Glycerol Flow through a Shielded Coil Induces Aggregation and Activity Enhancement of Horseradish Peroxidase
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals and Protein
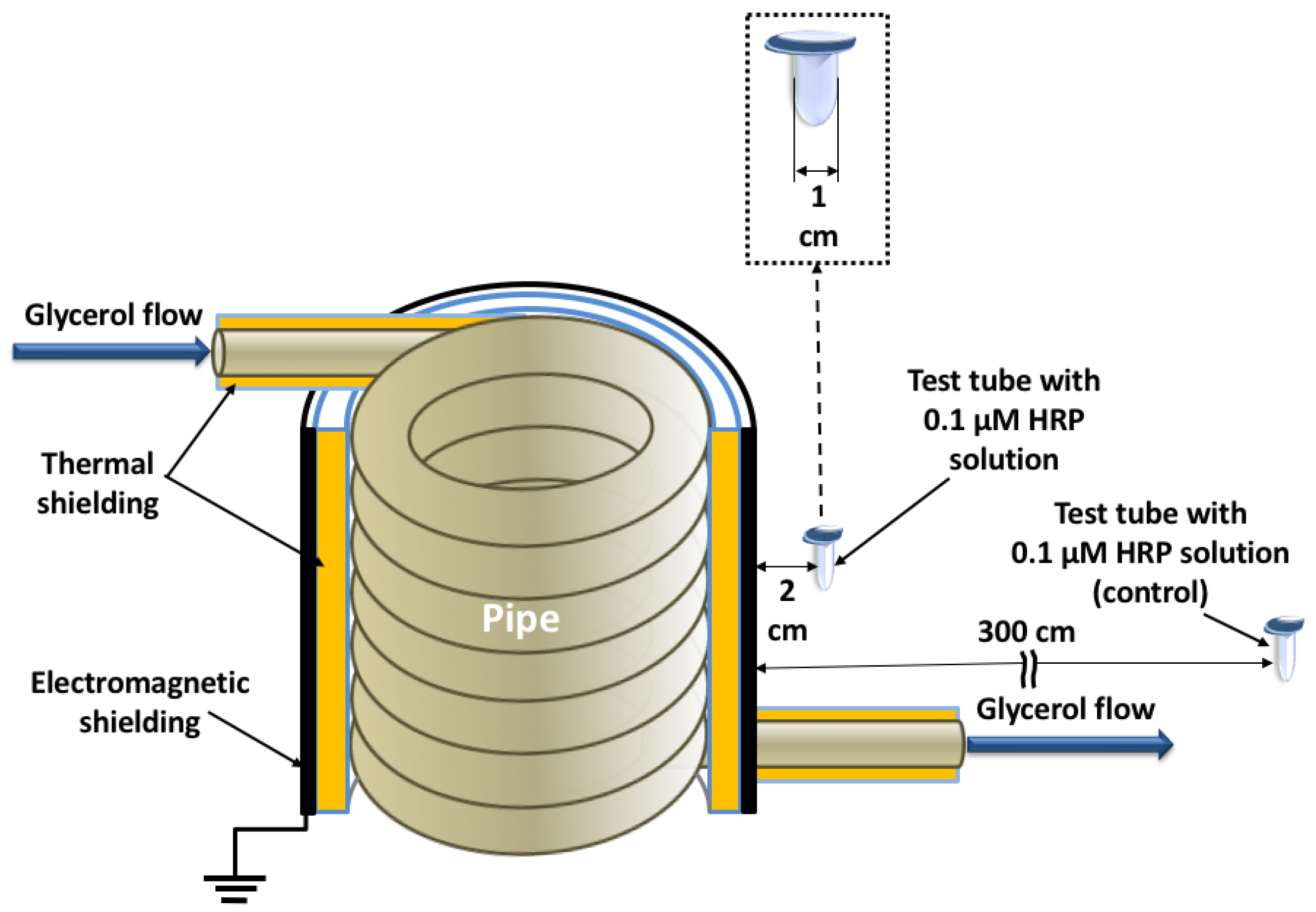
2.2. Experimental Setup
2.3. AFM Experiments—Preparation of Substrates for AFM
2.4. AFM Experiments—AFM Scanning
2.5. Spectrophotometric Determination of HRP Enzymatic Activity
3. Results
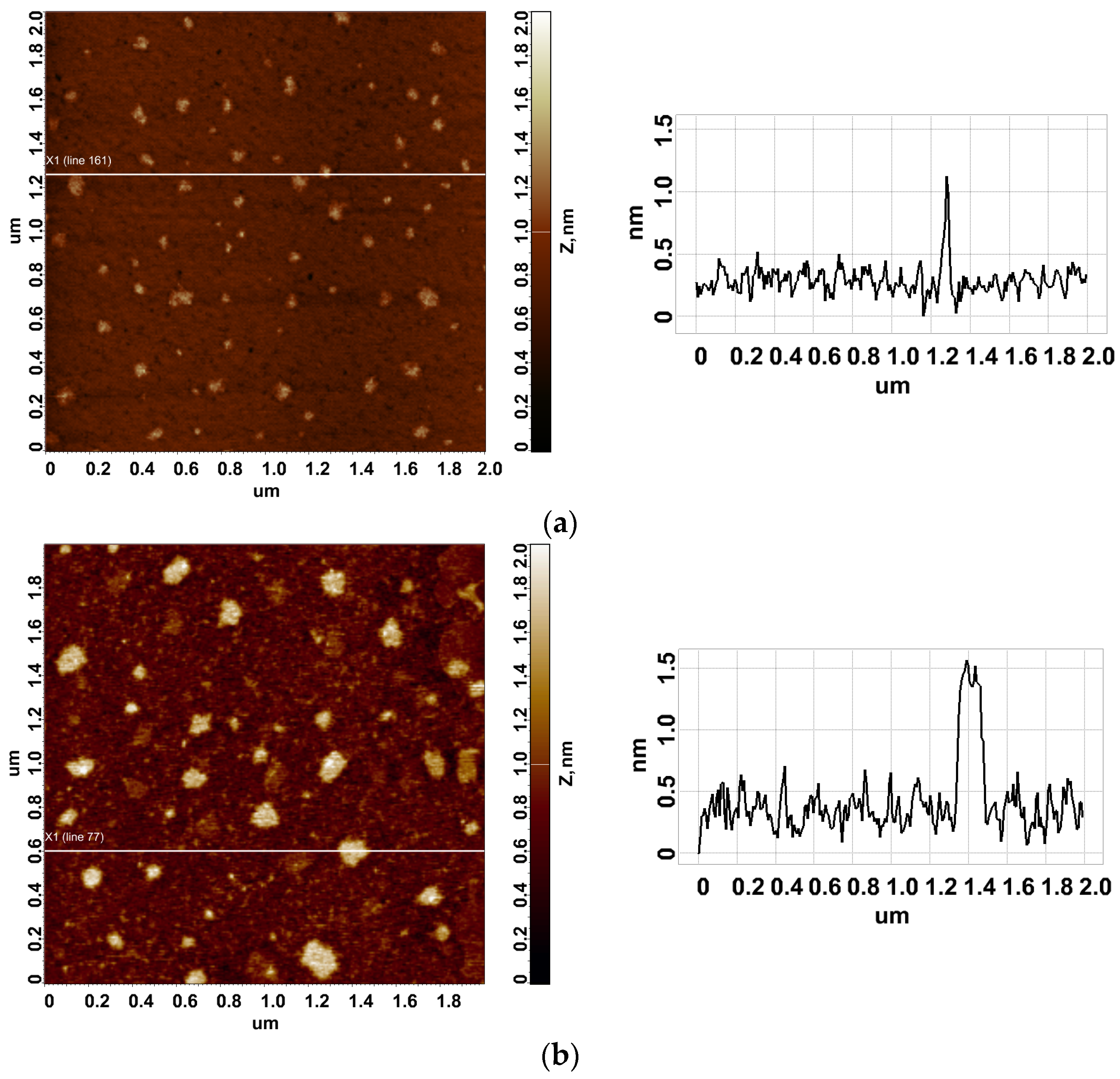
3.1. Atomic Force Microscopy
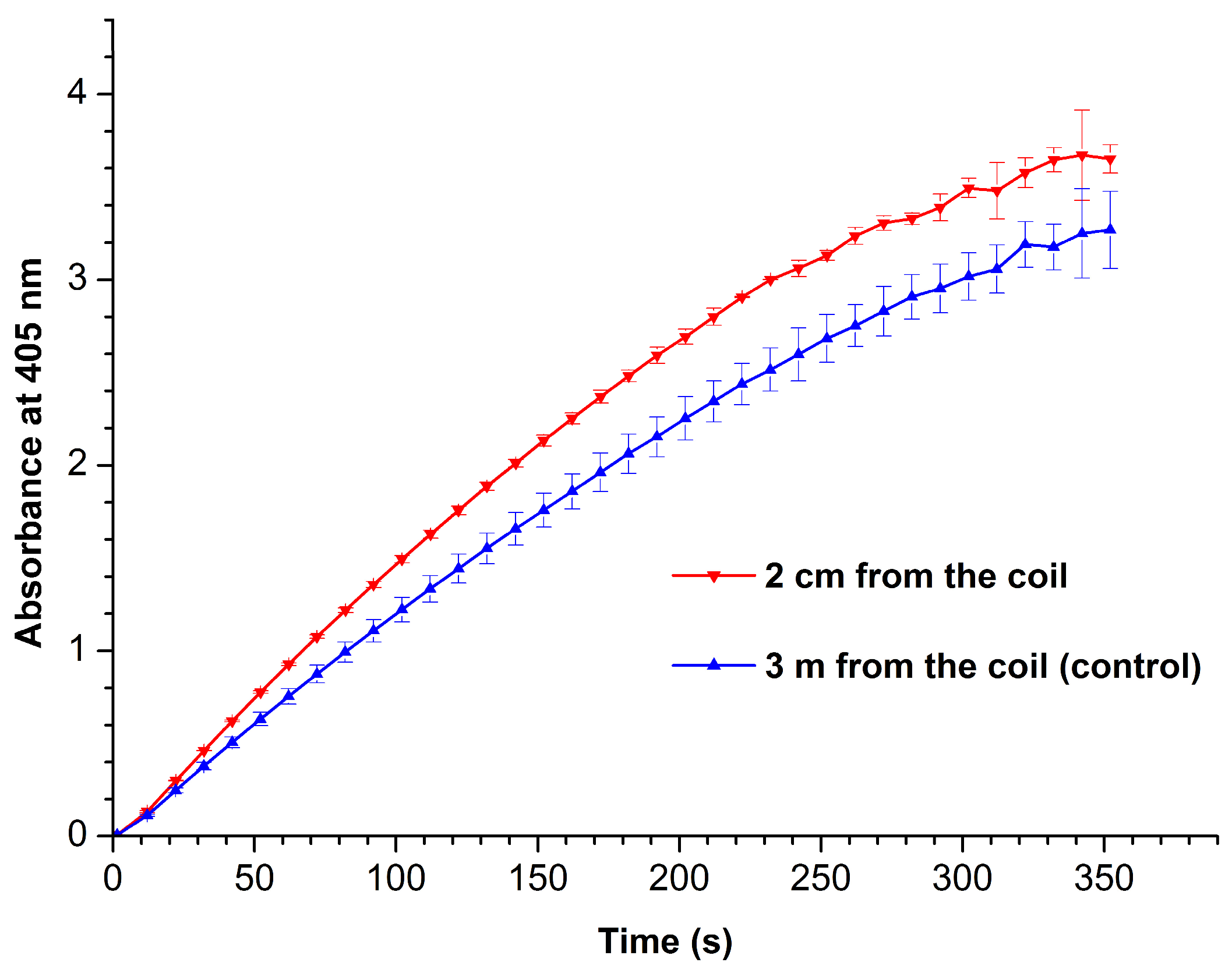
3.2. Spectrophotometric Determination of HRP Enzymatic Activity
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhang, T.; Liu, C.; Gu, Y.; Jérôme, F. Glycerol in Energy Transportation: A State-of-the-art Review. Green Chem. 2021, 23, 7865–7889. [Google Scholar] [CrossRef]
- Jacobsen, D.; McMartin, K.E. Alcohols and glycols. In Human Toxicology; Descotes, J., Ed.; Elsevier Science B.V.: Amsterdam, The Netherlands, 1996; pp. 623–648. [Google Scholar] [CrossRef]
- Vijayendran, R.A.; Ligler, F.S.; Leckband, D.E. A computational reaction-diffusion model for the analysis of transport-limited kinetics. Anal. Chem. 1998, 71, 5405–5412. [Google Scholar] [CrossRef]
- Teeparuksapun, K.; Hedström, M.; Mattiasson, B. A Sensitive Capacitive Biosensor for Protein a Detection Using Human IgG Immobilized on an Electrode Using Layer-by-Layer Applied Gold Nanoparticles. Sensors 2022, 22, 99. [Google Scholar] [CrossRef] [PubMed]
- Kamat, V.; Rafique, A. Exploring sensitivity & throughput of a parallel flow SPRi biosensor for characterization of antibody-antigen interaction. Anal. Biochem. 2017, 525, 8–22. [Google Scholar] [CrossRef]
- Yang, D.; Singh, A.; Wu, H.; Kroe-Barrett, R. Comparison of biosensor platforms in the evaluation of high affinity antibody-antigen binding kinetics. Anal. Biochem. 2016, 508, 78–96. [Google Scholar] [CrossRef] [Green Version]
- Zhao, H.; Gorshkova, I.I.; Fu, G.L.; Schuck, P. A comparison of binding surfaces for SPR biosensing using an antibody–antigen system and affinity distribution analysis. Methods 2013, 59, 328–335. [Google Scholar] [CrossRef] [Green Version]
- Dobson, J.; Kumar, A.; Willis, L.F.; Tuma, R.; Higazi, D.R.; Turner, R.; Lowe, D.C.; Ashcroft, A.E.; Radford, S.E.; Kapur, N.; et al. Inducing protein aggregation by extensional flow. Proc. Natl. Acad. Sci. USA 2017, 114, 4673–4678. [Google Scholar] [CrossRef] [Green Version]
