Abstract
Microwave (MW) ablation is becoming a routine technology in the interventional radiology field. A new approach combining MW ablation and chemical ablation is developed in this paper. The rationale for the development of this Microwave-Assisted Chemical Ablation (MA-CA) technology was to improve the utility of thermal ablation as a minimally invasive treatment for cancer. The experimental conditions for ex vivo bovine liver samples were: A—100 W (120 s) with no addition of ethanol; B—100 W (30 s), wait (60 s) (no power), and 100 W (90 s) with no addition of ethanol; C—100 W (30 s), wait (60 s), 100 W (30 s), and 100 W (60 s) with the addition of 5 mL ethanol; and D—100 W (30 s), wait (60 s), 100 W (30 s), 0 W (30 s) with the addition of 2.5 mL ethanol, and 100 W (60 s) with the addition of 5 mL ethanol (12,000 Joules Total). The results showed that with the use of ethanol, the ablation zone was enlarged and revealed improved sphericity. This novel combination has greater advantages than either technology individually. The objective is to increase the precision and efficiency of MW ablation and to broaden the range of tissues and pathologies that can be treated using this new approach, and to validate the benefits that arise from combining the advantages of MW and chemical ablation in a relevant setting.
1. Introduction
MW energy is receiving ever-increasing attention from researchers as an alternative to conventional energy sources for a wide spectrum of applications. Recent publications highlight the utility of MWs in many areas. In the extraction domain, Uzel [1] reports on the use of MW-assisted extraction (MAE) as a green technology and a new extraction technique that can be used, with examples presented for the food industry. Applications of the technique in the food industry are diverse and can range from phytochemicals to lipophilic and phenolic compounds, free amino acids, flavonoids, and anthocyanin, to name a few. Moret et al. [2] focused on the use of MW for the extraction of organic contaminants in food, as well as the determination of hydrocarbon contaminants. Another domain of interest has been the removal of cyclohexane in oil sands gangue [3]. Further examples are the use of MAE for the removal of bioactive compounds in herbal or food samples [4,5]. Recently, MAE has shown growing usage potential for cannabinoids [6].
Medical applications are an emerging field of interest for MW energy, specifically as a tool for tumour ablation. Some of the technology’s benefits include minimal invasiveness, short recovery time, repeatability, and low blood loss for patients. The following are examples of the interest in MWA. A literature review by Hernandez et al. [7] reports on the use of MWA for breast, liver, lung, and kidney tumours. Meloni et al. [8] described technical and clinical approaches whereby MWA was used for primary and secondary liver tumours, reviewing the physics related to MWA and its effect on tissue heating. Pusceddu et al. [9] presented a clinical study on patients who underwent tomography-guided percutaneous MWA and cementoplasty and concluded that given the promising results, larger investigations were indicated. An area where cooled MWA was investigated is in thyroid nodules, in which Korkusuz et al. [10] concluded that the method was safe and effective. In another example of the use of MWA in tumour ablation, the authors report on patients with hepatocellular carcinoma [11]. MWA can also be used for lung cancer patients who are not surgical candidates [12] and offers a potentially superior treatment option to Radio Frequency Ablation (RFA). In recent years, this minimally invasive treatment option has also been increasingly used for the ablation of hepatic adenomas, with success [13]. Gao et al. [14] produced a study where MWA was found to be a promising method for the treatment of renal cell carcinoma adjacent to renal sinus, especially for tumours of less than 4 cm. In a study by Jiang et al. [15], the technique is described as effective and safe for the treatment of cirrhosis patients with hypersplenism. Other retrospective clinical studies have been presented regarding the use of MWA in the treatment of lung tumours [16], renal angiomyolipoma [17], and peridiaphragmatic hepatic tumours [18]. All these studies indicate that the technique is safe. Belfiore et al. [19] presented a retrospective study whereby patients were treated with RF or MW for head and neck cancer, and concluded that percutaneous thermal ablation is a promising alternative treatment. All these examples are presented to demonstrate the vast potential of MWA within various fields of application.
Ablation is a minimally invasive procedure that allows for the removal or elimination of biological tissues from the body. In the case of cancerous tissue, tumour ablation can be either thermal or chemical. Various devices are used for the ablation of diseased tissues by delivering energy by laser, electrical current, ultrasound, MW, RF, and other methods. Ablation systems consist of generators, needle-like antennas, catheters, and other monitoring equipment.
In their paper, Knavel et al. [20] provide a general discussion describing the above-mentioned techniques with their general principles, as well as giving information as to what technique to use under which circumstances. Other systems were presented in de Baere et al. [21], Hinshaw et al. [22], and Ryan et al. [23], where the authors present the most-used thermal ablation modalities and describe the advantages and disadvantages of using each technique to treat particular tumours.
It is important to note that ablation zone patterns, i.e., size and shape (oblong to spherical shapes) can vary depending on the device used, the applicator, and the tissue interactions. Therefore, a good understanding of the equipment is necessary to optimize each treatment. The size and shape of the ablation zone are dependent on the differences in the electromagnetic fields. Different time and power combinations create different ablation zone shapes and sizes. Performance can vary widely between systems, and there is still some room for improvement in thermal tumour ablation.
