of toxicity.
However, research into natural methods for combating viral diseases is still in its nascent phases. Research on secondary metabolites with health benefits has revealed the extensive utilization of polyphenols, which possess advantageous dietary impacts on humans’ well-being [146,147,148].
Various cellular pathways can be influenced by wine polyphenols at a biochemical level [149]. Research in epidemiology suggests that the polyphenols present in dietary sources, such as wine, have a considerable impact on human health. PVPP (polyvinylpolypyrrolidone) a type of water-insoluble polymer known as ‘synthetic’, and is utilized for adsorbing phenolic compounds from beverages. Additionally, it is also employed in the treatment of white wine (legal limit: 80 g.hL−1) in the Douro region of Portugal to prevent phenolic oxidation and polymerization. The stability of wine is enhanced by this polymer, which adsorbs phenolic compounds, including those that cause browning reactions and bitterness [150]. This mixture, known as PVPP–white wine extract, is obtained through selective adsorption and contains a high concentration of phenolic compounds, making up roughly 80% of its dry mass. Among these compounds, phenolic acids, such as caftaric acid, and proanthocyanidins, such as catechin oligomers, are the most dominant [150,151,152]. This blend of phenolics exhibited impressive antioxidant activity by effectively scavenging superoxide anion radicals and maintaining lipid peroxidation in biological membranes. As a result, it has the potential to prevent or decrease oxidative stress linked to degenerative mechanisms in both type 2 diabetes mellitus and Alzheimer’s disease [150].
According to various studies, flavonoids have been linked to the targeted decrease in the viability of cancer cells [153,154,155]. The presence of quercetin in all the extracts is believed to contribute to this effect. Quercetin has the potential to combat cancer, as it can trigger apoptosis and hinder the growth of various human cancer cell lines [156]. A study conducted by Hamza [157] suggests that grape seed extract may have anticancer properties through its ability to promote apoptosis, inhibit cell proliferation, and block inflammation in hepatocarcinoma. It is important to mention that grape seed extract has been discovered to possess significant anti-tumor properties against various types of cancers, such as lung, colon, breast, bladder, leukemia, and prostate tumors [158,159]. According to a recent study, antioxidants found in grape stems have been found to protect against DNA damage caused by ROS, as well as inhibit the growth of liver (HepG2) and cervical (HeLa) cancer cells [160]. A recent study has further validated the usefulness of V. vinifera by-products in the creation of effective oral hygiene products that can prevent and treat various oral infections. These by-products possess a high value in terms of their polyvalent effects [161]. Numerous in vitro studies have thoroughly documented the antimicrobial and antiplatelet properties of V. vinifera extracts. Various phenolic compounds, such as flavonols, gallic acid, hydroxycinnamic acid, trans-resveratrol, and epicatechin, are present in wine and V. vinifera extracts, contributing to their antimicrobial activity [162,163]. Several other researchers have investigated the antibacterial properties of red wine and various oenological extracts, such as grape seed extracts, on a distinct type of dental plaque biofilm. This biofilm model includes Actinomyces oris, F. nucleatum, Streptococcus oralis, S. mutans, and Veillonella dispar [164]. Among all the extracts studied, the highest antimicrobial activity was present in red wine extract solutions enriched with grape seed extracts. In comparison with other wine extracts, it has been observed that grape seed extracts possess potent antimicrobial properties, which can be attributed to their high concentration of flavonoids and their derivatives. These compounds are believed to be the primary contributors to the extracts’ strong antimicrobial activity [161,164].
In the studies presented in Table 2, the bioactive compounds found in wine production by-products mostly have an anti-aging and sun protect function [165]. Nevertheless, although the by-products of grape processing can be potential sources of bioactive compounds, they have not yet been sufficiently explored. For example, Douro SkinCare (Portugal) and DC Dermoteca Cosmetics (Portugal) are two new cosmetic brands on the market that use grape oils in their formulations [149]. However, these two companies do not use all the by-products or extracts from the grape. As a result, more companies that re-use grape by-products while implementing environmental standards would have a business advantage.
4.2. Feed and Food Industry
The different compounds extracted from wine by-products and winery by-products can represent a significant value to the food and feed industry [166] (Table 3). These molecules or extracts can be used to produce oenological additives [167], obtain natural dyes [43], and obtain bioactive compounds [168]. In Europe, grape pomace is a highly valued by-product that is traditionally used for oil extraction, as well as a source of protein and tannins for animal feed [34].
The utilization of a winery by-products as a potential source of phenolic compounds has achieved great interest due to the multiple biological effects of these compounds as antioxidants and antimicrobials [6]. Extracts derived from grape by-products possess functional properties that make them useful in the development of a wide array of products. These applications range from food products to the inhibition of spoilage and pathogenic microorganisms, as well as the prevention of lipid oxidation [169].
The effects of natural antioxidants obtained from by-products produced from the wine industry have been studied in different raw materials for food industries, such as sunflower oil, fish oil, seaweed oil emulsion, beef, chicken, pork, and turkey meat, as well as in pre-prepared food, such as hamburgers, restructured meatballs, sausages, and marinated patés [170]. One of the main effects was related to their ability to delay lipid oxidation of these products during storage [171,172].
Grape pomace (‘Arinto’ and ‘Touriga’, Monsaraz, Alentejo, Portugal) flour can be used in a natural, cost-effective, environmentally friendly, and socially beneficial way by employing it in the development of human food, such as crackers. In this way, traditional wheat flour was supplemented with grape pomace flour between 5% and 10% (w/w) to nutritionally enrich the product [166]. The findings indicate a preference for crackers with 10% pomace flour in both varieties regarding the parameters evaluated (color, taste, texture, purchase intention, and global appreciation) [166]. The crackers with the highest percentage of incorporated grape pomace flour were the most adopted and appreciated. Consequently, increasing the percentage of this ingredient in the future will promote improvement in the nutritional value of goods for human consumption [166]. Ortega-Heras et al. [173] tested the incorporation of 10 and 20% of grape pomace flour in the manufacture of muffins, and obtained a high level of acceptance for the 10% incorporation. The muffins produced had a lower protein content, were denser, and included more fiber and fat. Similarly, Theagarajan et al. [174] incorporated 6% of grape pomace flour in the manufacture of cookies, resulting in a better quality product with an improved flavor. Additionally, it was found that the phenol content and antioxidant potency of the cookie extract increased to 4.03 mg GAE.g−1 of the cookie and IC50 0.72 mg. mL−1 of the cookie extract, respectively, with increasing incorporation of pomace flour from 4 to 6%.
Various studies have been conducted involving the incorporation of grape by-products in wheat bread [175,176,177]. Šporin et al. [177] incorporated different percentages (6, 10, and 15%) of grape pomace flour in wheat bread dough. It was found that the use of a mixture of wheat flour with grape pomace had a great impact on the rheological properties of the dough. The concentration of bagasse used was negatively correlated with the brightness and firmness of the bread, and also resulted in a reduction in water absorption. They also found that the cultivar from which the pomace is derived also has an impact on the properties of the bread produced, with the cultivar ‘Zelen’ leading to better results. Many nutraceutical compounds, particularly polyphenols, can be found in dried or lyophilized grape skins and seeds, as well as unfermented/semi-fermented and fermented varieties. These are commonly used to make infusions [178]. Grape by-product infusion is a method for valorizing grape by-products. The method is simple, and the primary expense is related to the electricity utilized for drying the moist material.
Therefore, these infusions can be used as health-promoting compounds that function as nutraceutical components [178].
In contrast, the creation and advancement of new products through innovative processes have led to the emergence of a distinct category of items known as functional foods. In this context, grape pomace extract can be used in the preparation of chicken products. Furthermore, the bioactive compounds present in grape extracts positively contribute to their well-known antioxidant and protective properties. Therefore, Tournour et al. [179] evaluated the effect of adding grape pomace extract and mechanically deboned chicken meat on the nutritional and sensory properties of chicken nuggets. The results show that adding mechanically deboned chicken meat to the ingot formulation mainly increased the fat content. In addition, the conduction of a suitability analysis confirmed these results and identified positive attributes, such as ‘light color’. Based on the findings, it can be inferred that adding grape pomace extract up to 120 mg.kg−1 and mechanically deboned chicken up to 15 g.100 g−1 did not have a negative impact on the visual appeal of chicken nuggets. In another study, Libera et al. [180] studied whether the polyphenol chemicals found in grape seeds can be employed in the development of novel animal-derived goods as a source of natural antioxidants. This study examined how different concentrations (0.1%, 0.2%, and 0.5%) of a grape seed extract containing 40% ethanol (GSE) affected the quality of dry-cured pork neck. After conducting the tests, it was found that the use of extracts with higher concentrations resulted in better lipid stability compared to the lot containing only 0.1%. According to the study, the GSE extract effectively inhibited lipid hydrolysis and limited oxidative processes in the meats during curing when added in a concentration of at least 0.2% [180]. Gasiński et al. [181] studied the potential use of white grape pomace, the most abundant waste from the white wine industry, in modifying the volatilome and phenolic content of beer. The pomace was added to beer after primary fermentation in varying concentrations (10% and 20% w/w) and subjected to different pre-treatments (pasteurized and unpasteurized).
The results show that in all the beers tested, there was an increase in the concentration of phenolic compounds of 321.584 to 501,459 mg GAE.dm−3, with the insertion of white grape pomace. There was also, with the insertion of white grape pomace, a reduction in the composition of volatiles in beers (the concentration of the acetaldehyde content was 17.425–31.425 mg.dm−3, much lower than the control sample, with 134.050 mg.dm−3), and an increase in relation to the antioxidant activity of the beers against the DPPH•, FRAP, and ABTS+• assays [181].

Table 3.
Studies regarding the application of grape by-products and derived extracts in food products.
Table 3.
