Will Artificial Intelligence Provide Answers to Current Gaps and Needs in Chronic Heart Failure?
Abstract
:1. Introduction
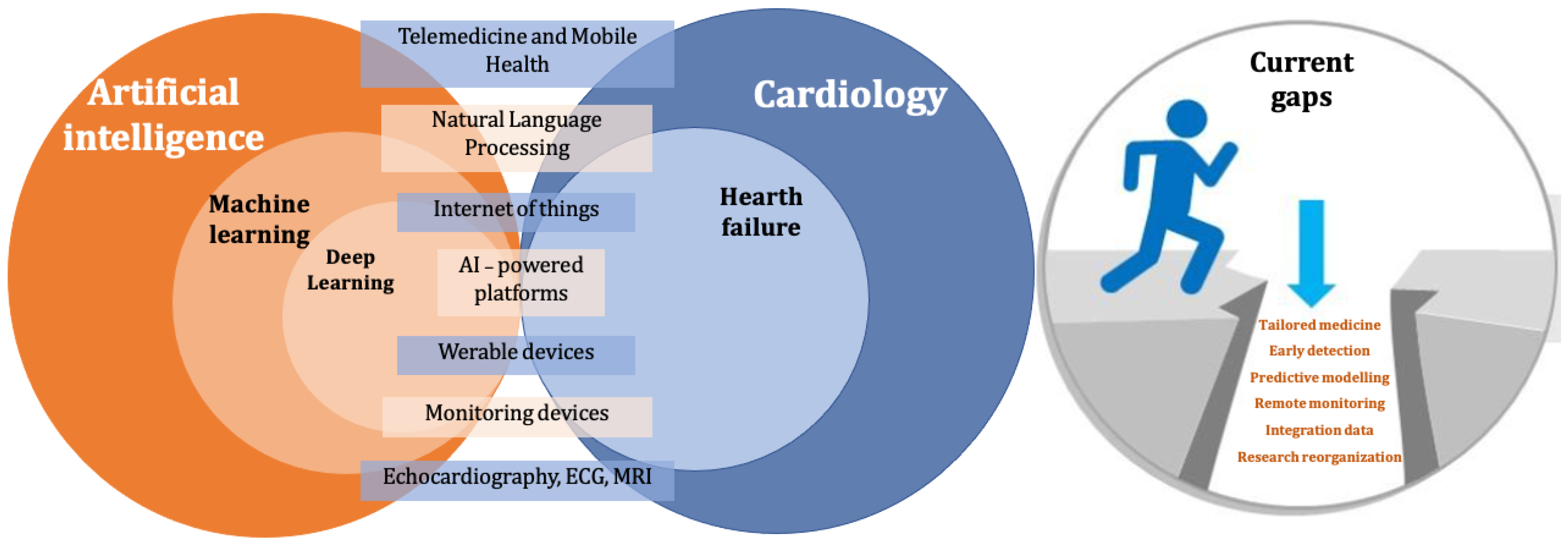
2. Current Gaps and Needs in Heart Failure Management
- Tailored medicine: despite advances in our understanding of the underlying causes of HF, there is still a lack of a truly personalised approach to treatment, taking into account individual factors such as genetics, lifestyle, and disease history [18]. It is essential to define the most appropriate therapeutic strategies depending on patients’ comorbidities, the specific etiology of CHF, the patient’s lifestyle, and specific disease subgroups (elderly individuals, women, patients with congenital heart disease) [19].
- Early detection: CHF is a chronic, progressive, and irreversible disease. In this context, improving our process for the early detection of HF is of key relevance to improve patient outcomes, especially at early stages [20].
- Remote monitoring: the development of effective and scalable remote monitoring solutions is paramount to improving HF management and reducing hospitalisation rates, with a relevant impact on the control of management costs for healthcare systems and to protect patients’ autonomy [21].
- Predictive modelling: to support decision-making and improve patient management, it is advisable to improve the prediction of HF progression and to define the underlying etiologies [22].
- Integration of data: methods for integrating and analyzing large amounts of data from various sources, including electronic health records, imaging, lab results, and wearable devices, to support the diagnosis and management of heart failure are paramount in the current context, where a growing number of clinical data are recorded and stored but often left unused [23].
- Research reorganisation: AI tools could help policy-makers and public payers to improve the prioritisation of research, to better focus on under-investigated and/or most promising topics. As an example, although it is recognised that the microbiome plays an essential role in the pathogenesis of HF, the exact mechanism of action in the development and progression of heart failure is still unknown. Similarly, there is a need to increase research resources on regenerative approaches to HF, including cell-based therapies, gene editing, and tissue engineering, to support the development of new treatments [24].