Various studies have demonstrated improvement in the effectiveness of thermal ablation. In a modelling study, Andreozzi et al. [24] presented results dealing with the application of pulsating heat sources applied to different tissue morphologies. They concluded that the advantages were that when using a pulsating heat source instead of a constant one, high temperature peaks were avoided, and at the same time, they achieved ablation of the same tumoural area as was achieved with a non-pulsing heat source. Along this line of thought of improving the target zone, although these authors used RF, Lim et al. [25] presented a study where four different types of RF waveform, i.e., half-sine, half-square, half-exponential, and damped-sine, were studied. With the use of numerical models, they concluded that their study provided information on the relationship between various RF waveforms and thermodynamic responses during the RFA process. Other researchers have used multiple antennas with various configurations for thermal ablation in their approach to improving ablation zones. In their investigation of the effects of single, double, and triple antenna configurations, Andreozzi et al. [26] used a mathematical model and concluded that the results obtained using multiple antennas offered potential for ablation zone enlargements. Trujillo-Romero et al. [27] evaluated the thermal performance of multi-antenna arrays with the goal of treating large bone tumours. With their model, they concluded that bone tumours of more than 3 cm3 could be treated using their antenna-array configuration.
MW-based ablation devices are relatively recent additions to the field. Paré et al. reported that it is possible to selectively heat one or more components of a material while leaving the others relatively cool [28], and that it is possible to selectively heat one phase of a multi-phase system while leaving other phases relatively cool [29,30,31,32]. While these techniques were initially developed to selectively destroy the microstructure of plant and animal tissues, their application can be transposed to in situ human tissues. Hence, this selective heating characteristic of MWs could have many important applications in the medical ablation field, and it is imperative to pursue the development of methods and apparatus that can take advantage of these properties. The advantages increase significantly when the selective heating can be augmented using chemical means as opposed to physical ones, as is the case with MW and other energy-based techniques. Taken collectively, there is a pressing need to harness the selective heating characteristics of MWs to enhance the efficiency of chemical ablation procedures and broaden the range of their applicability in the medical field, with special emphasis on the area of oncology [33,34].
In this study, a new approach combining MW ablation and chemical ablation is developed. Herein, a novel MW antenna concept, along with the use of ethanol as a susceptor, is presented, along with the preliminary results of this ablation technology, using MA-CA treatment on ex vivo bovine liver tissues.
2. Materials and Methods
This section will refer to the development of a “universal overall procedure” for various types of tissue. To determine the experimental conditions for the MA-CA, computer- assisted numerical models were obtained using commercial 3D electromagnetic (EM) simulation software. The applied parameters included dielectric conditions associated with the presence of a chemical agent in the target tissues simultaneously with the application of microwave energy. Although not reported here, these data were used to guide us in developing the ablation experimental conditions.
2.1. Microwaves in the Medical Field
The main benefit of utilizing MW energy resides in the fundamentally different method of transferring energy from the source to the sample. By directly delivering energy to MW-absorbing materials, conventional challenges such as long heating periods, establishing thermal gradients, and energy lost to the system’s environment can be avoided. Furthermore, the penetrating phenomenon of MW allows for the volumetric heating of samples.
The ability to heat specific materials depends on their dielectric properties. MWs are inherently unable to heat a combination of materials uniformly since different human tissues (e.g., liver and bones) all present different dielectric properties. Tissues that contain pathological areas, such as cancer, will also present different dielectric properties relative to the normal surrounding tissue. The unique attributes of MW energy have made the technology very attractive in medical applications as an alternative to conventional methods such as thermal heating or surgical ablations.
The goal of the technique is one of hyperthermia, where MW’s heat to cytotoxic conditions diseased tissues over healthy one. Natural blood flow serves as the basic cooling mechanism to avoid thermal diffusion to healthy tissues. Some applications of thermal ablation can be found in angioplasty, atrial fibrillation, benign prostatic hyperplasia, other urology-related disorders [35], dielectric blood coagulometry, stroke diagnostics [36], and drug efficiency enhancement.
In most cases, the utilization of MW energy has produced improved results compared to conventional methods while reducing heating time or reaction temperature. MW energy at 2.45 GHz is becoming the prime candidate for medical applications over 915 MHz and RF.
Until now, MW-assisted technology development has sought uniform heating—which is against the natural behaviour of MWs—and thus, efforts have mostly been devoted to developing “mechanical solutions”, which involve medical devices that remove the natural selective heating abilities of MWs [37]. No information relating to the development of dielectric-based methods and apparatus can therefore be found in the open access literature. However, the concept is beginning to make inroads. The goal of this research was to design better and more efficient dielectric-based heating strategies. As stated, it aims to make use of and capitalize on the unique selective heating characteristics of MWs by making use of microwave susceptors that are inert to human tissues and possess dielectric properties, such that enhanced temperature gradients will be created locally, while focusing the electric fields in the tissues to be treated. This is also important in the era of precision medicine, as each treatment can therefore be tailored to each patient.
2.2. Microwave-Assisted Chemical Ablation (MA-CA)
Microwave-Assisted Chemical Ablation refers to the use of MW energy combined with a chemical agent to achieve ablation. In this context, the chemical agent is called a susceptor. In our study, ethanol was chosen as the susceptor.