Studies regarding the application of grape by-products and derived extracts in food products.
| Grape By-Product | Variety/Origin | Food Product | Concentration | Effects | References |
|---|---|---|---|---|---|
| Pomace | Grape pomace (Ives noir cultivar, Vitis labrusca species) by the company Família Fardo, Quatro Barras/Paraná/Brazil | Rice | Addition of grape pomace flour in the process of parboiling the rice; GP:rice ratio 1:2. | -Improved the antioxidant activity. -Change in color. | [64] |
| Pomace | Vitis labrusca cv. Isabel | Salmon burger | Addition of 1 and 2% of grape pomace flour to the burger recipe. | -Increased dietary fiber content and storage stability. -Decrease in sensory properties. | [182] |
| Pomace | Vitis vinifera L. of the ‘Arinto’ and ‘Touriga Nacional’ | Crackers | 5%, 10%, and 15% | -‘High in fiber’, as per the Regulation (EC) No. 1924/2006, suggesting a functional food. | [183] |
| Pomace | ‘Syrah’, ‘Merlot’, and ‘Cabernet Sauvignon’ | Beef hamburger patties | 0%, 2%, and 4%. | -Provided hamburger patties with health promoting factors, such as antioxidant and other functional components. -Provided darker, sourer patties and a lower cooking yield. | [26] |
| Skin | ‘Pinot Noir’ (PN) and ‘Italian Riesling’ (IR) grape varieties from western Romania (Teremia Mare Winery, Timis County) | Pasta | Replacement of wheat flour with 3, 6, and 9% of grape skin flour. | -Increased antioxidant activity total phenolic content. -Better sensory evaluation. | [184] |
| Skin | Vitis vinifera L. of red variety Frankova modra from southwest Slovakia | Cookie dough | A total of 0, 5, 10, and 15% to weight of flour. | -Decreased dough consistency and stability. -Increased water absorption. -Volume and thickness of cookies decreased. | [185] |
| Seed | Grapes, Vitis vinifera L. of red variety Frankova modra; incorporation of grape skins and grape seeds | A total of 0, 5, 10, and 15% to weight of flour. | -Increased dough consistency and stability. -Decreased water absorption. -Volume and thickness of cookies decreased. | ||
| Skin | - | Butter biscuits | Grape powders were added to the dough in an amount of 15.0%. | -Increased the butter biscuits’ apparent dough viscosity. -Decreased the modulus of instantaneous springiness and the elasticity modulus. -Increased dough plastic viscosity. | [186] |
| Seed | Red wine grape pomace | Wheat bread dough | Different addition levels (0, 3, 5, 7, 9%) to the white wheat flour. | -Dough water absorption decreased with the increase of grape seed flour addition level, influencing dough development time and stability. -The falling number index showed a gradual decrease with the particle size decrease and addition level increase. | [175] |
| Seed | - | Meat emulsion | 50% | -Reduction of animal fat. | [187] |
| Seed | Ningxia Huahao winery (Yingchuan, China) | Noodle | 1% to 5% | -Replacement of flour. -Textural traits. | [188] |
| Seed | Tianjin Jianfeng Natural Product Co., Ltd. (Tianjin, China) | Seabass fillets | 0.5% | -Reduction of microbial growth and biogenic amines. -Color traits. | [189] |
| Seed | - | Roast chicken | 0.5% | -Reduction of microbial growth and oxidation. -Physical/color properties. | [190] |
| Seed | - | Minced Beef | 0.05% to 1% | -Reduction of microbial growth and oxidation. | [191] |
4.3. Food Packaging
In recent years, the demand for intelligent packaging systems for food products has risen significantly. This is because food manufacturers are trying to improve their products’ sustainability and environmental impact while maintaining their quality and safety [192,193]. Food packaging protects food from contamination and other impacts, such as odors, physical damage, shock, dust, temperature, light, moisture, and microorganisms. Therefore, ensuring the quality and safety of food is crucial, especially when trying to extend its shelf life and minimize food loss and waste. Using natural colorant indicators, such as anthocyanins, can effectively monitor the freshness and shelf life of perishable food items, thereby ensuring their quality control [193,194,195].
Ferreira et al. [196] studied the incorporation of grape pomace extracts containing 0.15% aqueous extract (mainly polysaccharides), 0.15–0.3% grape skin extract (wax), and 0.3–0.75% grape seed oil into chitosan films. The films made of chitosan that contained the aqueous extract were found to be highly hydrophilic and smooth. Additionally, these films demonstrated enhanced antioxidant properties, while maintaining their water solubility and mechanical strength. By incorporating wax into the chitosan solution, the resulting film had a more uneven and rough appearance, but its antioxidant properties were enhanced without affecting its solubility. The addition of wax resulted in improved flexibility and reduced stiffness of the films. Among the chitosan-based films with oil, those containing wax were the most hydrophobic and exhibited greater antioxidant activity. These films also had slightly lower water solubility. The findings suggest that grape pomace extract films made with chitosan are a viable alternative to synthetic materials. These films can also serve as carriers for functional compounds, which may have biological applications. Furthermore, they have the potential to extend the shelf life of food products.
In their study, Shahbazi [197] investigated the possible uses of GSE (1% w/v) on its own and in conjunction with ZEO essential oil from the Ziziphora clinopodioides plant in films made of chitosan and gelatin. According to the results, both films showed good antibacterial and antioxidant effects due to the high phenolic content. Biodegradable active packaging is crucial in the food industry as it provides a significant defense against microbial and chemical contamination. In a recent study, a new active packaging with antioxidant properties was developed using extracts from red grape pomace and blueberries as two distinct biological resources [198,199]. The extracts obtained by MAE were incorporated into chitosan and carboxymethylcellulose, respectively. Films that were enriched with red grape skin extract displayed superior antioxidant activity compared to those enriched with blueberry extract [199].
Cejudo-Bastante et al. [200] recently reported that using natural jute fibers impregnated with a grape pomace extract was proposed as a new active food packaging material. Because of the results, pomace extract’s antioxidant and antimicrobial properties were successfully transferred to natural jute tissues through supercritical impregnation. The authors compared the results with others obtained in polymeric matrices and offer a very optimistic view of using this natural fiber as an active food packaging [200].
In another study by Guo et al. [190], the goal was to determine the optimal concentration of a grape seed extract solution and investigate how it affects the physical and chemical properties of roasted chicken when combined with modified atmosphere packaging during storage at 4 °C (Table 3).
5. Conclusions
Winery by-products are important sources of bioactive compounds. These compounds with high added value are present in several winery by-products, namely, wine lees, grape pomace, and grape stems, and show antioxidant, antimicrobial, anti-inflammatory, or anticancer activity. Moreover, these bioactive compounds may have promising benefits in the pharmaceutical, cosmetic, and feed and food industries.
It is important to note the current and upcoming technological advancements that enable us to replace traditional methods with innovative ones for extracting valuable compounds from winery by-products. New technologies offer many benefits, such as reducing processing time, energy consumption, and the use of harmful and costly solvents. This promotes environmental benefits and significantly increases the conservation and yield of bioactive ingredients. Howeve.r, the enhanced extraction yields align perfectly with the principles of eco-friendly extraction techniques. Therefore, the importance of looking to the future cannot rule out what is already being accomplished in the present in the European Union and around the world, where several companies are shaping their values more sustainably and circularly, both in the development of products in closed cycles and the creation of services, which have reflected positively on their brands, attracting more customers.
All these examples show how adopting a circular bioeconomy approach is essential to achieving responsible and competitive production models in the wine industry. Considering the amount of winery by-products produced annually in Portugal and Europe, the wine industry’s by-products are considered to have enormous potential. In short, the presented solution aims to upcycle the wine industry’s by-products in a circular economy perspective through the use of green extraction technologies and the digitalization of the industry to obtain value-added products.
Author Contributions
A.R.M. and J.A.P.P.: Conceptualization. A.R.M., T.A., J.S. and E.M.C.A.: Writing—Original draft. M.E.P., J.A.P.P. and J.N.: Writing—Review and editing. M.E.P. and J.A.P.P.: Supervision. All authors have read and agreed to the published version of the manuscript.
Funding
For this research, Adriana R. Machado, Maintain Tugba Atatoprak, Joana Santos, and Jorge A. P. Paiva were supported by the research contract funded by Fundação para a Ciência e Tecnologia (FCT) and project CENTRO-04-3559-FSE-000095—Centro Portugal Regional Operational Programme (Centro2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Author Elisabete M.C. Alexandre was supported by the financial support of this work funded by national funds (OE) through FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of article 23 of the Decree-Law 57/2016, of 29 August, changed by Law 57/2017, of 19 July. The authors acknowledge Universidade Católica Portuguesa for the financial support of the CBQF Associate Laboratory under the FCT project UID/Multi/50016/2019; the University of Aveiro and FCT/MCT for the financial support for the QOPNA research Unit (FCT UID/QUI/00062/2019); and to the funding from Laboratório Associado LAQV-REQUIMTE (UIDB/50006/2020) through national funds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
Adriana R. Machado, Maintain Tugba Atatoprak, Joana Santos, and Jorge A. P. Paiva give thanks for their research contract funded by Fundação para a Ciência e Tecnologia (FCT) and project CENTRO-04-3559-FSE-000095—Centro Portugal Regional Operational Programme (Centro2020), under the PORTUGAL 2020 Partnership Agreement, through the European Regional Development Fund (ERDF). Author Elisabete M.C. Alexandre is also grateful for the financial support of this work funded by national funds (OE) through FCT—Fundação para a Ciência e a Tecnologia, I.P., in the scope of the framework contract foreseen in the numbers 4, 5, and 6 of article 23 of the Decree-Law 57/2016, of 29 August, changed by Law 57/2017, of 19 July. Thanks are due to the Universidade Católica Portuguesa for the financial support of the CBQF Associate Laboratory under the FCT project UID/Multi/50016/2019; to the University of Aveiro and FCT/MCT for the financial support for the QOPNA research Unit (FCT UID/QUI/00062/2019); and to Laboratório Associado LAQV-REQUIMTE (UIDB/50006/2020) for funding through national funds and, where applicable, co-financed by the FEDER, within the PT2020 Partnership Agreement. João Nunes thanks the Portuguese Foundation for Science and Technology (FCT), I.P./MCTES, for financial support through national funds (PIDDAC) in the scope of the Centre Bio R&D Unit (UIDB/05083/2020).
Conflicts of Interest
The authors declare no conflict of interest.
References
- Piasecka, I.; Gorska, A. Possible Uses of Fruit Pomaces in Food Technology as a Fortifying Additive—A Review. Zesz. Probl. Postępów Nauk. Rol. 2020, 600, 43–54. [Google Scholar] [CrossRef]
- FAO Organização Das Nações Unidas Para Agricultura e Alimentação: FAO Apresenta Avanços No Combate ÀS Perdas e Ao Desperdício de Alimentos|FAO No Brasil|Food and Agriculture Organization of the United Nations. Available online: https://www.fao.org/brasil/noticias/detail-events/pt/c/1062706/ (accessed on 25 January 2023).
