Exploring the Potential of Microextraction in the Survey of Food Fruits and Vegetable Safety
Abstract
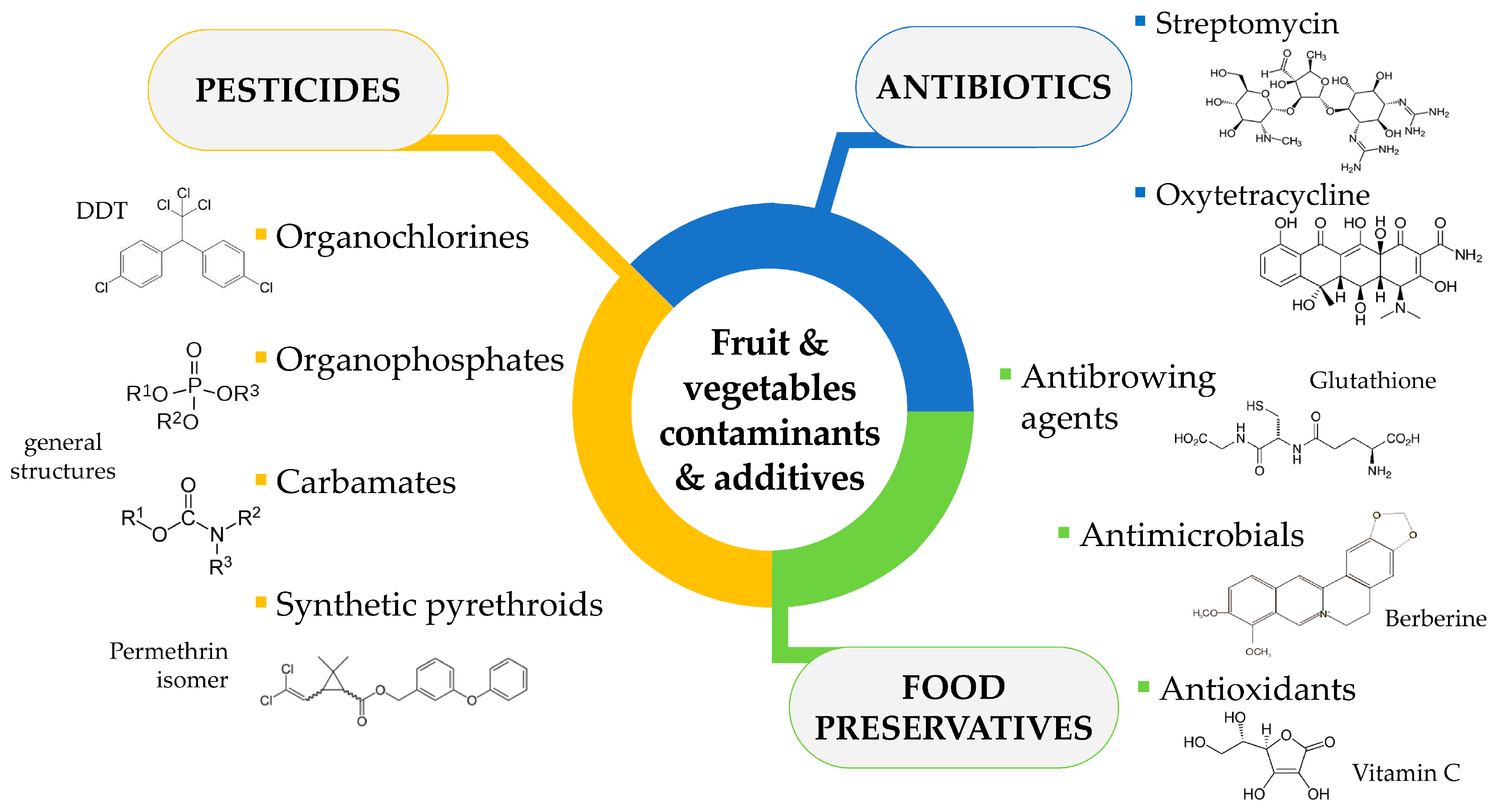
1. Introduction
1.1. Food Safety—Major Contaminants of Fresh Fruits and Vegetables and Their Toxicity
1.1.1. Pesticides
1.1.2. Antibiotics
1.1.3. Food Preservatives
1.2. The Current Food Safety Regulatory Framework
2. Extraction Approaches Used to Assess Food Safety
2.1. Conventional Extraction Approaches
| Extraction Method | Description/Analytical Technique | Contaminants/LODs | Matrix | Ref. |
|---|---|---|---|---|
| Pesticides | ||||
| SLE/SALLE-dSPE | 5 g spiked sample; 10 mL phosphate buffer (pH 6.7); ACN/H2O (83:17; v/v); NaCl/Na2SO4 (40.50; w/w); upper layer collection; dry. Clean-up step: redissolved (10 mL H2O); Florisil cartridge; elution (20 mL DCM); dry; resuspension (1 mL MP)/HPLC-DAD | Thiacloprid/0.03 µg mL−1 | Citrus, guava, tomato, cauliflower, and okra | [74] |
| SLE/LTP | 4 g sample; 4 mL ACN; agitation (10 min); centrifugation (3 min); freezer (8.5 h)/GC-ECD | Azoxystrobin, difenoconazole, chlorothalonil/0.12–1.73 mg kg−1 | Bell peppers | [77] |
| SLE/SPE | 15 g of sample; 15 mL of 0.1% ac ACN; 2 min vortex; addition of MgSO4, NaCl, CH3NaO2; 2 min vortex; 5 min centrifugation; 400 mg activated charcoal; 600 mg PSA; evaporation; reconstitution (1 mL n-hexane)/GC-MS | 30 pesticides/0.019–0.5 mg kg−1 | Peach | [75] |
| SLE/LLE-SPE | A 150 g sample; 300 mL ACN and 25 g celite; 120 mL PE; shaking; 12 mL NaCl saturated solution and H2O; upper layer extraction. Preconcentration: Florisil cartridge; 200 mL PE and DEE (50:50; v/v) elution/UPLC-MS/MS | Carbaryl/0.0003 µg mL−1 | Lettuce, cucumber, and spinach | [76] |
| SLE/SPE-MTMOS-CPTES | A 0.5 g sample; 2 mL MeOH + acetone (1:1, v/v); 1 mL NaCl (10%; w/v); H2O; 1 mL of sample through the cartridge; elution (1 mL EtAc); dry; reconstitution (100 µL ACN)/GC-MS | Malathion, chlorpyrifos, profenofos/0.01–0.07 μg mL−1 | Red apple and purple grape | [79] |
| SLE/SPE | A 50 g sample; centrifugation 10 min; mix the precipitate with 5 mL ACN; vortex 5 min; centrifugate; supernatant collection and dilution (H2O); 150 mL through SPE cartridge; elution (0.9 mL ACN)/HPLC-DAD | Metoxuron, monuron, chlortoluron, monolinuron, buturon/0.06–0.15 ng g−1 | Tomato | [102] |
| SLE/ILPR-SPE | A 20 g sample; centrifugation 15 min; acetate solution; supernatant freeze-dried overnight; redissolved (20 mL MeOH); dry; redissolved (20 mL H2O); 1 mL sample through ILPR cartridge; elution (9:1 v/v, 1.5 mL); dry; redissolved (0.5 mL MP)/HPLC-UV | Thidiazuron, forchlorfenuron/0.00169–0.00195 µg g−1 | Cucumber | [103] |
| SLE/LTP | A 4 g sample; 4 mL ACN; agitation (10 min); centrifugation (3 min); freezer (6 h); organic liquid phase collection; spike/GC-ECD | Azoxystrobin, difenoconazole, chlorothalonil/1.1–3.5 μg kg−1 | Bell peppers | [104] |
| SFE | A 0.3 g sample; 1.5 g Na2SO4; 25% (v/v) MeOH/SC-CO2 at 80 °C; 25 MPa for 5 min for dynamic extraction; ODS column; elution (5 mL acetone); dry; resuspension (1 mL MeOH, and 3 mL ACN/toluene (3:1; v/v))/LC-MS | Acetamiprid, dinotefuran, imidacloprid, and thiamethoxam/--- | Green onion | [80] |
| UAE-DES/MSPE | A 0.2 g sample; 2 mL DES (proline and propylene glycol at 1:3 M ratio); 50 min US; 5 min centrifugation; 10 mL dilution (H2O); pH 8.0; 25 mg of MWCNTs addition; dried; ACN resuspension/HPLC-UV | Fipronil, metalaxyl, paclobutrazol, myclobutanil, napropamide, thiacloprid, penconazole/0.02–0.05 μg mL−1 | Apple, pear, carrot, and cucumber | [84] |
| UAE-DES/LLE | A 0.5 g sample; DES (glycerol-proline 5 at 9:4 ratio); 1 min vortex; 20 min US; 5 min centrifugation; supernatant collection; 1 mL PE; 20 s vortex; 3 min centrifugation; PE elimination; re-extraction (DCM); DCM layer collection; dry; resuspended in MeOH/HPLC-DAD | Carbendazim, thiophanate-methyl, imidacloprid/0.05–0.2 µg mL−1 | Apple, grape, and tomato | [85] |
| QuEChERS/dSPE | A 10 g sample; 10 mL of ACN with 0.1% AcAc; shaken for 1 min manually; 4.0 g of MgSO4 and 1.7 g of NaOAC; shaken for 1 min with vortexing; centrifugation (4000 rpm, 8 min); 2 mL of supernatant; 150 mg of MgSO4 and 50 mg of PSA; centrifugation (4000 rpm, 5 min)/HPLC-HRMS (1 mL) | 82 pesticides/0.3–5 µg kg−1 | Organic carrots (pesticide-free) | [87] |
| QuEChERS/dSPE-IL-DLLME | IL-DLLME: 1 mL sample from dSPE clean-up, 10% NaCl (w/v) in 9 mL distilled H2O, 130 μL of IL (5 min; 7000 rpm stirring)/LC-MS/MS (100 μL) | Multi-pesticide residues/0.02–0.32 µg kg−1 | Bananas, oranges, cabbage, tomato, and onions | [105] |
| QuEChERS/dSPE (a) QuEChERS/d-SLE (b) | A (a) 2 g sample; 5 mL H2O (0.1% FAc); 10 mL ACN; agitation (5 min); 2.5 g NH4HCO2; agitation (2 min); centrifugation (5 min); dSPE: 1 mL supernatant + 150 mg MgSO4 + 50 mg PSA + 50 mg C18 + 7.5 mg MWCNTs; vortex (1 min); centrifugate (3 min); LC-MS/MS; (b) 4 mL ACN (remaining steps are the same); d-SLE: 0.1 mL supernatant + 100 mg C18 + 0.9 mL H2O; agitation (1 min); centrifugation (2 min); supernatant + 50 mg MgSO4 + 1 mL ACN agitation (1 min); centrifugate (2 min)/LC-MS/MS | 34 pesticides/(a) 0.5–1 μg kg−1; (b) 0.5–2.5 μg kg−1 | Black peppers | [88] |
| QuEChERS/DLLME-SFO | n-hexadecane added to the PSA mixture; US extraction and centrifugation (40 °C); solidification of the organic phase in ice bath; high-speed centrifugation of supernatant/GC-MS (1 μL) | Malathion, chlorpyrifos, parathion, bifenthrin, cyhalothrin, permethrin, fenvalerate, deltamethrin/0.3–1.5 μg kg−1 | Lettuce, long bean, broccoli, tomato, carrot, pumpkin, siew pak choy, sweet choy sum, sweet pak choy, celery, amaranth, spinach, cabbage, mushroom, and cucumber | [106] |
| QuEChERS/HS-SPME | PDMS (DDT) and PDMS/DVB (HCH) fibre extraction (headspace 5 min (DDT) and 30 min (HCH), 80 °C, 1000 rpm stirring)/GC-MS | DDT congeners/0.1 µg kg−1 Four HCH isomers/0.15–0.45 µg kg−1 | Khat | [94] |
| QuEChERS/SPE | A 2 g sample; 10 mL ACN, 4 g MgSO4, 1 g NaCl; 2 min vortex; 5 min centrifugation; re-extraction; dry; 2 mL PBS (pH 8.0); MIP-SPE cartridge; elution with 3 mL MeOH followed by 2 mL MeOH/AcAc (90:10, v/v); dry and reconstituted (ACN)/LC-MS/MS | 29 pesticides/0.005–0.07 μg L−1 | Corn, soybean oil, cucumber, and pear | [107] |
