Abstract
Digital therapeutics, evidence-based treatments delivered through software programs, are revolutionizing healthcare by utilizing cutting-edge computing technologies. Unlike conventional medical treatment methods, digital therapeutics are based on multiple information technologies, from data collection to analysis algorithms, and treatment support approaches. In this research, we provide a comprehensive overview of the latest technologies involved in the development of digital therapeutics and highlight specific technologies necessary for the future growth of the rapidly evolving digital therapeutics market. Furthermore, we present a system design of digital therapeutics for depression, currently being developed by our research team, to provide a detailed explanation of the technical process. Digital therapeutics require various technical supports, such as collecting user data in a security-enhanced medical environment, processing and analyzing the collected data, and providing personalized treatment methods to the user. The findings from this research will enable digital therapeutic companies to enhance their product performance, consequently bolstering their market competitiveness. Additionally, the research can be further extended to explore applicable methodologies at different stages of digital therapeutic environments.
1. Introduction
An international nonprofit organization, the Digital Therapy Alliance (DTA), defines digital therapy as evidence-based therapy interventions driven by high-quality software programs to prevent, manage, or treat a medical disorder or disease [1]. In essence, digital therapy aims to alleviate symptoms or continuously manage certain disorders by providing users with clinically validated medical software programs. Recently, the digital therapeutics (DTx) market has experienced rapid growth, particularly in the wake of the COVID-19 pandemic.
As defined by the DTA, DTx includes technology that provides disease prevention, management, and treatment [2]. In detail, DTx may require a predictive machine learning model for prevention, a recommendation system for providing treatment, and a user classification algorithm that can offer treatment according to the user’s continuously updated condition for management. Currently, most of the Food and Drug Administration (FDA)-certified DTx products are in the form of prescribing treatments for patients diagnosed by physicians in hospitals and treating their diseases [3]. Therefore, even if the provided DTx is a system for treatment, diagnostic technology will also be important to understand the current user’s condition in order to take advantage of software that can adapt to varying situations. To summarize, DTx may not be easily categorized as prevention, diagnosis, or treatment, as seen in the current medical system, and a comprehensive system should be pursued regardless of the specific focus.
The outbreak of COVID-19 has become an opportunity for the global digital therapeutic market to experience explosive growth and attract increased attention. According to recent meta-analysis research [4], the average number of patients suffering from mental illnesses such as major depressive episodes and anxiety disorders has increased by more than 15 percent due to the COVID-19 pandemic, and the demand for digital healthcare has risen due to limited medical access. Owing to these circumstances, countries such as the United Kingdom, Germany, and Australia have implemented measures such as easing regulations on digital treatments related to mental illness or even providing affordable digital treatments through national medical insurance budgets [5,6]. In addition, the U.S. has accelerated market growth by loosening the regulation restrictions on FDA’s approval procedures applicable to existing medical devices.
In response to these market changes, Pear Therapeutics [7] received FDA approval for the first time, and many companies worldwide are also developing digital therapeutics products to diagnose and treat human diseases. For example, Pear Therapeutics’ substance use disorder (SUD) treatment product, reSET, provides computerized behavioral therapy as a mobile application. reSET has been approved as an FDA De Novo and can be supplied in the prescription form to users aged 18 or older. It is a 90-day program that provides users with treatment and management of addictions such as cocaine and alcohol. Pear Therapeutics is also developing other DTx products to treat mental illnesses such as insomnia, schizophrenia, and post-traumatic stress disorder (PTSD). Another leading DTx company approved by the U.S. FDA is Akili Interactive. Akili provides Endeavor Rx for children with attention deficit hyperactivity disorder (ADHD) [8]. The product uses various gamified content to help control their attention, work memory, and execution skills through selective stimulus management engine (SSME) technology [9].
Most of the digital therapeutics products currently available in the global market are in the form of exclusive software such as mobile applications or medical platforms. At present, there are two primary forms of digital treatments: (1) software as a medical device (SaMD), which serves various medical purposes through standalone software, as defined by the International Medical Device Regulators Forum (IMDRF). SaMD encompasses healthcare platforms and networks that do not require specific hardware devices. (2) software in a medical device (SiMD), which shares a similar purpose but necessitates particular hardware.
For the definition of digital therapeutics, digital health and digital medicine are similar terms that are often misused in research. Digital health has the most comprehensive scope, and digital medicine and digital therapeutics are included in it. According to Figure 1, clear clinical evidence is required to classify digital health medicine from digital health [10]. Digital health refers to technologies aimed at improving the user’s well-being or health in everyday life, but it does not necessarily have to include clinical evidence. However, both digital medicine and digital therapeutics require a definite medical evaluation. In particular, to be classified as a digital therapeutic product, the medical technology must prove its effectiveness with real-world data results through clinical trials.
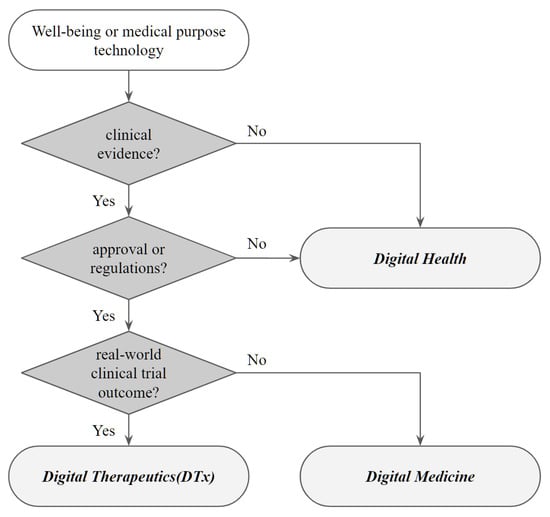
Figure 1.
