Peptide Mapping with LC-HRMS and In Silico Tools for Evaluation of Potential Allergenicity of Commercially Available Whey Protein Hydrolysates
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Size-Exclusion Ultra Performance Liquid Chromatography (SE-UPLC) of Proteins
2.3. LC-HRMS Analysis
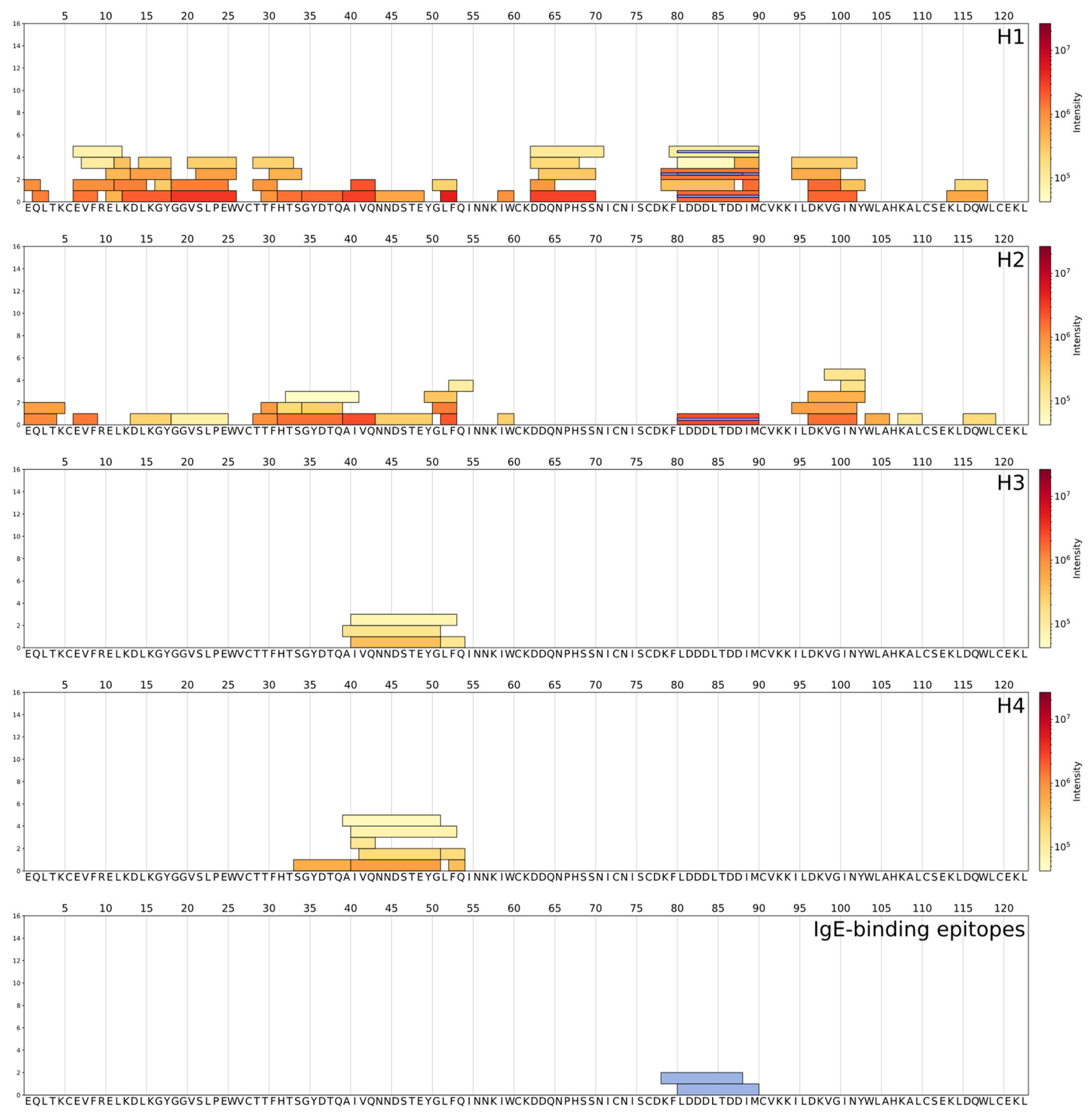
3. Results
3.1. Analysis of Protein Molecular Weight Distribution Using SE-UPLC
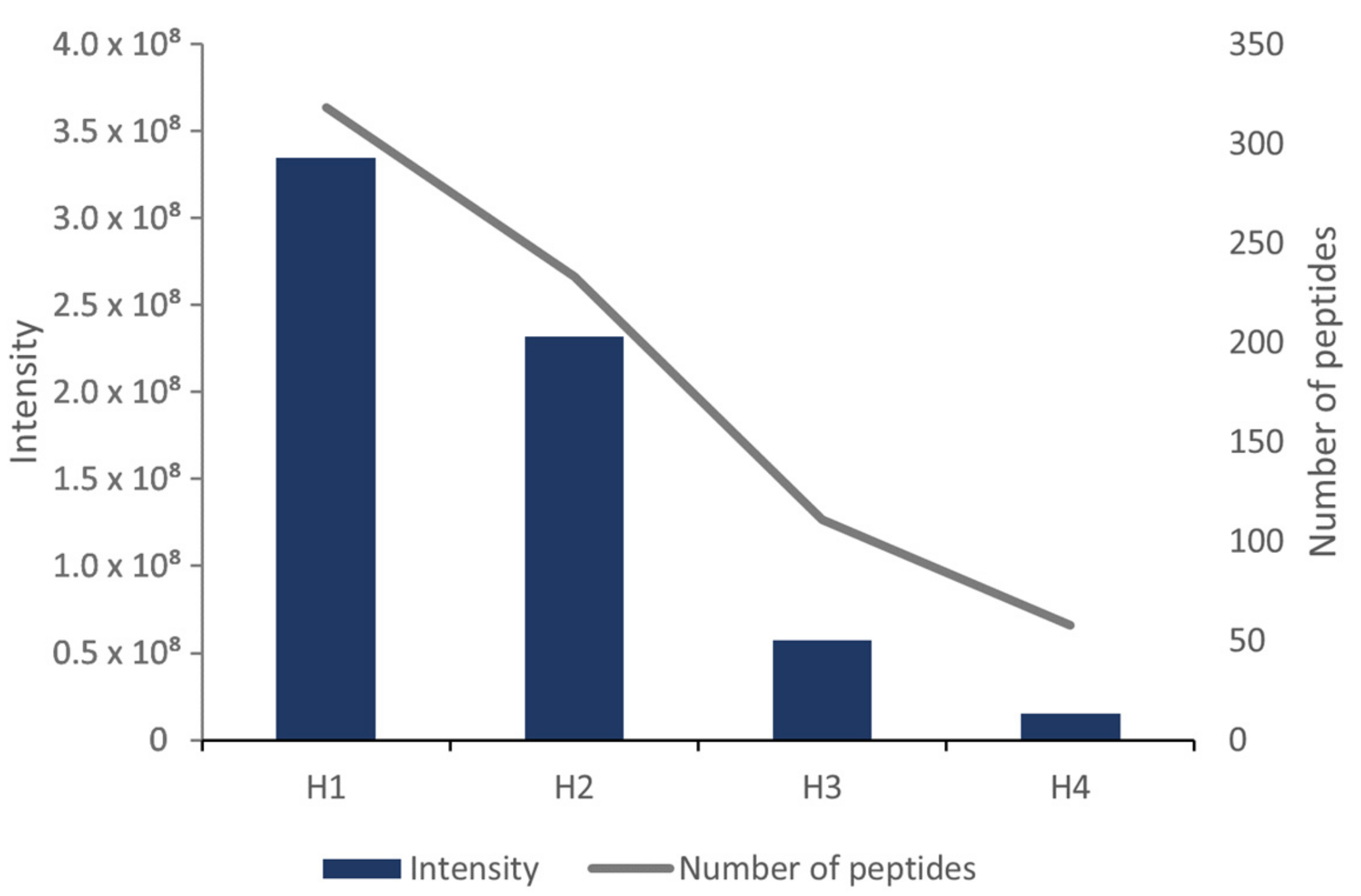
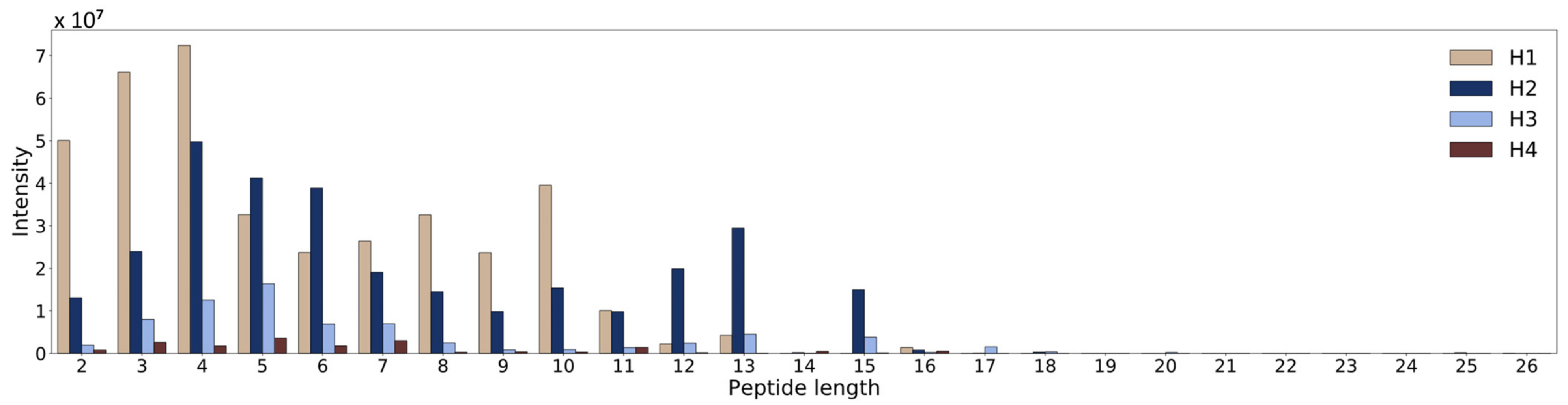
3.2. Determination of Peptides with LC-HRMS
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Knol, E.F.; de Jong, N.W.; Ulfman, L.H.; Tiemessen, M.M. Management of Cow’s Milk Allergy from an Immunological Perspective: What Are the Options? Nutrients 2019, 11, 2734. [Google Scholar] [CrossRef] [PubMed]
- Monaci, L.; Tregoat, V.; van Hengel, A.J.; Anklam, E. Milk allergens, their characteristics and their detection in food: A review. Eur. Food Res. Technol. 2006, 223, 149–179. [Google Scholar] [CrossRef]
- Sharma, S.; Kumar, P.; Betzel, C.; Singh, T.P. Structure and function of proteins involved in milk allergies. J. Chromatogr. B Biomed. Sci. Appl. 2001, 756, 183–187. [Google Scholar] [CrossRef] [PubMed]
- Peñas, E.; Préstamo, G.; Luisa Baeza, M.; Martínez-Molero, M.I.; Gomez, R. Effects of combined high pressure and enzymatic treatments on the hydrolysis and immunoreactivity of dairy whey proteins. Int. Dairy J. 2006, 16, 831–839. [Google Scholar] [CrossRef]
- Bu, G.; Luo, Y.; Chen, F.; Liu, K.; Zhu, T. Milk processing as a tool to reduce cow’s milk allergenicity: A mini-review. Dairy Sci. Technol. 2013, 93, 211–223. [Google Scholar] [CrossRef] [PubMed]
- Jeewanthi, R.K.C.; Lee, N.-K.; Paik, H.-D. Improved Functional Characteristics of Whey Protein Hydrolysates in Food Industry. Korean J. Food Sci. Anim. Resour. 2015, 35, 350–359. [Google Scholar] [CrossRef] [PubMed]
- Hays, T.; Wood, R.A. A Systematic Review of the Role of Hydrolyzed Infant Formulas in Allergy Prevention. Arch. Pediatr. Adolesc. Med. 2005, 159, 810. [Google Scholar] [CrossRef]
- Host, A.; Halken, S. Hypoallergenic formulas—When, to whom and how long: After more than 15 years we know the right indication! Allergy 2004, 59, 45–52. [Google Scholar] [CrossRef]
- Bøgh, K.L.; Barkholt, V.; Madsen, C.B. Characterization of the Immunogenicity and Allergenicity of Two Cow’s Milk Hydrolysates—A Study in Brown Norway Rats. Scand. J. Immunol. 2015, 81, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Wang, Z.; Yang, H.; Luo, X.; Sun, J.; Yang, M.; Shi, X.; Yue, X.; Zheng, Y. Evaluation of allergenicity of cow milk treated with enzymatic hydrolysis through a mouse model of allergy. J. Dairy Sci. 2022, 105, 1039–1050. [Google Scholar] [CrossRef]