3. Available AI Resources and Tools
4. Clinical Applications of AI to CHF Management
4.1. Telemedicine and Mobile Health
4.2. Monitoring Devices and AI-Powered Platforms
4.3. Internet of Things (IoT)
4.4. Natural Language Processing
4.5. Application of AI to Echocardiography, ECG, and Cardiac MRI
5. Smart Clinics
6. Current Research Focus
7. Limitation
8. Future Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| MDPI | Multidisciplinary Digital Publishing Institute |
| CHF | Chronic Heart Failure |
| CVD | Cardiovascular Diseases |
| NYHA | New York Heart Association |
| HF | Hearth Failure |
| AI | Artificial Intelligence |
| FD | Fractal Dimension |
| ML | Machine Learning |
| DL | Deep Learning |
| GPUs | Graphical Processing Units |
| mHealth | Mobile Health |
| PDAs | Personal Digital Assistants |
| PAP | Pulmonary Artery Pressure |
| ReDS | Remote Dielectic Sensing |
| IoT | Internet of Things |
| NLP | Natural Language Processing |
| SM | Self Management |
| EF | Ejection Fraction |
| GLS | Global Longitudinal Strain |
| CHART | Cardio-HART |
| PCG | Phonocardiography |
| MRI | Magnetic Resonance Imaging |
| MCG | Mechanical Force Bio-signal |
| HFpEF | Hearth Failure with Preserved Ejection Fraction |
| HFmrEF | Hearth Failure with Midly Reduced Ejection Fraction |
| HFrEF | Hearth Failure with Reduced Ejection Fraction |
| ECG | Electrocardiogram |
| DNNs | Deep Neural Networks |
| ICMs | Insertable cardiac monitors |
| ICD | Implantable Cardiac Defibrillator |
| AIHFMS | AI-Powered Heart Failure Management System |
| MultiSENSE | Multisensor Chronic Evaluation in Ambulatory Heart Failure Patients |
| HFRS | Heart Failure Risk Status |
References
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: Developed by the Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC) With the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [PubMed]
- Bytyçi, I.; Bajraktari, G. Mortality in heart failure patients. Anatol. J. Cardiol. 2015, 15, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Briasoulis, A.; Androulakis, E.; Christophides, T.; Tousoulis, D. The role of inflammation and cell death in the pathogenesis, progression and treatment of heart failure. Heart Fail. Rev. 2016, 21, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Ambrosy, A.P.; Fonarow, G.C.; Butler, J.; Chioncel, O.; Greene, S.J.; Vaduganathan, M.; Nodari, S.; Lam, C.S.; Sato, N.; Shah, A.N.; et al. The global health and economic burden of hospitalizations for heart failure: Lessons learned from hospitalized heart failure registries. J. Am. Coll. Cardiol. 2014, 63, 1123–1133. [Google Scholar] [CrossRef]
- Stewart, S.; MacIntyre, K.; Capewell, S.; McMurray, J.J. Heart failure and the aging population: An increasing burden in the 21st century? Heart 2003, 89, 49–53. [Google Scholar] [CrossRef]
- Tanaka, H. Utility of strain imaging in conjunction with heart failure stage classification for heart failure patient management. J. Echocardiogr. 2019, 17, 17–24. [Google Scholar] [CrossRef]
- Baker, D.W. Prevention of heart failure. J. Card. Fail. 2002, 8, 333–346. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, P.; Gaggin, H.K.; Dec, G.W. ACC/AHA versus ESC guidelines on heart failure: JACC guideline comparison. J. Am. Coll. Cardiol. 2019, 73, 2756–2768. [Google Scholar] [CrossRef]
- Osenenko, K.M.; Kuti, E.; Deighton, A.M.; Pimple, P.; Szabo, S.M. Burden of hospitalization for heart failure in the United States: A systematic literature review. J. Manag. Care Spec. Pharm. 2022, 28, 157–167. [Google Scholar] [CrossRef]
- Savarese, G.; Lund, L.H. Global public health burden of heart failure. Card. Fail. Rev. 2017, 3, 7. [Google Scholar] [CrossRef]
- Johnson, K.W.; Torres Soto, J.; Glicksberg, B.S.; Shameer, K.; Miotto, R.; Ali, M.; Ashley, E.; Dudley, J.T. Artificial intelligence in cardiology. J. Am. Coll. Cardiol. 2018, 71, 2668–2679. [Google Scholar] [CrossRef] [PubMed]