| QuEChERS (a) SLE/dSPE (b) | A (a) 10 g sample; (10 mL H2O in oranges); 10 mL ACN; 4 g MgSO4 + 1 g NaCl; agitation (1 min); centrifugation (10 min); UHPLC-Q-Orbitrap-MS2; (b) 10 g sample; (10 mL H2O in orange samples); 10 mL ACN (or 10 mL ACN containing 1% (v/v) FAc; agitation (1 min); centrifugation (10 min); (dSPE clean-up in oranges)/UHPLC-Q-Orbitrap-MS2 | 21 triazoles and 5 pesticides metabolites/0.05–2 mg kg−1 | Orange, grape, courgette, and strawberry | [108] |
| QuEChERS/dSPE | A 10 g sample; 10 mL ACN; agitation (10 min); agitation (1 min); 4 g MgSO4 + 1 g NaCl + 1 g C6H9Na3O9 + 0.5 g C6H8Na2O8; agitation (1 min); centrifugation (5 min); 2 mL supernatant + 25 mg PSA + 5 mg GCB + 150 mg MgSO4; vortex (30 s); centrifugation (2 min); dry; redissolve (acetone)/GC-MS/MS | 164 pesticides/--- | Basil, broccoli, Chinese cabbage, chives, dill, kale, leek, lettuce, parsley, and spinach | [89] |
| QuEChERS/dSPE | A 10 g sample; 0.1% FAc in ACN; agitation; 1.5 g NaOAC + 6 g MgSO4; agitation (1 min); centrifugation (5 min); 1 mL supernatant + 25 mg PSA + 150 mg MgSO4; vortex (1 min); centrifugation (5 min)/LC-MS/MS | 287 pesticides/--- | Mandarin orange and grapefruit | [90] |
| QuEChERS/dSPE | A 10 g sample; 10 mL ACN; agitation (10 min); 4 g MgSO4 + 1 g NaCl + 0.5 g C6H5Na3O7 + 1 g C6H8O7; vortex (1 min); centrifugation (2 min); 2 mL supernatant + 25 mg PSA + 5 mg GCB + 150 mg MgSO4; vortex (30 s); centrifugation (2 min)/LC-MS/MS | 45 pesticides/0.02–1.90 µg kg−1 | Banana, grape, strawberry, peach, kiwifruit, plum, pepper, cabbage, eggplant, cucumber, tomato, leek, cowpea, and lettuce | [91] |
| QuEChERS/dSPE | A 5 g sample; 20 mL ACN (+10 mL H2O for longan and Chinese cabbage); agitation (2 min); 4 g MgSO4 + 1 g NaCl + 0.25 g C6H6Na2O7 + 0.5 g C6H5Na3O7; agitation (1 min); centrifugation (5 min); 2 mL supernatant + 150 mg C18 + 150 mg MgSO4; agitation (1 min); centrifugation (5 min)/HPLC-MS/MS | Indaziflam, spirotetramat, cyantraniliprole, and their metabolites/0.3−1.5 μg kg−1 | Papaya, litchi, grape, longan, cucumber, and Chinese cabbage | [92] |
| QuEChERS/dSPE | A 5 g sample; 10 mL ACN; vortex (5 min); 4 g NaCl; agitation (1 min); centrifugation (5 min); 1 mL supernatant + 20 mg GCB; vortex (1 min); stand (1 min)/LC-MS/MS | Methoxyfenozid, chlorantraniliprole, indoxacarb, lufenuron, and chlorfenapyr/3.0 mg kg−1 | Spinach | [93] |
| Antibiotics | ||||
| UAE/SPE | A 1 g sample + 10 mL MeOH + 15 min US; centrifugation 15 min; supernatant collection; evaporation to 1 mL; + 10 mL H2O; Strata-X cartridge; elution with 2 mL MeOH; dry; reconstitution (H2O); spiked/UPLC-TQ | A total of 10 antibiotics (fluoroquinolones, sulfonamides, lincosamides, and metoxybenzylpyrimidines) and 6 of their metabolites/0.1–5.8 ng g−1 | Lettuce, tomato, cauliflower, and broad beans | [95] |
| UAE/SPE | A 1 g sample; spiked; 10 mL MeOH/H2O (4:1, v/v) acidified with 0.2% FA; 10 min US; centrifugation 10 min (*repeated two more times but in the second step, DisQue salt was added); evaporated to 6 mL; Oasis PRiME HLB cartridge; eluate was dried and reconstituted with 0.5 mL MeOH/UHPLC-MS/MS | 81 antibiotics/0.001–0.26 ng g−1 | Banana and cabbage | [9] |
| UAE/SPE | A 0.25–0.5 g sample; lyophilized; 3 mL 20% w/v Mg(NO3)2 + 2% v/v NH3; 10 min (×2) US; spiked; diluted to 25 mL (H2O); pH 3; HLB cartridges; elution (2 × 2.5 mL 0.1% FAc-MeOH (80:20, v/v))/HPLC-MS/MS | Marbofloxacin, levofloxacin, norfloxacin, ciprofloxacin, danofloxacin, enrofloxacin, and orbifloxacin/1–3 ng g−1 | Spinach, lettuce, and cucumber | [96] |
| Preservatives | ||||
| SLE | A2 g sample; 2 × 5 mL of MeOH solvent; sonication (30 min); centrifugation; supernatant elimination; addition of 5 mL of MeOH to the extracts; filtration/HPLC-PDA | Parabens (P)—MeP, EtP, PrP, BuP, and isoBuP/0.005–0.05 µg g−1 | Fruits and vegetables from the local market in Riyadh, Saudi Arabia | [98] |
| SLE | Sample-25% EtOH (solvent); stirring (1 h) at 45 °C; centrifugation (15 min); supernatant collection; dry; resuspension (H2O)/HPLC-DAD | Gallic acid, rutin, and quercetin/--- | Tomato | [99] |
| UAS/SPE | A 2 g sample; 6 mL ACN; homogenization; 10 min US; centrifugation (10 min); supernatant collection; dry to 200 µL; reconstitution (10 mL H2O); pH 4; SPE cartridge; elution (400 µL ACN); evaporation to 25 µL; 70 µL + 1% TMCS mixture for derivatization/GC-MS | A total of 24 EDCs including alkylphenols, BPA, phenylphenols, parabens, organophosphorus pesticides and triclosan/--- | Banana, apple, pear, kiwi, orange, mandarin orange, lemon, potato, onion, garlic, tomato, carrot, zucchini, eggplant, lettuce, pepper, white turnip, and cucumber | [100] |
| QuEChERS/dSPE | Juices vortexed (5 min) and centrifugated (5 min); 5 mL supernatant; pH 6; 1.5 g MgSO4 + 250 mg NaCl; vortex (1 min); 4 mL ACN; vortex (5 min); centrifugation (10 min); collection (1 mL); 200 mg PSA; vortex (1 min); centrifugation (5 min)/GC-MS/MS | PG, TBHQ, NDGA, 3-BHA, 2-BHA, 2,6-BHP, 3,5-BHT, 4-HRC, FA, LG, and OG/8.14–25.45 ng L−1 | Fruit juice samples (apple, pineapple, mango, litchi, orange, and mixed fruit) | [101] |
2.2. Microextraction Approaches
2.2.1. Sorbent-Based Microextraction Techniques
2.2.2. Liquid-Based Microextraction Techniques
2.2.3. The Potential of Microextraction Approaches
| Extraction Method | Description/Analytical Technique | Contaminants/LODs | Matrix | Ref. |
|---|---|---|---|---|
| Pesticides | ||||
| DI-SPME | TpPaNO2-coated fibre extraction (45 min RT immersion, 1000 rpm stirring)/GC-ECD | A total of 11 pesticides/0.04–0.25 μg kg−1 | Apple, pear, melon, peach, plum, cucumber, oilseed rape, lettuce, summer squash, and celery cabbage | [113] |
| DI-SPME | Triazine-based conjugated microporous polymer-coated fibre extraction (immersion; 40 min, 55 °C, 1000 rpm stirring)/GC-ECD | A total of 12 halogens-containing environmental hormones/0.02–0.04 ng g−1 | Apple, nectarine, pear, Chinese cabbage, pakchoi, baby cabbage, rape, and lettuce | [2] |
| DI-SPME | A total of 20% acetone (v/v), 10% NaCl (w/w), 0.02% NaN3, DI extraction (60 min, 55 °C)/GC-MS | PAHs, PCBs, and pesticide residues/1–30 μg kg−1 | Edible seaweeds | [114] |
| HF-SPME | A 10 mL sample (extraction device); MIL-101@GO-HF withdrawn from the vial; analytes placed in another desorption vial with MeOH; desorption (sonication); elution (solvent)/HPLC-UV (25 µL) | Diazinon, chlorpyrifos/0.21, 0.27 mg L−1 | Tomato and cucumber | [115] |