- Mäkinen-Kiljunen, S.; Palosuo, T. A sensitive enzyme-linked immunosorbent assay for determination of bovine ?—Lactoglobulin in infant feeding formulas and in human milk. Allergy 1992, 47, 347–352. [Google Scholar] [CrossRef]
- Rosendal, A.; Barkholt, V. Detection of Potentially Allergenic Material in 12 Hydrolyzed Milk Formulas. J. Dairy Sci. 2000, 83, 2200–2210. [Google Scholar] [CrossRef] [PubMed]
- Puerta, A.; Diez-Masa, J.C.; de Frutos, M. Immunochromatographic determination of β-lactoglobulin and its antigenic peptides in hypoallergenic formulas. Int. Dairy J. 2006, 16, 406–414. [Google Scholar] [CrossRef]
- Oldæus, G.; Björkstén, B.; Jenmalm, M.; Kjellman, N.-I.M. Cow’s milk IgE and IgG antibody responses to cow’s milk formulas. Allergy 1999, 54, 352–357. [Google Scholar] [CrossRef] [PubMed]
- Bahna, S.L. Hypoallergenic formulas: Optimal choices for treatment versus prevention. Ann. Allergy Asthma Immunol. 2008, 101, 453–459. [Google Scholar] [CrossRef]
- Chan, Y.; Shek, L.; Aw, M.; Quak, S.; Lee, B. Use of hypoallergenic formula in the prevention of atopic disease among Asian children. J. Paediatr. Child Health 2002, 38, 84–88. [Google Scholar] [CrossRef] [PubMed]
- Siemensma, A.D.; Weijer, W.J.; Bak, H.J. The importance of peptide lengths in hypoallergenic infant formulae. Trends Food Sci. Technol. 1993, 4, 16–21. [Google Scholar] [CrossRef]
- Van Hoeyveld, E.M.; Escalona-Monge, M.; DE Swert, L.F.; Stevens, E.A. Allergenic and antigenic activity of peptide fragments in a whey hydrolysate formula: Allergenic and antigenic activity. Clin. Exp. Allergy 1998, 28, 1131–1137. [Google Scholar] [CrossRef]
- Businco, L.; Dreborg, S.; Einarsson, R.; Giampietro, P.G.; Hest, A.; Keller, K.M.; Strobel, S.; Wahn, U. Hydrolysed cow’s milk formulae: Allergenicity and use in treatment and prevention. An ESPACI position paper. Pediatr. Allergy Immunol. 1993, 4, 101–111. [Google Scholar] [CrossRef]
- Adams, S.L.; Barnett, D.; Walsh, B.J.; Pearce, R.J.; Hill, D.J.; Howden, M.E.H. Human IgE-binding synthetic peptides of bovine β-lactoglobulin and α-lactalbumin. In vitro cross-reactivity of the allergens. Immunol. Cell Biol. 1991, 69, 191–197. [Google Scholar] [CrossRef]
