Featured Application
As sacha inchi meal protein hydrolysates have significant antioxidant and hypoglycemic activity in vitro, the hydrolysates obtained could be used as for functional food and pharmaceutical development.
Abstract
Sacha inchi meal (SIM) is the residue from the processing of the oil crop sacha inchi. In the present study, biological enzymolysis was performed on SIM protein to obtain hydrolysates, and then the antioxidant and hypoglycemic activities were evaluated. The results showed that the scavenging rates of alkaline protease hydrolysate (SAl) and protamex hydrolysate (SPr) to ABTS free radicals were close to that of vitamin C at 5 mg/mL, amounting to 99.83 ± 0.33% and 100.00 ± 0.09%, respectively. The dipeptidyl peptidase (DPP-IV) inhibitory activities of SPr and SAl were also the highest, at the concentration of 2.5 mg/mL, their inhibition rates were 74.15 ± 0.68% and 56.38 ± 1.51%, respectively, with the IC50 values of 1.007 mg/mL and 2.130 mg/mL, respectively. Moreover, compared with the model group, all the hydrolysates increased the glucose consumption by 187.01–348.79% (at 800 μg/mL) in insulin-resistant HepG2 cells, which were better than positive drug Metformin. In conclusion, SIM protein hydrolysates have significant antioxidant and hypoglycemic activity in vitro; therefore, the hydrolysates could be used as for functional food and pharmaceutical development.
1. Introduction
Sacha inchi, Plukenetia volubilis L., is a perennial climbing woody vine of Euphorbiaceae, which grows mostly in the tropical rainforest area of the Andes in South America at an altitude of 80–1700 m [1]. The fruit capsule contains edible dark brown oval seeds with milky white kernels. The protein content of kernel is 24.2–27.0%, similar to those of soybeans (28%), cottonseed (33%), sunflower seeds (24%) and peanuts (23%) [2]. As a by-product after oil extraction, the protein content of sacha inchi meal (SIM) was up to 55%, which was not effectively used except for the aquatic animal feed [3].
The research on protein hydrolysates and bioactive peptides used for the added-value products has attracted great attention of food research and the food industry. Food-derived hydrolysates or peptides exhibited various bioactivities, including antioxidant, antimicrobial, anti-diabetic, and anti-hypertensive activities, etc. [4,5,6]. Compared with drugs, hydrolysates or polypeptides derived from food protein have the advantages of low toxicity, no side effects, low cost and easy production (enzymatic hydrolysis was usually employed) [7,8]. The purpose of the present work was to investigate the antioxidant and dipeptidyl peptidase (DPP-IV) inhibitory activities of SIM-derived protein hydrolysates as well as their effects on glucose consumption, which provides a foundation for value-added processing of SIM.
2. Materials and Methods
2.1. Materials
SIM was provided by Lianzhong Biotechnol. Co., Ltd. (Pu Er, Yunnan, China), which has been engaging in the cultivation, production and sales of sacha inchi since 2009. Pepsin (3000 U/mg) and trypsin (250 U/mg) were from Qiyun Biotechnol. Co., Ltd. (Guangzhou, China). Papain (800 U/mg) and protamex (120 U/mg) were purchased from Yuanye Biotechnol. Co., Ltd. (Guangzhou, China). Liver cancer cells (HepG2) were purchased from the Animal Experimental Center of Sun Yat-Sen University, Guangzhou, China. Alkaline protease (200 U/mg), bromelain (300 U/mg), α-amylase (>500 U/mg protein) and dipeptidyl peptidase (DPP-IV) (>4000 U/mg protein) were purchased from Sigma-Aldrich (Shanghai, China). Experiments were carried out at the South China University of Technology.