| MD-SPME | pesticide standard solutions (10 mL each, 15 ng mL−1); 15 mg rGOQDs@ Fe (sorbent); 5 min ultrasonication; extraction; sorbent separated from the solution under external magnetic field; 0.4 mL of acetone under sonication for 3.5 min (elution)/GC-MS (10 μL) | Sevin, fenitrothion, malathion, parathion, diazinon/0.04–0.07 mg L−1 | Fruit juices | [116] |
| Online SPE | Monolith column for on-line solid-phase extraction coupled with LC-MS/MS | A total of 15 amide herbicides | Rice | [117] |
| dSPE | dSPE using PSA and MWCNTs as a mixed sorbent material/GC-MS | Multiple pesticide residues analysis | Strawberry, raspberry, blueberry, and blackberry | [118] |
| dSPE | ACN extraction, octadecylsilane-dispersive SPE (clean-up); UPLC HSS T3 column connected to a MS/MS via an electrospray ionisation source | Thiamethoxam, clothianidin, fipronil, fipronil sulfone, fipronil sulfide, fipronil desulfinyl, and pyraclostrobin/10 μg kg–1 | Rice, corn, cucumber, tomato, apple, and banana | [119] |
| MEPS | Metal-organic framework MIL-101(Cr) | Triazine herbicides (desmetryn, prometon, ametryn, prometryn, atraton, and dipropetryn)/0.01–0.12 ng g−1 | Corn | [121] |
| DLLME | Glyphosate converted to dithiocarbamic acid with carbon disulphide, followed by copper in the presence of ammonia; complex formation; collection in dichloromethane drop/UV-Vis | Glyphosate (herbicide)/0.21 mg L−1 | Legumes | [122] |
| DLLME | A total of 0.6 mL of CTAB (0.01 mol L−1), ziram, 0.6 mL ascorbic acid (0.01 mol L−1); 4.5 mL H2O (dilution);400 μL carbon tetrachloride, 2.75 mL ethanol, 0.75 mL of HAuCl4 (1.52 × 10–4 mol L−1); cloudy mixture centrifugation (80 s, 6000 rpm)/UV-Vis analysis (530 nm) of red sediment phase | Ziram/0.06 ng mL−1 | Potato, carrot, and wheat | [123] |
| GA-DLLME | A gas bubbled into a test tube to disperse the DES (extraction solvent); cloudy solution formation; centrifugation; collection of the sedimented phase/GC-FID (1 μL) | Diazinon, penconazole, haloxyfop-R-methyl, hexaconazole, diniconazole, clodinafop-propargyl, tebuconazole, bromopropylate, and fenazaquin/0.24–1.4 μg L−1 | Grape juice, apple, fresh cucumber, tomato, and onion | [124] |
| MCNOs-DLLME | Magnetic carbon nano-onions absorbed the analytes; clean-up and preconcentration by DLLME/GC-MS | Several pesticides/0.001–0.005 ng mL−1 | Fruit juices and vegetables | [125] |
| VA-DLLME | VA extraction; DDLME of the supernatant by chloroform/LC-MS/MS (10 μL) | Fipronil/0.07 μg kg−1 fipronil sulfone/0.04 μg kg−1 | Tomato | [126] |
| dSPE–DLLME | Analytes adsorbed into the poly (ε–caprolactone) grafted graphene quantum dots sorbent; centrifugation and elution by MeOH; eluent mixed with 1,1,2-TCE; centrifugation/GC-FID (1 μL) | Penconazole, chlorpyrifos, haloxyfop–R–methyl, oxadiazon, clodinafop–propargyl, diniconazole, fenazaquin, fenpropathri, and fenoxaprop–P–ethyl/0.32–0.76 ng mL–1 | Pomegranate, watermelon, apricot, grape, sour cherry, apple, and orange packed fruit juices | [127] |
| CNPs-dSPE-DLLME | Analytes adsorbed into the amorphous CNPs sorbent; centrifugation and elution of supernatant with iso-propanol under sonication; eluent mixed with 1,2-DBE; cloudy solution formation; centrifugation/GC-FID (1 μL) | Fenpropathrin and chlorpyrifos (insecticide), clodinafop–propargyl and oxadiazon (herbicide), and tebuconazole, penconazole, and diniconazole (fungicide)/0.83–1.16 ng mL–1 | Apricot, grape, peach, pear, pomegranate, sour cherry, and orange juices | [128] |
| IL-DLLME | A 5.0 mL sample diluted with ACN (0.3–280 µg L−1); spiked with 450 µL of Cu(II) (5 mg L−1) at pH 5.5; TBZ-Cu complex formation; 15 mg [C4mim][PF6] (extraction solvent); 300 µL ACN (disperser solvent); extraction of the complex to IL phase; 15 mL H2O; clouding by ultrasonication (40 °C, 10 min); analyte extraction into droplets of [C4mim][PF6]; centrifugation (3500 rpm 2 min); IL phase dilution with 400 µL MeOH/UV-Vis (340 nm) | TBZ/0.8–50 μg L−1 | Mushroom, cherry tomato, green pepper, corn, carrot, grape, apple, banana, orange, lemon, and apricot | [129] |
| dSPE- SFOME | ACN extraction; clean-up and concentration by SFOME/GC-ECD | Organochlorine pesticides/0.45–1.33 μg kg−1 | Cabbage, spinach, and lettuce | [130] |
| QuEChERS-MSPE- DLLME | MSPE: 5 mL of sample absorbed in 50 mg of Fe3O4@SiO2 magnetic nanoparticles; ACN desorption; DLLME: 40 μL 1,1,2-TCE (extraction solvent)/GC-FID (1 μL) | Diazinon, penconazole, oxadiazon, diniconazole, fenazaquin/5–200 μg L−1 | Peach, grape, apricot, grape, sour cherry, peach, and tomato | [131] |
| Preservatives | ||||
| LDS-DLLME-MSPE | LDS-DLLME: 5 mL sample, 50 μL 1-heptanol (30 s; 2000 rpm); MSPE: 160 μL DA@Fe3O4 nanoparticles (30 s; 2000 rpm); 200 μL acidified ACN desorbed 1-heptanol by vortex (20 s)/HPLC (20 μL) | Synthetic phenolic antioxidants/1.2–5.8 ng mL−1 | Olive oil | [132] |
| DLLME | A 4 mL sample, 0.50 NaCl, 0.50 mL acetone (dispenser), 50 μL trichloromethane (extraction solvent); cloudy solution formation; centrifugation (6 min at 1789 g)/GC-MS (1 μL) | A total of 7 preservatives and 4 parabens/0.15–0.50 mg kg−1 | Fruit juices | [133] |
| DLLME | A 5 mL sample, 0.85 g NaCl, 625 μL acetone (dispenser), 350 μL chloroform (extraction solvent); cloudy solution formation; centrifugation (10 min, 4500 rpm)/HPLC-DAD (20 μL) | Benzoic acid, sorbic acid, butylated hydroxyanisole, butylated hydroxytoluene/0.03 μg mL−1 | Fruit juices | [134] |
| QuEChERS-MSPE | QuEChERS extraction; MSPE by magnetic graphene nanoparticles/GC-MS | A total of 16 preservatives/0.21–11.50 μg kg−1 | Cabbage | [135] |
| QuEChERS-SPE | A 1.5 g sample, 10 mL distilled H2O, 150 μL AcAc, 10 mL ACN; sonication (1.5 min); 4.0 g MgSO4, 1.0 g NaCl; centrifugation (5 min, 5500 rpm); SPE with chitosan/HPLC-UV | Sodium benzoate, potassium sorbate, propylparaben, methylparaben/1.1–2.4 mg kg−1 | Fruit juices | [136] |
| HS-SPME | PA fibre extraction (headspace, 30 min, 50 °C, 600 rpm)/GC-FID | Benzoic acid, sorbic acid, propionic acid/1.1–1.7 mg L−1 | Pickled vegetables and soy sauce | [73] |
| UALLME | A 0.10 g sample, 3 mL n-hexane, 600 μL NADES; US (30 min); centrifugation (10 min, 3000 rpm); two clear phases formation; NADES-rich phase withdrawn/HPLC-UV (10 μL) | TBHQ/0.02 mg kg−1 | A total of 12 kinds of edible oils | [137] |