- Ball, G.; Shelton, M.J.; Walsh, B.J.; Hill, D.J.; Hosking, C.S.; Howden, M.E.H. A major continuous allergenic epitope of bovine beta-lactoglobulin recognized by human IgE binding. Clin. Htmlent Glyphamp Asciiamp Exp. Allergy 1994, 24, 758–764. [Google Scholar] [CrossRef] [PubMed]
- Heinzmann, A.; Blattmann, S.; Spuergin, P.; Forster, J.; Deichmann, K.A. The Recognition Pattern of Sequential B Cell Epitopes of Beta–Lactoglobulin Does Not Vary with the Clinical Manifestations of Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 1999, 120, 280–286. [Google Scholar] [CrossRef] [PubMed]
- Sélo, I.; Clément, G.; Bernard, H.; Chatel, J.; Créminon, C.; Peltre, G.; Wal, J. Allergy to bovine β-lactoglobulin: Specificity of human IgE to tryptic peptides. Clin. Exp. Allergy 1999, 29, 1055–1063. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, K.-M.; Chatchatee, P.; Bardina, L.; Beyer, K.; Sampson, H.A. IgE and IgG Binding Epitopes on α-Lactalbumin and β-Lactoglobulin in Cow’s Milk Allergy. Int. Arch. Allergy Immunol. 2001, 126, 111–118. [Google Scholar] [CrossRef]
- Cerecedo, I.; Zamora, J.; Shreffler, W.G.; Lin, J.; Bardina, L.; Dieguez, M.C.; Wang, J.; Muriel, A.; de la Hoz, B.; Sampson, H.A. Mapping of the IgE and IgG4 sequential epitopes of milk allergens with a peptide microarray–based immunoassay. J. Allergy Clin. Immunol. 2008, 122, 589–594. [Google Scholar] [CrossRef]
- Wang, J.; Lin, J.; Bardina, L.; Goldis, M.; Nowak-Węgrzyn, A.; Shreffler, W.G.; Sampson, H.A. Correlation of IgE/IgG4 milk epitopes and affinity of milk-specific IgE antibodies with different phenotypes of clinical milk allergy. J. Allergy Clin. Immunol. 2010, 125, 695–702.e6. [Google Scholar] [CrossRef]
- Benedé, S.; López-Expósito, I.; Giménez, G.; Grishina, G.; Bardina, L.; Sampson, H.A.; López-Fandiño, R.; Molina, E. Mapping of IgE epitopes in in vitro gastroduodenal digests of β-lactoglobulin produced with human and simulated fluids. Food Res. Int. 2014, 62, 1127–1133. [Google Scholar] [CrossRef]
- Oliveira, J.P.B.; Candreva, A.M.; Rizzo, G.; Ramos, M.V.; Oliveira, J.S.; Oliveira, H.D.; Ary, M.B.; Docena, G.; Freitas, C.D.T. Allergenicity reduction of cow’s milk proteins using latex peptidases. Food Chem. 2019, 284, 245–253. [Google Scholar] [CrossRef]
- Zhang, Q.; Chen, Q.-H.; He, G.-Q. Effect of ultrasonic-ionic liquid pretreatment on the hydrolysis degree and antigenicity of enzymatic hydrolysates from whey protein. Ultrason. Sonochem. 2020, 63, 104926. [Google Scholar] [CrossRef]