2.2. Protein Extraction
Sacha inchi meal protein was extracted using ultrasonic alkali dissolution and acid precipitation [9]. Briefly, sacha inchi meal was degreased, powdered and dispersed in distilled water (1:10, g/mL, pH = 10), then ultrasonic treatment (50 °C, 400 W, 30 min) and centrifuge (537× g, 30 min) were performed, adjusting pH = 4.5 by HCl (1 M), and precipitating the protein for 24 h at 4 °C. After centrifuge (537× g, 15 min) and washing with pure water (3 times), the resolved protein was subjected to dialysis (5000 Da dialysis bag, 48 h) for removing salt, freeze-dried and stored at −20 °C. Kjeldahl method (KDY-9820, Guangzhou, China) was used to determine the total nitrogen content of the crude protein (the conversion factor was 6.25).
2.3. Enzymatic Hydrolysis
The extracted protein was hydrolyzed by six proteases under their optimal conditions [5] (optimal temperature, optimal pH): pepsin (37 °C, 2), papain (55 °C, 7), trypsin (37 °C, 8), protamex (55 °C, 7), alkaline protease (50 °C, 8), bromelain (55 °C, 7). The ratio of material to liquid was 1:20 (mg/mL), and the additional amount of protease was 5% (dry basis mass ratio). After 4 h of enzymolysis, the solution (pH = 7) was boiled to inactivate the enzyme, centrifuged (1074× g, 4 °C, 10 min), and then the supernatant was collected and freeze-dried to obtain SIM protein hydrolysis product. Peptide content was determined by BCA kit (150 T, Biyuntian Biotechnology Co., Ltd., Guangzhou, China), and degree of hydrolysis (DH) was calculated by the formula:
where FAN is free amino nitrogen content (mg/mg) of sample.
2.4. Molecular Weight Distribution
As described in [10], a crude peptide solution (5 mg/mL) and a standard peptide sample solution (1 mg/mL) were prepared, passing 0.22 μM filter membrane, and sample measurement was performed. Chromatographic column: TSK gel 2000SWXL (300 mm × 7.8 mm); mobile phase A: water containing 0.1% trifluoroacetic acid; mobile phase B: acetonitrile containing 0.1% trifluoroacetic acid; detector: UV detector; detection wavelength: 220 nm. Chromatographic conditions: 20 μL of sample solution was injected to the column (25 °C) at a rate of 0.5 mL/min. Elution conditions: equal elution; mobile phase A: 80%; mobile phase B: 20%; elution time: 30 min. A standard curve was created using the LogM (M: molecular weight) of the standard sample as the X-axis and the retention time as the Y-axis. The relative molecular weight of SIM protein hydrolysis product can be calculated based on fitting equation.
2.5. Antioxidant Activity
According to the method described before [11], ABTS and DPPH scavenging activities were determined. Ultra-pure water was used as the negative control instead of the sample solution, blank group was phosphate buffer solution (0.01 M) instead of ABTS or DPPH, negative blank group was phosphate buffer solution (0.01 M) instead of ABTS or DPPH and ultrapure water instead of sample, and Vc was used as the positive control. Two concentrations of hydrolysates and Vc were used: 1 mg/mL and 5 mg/mL for ABTS; 5 mg/mL and 10 mg/mL for DPPH. The experiment was repeated three times in each group. The calculation formula of ABTS and DPPH radical clearance was as follows:
2.6. Inhibitory Activity of α-Amylase
The inhibitory activity of α-amylase was assayed by previous methods [6]. The control group was buffer (20 μL) and enzyme solution (10 μL). Background group was sample solution (20 μL) and buffer (10 μL). Negative control group was the control group without sample, and negative background group is the background control group. Each group of experiments was repeated 3 times. The formula of α amylase inhibition rate is calculated as follows (A is absorbance value):
2.7. Inhibitory Activity of Dipeptidyl Peptidase (DPP-IV)
DPP-IV inhibitory activity was performed according to [6]. S was the experimental group, B was the sample background group, negative control group (NC) was the control group without samples, negative background group (NB) was the background of the control group. The inhibition rate of enzymolysis products on DPP-IV was calculated as below (A is absorbance value):
2.8. Inhibitory Type of Hydrolysates on DPP-IV
DPP-IV was diluted to different folds: 32/6 U/L, 32/5 U/L, 32/4 U/L, 32/3, 32/2 U/L. Peptide sample (SPr and Sal) solutions (25 μL) with different concentrations, DPP-IV (25 μL) with different concentrations and substrate solution (50 μL) were mixed to each hole of a 96-well plate, and the fluorescence values every 1 min within 30 min were immediately measured, the reaction rate of enzyme relative to the enzyme concentration was obtained under different sample concentrations, and the inhibition type of hydrolysates on DPP-IV was determined.