The use of electromagnetic susceptors to focus the energy on a predefined area is the central scientific theme on which our R&D activities are based. The incorporation of susceptors allows us to limit the loss of energy to surrounding healthy tissues. They may be chosen due to their individual properties, which allows us to enhance the temperature gradient created through the application of electromagnetic energy. Furthermore, the susceptors can be selected strategically to provide a combination of thermal and chemical ablation effects. For example, ethanol can be used as it possesses favourable thermal and dielectric properties while also having a toxicity threshold in living cells.
The energy emitting sources can be controlled in response to real-time measurements provided by the energy delivery devices. This allows the operator to maintain constant energy flow to the target area while the impedance properties change during the course of ablative treatment. Additionally, the energy delivery devices are mobile and can move in real time as the dielectric properties of the tissues evolve during treatment and thereby maintain conditions of constant impedance.
In the design reported here, the susceptor is delivered via the central shaft of the MW-emitting device. We found that this ensures minimal field perturbations as the material is introduced. In this case, we combine the chemical toxicity of ethanol with the thermal necrosis caused by the MW energy. Controlling the flow of the ethanol allows for a certain level of control of the impedance conditions in real time, and allows for a certain level of control of the temperature, thus further providing control of the impedance while providing some cooling of the microwave-emitting device.
2.3. Innovative Aspects of This MW-Assisted Ablation Technology Approach
Ethanol was chosen since it can be an effective chemical ablation substance on its own. However, when combined with MW energy, it becomes more effective. The toxicity of ethanol also increases as the temperature increases. Its permittivity varies from ca. 10 to 1 as the temperature varies from ca. 35 to ca. 80 degrees, which augments the field by a factor of ca. 5 over liver tissues (ε′ of ca. 45) at the ethanol introduction point. The loss factor (ε″) of ethanol is ca. 7.7 and varies little as the temperature varies from ca. 35 to its boiling point. Thus, its ability to heat remains constant until it reaches its boiling point; then, the surrounding tissues act as a natural microwave reflector.
The boiling point of ethanol (78 °C) is lower than that of water (100 °C); thus, the maximum temperature attainable during ablation will be lower. This property of ethanol is beneficial for ablation as it reduces the potential of off-target tissue damage due to thermal diffusion. Additionally, the heat capacity of ethanol (2.3 J/g·K) is about half that of water (4.2 J/g·K) which further reduces the potential for collateral damage due to thermal diffusion. Its density is about 80% that of water; thus, a smaller mass of ethanol is required per unit volume to be treated. The flow rate of ethanol allows a single protocol to adapt to the varying dielectric characteristics of tissues.
In MW ablation procedures, susceptors are used to enhance the heating speed and to increase the temperature gradient. Variable frequency is used to adapt to the tissues’ influence on field propagation, and a variable phase is used to adapt to the tissues’ influence on field patterns.
This technology comprises innovative features to enhance the efficiency of ablation procedures. As stated, one of these features is an additional channel in the antenna that is located directly in the centre of the coaxial emitting unit and used to deliver substances that enhance the ablation efficiency. Optionally, the antenna can comprise a resonator, with the latter enabling matching of the impedance of each tissue. This reduction in the loss of energy delivery also reduces the time required to complete the ablation process with reduced collateral damage.
For this research project, the antennas were designed specifically for the delivery of susceptors during the IR procedure. In certain cases, a susceptor may be chosen with the intent to improve the delivery of thermal energy; however, we believe that the added benefit of chemical toxicity may be advantageous in many applications. Ideally, an ablative approach would include a susceptor to project the field where required, and thus, offer additional thermal energy while allowing for enhanced control within the ablation zone. In many instances, the addition of a chemical ablation agent would result in the combined benefits of chemical toxicity and thermal necrosis. Another benefit is that once the ablation procedure is completed, the centre channel can then be used for injecting additional substances to accelerate the healing process or to enhance the long-term efficiency of the treatment, thus reducing the risk for recurrence. Specific examples of this would include the delivery of antibiotic prophylaxis and/or immune modulating agents.
2.4. Experimental Ex Vivo Ablation Conditions
The experimental set-up used for all the experiments is presented in Figure 1a. Figure 1b is a schematic representation of how the experimental set-up works. Bovine liver was used for the preliminary ex vivo ablative trials. Fresh beef liver was purchased from a local provider. All tissue samples for each set of experiments were cut from a single-animal liver in order to minimize variations in the dielectric properties of the various tissue samples. While this addresses the potential for inter-individual variations, it does not preclude that the placement of the ablation antenna may be affected by intra-tissue variations, such as the proximity of large empty blood vessels or fat pockets. These anatomical structures will be shown at the dissection step. During actual clinical procedures, these structures may be visualized and avoided using radiological imaging techniques such as ultrasound, computer tomography scanning, or magnetic resonance imaging.
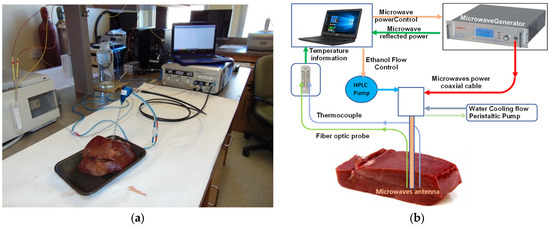
Figure 1.