- Dąbrowska, A.; Bajzert, J.; Babij, K.; Szołtysik, M.; Stefaniak, T.; Willak-Janc, E.; Chrzanowska, J. Reduced IgE and IgG antigenic response to milk proteins hydrolysates obtained with the use of non-commercial serine protease from Yarrowia lipolytica. Food Chem. 2020, 302, 125350. [Google Scholar] [CrossRef]
- Lozano-Ojalvo, D.; Pérez-Rodríguez, L.; Pablos-Tanarro, A.; López-Fandiño, R.; Molina, E. Pepsin treatment of whey proteins under high pressure produces hypoallergenic hydrolysates. Innov. Food Sci. Emerg. Technol. 2017, 43, 154–162. [Google Scholar] [CrossRef]
- Ji, J.; Zhu, P.; Pi, F.; Sun, C.; Sun, J.; Jia, M.; Ying, C.; Zhang, Y.; Sun, X. Development of a liquid chromatography-tandem mass spectrometry method for simultaneous detection of the main milk allergens. Food Control 2017, 74, 79–88. [Google Scholar] [CrossRef]
- Pescuma, M.; Hébert, E.M.; Rabesona, H.; Drouet, M.; Choiset, Y.; Haertlé, T.; Mozzi, F.; de Valdez, G.F.; Chobert, J.-M. Proteolytic action of Lactobacillus delbrueckii subsp. bulgaricus CRL 656 reduces antigenic response to bovine β-lactoglobulin. Food Chem. 2011, 127, 487–492. [Google Scholar] [CrossRef] [PubMed]
- Zhao, Q.; Wang, Y.; Zhu, Z.; Zhao, Q.; Zhu, L.; Jiang, L. Efficient reduction of β-lactoglobulin allergenicity in milk using Clostridium tyrobutyricum Z816. Food Sci. Hum. Wellness 2023, 12, 809–816. [Google Scholar] [CrossRef]
- Planque, M.; Arnould, T.; Dieu, M.; Delahaut, P.; Renard, P.; Gillard, N. Advances in ultra-high performance liquid chromatography coupled to tandem mass spectrometry for sensitive detection of several food allergens in complex and processed foodstuffs. J. Chromatogr. A 2016, 1464, 115–123. [Google Scholar] [CrossRef]
- Fan, S.; Ma, J.; Liu, Z.; Ning, Y.; Cao, M.; Li, Q.; Zhang, Y. Determination of egg and milk allergen in food products by liquid chromatography-tandem mass spectrometry based on signature peptides and isotope-labeled internal standard. Food Sci. Hum. Wellness 2023, 12, 728–736. [Google Scholar] [CrossRef]
- Ma, X.; Li, H.; Zhang, J.; Huang, W.; Han, J.; Ge, Y.; Sun, J.; Chen, Y. Comprehensive quantification of sesame allergens in processed food using liquid chromatography-tandem mass spectrometry. Food Control 2020, 107, 106744. [Google Scholar] [CrossRef]
- Kang, W.; Zhang, J.; Li, H.; Yu, N.; Tang, R.; Sun, X.; Wei, L.; Sun, J.; Chen, Y. Quantification of major allergens in peach based on shotgun proteomics using liquid chromatography-tandem mass spectrometry. LWT 2022, 160, 113234. [Google Scholar] [CrossRef]