- Bachtiger, P.; Plymen, C.M.; Pabari, P.A.; Howard, J.P.; Whinnett, Z.I.; Opoku, F.; Janering, S.; Faisal, A.A.; Francis, D.P.; Peters, N.S. Artificial intelligence, data sensors and interconnectivity: Future opportunities for heart failure. Card. Fail. Rev. 2020, 6, e11. [Google Scholar] [CrossRef]
- Bruno, P.; Zaffino, P.; Scaramuzzino, S.; De Rosa, S.; Indolfi, C.; Calimeri, F.; Spadea, M.F. Using cnns for designing and implementing an automatic vascular segmentation method of biomedical images. In Proceedings of the AI* IA 2018–Advances in Artificial Intelligence: XVIIth International Conference of the Italian Association for Artificial Intelligence, Trento, Italy, 20–23 November 2018; Springer: Berlin/Heidelberg, Germany, 2018; pp. 60–70. [Google Scholar]
- Bruno, P.; Spadea, M.F.; Scaramuzzino, S.; De Rosa, S.; Indolfi, C.; Gargiulo, G.; Giugliano, G.; Esposito, G.; Calimeri, F.; Zaffino, P. Assessing vascular complexity of PAOD patients by deep learning-based segmentation and fractal dimension. Neural Comput. Appl. 2022, 34, 22015–22022. [Google Scholar] [CrossRef]
- Bruno, P.; Zaffino, P.; Scaramuzzino, S.; De Rosa, S.; Indolfi, C.; Calimeri, F.; Spadea, M.F. Segmentation of vessel tree from cine-angiography images for intraoperative clinical evaluation. In Proceedings of the CEUR WORKSHOP PROCEEDINGS. CEUR-WS, Trento, Italy, 22 November 2019; Volume 2272. [Google Scholar]
- Yasmin, F.; Shah, S.M.I.; Naeem, A.; Shujauddin, S.M.; Jabeen, A.; Kazmi, S.; Siddiqui, S.A.; Kumar, P.; Salman, S.; Hassan, S.A.; et al. Artificial intelligence in the diagnosis and detection of heart failure: The past, present, and future. Rev. Cardiovasc. Med. 2021, 22, 1095–1113. [Google Scholar] [CrossRef] [PubMed]
- Topol, E.J. High-performance medicine: The convergence of human and artificial intelligence. Nat. Med. 2019, 25, 44–56. [Google Scholar] [CrossRef] [PubMed]
- Ruberg, S.J.; Shen, L. Personalized medicine: Four perspectives of tailored medicine. Stat. Biopharm. Res. 2015, 7, 214–229. [Google Scholar] [CrossRef]
- Colvin, M.; Sweitzer, N.K.; Albert, N.M.; Krishnamani, R.; Rich, M.W.; Stough, W.G.; Walsh, M.N.; Canary, C.A.W.; Allen, L.A.; Bonnell, M.R.; et al. Heart failure in non-Caucasians, women, and older adults: A white paper on special populations from the Heart Failure Society of America Guideline Committee. J. Card. Fail. 2015, 21, 674–693. [Google Scholar] [CrossRef]
- De Couto, G.; Ouzounian, M.; Liu, P.P. Early detection of myocardial dysfunction and heart failure. Nat. Rev. Cardiol. 2010, 7, 334–344. [Google Scholar] [CrossRef]
- Klersy, C.; De Silvestri, A.; Gabutti, G.; Regoli, F.; Auricchio, A. A meta-analysis of remote monitoring of heart failure patients. J. Am. Coll. Cardiol. 2009, 54, 1683–1694. [Google Scholar] [CrossRef] [Green Version]
- Błaziak, M.; Urban, S.; Wietrzyk, W.; Jura, M.; Iwanek, G.; Stańczykiewicz, B.; Kuliczkowski, W.; Zymliński, R.; Pondel, M.; Berka, P.; et al. An Artificial Intelligence Approach to Guiding the Management of Heart Failure Patients Using Predictive Models: A Systematic Review. Biomedicines 2022, 10, 2188. [Google Scholar] [CrossRef]
- Byrd, T.F.; Ahmad, F.S.; Liebovitz, D.M.; Kho, A.N. Defragmenting heart failure care: Medical records integration. Heart Fail. Clin. 2020, 16, 467–477. [Google Scholar] [CrossRef] [PubMed]
- Lopes, L.R.; Elliott, P.M. Genetics of heart failure. Biochim. Et Biophys. Acta (BBA)-Mol. Basis Dis. 2013, 1832, 2451–2461. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cannataro, M.; Guzzi, P.H.; Agapito, G.; Zucco, C.; Milano, M. Artificial Intelligence in Bioinformatics: From Omics Analysis to Deep Learning and Network Mining; Elsevier: Amsterdam, The Netherlands, 2022. [Google Scholar]
- Baldi, P. Deep learning in biomedical data science. Annu. Rev. Biomed. Data Sci. 2018, 1, 181–205. [Google Scholar] [CrossRef]