Classification of Digital Health, Digital Medicine, and Digital Therapeutics.
Digital therapeutics is not just about digitizing existing medical treatment methods, but it represents a new type of treatment software with various new computer technologies applied in each process. From identifying specific user medical conditions to treating and managing diagnosed disorders, DTx requires multiple cutting-edge technologies [11].
In this paper, we explain the latest technologies that should be considered for developing DTx and provide examples of major depressive disorder DTx designs. The products in DTx consist of four main categories: data collection and preprocessing, data analysis algorithms, HCI (human–computer interaction) factors, and recommendation systems for treatment.
The core technologies in DTx consist of four main categories: data collection and preprocessing, data analysis algorithms, HCI, and recommendation systems for treatment. MDDs include both diagnosis and treatment of diseases that digital treatments can perform, and all four categories are essential to consider in each process. With the development of smart devices, it is possible to identify symptoms in daily life. It also has the characteristics of mental illnesses that require the daily application of various nondrug treatments, so an expansion of the DTx system can be applied. The demand for mental illness treatment has soared worldwide after the pandemic, and non-face-to-face systems have increased, so the suggested DTx architecture will be a good stepping stone for human well-being. We expect DTx companies to improve their product performance and digital health researchers to expand the research scope to applicable methods in the growing digital therapeutics market.
2. Technologies in DTx
Papers on digital therapeutics published after 2019 organized the concept and trend of the market and listed what types of latest technologies can be applied for future development. We used the following search terms: “digital therapeutics”, “digital health”, “digital medicine”. A total of 26 different research papers from Q1 journals, Q2 journals, and conferences were selected to generate the foundation for this study. Subsequently, for each technical section listed in this paper, more than 30 studies for each area were searched under the same conditions. From the list, the optimal and most supporting studies for the digital therapeutics field were found and listed. Finally, all papers referred to in this study were searched on Google Scholar and PubMed.
Digital therapeutics have been actively applied in various medical fields, such as chronic, physical, and mental diseases. Rather than simply digitizing existing diagnostic and therapeutic methods, digital therapeutics companies have begun to incorporate treatments that utilize computer technologies such as big data analysis, artificial intelligence (AI) techniques, and machine learning (ML) algorithms.
The technology used in digital therapeutics can be mainly viewed from the perspective of developers and actual service users. The left side of Figure 2 shows the essential steps from the DTx service developer’s point of view and the technologies to be considered for each implementation step. On the other hand, the right side of the Figure 2 presents the technologies faced from the service user’s point of view. This section summarizes the technologies that need to be considered in DTx development and commercialization into four core categories.
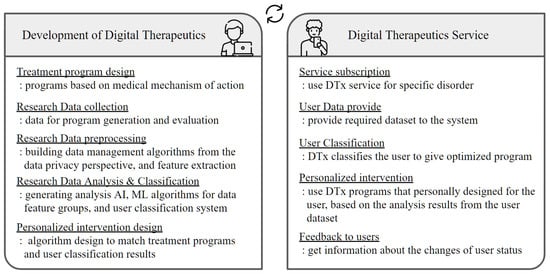
Figure 2.
DTx technologies from two perspectives: system developer and system user.
2.1. Data Collection
In terms of data collection, accurate symptoms and severity must be identified first before providing medical treatment to users. Although there are differences in diagnostic criteria and methods for each disease, physical disorder diagnoses are usually made through medical data collected in hospitals, while various rating scales are widely used for mental illnesses. Medical image data or biosignal data have the advantage of observing the objective state of the patient, but only the data of the specific day can be viewed [12]. In addition, the medical rating scales include questionnaires that can determine past conditions, but it is less objective because the score highly depends on the patient’s self-response. However, digital therapeutics can consider objectivity and time series characteristics through various types of data collection.
Many digital therapeutic products are currently developed in a mobile application format. One of the valuable aspects of the mobile applications of DTx is that it can bring additional data through the user’s wearable device. Various wearable sensor devices, such as smartwatch types, bracelets, rings, necklaces, chest bands, and shoe insoles, have been developed to gather human biosignal data. Photoplethysmogram (PPG) sensors are the most widely utilized body indicators of heart-related data such as heart rate, heart rate variability, and electrocardiogram [13]. In addition, user data can be collected with various sensors of wearable devices, such as user activity detection using a gyroscope and accelerometer, biological fluid calculation with a sweat-rate sensor [14], user motion recognition using pressure sensors, and emotional state prediction using skin conductivity and electromyography [15]. DTx products can optimize these user data to provide a more objective treatment according to their target disorder.
In addition to collecting user data using mobile devices, disease-related data using virtual reality (VR) devices have also been actively developed recently. VR devices were adopted as exposure therapy devices provided to patients with mental illness in [16], where they could create specific spaces and situations similar to reality. The treatment and diagnosis of mental disorders are also continuously developing so that they can be provided through VR devices, and as one of the representative examples, there is research to identify and determine attention deficit and hyperactivity disorder (ADHD) symptoms in children based on collected VR data [17]. That study generated a virtual environment to collect data from users in multiple VR scenes, and an ADHD classification was conducted with an AI model created through a DSM-5-based rating scale.
Data Preprocessing
Since the data accumulated from wearable sensors and smart devices cannot be directly applied to algorithm analysis due to their high dimensionality and high noise rate [18], it must first undergo a noise filtering, feature selection, and extraction process to obtain representative data features from the original dataset.