- Melo, P.S.; Bergamaschi, K.B.; Tiveron, A.P.; Massarioli, A.P.; Oldoni, T.L.C.; Zanus, M.C.; Pereira, G.E.; de Alencar, S.M. Composição fenólica e atividade antioxidante de resíduos agroindustriais. Cienc. Rural 2011, 41, 1088–1093. [Google Scholar] [CrossRef]
- Saraiva, B.R.; Vital, A.C.P.; Anjo, F.A.; de Cesaro, E.; Matumoto-Pintro, P.T. Valorização de resíduos agroindustriais: Fontes de nutrientes e compostos bioativos para a alimentação humana. Pubsaúde 2018, 1, 1–10. [Google Scholar] [CrossRef]
- Trevizan, J.A.C.; Bido, G.D.S.; Ferrari, A.; Felipe, D.F. Nutritional Composition of Malted Barley Residue from Brewery. J. Mgmt. Sustain. 2021, 11, 27–34. [Google Scholar] [CrossRef]
- Jesus, M.S.; Genisheva, Z.; Romaní, A.; Pereira, R.N.; Teixeira, J.A.; Domingues, L. Bioactive Compounds Recovery Optimization from Vine Pruning Residues Using Conventional Heating and Microwave-Assisted Extraction Methods. Ind. Crops Prod. 2019, 132, 99–110. [Google Scholar] [CrossRef]
- Teixeira, A.; Baenas, N.; Dominguez-Perles, R.; Barros, A.; Rosa, E.; Moreno, D.A.; Garcia-Viguera, C. Natural Bioactive Compounds from Winery By-Products as Health Promoters: A Review. Int. J. Mol. Sci. 2014, 15, 15638–15678. [Google Scholar] [CrossRef]
- Agostini, F. Obtenção e Análise de Óleo e Compostos Fenólicos de Sementes de Diferentes Variedades de uva (Vitis vinifera e Vitis labrusca) Cultivadas no Rio Grande do Sul. Ph.D. Thesis, Universidade de Caxias do Sul, Caxias do Sul, Brazil, 2014. [Google Scholar]
- Campos, D.A.; Gómez-García, R.; Vilas-Boas, A.A.; Madureira, A.R.; Pintado, M.M. Management of Fruit Industrial By-Products—A Case Study on Circular Economy Approach. Molecules 2020, 25, 320. [Google Scholar] [CrossRef] [PubMed]
- Salgado-Ramos, M.; Mariatti, F.; Tabasso, S.; Sánchez-Verdú, M.P.; Moreno, A.; Cravotto, G. Sustainable and Non-Conventional Protocols for the Three-Way Valorisation of Lignin from Grape Stalks. Chem. Eng. Process. Process Intensif. 2022, 178, 109027. [Google Scholar] [CrossRef]
- Schwartz, C.G.K.; de Jesus, J.L.L.; Ramos, F.A.P.; Mezalira, T.S.; Ferreira, R.G.; Otutumi, L.K.; Soares, A.A. Compostos bioativos do bagaço de uva (Vitis vinífera) seus benefícios e perspectivas para o desenvolvimento sustentável. In Tecnologia de Alimentos: Tópicos Físicos, Químicos e Biológicos; Científica: Guarujá, Brazil, 2020; pp. 483–505. [Google Scholar]
- Sirohi, R.; Tarafdar, A.; Singh, S.; Negi, T.; Gaur, V.K.; Gnansounou, E.; Bharathiraja, B. Green Processing and Biotechnological Potential of Grape Pomace: Current Trends and Opportunities for Sustainable Biorefinery. Bioresour. Technol. 2020, 314, 123771. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. World Wine Production Outlook OIV First Estimates 4 November 2021; International Organisation of Vine and Wine: Dijon, France, 2021. [Google Scholar]
- Nanni, A.; Parisi, M.; Colonna, M. Wine By-Products as Raw Materials for the Production of Biopolymers and of Natural Reinforcing Fillers: A Critical Review. Polymers 2021, 13, 381. [Google Scholar] [CrossRef]
- International Organisation of Vine and Wine. World Wine Production Outlook. OIV First Estimates. 2022. Available online: https://www.greatwinecapitals.com/wpfd_file/en-oiv-2022-world-wine-production-outlook-1/ (accessed on 21 March 2023).
- Makris, D.P.; Boskou, G.; Andrikopoulos, N.K. Polyphenolic Content and in Vitro Antioxidant Characteristics of Wine Industry and Other Agri-Food Solid Waste Extracts. J. Food Compos. Anal. 2007, 20, 125–132. [Google Scholar] [CrossRef]
- Melo, P.S.; Massarioli, A.P.; Denny, C.; dos Santos, L.F.; Franchin, M.; Pereira, G.E.; de Souza Vieira, T.M.F.; Rosalen, P.L.; de Alencar, S.M. Winery By-Products: Extraction Optimization, Phenolic Composition and Cytotoxic Evaluation to Act as a New Source of Scavenging of Reactive Oxygen Species. Food Chem. 2015, 181, 160–169. [Google Scholar] [CrossRef] [PubMed]
- Rubilar, M.; Pinelo, M.; Shene, C.; Sineiro, J.; Nuñez, M.J. Separation and HPLC-MS Identification of Phenolic Antioxidants from Agricultural Residues: Almond Hulls and Grape Pomace. J. Agric. Food Chem. 2007, 55, 10101–10109. [Google Scholar] [CrossRef]
- da Silveira, M.A.G.; Meira, S.M.M.; Felix, S.; Gautério, F.G.A.; de los Santos, J.R.G. A sustentabilidade do destino do bagaço da vinificação no Brasil. Res. Soc. Dev. 2020, 9, e247997197. [Google Scholar] [CrossRef]
- Fontana, M.; Murowaniecki Otero, D.; Pereira, A.M.; Santos, R.B.; Gularte, M.A. Grape Pomace Flour for Incorporation into Cookies: Evaluation of Nutritional, Sensory and Technological Characteristics. J. Culin. Sci. Technol. 2022, 1–20. [Google Scholar] [CrossRef]
- Moreira, M.M.; Rodrigues, F.; Dorosh, O.; Pinto, D.; Costa, P.C.; Švarc-Gajić, J.; Delerue-Matos, C. Vine-Canes as a Source of Value-Added Compounds for Cosmetic Formulations. Molecules 2020, 25, 2969. [Google Scholar] [CrossRef]
- Leal, C.; Santos, R.A.; Pinto, R.; Queiroz, M.; Rodrigues, M.; José Saavedra, M.; Barros, A.; Gouvinhas, I. Recovery of Bioactive Compounds from White Grape (Vitis vinifera L.) Stems as Potential Antimicrobial Agents for Human Health. Saudi J. Biol. Sci. 2020, 27, 1009–1015. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Pinto, R.; Santos, R.; Saavedra, M.J.; Barros, A.I. Enhanced Phytochemical Composition and Biological Activities of Grape (Vitis vinifera L.) Stems Growing in Low Altitude Regions. Sci. Hortic. 2020, 265, 109248. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Santos, R.A.; Queiroz, M.; Leal, C.; Saavedra, M.J.; Domínguez-Perles, R.; Rodrigues, M.; Barros, A.I. Monitoring the Antioxidant and Antimicrobial Power of Grape (Vitis vinifera L.) Stems Phenolics over Long-Term Storage. Ind. Crops Prod. 2018, 126, 83–91. [Google Scholar] [CrossRef]
- Chen, J.; Thilakarathna, W.P.D.W.; Astatkie, T.; Rupasinghe, H.P.V. Optimization of Catechin and Proanthocyanidin Recovery from Grape Seeds Using Microwave-Assisted Extraction. Biomolecules 2020, 10, 243. [Google Scholar] [CrossRef]
- Pereira, A.; Lee, H.C.; Lammert, R., Jr.; Wolberg, C., Jr.; Ma, D.; Immoos, C.; Casassa, F.; Kang, I. Effects of Red-Wine Grape Pomace on the Quality and Sensory Attributes of Beef Hamburger Patty. Int. J. Food Sci. Technol. 2022, 57, 1814–1823. [Google Scholar] [CrossRef]
- Yu, J.; Smith, I.; Carver, J.; Holmes, B. Fatty Acid Composition of Grape Seed Oil as Affected by Grape Variety and Extraction Solvent. EC Nutr. 2021, 16, 51–58. [Google Scholar]
- Sahpazidou, D.; Geromichalos, G.D.; Stagos, D.; Apostolou, A.; Haroutounian, S.A.; Tsatsakis, A.M.; Tzanakakis, G.N.; Hayes, A.W.; Kouretas, D. Anticarcinogenic Activity of Polyphenolic Extracts from Grape Stems against Breast, Colon, Renal and Thyroid Cancer Cells. Toxicol. Lett. 2014, 230, 218–224. [Google Scholar] [CrossRef] [PubMed]
- Ferreyra, S.; Bottini, R.; Fontana, A. Background and Perspectives on the Utilization of Canes’ and Bunch Stems’ Residues from Wine Industry as Sources of Bioactive Phenolic Compounds. J. Agric. Food Chem. 2023, 71, 8699–8730. [Google Scholar] [CrossRef]
- Haas, I.C.D.S. Resíduo Obtido Do Processamento de Sucos de Uva (Vitis labrusca L.): Composição Fenólica, Bioacessibilidade In Vitro e Potencial Biológico Em Células Tumorais. Ph.D. Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2019. [Google Scholar]
- Kandemir, K.; Piskin, E.; Xiao, J.; Tomas, M.; Capanoglu, E. Fruit Juice Industry Wastes as a Source of Bioactives. J. Agric. Food Chem. 2022, 70, 6805–6832. [Google Scholar] [CrossRef] [PubMed]
- Carvalho, M.T.D.; Bergamasco, R.; Gomes, R.G. Métodos de extração de compostos bioativos: Aproveitamento de subprodutos na agroindústria. Uningá Rev. 2018, 33, 66–84. [Google Scholar]
- Zwingelstein, M.; Draye, M.; Besombes, J.-L.; Piot, C.; Chatel, G. Viticultural Wood Waste as a Source of Polyphenols of Interest: Opportunities and Perspectives through Conventional and Emerging Extraction Methods. Waste Manag. 2020, 102, 782–794. [Google Scholar] [CrossRef]
- Barba, F.J.; Zhu, Z.; Koubaa, M.; Sant’Ana, A.S.; Orlien, V. Green Alternative Methods for the Extraction of Antioxidant Bioactive Compounds from Winery Wastes and By-Products: A Review. Trends Food Sci. Technol. 2016, 49, 96–109. [Google Scholar] [CrossRef]