- Litjens, G.; Kooi, T.; Bejnordi, B.E.; Setio, A.A.A.; Ciompi, F.; Ghafoorian, M.; Van Der Laak, J.A.; Van Ginneken, B.; Sánchez, C.I. A survey on deep learning in medical image analysis. Med. Image Anal. 2017, 42, 60–88. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Averbuch, T.; Sullivan, K.; Sauer, A.; Mamas, M.A.; Voors, A.A.; Gale, C.P.; Metra, M.; Ravindra, N.; Van Spall, H.G. Applications of artificial intelligence and machine learning in heart failure. Eur. Heart J.-Digit. Health 2022, 3, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Corotto, P.S.; McCarey, M.M.; Adams, S.; Khazanie, P.; Whellan, D.J. Heart failure patient adherence: Epidemiology, cause, and treatment. Heart Fail. Clin. 2013, 9, 49–58. [Google Scholar] [CrossRef]
- D’Amario, D.; Canonico, F.; Rodolico, D.; Borovac, J.A.; Vergallo, R.; Montone, R.A.; Galli, M.; Migliaro, S.; Restivo, A.; Massetti, M.; et al. Telemedicine, artificial intelligence and humanisation of clinical pathways in heart failure management: Back to the future and beyond. Card. Fail. Rev. 2020, 6, e16. [Google Scholar] [CrossRef]
- Gjeka, R.; Patel, K.; Reddy, C.; Zetsche, N. Patient engagement with digital disease management and readmission rates: The case of congestive heart failure. Health Inform. J. 2021, 27, 14604582211030959. [Google Scholar] [CrossRef]
- Martínez-Pérez, B.; De La Torre-Díez, I.; López-Coronado, M. Mobile health applications for the most prevalent conditions by the World Health Organization: Review and analysis. J. Med. Internet Res. 2013, 15, e120. [Google Scholar] [CrossRef]
- Kitsiou, S.; Vatani, H.; Paré, G.; Gerber, B.S.; Buchholz, S.W.; Kansal, M.M.; Leigh, J.; Creber, R.M.M. Effectiveness of mobile health technology interventions for patients with heart failure: Systematic review and meta-analysis. Can. J. Cardiol. 2021, 37, 1248–1259. [Google Scholar] [CrossRef]
- Goldenthal, I.L.; Sciacca, R.R.; Riga, T.; Bakken, S.; Baumeister, M.; Biviano, A.B.; Dizon, J.M.; Wang, D.; Wang, K.C.; Whang, W.; et al. Recurrent atrial fibrillation/flutter detection after ablation or cardioversion using the AliveCor KardiaMobile device: IHEART results. J. Cardiovasc. Electrophysiol. 2019, 30, 2220–2228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yenikomshian, M.; Jarvis, J.; Patton, C.; Yee, C.; Mortimer, R.; Birnbaum, H.; Topash, M. Cardiac arrhythmia detection outcomes among patients monitored with the Zio patch system: A systematic literature review. Curr. Med Res. Opin. 2019, 35, 1659–1670. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schmier, J.K.; Ong, K.L.; Fonarow, G.C. Cost-effectiveness of remote cardiac monitoring with the CardioMEMS heart failure system. Clin. Cardiol. 2017, 40, 430–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amir, O.; Ben-Gal, T.; Weinstein, J.M.; Schliamser, J.; Burkhoff, D.; Abbo, A.; Abraham, W.T. Evaluation of remote dielectric sensing (ReDS) technology-guided therapy for decreasing heart failure re-hospitalizations. Int. J. Cardiol. 2017, 240, 279–284. [Google Scholar] [CrossRef] [Green Version]
- Stehlik, J.; Schmalfuss, C.; Bozkurt, B.; Nativi-Nicolau, J.; Wohlfahrt, P.; Wegerich, S.; Rose, K.; Ray, R.; Schofield, R.; Deswal, A.; et al. Continuous wearable monitoring analytics predict heart failure hospitalization: The LINK-HF multicenter study. Circ. Heart Fail. 2020, 13, e006513. [Google Scholar] [CrossRef]
- Gardner, R.S.; Singh, J.P.; Stancak, B.; Nair, D.G.; Cao, M.; Schulze, C.; Thakur, P.H.; An, Q.; Wehrenberg, S.; Hammill, E.F.; et al. HeartLogic multisensor algorithm identifies patients during periods of significantly increased risk of heart failure events: Results from the MultiSENSE study. Circ. Heart Fail. 2018, 11, e004669. [Google Scholar] [CrossRef]
- Hernandez, A.F.; Albert, N.M.; Allen, L.A.; Ahmed, R.; Averina, V.; Boehmer, J.P.; Cowie, M.R.; Chien, C.V.; Galvao, M.; Klein, L.; et al. Multiple cArdiac seNsors for mAnaGEment of Heart Failure (MANAGE-HF)–Phase I Evaluation of the Integration and Safety of the HeartLogic Multisensor Algorithm in Patients With Heart Failure. J. Card. Fail. 2022, 28, 1245–1254. [Google Scholar] [CrossRef]