However, when collecting and applying data at both the development stage and the actual service stage, it is essential to check whether the data contain critical personal information that can infer a specific individual or not. Physical and psychological data that determine the user’s medical condition are classified as personal health information (PHI), which must be controlled under strict data protection regulations, especially in the United States through the Health Insurance Portability and Accountability Act (HIPAA) [19]. Therefore, user data should be collected without personal identification information or need to be deidentified [20,21,22] through separate data preprocessing after the data collection step.
For example, Neamatullah et al. [20] developed a pattern-matching deidentification algorithm based on the Perl language, to deidentify patients’ critical information from medical records. With the program, they achieved an overall recall of 0.967 in deidentification performance. On the other hand, Dernoncourt et al. [23] adopted a recurrent neural network model to detect and deidentify PHI from patient medical notes. With a bidirectional long short-term memory model structure, they increased the deidentification performance to 0.9923 for the F1-score on the Multi-parameter Intelligent Monitoring for Intensive Care (MIMIC) clinical dataset.
Including the above two methods, most of the deidentification processing techniques currently applied in medical data analysis research are pseudonymization, aggregation, data reduction, data suppression, and data-masking procedures. In public medical datasets, a method of leaving only the data variables necessary for analysis is generally applied through a data reduction method that removes all identifier information. When analyzing the data collected by each medical institution, individual user information is modified through pseudonymization, and critical features are deleted through data reduction. After that, if necessary, data values such as age, height, and weight may be categorized before use. The deidentification items of medical data are applied equally to comply with international standards in almost all institutions and differ only in their methods. In general, deidentification is applied to medical record data simply by tagging, and artificial-neural-network-based algorithms are applied to data with complex structures and dimensions, such as medical images.
2.2. Data Analysis
Digital therapeutics require an accurate data analysis in both the development and service steps, as shown in Figure 2. Since DTx utilizes medical datasets, a high rate of sensitivity and specificity performance is required from the data analysis. For example, during the development stage of DTx, it is necessary to analyze the processed research data to generate algorithms for diagnosis, classification, and treatment recommendation. Moreover, from the perspective of service users who wish to use DTx, they expect accurate analysis results on their medical condition and optimally personalized treatments based on their data. Therefore, AI technology using various machine learning techniques is applied to each step.
2.2.1. Machine Learning for DTx
In previous research fields, medical data analysis has mainly focused on statistical analysis techniques. However, the medical AI field is expanding with the development of various machine learning algorithms that show higher classification performance by repeatedly learning hidden data features that human eyes cannot recognize. By training data features through deep neural network structures, it expanded its scope to specific medical fields such as radiology [24], pathology [25], neuroscience [26], genetics [27], mental illness [28], and various other chronic diseases.
DTx products utilize diagnostic or classification algorithms to analyze targeted illnesses through medical AI technology and design their systems. When analyzing medical image data, convolutional neural network (CNN)-based techniques such as prediction, classification, segmentation, and augmentation are performed to obtain meaningful information and make decisions from the original input image. In addition, recurrent neural network (RNN)-based algorithms and transformer-based encoder–decoder-structured algorithms that can be applied to time-series data analysis are used because it is important to check the state changes rather than diagnosing with a user’s static state.
Medical imaging is where machine learning techniques that identify data characteristics and help make final decisions are currently being applied the most [29]. The paper shows that learning techniques such as support vector machines and relevance vector machines are commonly used in the fields of computer-aided diagnosis (CAD) and functional brain mapping. Through this, lesions and abnormalities are efficiently found in medical images containing noise. In addition, similar machine learning algorithms have been used in various ways in the field of lung disease brought on by COVID-19. Research has emerged to find infected parts, evaluate the severity [30], and see accurate lesion positions through lung CT images and X-ray images [31,32]. With this research, it has been confirmed that machine learning algorithms can efficiently help humans to make medical decisions.
2.2.2. Federated Learning for DTx
Since digital therapeutics is a field that deals with sensitive medical data, it is required to implement a deidentification process for the collected user data. In particular, in a model training structure for machine learning, a method of collecting data at a single server site or institution is required. Data leakage, invasion, or security attacks will likely occur in this procedure. However, using a federated learning structure allows the researcher to be relieved of data security concerns.
A federated learning (FL) algorithm does not collect data from users [33]. Instead, the central server generates one initial global model. Then, it distributes the model weights to the devices participating in the learning procedure, and each device learns the received model weights in its data. After that, the updated model weight is transmitted back to the central server from each user. The server finally integrates the collected model weights to create a new global model. This whole process repeats until the model converges. As seen from its structure, there is no movement of sensitive information from personal medical records when using federated learning. Therefore, the server is secure in terms of user data security or user data ownership because it cannot access or verify user information [34].
Various research studies have been conducted to use medical artificial intelligence technology that applies federated learning in multiple directions to solve the limitations of data regulations, such as the General Data Protection Regulation (GDPR) [35] and California’s Privacy Rights Act (CPRA). For example, a study that applied a FL environment that classified pediatric chest radiographs based on patients’ lung imaging data demonstrated that using the FL structure could lead to a similar performance compared to existing machine learning techniques while maintaining compliance with data security regulations. Medical federated learning research about diagnosing hypertrophic cardiomyopathy also showed a similar diagnostic performance to the machine learning algorithm for collecting sensitive medical datasets [36]. In addition to applying FL to medical image data, some studies adopt patient biosignal datasets to analyze their diseases. Representative research examples include classifying arrhythmia by applying federated learning into the CNN analysis of electrocardiogram data, the Fed-REMCS (Real-time Emotion State Classification from Multi-modal Streaming) study [37] that classified human emotions by analyzing physiological signals, and the PFCM (Personalized Federated Cluster Model) study [38] that analyzed depression severity through HRV analysis.