- Ravelo, B.; Duval, F.; Kane, S.; Nsom, B. Demonstration of the triboelectricity effect by the flow of liquid water in the insulating pipe. J. Electrost. 2011, 69, 473–478. [Google Scholar] [CrossRef]
- Choi, D.; Lee, H.; Im, D.J.; Kang, I.S.; Lim, G.; Kim, D.S.; Kang, K.H. Spontaneous electrical charging of droplets by conventional pipetting. Sci. Rep. 2013, 3, 2037. [Google Scholar] [CrossRef] [Green Version]
- Cheedarala, R.K.; Song, J.I. Harvesting of flow current through implanted hydrophobic PTFE surface within silicone-pipe as liquid nanogenerator. Sci. Rep. 2022, 12, 3700. [Google Scholar] [CrossRef]
- Xu, W.; Zheng, H.; Liu, Y.; Zhou, X.; Zhang, C.; Song, Y.; Deng, X.; Leung, M.; Yang, Z.; Xu, R.X.; et al. A droplet-based electricity generator with high instantaneous power density. Nature 2020, 578, 392–396. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, L.; Yang, X.; Hong, H.; Yang, Q.; Wang, J.; Tang, Q. Cumulative charging behavior of water droplets driven freestanding triboelectric nanogenerator toward hydrodynamic energy harvesting. J. Mater. Chem. A 2020, 8, 7880–7888. [Google Scholar] [CrossRef]
- Haque, R.I.; Arafat, A.; Briand, D. Triboelectric effect to harness fluid flow energy. J. Phys. Conf. Ser. 2019, 1407, 012084. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Romanova, T.S.; Valueva, A.A.; Tatur, V.Y.; Stepanov, I.N.; Ziborov, V.S. Investigation of the Influence of Liquid Motion in a Flow-based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Water Flow. Appl. Sci. 2020, 10, 4560. [Google Scholar] [CrossRef]
- Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Negodailov, A.N.; Lukyanitsa, A.A.; Ivanov, Y.D. Investigation of the Influence of Liquid Motion in a Flow-Based System on an Enzyme Aggregation State with an Atomic Force Microscopy Sensor: The Effect of Glycerol Flow. Appl. Sci. 2020, 10, 4825. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Ershova, M.O.; Tatur, V.Y.; Ziborov, V.S. AFM Study of the Influence of Glycerol Flow on Horseradish Peroxidase near the in/out Linear Sections of a Coil. Appl. Sci. 2021, 11, 1723. [Google Scholar] [CrossRef]