2.9. Cell Viability and Hypoglycemic Activity
IR-HepG2 model establishment and cytotoxicity evaluation were described as before [6]. Briefly, cells were cultured in CO2 incubator with DMEM medium. S was the sample treatment group, SB was the sample group without cells, N was the negative control group, and NB was the negative group without cells. MTT assay was performed, the calculation formula of cell growth inhibition rate is as follows (C is the light absorption value):
In the established IR-HepG2 model, blank group was low sugar medium DMEM (200 μL) containing 2% serum, model group was palmitic acid high glucose medium (200 μL, 0.25 mM) containing 2% serum, control group was model group containing 2 mM metformin, sample treatment group was model group containing different concentration of samples. After incubation for 24 h, the consumption of glucose in the medium supernatant was measured by glucose assay kit (Rongsheng Bio. Co., Ltd., Shanghai, China):
2.10. Statistical Analysis
Each experiment was repeated at least 3 times. The data were given in the form of mean ± standard error. Origin 9.0 software (OriginLab Corporation, Northampton, MA, USA) was used to calculate IC50 values using logistic equation. SPSS 20.0 (IBM, Chicargo, IL, USA) was used to compare the significant difference, p < 0.05.
3. Results
3.1. Hydrolysis of SIM Protein
The extracted protein was hydrolyzed under the optimal hydrolysis conditions of six proteases (Figure 1). The protomax-digested hydrolysate (SPr) and alkaline-digested hydrolysate (SAl) had significantly higher degrees of hydrolysis, up to 20.74 ± 0.88% and 25.15 ± 1.37%, respectively, compared with other hydrolysates. The polypeptide contents of SPr and SAl were 44.33 ± 0.38% and 31.96 ± 0.04%, respectively. The primary components of SPr and SAl were the small molecular weight fractions (<0.5 kD), with the percentages of 69.28 ± 0.11% and 79.97 ± 0.07%, respectively (Table 1, Figure 2).
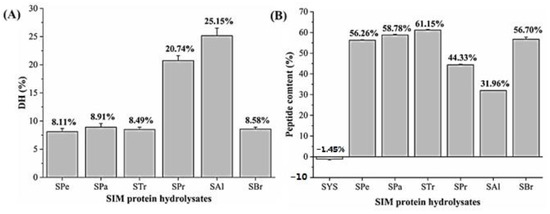
Figure 1.
(A) Hydrolysis degree DH (%) of SIM protein hydrolysates. (B) Peptide contents (%) of SIM protein hydrolysates. SYS: sacha inchi meal protein (SIMP); SPe: SIMP enzymolysis product by pepsin; Spa: SIMP enzymolysis product by papain; STr: SIMP enzymolysis product by trypsin; SBr: SIMP enzymolysis product by bromelain; SPr: SIMP enzymolysis product by protamex; SAl: SIMP enzymolysis product by alkaline. p < 0.05 was considered to be significant.

Table 1.
Different components of sacha inchi meal protein hydrolysis product.
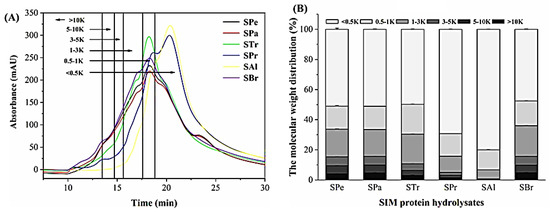
Figure 2.