The analysis results through medical AI are incorporated into digital therapeutics, and the system manages and treats the confirmed disease symptoms. However, there are still limitations to applying federated learning to digital therapeutics beyond the field of simple medical artificial intelligence research. Since most DTx products are mobile software, the first consideration must be understanding the different hardware devices used by each user. To apply digital therapeutics, it is necessary to use a hospital-to-patient structure for federated learning, as shown in Figure 3, among the structures presented in the previous research [39]. Yet, efficient data processing through machine learning becomes problematic since it uses a mobile platform. Recently, lightweight machine learning models such as MobileNets [40] have also been developed for limited-resource environments. However, it is still difficult to consider all the mobile user environments. In addition to resource limitations, issues such as client management, responsibility, and traceability still need to be solved in medical federated learning [41]. Furthermore, collecting and analyzing user data will require implementing an FL system that can be comprehensively applied to the DTx system, as data security or data ownership problems remain regardless of how deidentifiable processing is adopted.
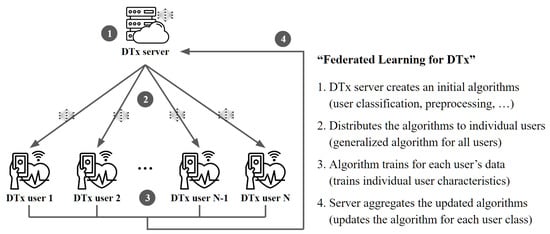
Figure 3.
Top-down federated learning structure for digital therapeutics. The server distributes a model to the individual clients to update the global model.
2.3. HCI Factors
Regardless of the kinds of technology implied in DTx, user interactivity must be sufficiently satisfied. In the case of DTx, unlike conventional medical services, it is possible to empower users to participate actively in treatment programs. However, at the same time, since self-management is required, motivation must be provided for appropriate compliance [42,43]. This empowerment and motivation can be provided through sufficient interactivity of the services. For this, there are a few suggestions about user-centered human–computer interaction components from earlier DTx studies. Furthermore, the therapeutic alliance is also considered necessary, especially in mental-healthcare-related areas where DTx has been most widely applied in recent years. The therapeutic alliance (TA), which refers to the rapport and the relationship between physicians and patients in existing mental health treatment programs, remains a crucial factor in digital formation [44]. Therefore, to secure such a therapeutic alliance, the HCI factors must be considered [45].
To establish a TA, the difficulty of non-face-to-face conditions in DTx can be offset by several HCI theories suggested by Tong et al. [46]. The persuasive system design (PSD) contains four factors; primary task support, dialogue support, system credibility support, and social support, which can advocate empathy and build the relationship between DTx and the users [45,46].
For primary task support, minimizing the user’s participation effort must be accompanied by plenty of guides and explanations to change the user’s attitude. In addition, it must provide personalized and trackable content to allow users to self-monitor their activities. In dialogue support, patients need to be provided feedback through words, images, symbols, or sounds, and it should be designed to appeal to users. Moreover, rewards, reminders, and suggestions that help to accomplish the task are essential in dialogue support. On the other support, system credibility, the information provided in the service must be truthful and appear knowledgeable and experienced. Moreover, although it is digitized, it still needs to deliver real-world feelings behind the service. At last, for social support, providing shared services that can promote social learning, comparison, cooperation and recognition are recommended [47]. If these HCI elements based on the PSD theory are incorporated into digital therapeutics, it will form a therapeutic alliance, which will increase the effectiveness of digital therapeutics.
In addition to forming a TA, long-term regular use of DTx requires user satisfaction [48]. For this sustainability, the perceived usefulness, perceived ease of use, and attitude are suggested in the existing technology acceptance model (TAM) [49]. The TAM model states that in the acceptance of new technologies, the perceived usefulness and perceived ease of use influence the attitudes in a positive or negative direction toward the service. These attitudes are mediated to ultimately determine the intention to continue using the service or product [50]. Depending on the characteristics of the service or product to be applied, the variable of TAM is often expanded. In the DTx context, perceived enjoyment is often considered as a compelling variable, and it has been proved that it does affect the intention to use [51]. In this regard, successful digital treatment requires users to perceive the product as easy to use and recognize that the program is beneficial to them. In addition, if a positive attitude toward digital therapeutics is secured by delivering the perception that digital therapeutics is enjoyable, users’ intention to continue using the product can be promoted. To sum up, these perceptions will further induce appropriate compliance and regular use of the DTx from users. Encompassing the HCI-related elements, the user system flowchart shown in Figure 4 demonstrates the adjusted features specifically applicable to DTx.
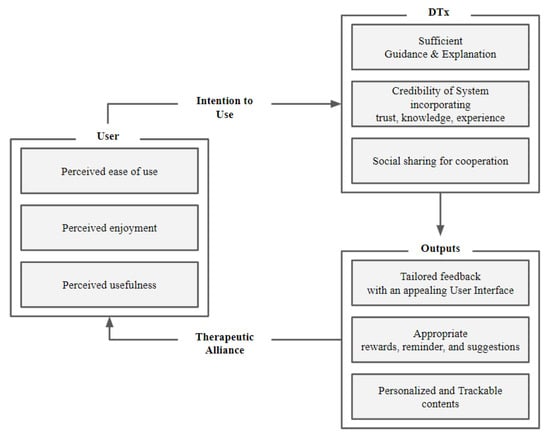
Figure 4.
Human-centered system flowchart proposed to increase the effectiveness of DTx.
2.4. Recommendation System
One of the most representative advantages of digital therapeutics compared to traditional treatment methods is that it can provide the most optimized treatment for individual users based on the users’ data or record histories. This user customization is highly related to a recommendation system (RS) that provides a personalized treatment design based on user data. It is possible to suggest more dynamic treatments by collecting users’ daily data from DTx products and not just relying on the treatment provided by hospitals collectively.