(a) MA-CA experimental set up—real-time alcohol diffusion/ablation. Represented in the set up are: a data recorder, a microwave generator, an ablation antenna that is water cooled, thermocouples, fibre optics, and an HPLC pump to deliver the ethanol. (b) A schematic representation of the experimental set-up.
A commercial MW generator (SurBlate, Vison medical) was used for the preliminary evaluation of this novel MA-CA technology. Additionally, a commercially available cyst MW ablation probe (Vison Medical) was used as pre-beta prototype. The hollow lumens were used to dispense the chemical ablation agent into the target tissues simultaneously with the emission of the MW energy. The same antenna was used for each set of ablation conditions. After each experiment, ethanol was allowed to flow through the antenna at 5 mL/min to ensure that the channels were not clogged. Following this, the antenna was emptied of all residual ethanol using an air-filled syringe.
The ablation procedures were performed using the conditions presented in Table 1. Various experiments were carried out at different durations and power levels, with and without ethanol. The optimum conditions obtained were the ones presented in Table 1. Additionally, 100 W was the typical practical value, and the other conditions were used to analyze, in the presence or absence of ethanol, the impact on the distribution of power in the irradiated zone. We also used simulation software (Ansys HFSS) and observed that the results obtained from the ex vivo samples were very similar to the results obtained with the simulation software. The modelling results showed, in the presence of ethanol, that the electric field was significantly concentrated around the emitter compared to the scenario without ethanol. All experiments were performed using the same total energy of 12,000 Joules. The first two sets of experiments, i.e., experimental conditions 1 and 2, were used to test the technology under MWA conditions, and no ethanol was added. Under experimental conditions 3 and 4, different sequences and different ethanol volumes were used. Experimental results where more power and less time were used gave better results.

Table 1.
Ablation experimental conditions for MWA and MA-CA. These conditions are a sequence of power and time, maintaining a total energy of 12,000 Joules for each set of experiments, with or without ethanol.
For the elution of ethanol, volume and flow rate were selected to control the spatial evolution of the electrical field and the resulting ablative process over time. The volume of ethanol dispensed was calculated as a function of its thermal properties to offer good control over thermal diffusion to the surrounding non-diseased tissue, and thus, reduce the potential for collateral damage.
The effects of various parameters, including conventional ones such as power over time, were applied, along with MA-CA-specific ones such as volume of ethanol dispensed, and the ethanol elution rate was measured and monitored. The evolution of the temperature at various locations surrounding the emission point was monitored, as was the applied and reflected power over time as the ablation proceeded. The resulting ablation zones were characterized by the surface and volumetric measurements, colour, and consistency of the necrosed tissues.
After dissection, measurements of the ablation zones were performed using a Mitutoyo Digimatic CaliperTM (Made in Japan). Figure 2 shows the axis along which the measurements were taken, along with the placement of the fibre optics and thermocouples. Thermocouples are provided with the generator and used in medical procedures. We verified that they were not interfering with the field. Additionally, fibre optics were used as a backup to check the consistency of the measurements given by the thermocouples. A FlirTM C2 thermal imaging camera was used to record the thermal gradients of post-treatment dissected tissue. These measurements were performed within one minute after the end of the treatment itself.
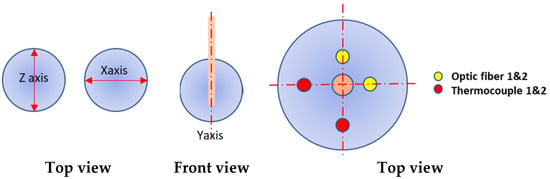
Figure 2.
Measurement of the ablation zone following MW delivery. Dimensions were measured along the x axis for the width, z axis for the height, and y axis for the length. Fibre optic probes and thermocouples were used to monitor the temperature during the ablation and were placed at 1 cm from the ablation probe.
3. Results
The measurements of the ablation results obtained for each of the cited conditions are reported in Table 2.

Table 2.
Ablation results—measurement of ablation zones along the x, y, and z axes for each set of conditions.
Ex Vivo Ablation Work
Figure 3, Figure 4, Figure 5 and Figure 6 are representative ablations created in ex vivo bovine liver tissues using MWA and MA-CA experimental conditions. Thermal and photographic imaging are presented. It is well known that the results of microwave ablation devices are influenced by the size and shape of the ex vivo sample [38]. For these experiments, the ex vivo sample always had dimensions large enough to proceed with multiple microwave ablations on the same piece of tissue. We observed that with ethanol present, the results gave a more spherical ablation zone, which confirms the modelling results. The form of impact mainly depends on the dielectric characteristics of the irradiated zone. These vary according to the density of the tissue, the temperature, and the density of the treated medium. They can also vary depending on the patient. The shape of the impact may also depend on the design of the ablation antenna. In fact, certain embodiments more or less limit field leakage along the body of the antenna, towards the surface of the treated tissue where the antenna is implanted. In the trials presented, the ablation zone was well defined, with a rubbery texture with a hard charred area. In some samples, some veins were present. Comments on the ablation zone shape are presented in Section 4.
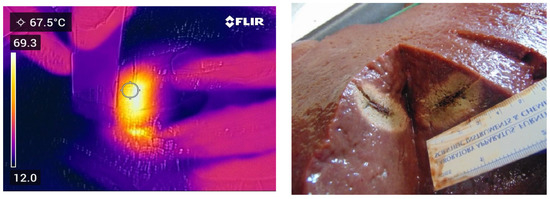
Figure 3.