- Arju, G.; Berg, H.Y.; Lints, T.; Nisamedtinov, I. Methodology for Analysis of Peptide Consumption by Yeast during Fermentation of Enzymatic Protein Hydrolysate Supplemented Synthetic Medium Using UPLC-IMS-HRMS. Fermentation 2022, 8, 145. [Google Scholar] [CrossRef]
- Vita, R.; Mahajan, S.; Overton, J.A.; Dhanda, S.K.; Martini, S.; Cantrell, J.R.; Wheeler, D.K.; Sette, A.; Peters, B. The Immune Epitope Database (IEDB): 2018 update. Nucleic Acids Res. 2019, 47, D339–D343. [Google Scholar] [CrossRef]
- Järvinen, K.-M.; Beyer, K.; Vila, L.; Chatchatee, P.; Busse, P.J.; Sampson, H.A. B-cell epitopes as a screening instrument for persistent cow’s milk allergy. J. Allergy Clin. Immunol. 2002, 110, 293–297. [Google Scholar] [CrossRef]
- Hattori, M.; Ametani, A.; Katakura, Y.; Shimizu, M.; Kaminogawa, S. Unfolding/refolding studies on bovine beta-lactoglobulin with monoclonal antibodies as probes. Does a renatured protein completely refold? J. Biol. Chem. 1993, 268, 22414–22419. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Yuan, S.; He, S.; Gao, J.; Chen, H. Identification and characterization of the antigenic site (epitope) on bovine β-lactoglobulin: Common residues in linear and conformational epitopes. J. Sci. Food Agric. 2015, 95, 2916–2923. [Google Scholar] [CrossRef] [PubMed]
- Cong, Y.J.; Li, L.F. Identification of the critical amino acid residues of immunoglobulin E and immunoglobulin G epitopes in β-lactoglobulin by alanine scanninapplsci-13-07402g analysis. J. Dairy Sci. 2012, 95, 6307–6312. [Google Scholar] [CrossRef] [PubMed]
- Totsuka, M.; Ametani, A.; Kaminogawa, S. Fine mapping of T-cell determinants of bovine β-lactoglobulin. Cytotechnology 1997, 25, 101–113. [Google Scholar] [CrossRef]
- Gouw, J.W.; Jo, J.; Meulenbroek, L.A.P.M.; Heijjer, T.S.; Kremer, E.; Sandalova, E.; Knulst, A.C.; Jeurink, P.V.; Garssen, J.; Rijnierse, A.; et al. Identification of peptides with tolerogenic potential in a hydrolysed whey-based infant formula. Clin. Exp. Allergy 2018, 48, 1345–1353. [Google Scholar] [CrossRef]
- Chicón, R.; Belloque, J.; Alonso, E.; López-Fandiño, R. Antibody binding and functional properties of whey protein hydrolysates obtained under high pressure. Food Hydrocoll. 2009, 23, 593–599. [Google Scholar] [CrossRef]
- Svenning, C.; Brynhildsvold, J.; Molland, T.; Langsrud, T.; Elisabeth Vegarud, G. Antigenic response of whey proteins and genetic variants of β-lactoglobulin—The effect of proteolysis and processing. Int. Dairy J. 2000, 10, 699–711. [Google Scholar] [CrossRef]