Typically, an RS provides customized systems to users using algorithms that are content-based, collaborative, or hybrid filtering [52]. In particular, hybrid filtering has been applied to extract hidden data features, program usage, and user information to calculate the similarity between users and systems. For example, in the RS field, there are studies on content filtering systems that recommend subsequent media through simple user usage records [53,54]. Many studies suggest movies or media by analyzing user review texts, and image features using deep-neural-network-based algorithms [55,56,57].
The digital therapeutics products may apply a hybrid-filtering recommendation system among the structures as shown in Figure 5. For example, Bhatti designed the clinical care system, which used an fb-KNN algorithm-based RS for data collected when diagnosing and treating diseases [58]. Furthermore, Ihnaini presented a diabetes prediction method, which adopted a machine-learning-based smart healthcare RS for electronic health records [59]. As such, DTx can collect information about users from devices and mobile applications and apply recommendation systems in more detail than pharmaceutical approaches or offline treatment programs. In the future, the performance of DTx products will be determined based on how detailed the provided user-specific treatment is.
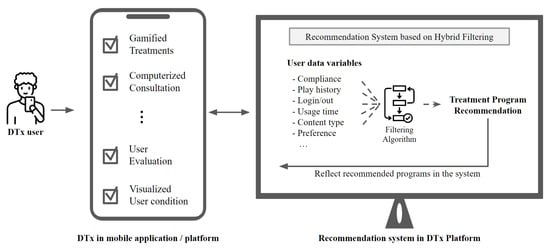
Figure 5.
Recommendation System for Digital Therapeutics.
Moreover, it should be continuously developed to use an improved hybrid-filtering recommendation system based on multiple data features extracted through machine learning algorithms.
When applying this recommendation system, personal health information protection technologies should be considered together. Providing personalized therapeutic content based on each user’s data variables and attributes means checking their own data. However, this can make users feel that their information has been violated. Therefore, a recommendation algorithm based on federated learning described in Section 2.2.2 should be considered. Rather than collecting and analyzing each user’s data, a structure that develops into a separate recommendation algorithm with a personalized federated learning structure by forming an initial global algorithm should be designed.
3. Major Depressive Disorder DTx
As an extension of the research from this paper, our research team is currently developing a digital therapeutics platform based on the aforementioned cutting-edge technologies. The digital therapeutics platform is developed to efficiently diagnose major depressive disorder (MDD) and provide customized management programs based on diagnosis results. The entire architecture is designed by incorporating technologies that can cover the possible issues of traditional medical therapies while ensuring a high performance at each stage of development. Based on this, we are conducting research and development with the goal of delivering SaMD-format digital therapeutics to patients diagnosed with major depressive disorder.
Major depressive disorder (MDD) is a mental disorder with symptoms that maintain for more than two weeks of persistent depressed mood, changes in appetite, lethargy, and sleep disorders. It is one of the most common disorders that occur in modern people. According to reports from the World Health Organization (WHO), the number of people suffering from MDD worldwide exceeds 280 million, and approximately 60% of suicide deaths worldwide are from people suffering from major depressive disorder. As a diagnostic method, complex psychometric tests such as the Hamilton Depression Rating Scale (HDRS), Hamilton Anxiety Rating Scale (HARS), Beck Depression Inventory (BDI), and Patient Health Questionnaire-9 (PHQ-9), are performed under the supervision of psychiatrists. Representative treatments for depression include antidepressant medications, electroconvulsive therapy (ECT), repetitive transcranial magnetic stimulation therapy (rTMS), and cognitive behavioral therapy (CBT). However, due to the low access to medical healthcare and concerns about possible side effects from treatment methods, there is a need for an adequately designed interactive system to provide comprehensive social care for individuals with depression.
Therefore, we determined that a digital therapeutics platform was well-suited to address the medical gaps in the care of patients with major depressive disorders and assist psychiatrists in gaining more detailed insights into their patients’ daily conditions. By leveraging users’ everyday life data, we aimed to diagnose and recognize the severity and characteristics of depression, with the ultimate goal of providing personalized treatment interventions supported by AI technologies. In addition to technological considerations, we also took into account four key points when designing this system.
- Real-time user management.
- Daily life management.
- System usability.
- Personalized treatment.
To this end, we created a complete DTx service that includes the use of hardware devices, data categorization, preprocessing, analysis, user classification, and provision of customized care. The specific process of the system is shown in Figure 6.
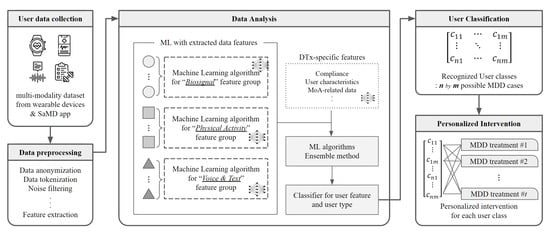
Figure 6.
Overall Architecture of Major Depressive Disorder Digital Therapeutics, Under Development.
First, user data are collected to determine depression severity and characteristics. Then, the multimodal data preprocessing method is adopted to more accurately predict user status, including information collected from multiple wearable devices and our mobile application. As mentioned above, various depression rating scales have been used for patient diagnosis tools. Still, they have a critical disadvantage in that they have to depend on scale score results, which are easily affected by the patient’s daily condition. For example, for depression, it is necessary to check that specific depression-related symptoms last for more than two weeks. However, the diagnosis through a rating scale asks patients to judge their condition for the past two weeks. Therefore, observing time-series changes in the objective state is more accurate and can be continuously collected through wearable devices and our SaMD mobile application.