Thermal and photographic imaging of ablation. Ablation conditions: 100 W for 120 s with no ethanol addition.
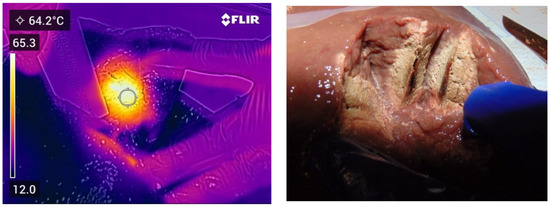
Figure 4.
Thermal and photographic imaging of ablation. Ablation conditions: 100 W for 30 s with no ethanol addition; 0 W for 60 s with no ethanol addition; and 100 W for 90 s with no ethanol addition.
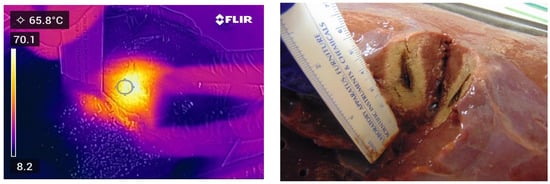
Figure 5.
Thermal and photographic imaging of ablation. Ablation conditions: 100 W for 30 s with no ethanol addition; 0 W for 60 s with no ethanol addition; 100 W for 30 s with no ethanol addition; and 100 W for 60 s with an elution of 5.0 mL of ethanol.
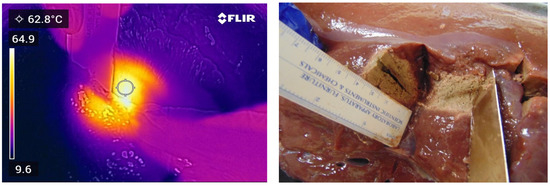
Figure 6.
Thermal and photographic imaging of ablation. Ablation conditions: 100 W for 30 s with no ethanol addition; 0 W for 60 s with no ethanol addition; 100 W for 30 s with no ethanol addition; 0 W for 30 s with elution of 2.5 mL of ethanol; and 100 W for 60 s with an elution of 5.0 mL of ethanol.
4. Discussion
In performing the ablation procedure, one of the key aims was to ensure adequate control of the ethanol elution in order to maintain good control over the ablated volume. In the ablations performed with ethanol, we first created a small cavity in the tissue before dispensing the ethanol. The cavity was formed by applying microwave power via the ablation antenna for a determined period of time prior to beginning the addition of the ethanol. The strategy was to create a small “empty” sphere surrounded by necrosed tissues. The latter were hard and used to control, or at least limit, the diffusion of the ethanol when it was being dispensed. We found that there were numerous advantages to this method. From empirical data, we know the size of the cavity formed by this initial energy impulse. The ethanol can be added prior to or while applying the power. The ethanol diffusion away from the dispensing location is greatly hindered, thus reducing the potential for harming tissues that are remote from the target area. We know that we induced a significantly lower value of permittivity at the microwave-emitting point, thus effectively creating a reflection “mirror” from the untreated tissues surrounding the ethanol-filled cavity; this would lead to more spherical ablation zones and reduced collateral damage to healthy tissue.
All trials were performed using a single pre-beta antenna type. This probe was equipped with an additional hollow lumen surrounding the MW-emitting coaxial antenna. While this is not the optimal design configuration, it allowed for preliminary validation of the theory. In our view, the optimal configuration would incorporate a hollow lumen at the very centre of the MW-emitting coaxial antenna.
A single application of MW power of 100 W (120 s) with no addition of ethanol was performed as a basic reference; a non-spherical ablation zone was created showing elongation along the shaft of the antenna. This is typical of an antenna showing significant reflection. All the following comparisons were made with respect to this trial.
A test was performed where a MW power of 100 W was applied for 30 s, followed by a period of 60 s with no applied power, and then, followed by another application of 100 W for 90 s (same 12,000 Joules applied) with no addition of ethanol. This demonstrates the effect resulting from the pause period, which caused the tissue to harden and contract. An enlarged non-spherical ablation zone was created showing elongation along the shaft of the antenna, which is also typical of an antenna showing significant reflection.
A test with similar MW power parameters to the previous one was performed, this time with 5.0 mL of ethanol being introduced during the final application of MW energy (60 s) (same energy of 12,000 Joules). The ablation zone was enlarged and revealed improved sphericity. Energy reflection along the antenna path was still visible.
A multi-step test with an initial MW power of 100 W for 30 s, followed by a pause period of 60 s, and then, 100 W of MW for 30 s, followed by no power for 30 s and the addition of 2.5 mL of ethanol during that period, and a final MW application of 100 W for 60 s with a further addition of 5.0 mL of ethanol during that period (same energy of 12,000 Joules). These conditions produced a much larger ablation zone, better sphericity, and clear evidence of the limiting effect of ethanol on thermal runaway.
Some of the main findings in these experiments were as follows:
- Typical experiments were performed for a single value of applied power (100 W) and applied energy (12,000 Joules)—only the sequence of the applied power differed.
- The volume of the ablated tissue was calculated as an ellipsoid (not a sphere), although MA-CA was closer to a sphere than MWA alone.
- The ablated volumes of MA-CA were larger than MWA alone by 20–50%.