- Poojary, M.M.; Dellarosa, N.; Roohinejad, S.; Koubaa, M.; Tylewicz, U.; Gómez-Galindo, F.; Saraiva, J.A.; Rosa, M.D.; Barba, F.J. Influence of Innovative Processing on γ-Aminobutyric Acid (GABA) Contents in Plant Food Materials. Compr. Rev. Food Sci. Food Saf. 2017, 16, 895–905. [Google Scholar] [CrossRef]
- Jin, S.; Yang, B.; Cheng, Y.; Tan, J.; Kuang, H.; Fu, Y.; Bai, X.; Xie, H.; Gao, Y.; Lv, C.; et al. Improvement of Resveratrol Production from Waste Residue of Grape Seed by Biotransformation of Edible Immobilized Aspergillus Oryzae Cells and Negative Pressure Cavitation Bioreactor Using Biphasic Ionic Liquid Aqueous System Pretreatment. Food Bioprod. Process. 2017, 102, 177–185. [Google Scholar] [CrossRef]
- Santana, M.T.A. Caracterização Físico-Química, Química e Sensorial de Frutos e Vinho da cv. Patrícia (Vitis labrusca L.). Ph.D. Thesis, Universidade Federal de Lavras, Lavras, Brazil, 2005. [Google Scholar]
- Spinei, M.; Oroian, M. The Potential of Grape Pomace Varieties as a Dietary Source of Pectic Substances. Foods 2021, 10, 867. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; Gonzaga, L.V.; Rizelio, V.M.; Gonçalves, A.E.D.S.S.; Genovese, M.I.; Fett, R. Phenolic Compounds and Antioxidant Activity of Seed and Skin Extracts of Red Grape (Vitis vinifera and Vitis labrusca) Pomace from Brazilian Winemaking. Food Res. Int. 2011, 44, 897–901. [Google Scholar] [CrossRef]
- Haas, I.C.D.S. Resíduo Obtido Do Processamento Do Suco de Uva: Caracterização e Cinética de Secagem. Master’s Thesis, Universidade Federal de Santa Catarina, Florianópolis, Brazil, 2015. [Google Scholar]
- Versari, A.; Boulton, R.B.; Parpinello, G.P. A Comparison of Analytical Methods for Measuring the Color Components of Red Wines. Food Chem. 2008, 106, 397–402. [Google Scholar] [CrossRef]
- Baaka, N.; Ben Ticha, M.; Haddar, W.; Hammami, S.; Mhenni, M.F. Extraction of Natural Dye from Waste Wine Industry: Optimization Survey Based on a Central Composite Design Method. Fibers Polym. 2015, 16, 38–45. [Google Scholar] [CrossRef]
- De Oliveira Filho, J.F. Extração de Corante Natural a Partir do Resíduo da Uva. Bachelor’s Thesis, Universidade Federal do Rio Grande do Norte, Natal, Brazil, 2017; p. 44. [Google Scholar]
- Blackford, M.; Comby, M.; Zeng, L.; Dienes-Nagy, Á.; Bourdin, G.; Lorenzini, F.; Bach, B. A Review on Stems Composition and Their Impact on Wine Quality. Molecules 2021, 26, 1240. [Google Scholar] [CrossRef]
- Romaní, A.; Michelin, M.; Domingues, L.; Teixeira, J.A. Chapter 16—Valorization of Wastes from Agrofood and Pulp and Paper Industries Within the Biorefinery Concept: Southwestern Europe Scenario. In Waste Biorefinery; Bhaskar, T., Pandey, A., Mohan, S.V., Lee, D.-J., Khanal, S.K., Eds.; Elsevier: Amsterdam, The Netherlands, 2018; pp. 487–504. ISBN 978-0-444-63992-9. [Google Scholar]
- Cordeiro, A.I.; Moreno, L.; Espejo, A.; Machuca, S.; Almeida, T.; Santos, M.; Mondragão-Rodrigues, F.; Pacheco de Carvalho, G.; Paulo, M.; Sanchez, R. Valorização dos Subprodutos Produzidos nas Adegas da Zona Euroace. 2020. Available online: https://www.rederural.gov.pt/centro-de-recursos/send/10-inovacao/1885-artigo-cientifico-valorizacao-dos-subprodutos-produzidos-nas-adegas-da-zona-euroace (accessed on 21 March 2023).
- Bharathiraja, B.; Iyyappan, J.; Jayamuthunagai, J.; Kumar, R.P.; Sirohi, R.; Gnansounou, E.; Pandey, A. Critical Review on Bioconversion of Winery Wastes into Value-Added Products. Ind. Crops Prod. 2020, 158, 112954. [Google Scholar] [CrossRef]
- Rezende, F.A.; dos Santos, B.; do Nascimento, I.J.; Candiootto, A. Processo de Fabricação de Vinho. In Proceedings of the VIII EEPA—Encontro de Engenharia de Produção Agroindustrial; 2015. Available online: http://www.fecilcam.br/anais/viii_eepa/arquivos/11-01.pdf (accessed on 21 March 2023).
- Salazar, P.B.; Romero, V.L.; Minahk, C.J.; Vaquero, M.J.R. 3 Winery-Derived By-Products: Valorization and Potential. Emerg. Environ. Technol. Health Prot. 2018, 17, 31–46. [Google Scholar]
- Ramawat, K.G.; Merillon, J.M.; Arora, J. Agricultural Waste: Environmental Impact, Useful Metabolites and Energy Production; Spinger: Berlin/Heidelberg, Germany, 2023; ISBN 978-981-19877-3-1. [Google Scholar]
- Navarro, P.; Sarasa, J.; Sierra, D.; Esteban, S.; Ovelleiro, J.L. Degradation of Wine Industry Wastewaters by Photocatalytic Advanced Oxidation. Water Sci. Technol. 2005, 51, 113–120. [Google Scholar] [CrossRef]
- Bustamante, M.A.; Said-Pullicino, D.; Agulló, E.; Andreu, J.; Paredes, C.; Moral, R. Application of Winery and Distillery Waste Composts to a Jumilla (SE Spain) Vineyard: Effects on the Characteristics of a Calcareous Sandy-Loam Soil. Agric. Ecosyst. Environ. 2011, 140, 80–87. [Google Scholar] [CrossRef]
- Oliveira, R.M.C. Valorização do Bagaço de Uva: Avaliação da Potencialidade de Produção de Biogás. Ph.D. Thesis, Universidade da Beira Interior, Covilhã, Portugal, 2011. [Google Scholar]
- The Council of the European Union. Council Regulation (EC), No 479/2008 of 29 April 2008 on the common organisation of the market in wine, amending Regulations (EC) No 1493/1999, (EC) No 1782/2003, (EC) No 1290/2005, (EC) No 3/2008 and repealing Regulations (EEC) No 2392/86 and (EC) No 1493/1999. (06.06.2008). Off. J. Eur. Union 2008, 148, 1–61. [Google Scholar]
- Patel, S. Treatment of Distillery Waste Water: A Review. Int. J. Theor. Appl. Sci. 2018, 10, 23. [Google Scholar]
- Güneş Bayir, A.; Aksoy, A.N.; KoçyiğiT, A. The Importance of Polyphenols as Functional Food in Health. Bezmialem Sci. 2019, 7, 157–163. [Google Scholar] [CrossRef]
- Pandey, K.B.; Rizvi, S.I. Plant Polyphenols as Dietary Antioxidants in Human Health and Disease. Oxidative Med. Cell. Longev. 2009, 2, 270–278. [Google Scholar] [CrossRef] [PubMed]
- Rasouli, H.; Farzaei, M.H.; Khodarahmi, R. Polyphenols and Their Benefits: A Review. Int. J. Food Prop. 2017, 20, 1700–1741. [Google Scholar] [CrossRef]
- Rotili, M.C.C.; Villa, F.; Braga, G.C.; de França, D.L.B.; Rosanelli, S.; Laureth, J.C.U.; da Silva, D.F.; Rotili, M.C.C.; Villa, F.; Braga, G.C.; et al. Bioactive Compounds, Antioxidant and Physic-Chemical Characteristics of the Dovyalis Fruit. Acta Scientiarum. Agron. 2018, 40, e35465. [Google Scholar] [CrossRef]
- Voss, G.B.; Oliveira, A.L.S.; Alexandre, E.M.d.C.; Pintado, M.E. Chapter 1—Importance of Polyphenols: Consumption and Human Health. In Technologies to Recover Polyphenols from AgroFood By-Products and Wastes; Pintado, M.E., Saraiva, J.M.A., Alexandre, E.M.d.C., Eds.; Academic Press: Cambridge, MA, USA, 2022; pp. 1–23. ISBN 9780323852739. [Google Scholar]
- Nieto, J.A.; Santoyo, S.; Prodanov, M.; Reglero, G.; Jaime, L. Valorisation of Grape Stems as a Source of Phenolic Antioxidants by Using a Sustainable Extraction Methodology. Foods 2020, 9, 604. [Google Scholar] [CrossRef]
- Abreu, B.B.; Sousa, C.R.N.; Passos, J.C.; Marinho, A.R.S.; Brandão, A.C.A.S.; de Oliveira, M.L.V.S.; Moreira-Araújo, R.S.d.R. Composição centesimal, compostos bioativos e atividade antioxidante em cálice de hibisco (Hibiscus sabdariffa L.). J. Interdiscip. Biociênc. 2019, 4, 1–4. [Google Scholar] [CrossRef]
- Schneuer, F.J.; Nassar, N.; Tasevski, V.; Morris, J.M.; Roberts, C.L. Association and Predictive Accuracy of High TSH Serum Levels in First Trimester and Adverse Pregnancy Outcomes. J. Clin. Endocrinol. Metab. 2012, 97, 3115–3122. [Google Scholar] [CrossRef]
- Balbinoti, T.C.V.; Stafussa, A.P.; Haminiuk, C.W.I.; Maciel, G.M.; Sassaki, G.L.; Jorge, L.M.d.M.; Jorge, R.M.M. Addition of Grape Pomace in the Hydration Step of Parboiling Increases the Antioxidant Properties of Rice. Int. J. Food Sci. Technol. 2020, 55, 2370–2380. [Google Scholar] [CrossRef]
- Teixeira, N.; Mateus, N.; de Freitas, V.; Oliveira, J. Wine Industry By-Product: Full Polyphenolic Characterization of Grape Stalks. Food Chem. 2018, 268, 110–117. [Google Scholar] [CrossRef]
- Wichienchot, S.; Chakkaravarthi, S. Chapter 42—Polyphenols from Food Processing Byproducts and Their Microbiota–Gut–Brain Axis-Based Health Benefits. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 855–880. ISBN 978-0-12-824044-1. [Google Scholar]
- Baroi, A.M.; Popitiu, M.; Fierascu, I.; Sărdărescu, I.-D.; Fierascu, R.C. Grapevine Wastes: A Rich Source of Antioxidants and Other Biologically Active Compounds. Antioxidants 2022, 11, 393. [Google Scholar] [CrossRef]