Data collected from each device through our MDD DTx application go through a preprocessing process at the server site. As described above, the data collected from the application are classified into personal health information (PHI) and may include users’ sensitive information. Therefore, our platform primarily reduces security risks through data tokenization and anonymization in the data transmission process. In addition, critical user information is excluded, and unnecessary signals generated from wearable device data are removed through noise filtering from the tokenized data. Finally, as the last step in the data processing, our platform extracts and observes representative data features from the entire collected data through the feature extraction step for training.
To understand the user’s detailed characteristics through the multimodal approach, data features that have undergone the preprocessing step are grouped into similar clusters before the data analysis. Then, each feature group is analyzed through a machine learning algorithm that fits its data structure. Since the disorder-related variables for each feature group are different, our team did not integrate extracted data features into one embedding layer. Therefore, each feature group with a biosignal, physical activity, voice, and text separately has a corresponding machine learning algorithm. The trained result leads to generating an integrated classifier, and at that time, the feature-trained result for the specific feature collected from the DTx application is also added. At the beginning of the system, the DTx usage information does not come in due to the lack of stored data, but the importance of this section increases as the user continues to use the DTx application. These results significantly impact user classification and personalized-intervention-providing algorithms.
As mentioned in the previous section, one of the most significant advantages of digital therapeutics is that it can provide individually customized treatments to each user, unlike conventional treatment methods. Pharmacological treatment can also be personalized, such as changing the type of drug or dosage according to user experience or treatment effect, but it cannot reflect patient changes according to treatment effect or customize according to patient characteristics. Therefore, our research team used a hybrid-filtering recommendation system based on collected user data to provide user-customized treatment programs. For example, MDD has a huge variance in cause and severity from person to person, and the optimal treatment methods based on these factors are all different. We provide personalized depression intervention, which is strictly implemented based on a medically proven mechanism of action (MoA), to our users. It is designed to suggest individualized treatment processes to all users by considering their biological, physical, and psychological factors through data analysis and user classification algorithms.
In addition, a human-centered design that can be used for a long time is also reflected in our MDD DTx in consideration of the characteristics of diseases that are difficult to treat in a short time. For this, virtual agents are provided within the system to form a therapeutic alliance. In addition, in order to provide a better understanding of the system, the content for explanations and guides is provided separately. Moreover, because the secure credibility of the system is important, the image of a doctor who can be easily trusted is used in the system. These elements are reflected in our DTx system, and as an output, appropriate rewards, reminders, and suggestions are provided to users according to the use of the system. There is also a page where users can always see their usage data. The developing DTx for MDD followed the human-centered system flowchart suggested earlier. We expect that through this human-centered design, we can secure ease, usefulness, and enjoyment, leading to users’ continuous use.
Currently, antidepressant treatment is provided to patients as the most effective treatment for major depressive disorders, and patients visit medical institutions regularly to continue treatment. Still, the need for daily life management during long visit cycles remains a problem to be solved. With the DTx architecture presented in the study, it will be possible to check and analyze the user’s condition in real time to derive the optimal treatment effect for the user and prevent potential accidents. As a result, the spread of DTx systems is expected to help innovate in public healthcare by providing solutions for mental illness, which causes high suicide rates worldwide.
4. Discussion
With the development of computer technology and software platforms such as mobile applications worldwide, diagnostic and treatment methods for human diseases are also changing in an innovative direction, digital therapeutics. The DTx market is expected to expand to USD 7.1 billion by 2025. However, so far, research on DTx only addresses the limitations of clinical regulation or the effectiveness of its own clinical solutions. Therefore, continuous development will be needed to provide optimal solutions to meet growing demand.
The field is gradually developing through applying the latest technologies, from collecting user data in the DTx development and service to providing treatment back to the user, using data anonymization, deidentification, and federated learning for secure data processing, feature extraction for observing hidden data characteristics, various machine learning analysis for efficient results, and recommendation systems for providing users with optimized treatment processes. This way, the technologies are integrated at each stage to complete a new medical platform for the target disease.
This paper described the latest technologies to be applied in the process of developing solutions. Differentiated digital therapeutic solutions can only be created when advanced technologies are involved, from data collection to solution provision, beyond simply digitizing existing treatments. We used a comprehensive architecture of major depression digital treatments under development as an example to convey clear technical directions that markets should pursue.
The DTx platform for major depressive disorders introduced in this paper is currently undergoing software certification in the development stages and is nearing the commencement of clinical trials in the Republic of Korea. Therefore, detailed information regarding the extracted data feature types, examples of customized treatment programs, and the specific number of classes used for the DTx user classification was not disclosed in this paper, as it falls beyond the scope of the overall architecture introduction. Subsequent studies will focus on the effectiveness and performance of digital treatments for major depressive disorders based on the results of the clinical trial conducted on our MDD platform. Furthermore, our team will also explore specific advantages of DTx, including user satisfaction and compliance, in addition to assessing the treatment performance of MDD DTx on patients.
5. Conclusions
Unlike conventional clinical methods, the digital therapeutics market provides complex solutions based on hardware and software technologies. It is necessary to develop a way that can collect data in various situations and dynamically provide optimal diagnostic, management, and treatment solutions. This research summarized technologies and examples that need to be applied at each stage and proposed an entire architecture to inform solution developers and providers.