- Leksrisompong, P.P.; Miracle, R.E.; Drake, M. Characterization of Flavor of Whey Protein Hydrolysates. J. Agric. Food Chem. 2010, 58, 6318–6327. [Google Scholar] [CrossRef]
- Miraglia Del Giudice, M.; D’Auria, E.; Peroni, D.; Palazzo, S.; Radaelli, G.; Comberiati, P.; Galdo, F.; Maiello, N.; Riva, E. Flavor, relative palatability and components of cow’s milk hydrolysed formulas and amino acid-based formula. Ital. J. Pediatr. 2015, 41, 42. [Google Scholar] [CrossRef]
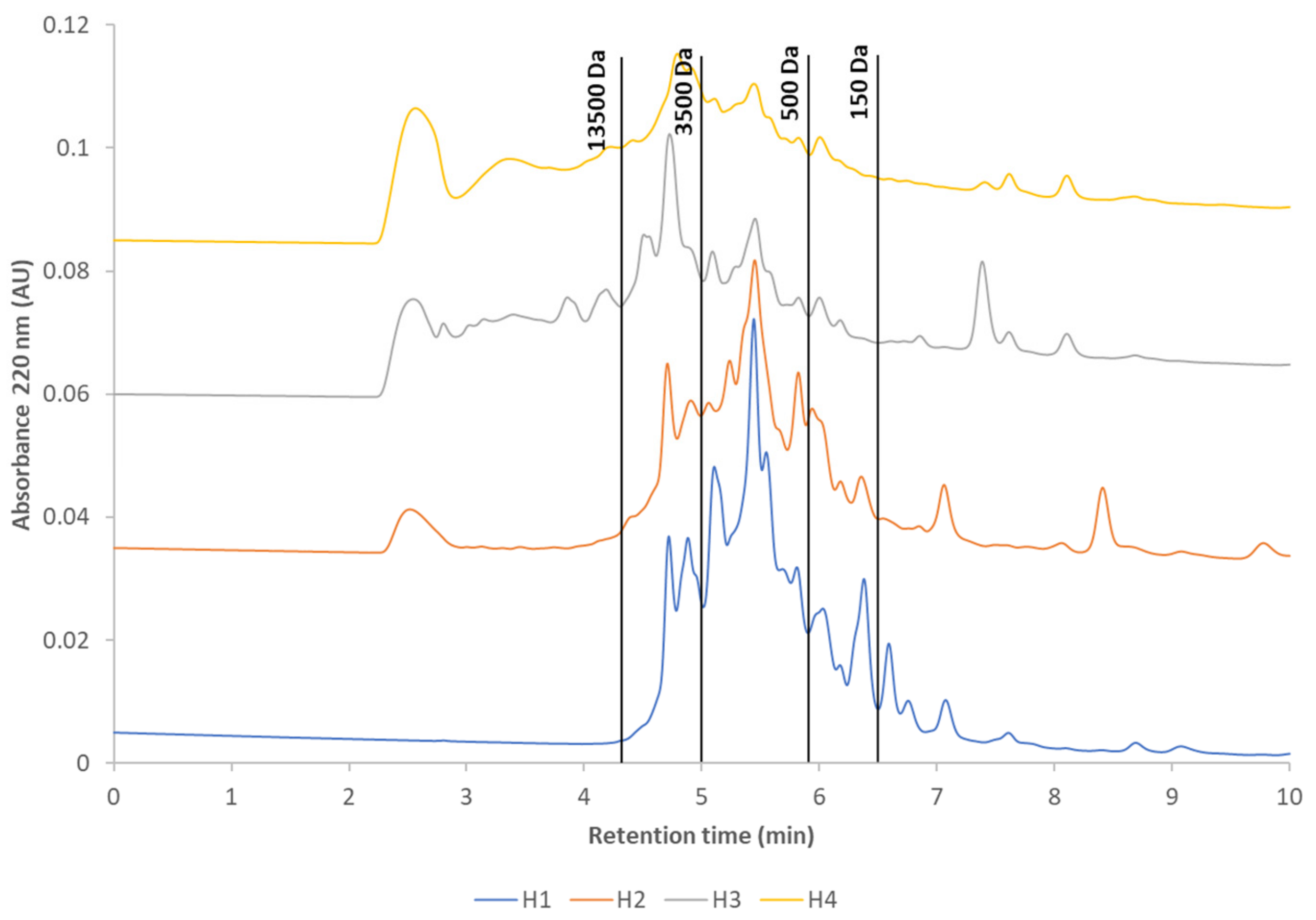
| % of Total Area (Mean ± SD) | |||||
|---|---|---|---|---|---|
| MW, Da | RT, Min * | H1 | H2 | H3 | H4 |
| >13,500 | 0–4.4 | 0.0 ± 0.0 | 6.0 ± 0.4 | 35.4 ± 0.7 | 34.0 ± 1.0 |
| 3500–13,500 | 4.4–5.0 | 17.8 ± 0.1 | 19.5 ± 0.2 | 24.4 ± 0.8 | 20.3 ± 0.0 |
| 500–3500 | 5.0–5.9 | 54.2 ± 0.2 | 42.8 ± 1.0 | 21.5 ± 0.2 | 21.1 ± 0.1 |
| 150–500 | 5.9–6.5 | 16.9 ± 0.1 | 15.7 ± 1.1 | 7.5 ± 0.3 | 8.1 ± 0.1 |
| <150 | 6.5–15.0 | 11.1 ± 0.0 | 16.1 ± 0.3 | 11.2 ± 1.0 | 16.5 ± 0.8 |
| % of the Total Intensity | ||||
|---|---|---|---|---|
| Protein | H1 | H2 | H3 | H4 |
| α-LA | 12.8 | 6.7 | 1.0 | 13.0 |
| β-LG | 61.3 | 85.1 | 73.3 | 80.0 |
| BSA | 4.9 | 1.7 | 1.8 | 0.9 |
| Apolipoprotein | 1.5 | 0.8 | 3.9 | 1.3 |
| Complement C3 | 5.3 | 2.1 | 6.3 | 4.4 |
| Fibrinogen alpha | 1.5 | 0.5 | 2.0 | 0.0 |
| Lactoperoxidase | 2.5 | 1.0 | 8.3 | 0.0 |
| Lactotransferrin | 8.0 | 1.1 | 2.1 | 0.5 |
| Osteopontin | 2.4 | 0.9 | 1.4 | 0.0 |
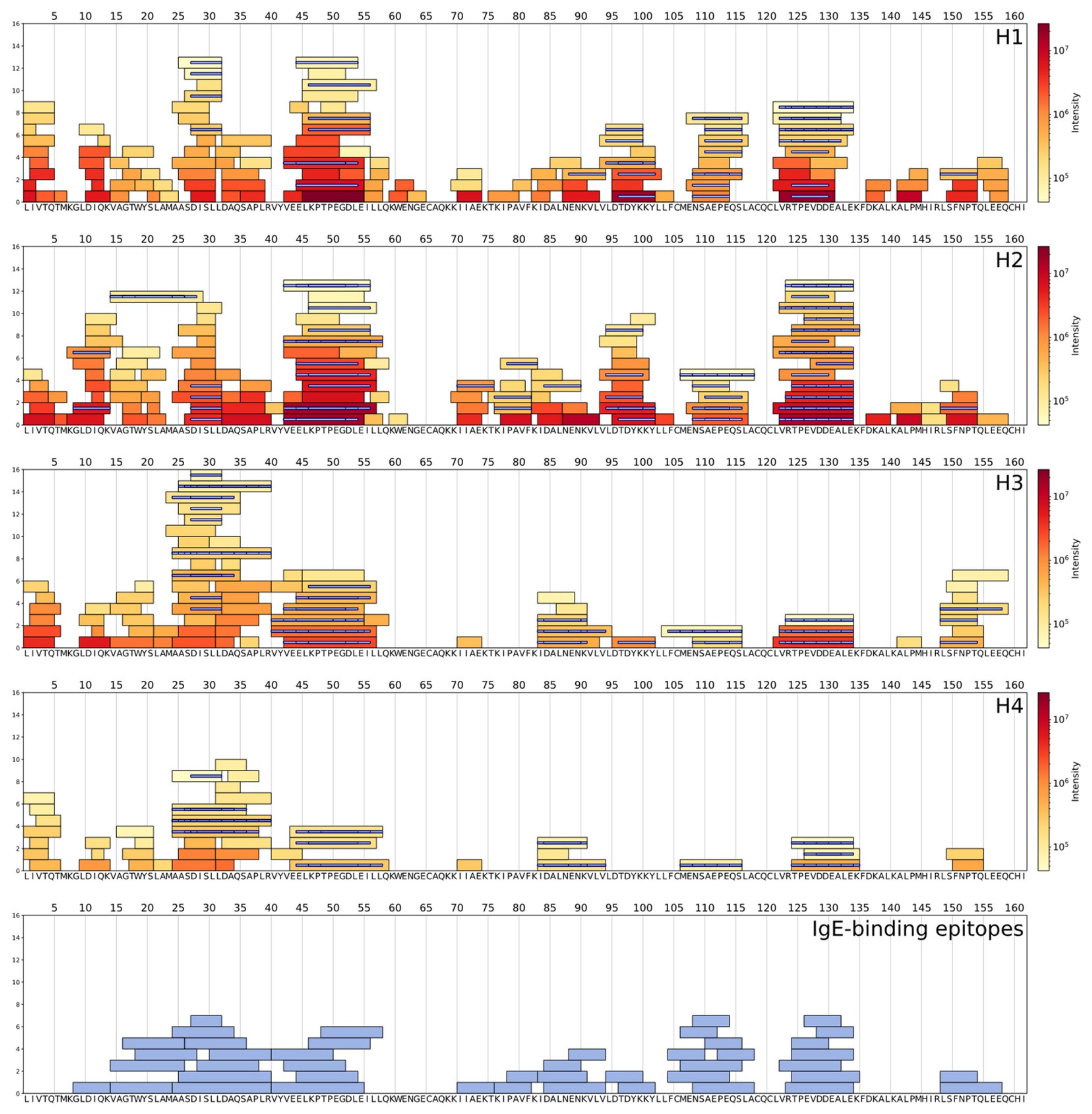
| Protein (UniProt Database ID) | Identified Peptides | WPH | Epitope Sequence | Epitope Location in Protein Chain | Immune Epitope Database ID | References |
|---|---|---|---|---|---|---|
| α-LA (P00711) | f79-90 | H1 | KFLDDDLTDD | f79-88 | 115308 | [24] |
| f81-90 f80-90 f79-90 | H1, H2 H1 H1 | LDDDLTDDIM | f81-90 | 115340 | [24] | |
| β-LG (P02754) | f25-40 | H3, H4 | AASDISLLDAQSAPLR | f25-40 | 426 | [23] |
| f9-14 f8-14 | H2 H2 | GLDIQK | f9-14 | 20801 | [23] | |
| f84-91 f84-93 f84-95 f84-94 | H3, H4 H3 H3 H4 | IDALNENK | f84-91 | 25535 | [23] | |
| f46-56 f46-57 f47-57 f45-57 f43-56 f43-57 f43-58 f41-57 f44-57 f44-58 f44-59 | H1, H2 H1, H2, H3 H2 H2, H3 H2 H2, H3 H2 H3 H4 H4 H4 | KPTPEGDLEI | f47-56 | 32907 | [24,41] | |
| f78-83 | H2 | PAVFK | f79-83 | 46987 | [23] | |
| f123-134 f122-134 f124-134 | H1, H2, H3 H1, H2, H3 H2, H3 | RTPEVDDEALE | f124-134 | 56145 | [20] | |
| f125-135 | H2, H4 | TPEVDDEALEK | f125-135 | 65565 | [22,23,42] | |
| f41-55 f41-57 | H3 H3 | VYVEELKPTP | f41-50 | 72177 | [24] | |