- Ribéreau-Gayon, P.; Dubourdieu, D.; Donèche, B.; Lonvaud, A. Handbook of Enology, Volume 1: The Microbiology of Wine and Vinifications; John Wiley & Sons: Hoboken, NJ, USA, 2006; ISBN 9780470010358. [Google Scholar]
- Tralhão, G.M.d.S. Propriedades Antioxidantes e Compostos Bioactivos em Vinhos Portugueses Monocasta. Ph.D. Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2015. [Google Scholar]
- Dengo, B.L.; Ferreira, J.R.N. Avaliação in vitro do potencial fotoprotetor do extrato do bagaço da uva Isabel (Vitis labrusca L.). Evidencia 2017, 17, 45–56. [Google Scholar] [CrossRef]
- Ribeiro, L.F. Avaliação Dos Compostos Bioativos e Atividade Antioxidante In Vitro e in Vivo Em Bagaços de Uvas (Vitis Vinífera e Vitis labrusca). Ph.D. Thesis, Departamento de Engenharia Química, Setor de Tecnologia, Universidade Federal do Paraná, Curitiba, Brazil, 2016. [Google Scholar]
- Ferrari, V. A Sustentabilidade da Vitivinicultura Através de Seus Próprios Resíduos. Bachelor’s Thesis, Universidade de Caxias do Sul, Campus Universitário da Região dos Vinhedos, Bento Gonçalves, Brazil, 2010; p. 27. [Google Scholar]
- Santos, P.V.S.; Leite, Â.A.M. Identificação de produtos secundários da vinificação: Um estudo de caso. Rev. Gestão Sustentabilidade Ambient. 2020, 9, 650–666. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Rosselló, C.; Simal, S.; Garau, M.C.; López, F.; Femenia, A. Physico-Chemical Properties of Cell Wall Materials Obtained from Ten Grape Varieties and Their Byproducts: Grape Pomaces and Stems. LWT Food Sci. Technol. 2010, 43, 1580–1586. [Google Scholar] [CrossRef]
- Rajković, M.; Popović-Minić, D.; Milincić, D.D.; Zdravković, M. Circular Economy in Food Industry. Zaštita Mater. 2020, 61, 229–250. [Google Scholar] [CrossRef]
- Gouvinhas, I.; Barros, A. Winery By-Products as Source of Bioactive Compounds for Pharmaceutical and Cosmetic Industries; IntechOpen: London, UK, 2021; ISBN 9781838806835. [Google Scholar]
- Peixoto, C.M.; Dias, M.I.; Alves, M.J.; Calhelha, R.C.; Barros, L.; Pinho, S.P.; Ferreira, I.C.F.R. Grape Pomace as a Source of Phenolic Compounds and Diverse Bioactive Properties. Food Chem. 2018, 253, 132–138. [Google Scholar] [CrossRef]
- Debien, I.C.d.N. Process Study of Extraction and Phase Equilibrium Data at High Pressures: Obtaining of Beta-Ecdysone from Brazilian Ginseng (Pfaffia glomerata) and Phase Equilibrium Data of Systems Containing L-Lactic Acid, CO2, Propane and Ethanol. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2014. [Google Scholar]
- Júnior, F.D.B.; Marquezi, L.C.; Sakai, O.A.; Terhaag, M.M. Efeitos de diferentes técnicas extrativas na obtenção da β-ecdisona proveniente de Pfaffia glomerata: Um estudo de revisão. Res. Soc. Dev. 2021, 10, e24610414147. [Google Scholar] [CrossRef]
- Rockenbach, I.I.; da Silva, G.L.; Rodrigues, E.; Gonzaga, L.V.; Fett, R. Revista Atividade antioxidante de extratos de bagaço de uva das variedades Regente e Pinot Noir (Vitis vinifera). Rev. Do Inst. Adolfo Lutz 2007, 66, 158–163. [Google Scholar]
- Okunowo, W.O.; Oyedeji, O.; Afolabi, L.O.; Matanmi, E. Essential Oil of Grape Fruit (Citrus Paradisi) Peels and Its Antimicrobial Activities. Am. J. Plant Sci. 2013, 2013, 34556. [Google Scholar] [CrossRef]
- Oliveira, V.P.F. Valorização de Subprodutos da Vinha e do Vinho-Composição Fenólica e Atividade Antioxidante. Master’s Thesis, Universidade Lusófona de Humanidades e Tecnologias, Lisbon, Portugal, 2016. [Google Scholar]
- Rombaut, N.; Savoire, R.; Thomasset, B.; Castello, J.; Van Hecke, E.; Lanoisellé, J.-L. Optimization of Oil Yield and Oil Total Phenolic Content during Grape Seed Cold Screw Pressing. Ind. Crops Prod. 2015, 63, 26–33. [Google Scholar] [CrossRef]
- Rodrigues, B.; Rodrigues, R.; Soares, R. Extração Sólido-Líquido de Compostos Fenólicos do Pericarpo da Noz; Politecnico de Coimbra Escola Superior Agraria: Coimbra, Portugal, 2018; p. 14. [Google Scholar]
- Veggi, P.C. Obtenção de Extratos Vegetais Por Diferentes Métodos de Extração: Estudo Experimental e Simulação Dos Processos. Ph.D. Thesis, Universidade Estadual de Campinas, Campinas, Brazil, 2009. [Google Scholar]
- Conde, E.; Moure, A.; Domínguez, H.; Parajó, J.C. 18—Extraction of Natural Antioxidants from Plant Foods. In Separation, Extraction and Concentration Processes in the Food, Beverage and Nutraceutical Industries; Rizvi, S.S.H., Ed.; Woodhead Publishing Series in Food Science, Technology and Nutrition; Woodhead Publishing: Sawston, UK, 2013; pp. 506–594. ISBN 9781845696450. [Google Scholar]
- Saponea, V.; Ciccib, A.; Franceschic, D.; Vincenzic, S.; Bravia, M. Antioxidant Extraction and Bioactivity Preservation from Winery By-Products by Natural Deep Eutectic Solvents (Nades). Chem. Eng. Trans. 2020, 79, 157–162. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Araújo, P.; Duarte, M.F.; de Freitas, V.; Pintado, M.; Saraiva, J.A. High-Pressure Assisted Extraction of Bioactive Compounds from Industrial Fermented Fig by-Product. J. Food Sci. Technol. 2017, 54, 2519–2531. [Google Scholar] [CrossRef] [PubMed]
- Alexandre, E.M.C.; Moreira, S.A.; Castro, L.M.G.; Pintado, M.; Saraiva, J.A. Emerging Technologies to Extract High Added Value Compounds from Fruit Residues: Sub/Supercritical, Ultrasound-, and Enzyme-Assisted Extractions. Food Rev. Int. 2018, 34, 581–612. [Google Scholar] [CrossRef]
- Sánchez-Gómez, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Vine-Shoot Waste Aqueous Extracts for Re-Use in Agriculture Obtained by Different Extraction Techniques: Phenolic, Volatile, and Mineral Compounds. J. Agric. Food Chem. 2014, 62, 10861–10872. [Google Scholar] [CrossRef] [PubMed]
- Dabetić, N.; Todorović, V.; Panić, M.; Radojčić Redovniković, I.; Šobajić, S. Impact of Deep Eutectic Solvents on Extraction of Polyphenols from Grape Seeds and Skin. Appl. Sci. 2020, 10, 4830. [Google Scholar] [CrossRef]
- Panić, M.; Gunjević, V.; Cravotto, G.; Radojčić Redovniković, I. Enabling Technologies for the Extraction of Grape-Pomace Anthocyanins Using Natural Deep Eutectic Solvents in up-to-Half-Litre Batches Extraction of Grape-Pomace Anthocyanins Using NADES. Food Chem. 2019, 300, 125185. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.N.; Teixeira, J.A.; Vicente, A.A.; Cappato, L.P.; Ferreira, M.V.S.; Rocha, R.S.; da Cruz, A.G. Ohmic Heating for the Dairy Industry: A Potential Technology to Develop Probiotic Dairy Foods in Association with Modifications of Whey Protein Structure. Curr. Opin. Food Sci. 2018, 22, 95–101. [Google Scholar] [CrossRef]
- Da Rocha, C.B.; Noreña, C.P.Z. Microwave-Assisted Extraction and Ultrasound-Assisted Extraction of Bioactive Compounds from Grape Pomace. Int. J. Food Eng. 2020, 16. [Google Scholar] [CrossRef]
- Nicolai, M.; Pereira, P.; Rijo, P.; Amaral, O.; Amaral, A.; Palma, L. Vitis vinera L. Pomace: Chemical and Nutritional Characterization (Caracterização Química e Nutricional Do Bagaço de Vitis vinera L.). J. Biomed. Biopharm. Res. 2018, 15, 156–166. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Comas-Serra, F.; Femenia, A.; Rosselló, C.; Simal, S. Effect of Power Ultrasound Application on Aqueous Extraction of Phenolic Compounds and Antioxidant Capacity from Grape Pomace (Vitis vinifera L.): Experimental Kinetics and Modeling. Ultrason. Sonochemistry 2015, 22, 506–514. [Google Scholar] [CrossRef]
- Pezzini, V.; Agostini, F.; Smiderle, F.; Touguinha, L.; Salvador, M.; Moura, S. Grape Juice By-Products Extracted by Ultrasound and Microwave-Assisted with Different Solvents: A Rich Chemical Composition. Food Sci. Biotechnol. 2018, 28, 691–699. [Google Scholar] [CrossRef] [PubMed]
- Jain, T.; Jain, V.; Pandey, R.; Vyas, A.; Shukla, S.S. Microwave Assisted Extraction for Phytoconstituents—An Overview. Asian J. Res. Chem. (AJRC) 2009, 2, 19–25. [Google Scholar]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green Extraction of Natural Products: Concept and Principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Cravotto, G.; Boffa, L.; Mantegna, S.; Perego, P.; Avogadro, M.; Cintas, P. Improved Extraction of Vegetable Oils under High-Intensity Ultrasound and/or Microwaves. Ultrason. Sonochemistry 2008, 15, 898–902. [Google Scholar] [CrossRef] [PubMed]
- Balasubramaniam, V.M.; Martínez-Monteagudo, S.I.; Gupta, R. Principles and Application of High Pressure–Based Technologies in the Food Industry. Annu. Rev. Food Sci. Technol. 2015, 6, 435–462. [Google Scholar] [CrossRef]