Author Contributions
J.H.Y. and H.J. contributed to the composition and preparation of the paper. T.-M.C. proposed the research and theorem in the field of digital therapeutics technologies. J.H.Y., H.J. and T.-M.C. discussed the suggested research idea. J.H.Y. and H.J. wrote the overall manuscript. T.-M.C. provided invaluable guidance throughout the research and the writing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Technology Innovation Program (or Industrial Strategic Technology Development Program-Source Technology Development and Commercialization of Digital Therapeutics) (20014967, Development of Digital Therapeutics for Depression from COVID-19) funded By the Ministry of Trade, Industry & Energy (MOTIE, Republic of Korea).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
This research was supported by Institute of Information & communications Technology Planning & Evaluation (IITP) grant funded by the Korea government (MSIT) (no. 2019-0-00421, AI Graduate School Support Program (Sungkyunkwan University)).
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
Abbreviations
The following abbreviations are used in this manuscript:
| FL | Federated learning |
| DTx | Digital therapeutics |
| MDD | Major depressive disorder |
References
- Digital Therapeutics Alliance. Digital Therapeutics Definition and Core Principles. Technical Report. 2019. Available online: https://dtxalliance.org/wp-content/uploads/2021/01/DTA_DTx-Definition-and-Core-Principles.pdf (accessed on 1 January 2023).
- Digital Therapeutics Alliance. Digital Therapeutics Product Categories. Technical Report. 2021. Available online: https://dtxalliance.org/wp-content/uploads/2021/01/DTA_FS_DTx-Product-Categories_010521.pdf (accessed on 1 January 2023).
- Hong, J.S.; Wasden, C.; Han, D.H. Introduction of digital therapeutics. Comput. Methods Programs Biomed. 2021, 209, 106319. [Google Scholar] [CrossRef] [PubMed]
- Leung, C.; Ho, M.K.; Bharwani, A.A.; Cogo-Moreira, H.; Wang, Y.; Chow, M.S.; Fan, X.; Galea, S.; Leung, G.M.; Ni, M.Y. Mental disorders following COVID-19 and other epidemics: A systematic review and meta-analysis. Transl. Psychiatry 2022, 12, 205. [Google Scholar] [CrossRef] [PubMed]
- McKinsey & Company. The European Path to Reimbursement for Digital Health Solutions. Technical Report. 2020. Available online: https://www.mckinsey.com/industries/life-sciences/our-insights/the-european-path-to-reimbursement-for-digital-health-solutions (accessed on 1 January 2023).
- Health IT News. Germany Introduces Digital Supply Act to Digitalise Healthcare. Technical Report. 2019. Available online: https://www.healthcareitnews.com/news/emea/germany-introduces-digital-supply-act-digitalise-healthcare (accessed on 1 January 2023).
- Velez, F.F.; Colman, S.; Kauffman, L.; Ruetsch, C.; Anastassopoulos, K. Real-world reduction in healthcare resource utilization following treatment of opioid use disorder with reSET-O, a novel prescription digital therapeutic. Expert Rev. Pharmacoecon. Outcomes Res. 2021, 21, 69–76. [Google Scholar] [CrossRef] [PubMed]
- Kollins, S.H.; DeLoss, D.J.; Cañadas, E.; Lutz, J.; Findling, R.L.; Keefe, R.S.; Epstein, J.N.; Cutler, A.J.; Faraone, S.V. A novel digital intervention for actively reducing severity of paediatric ADHD (STARS-ADHD): A randomised controlled trial. Lancet Digit. Health 2020, 2, e168–e178. [Google Scholar] [CrossRef]
- Bove, R.M.; Rush, G.; Zhao, C.; Rowles, W.; Garcha, P.; Morrissey, J.; Schembri, A.; Alailima, T.; Langdon, D.; Possin, K.; et al. A videogame-based digital therapeutic to improve processing speed in people with multiple sclerosis: A feasibility study. Neurol. Ther. 2019, 8, 135–145. [Google Scholar] [CrossRef]
- Elenko, E.; Underwood, L.; Zohar, D. Defining digital medicine. Nat. Biotechnol. 2015, 33, 456–461. [Google Scholar] [CrossRef]
- Dang, A.; Arora, D.; Rane, P. Role of digital therapeutics and the changing future of healthcare. J. Fam. Med. Prim. Care 2020, 9, 2207. [Google Scholar] [CrossRef]
- Sajda, P. Machine learning for detection and diagnosis of disease. Annu. Rev. Biomed. Eng. 2006, 8, 537–565. [Google Scholar] [CrossRef]
- Temko, A. Accurate heart rate monitoring during physical exercises using PPG. IEEE Trans. Biomed. Eng. 2017, 64, 2016–2024. [Google Scholar] [CrossRef]
- Salvo, P.; Di Francesco, F.; Costanzo, D.; Ferrari, C.; Trivella, M.G.; De Rossi, D. A wearable sensor for measuring sweat rate. IEEE Sens. J. 2010, 10, 1557–1558. [Google Scholar] [CrossRef]
- Nakasone, A.; Prendinger, H.; Ishizuka, M. Emotion recognition from electromyography and skin conductance. In Proceedings of the 5th international Workshop on Biosignal Interpretation, Tokyo, Japan, 6–8 September 2005; pp. 219–222. [Google Scholar]