| CPE | Tergitol (19% v/v), NaCl (0.83 g), ultrasonic stirring (15 min; 36 °C)/HPLC-ECD | TBZ/5.4 μg L−1 | Tomato | [138] |
| SERS | Paper-based SERS 12 μL sample; spectra taken during the transition process from wet state to dry state in a dispersive spectrometer-CCD and a laser diode (785 nm, 250 mW, 30 s exposure time, 4 laser accumulations, between 3200 and 200 cm−1, 2 cm−1 spectral resolution, 100% nominal power)/HPLC-DAD (296 nm) | TBZ/>2 ppm | Mango peels | [139] |
| UHPSFE | A 0.1 g sample; 1.0 g glass beads; 5 min heating at 53 °C; dried; reconstituted in n-heptane (100 µL)/GC-MS | Atrazine, 2,4′-DDD, 4,4′-DDT, and endrin/0.2–2.0 ng g−1 | Onion | [120] |
3. Concluding Remarks
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Green, H.; Broun, P.; Cakmak, I.; Condon, L.; Fedoroff, N.; Gonzalez-Valero, J.; Graham, I.; Lewis, J.; Moloney, M.; Oniang’o, R.K.; et al. Planting seeds for the future of food. J. Sci. Food Agric. 2016, 96, 1409–1414. [Google Scholar] [CrossRef] [PubMed]
- Wan, N.; Chang, Q.; Hou, F.; Li, J.; Zang, X.; Zhang, S.; Wang, C.; Wang, Z. Efficient solid-phase microextraction of twelve halogens-containing environmental hormones from fruits and vegetables by triazine-based conjugated microporous polymer coating. Anal. Chim. Acta 2022, 1195, 339458. [Google Scholar] [CrossRef] [PubMed]
- OECD/FAO. OECD-FAO Agricultural Outlook 2012–2021; OECD Publishing and FAO: Paris, France, 2012. [Google Scholar] [CrossRef]
- Reeves, W.R.; McGuire, M.K.; Stokes, M.; Vicini, J.L. Assessing the Safety of Pesticides in Food: How Current Regulations Protect Human Health. Adv. Nutr. 2019, 10, 80–88. [Google Scholar] [CrossRef]
- EU Legislation on MRLs. Available online: https://food.ec.europa.eu/plants/pesticides/maximum-residue-levels/eu-legislation-mrls_en#:~:text=A%20general%20default%20MRL%20of,e.g.%20babies%2C%20children%20and%20vegetarians (accessed on 18 March 2023).
- The 2019 European Union Report on Pesticide Residues in Food. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/6491 (accessed on 19 March 2023).
- Okafor-Elenwo, E.J.; Imade, O.S. Ready-to-eat vegetable salads served in Nigerian restaurants: A potential source of multidrug-resistant bacteria. J. Appl. Microbiol. 2020, 129, 1402–1409. [Google Scholar] [CrossRef] [PubMed]
- Cho, I.; Blaser, M.J. The human microbiome: At the interface of health and disease. Nat. Rev. Genet. 2012, 13, 260–270. [Google Scholar] [CrossRef] [PubMed]
- Hu, M.; Ben, Y.; Wong, M.H.; Zheng, C. Trace Analysis of Multiclass Antibiotics in Food Products by Liquid Chromatography-Tandem Mass Spectrometry: Method Development. J. Agric. Food. Chem. 2021, 69, 1656–1666. [Google Scholar] [CrossRef]
- Gutierrez-del-Rio, I.; Fernandez, J.; Lombo, F. Plant nutraceuticals as antimicrobial agents in food preservation: Terpenoids, polyphenols and thiols. Int. J. Antimicrob. Agents 2018, 52, 309–315. [Google Scholar] [CrossRef]
- Carocho, M.; Morales, P.; Ferreira, I.C.F.R. Antioxidants: Reviewing the chemistry, food applications, legislation and role as preservatives. Trends Food Sci. Technol. 2018, 71, 107–120. [Google Scholar] [CrossRef]
- Camara, J.S.; Perestrelo, R.; Berenguer, C.V.; Andrade, C.F.P.; Gomes, T.M.; Olayanju, B.; Kabir, A.; Rocha, C.M.R.; Teixeira, J.A.; Pereira, J.A.M. Green Extraction Techniques as Advanced Sample Preparation Approaches in Biological, Food, and Environmental Matrices: A Review. Molecules 2022, 27, 2953. [Google Scholar] [CrossRef]
- Sulaiman, N.S.; Rovina, K.; Joseph, V.M. Classification, extraction and current analytical approaches for detection of pesticides in various food products. J. Consum. Prot. Food Saf. 2019, 14, 209–221. [Google Scholar] [CrossRef]
- Marete, G.M.; Shikuku, V.O.; Lalah, J.O.; Mputhia, J.; Wekesa, V.W. Occurrence of pesticides residues in French beans, tomatoes, and kale in Kenya, and their human health risk indicators. Environ. Monit. Assess. 2020, 192, 692. [Google Scholar] [CrossRef] [PubMed]
- Botitsi, H.V.; Garbis, S.D.; Economou, A.; Tsipi, D.F. Current mass spectrometry strategies for the analysis of pesticides and their metabolites in food and water matrices. Mass Spectrom. Rev. 2011, 30, 907–939. [Google Scholar] [CrossRef] [PubMed]
- Yadav, I.C.; Devi, N.L. Pesticides classification and its impact on human and environment. Environ. Sci. Eng. 2017, 6, 140–158. [Google Scholar]
- Jayaraj, R.; Megha, P.; Sreedev, P. Organochlorine pesticides, their toxic effects on living organisms and their fate in the environment. Interdiscip. Toxicol. 2016, 9, 90–100. [Google Scholar] [CrossRef]
- Yan, X.; Li, H.; Yan, Y.; Su, X. Selective detection of parathion-methyl based on near-infrared CuInS2 quantum dots. Food Chem. 2015, 173, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Fahimi-Kashani, N.; Hormozi-Nezhad, M.R. Gold-Nanoparticle-Based Colorimetric Sensor Array for Discrimination of Organophosphate Pesticides. Anal. Chem. 2016, 88, 8099–8106. [Google Scholar] [CrossRef] [PubMed]
- Chawla, P.; Kaushik, R.; Shiva Swaraj, V.J.; Kumar, N. Organophosphorus pesticides residues in food and their colorimetric detection. Environ. Nanotechnol. Monit. Manag. 2018, 10, 292–307. [Google Scholar] [CrossRef]
- Dias, E.; Garcia e Costa, F.; Morais, S.; de Lourdes Pereira, M. A Review on the Assessment of the Potential Adverse Health Impacts of Carbamate Pesticides. Top. Public Health 2015, 1, 197–212. [Google Scholar] [CrossRef]
- Goel, A.; Aggarwal, P. Pesticide poisoning. Natl. Med. J. India 2007, 20, 182–191. [Google Scholar]
- Bonerba, E.; Panseri, S.; Arioli, F.; Nobile, M.; Terio, V.; Di Cesare, F.; Tantillo, G.; Maria Chiesa, L. Determination of antibiotic residues in honey in relation to different potential sources and relevance for food inspection. Food Chem. 2021, 334, 127575. [Google Scholar] [CrossRef]
- Kim, C.; Ryu, H.D.; Chung, E.G.; Kim, Y.; Lee, J.K. A review of analytical procedures for the simultaneous determination of medically important veterinary antibiotics in environmental water: Sample preparation, liquid chromatography, and mass spectrometry. J. Environ. Manag. 2018, 217, 629–645. [Google Scholar] [CrossRef]