- Santini, L.; D’Onofrio, A.; Dello Russo, A.; Calò, L.; Pecora, D.; Favale, S.; Petracci, B.; Molon, G.; Bianchi, V.; De Ruvo, E.; et al. Prospective evaluation of the multisensor HeartLogic algorithm for heart failure monitoring. Clin. Cardiol. 2020, 43, 691–697. [Google Scholar] [CrossRef]
- Capucci, A.; Santini, L.; Favale, S.; Pecora, D.; Petracci, B.; Calò, L.; Molon, G.; Cipolletta, L.; Bianchi, V.; Schirripa, V.; et al. Preliminary experience with the multisensor HeartLogic algorithm for heart failure monitoring: A retrospective case series report. ESC Heart Fail. 2019, 6, 308–318. [Google Scholar] [CrossRef] [Green Version]
- Treskes, R.W.; Beles, M.; Caputo, M.L.; Cordon, A.; Biundo, E.; Maes, E.; Egorova, A.D.; Schalij, M.J.; Van Bockstal, K.; Grazioli-Gauthier, L.; et al. Clinical and economic impact of HeartLogic™ compared with standard care in heart failure patients. ESC Heart Fail. 2021, 8, 1541–1551. [Google Scholar] [CrossRef]
- De Juan Bagudá, J.; Gómez, J.J.G.; Iglesias, M.P.; León, R.C.; Pérez, V.E.; Fernández, Ó.G.; Gándara, N.R.; Artaza, J.G.; Molina, B.D.; Gallego, A.M.; et al. Remote heart failure management using the HeartLogic algorithm. RE-HEART Registry. Rev. Espa Nola De Cardiol. 2022, 75, 709–716. [Google Scholar] [CrossRef]
- D’Onofrio, A.; Solimene, F.; Calò, L.; Calvi, V.; Viscusi, M.; Melissano, D.; Russo, V.; Rapacciuolo, A.; Campana, A.; Caravati, F.; et al. Combining home monitoring temporal trends from implanted defibrillators and baseline patient risk profile to predict heart failure hospitalizations: Results from the SELENE HF study. EP Eur. 2022, 24, 234–244. [Google Scholar] [CrossRef] [PubMed]
- Cowie, M.R.; Sarkar, S.; Koehler, J.; Whellan, D.J.; Crossley, G.H.; Tang, W.H.W.; Abraham, W.T.; Sharma, V.; Santini, M. Development and validation of an integrated diagnostic algorithm derived from parameters monitored in implantable devices for identifying patients at risk for heart failure hospitalization in an ambulatory setting. Eur. Heart J. 2013, 34, 2472–2480. [Google Scholar] [CrossRef] [PubMed]
- Virani, S.A.; Sharma, V.; McCann, M.; Koehler, J.; Tsang, B.; Zieroth, S. Prospective evaluation of integrated device diagnostics for heart failure management: Results of the TRIAGE-HF study. ESC Heart Fail. 2018, 5, 809–817. [Google Scholar] [CrossRef] [PubMed]
- Brugada, J.; Delnoy, P.P.; Brachmann, J.; Reynolds, D.; Padeletti, L.; Noelker, G.; Kantipudi, C.; Rubin Lopez, J.M.; Dichtl, W.; Borri-Brunetto, A.; et al. Contractility sensor-guided optimization of cardiac resynchronization therapy: Results from the RESPOND-CRT trial. Eur. Heart J. 2017, 38, 730–738. [Google Scholar] [CrossRef] [Green Version]
- Whellan, D.J.; O’Connor, C.M.; Ousdigian, K.T.; Lung, T.H.; PARTNERS HF Study Investigators. Rationale, design, and baseline characteristics of a Program to Assess and Review Trending INformation and Evaluate CorRelation to Symptoms in Patients with Heart Failure (PARTNERS HF). Am. Heart J. 2008, 156, 833–839. [Google Scholar] [CrossRef]
- Stolen, C.; Rosman, J.; Manyam, H.; Kwan, B.; Kelly, J.; Perschbacher, D.; Garner, J.; Richards, M. Preliminary results from the LUX-Dx insertable cardiac monitor remote programming and performance (LUX-Dx PERFORM) study. Clin. Cardiol. 2023, 46, 100–107. [Google Scholar] [CrossRef]
- Maurya, M.R.; Riyaz, N.U.S.; Reddy, M.S.B.; Yalcin, H.C.; Ouakad, H.M.; Bahadur, I.; Al-Maadeed, S.; Sadasivuni, K.K. A review of smart sensors coupled with Internet of Things and Artificial Intelligence approach for heart failure monitoring. Med. Biol. Eng. Comput. 2021, 59, 2185–2203. [Google Scholar] [CrossRef]
- Singhal, A.; Cowie, M.R. The role of wearables in heart failure. Curr. Heart Fail. Rep. 2020, 17, 125–132. [Google Scholar] [CrossRef]
- Mocker, M.; Ross, J. Digital Transformation at Royal Philips. In Proceedings of the Thirty ninth International Conference on Information Systems, San Francisco, CA, USA, 13–16 December 2018. [Google Scholar]