High performance liquid chromatography profile (A) of sacha inchi meal protein (SIMP) enzymolysis product and distribution of components (B). SPe: SIMP enzymolysis product by pepsin; Spa: SIMP enzymolysis product by papain; STr: SIMP enzymolysis product by trypsin; SBr: SIMP enzymolysis product by bromelain; SPr: SIMP enzymolysis product by protamex; SAl: SIMP enzymolysis product by alkaline.
3.2. Antioxidant Activity
The scavenging ability of the six hydrolysates to ABTS and DPPH free radicals was shown in Figure 3; Vc is the positive control. The results showed that each component has a high scavenging capacity for ABTS free radicals, but it is difficult to scavenge DPPH free radicals (100% for Vc, data not shown). The scavenging ability of each component to ABTS free radicals was ordered as below: SAl > SPr > STr > SPe > SBr > SPa > SIM protein. Among them, the scavenging rates of SAl and SPr to ABTS free radicals at 5 mg/mL were close to Vc, which were 99.83 ± 0.33% and 100.00 ± 0.09%, respectively.
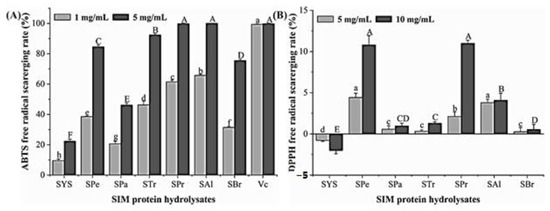
Figure 3.
Antioxidant activity of enzymolysis products. The concentrations of enzymolysis product and Vc are the same. SYS: sacha inchi meal protein (SIMP); SPe: SIMP enzymolysis product by pepsin; Spa: SIMP enzymolysis product by papain; STr: SIMP enzymolysis product by trypsin; SBr: SIMP enzymolysis product by bromelain; SPr: SIMP enzymolysis product by protamex; SAl: SIMP enzymolysis product by alkaline. Significant differences (p < 0.05) were shown for high concentrations (capital letters) and for low concentrations (lowercase letters). (A) ABTS free radical scavenging rate (%) of SIM protein hydrolysates. (B) DPPH free radical scavenging rate (%) of SIM protein hydrolysates.
3.3. Inhibitory Activity on α-Amylase
Figure 4A showed that the crude protein and enzymatic hydrolysates had a certain inhibitory effect on α-amylase, and the inhibitory activity was positively correlated with the concentration, but the inhibitory activity of all components did not exceed 40% at 5 mg/mL. Among which the activity of crude protein was the highest, only 34.73 ± 1.72%, the inhibition rate of hydrolysates on α- amylase was lower than that of crude protein, indicating the potentially synergistic effects among hydrolysates.
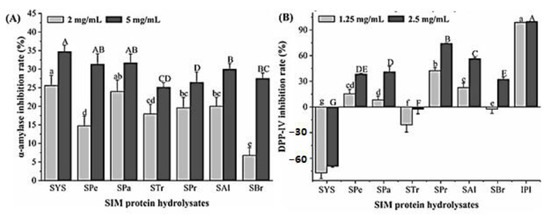
Figure 4.
Inhibition of crude protein and hydrolysates on α-amylase (A) and DPP-IV (B). SYS: sacha inchi meal protein (SIMP); SPe: SIMP enzymolysis product by pepsin; Spa: SIMP enzymolysis product by papain; STr: SIMP enzymolysis product by trypsin; SBr: SIMP enzymolysis product by bromelain; SPr: SIMP enzymolysis product by protamex; SAl: SIMP enzymolysis product by alkaline. Significant differences (p < 0.05) were shown for high concentrations (capital letters) and for low concentrations (lowercase letters).