- The volumes of ethanol used were small, at less than 10 mL. This was important as it led to no significant loss of ethanol outside the target zone. This is also an important factor when in vivo ablation procedures are performed.
We also experienced issues with the antennas. In our pre-beta prototype antenna, the lumen for dispensing the ethanol was not in the very centre of the antenna; thus, we obtained significantly distorted microwave-emitting patterns. The antenna also lost efficiency over time; therefore, this affected the quality of the data in terms of reproducibility. The current antenna design is not optimized for the approach presented, but eventually, with technological developments, an antenna that meets these needs may emerge.
The impact of the chemical ablation agent on the procedure is significant, as stated earlier. The idea is for the ablation antenna to always “see” an area whose dielectric characteristics are kept almost constant. By using ethanol, the emitting antenna always sees a mixture of susceptor/malignant tissue whose dielectric characteristics are kept approximately stable. This dampens the dielectric variations in the tissues under the temperature increase effect, which itself is limited to the evaporation temperature of the alcohol, which is always higher than the lethal temperature of the cells.
5. Conclusions
A novel MW-assisted tissue ablation procedure is described. Its design incorporates the introduction of a chemical ablation agent at the emission point of the MW antenna. Its design parameters were established and an assessment of its efficiency relative to current MW-assisted ablation procedures was performed using ethanol as the chemical ablation agent. Using varied conditions of energy and ethanol delivery, we observed significant improvement in the desired ablation effect. These results suggest that the technique can lead to improved control over the ablation zone while reducing the collateral damage, thus increasing the precision of the minimally invasive intervention. MA-CA is a dielectric-based method that selectively and safely treats affected tissues, irrespective of the nature of the tissues.
6. Patents
Patents resulting from the work reported in this manuscript [33,34]:
Atlantic Cancer Research Institute, Inventors: Paré, J.R.J.; Bélanger, J.M.R.; Microwave-Assisted Medical Technologies and Apparatus Therefor, Canadian Patent No. 3,017,029 (3 November 2020); Japanese Patent No. 6,854,827 (18 March 2021).
Author Contributions
Conceptualization, J.R.J.P., J.M.R.B. and J.-F.R.; formal analysis, J.R.J.P., J.M.R.B., D.F., A.T. and J.-C.S.; funding acquisition, J.R.J.P.; investigation, J.R.J.P., A.T., J.-C.S. and J.-F.R.; methodology, J.R.J.P., J.M.R.B., G.C., D.F., A.T., J.-C.S. and J.-F.R.; project administration, J.R.J.P.; resources, J.R.J.P.; supervision, J.R.J.P., J.M.R.B., G.C. and D.F.; validation, J.R.J.P., J.M.R.B. and G.C.; writing—original draft, J.M.R.B.; writing—review and editing, J.M.R.B., G.C., D.F., A.T., J.-C.S. and J.-F.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the following organizations: J.R.J.P. was the recipient of the New Brunswick Innovation Foundation (NBIF) Innovation Research Chair in Medical Technologies, funded by the NBIF, and the NBIF-RIF contribution 2016-039-RIT, as well as funding from the ACOA Business Development Project (BDP) of the Atlantic Canada Opportunity Agency (ACOA) 210900.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We are indebted to the Atlantic Cancer Research Institute (ACRI) host organization’s Chair and its Founder and Senior Scientist Rodney Ouellette. We thank Vison Medical (China) and its Canadian subsidiary MIMA-Pro Scientific, Inc., and Sairem (France) who graciously provided the equipment. We also thank R. Ouellette for his valuable and constructive feedback on the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Uzel, R.A. Microwave-Assisted Green Extraction Technology for Sustainable Food Processing. In Emerging Microwave Technologies in Industrial, Agricultural, Medical and Food Processing; You, K.Y., Ed.; Chapter 9; IntechOpen: Rijeka, Croatia, 2018. [Google Scholar] [CrossRef]
- Moret, S.; Conchione, C.; Srbinovska, A.; Lucci, P. Microwave-Based Technique for Fast and Reliable Extraction of Organic Contaminants from Food, with a Special Focus on Hydrocarbon Contaminants. Foods 2019, 8, 503. [Google Scholar] [CrossRef] [PubMed]