- Lopatto, E.; Choi, J.; Colina, A.; Ma, L.; Howe, A.; Hinsa-Leasure, S. Characterizing the soil microbiome and quantifying antibiotic resistance gene dynamics in agricultural soil following swine CAFO manure application. PLoS ONE 2019, 14, e0220770. [Google Scholar] [CrossRef]
- Cerqueira, F.; Matamoros, V.; Bayona, J.M.; Berendonk, T.U.; Elsinga, G.; Hornstra, L.M.; Pina, B. Antibiotic resistance gene distribution in agricultural fields and crops. A soil-to-food analysis. Environ. Res. 2019, 177, 108608. [Google Scholar] [CrossRef]
- Tadić, Đ.; Bleda Hernandez, M.J.; Cerqueira, F.; Matamoros, V.; Piña, B.; Bayona, J.M. Occurrence and human health risk assessment of antibiotics and their metabolites in vegetables grown in field-scale agricultural systems. J. Hazard. Mater. 2021, 401, 123424. [Google Scholar] [CrossRef]
- Liu, X.; Steele, J.C.; Meng, X.-Z. Usage, residue, and human health risk of antibiotics in Chinese aquaculture: A review. Environ. Pollut. 2017, 223, 161–169. [Google Scholar] [CrossRef] [PubMed]
- Benelli, G.; Canale, A.; Toniolo, C.; Higuchi, A.; Murugan, K.; Pavela, R.; Nicoletti, M. Neem (Azadirachta indica): Towards the ideal insecticide? Nat. Prod. Res. 2017, 31, 369–386. [Google Scholar] [CrossRef]
- Jones, A.L.; Schnabel, E.L. The Development of Streptomycin-Resistant Strains of Erwinia amylovora; CABI: Wallingford, UK, 2000; pp. 235–251. [Google Scholar] [CrossRef]
- Stockwell, V.O.; Duffy, B. Use of antibiotics in plant agriculture. OIE Rev. Sci. Tech. 2012, 31, 199–210. [Google Scholar] [CrossRef] [PubMed]
- McManus, P.S.; Stockwell, V.O.; Sundin, G.W.; Jones, A.L. Antibiotic use in plant agriculture. Annu. Rev. Phytopathol. 2002, 40, 443–465. [Google Scholar] [CrossRef] [PubMed]
- Bensid, A.; El Abed, N.; Houicher, A.; Regenstein, J.M.; Ozogul, F. Antioxidant and antimicrobial preservatives: Properties, mechanism of action and applications in food—A review. Crit. Rev. Food Sci. Nutr. 2022, 62, 2985–3001. [Google Scholar] [CrossRef]
- Baptista, R.C.; Horita, C.N.; Sant’Ana, A.S. Natural products with preservative properties for enhancing the microbiological safety and extending the shelf-life of seafood: A review. Food Res. Int. 2020, 127, 108762. [Google Scholar] [CrossRef]
- Carocho, M.; Ferreira, I.C. A review on antioxidants, prooxidants and related controversy: Natural and synthetic compounds, screening and analysis methodologies and future perspectives. Food Chem. Toxicol. 2013, 51, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Salmanzadeh, R.; Eskandani, M.; Mokhtarzadeh, A.; Vandghanooni, S.; Ilghami, R.; Maleki, H.; Saeeidi, N.; Omidi, Y. Propyl gallate (PG) and tert-butylhydroquinone (TBHQ) may alter the potential anti-cancer behavior of probiotics. Food Biosci. 2018, 24, 37–45. [Google Scholar] [CrossRef]
- Eskandani, M.; Hamishehkar, H.; Ezzati Nazhad Dolatabadi, J. Cytotoxicity and DNA damage properties of tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2014, 153, 315–320. [Google Scholar] [CrossRef] [PubMed]
- Kashanian, S.; Dolatabadi, J.E.N. DNA binding studies of 2-tert-butylhydroquinone (TBHQ) food additive. Food Chem. 2009, 116, 743–747. [Google Scholar] [CrossRef]
- Dolatabadi, J.E.N.; Kashanian, S. A review on DNA interaction with synthetic phenolic food additives. Food Res. Int. 2010, 43, 1223–1230. [Google Scholar] [CrossRef]
- Hamishehkar, H.; Khani, S.; Kashanian, S.; Ezzati Nazhad Dolatabadi, J.; Eskandani, M. Geno- and cytotoxicity of propyl gallate food additive. Drug Chem. Toxicol. 2014, 37, 241–246. [Google Scholar] [CrossRef]
- Negi, P.S. Plant extracts for the control of bacterial growth: Efficacy, stability and safety issues for food application. Int. J. Food Microbiol. 2012, 156, 7–17. [Google Scholar] [CrossRef]
- Davidson, P.M.; Zivanovic, S. The Use of Natural Antimicrobials; Elsevier: Amsterdam, The Netherlands, 2003; pp. 5–30. [Google Scholar] [CrossRef]
- Arioglu-Tuncil, S.; Voelker, A.L.; Taylor, L.S.; Mauer, L.J. Amorphization of Thiamine Chloride Hydrochloride: Effects of Physical State and Polymer Type on the Chemical Stability of Thiamine in Solid Dispersions. Int. J. Mol. Sci. 2020, 21, 5935. [Google Scholar] [CrossRef]
- Oms-Oliu, G.; Rojas-Graü, M.A.; González, L.A.; Varela, P.; Soliva-Fortuny, R.; Hernando, M.I.H.; Munuera, I.P.; Fiszman, S.; Martín-Belloso, O. Recent approaches using chemical treatments to preserve quality of fresh-cut fruit: A review. Postharvest Biol. Technol. 2010, 57, 139–148. [Google Scholar] [CrossRef]
- Ru, X.; Tao, N.; Feng, Y.; Li, Q.; Wang, Q. A novel anti-browning agent 3-mercapto-2-butanol for inhibition of fresh-cut potato browning. Postharvest Biol. Technol. 2020, 170, 111324. [Google Scholar] [CrossRef]
- He, Q.; Luo, Y. Enzymatic browning and its control in fresh-cut produce. Stewart Postharvest Rev. 2007, 6, 3. [Google Scholar] [CrossRef]
- Afoakwah, A.N. Anti-browning methods on fresh-cut fruits and fruit juice: A Review. Afr. J. Biol. Sci. 2020, 2, 27. [Google Scholar] [CrossRef]
- Jang, J.-H.; Moon, K.-D. Inhibition of polyphenol oxidase and peroxidase activities on fresh-cut apple by simultaneous treatment of ultrasound and ascorbic acid. Food Chem. 2011, 124, 444–449. [Google Scholar] [CrossRef]
- Ding, C.K.; Chachin, K.; Ueda, Y.; Wang, C.Y. Inhibition of loquat enzymatic browning by sulfhydryl compounds. Food Chem. 2002, 76, 213–218. [Google Scholar] [CrossRef]
- Loizzo, M.R.; Tundis, R.; Menichini, F. Natural and Synthetic Tyrosinase Inhibitors as Antibrowning Agents: An Update. Compr. Rev. Food Sci. Food Saf. 2012, 11, 378–398. [Google Scholar] [CrossRef]
- Lim, W.Y.; Cheun, C.F.; Wong, C.W. Inhibition of enzymatic browning in sweet potato (Ipomoea batatas (L.)) with chemical and natural anti-browning agents. J. Food Process. Preserv. 2019, 43, 14195. [Google Scholar] [CrossRef]
- Zikankuba, V.L.; Mwanyika, G.; Ntwenya, J.E.; James, A. Pesticide regulations and their malpractice implications on food and environment safety. Cogent Food Agric. 2019, 5, 1601544. [Google Scholar] [CrossRef]
- Thompson, L.A.; Darwish, W.S. Environmental Chemical Contaminants in Food: Review of a Global Problem. J. Toxicol. 2019, 2019, 2345283. [Google Scholar] [CrossRef]
- Codex Alimentarius Commission—Procedural Manual, 27th ed.; FAO: Rome, Italy, 2019.