- Castro, L.M.G.; Alexandre, E.M.C.; Saraiva, J.A.; Pintado, M. Impact of High Pressure on Starch Properties: A Review. Food Hydrocoll. 2020, 106, 105877. [Google Scholar] [CrossRef]
- Huang, H.-W.; Hsu, C.-P.; Yang, B.B.; Wang, C.-Y. Advances in the Extraction of Natural Ingredients by High Pressure Extraction Technology. Trends Food Sci. Technol. 2013, 33, 54–62. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Silva, S.; Santos, S.A.O.; Silvestre, A.J.D.; Duarte, M.F.; Saraiva, J.A.; Pintado, M. Antimicrobial Activity of Pomegranate Peel Extracts Performed by High Pressure and Enzymatic Assisted Extraction. Food Res. Int. 2019, 115, 167–176. [Google Scholar] [CrossRef]
- Prasad, K.N.; Yang, E.; Yi, C.; Zhao, M.; Jiang, Y. Effects of High Pressure Extraction on the Extraction Yield, Total Phenolic Content and Antioxidant Activity of Longan Fruit Pericarp. Innov. Food Sci. Emerg. Technol. 2009, 10, 155–159. [Google Scholar] [CrossRef]
- Xi, J.; Shen, D.; Zhao, S.; Lu, B.; Li, Y.; Zhang, R. Characterization of Polyphenols from Green Tea Leaves Using a High Hydrostatic Pressure Extraction. Int. J. Pharm. 2009, 382, 139–143. [Google Scholar] [CrossRef]
- Putnik, P.; Kovačević, D.B.; Ježek, D.; Šustić, I.; Zorić, Z.; Dragović-Uzelac, V. High-Pressure Recovery of Anthocyanins from Grape Skin Pomace (Vitis vinifera Cv. Teran) at Moderate Temperature. J. Food Process. Preserv. 2018, 42, e13342. [Google Scholar] [CrossRef]
- Khan, M.K.; Ahmad, K.; Hassan, S.; Imran, M.; Ahmad, N.; Xu, C. Effect of Novel Technologies on Polyphenols during Food Processing. Innov. Food Sci. Emerg. Technol. 2018, 45, 361–381. [Google Scholar] [CrossRef]
- Ramírez-Jiménez, A.K.; Rangel-Hernández, J.; Morales-Sánchez, E.; Loarca-Piña, G.; Gaytán-Martínez, M. Changes on the Phytochemicals Profile of Instant Corn Flours Obtained by Traditional Nixtamalization and Ohmic Heating Process. Food Chem. 2019, 276, 57–62. [Google Scholar] [CrossRef]
- El Darra, N.; Grimi, N.; Vorobiev, E.; Louka, N.; Maroun, R. Extraction of Polyphenols from Red Grape Pomace Assisted by Pulsed Ohmic Heating. Food Bioprocess Technol. 2013, 6, 1281–1289. [Google Scholar] [CrossRef]
- Koubaa, M.; Roselló-Soto, E.; Šic Žlabur, J.; Režek Jambrak, A.; Brnčić, M.; Grimi, N.; Boussetta, N.; Barba, F.J. Current and New Insights in the Sustainable and Green Recovery of Nutritionally Valuable Compounds from Stevia Rebaudiana Bertoni. J. Agric. Food Chem. 2015, 63, 6835–6846. [Google Scholar] [CrossRef]
- Barbosa-Canovas, G.V.; Pierson, M.D.; Zhang, Q.H.; Schaffner, D.W. Pulsed Electric Fields. J. Food Sci. 2000, 65, 65–79. [Google Scholar] [CrossRef]
- Gil, K.A.; Tuberoso, C.I.G. Crucial Challenges in the Development of Green Extraction Technologies to Obtain Antioxidant Bioactive Compounds from Agro-Industrial By–Products. Chem. Biochem. Eng. Q. 2021, 35, 105–138. [Google Scholar] [CrossRef]
- Vicas, S.I.; Bandici, L.; Teusdea, A.C.; Bandici, G.E. Extraction of Bioactive Compounds from Two Grape Varieties Using Pulsed Electric Field. Bull. Univ. Agric. Sci. Vet. Med. Cluj-Napoca Food Sci. Technol. 2016, 73, 85–92. [Google Scholar] [CrossRef]
- Ghafoor, K.; AL-Juhaimi, F.Y.; Choi, Y.H. Supercritical Fluid Extraction of Phenolic Compounds and Antioxidants from Grape (Vitis labrusca B.) Seeds. Plant Foods Hum. Nutr. 2012, 67, 407–414. [Google Scholar] [CrossRef]
- Barbosa, H.M.A.; de Melo, M.M.R.; Coimbra, M.A.; Passos, C.P.; Silva, C.M. Optimization of the Supercritical Fluid Coextraction of Oil and Diterpenes from Spent Coffee Grounds Using Experimental Design and Response Surface Methodology. J. Supercrit. Fluids 2014, 85, 165–172. [Google Scholar] [CrossRef]
- Berk, Z. Food Process Engineering and Technology; Academic Press: Cambridge, MA, USA, 2018; ISBN 978-0-12-812054-5. [Google Scholar]
- Borges, M.E.; Tejera, R.L.; Díaz, L.; Esparza, P.; Ibáñez, E. Natural Dyes Extraction from Cochineal (Dactylopius coccus). New Extraction Methods. Food Chem. 2012, 132, 1855–1860. [Google Scholar] [CrossRef]
- Plaza, M.; Turner, C. Pressurized Hot Water Extraction of Bioactives. TrAC Trends Anal. Chem. 2015, 71, 39–54. [Google Scholar] [CrossRef]
- Otero-Pareja, M.J.; Casas, L.; Fernández-Ponce, M.T.; Mantell, C.; de la Ossa, E.J.M. Green Extraction of Antioxidants from Different Varieties of Red Grape Pomace. Molecules 2015, 20, 9686–9702. [Google Scholar] [CrossRef]
- Pedras, B.; Salema-Oom, M.; Sá-Nogueira, I.; Simões, P.; Paiva, A.; Barreiros, S. Valorization of White Wine Grape Pomace through Application of Subcritical Water: Analysis of Extraction, Hydrolysis, and Biological Activity of the Extracts Obtained. J. Supercrit. Fluids 2017, 128, 138–144. [Google Scholar] [CrossRef]
- Wang, L.; Huang, J.; Li, Z.; Liu, D.; Fan, J. A Review of the Polyphenols Extraction from Apple Pomace: Novel Technologies and Techniques of Cell Disintegration. Crit. Rev. Food Sci. Nutr. 2022, 1–14. [Google Scholar] [CrossRef]
- Roselló-Soto, E.; Parniakov, O.; Deng, Q.; Patras, A.; Koubaa, M.; Grimi, N.; Boussetta, N.; Tiwari, B.K.; Vorobiev, E.; Lebovka, N.; et al. Application of Non-Conventional Extraction Methods: Toward a Sustainable and Green Production of Valuable Compounds from Mushrooms. Food Eng. Rev. 2016, 8, 214–234. [Google Scholar] [CrossRef]
- Patist, A.; Bates, D. Ultrasonic Innovations in the Food Industry: From the Laboratory to Commercial Production. Innov. Food Sci. Emerg. Technol. 2008, 9, 147–154. [Google Scholar] [CrossRef]
- González-Centeno, M.R.; Knoerzer, K.; Sabarez, H.; Simal, S.; Rosselló, C.; Femenia, A. Effect of Acoustic Frequency and Power Density on the Aqueous Ultrasonic-Assisted Extraction of Grape Pomace (Vitis vinifera L.)—A Response Surface Approach. Ultrason. Sonochemistry 2014, 21, 2176–2184. [Google Scholar] [CrossRef]
- Mouratoglou, E.; Malliou, V.; Makris, D.P. Novel Glycerol-Based Natural Eutectic Mixtures and Their Efficiency in the Ultrasound-Assisted Extraction of Antioxidant Polyphenols from Agri-Food Waste Biomass. Waste Biomass Valorization 2016, 7, 1377–1387. [Google Scholar] [CrossRef]
- Patsea, M.; Stefou, I.; Grigorakis, S.; Makris, D.P. Screening of Natural Sodium Acetate-Based Low-Transition Temperature Mixtures (LTTMs) for Enhanced Extraction of Antioxidants and Pigments from Red Vinification Solid Wastes. Environ. Process. 2017, 4, 123–135. [Google Scholar] [CrossRef]
- Frum, A.; Dobrea, C.M.; Rus, L.L.; Virchea, L.-I.; Morgovan, C.; Chis, A.A.; Arseniu, A.M.; Butuca, A.; Gligor, F.G.; Vicas, L.G.; et al. Valorization of Grape Pomace and Berries as a New and Sustainable Dietary Supplement: Development, Characterization, and Antioxidant Activity Testing. Nutrients 2022, 14, 3065. [Google Scholar] [CrossRef] [PubMed]
- Drevelegka, I.; Goula, A.M. Recovery of Grape Pomace Phenolic Compounds through Optimized Extraction and Adsorption Processes. Chem. Eng. Process. Process Intensif. 2020, 149, 107845. [Google Scholar] [CrossRef]
- Loarce, L.; Oliver-Simancas, R.; Marchante, L.; Díaz-Maroto, M.C.; Alañón, M.E. Implementation of Subcritical Water Extraction with Natural Deep Eutectic Solvents for Sustainable Extraction of Phenolic Compounds from Winemaking By-Products. Food Res. Int. 2020, 137, 109728. [Google Scholar] [CrossRef]
- Radošević, K.; Ćurko, N.; Gaurina Srček, V.; Cvjetko Bubalo, M.; Tomašević, M.; Kovačević Ganić, K.; Radojčić Redovniković, I. Natural Deep Eutectic Solvents as Beneficial Extractants for Enhancement of Plant Extracts Bioactivity. LWT 2016, 73, 45–51. [Google Scholar] [CrossRef]
- Pagano, I.; Campone, L.; Celano, R.; Piccinelli, A.L.; Rastrelli, L. Green Non-Conventional Techniques for the Extraction of Polyphenols from Agricultural Food by-Products: A Review. J. Chromatogr. A 2021, 1651, 462295. [Google Scholar] [CrossRef]
- Alexandre, E.M.C.; Pinto, C.A.; Moreira, S.A.; Pintado, M.; Saraiva, J.A. 5—Nonthermal Food Processing/Preservation Technologies. In Saving Food; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2019; pp. 141–169. ISBN 978-0-12-815357-4. [Google Scholar]
- Vieira, P.; Ribeiro, C.; Pinto, C.A.; Saraiva, J.A.; Barba, F.J. Chapter 15—Application of HPP in Food Fermentation Processes. In Present and Future of High Pressure Processing; Barba, F.J., Tonello-Samson, C., Puértolas, E., Lavilla, M., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 329–351. ISBN 978-0-12-816405-1. [Google Scholar]