- Khalkhali, R.; Peyravi, A.; Hashisho, Z.; Choi, P. Microwave-Assisted Removal of Cyclohexane from Oil Sands Gangue. Ind. Eng. Chem. Res. 2022, 61, 6611–6617. [Google Scholar] [CrossRef]
- Bagade, S.B.; Patil, M. Recent Advances in Microwave Assisted Extraction of Bioactive Compounds from Complex Herbal Samples: A Review. Crit. Rev. Anal. Chem. 2021, 51, 138–149. [Google Scholar] [CrossRef] [PubMed]
- Sadeghi, A.; Hakimzadeh, V.; Karimifar, B. Microwave Assisted Extraction of Bioactive Compounds from Food: A Review. Int. J. Food Sci. Nutr. Eng. 2017, 7, 19–27. [Google Scholar]
- Valizadehderakhshan, M.; Shahbazi, A.; Kazem-Rostami, M.; Todd, M.S.; Bhowmil, A.; Wang, L. Extraction of Cannabinoids from Cannabis sativa L. (Hemp)—Review. Agriculture 2021, 11, 384. [Google Scholar] [CrossRef]
- Hernàndez, J.I.; Cepeda, M.F.J.; Valdés, F.; Guerrero, G.D. Microwave Ablation: State-of the-Art Review. Onco Targets Ther. 2015, 8, 1627–1632. [Google Scholar]
- Meloni, M.F.; Chiang, J.; Laeseke, P.F.; Dietrich, C.F.; Sannino, A.; Solbiati, M.; Nocerino, E.; Brace, C.; Lee, F.T. Microwave ablation in primary and secondary liver tumours: Technical and clinical approaches. Int. J. Hyperth. 2017, 33, 15–24. [Google Scholar] [CrossRef]
- Pusceddu, C.; Sotgia, B.; Fele, R.M.; Ballicu, N.; Melis, L. Combined Microwave Ablation and Cementoplasty in Patients with Painful Bone Metastases at High Risk of Fracture. Cardiovasc. Interv. Radiol. 2016, 39, 74–80. [Google Scholar] [CrossRef]
- Korkusuz, Y.; Mader, O.M.; Kromen, W.; Happel, C.; Ahmad, S.; Gröner, D.; Koca, M.; Mader, A.; Grünwald, F.; Korkusuz, H. Cooled microwave ablation of thyroid nodules: Initial experience. Eur. J. Radiol. 2016, 85, 2127–2132. [Google Scholar] [CrossRef]
- Chiang, J.; Cristescu, M.; Lee, M.H.; Moreland, A.; Hinshaw, J.L.; Lee, F.T.; Brace, C. Effects of Microwave Ablation on Arterial and Venous Vasculature after Treatment of Hepatocellular Carcinoma. Radiology 2016, 281, 617–624. [Google Scholar] [CrossRef]
- Xiong, L.; Dupuy, D.E. Lung Ablation Whats New? J. Thorac. Imaging 2016, 31, 228–237. [Google Scholar] [CrossRef]
- Smolock, A.R.; Cristescu, M.M.; Potretzke, T.A.; Ziemlewicz, T.J.; Lubner, M.G.; Hinshaw, J.L.; Brace, C.L.; Lee, F.T., Jr. Microwave Ablation for the Treatment of Hepatic Adenomas. J. Vasc. Interv. Radiol. 2016, 27, 244–249. [Google Scholar] [CrossRef]
- Gao, Y.; Liang, P.; Yu, X.; Yu, J.; Cheng, Z.; Han, Z.; Duan, S.; Huang, H. Microwave Treatment of Renal Cell Carcinoma Adjacent to Renal Sinus. Eur. J. Radiol. 2016, 85, 2083–2089. [Google Scholar] [CrossRef]
- Jiang, X.W.; Gao, F.; Ma, Y.; Feng, S.F.; Liu, X.L.; Zhou, H.K. Percutaneous Microwave Ablation in the Spleen for Treatment of Hypersplenism in Cirrhosis Patients. Dig. Dis. Sci. 2016, 61, 287–292. [Google Scholar] [CrossRef]
- Egashira, Y.; Singh, S.; Bandula, S.; Illing, R. Percutaneous High-Energy Microwave Ablation for the Treatment of Pulmonary Tumors: A Retrospective Single-Center Experience. J. Vasc. Interv. Radiol. 2016, 27, 474–479. [Google Scholar] [CrossRef]
- Cristescu, M.; Abel, E.J.; Wells, S.; Ziemlewicz, T.J.; Hedican, S.P.; Lubner, M.G.; Hinshaw, J.L.; Brace, C.L.; Lee, F.T., Jr. Percutaneous Microwave Ablation of Renal Angiomyolipomas. Cardiovasc. Interv. Radiol 2016, 39, 433–440. [Google Scholar] [CrossRef]
- Smolock, A.R.; Lubner, M.G.; Ziemlewicz, T.J.; Hinshaw, J.L.; Kitchin, D.R.; Brace, C.L.; Lee, F.T., Jr. Microwave Ablation of Hepatic Tumors Abutting the Diaphragm Is Safe and Effective. Am. J. Roentgenol. 2015, 204, 197–203. [Google Scholar] [CrossRef]
- Belfiore, M.P.; Sciandra, M.; Romano, F.; Tartaglione, T.; De Lucia, G.; Volpe, T.D.; Buonomo, C.; Cappabianca, S.; Rotondo, A.; Belfiore, G. Preliminary Results in Unresectable Head and Neck Cancer Treated by Radiofrequency and Microwave Ablation: Feasibility, Efficacy, and Safety. J. Vasc. Interv. Radiol. 2015, 26, 1189–1196. [Google Scholar] [CrossRef]
- Knavel, E.M.; Brace, C.L. Tumor Ablation: Common Modalities and General Practices. Tech. Vasc. Interv. Radiol. 2013, 16, 192–200. [Google Scholar] [CrossRef]
- de Baere, T.; Deschamps, F. New tumor ablation techniques for cancer treatment (microwave, electroporation). Diagn. Interv. Imaging 2014, 95, 677–682. [Google Scholar] [CrossRef]