- Islam, M.N.; Bint-E-Naser, S.F.; Khan, M.S. Pesticide Food Laws and Regulations; Springer International Publishing: Cham, Switzerland, 2017; pp. 37–51. [Google Scholar] [CrossRef]
- Abdelraheem, E.M.; Hassan, S.M.; Arief, M.M.; Mohammad, S.G. Validation of quantitative method for azoxystrobin residues in green beans and peas. Food Chem. 2015, 182, 246–250. [Google Scholar] [CrossRef]
- Paoloni, A.; Alunni, S.; Pelliccia, A.; Pecorelli, I. Rapid determination of residues of pesticides in honey by µGC-ECD and GC-MS/MS: Method validation and estimation of measurement uncertainty according to document No. SANCO/12571/2013. J. Environ. Sci. Health Part B 2016, 51, 133–142. [Google Scholar] [CrossRef]
- Risk profile related to production and consumption of insects as food and feed. EFSA J. 2015, 13, 4257. [CrossRef]
- Wanwimolruk, S.; Phopin, K.; Boonpangrak, S.; Prachayasittikul, V. Food safety in Thailand 4: Comparison of pesticide residues found in three commonly consumed vegetables purchased from local markets and supermarkets in Thailand. PeerJ 2016, 4, e2432. [Google Scholar] [CrossRef]
- van der Velde-Koerts, T.; Margerison, S.; Breysse, N.; Lutze, J.; Mahieu, K.; Reich, H.; Rietveld, A.; Sarda, X.; Sieke, C.; Vial, G.; et al. Impact of proposed changes in IESTI equations for short-term dietary exposure to pesticides from Australian and Codex perspective. J. Environ. Sci. Health Part B 2018, 53, 366–379. [Google Scholar] [CrossRef]
- Han, Y.; Song, L.; Liu, S.; Zou, N.; Li, Y.; Qin, Y.; Li, X.; Pan, C. Simultaneous determination of 124 pesticide residues in Chinese liquor and liquor-making raw materials (sorghum and rice hull) by rapid Multi-plug Filtration Cleanup and gas chromatography–tandem mass spectrometry. Food Chem. 2018, 241, 258–267. [Google Scholar] [CrossRef]
- Yang, X.; Luo, J.; Duan, Y.; Li, S.; Liu, C. Simultaneous analysis of multiple pesticide residues in minor fruits by ultrahigh-performance liquid chromatography/hybrid quadrupole time-of-fight mass spectrometry. Food Chem. 2018, 241, 188–198. [Google Scholar] [CrossRef]
- Nambirajan, K.; Muralidharan, S.; Manonmani, S.; Kirubhanandhini, V.; Ganesan, K. Incidences of mortality of Indian peafowl Pavo cristatus due to pesticide poisoning in India and accumulation pattern of chlorinated pesticides in tissues of the same species collected from Ahmedabad and Coimbatore. Environ. Sci. Pollut. Res. 2018, 25, 15568–15576. [Google Scholar] [CrossRef]
- Handford, C.E.; Elliott, C.T.; Campbell, K. A review of the global pesticide legislation and the scale of challenge in reaching the global harmonization of food safety standards. Integr. Environ. Assess. Manag. 2015, 11, 525–536. [Google Scholar] [CrossRef]
- Qian, J.; Shi, C.; Wang, S.; Song, Y.; Fan, B.; Wu, X. Cloud-based system for rational use of pesticide to guarantee the source safety of traceable vegetables. Food Control 2018, 87, 192–202. [Google Scholar] [CrossRef]
- Xianxia, W.; Yunxi, Z. Farmers’ Dual Roles in Food Safety: Perceptions and Countermeasures. J. Resour. Ecol. 2018, 9, 78. [Google Scholar] [CrossRef]
- Hamilton, D.; Yoshida, M.; Wolterink, G.; Solecki, R. Evaluation of Pesticide Residues by FAO/WHO JMPR; World Scientific (Europe): London, UK, 2017; pp. 113–196. [Google Scholar] [CrossRef]
- Yamada, Y. Importance of Codex Maximum Residue Limits for Pesticides for the Health of Consumers and International Trade; World Scientific (Europe): London, UK, 2017; pp. 269–282. [Google Scholar] [CrossRef]
- Yeung, M.T.; Kerr, W.A.; Coomber, B.; Lantz, M.; McConnell, A. Why Maximum Residue Limits for Pesticides Are an Important International Issue; Springer International Publishing: Cham, Switzerland, 2017; pp. 1–9. [Google Scholar] [CrossRef]
- Chen, J.; Ying, G.G.; Deng, W.J. Antibiotic Residues in Food: Extraction, Analysis, and Human Health Concerns. J. Agric. Food Chem. 2019, 67, 7569–7586. [Google Scholar] [CrossRef]
- Simjee, S.; Henninger, M.; Ippolito, G.; Atkinson, J. Can we align antibiotic policies at an international level in the absence of harmonized definitions? J. Antimicrob. Chemother. 2022, 77, 549–555. [Google Scholar] [CrossRef]
- Cox, S.; Sandall, A.; Smith, L.; Rossi, M.; Whelan, K. Food additive emulsifiers: A review of their role in foods, legislation and classifications, presence in food supply, dietary exposure, and safety assessment. Nutr. Rev. 2021, 79, 726–741. [Google Scholar] [CrossRef]
- Tungkijanansin, N.; Alahmad, W.; Nhujak, T.; Varanusupakul, P. Simultaneous determination of benzoic acid, sorbic acid, and propionic acid in fermented food by headspace solid-phase microextraction followed by GC-FID. Food Chem. 2020, 329, 127161. [Google Scholar] [CrossRef]
- Akram, S.; Sultana, B.; Asi, M.R.; Mushtaq, M. Salting-out-assisted liquid-liquid extraction and reverse-phase high-performance liquid chromatographic monitoring of thiacloprid in fruits and vegetables. Sep. Sci. Technol. 2018, 53, 1563–1571. [Google Scholar] [CrossRef]
- Samad, A.; Akhtar, S.; Shahid, M.M.; Ahad, K. Determination of pesticide residues in peaches by using gas chromatography and mass spectrometric detection. Int. J. Environ. Anal. Chem. 2019, 99, 1446–1458. [Google Scholar] [CrossRef]
- AlFaris, N.A.; ALTamimi, J.Z.; ALOthman, Z.A.; Wabaidur, S.M.; Ghafar, A.A.; Aldayel, T.S. Development of a sensitive liquid-liquid extraction and ultra-performance liquid chromatography-tandem mass spectrometry method for the analysis of carbaryl residues in fresh vegetables sold in Riyadh. J. King Saud Univ. Sci. 2020, 32, 2414–2418. [Google Scholar] [CrossRef]
- Heleno, F.F.; Rodrigues, A.A.Z.; Queiroz, M.E.L.R.; Neves, A.A.; Oliveira, A.F.; Libardi, V.M. Determination of fungicides in bell pepper using solid-liquid extraction with low temperature partitioning. Microchem. J. 2019, 148, 79–84. [Google Scholar] [CrossRef]
- Perestrelo, R.; Silva, P.; Porto-Figueira, P.; Pereira, J.A.M.; Silva, C.; Medina, S.; Camara, J.S. QuEChERS—Fundamentals, relevant improvements, applications and future trends. Anal. Chim. Acta 2019, 1070, 1–28. [Google Scholar] [CrossRef]
- Veloo, K.V.; Ibrahim, N.A.S. Solid-phase extraction using chloropropyl functionalized sol-gel hybrid sorbent for simultaneous determination of organophosphorus pesticides in selected fruit samples. J. Sep. Sci. 2020, 43, 3027–3035. [Google Scholar] [CrossRef]
- Nakamura, K.; Otake, T.; Hanari, N. Evaluation of supercritical fluid extraction for the determination of neonicotinoid pesticides in green onion. J. Environ. Sci. Health Part B 2020, 55, 604–612. [Google Scholar] [CrossRef]
- Abbott, A.P.; Capper, G.; Davies, D.L.; Rasheed, R.K.; Tambyrajah, V. Novel solvent properties of choline chloride/urea mixtures. Chem. Commun. 2003, 39, 70–71. [Google Scholar] [CrossRef]
- Jouyban, A.; Farajzadeh, M.A.; Mogaddam, M.R.A. A lighter-than-water deep eutectic-solvent-based dispersive liquid-phase microextraction method in a U-shaped homemade device. New J. Chem. 2018, 42, 10100–10110. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Hojghan, A.S.; Mogaddam, M.R.A. Development of a new temperature-controlled liquid phase microextraction using deep eutectic solvent for extraction and preconcentration of diazinon, metalaxyl, bromopropylate, oxadiazon, and fenazaquin pesticides from fruit juice and vegetable samples followed by gas chromatography-flame ionization detection. J. Food Compos. Anal. 2018, 66, 90–97. [Google Scholar] [CrossRef]
- Zhao, J.; Meng, Z.; Zhao, Z.; Zhao, L. Ultrasound-assisted deep eutectic solvent as green and efficient media combined with functionalized magnetic multi-walled carbon nanotubes as solid-phase extraction to determine pesticide residues in food products. Food Chem. 2020, 310, 125863. [Google Scholar] [CrossRef]
- Chen, S.; An, Q.; Sun, H.; Mao, M.Q. Application of ultrasound-assisted deep eutectic solvent extraction combined with liquid-liquid extraction method to the extraction of three pesticide residues from fruit and vegetable samples. Acta Chromatogr. 2021, 33, 30–36. [Google Scholar] [CrossRef]
- Anastassiades, M.; Lehotay, S.J.; Stajnbaher, D.; Schenck, F.J. Fast and easy multiresidue method employing acetonitrile extraction/partitioning and “dispersive solid-phase extraction” for the determination of pesticide residues in produce. J. AOAC Int. 2003, 86, 412–431. [Google Scholar] [CrossRef]