- Leal, C.; Gouvinhas, I.; Santos, R.A.; Rosa, E.; Silva, A.M.; Saavedra, M.J.; Barros, A.I. Potential Application of Grape (Vitis vinifera L.) Stem Extracts in the Cosmetic and Pharmaceutical Industries: Valorization of a by-Product. Ind. Crops Prod. 2020, 154, 112675. [Google Scholar] [CrossRef]
- Mota, M.F. Caracterização de óleo de Grainha de Uva de Distintas Castas Cultivadas sob as Mesmas Condições Edafo-Climáticas. Ph.D. Thesis, Universidade de Lisboa, Lisbon, Portugal, 2018; p. 78. [Google Scholar]
- Syed, U.T.; Brazinha, C.; Crespo, J.G.; Ricardo-da-Silva, J.M. Valorisation of Grape Pomace: Fractionation of Bioactive Flavan-3-Ols by Membrane Processing. Sep. Purif. Technol. 2017, 172, 404–414. [Google Scholar] [CrossRef]
- Figueira, D.R. Valorization of Agro-Industrial Waste through Chemical and Microbiological Approaches. Ph.D. Thesis, Universidade Nova de Lisboa, Lisbon, Portugal, 2017. [Google Scholar]
- Silva, V.; Igrejas, G.; Falco, V.; Santos, T.P.; Torres, C.; Oliveira, A.M.P.; Pereira, J.E.; Amaral, J.S.; Poeta, P. Chemical Composition, Antioxidant and Antimicrobial Activity of Phenolic Compounds Extracted from Wine Industry by-Products. Food Control. 2018, 92, 516–522. [Google Scholar] [CrossRef]
- Luchian, C.E.; Cotea, V.V.; Vlase, L.; Toiu, A.M.; Colibaba, L.C.; Răschip, I.E.; Nadăş, G.; Gheldiu, A.M.; Tuchiluş, C.; Rotaru, L. Antioxidant and Antimicrobial Effects of Grape Pomace Extracts. BIO Web Conf. 2019, 15, 04006. [Google Scholar] [CrossRef]
- Casagrande, M.; Zanela, J.; Pereira, D.; de Lima, V.A.; Oldoni, T.L.C.; Carpes, S.T. Optimization of the Extraction of Antioxidant Phenolic Compounds from Grape Pomace Using Response Surface Methodology. J. Food Meas. Charact. 2019, 13, 1120–1129. [Google Scholar] [CrossRef]
- Kalli, E.; Lappa, I.; Bouchagier, P.; Tarantilis, P.A.; Skotti, E. Novel Application and Industrial Exploitation of Winery By-Products. Bioresour. Bioprocess. 2018, 5, 46. [Google Scholar] [CrossRef]
- Maia, M.; Ferreira, A.E.N.; Laureano, G.; Patrícia Marques, A.; Torres, V.M.; Bernardes Silva, A.; Rita Matos, A.; Cordeiro, C.; Figueiredo, A.; Silva, M.S. Vitis vinifera ‘Pinot Noir’ Leaves as a Source of Bioactive Nutraceutical Compounds. Food Funct. 2019, 10, 3822–3827. [Google Scholar] [CrossRef]
- Milinčić, D.D.; Stanisavljević, N.S.; Kostić, A.Ž.; Soković Bajić, S.; Kojić, M.O.; Gašić, U.M.; Barać, M.B.; Stanojević, S.P.; Lj Tešić, Ž.; Pešić, M.B. Phenolic Compounds and Biopotential of Grape Pomace Extracts from Prokupac Red Grape Variety. LWT 2021, 138, 110739. [Google Scholar] [CrossRef]
- Paraiso, I.L.; Revel, J.S.; Stevens, J.F. Potential Use of Polyphenols in the Battle against COVID-19. Curr. Opin. Food Sci. 2020, 32, 149–155. [Google Scholar] [CrossRef]
- Sridhar, A.; Ponnuchamy, M.; Kumar, P.S.; Kapoor, A.; Vo, D.-V.N.; Prabhakar, S. Techniques and Modeling of Polyphenol Extraction from Food: A Review. Environ. Chem. Lett. 2021, 19, 3409–3443. [Google Scholar] [CrossRef] [PubMed]
- Piccolella, S.; Crescente, G.; Candela, L.; Pacifico, S. Nutraceutical Polyphenols: New Analytical Challenges and Opportunities. J. Pharm. Biomed. Anal. 2019, 175, 112774. [Google Scholar] [CrossRef]
- Theodorou, M.K.; Kingston-Smith, A.H.; Winters, A.L.; Lee, M.R.F.; Minchin, F.R.; Morris, P.; MacRae, J. Polyphenols and Their Influence on Gut Function and Health in Ruminants: A Review. Environ. Chem. Lett. 2006, 4, 121–126. [Google Scholar] [CrossRef]
- Nunes, M.A.; Rodrigues, F.; Oliveira, M.B.P.P. 11—Grape Processing By-Products as Active Ingredients for Cosmetic Proposes. In Handbook of Grape Processing By-Products; Galanakis, C.M., Ed.; Academic Press: Cambridge, MA, USA, 2017; pp. 267–292. ISBN 978-0-12-809870-7. [Google Scholar]
- da Rocha, L.C.A. Phenolic Compounds of White Wine from the Douro Region: The Relationship between Chemistry and Bioactivity in the Context of Alzheimer’s Disease and Type 2 Diabetes. Master’s Thesis, Universidade do Porto, Porto, Portugal, 2018. [Google Scholar]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine Polyphenol Content and Its Influence on Wine Quality and Properties: A Review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Versari, A.; Ricci, A.; Brioni, A.; Galaz Torres, C.; Pavez Moreno, C.A.; Concha García, J.; Parpinello, G.P. Evaluation of Plant-Based Byproducts as Green Fining Agents for Precision Winemaking. Molecules 2022, 27, 1671. [Google Scholar] [CrossRef] [PubMed]
- Mahmoudi, S.; Ghorbani, M.; Sabzichi, M.; Ramezani, F.; Hamishehkar, H.; Samadi, N. Targeted Hyaluronic Acid-Based Lipid Nanoparticle for Apigenin Delivery to Induce Nrf2-Dependent Apoptosis in Lung Cancer Cells. J. Drug Deliv. Sci. Technol. 2019, 49, 268–276. [Google Scholar] [CrossRef]
- Tavsan, Z.; Kayali, H.A. Flavonoids Showed Anticancer Effects on the Ovarian Cancer Cells: Involvement of Reactive Oxygen Species, Apoptosis, Cell Cycle and Invasion. Biomed. Pharmacother. 2019, 116, 109004. [Google Scholar] [CrossRef]
- Zhu, Z.-Y.; Wang, F.; Jia, C.-H.; Xie, M.-L. Apigenin-Induced HIF-1α Inhibitory Effect Improves Abnormal Glucolipid Metabolism in AngⅡ/Hypoxia-Stimulated or HIF-1α-Overexpressed H9c2 Cells. Phytomedicine 2019, 62, 152713. [Google Scholar] [CrossRef] [PubMed]
- Rauf, A.; Imran, M.; Khan, I.A.; ur-Rehman, M.-; Gilani, S.A.; Mehmood, Z.; Mubarak, M.S. Anticancer Potential of Quercetin: A Comprehensive Review. Phytother. Res. 2018, 32, 2109–2130. [Google Scholar] [CrossRef]
- Hamza, A.A.; Heeba, G.H.; Elwy, H.M.; Murali, C.; El-Awady, R.; Amin, A. Molecular Characterization of the Grape Seeds Extract’s Effect against Chemically Induced Liver Cancer: In Vivo and in Vitro Analyses. Sci. Rep. 2018, 8, 1270. [Google Scholar] [CrossRef]
- Dinicola, S.; Cucina, A.; Antonacci, D.; Bizzarri, M. Anticancer Effects of Grape Seed Extract on Human Cancers: A Review. J. Carcinog. Mutagen. 2014, 8. [Google Scholar] [CrossRef]
- Tsiviki, M.; Goula, A.M. Chapter 16—Valorization of Grape Seeds. In Valorization of Agri-Food Wastes and By-Products; Bhat, R., Ed.; Academic Press: Cambridge, MA, USA, 2021; pp. 331–346. ISBN 9780128240441. [Google Scholar]
- Apostolou, A.; Stagos, D.; Galitsiou, E.; Spyrou, A.; Haroutounian, S.; Portesis, N.; Trizoglou, I.; Wallace Hayes, A.; Tsatsakis, A.M.; Kouretas, D. Assessment of Polyphenolic Content, Antioxidant Activity, Protection against ROS-Induced DNA Damage and Anticancer Activity of Vitis vinifera Stem Extracts. Food Chem. Toxicol. 2013, 61, 60–68. [Google Scholar] [CrossRef] [PubMed]
- Bogdan, C.; Pop, A.; Iurian, S.M.; Benedec, D.; Moldovan, M.L. Research Advances in the Use of Bioactive Compounds from Vitis vinifera By-Products in Oral Care. Antioxidants 2020, 9, 502. [Google Scholar] [CrossRef] [PubMed]
- Kouidhi, B.; Al Qurashi, Y.M.A.; Chaieb, K. Drug Resistance of Bacterial Dental Biofilm and the Potential Use of Natural Compounds as Alternative for Prevention and Treatment. Microb. Pathog. 2015, 80, 39–49. [Google Scholar] [CrossRef]
- Oliveira, D.A.; Salvador, A.A.; Smânia, A.; Smânia, E.F.A.; Maraschin, M.; Ferreira, S.R.S. Antimicrobial Activity and Composition Profile of Grape (Vitis vinifera) Pomace Extracts Obtained by Supercritical Fluids. J. Biotechnol. 2013, 164, 423–432. [Google Scholar] [CrossRef]
- Muñoz-González, I.; Thurnheer, T.; Bartolomé, B.; Moreno-Arribas, M.V. Red Wine and Oenological Extracts Display Antimicrobial Effects in an Oral Bacteria Biofilm Model. J. Agric. Food Chem. 2014, 62, 4731–4737. [Google Scholar] [CrossRef]
- Chakka, A.K.; Babu, A.S. Bioactive Compounds of Winery By-Products: Extraction Techniques and Their Potential Health Benefits. Appl. Food Res. 2022, 2, 100058. [Google Scholar] [CrossRef]
- Palma, L.; Nunes, C.; Gameiro, R.; Rodrigues, M.; Gothe, S.; Tavares, N.; Pego, C.; Nicolai, M.; Pereira, P. Preliminary Sensory Evaluation of Salty Crackers with Grape Pomace Flour (Avaliação Sensorial Preliminar de Bolachas Salgadas de Farinha de Bagaço de Uva). Biomed. Biopharm. Res. 2020, 17, 33–43. [Google Scholar] [CrossRef]
- Cebrián-Tarancón, C.; Sánchez-Gómez, R.; Cabrita, M.J.; García, R.; Zalacain, A.; Alonso, G.L.; Salinas, M.R. Winemaking with Vine-Shoots. Modulating the Composition of Wines by Using Their Own Resources. Food Res. Int. 2019, 121, 117–126. [Google Scholar] [CrossRef]