- Almalki, T.S.; Alshuhri, A.M.; Almaymoni, S.H.; Almelabi, T.K.; Alshamrani, Y.S.; Alshamrani, M.S. Current concepts and future perspectives of artificial intelligence in the pharmaceutical industry. A scoping review. Eur. J. Mol. Clin. Med. 2022, 9, 2022. [Google Scholar]
- Choi, E.; Schuetz, A.; Stewart, W.F.; Sun, J. Using recurrent neural network models for early detection of heart failure onset. J. Am. Med. Inform. Assoc. 2017, 24, 361–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barry, T.; Farina, J.M.; Chao, C.J.; Ayoub, C.; Jeong, J.; Patel, B.N.; Banerjee, I.; Arsanjani, R. The Role of Artificial Intelligence in Echocardiography. J. Imaging 2023, 9, 50. [Google Scholar] [CrossRef] [PubMed]
- Chen, H.; Zheng, Y.; Park, J.H.; Heng, P.A.; Zhou, S.K. Iterative multi-domain regularized deep learning for anatomical structure detection and segmentation from ultrasound images. In Proceedings of the Medical Image Computing and Computer-Assisted Intervention–MICCAI 2016: 19th International Conference, Athens, Greece, 17–21 October 2016; Part II 19. Springer: Berlin/Heidelberg, Germany, 2016; pp. 487–495. [Google Scholar]
- Tromp, J.; Seekings, P.J.; Hung, C.L.; Iversen, M.B.; Frost, M.J.; Ouwerkerk, W.; Jiang, Z.; Eisenhaber, F.; Goh, R.S.; Zhao, H.; et al. Automated interpretation of systolic and diastolic function on the echocardiogram: A multicohort study. Lancet Digit. Health 2022, 4, e46–e54. [Google Scholar] [CrossRef] [PubMed]
- Grün, D.; Rudolph, F.; Gumpfer, N.; Hannig, J.; Elsner, L.K.; von Jeinsen, B.; Hamm, C.W.; Rieth, A.; Guckert, M.; Keller, T. Identifying heart failure in ECG data with artificial intelligence—A meta-analysis. Front. Digit. Health 2021, 2, 584555. [Google Scholar] [CrossRef]
- Ballinger, B.; Hsieh, J.; Singh, A.; Sohoni, N.; Wang, J.; Tison, G.; Marcus, G.; Sanchez, J.; Maguire, C.; Olgin, J.; et al. DeepHeart: Semi-supervised sequence learning for cardiovascular risk prediction. In Proceedings of the AAAI conference on artificial intelligence, New Orleans, LA, USA, 2–7 February 2018; Volume 32. [Google Scholar]
- Karaoğuz, M.R.; Yurtseven, E.; Aslan, G.; Deliormanlı, B.G.; Adıgüzel, Ö.; Gönen, M.; Li, K.M.; Yılmaz, E.N. The quality of ECG data acquisition, and diagnostic performance of a novel adhesive patch for ambulatory cardiac rhythm monitoring in arrhythmia detection. J. Electrocardiol. 2019, 54, 28–35. [Google Scholar] [CrossRef]
- Attia, Z.I.; Noseworthy, P.A.; Lopez-Jimenez, F.; Asirvatham, S.J.; Deshmukh, A.J.; Gersh, B.J.; Carter, R.E.; Yao, X.; Rabinstein, A.A.; Erickson, B.J.; et al. An artificial intelligence-enabled ECG algorithm for the identification of patients with atrial fibrillation during sinus rhythm: A retrospective analysis of outcome prediction. Lancet 2019, 394, 861–867. [Google Scholar] [CrossRef]
- Fiorina, L.; Maupain, C.; Gardella, C.; Manenti, V.; Salerno, F.; Socie, P.; Li, J.; Henry, C.; Plesse, A.; Narayanan, K.; et al. Evaluation of an Ambulatory ECG Analysis Platform Using Deep Neural Networks in Routine Clinical Practice. J. Am. Heart Assoc. 2022, 11, e026196. [Google Scholar] [CrossRef]
- Harmon, D.M.; Carter, R.E.; Cohen-Shelly, M.; Svatikova, A.; Adedinsewo, D.A.; Noseworthy, P.A.; Kapa, S.; Lopez-Jimenez, F.; Friedman, P.A.; Attia, Z.I. Real-world performance, long-term efficacy, and absence of bias in the artificial intelligence enhanced electrocardiogram to detect left ventricular systolic dysfunction. Eur. Heart J.-Digit. Health 2022, 3, 238–244. [Google Scholar] [CrossRef]
- Nogueira, M.A.; Calcagno, S.; Campbell, N.; Zaman, A.; Koulaouzidis, G.; Jalil, A.; Alam, F.; Stankovic, T.; Szabo, E.; Szabo, A.B.; et al. Detecting heart failure using novel bio-signals and a knowledge enhanced neural network. Comput. Biol. Med. 2023, 154, 106547. [Google Scholar] [CrossRef]
- Calcagno, S.; Biondi-Zoccai, G.; Stankovic, T.; Szabo, E.; Szabo, A.B.; Kecskes, I. Novel tech throws knock-out punch to ECG improving GP referral decisions to cardiology. Open Heart 2022, 9, e001852. [Google Scholar] [CrossRef]