- Chiarello, M.; Moura, S. Determination of Pesticides in Organic Carrots by High-Performance Liquid Chromatography/High-Resolution Mass Spectrometry. Anal. Lett. 2018, 51, 2561–2574. [Google Scholar] [CrossRef]
- Yao, W.; Zhang, Z.; Song, S.; Hao, X.; Xu, Y.; Han, L. Multi-residue Analysis of 34 Pesticides in Black Pepper by QuEChERS with d-SPE Vs. d-SLE Cleanup. Food Anal. Methods 2019, 12, 176–189. [Google Scholar] [CrossRef]
- Pszczolinska, K.; Shakeel, N.; Barchanska, H. A simple approach for pesticide residues determination in green vegetables based on QuEChERS and gas chromatography tandem mass spectrometry. J. Food Compos. Anal. 2022, 114, 04783. [Google Scholar] [CrossRef]
- Yuan, X.; Kim, C.J.; Lee, R.; Kim, M.; Shin, H.J.; Kim, L.; Jeong, W.T.; Shin, Y.; Kyung, K.S.; Noh, H.H. Validation of a Multi-Residue Analysis Method for 287 Pesticides in Citrus Fruits Mandarin Orange and Grapefruit Using Liquid Chromatography-Tandem Mass Spectrometry. Foods 2022, 11, 3522. [Google Scholar] [CrossRef]
- Zheng, K.; Wu, X.; Chen, J.; Chen, J.; Lian, W.; Su, J.; Shi, L. Establishment of an LC-MS/MS Method for the Determination of 45 Pesticide Residues in Fruits and Vegetables from Fujian, China. Molecules 2022, 27, 8674. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Wang, X.; Chang, H.; Sun, H.; Liu, Y. Establishment of a Method for the Detection of Indaziflam, Spirotetramat, Cyantraniliprole, and Their Metabolites and Application for Fruit and Vegetable Risk Assessment. J. Agric. Food Chem. 2022, 70, 16369–16381. [Google Scholar] [CrossRef]
- Feng, Y.Z.; Zhang, G.F.; Zhang, A.J.; Zhou, L.; Bian, Y.L.; Pan, J.J.; Yang, S.M.; Han, J.F.; Ma, X.G.; Qi, X.X.; et al. Dissipation, Residue, and Dietary Risk Assessment of Methoxyfenozide, Chlorantraniliprole, Indoxacarb, Lufenuron, and Chlorfenapyr in Spinach Using a Modified QuEChERS Method Combined with a Tandem Mass Spectrometry Technique. Agronomy 2022, 12, 3173. [Google Scholar] [CrossRef]
- Woldetsadik, D.; Simon, M.P.; Knuth, D.; Hailu, H.; Gebresilassie, A.; Dejen, A.; Düring, R.-A. Exposure to DDT and HCH congeners and associated potential health risks through khat (Catha edulis) consumption among adults in South Wollo, Ethiopia. Environ. Geochem. Health 2021, 43, 3597–3613. [Google Scholar] [CrossRef]
- Tadic, D.; Matamoros, V.; Bayona, J.M. Simultaneous determination of multiclass antibiotics and their metabolites in four types of field-grown vegetables. Anal. Bioanal. Chem. 2019, 411, 5209–5222. [Google Scholar] [CrossRef]
- Merlo, F.; Centenaro, D.; Maraschi, F.; Profumo, A.; Speltini, A. Green and Efficient Determination of Fluoroquinolone Residues in Edible Green Fruits and Leafy Vegetables by Ultrasound-Assisted Extraction Followed by HPLC-MS/MS. Molecules 2022, 27, 6595. [Google Scholar] [CrossRef]
- Petric, Z.; Ružić, J.; Žuntar, I. The controversies of parabens—An overview nowadays. Acta Pharm. 2021, 71, 17–32. [Google Scholar] [CrossRef]
- Maher, H.M.; Alzoman, N.Z.; Almeshal, M.A.; Alotaibi, H.A.; Alotaibi, N.N.; Al-Showiman, H. Quantitative screening of parabens in Ready-to-eat foodstuffs available in the Saudi market using high performance liquid chromatography with photodiode array detection. Arab. J. Chem. 2020, 13, 2897–2911. [Google Scholar] [CrossRef]
- De Souza, P.G.; Toci, A.T.; Mafra, M.R.; Farias, F.O.; Igarashi-Mafra, L. Natural Extracts from Eugenia brasiliensis Lam Leaves to Improve the Shelf-Life of Fresh Tomatoes. Waste Biomass Valorization 2022, 14, 1293–1304. [Google Scholar] [CrossRef]
- Hejji, L.; Azzouz, A.; Palacios Colon, L.; Souhail, B.; Ballesteros, E. A multi-residue method for determining twenty-four endocrine disrupting chemicals in vegetables and fruits using ultrasound-assisted solid-liquid extraction and continuous solid-phase extraction. Chemosphere 2021, 263, 128158. [Google Scholar] [CrossRef] [PubMed]
- Gupta, M.K.; Anand, A.; Asati, A.; Thati, R.; Katragunta, K.; Agarwal, R.; Mudiam, M.K.R. Quantitative determination of phenolic antioxidants in fruit juices by GC-MS/MS using automated injector port silylation after QuEChERS extraction. Microchem. J. 2021, 160, 105705. [Google Scholar] [CrossRef]
- Wang, Q.; Wang, C.; Wang, J.; Liu, W.; Hao, L.; Zhou, J.; Wang, Z.; Wu, Q. Sensitive determination of phenylurea herbicides in soybean milk and tomato samples by a novel hypercrosslinked polymer based solid-phase extraction coupled with high performance liquid chromatography. Food Chem. 2020, 317, 126410. [Google Scholar] [CrossRef]
- Li, P.; Lu, Y.; Cao, J.; Li, M.; Yang, C.; Yan, H. Imidazolium ionic-liquid-modified phenolic resin for solid-phase extraction of thidiazuron and forchlorfenuron from cucumbers. J. Chromatogr. A 2020, 1623, 461192. [Google Scholar] [CrossRef]
- Zinato Rodrigues, A.A.; Ribeiro de Queiroz, M.E.L.; D’Antonino Faroni, L.R.; Figueiredo Prates, L.H.; Neves, A.A.; de Oliveira, A.F.; de Freitas, J.F.; Heleno, F.F.; Zambolim, L. The efficacy of washing strategies in the elimination of fungicide residues and the alterations on the quality of bell peppers. Food Res. Int. 2021, 147, 110579. [Google Scholar] [CrossRef]
- Lawal, A.; Wong, R.C.S.; Tan, G.H.; Abdulra’uf, L.B.; Alsharif, A.M.A. Multi-pesticide Residues Determination in Samples of Fruits and Vegetables Using Chemometrics Approach to QuEChERS-dSPE Coupled with Ionic Liquid-Based DLLME and LC–MS/MS. Chromatographia 2018, 81, 759–768. [Google Scholar] [CrossRef]
- Mao, X.; Wan, Y.; Li, Z.; Chen, L.; Lew, H.; Yang, H. Analysis of organophosphorus and pyrethroid pesticides in organic and conventional vegetables using QuEChERS combined with dispersive liquid-liquid microextraction based on the solidification of floating organic droplet. Food Chem. 2020, 309, 125755. [Google Scholar] [CrossRef]
- Feng, G.; Sun, J.; Wang, M.; Wang, M.; Li, Z.; Wang, S.; Zheng, L.; Wang, J.; She, Y.; Abd El-Aty, A.M. Preparation of molecularly imprinted polymer with class-specific recognition for determination of 29 sulfonylurea herbicides in agro-products. J. Chromatogr. A 2021, 1647, 462143. [Google Scholar] [CrossRef] [PubMed]
- Hergueta-Castillo, M.E.; Lopez-Rodriguez, E.; Lopez-Ruiz, R.; Romero-Gonzalez, R.; Garrido Frenich, A. Targeted and untargeted analysis of triazole fungicides and their metabolites in fruits and vegetables by UHPLC-orbitrap-MS(2). Food Chem. 2022, 368, 130860. [Google Scholar] [CrossRef] [PubMed]
- Kabir, A.; Locatelli, M.; Ulusoy, H.I. Recent Trends in Microextraction Techniques Employed in Analytical and Bioanalytical Sample Preparation. Separations 2017, 4, 36. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Casado, N.; Porto-Figueira, P.; Câmara, J.S. The Potential of Microextraction Techniques for the Analysis of Bioactive Compounds in Food. Front. Nutr. 2022, 9, 825519. [Google Scholar] [CrossRef]
- Arthur, C.L.; Pawliszyn, J. Solid phase microextraction with thermal desorption using fused silica optical fibers. Anal. Chem. 1990, 62, 2145–2148. [Google Scholar] [CrossRef]
- Pereira, J.A.M.; Gonçalves, J.; Porto-Figueira, P.; Figueira, J.A.; Alves, V.; Perestrelo, R.; Medina, S.; Câmara, J.S. Current trends on microextraction by packed sorbent—Fundamentals, application fields, innovative improvements and future applications. Analyst 2019, 144, 5048–5074. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Zhou, X.; Zang, X.; Pang, Y.; Chang, Q.; Wang, C.; Wang, Z. Determination of pesticides residues in vegetable and fruit samples by solid-phase microextraction with a covalent organic framework as the fiber coating coupled with gas chromatography and electron capture detection. J. Sep. Sci. 2018, 41, 4038–4046. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Gionfriddo, E.; Acquaro, V., Jr.; Pawliszyn, J. Direct immersion solid-phase microextraction analysis of multi-class contaminants in edible seaweeds by gas chromatography-mass spectrometry. Anal. Chim. Acta 2018, 1031, 83–97. [Google Scholar] [CrossRef]
- Darvishnejad, F.; Raoof, J.B.; Ghani, M. MIL-101 (Cr) @ graphene oxide-reinforced hollow fiber solid-phase microextraction coupled with high-performance liquid chromatography to determine diazinon and chlorpyrifos in tomato, cucumber and agricultural water. Anal. Chim. Acta 2020, 1140, 99–110. [Google Scholar] [CrossRef]