| f111-116 f110-116 f110-117 f109-116 f108-117 f109-117 f107-118 f104-116 f107-116 | H1 H1 H1, H2 H1, H3 H1, H2 H2 H2 H3 H4 | AEPEQS | f111-116 | 95218 | [21] | |
| f83-90 f84-91 f84-93 f84-95 f84-94 | H2 H3, H4 H3 H3 H4 | DALNEN | f85-90 | 95300 | [21] | |
| f123-134 f122-134 f128-134 f127-134 f125-134 f125-135 f124-134 f127-135 | H1, H2, H3 H1, H2, H3 H2 H2 H2, H4 H2, H4 H2, H3 H4 | DDEALE | f129-134 | 95306 | [21] | |
| f88-94 f84-95 f84-94 | H1 H3 H4 | ENKVLV | f89-94 | 95357 | [21] | |
| f122-132 f123-133 f123-134 f122-134 f127-134 f125-134 f125-135 f124-134 f127-135 | H1 H1 H1, H2, H3 H1, H2, H3 H2 H2, H4 H2, H4 H2, H3 H4 | EVDDEA | f127-132 | 95369 | [21] | |
| f104-116 | H3 | FCMENS | f105-110 | 95378 | [21] | |
| f71-76 | H2 | IIAEKT | f71-76 | 95461 | [21] | |
| f77-82 f76-82 | H2 H2 | KIPAVF | f77-82 | 95498 | [21,43] | |
| f95-100 f94-100 f94-102 f94-101 | H1, H2 H1, H2 H1, H2 H2 | LDTDYK | f95-100 | 95545 | [21] | |
| f149-154 f149-155 f149-159 | H1, H2, H3 H3 H3 | LSFNPT | f149-154 | 95574 | [21] | |
| f107-118 f104-116 f107-116 | H2 H3 H4 | MENSAE | f107-112 | 95590 | [21] | |
| f109-114 f108-114 f109-116 f108-117 f109-117 f107-118 f104-116 f107-116 | H1, H2 H1 H1, H3 H1, H2 H2 H2 H3 H4 | NSAEPE | f109-114 | 95638 | [21] | |
| f107-118 | H2 | PEQSLA | f113-118 | 95652 | [21] | |
| f96-102 f96-103 f94-102 | H1, H2, H3 H1 H1, H2 | TDYKKY | f97-102 | 95898 | [21] | |
| f124-131 f123-131 f122-131 f122-132 f123-133 f123-134 f122-134 f125-134 f125-135 f124-134 | H1, H2 H1, H2 H1, H2 H1 H1 H1, H2, H3 H1, H2, H3 H2, H4 H2, H4 H2, H3 | TPEVDD | f125-130 | 95922 | [21] | |
| f15-29 | H2 | VAGTWYSLAMAA | f15-26 | 95946 | [21] | |
| f26-40 f25-40 | H3 H3, H4 | ASDISLLDAQSAPLR | f26-40 | 96113 | [44] | |
| f41-55 f41-57 | H3 H3 | VYVEELKPTPEGDLE | f41-55 | 97098 | [45] | |
| f25-35 f24-35 f25-40 f25-36 f25-38 | H3 H3 H3, H4 H4 H4 | AASDISLLDA | f25-34 | 98677 | [24] | |
| f84-95 f84-94 | H3 H4 | DALNENKVLV | f85-94 | 98723 | [24] | |
| f45-54 f45-55 f43-55 f45-57 f43-56 f43-57 f43-58 f41-55 f41-57 f44-57 f44-58 f44-59 | H1 H1, H2 H1, H2, H3 H2, H3 H2 H2, H3 H2 H3 H3 H4 H4 H4 | ELKPTPEGDL | f45-54 | 98760 | [24] | |
| f104-116 | H3 | FCMENSAEPE | f105-114 | 98772 | [24] | |
| f26-40 f25-40 f25-38 | H3 H3, H4 H4 | ISLLDAQSAP | f29-38 | 98836 | [24] | |
| f26-40 f25-40 | H3 H3, H4 | LLDAQSAPLR | f31-40 | 98881 | [24] | |
| f149-159 | H3 | LSFNPTQLEE | f149-158 | 98893 | [24] | |
| f107-118 f104-116 f107-116 | H2 H3 H4 | MENSAEPEQS | f107-116 | 98902 | [24] | |