3.4. Inhibitory Activity and Inhibition Type on DPP-IV
Figure 4B showed the inhibition of crude protein and hydrolysates on DPP-IV, dipriotin A (IPI) is the positive control. Crude protein has no inhibitory effect on DPP-IV, and the inhibition activities of SPr and SAl were the highest, which were concentration dependent, at the concentration of 2.5 mg/mL, their inhibition rates were 74.15 ± 0.68% and 56.38 ± 1.51%, respectively, with the IC50 values of 1.007 mg/mL and 2.130 mg/mL, respectively. The inhibition activity of each enzymatic hydrolysate on DPP-IV was ordered as below: SPr > SAl > SPa > SPe > SBr > STr.
According to Figure 5, under different concentrations of SPr and SAl, the fitting line passed through the origin of the coordinate axis, and the slope decreased with the increased concentration of the hydrolysates. This indicated that the inhibitory effect of the hydrolysates on DPP-IV was reversible.
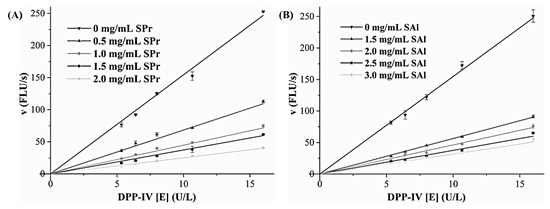
Figure 5.
Inhibition type of enzymolysis product SPr (A) and SAl (B) to DPP-IV. SPr: sacha inchi meal protein (SIMP) enzymolysis product by protamex; SAl: SIMP enzymolysis product by alkaline.
3.5. Effects of the Hydrolysates on Glucose Uptake
Figure 6A indicated that all the hydrolysates had no toxic effect on HepG2 cells at the concentrations of 200–1000 μg/mL. The results of glucose consumption in IR-HepG2 cells are displayed in Figure 6B. The data showed that HepG2 cells had an obvious insulin resistance effect after the induction of 0.25 mM palmitic acid. After 24 h, compared with the model group, crude protein did not significantly promote the glucose consumption of IR- HepG2 cells, but the hydrolysates had a very significant upward trend (p < 0.01), which was much higher than the positive control metformin, and even exceeded the glucose consumption of the normal group at a high concentration. The glucose consumption was positively correlated with the concentrations of SPa, STr and Sal in IR-HepG2 cells. Compared with the model group, at 800 μg/mL, the hydrolysates increased the glucose consumption of IR-HepG2 cells by 187.01–348.79%, even exceeding the normal group by 40.47%, indicating that the SIM protein hydrolysates have significant hypoglycemic activity in vitro.
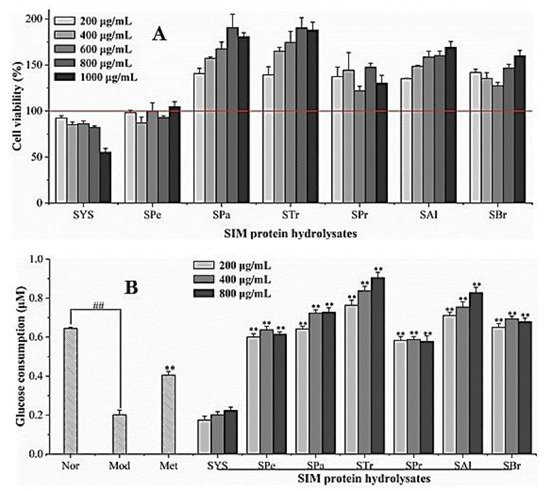
Figure 6.