- Akbarzade, S.; Chamsaz, M.; Rounaghi, G.H.; Ghorbani, M. Zero valent Fe-reduced graphene oxide quantum dots as a novel magnetic dispersive solid phase microextraction sorbent for extraction of organophosphorus pesticides in real water and fruit juice samples prior to analysis by gas chromatography-mass spectrometry. Anal. Bioanal. Chem. 2018, 410, 429–439. [Google Scholar] [CrossRef]
- Zhang, H.; Ai, L.; Ma, Y.; Wang, J.; Li, X. Determination of 15 amide herbicides in rice using monolith column for on-line solid-phase extraction coupled with liquid chromatography-tandem mass spectrometry. Chin. J. Chromatogr. 2018, 36, 991–998. [Google Scholar] [CrossRef]
- Zhao, P.; Alvarez, P.J.; Li, X.; Pan, C. Development of an analytical method for pesticide residues in berries with dispersive solid phase extraction using multiwalled carbon nanotubes and primary secondary amine sorbents. Anal. Methods 2018, 10, 757–766. [Google Scholar] [CrossRef]
- Rong, L.; Wu, X.; Xu, J.; Dong, F.; Liu, X.; Pan, X.; Du, P.; Wei, D.; Zheng, Y. Simultaneous determination of three pesticides and their metabolites in unprocessed foods using ultraperformance liquid chromatography-tandem mass spectrometry. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk Assess. 2018, 35, 273–281. [Google Scholar] [CrossRef]
- Tolcha, T.; Gemechu, T.; Al-Hamimi, S.; Megersa, N.; Turner, C. High Density Supercritical Carbon Dioxide for the Extraction of Pesticide Residues in Onion with Multivariate Response Surface Methodology. Molecules 2020, 25, 1012. [Google Scholar] [CrossRef]
- Jiang, Y.; Ma, P.; Li, X.; Piao, H.; Li, D.; Sun, Y.; Wang, X.; Song, D. Application of metal-organic framework MIL-101(Cr) to microextraction in packed syringe for determination of triazine herbicides in corn samples by liquid chromatography-tandem mass spectrometry. J. Chromatogr. A 2018, 1574, 36–41. [Google Scholar] [CrossRef] [PubMed]
- Cetin, E.; Sahan, S.; Ulgen, A.; Sahin, U. DLLME-spectrophotometric determination of glyphosate residue in legumes. Food Chem. 2017, 230, 567–571. [Google Scholar] [CrossRef] [PubMed]
- Hashemi, F.; Rastegarzadeh, S.; Pourreza, N. A combination of dispersive liquid-liquid microextraction and surface plasmon resonance sensing of gold nanoparticles for the determination of ziram pesticide. J. Sep. Sci. 2018, 41, 1156–1163. [Google Scholar] [CrossRef] [PubMed]
- Farajzadeh, M.A.; Sattari Dabbagh, M.; Yadeghari, A. Deep eutectic solvent based gas-assisted dispersive liquid-phase microextraction combined with gas chromatography and flame ionization detection for the determination of some pesticide residues in fruit and vegetable samples. J. Sep. Sci. 2017, 40, 2253–2260. [Google Scholar] [CrossRef] [PubMed]
- Marzi Khosrowshahi, E.; Farajzadeh, M.A.; Tuzen, M.; Afshar Mogaddam, M.R.; Nemati, M. Application of magnetic carbon nano-onions in dispersive solid-phase extraction combined with DLLME for extraction of pesticide residues from water and vegetable samples. Anal. Methods 2021, 13, 3592–3604. [Google Scholar] [CrossRef]
- Abdallah, O.I.; Ahmed, N.S. Development of a Vortex-Assisted Dispersive Liquid-Liquid Microextraction (VA-DLLME) and LC-MS/MS Procedure for Simultaneous Determination of Fipronil and its Metabolite Fipronil Sulfone in Tomato Fruits. Food Anal. Methods 2019, 12, 2314–2325. [Google Scholar] [CrossRef]
- Mohebbi, A.; Farajzadeh, M.A.; Mahmoudzadeh, A.; Etemady, A. Combination of poly (ε-caprolactone) grafted graphene quantum dots–based dispersive solid phase extraction followed by dispersive liquid-liquid microextraction for extraction of some pesticides from fruit juices prior to their quantification by gas chroma. Microchem. J. 2020, 153, 104328. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Mohebbi, A.; Fouladvand, H.; Mogaddam, M.R.A. A new and facile method for preparation of amorphous carbon nanoparticles and their application as an efficient and cheap sorbent for the extraction of some pesticides from fruit juices. Microchem. J. 2020, 155, 104795. [Google Scholar] [CrossRef]
- Altunay, N.; Uluzger, D.; Gurkan, R. Simple and fast spectrophotometric determination of low levels of thiabendazole residues in fruit and vegetables after pre-concentration with ionic liquid phase microextraction. Food Addit. Contam. Part A Chem. Anal. Control. Expo Risk Assess. 2018, 35, 1139–1154. [Google Scholar] [CrossRef]
- Qiu, B.; Wang, X.C.; Li, H.P.; Shu, B.; Li, S.; Yang, Z.G. Dispersive-Solid-Phase Extraction Cleanup Integrated to Dispersive Liquid-Liquid Microextraction Based on Solidification of Floating Organic Droplet for Determination of Organochlorine Pesticides in Vegetables. Food Anal. Methods 2018, 11, 693–702. [Google Scholar] [CrossRef]
- Farajzadeh, M.A.; Safi, R.; Yadeghari, A. Combination of QuEChERS extraction with magnetic solid phase extraction followed by dispersive liquid-liquid microextraction as an efficient procedure for the extraction of pesticides from vegetable, fruit, and nectar samples having high content of solids. Microchem. J. 2019, 147, 571–581. [Google Scholar] [CrossRef]
- Li, X.; Meng, D.; Zhang, L.; Zhao, J.; Yang, Y. Low-density solvent-based dispersive liquid–liquid microextraction coupled with hydrophobic magnetic nanoparticles for determination of synthetic phenolic antioxidants in vegetable oils by high-performance liquid chromatography. Sep. Sci. Technol. 2018, 53, 2224–2231. [Google Scholar] [CrossRef]
- Ding, M.; Liu, W.; Peng, J.; Liu, X.; Tang, Y. Simultaneous determination of seven preservatives in food by dispersive liquid-liquid microextraction coupled with gas chromatography-mass spectrometry. Food Chem. 2018, 269, 187–192. [Google Scholar] [CrossRef]
- Tighrine, A.; Pinto, E.; Melo, A.; Ferreira, I.M.P.L.V.O.; Mamou, M.; Amir, Y. Simultaneous Extraction and Determination of Preservatives and Antioxidants in Juice Samples by an Optimized Microextraction Method Using Central Composite Design and Validated with Accuracy Profile. J. AOAC Int. 2019, 102, 208–216. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.L.; Cao, S.R.; Zhang, L.; Xi, C.X.; Chen, Z.Q. Modified QuEChERS Combination with Magnetic Solid-Phase Extraction for the Determination of 16 Preservatives by Gas Chromatography-Mass Spectrometry. Food Anal. Methods 2017, 10, 587–595. [Google Scholar] [CrossRef]
- Abdelghani, J.I.; Al-Degs, Y.S. Spectroscopic quantifiication of preservatives in different food matrices using QuEChERS extraction and multivariate calibration with comparison against liquid chromatography. Arab. J. Chem. 2022, 15, 103462. [Google Scholar] [CrossRef]
- Liu, W.; Zhang, K.; Chen, J.; Yu, J. Ascorbic acid and choline chloride: A new natural deep eutectic solvent for extracting tert-butylhydroquinone antioxidant. J. Mol. Liq. 2018, 260, 173–179. [Google Scholar] [CrossRef]
- Caixeta-Neta, A.; Ribeiro, G.C.; De Amorim, K.P.; Andrade, L.S. Electrochemical determination of thiabendazole pesticide extracted and preconcentrated from tomato samples by cloud point extraction. Anal. Methods 2020, 12, 5823–5832. [Google Scholar] [CrossRef]
- Teixeira, C.A.; Poppi, R.J. Paper-based SERS substrate and one-class classifier to monitor thiabendazole residual levels in extracts of mango peels. Spectrochim. Acta. A Mol. Biomol. Spectrosc. 2020, 229, 117913. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Berenguer, C.V.; García-Cansino, L.; García, M.Á.; Marina, M.L.; Câmara, J.S.; Pereira, J.A.M. Exploring the Potential of Microextraction in the Survey of Food Fruits and Vegetable Safety. Appl. Sci. 2023, 13, 7117. https://doi.org/10.3390/app13127117
Berenguer CV, García-Cansino L, García MÁ, Marina ML, Câmara JS, Pereira JAM. Exploring the Potential of Microextraction in the Survey of Food Fruits and Vegetable Safety. Applied Sciences. 2023; 13(12):7117. https://doi.org/10.3390/app13127117
Chicago/Turabian StyleBerenguer, Cristina V., Laura García-Cansino, María Ángeles García, María Luisa Marina, José S. Câmara, and Jorge A. M. Pereira. 2023. "Exploring the Potential of Microextraction in the Survey of Food Fruits and Vegetable Safety" Applied Sciences 13, no. 12: 7117. https://doi.org/10.3390/app13127117
APA StyleBerenguer, C. V., García-Cansino, L., García, M. Á., Marina, M. L., Câmara, J. S., & Pereira, J. A. M. (2023). Exploring the Potential of Microextraction in the Survey of Food Fruits and Vegetable Safety. Applied Sciences, 13(12), 7117. https://doi.org/10.3390/app13127117