- Xiao, S.; Wu, H.; Li, N.; Tan, X.; Deng, H.; Zhang, X.; Tang, J.; Li, Y. Triboelectric Mechanism of Oil-Solid Interface Adopted for Self-Powered Insulating Oil Condition Monitoring. Adv. Sci. 2023, 10, 2207230. [Google Scholar] [CrossRef]
- Tanasescu, F.; Cramariuc, R. Electroststica în Technica; Editura Technica: Bucharest, Romania, 1977. [Google Scholar]
- Balmer, R. Electrostatic Generation in Dielectric Fluids: The Viscoelectric Effect. In Proceedings of the WTC2005 World Tribology Congress III, Washington, DC, USA, 12–16 September 2005. Paper No. WTC2005-63806. [Google Scholar] [CrossRef] [Green Version]
- Yoo, D.; Jang, S.; Cho, S.; Choi, D.; Kim, D.S. A Liquid Triboelectric Series. Adv. Mater. 2023, 2300699. [Google Scholar] [CrossRef]
- Kushchev, L.A.; Okuneva, G.L.; Suslov, D.Y.; Gravin, A.A. Modeling biogas production in bubbling bioreactors. Chem. Petrol. Eng. 2012, 47, 613–618. [Google Scholar] [CrossRef]
- Steudler, S.; Werner, A.; Cheng, J.J. (Eds.) Solid State Fermentation. Research and Industrial Applications; Springer Nature: Cham, Switzerland, 2019. [Google Scholar] [CrossRef]
- Pino, M.S.; Rodríguez-Jasso, R.M.; Michelin, M.; Flores-Gallegos, A.C.; Morales-Rodriguez, R.; Teixeira, J.A.; Ruiz, H.A. Bioreactor design for enzymatic hydrolysis of biomass under the biorefinery concept. Chem. Eng. J. 2018, 347, 119–136. [Google Scholar] [CrossRef] [Green Version]
- Ruggeri, F.S.; Šneideris, T.; Vendruscolo, M.; Knowles, T.P.J. Atomic force microscopy for single molecule characterisation of protein aggregation. Arch. Biochem. Biophys. 2019, 664, 134–148. [Google Scholar] [CrossRef]
- Cawood, E.E.; Karamanos, T.K.; Wilson, A.J.; Radford, S.E. Visualizing and trapping transient oligomers in amyloid assembly pathways. Biophys. Chem. 2021, 268, 106505. [Google Scholar] [CrossRef]
- Yang, H.; Wang, Y.; Lai, S.; An, H.; Li, Y.; Chen, F. Application of Atomic Force Microscopy as a Nanotechnology Tool in Food Science. J. Food Sci. 2007, 72, R65–R75. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Ivanova, I.A.; Valueva, A.A.; Tatur, V.Y.; Smelov, M.V.; Ivanova, N.D.; Ziborov, V.S. AFM imaging of protein aggregation in studying the impact of knotted electromagnetic field on a peroxidase. Sci. Rep. 2020, 10, 9022. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Tatur, V.Y.