Effect of SIM protein hydrolysates on HepG2 viability (A). Effects of SIM protein hydrolysates on glucose uptake of IR-HepG2 (B). SYS: sacha inchi meal protein (SIMP); SPe: SIMP enzymolysis product by pepsin; Spa: SIMP enzymolysis product by papain; STr: SIMP enzymolysis product by trypsin; SBr: SIMP enzymolysis product by bromelain; SPr: SIMP enzymolysis product by protamex; SAl: SIMP enzymolysis product by alkaline. Significant differences were shown for normal Vs. model groups (## p < 0.01) and for sample Vs. model groups (** p < 0.01).
4. Discussion
Previous reports indicated that low molecular weight peptides were better than high molecular weight peptides in donating hydrogen atoms for ABTS•+ reduction, i.e., the low molecular weight peptide fractions could have the superior ABTS•+ scavenging ability to the high molecular weight peptide fractions [12]. For example, He et al. [13] indicated that the smaller-sized peptides (<3 kDa) had a stronger potency as ABTS•+ scavengers when compared with the larger-sized peptides (>3 kDa). Nasir et al. [14] demonstrated that smaller peptides (<3 kDa) from shortfin scad (Decapterus macrosoma) muscle protein hydrolysate possessed stronger antioxidant activity than the larger peptides (>3 kDa). Karimi et al. [15] showed that the corn germ protein hydrolysate fraction with the highest ABTS•+ scavenging activity was the fraction containing low molecular weight peptides. In this study, SIM protein hydrolysates exhibited stronger antioxidant activity than the crude protein. In particular, the protamex-treated (SPr) and the alkaline protease-treated (SAl) hydrolysates had the strongest ABTS free radicals scavenging activity. Moreover, the inhibition activities of SPr and SAl were also the highest, at the concentration of 2.5 mg/mL, and their inhibition rates were 74.15 ± 0.68% and 56.38 ± 1.51%, respectively, with the IC50 values of 1.007 mg/mL and 2.130 mg/mL, respectively. According to Table 1, SPr and Sal contained much more small peptides (<0.5 kDa), up to 69.28 ± 0.11% and 79.97 ± 0.07%, respectively, compared with other hydrolysates. This means that the present observation was similar to previous reports, confirming that lower molecular weight peptides could tend to provide greater ABTS radical scavenging activity than higher molecular weight peptides.
On the other hand, one of the most crucial factors that affects the bioactivity of protein hydrolysates is that the species of the used enzyme, depending on which functional groups the enzyme cleave to, in turn, increases bioactivity. For example, the peptides containing Pro, Gly, Ala, Val and Leu in their peptides or having Arg at the third amino acid position next to the N-terminus exhibited strong antioxidant activity [16,17]. The peptides with branched chains (such as Lys, Phe, Tyr and Trp) and cationic residues were considered to be strong alpha-amylase inhibitors [18]. Gly or Phe at the N-terminal and Phe or Leu at the C-terminal were considered to be important for alpha-amylase inhibition [19]. de Souza Rocha et al. [20] indicated that the alcalase-digested Vigna unguiculata hydrolysates had the highest DPP-IV inhibition (IC50 = 0.58 mg soluble protein /mL). Han et al. [21] compared alcalase- and pepsin-treated oilseed protein hydrolysates and demonstrated that alcalase-treated whey protein hydrolysate exhibited the strongest DPP-IV inhibition (43.9 ± 6.0% at 10 mg/mL). Kong et al. [22] showed that the alcalase-derived hydrolysate from walnut (Juglans regia L.) protein exhibited better DPP-IV inhibitory activity of 33.90% (at 0.50 mg/mL). The present data showed that the alkaline protease-treated (SAl) hydrolysates also had stronger DPP-IV inhibitory activity. This suggests that alkaline protease could be a good choice for generating DPP-IV inhibitory hydrolysates. This was probably attributed to the fact that the cleavage sites of alkaline proteases are mainly peptide bonds with aromatic or hydrophobic amino acids on the carboxyl side hydrophobic N-terminal, and the hydrophobic N-terminal such as tryptophan, and proline or alanine were important features of DPP-IV inhibitory peptides [23]. Moreover, small peptides could be absorbed via mucosa of the small intestine [24], so the fractions with molecular weight <3 kDa are suitable for functional food applications.