| f107-118 | H2 | NSAEPEQSLA | f109-118 | 98919 | [24] | |
| f26-40 f25-40 f25-36 f25-38 | H3 H3, H4 H4 H4 | SDISLLDAQS | f27-36 | 98997 | ||
| f43-58 f44-58 f44-59 | H2 H4 H4 | TPEGDLEILL | f49-58 | 99028 | [24] | |
| f43-55 f43-56 f43-57 f43-58 f41-55 f41-57 | H1, H2, H3 H2 H2, H3 H2 H3 H3 | VEELKPTPEG | f43-52 | 99036 | [24] | |
| f122-132 f123-133 f123-134 f122-134 | H1 H1 H1, H2, H3 H1, H2, H3 | VRTPEVDDEA | f123-132 | 99049 | [24] | |
| f15-29 | H2 | WYSLAMAASD | f19-28 | 99061 | [24] | |
| f15-29 | H2 | GTWYSLAMAA | f17-26 | 115275 | [24] | |
| f123-134 f122-134 f125-134 f125-135 f124-134 | H1, H2, H3 H1, H2, H3 H2, H4 H2, H4 H2, H3 | TPEVDDEALE | f125-134 | 115519 | [24] | |
| f15-29 | H2 | VAGTWYSLAM | f15-24 | 115530 | [24] | |
| f28-32 f27-32 f26-32 f25-32 f26-35 f25-35 f24-35 f26-40 f25-40 f25-36 f25-38 | H1, H2, H3 H1, H2, H3 H1, H2, H3 H2, H3, H4 H3 H3 H3 H3 H3, H4 H4 H4 | DISLL | f28-32 | 851640 | [46] |
| WPH | µg/g (Mean ± SD) | µg/g IF Powder | µg/L in Ready-to-Drink IF |
|---|---|---|---|
| H1 | 0.13 ± 0.07 | 0.02 | 2.45 |
| H2 | 237 ± 118 | 39 | 5049 |
| H3 | 86,922 ± 43,461 | 13,038 | 1,786,252 |
| H4 | 68,786 ± 34,392 | 9380 | 1,285,042 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Taivosalo, A.; Stulova, I.; Kütt, M.-L.; Kriščiunaite, T.; Lints, T.; Gimaeva, T.; Tamm, M. Peptide Mapping with LC-HRMS and In Silico Tools for Evaluation of Potential Allergenicity of Commercially Available Whey Protein Hydrolysates. Appl. Sci. 2023, 13, 7402. https://doi.org/10.3390/app13137402
Taivosalo A, Stulova I, Kütt M-L, Kriščiunaite T, Lints T, Gimaeva T, Tamm M. Peptide Mapping with LC-HRMS and In Silico Tools for Evaluation of Potential Allergenicity of Commercially Available Whey Protein Hydrolysates. Applied Sciences. 2023; 13(13):7402. https://doi.org/10.3390/app13137402
Chicago/Turabian StyleTaivosalo, Anastassia, Irina Stulova, Mary-Liis Kütt, Tiina Kriščiunaite, Taivo Lints, Tatjana Gimaeva, and Martti Tamm. 2023. "Peptide Mapping with LC-HRMS and In Silico Tools for Evaluation of Potential Allergenicity of Commercially Available Whey Protein Hydrolysates" Applied Sciences 13, no. 13: 7402. https://doi.org/10.3390/app13137402
APA StyleTaivosalo, A., Stulova, I., Kütt, M.-L., Kriščiunaite, T., Lints, T., Gimaeva, T., & Tamm, M. (2023). Peptide Mapping with LC-HRMS and In Silico Tools for Evaluation of Potential Allergenicity of Commercially Available Whey Protein Hydrolysates. Applied Sciences, 13(13), 7402. https://doi.org/10.3390/app13137402