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Repnikov, V.V.; et al. Effect of Spherical Elements of Biosensors and Bioreactors on the Physicochemical Properties of a Peroxidase Protein. Polymers 2021, 13, 1601. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Frantsuzov, P.A.; Zöllner, A.; Medvedeva, N.V.; Archakov, A.I.; Reinle, W.; Bernhardt, R. Atomic Force Microscopy Study of Protein–Protein Interactions in the Cytochrome CYP11A1 (P450scc)-Containing Steroid Hydroxylase System. Nanoscale Res. Lett. 2011, 6, 54. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, Y.D.; Bukharina, N.S.; Frantsuzov, P.A.; Pleshakova, T.O.; Kanashenko, S.L.; Medvedeva, N.V.; Argentova, V.V.; Zgoda, V.G.; Munro, A.W.; Archakov, A.I. AFM study of cytochrome CYP102A1 oligomeric state. Soft Matter 2012, 8, 4602–4608. [Google Scholar] [CrossRef]
- Baron, A.M.; Lubambo, A.F.; Lima, V.M.G.; de Camargo, P.C.; Mitchell, D.A.; Krieger, N. Atomic Force Microscopy: A Useful Tool for Evaluating Aggregation of Lipases. Microsc. Microanal. 2005, 11 (Suppl. 3), 74–77. [Google Scholar] [CrossRef]
- Blasi, L.; Longo, L.; Vasapollo, G.; Cingolani, R.; Rinaldi, R.; Rizzello, T.; Acierno, R.; Maffia, M. Characterization of glutamate dehydrogenase immobilization on silica surface by atomic force microscopy and kinetic analyses. Enz. Microbial Technol. 2005, 36, 818–823. [Google Scholar] [CrossRef]
- Zhang, P.; Tan, W. Atomic force microscopy for the characterization of immobilized enzyme molecules on biosensor surfaces. Fresenius J. Anal. Chem. 2001, 369, 302–307. [Google Scholar] [CrossRef]
- Neeli, R.; Girvan, H.M.; Lawrence, A.; Warren, M.J.; Leys, D.; Scrutton, N.S.; Munro, A.W. The dimeric form of flavocytochrome P450 BM3 is catalytically functional as a fatty acid hydroxylase. FEBS Lett. 2005, 579, 5582–5588. [Google Scholar] [CrossRef]
- Davies, P.F.; Rennke, H.G.; Cotran, R.S. Influence of molecular charge upon the endocytosis and intracellular fate of peroxidase activity in cultured arterial endothelium. J. Cell Sci. 1981, 49, 69–86. [Google Scholar] [CrossRef]
- Welinder, K.G. Amino acid sequence studies of horseradish peroxidase. amino and carboxyl termini, cyanogen bromide and tryptic fragments, the complete sequence, and some structural characteristics of horseradish peroxidase C. Eur. J. Biochem. 1979, 96, 483–502. [Google Scholar] [CrossRef]