5. Conclusions
Sacha inchi meal-derived hydrolysates, especially alkaline protease hydrolysate and protamex hydrolysate, exhibited potent hypoglycemic activity, which can be applied to functional food development.
Author Contributions
T.S. wrote the manuscript; K.W. performed the experiment; X.Z. designed the experiment and revised the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Available upon request.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Cai, Z.Q. Shade delayed flowering and decreased photosynthesis, growth and yield of Sacha Inchi (Plukenetia volubilis) plants. Ind. Crop. Prod. 2011, 34, 1235–1237. [Google Scholar] [CrossRef]
- Wang, S.; Zhu, F.; Kakuda, Y. Sacha inchi (Plukenetia volubilis L.): Nutritional composition, biological activity, and uses. Food Chem. 2018, 265, 316–328. [Google Scholar] [CrossRef]
- Rawdkuen, S.; D’Amico, S.; Schoenlechner, R. Physicochemical, Functional, and In Vitro Digestibility of Protein Isolates from Thai and Peru Sacha Inchi (Plukenetia volubilis L.) Oil Press-Cakes. Foods 2022, 11, 1869. [Google Scholar] [CrossRef]
- Roy, F.; Boye, J.I.; Simpson, B.K. Bioactive proteins and peptides in pulse crops: Pea, chickpea and lentil. Food Res. Int. 2010, 43, 432–442. [Google Scholar] [CrossRef]
- Liu, Q.; Yao, Y.S.; Ibrahim, M.A.A.; El Halawany, A.M.; Yang, L.; Zhang, X.W. Production of Dual Inhibitory Hydrolysate by Enzymatic Hydrolysis of Squid Processing By-product. Mar. Biotechnol. 2022, 24, 293–302. [Google Scholar] [CrossRef]
- Hu, S.F.; Fan, X.D.; Qi, P.; Zhang, X.W. Identification of anti-diabetes peptides from Spirulina platensis. J. Funct. Foods 2019, 56, 333–341. [Google Scholar] [CrossRef]
- Alashi, A.M.; Blanchard, C.L.; Mailer, J.R.; Agboola, S.; Mawson, J.A.; He, R.; Girgih, A.; Aluko, R.E. Antioxidant properties of Australian canola meal protein hydrolysates. Food Chem. 2014, 146, 500–506. [Google Scholar] [CrossRef]
- Onuh, J.O.; Girgih, A.T.; Aluko, R.E.; Aliani, M. Inhibitions of renin and angiotensin converting enzyme activities by enzymatic chicken skin protein hydrolysates. Food Res. Int. 2014, 53, 260–267. [Google Scholar] [CrossRef]
- Ding, Y.X.; Gao, Y.H.; Sun, J.X.; Li, C.Q.; Yue, X.Q.; Shao, J.H. Influence of ultrasonic treatment on functional properties and structure of tussah pupa protein isolate. J. Insects Food Feed 2022, 8, 1133–1148. [Google Scholar] [CrossRef]
- Fan, X.D.; Hu, S.F.; Wang, K.; Yang, R.F.; Zhang, X.W. Coupling of ultrasound and subcritical water for peptides production from Spirulina platensis. Food Prod. Process. 2020, 121, 105–112. [Google Scholar] [CrossRef]
- Evangelho, J.A.; Berrios, J.J.; Pinto, V.Z.; Antunes, M.D.; Vanier, N.L.; Zavareze, E.R. Antioxidant activity of black bean (Phaseolus vulgaris L.) protein hydrolysates. Food Sci. Technol. 2016, 36 (Suppl. 1), 23–27. [Google Scholar] [CrossRef]
- Khositanon, P.; Panya, N.; Roytrakul, S.; Krobthong, S.; Chanroj, S.; Choksawangkarn, W. Effects of fermentation periods on antioxidant and angiotensin I-converting enzyme inhibitory activities of peptides from fish sauce by-products. LWT Food Sci. Technol. 2021, 135, 110122. [Google Scholar] [CrossRef]