- Hinshaw, J.L.; Lubner, M.G.; Ziemlewicz, T.J.; Lee, F.T.; Brace, C.L. Percutaneous Tumor Ablation Tools: Microwave, Radiofrequency, or Cryoablation—What Should You Use and Why? Vasc. Interv. Radiol. 2014, 34, 1344–1362. [Google Scholar] [CrossRef] [PubMed]
- Ryan, M.J.; Willatt, J.; Majdalany, B.S.; Kielar, A.Z.; Chong, S.; Ruma, J.A.; Pandya, A. Ablation Techniques for Primary and Metastatic Liver Tumors. World J. Hepatol. 2016, 8, 191–199. [Google Scholar] [CrossRef] [PubMed]
- Andreozzi, A.; Brunese, L.; Iasiello, M.; Tucci, C. Numerical Analysis of the Pulsating Heat Source Effects in a Tumor Tissue. Comput. Methods Programs Biomed. 2021, 200, 105887. [Google Scholar] [CrossRef] [PubMed]
- Lim, D.; Namgung, B.; Woo, D.G.; Choi, J.S.; Kim, H.S.; Tack, G.R. Effect of Input Waveform Pattern and Large Blood Vessel Existence on Destruction of Liver Tumor Using Radiofrequency Ablation: Finite Element Analysis. J. Biomech. Eng. 2010, 132, 061003-1. [Google Scholar] [CrossRef]
- Andreozzi, A.; Brunese, L.; Iasiello, M.; Tucci, C.; Vanoli, G.P. A Novel Local Thermal Non-Equilibrium Model for Biological Tissue Applied to Multiple-Antennas Configurations for Thermal Ablation. Numer. Heat Transf. Part A Appl. 2021, 79, 111–121. [Google Scholar] [CrossRef]
- Trujillo-Romero, C.J.; Merida, J.D.; Ramirez-Guzman, T.J.; Martinez-Valdez, R.; Leija-Salas, L.; Vera-Hernandez, A.; Rico-Martinez, G.; Flores-Cuautle, J.J.A.; Gutierrez-Martinez, J.; Sacristan-Rock, E. Thermal Evaluation of Multi-Antenna Systems Proposed to Treat Bone Tumors: Finite Element Analysis. Sensors 2022, 22, 7604. [Google Scholar] [CrossRef]
- Paré, J.R.J.; Sigouin, M.; Lapointe, J.; Her Majesty the Queen in right of Canada, as represented by the Minister of the Environment, Ottawa, Canada. Microwave Assisted Natural Products Extraction. U.S. Patent 5,002,784, 26 March 1991. [Google Scholar]
- Paré, J.R.J.; Her Majesty the Queen in right of Canada, as represented by the Minister of the Environment, Ottawa, Canada. Microwave-Assisted Generation of Volatiles, of Supercritical Fluid, and Apparatus Therefor. U.S. Patent 5,377,426, 3 January 1995. [Google Scholar]
- Paré, J.R.J.; Her Majesty the Queen in right of Canada, as represented by the Minister of the Environment, Ottawa, Canada. Microwave-Assisted Generation of Volatiles, of Supercritical Fluid, and Apparatus Therefor. U.S. Patent 5,519,947, 28 May 1996. [Google Scholar]
- Paré, J.R.J.; Her Majesty the Queen in right of Canada, as represented by the Minister of the Environment, Ottawa, Canada. Microwave-Assisted Separations Using Volatiles. U.S. Patent 5,675,909, 14 October 1997. [Google Scholar]
- Paré, J.R.J.; Her Majesty the Queen in right of Canada, as represented by the Minister of the Environment, Ottawa, Canada. Microwave-Assisted Separation using Volatiles, and Apparatus Therefor. U.S. Patent 5,732,476, 31 March 1998. [Google Scholar]
- Paré, J.R.J.; Bélanger, J.M.R.; Atlantic Cancer Research Institute. Microwave-Assisted Medical Technologies and Apparatus Therefore. Canadian Patent No. 3,017,029, 3 November 2020. [Google Scholar]
- Paré, J.R.J.; Bélanger, J.M.R.; Atlantic Cancer Research Institute. Microwave-Assisted Medical Technologies and Apparatus Therefore. Japanese Patent No. 6,854,827, 18 March 2021. [Google Scholar]
- Clark, T.; Sabharwal, T.; Lee, M.J.; Watkinson, A.F. (Eds.) Interventional Radiology Techniques in Ablation; Springer: London, UK, 2013; pp. 1–188. [Google Scholar]
- Persson, M.; Fhager, A.; Dobsicek Trefna, H.; Yu, Y.; McKelvey, T.; Pegenius, G.; Karlsson, J.-E.; Elam, M. Microwave-Based Stroke Diagnosis Making Global Prehospital Thrombolytic Treatment Possible. IEEE Trans. Biomed. Eng. 2014, 61, 2806–2817. [Google Scholar] [CrossRef]
- Paré, J.R.J.; Bélanger, J.M.R.; Punt, M.M.; Her Majesty the Queen in right of Canada as represented by the Minister of the Environment, Ottawa, Canada. Controlled Energy Density Microwave-Assisted Processes. U.S. Patent 6,061,926, 16 May 2000. [Google Scholar]
- Cavagnaro, M.; Amabile, C.; Cassarino, S.; Tosoratti, N.; Pinto, R.; Lopresto, V. Influence of the Target Tissue Size on the Shape of ex vivo Microwave Ablation Zones. Int. J. Hyperth. 2015, 31, 48–57. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).