- Tams, J.W.; Welinder, K.G. Mild chemical deglycosylation of horseradish peroxidase yields a fully active, homogeneous enzyme. Anal. Biochem. 1995, 228, 48–55. [Google Scholar] [CrossRef]
- Veitch, N.C. Horseradish peroxidase: A modern view of a classic enzyme. Phytochemistry 2004, 65, 249–259. [Google Scholar] [CrossRef]
- Ohshima, T.; Tamura, T.; Sato, M. Influence of pulsed electric field on various enzyme activities. J. Elstat. 2007, 65, 156–161. [Google Scholar] [CrossRef]
- Wasak, A.; Drozd, R.; Jankowiak, D.; Rakoczy, R. The influence of rotating magnetic field on bio-catalytic dye degradation using the horseradish peroxidase. Biochem. Eng. J. 2019, 147, 81–88. [Google Scholar] [CrossRef]
- Emamdadi, N.; Gholizadeh, M.; Housaindokht, M.R. Investigation of static magnetic field effect on horseradish peroxidase enzyme activity and stability in enzymatic oxidation process. Int. J. Biol. Macromol. 2021, 170, 189–195. [Google Scholar] [CrossRef]
- Sun, J.; Sun, F.; Xu, B.; Gu, N. The quasi-one-dimensional assembly of horseradish peroxidase molecules in presence of the alternating magnetic field. Coll. Surf. A Physicochem. Eng. Aspects 2010, 360, 94–98. [Google Scholar] [CrossRef]
- Sun, J.; Zhou, H.; Jin, Y.; Wang, M.; Gu, N. Magnetically enhanced dielectrophoretic assembly of horseradish peroxidase molecules: Chaining and molecular monolayers. Chem. Phys. Chem. 2008, 9, 1847–1850. [Google Scholar] [CrossRef]
- Fortune, J.A.; Wu, B.-I.; Klibanov, A.M. Radio Frequency Radiation Causes No Nonthermal Damage in Enzymes and Living Cells. Biotechnol. Prog. 2010, 26, 1772–1776. [Google Scholar] [CrossRef]
- Yao, Y.; Zhang, B.; Pang, H.; Wang, Y.; Fu, H.; Chen, X.; Wang, Y. The effect of radio frequency heating on the inactivation and structure of horseradish peroxidase. Food Chem. 2023, 398, 133875. [Google Scholar] [CrossRef]
- Lopes, L.C.; Barreto, M.T.; Gonçalves, K.M.; Alvarez, H.M.; Heredia, M.F.; De Souza, R.O.M.; Cordeiro, Y.; Dariva, C.; Fricks, A.T. Stability and structural changes of horseradish peroxidase: Microwave versus conventional heating treatment. Enzym. Microb. Technol. 2015, 69, 10–18. [Google Scholar] [CrossRef]