- He, R.; Girgih, A.T.; Malomo, S.A.; Ju, X.; Aluko, R.E. Antioxidant activities of enzymatic rapeseed protein hydrolysates and the membrane ultrafiltration fractions. J. Funct. Foods 2013, 5, 219–227. [Google Scholar] [CrossRef]
- Nasir, N.A.M.; Sarbon, N.M. Angiotensin converting enzyme (ACE), antioxidant activity and functional properties of shortfin scad (Decapterus macrosoma) muscle protein hydrolysate at different molecular weight variations. Biocatal. Agric. Biotechnol. 2019, 20, 101254. [Google Scholar] [CrossRef]
- Karimi, A.; Azizi, M.H.; Ahmadi Gavlighi, H. Fractionation of hydrolysate from corn germ protein by ultrafiltration: In vitro antidiabetic and antioxidant activity. Food Sci. Nutr. 2020, 8, 2395–2405. [Google Scholar] [CrossRef]
- Kim, B.S.K. Investigation of jumbo squid (Dosidicus gigas) skin gelatin peptides for their in vitro antioxidant effects. Life Sci. 2005, 77, 2166–2178. [Google Scholar]
- Sampath Kumar, N.S.; Nazeer, R.A.; Jaiganesh, R. Purification and identification of antioxidant peptides from the skin protein hydrolysate of two marine fishes, horse mackerel (Magalaspis cordyla) and croaker (Otolithes ruber). Amino Acids 2012, 42, 1641–1649. [Google Scholar] [CrossRef] [PubMed]
- Ngoh, Y.-Y.; Tye, G.J.; Gan, C.-Y. The investigation of alpha-amylase inhibitory activity of selected Pinto bean peptides via preclinical study using AR42J cell. J. Funct. Foods 2017, 35, 641–647. [Google Scholar] [CrossRef]
- Ngoh, Y.-Y.; Gan, C.-Y. Enzyme-assisted extraction and identification of antioxidative and alpha-amylase inhibitory peptides from Pinto beans (Phaseolus vulgaris cv. Pinto). Food Chem. 2016, 190, 331–337. [Google Scholar] [CrossRef]
- de Souza Rocha, T.; Hernandez, L.M.R.; Chang, Y.K.; de Mejía, E.G. Impact of germination and enzymatic hydrolysis of cowpea bean (Vigna unguiculata) on the generation of peptides capable of inhibiting dipeptidyl peptidase IV. Food Res Int. 2014, 64, 799–809. [Google Scholar] [CrossRef]
- Han, R.; Hernández Álvarez, A.J.; Maycock, J.; Murray, B.S.; Boesch, C. Comparison of alcalase- and pepsin-treated oilseed protein hydrolysates—Experimental validation of predicted antioxidant, antihypertensive and antidiabetic properties. Curr. Res. Food Sci. 2021, 4, 141–149. [Google Scholar] [CrossRef] [PubMed]
- Kong, X.; Zhang, L.; Song, W.; Zhang, C.; Hua, Y.; Chen, Y.; Li, X. Separation, identification and molecular binding mechanism of dipeptidyl peptidase IV inhibitory peptides derived from walnut (Juglans regia L.) protein. Food Chem. 2021, 347, 129062. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Cheng, J.; Wu, H. Discovery of Food-Derived Dipeptidyl Peptidase IV Inhibitory Peptides: A Review. Int. J. Mol. Sci. 2019, 20, 463. [Google Scholar] [CrossRef]
- Tkaczewska, J.; Bukowski, M.; Mak, P. Identification of antioxidant peptides in enzymatic hydrolysates of carp (Cyprinus Carpio) skin gelatin. Molecules 2019, 24, 97. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).