- Rajha, H.N.; El Kantar, S.; Afif, C.; Boussetta, N.; Louka, N.; Maroun, R.G.; Vorobiev, E. Selective Multistage Extraction Process of Biomolecules from Vine Shoots by a Combination of Biological, Chemical, and Physical Treatments. Comptes Rendus Chim. 2018, 21, 581–589. [Google Scholar] [CrossRef]
- Mattos, G.N.; Tonon, R.V.; Furtado, A.A.; Cabral, L.M. Grape By-Product Extracts against Microbial Proliferation and Lipid Oxidation: A Review. J. Sci. Food Agric. 2017, 97, 1055–1064. [Google Scholar] [CrossRef] [PubMed]
- Escobar-Avello, D.; Lozano-Castellón, J.; Mardones, C.; Pérez, A.J.; Saéz, V.; Riquelme, S.; von Baer, D.; Vallverdú-Queralt, A. Phenolic Profile of Grape Canes: Novel Compounds Identified by LC-ESI-LTQ-Orbitrap-MS. Molecules 2019, 24, 3763. [Google Scholar] [CrossRef]
- Louli, V.; Ragoussis, N.; Magoulas, K. Recovery of Phenolic Antioxidants from Wine Industry By-Products. Bioresour. Technol. 2004, 92, 201–208. [Google Scholar] [CrossRef]
- Strapasson, G.C. Caracterização E Utilização Do Resíduo De Produção De Vinho No Desenvolvimento De Alimentos Com Propriedade Funcional. Ph.D. Thesis, Universidade Federal do Paraná, Curitiba, Brazil, 2016; p. 148. [Google Scholar]
- Ortega-Heras, M.; Gómez, I.; de Pablos-Alcalde, S.; González-Sanjosé, M.L. Application of the Just-About-Right Scales in the Development of New Healthy Whole-Wheat Muffins by the Addition of a Product Obtained from White and Red Grape Pomace. Foods 2019, 8, 419. [Google Scholar] [CrossRef]
- Theagarajan, R.; Malur Narayanaswamy, L.; Dutta, S.; Moses, J.A.; Chinnaswamy, A. Valorisation of Grape Pomace (Cv. Muscat) for Development of Functional Cookies. Int. J. Food Sci. Technol. 2019, 54, 1299–1305. [Google Scholar] [CrossRef]
- Mironeasa, S.; Iuga, M.; Zaharia, D.; Dabija, A.; Mironeasa, C. Influence of Particle Sizes and Addition Level of Grape Seeds on Wheat Flour Dough Rheological Properties. In Proceedings of the International Multidisciplinary Scientific GeoConference: SGEM; Surveying Geology & Mining Ecology Management (SGEM), Sofia, Bulgaria, 1–10 July 2017; Volume 17, pp. 265–272. [Google Scholar]
- Mironeasa, S.; Mironeasa, C. Dough Bread from Refined Wheat Flour Partially Replaced by Grape Peels: Optimizing the Rheological Properties. J. Food Process. Eng. 2019, 42, e13207. [Google Scholar] [CrossRef]
- Šporin, M.; Avbelj, M.; Kovač, B.; Možina, S.S. Quality Characteristics of Wheat Flour Dough and Bread Containing Grape Pomace Flour. Food Sci. Technol. Int. 2018, 24, 251–263. [Google Scholar] [CrossRef] [PubMed]
- Vilela, A.; Pinto, T. Grape Infusions: The Flavor of Grapes and Health-Promoting Compounds in Your Tea Cup. Beverages 2019, 5, 48. [Google Scholar] [CrossRef]
- Tournour, H.H.; Cunha, L.M.; Magalhães, L.M.; Lima, R.C.; Segundo, M.A. Evaluation of the Joint Effect of the Incorporation of Mechanically Deboned Meat and Grape Extract on the Formulation of Chicken Nuggets. Food Sci. Technol. Int. 2017, 23, 328–337. [Google Scholar] [CrossRef]
- Libera, J.; Kononiuk, A.; Kęska, P.; Wójciak, K. Use of Grape Seed Extract as a Natural Antioxidant Additive in Dry-Cured Pork Neck Technology. Biotechnol. Food Sci. 2018, 82, 141–150. [Google Scholar]
- Gasiński, A.; Kawa-Rygielska, J.; Mikulski, D.; Kłosowski, G.; Głowacki, A. Application of White Grape Pomace in the Brewing Technology and Its Impact on the Concentration of Esters and Alcohols, Physicochemical Parameteres and Antioxidative Properties of the Beer. Food Chem. 2022, 367, 130646. [Google Scholar] [CrossRef] [PubMed]
- Cilli, L.P.; Contini, L.R.F.; Sinnecker, P.; Lopes, P.S.; Andreo, M.A.; Neiva, C.R.P.; Nascimento, M.S.; Yoshida, C.M.P.; Venturini, A.C. Effects of Grape Pomace Flour on Quality Parameters of Salmon Burger. J. Food Process. Preserv. 2020, 44, e14329. [Google Scholar] [CrossRef]
- Marcos, J.; Carriço, R.; Sousa, M.J.; Palma, M.L.; Pereira, P.; Nunes, M.C.; Nicolai, M. Effect of Grape Pomace Flour in Savory Crackers: Technological, Nutritional and Sensory Properties. Foods 2023, 12, 1392. [Google Scholar] [CrossRef] [PubMed]
- Gaita, C.; Alexa, E.; Moigradean, D.; Conforti, F.; Poiana, M.A. Designing of High Value-Added Pasta Formulas by Incorporation of Grape Pomace Skins. Rom. Biotechnol. Lett. 2018, 25, 1607–1614. [Google Scholar] [CrossRef]
- Kuchtova, V.; Kohajdova, Z.; Karovicova, J.; Laukova, M. Physical, Textural and Sensory Properties of Cookies Incorporated with Grape Skin and Seed Preparations. Pol. J. Food Nutr. Sci. 2018, 68, 309–317. [Google Scholar] [CrossRef]
- Brykova, T.; Samohvalova, O.; Grevtseva, N.; Kasabova, K.; Grygorenko, A. The Influence of Grape Powders on the Rheological Properties of Dough and Characteristics of the Quality of Butter Biscuits. Food Sci. Technol. 2018, 12, 33–38. [Google Scholar] [CrossRef]
- Kim, T.-K.; Yong, H.-I.; Jung, S.; Kim, Y.-B.; Choi, Y.-S. Effects of Replacing Pork Fat with Grape Seed Oil and Gelatine/Alginate for Meat Emulsions. Meat Sci. 2020, 163, 108079. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.-X.; Ni, Z.-J.; Thakur, K.; Wang, S.; Zhang, J.-G.; Shang, Y.-F.; Wei, Z.-J. Effect of Grape Seed Power on the Structural and Physicochemical Properties of Wheat Gluten in Noodle Preparation System. Food Chem. 2021, 355, 129500. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Chen, L.; Wongmaneepratip, W.; He, Y.; Zhao, L.; Yang, H. Effect of Vacuum Impregnated Fish Gelatin and Grape Seed Extract on Moisture State, Microbiota Composition, and Quality of Chilled Seabass Fillets. Food Chem. 2021, 354, 129581. [Google Scholar] [CrossRef]
- Guo, Y.; Huang, J.; Chen, Y.; Hou, Q.; Huang, M. Effect of Grape Seed Extract Combined with Modified Atmosphere Packaging on the Quality of Roast Chicken. Poult. Sci. 2020, 99, 1598–1605. [Google Scholar] [CrossRef]
- Reham, A.A.; Shimaa, N.E. Grape Seed Extract as Natural Antioxidant and Antibacterial in Minced Beef. PSM Biol. Res. 2017, 2, 89–96. [Google Scholar]
- Sani, M.A.; Azizi-Lalabadi, M.; Tavassoli, M.; Mohammadi, K.; McClements, D.J. Recent Advances in the Development of Smart and Active Biodegradable Packaging Materials. Nanomaterials 2021, 11, 1331. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, Y.S. Anthocyanin—A Natural Dye for Smart Food Packaging Systems. Korean J. Packag. Sci. Technol. 2018, 24, 167–180. [Google Scholar] [CrossRef]
- Singh, S.; Gaikwad, K.K.; Lee, M.; Lee, Y.S. Microwave-Assisted Micro-Encapsulation of Phase Change Material Using Zein for Smart Food Packaging Applications. J. Therm. Anal. Calorim. 2018, 131, 2187–2195. [Google Scholar] [CrossRef]
- Yong, H.; Liu, J. Recent Advances in the Preparation, Physical and Functional Properties, and Applications of Anthocyanins-Based Active and Intelligent Packaging Films. Food Packag. Shelf Life 2020, 26, 100550. [Google Scholar] [CrossRef]
- Ferreira, A.S.; Nunes, C.; Castro, A.; Ferreira, P.; Coimbra, M.A. Influence of Grape Pomace Extract Incorporation on Chitosan Films Properties. Carbohydr. Polym. 2014, 113, 490–499. [Google Scholar] [CrossRef]
- Shahbazi, Y. The Properties of Chitosan and Gelatin Films Incorporated with Ethanolic Red Grape Seed Extract and Ziziphora Clinopodioides Essential Oil as Biodegradable Materials for Active Food Packaging. Int. J. Biol. Macromol. 2017, 99, 746–753. [Google Scholar] [CrossRef] [PubMed]
- Dilucia, F.; Lacivita, V.; Conte, A.; Del Nobile, M.A. Sustainable Use of Fruit and Vegetable By-Products to Enhance Food Packaging Performance. Foods 2020, 9, 857. [Google Scholar] [CrossRef] [PubMed]
- Kurek, M.; Hlupić, L.; Elez Garofulić, I.; Descours, E.; Ščetar, M.; Galić, K. Comparison of Protective Supports and Antioxidative Capacity of Two Bio-Based Films with Revalorised Fruit Pomaces Extracted from Blueberry and Red Grape Skin. Food Packag. Shelf Life 2019, 20, 100315. [Google Scholar] [CrossRef]
- Cejudo-Bastante, C.; Arjona-Mudarra, P.; Fernández-Ponce, M.T.; Casas, L.; Mantell, C.; Martínez de la Ossa, E.J.; Pereyra, C. Application of a Natural Antioxidant from Grape Pomace Extract in the Development of Bioactive Jute Fibers for Food Packaging. Antioxidants 2021, 10, 216. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).