- Segar, M.W.; Hall, J.L.; Jhund, P.S.; Powell-Wiley, T.M.; Morris, A.A.; Kao, D.; Fonarow, G.C.; Hernandez, R.; Ibrahim, N.E.; Rutan, C.; et al. Machine learning–based models incorporating social determinants of health vs traditional models for predicting in-hospital mortality in patients with heart failure. JAMA Cardiol. 2022, 7, 844–854. [Google Scholar] [CrossRef] [PubMed]
- Pana, M.; Vasilescu, E.; Busnatu, S.; Andrei, C.; Popescu, N.; Sinescu, C. Prediction of congestive heart failure in patients using artificial intelligence: Proof of concept. Eur. Heart J. 2021, 42, 724–2547. [Google Scholar] [CrossRef]
- Wang, J. Heart failure prediction with machine learning: A comparative study. In Journal of Physics: Conference Series; IOP Publishing: London, UK, 2021; Volume 2031, p. 012068. [Google Scholar]
- Tchoukina, I.; Smallfield, M.C.; Shah, K.B. Device management and flow optimization on left ventricular assist device support. Crit. Care Clin. 2018, 34, 453–463. [Google Scholar] [CrossRef]
- Koehler, F.; Koehler, K.; Deckwart, O.; Prescher, S.; Wegscheider, K.; Kirwan, B.A.; Winkler, S.; Vettorazzi, E.; Bruch, L.; Oeff, M.; et al. Efficacy of telemedical interventional management in patients with heart failure (TIM-HF2): A randomised, controlled, parallel-group, unmasked trial. Lancet 2018, 392, 1047–1057. [Google Scholar] [CrossRef] [PubMed]
- Dendale, P.; De Keulenaer, G.; Troisfontaines, P.; Weytjens, C.; Mullens, W.; Elegeert, I.; Ector, B.; Houbrechts, M.; Willekens, K.; Hansen, D. Effect of a telemonitoring-facilitated collaboration between general practitioner and heart failure clinic on mortality and rehospitalization rates in severe heart failure: The TEMA-HF 1 (TElemonitoring in the MAnagement of Heart Failure) study. Eur. J. Heart Fail. 2012, 14, 333–340. [Google Scholar] [CrossRef]
- Gelman, R.; Hurvitz, N.; Nesserat, R.; Kolben, Y.; Nachman, D.; Jamil, K.; Agus, S.; Asleh, R.; Amir, O.; Berg, M.; et al. A second-generation artificial intelligence-based therapeutic regimen improves diuretic resistance in heart failure: Results of a feasibility open-labeled clinical trial. Biomed. Pharmacother. 2023, 161, 114334. [Google Scholar] [CrossRef]
- Segar, M.W.; Vaduganathan, M.; Patel, K.V.; McGuire, D.K.; Butler, J.; Fonarow, G.C.; Basit, M.; Kannan, V.; Grodin, J.L.; Everett, B.; et al. Machine learning to predict the risk of incident heart failure hospitalization among patients with diabetes: The WATCH-DM risk score. Diabetes Care 2019, 42, 2298–2306. [Google Scholar] [CrossRef]
- Luštrek, M.; Bohanec, M.; Barca, C.C.; Ciancarelli, M.C.; Clays, E.; Dawodu, A.A.; Derboven, J.; De Smedt, D.; Dovgan, E.; Lampe, J.; et al. A personal health system for self-management of congestive heart failure (HeartMan): Development, technical evaluation, and proof-of-concept randomized controlled trial. JMIR Med. Inform. 2021, 9, e24501. [Google Scholar] [CrossRef]
- Chen, J.; Gao, Y. The role of deep learning-based echocardiography in the diagnosis and evaluation of the effects of routine anti-heart-failure Western medicines in elderly patients with acute left heart failure. J. Healthc. Eng. 2021, 2021, 4845792. [Google Scholar] [CrossRef]

| AI Tools | Function | |
|---|---|---|
| Telemedicine and Mobile Health | Veta Health | Allow remote monitoring and management of heart failure patients |
| Welby | Uses AI and machine learning algorithms to remotely manage heart failure patients monitoring their blood pressure levels and achieving weight loss | |
| Monitoring devices | KardiaMobile | Continuously monitor heart rhythm and are already used for detecting atrial fibrillation |
| iRhythm Zio XT | ||
| AI-powered platforms | CardioMEMS | Measure pulmonary artery pressure |
| ReDS | Detect the extent of pulmonary congestion | |
| LINK-HF | Record ECG, 3-axis accelerometry, skin impedance, body temperature, and posture | |