- Caliga, R.; Maniu, C.L.; Mihăşan, M. ELF-EMF exposure decreases the peroxidase catalytic efficiency in vitro. Open Life Sci. 2016, 11, 71–77. [Google Scholar] [CrossRef]
- Bilal, M.; Rasheed, T.; Zhao, Y.; Iqbal, H.M.N. Agarose-chitosan hydrogel-immobilized horseradish peroxidase with sustainable bio-catalytic and dye degradation properties. Int. J. Biol. Macromol. 2019, 124, 742–749. [Google Scholar] [CrossRef]
- Krainer, F.W.; Glieder, A. An updated view on horseradish peroxidases: Recombinant production and biotechnological applications. Appl. Microbiol. Biotechnol. 2015, 99, 1611–1625. [Google Scholar] [CrossRef] [Green Version]
- Maryskova, M.; Linhartova, L.; Novotny, V.; Rysova, M.; Cajthaml, T.; Sevcu, A. Laccase and horseradish peroxidase for green treatment of phenolic micropollutants in real drinking water and wastewater. Environ. Sci. Pollut. Res. 2021, 28, 31566–31574. [Google Scholar] [CrossRef]
- Bayramoğlu, G.; Arıca, M.Y. Enzymatic removal of phenol and p-chlorophenol in enzyme reactor: Horseradish peroxidase immobilized on magnetic beads. J. Hazard. Mater. 2008, 156, 148–155. [Google Scholar] [CrossRef]
- Zhao, Y.; Zheng, Y.; Kong, R.; Xia, L.; Qu, F. Ultrasensitive electrochemical immunosensor based on horseradish peroxidase (HRP)-loaded silica-poly(acrylic acid) brushes for protein biomarker detection. Biosens. Bioelectron. 2016, 75, 383–388. [Google Scholar] [CrossRef]
- Kiselyova, O.I.; Yaminsky, I.; Ivanov, Y.D.; Kanaeva, I.P.; Kuznetsov, V.Y.; Archakov, A.I. AFM study of membrane proteins, cytochrome P450 2B4, and NADPH–Cytochrome P450 reductase and their complex formation. Arch. Biochem. Biophys. 1999, 371, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Pleshakova, T.O.; Kaysheva, A.L.; Shumov, I.D.; Ziborov, V.S.; Bayzyanova, J.M.; Konev, V.A.; Uchaikin, V.F.; Archakov, A.I.; Ivanov, Y.D. Detection of hepatitis C virus core protein in serum using aptamer-functionalized AFM chips. Micromachines 2019, 10, 129. [Google Scholar] [CrossRef] [Green Version]
- Enzymatic Assay of Peroxidase (EC 1.11.1.7) 2,20-Azino-Bis(3-Ethylbenzthiazoline-6-Sulfonic Acid) as a Substrate Sigma Prod. No. P-6782. Available online: https://www.sigmaaldrich.com/RU/en/technical-documents/protocol/protein-biology/enzymeactivity-assays/enzymatic-assay-of-peroxidase-abts-as-substrate (accessed on 18 February 2022).