| Medicomp-Quippi | Help healthcare providers personalise treatment plans and drug dosing | |
| Natural Language Processing | Linguamatics’ NLP software | Extract data from electronic health records to provide insights on heart failure patients |
| EHR analytics platform | Analyse electronic health records to support the diagnosis and management of heart failure |
| Implantable Devices | Algorithm Name | Type of Device | Function |
|---|---|---|---|
| Boston | HeartLogic | ICD | Reveals signs of elevated filling pressures and weakened ventricular contraction. Measures pulmonary accumulation. Monitors rapid shallow breathing patterns. Indicated cardiac status and arrhythmias. Show activity levels |
| Medtronic | TriageHF | ICD | Thoracic impedance, detection of arrhythmias, atrial fibrillation burden, evaluation of heart rate, heart rate variability, blood pressure |
| Biotronik | HeartInsight | ICD | Atrial fibrillation burden, evaluation of heart rate variability, blood pressure, thoracic impedance, detection of arrhythmias, |
| Type of Wearable Device | Sensors | Measurements Available | Clinical Application |
|---|---|---|---|
| Ear buds | PPG | HR; BP; SaPO2; cardiac output; stroke volume; rhythm and sleep evaluation | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection Long QT diagnosis; HF management; Hypertension screening and management |
| Smart ring | PPG | HR; BP; SaPO2; cardiac output; stroke volume; rhythm and sleep evaluation | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
| Patch | ECG | Single-lead and multi-lead ECG; continuous ECG-monitoring; QTc measurement; arrhythmia detection | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
| Chest strap | ECG | Single-lead and multi-lead ECG; continuous ECG-monitoring; QTc measurement; arrhythmia detection | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
| Clothing and shoe sensors | ECG | Single-lead and multi-lead ECG; continuous ECG-monitoring; QTc measurement; arrhythmia detection | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
| Smart watch | PPG; ECG | HR; BP; SaPO2; cardiac output; stroke volume; rhythm and sleep evaluation. Single-lead and multi-lead ECG; continuous ECG-monitoring; QTc measurement; arrhythmia detection | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
| Smart band | PPG; ECG | HR; BP; SaPO2; cardiac output; stroke volume; rhythm and sleep evaluation. Single-lead and multi-lead ECG; continuous ECG-monitoring; QTc measurement; arrhythmia detection | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
| Smart ring | PPG; ECG | HR; BP; SaPO2; cardiac output; stroke volume; rhythm and sleep evaluation. Single-lead and multi-lead ECG; continuous ECG-monitoring; QTc measurement; arrhythmia detection | Risk assessment and prediction; Cardiac telerehabilitation; Arrhythmia detection; Long QT diagnosis; HF management; Hypertension screening and management |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Boccuto, F.; De Rosa, S.; Torella, D.; Veltri, P.; Guzzi, P.H. Will Artificial Intelligence Provide Answers to Current Gaps and Needs in Chronic Heart Failure? Appl. Sci. 2023, 13, 7663. https://doi.org/10.3390/app13137663
Boccuto F, De Rosa S, Torella D, Veltri P, Guzzi PH. Will Artificial Intelligence Provide Answers to Current Gaps and Needs in Chronic Heart Failure? Applied Sciences. 2023; 13(13):7663. https://doi.org/10.3390/app13137663
Chicago/Turabian StyleBoccuto, Fabiola, Salvatore De Rosa, Daniele Torella, Pierangelo Veltri, and Pietro Hiram Guzzi. 2023. "Will Artificial Intelligence Provide Answers to Current Gaps and Needs in Chronic Heart Failure?" Applied Sciences 13, no. 13: 7663. https://doi.org/10.3390/app13137663
APA StyleBoccuto, F., De Rosa, S., Torella, D., Veltri, P., & Guzzi, P. H. (2023). Will Artificial Intelligence Provide Answers to Current Gaps and Needs in Chronic Heart Failure? Applied Sciences, 13(13), 7663. https://doi.org/10.3390/app13137663