- Sanders, S.A.; Bray, R.C.; Smith, A.T. pH-dependent properties of a mutant horseradish peroxidase isoenzyme C in which Arg38 has been replaced with lysine. Eur. J. Biochem. 1994, 224, 1029–1037. [Google Scholar] [CrossRef]
- Drozd, M.; Pietrzak, M.; Parzuchowski, P.G.; Malinowska, E. Pitfalls and capabilities of various hydrogen donors in evaluation of peroxidase-like activity of gold nanoparticles. Anal. Bioanal. Chem. 2016, 408, 8505–8513. [Google Scholar] [CrossRef] [Green Version]
- Ivanov, Y.D.; Tatur, V.Y.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Ivanova, N.D.; Stepanov, I.N.; Lukyanitsa, A.A.; et al. The Effect of a Dodecahedron-Shaped Structure on the Properties of an Enzyme. J. Funct. Biomater. 2022, 13, 166. [Google Scholar] [CrossRef]
- Shipov, G.I. Changing the properties of biomolecules under the influence of the shape of objects and their movement. Zametki Uchenogo (Sci. Notes) 2021, 9-1, 66–69. [Google Scholar]
- Pouplin, A.; Masbernat, O.; Décarre, S.; Liné, A. Wall friction and effective viscosity of a homogeneous dispersed liquid–liquid flow in a horizontal pipe. AIChE J. Fluid Mech. Transp. Phenom. 2011, 57, 1119–1131. [Google Scholar] [CrossRef]
- Shin, E.-C.; Ko, J.-H.; Lyeo, H.-K.; Kim, Y.-H. Derivation of a governing rule in triboelectric charging and series from thermoelectricity. Phys. Rev. Res. 2022, 4, 023131. [Google Scholar] [CrossRef]
- Lee, D.W.; Kong, D.S.; Kim, J.H.; Park, S.H.; Hu, Y.C.; Ko, Y.J.; Jeong, C.B.; Lee, S.; Choi, J.I.J.; Lee, G.-H.; et al. Correlation between frictional heat and triboelectric charge: In operando temperature measurement during metal-polymer physical contact. Nano Energy 2022, 103 Pt A, 107813. [Google Scholar] [CrossRef]
- Ducati, T.R.; Simões, L.H.; Galembeck, F. Charge partitioning at gas-solid interfaces: Humidity causes electricity buildup on metals. Langmuir 2010, 26, 13763. [Google Scholar] [CrossRef]
- Nie, J.; Wang, Z.; Ren, Z.; Li, S.; Chen, X.; Wang, Z.L. Power generation from the interaction of a liquid droplet and a liquid membrane. Nat. Commun. 2019, 10, 2264. [Google Scholar] [CrossRef] [Green Version]
- Pan, S.; Zhang, Z. Fundamental theories and basic principles of triboelectric effect: A review. Friction 2019, 7, 2–17. [Google Scholar] [CrossRef]
- Ranada, A.F. Knotted solutions of the Maxwell equations in vacuum. J. Phys. A Math. Gen. 1990, 23, L815. [Google Scholar] [CrossRef]
- Lee, W.; Gheorghe, A.; Tiurev, K.; Ollikainen, T.; Möttönen, M.; Hall, D.S. Synthetic electromagnetic knot in a three-dimensional skyrmion. Sci. Adv. 2018, 4, eaao3820. [Google Scholar] [CrossRef] [Green Version]
- Smelov, M.V. Experimental study of nodular antennas in the form of shamrock and pentacle. Radio Eng. 2013, 2, 023–029. [Google Scholar]
- Nefedov, E.I.; Ermolaev, Y.M.; Smelov, M.V. Experimental study of excitation and propagation of nodular electromagnetic waves in various media. Radio Eng. 2014, 2, 31–34. [Google Scholar]
- Fogarty, A.C.; Laage, D. Water Dynamics in Protein Hydration Shells: The Molecular Origins of the Dynamical Perturbation. J. Phys. Chem. B 2014, 118, 7715–7729. [Google Scholar] [CrossRef]
- Verma, P.K.; Rakshit, S.; Mitra, R.K.; Pal, S.K. Role of hydration on the functionality of a proteolytic enzyme α-chymotrypsin under crowded environment. Biochimie 2011, 93, 1424–1433. [Google Scholar] [CrossRef]
- Laage, D.; Elsaesser, T.; Hynes, J.T. Water Dynamics in the Hydration Shells of Biomolecules. Chem. Rev. 2017, 117, 10694–10725. [Google Scholar] [CrossRef]
- Ignatenko, O.V.; Sjölander, A.; Hushpulian, D.M.; Kazakov, S.V.; Ouporov, I.V.; Chubar, T.A.; Poloznikov, A.A.; Ruzgas, T.; Tishkov, V.I.; Gorton, L.; et al. Electrochemistry of chemically trapped dimeric and monomeric recombinant horseradish peroxidase. Adv. Biosens. Bioelectron. 2013, 2, 25–34. [Google Scholar]
- Ivanova, I.A.; Ershova, M.O.; Shumov, I.D.; Valueva, A.A.; Ivanov, Y.D.; Pleshakova, T.O. Atomic Force Microscopy Study of the Temperature and Storage Duration Dependencies of Horseradish Peroxidase Oligomeric State. Biomedicines 2022, 10, 2645. [Google Scholar] [CrossRef]
- Schramm, F.D.; Schroeder, K.; Jonas, K. Protein aggregation in bacteria. FEMS Microbiol. Rev. 2020, 44, 54–72. [Google Scholar] [CrossRef]
- Gentile, K.; Bhide, A.; Kauffman, J.; Ghosh, S.; Maiti, S.; Adair, J.; Lee, T.-H.; Sen, A. Enzyme aggregation and fragmentation induced by catalysis relevant species. Phys. Chem. Chem. Phys. 2021, 23, 20709–20717. [Google Scholar] [CrossRef]
- Chattopadhyay, K.; Mazumdar, S. Structural and Conformational Stability of Horseradish Peroxidase: Effect of Temperature and pH. Biochemistry 2000, 39, 263–270. [Google Scholar] [CrossRef]
- Andrade, J.D.; Hlady, V.; Wei, A.P. Adsorption of complex proteins at interfaces. Pure Appl. Chem. 1992, 64, 1777–1781. [Google Scholar] [CrossRef] [Green Version]
- Ziborov, V.S.; Pleshakova, T.O.; Shumov, I.D.; Kozlov, A.F.; Valueva, A.A.; Ivanova, I.A.; Ershova, M.O.; Larionov, D.I.; Evdokimov, A.N.; Tatur, V.Y.; et al. The Impact of Fast-Rise-Time Electromagnetic Field and Pressure on the Aggregation of Peroxidase upon Its Adsorption onto Mica. Appl. Sci. 2021, 11, 11677. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ivanov, Y.D.; Shumov, I.D.; Kozlov, A.F.; Ershova, M.O.; Valueva, A.A.; Ivanova, I.A.; Tatur, V.Y.; Lukyanitsa, A.A.; Ivanova, N.D.; Ziborov, V.S. Glycerol Flow through a Shielded Coil Induces Aggregation and Activity Enhancement of Horseradish Peroxidase. Appl. Sci. 2023, 13, 7516. https://doi.org/10.3390/app13137516
Ivanov YD, Shumov ID, Kozlov AF, Ershova MO, Valueva AA, Ivanova IA, Tatur VY, Lukyanitsa AA, Ivanova ND, Ziborov VS. Glycerol Flow through a Shielded Coil Induces Aggregation and Activity Enhancement of Horseradish Peroxidase. Applied Sciences. 2023; 13(13):7516. https://doi.org/10.3390/app13137516
Chicago/Turabian StyleIvanov, Yuri D., Ivan D. Shumov, Andrey F. Kozlov, Maria O. Ershova, Anastasia A. Valueva, Irina A. Ivanova, Vadim Y. Tatur, Andrei A. Lukyanitsa, Nina D. Ivanova, and Vadim S. Ziborov. 2023. "Glycerol Flow through a Shielded Coil Induces Aggregation and Activity Enhancement of Horseradish Peroxidase" Applied Sciences 13, no. 13: 7516. https://doi.org/10.3390/app13137516
APA StyleIvanov, Y. D., Shumov, I. D., Kozlov, A. F., Ershova, M. O., Valueva, A. A., Ivanova, I. A., Tatur, V. Y., Lukyanitsa, A. A., Ivanova, N. D., & Ziborov, V. S. (2023). Glycerol Flow through a Shielded Coil Induces Aggregation and Activity Enhancement of Horseradish Peroxidase. Applied Sciences, 13(13), 7516. https://doi.org/10.3390/app13137516