- Freeman, D.; Reeve, S.; Robinson, A.; Ehlers, A.; Clark, D.; Spanlang, B.; Slater, M. Virtual reality in the assessment, understanding, and treatment of mental health disorders. Psychol. Med. 2017, 47, 2393–2400. [Google Scholar] [CrossRef] [PubMed]
- Son, H.M.; Lee, D.G.; Joung, Y.S.; Lee, J.W.; Seok, E.J.; Chung, T.M.; Oh, S. A novel approach to diagnose ADHD using virtual reality. Int. J. Web Inf. Syst. 2021, 17, 516–536. [Google Scholar] [CrossRef]
- Wang, S.; Celebi, M.E.; Zhang, Y.D.; Yu, X.; Lu, S.; Yao, X.; Zhou, Q.; Miguel, M.G.; Tian, Y.; Gorriz, J.M.; et al. Advances in data preprocessing for biomedical data fusion: An overview of the methods, challenges, and prospects. Inf. Fusion 2021, 76, 376–421. [Google Scholar] [CrossRef]
- Office for Civil Rights, HHS. Standards for privacy of individually identifiable health information. Final rule. Fed. Regist. 2002, 67, 53181–53273. [Google Scholar]
- Neamatullah, I.; Douglass, M.M.; Lehman, L.W.H.; Reisner, A.; Villarroel, M.; Long, W.J.; Szolovits, P.; Moody, G.B.; Mark, R.G.; Clifford, G.D. Automated de-identification of free-text medical records. BMC Med Inform. Decis. Mak. 2008, 8, 32. [Google Scholar] [CrossRef] [PubMed]
- Kushida, C.A.; Nichols, D.A.; Jadrnicek, R.; Miller, R.; Walsh, J.K.; Griffin, K. Strategies for de-identification and anonymization of electronic health record data for use in multicenter research studies. Med. Care 2012, 50, S82. [Google Scholar] [CrossRef]
- Meystre, S.M.; Friedlin, F.J.; South, B.R.; Shen, S.; Samore, M.H. Automatic de-identification of textual documents in the electronic health record: A review of recent research. BMC Med. Res. Methodol. 2010, 10, 70. [Google Scholar] [CrossRef]
- Dernoncourt, F.; Lee, J.Y.; Uzuner, O.; Szolovits, P. De-identification of patient notes with recurrent neural networks. J. Am. Med. Inform. Assoc. 2017, 24, 596–606. [Google Scholar] [CrossRef]
- Hosny, A.; Parmar, C.; Quackenbush, J.; Schwartz, L.H.; Aerts, H.J. Artificial intelligence in radiology. Nat. Rev. Cancer 2018, 18, 500–510. [Google Scholar] [CrossRef]
- Chang, H.Y.; Jung, C.K.; Woo, J.I.; Lee, S.; Cho, J.; Kim, S.W.; Kwak, T.Y. Artificial intelligence in pathology. J. Pathol. Transl. Med. 2019, 53, 1–12. [Google Scholar] [CrossRef]
- Ienca, M.; Ignatiadis, K. Artificial intelligence in clinical neuroscience: Methodological and ethical challenges. AJOB Neurosci. 2020, 11, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Libbrecht, M.W.; Noble, W.S. Machine learning applications in genetics and genomics. Nat. Rev. Genet. 2015, 16, 321–332. [Google Scholar] [CrossRef] [PubMed]
- Bzdok, D.; Meyer-Lindenberg, A. Machine learning for precision psychiatry: Opportunities and challenges. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Erickson, B.J.; Korfiatis, P.; Akkus, Z.; Kline, T.L. Machine learning for medical imaging. Radiographics 2017, 37, 505–515. [Google Scholar] [CrossRef]
- Gao, K.; Su, J.; Jiang, Z.; Zeng, L.L.; Feng, Z.; Shen, H.; Rong, P.; Xu, X.; Qin, J.; Yang, Y.; et al. Dual-branch combination network (DCN): Towards accurate diagnosis and lesion segmentation of COVID-19 using CT images. Med. Image Anal. 2021, 67, 101836. [Google Scholar] [CrossRef]
- Chen, C.; Zhou, K.; Zha, M.; Qu, X.; Guo, X.; Chen, H.; Wang, Z.; Xiao, R. An effective deep neural network for lung lesions segmentation from COVID-19 CT images. IEEE Trans. Ind. Inform. 2021, 17, 6528–6538. [Google Scholar] [CrossRef]
- Wang, X.; Jiang, L.; Li, L.; Xu, M.; Deng, X.; Dai, L.; Xu, X.; Li, T.; Guo, Y.; Wang, Z.; et al. Joint learning of 3D lesion segmentation and classification for explainable COVID-19 diagnosis. IEEE Trans. Med. Imaging 2021, 40, 2463–2476. [Google Scholar] [CrossRef]
- Konečnỳ, J.; McMahan, H.B.; Yu, F.X.; Richtárik, P.; Suresh, A.T.; Bacon, D. Federated learning: Strategies for improving communication efficiency. arXiv 2016, arXiv:1610.05492. [Google Scholar]
- Bonawitz, K.; Eichner, H.; Grieskamp, W.; Huba, D.; Ingerman, A.; Ivanov, V.; Kiddon, C.; Konečnỳ, J.; Mazzocchi, S.; McMahan, B.; et al. Towards federated learning at scale: System design. Proc. Mach. Learn. Syst. 2019, 1, 374–388. [Google Scholar]
- Voigt, P.; Von dem Bussche, A. The EU General Data Protection Regulation (GDPR), 1st ed.; A Practical Guide; Springer International Publishing: Cham, Switzerland, 2017; Volume 10, pp. 10–5555. [Google Scholar]
- Raza, A.; Tran, K.P.; Koehl, L.; Li, S. Designing ecg monitoring healthcare system with federated transfer learning and explainable ai. Knowl.-Based Syst. 2022, 236, 107763. [Google Scholar] [CrossRef]
- Nandi, A.; Xhafa, F. A Federated Learning Method for Real-time Emotion State Classification from Multi-modal Streaming. Methods 2022, 204, 340–347. [Google Scholar] [CrossRef] [PubMed]
- Yoo, J.H.; Son, H.M.; Jeong, H.; Jang, E.H.; Kim, A.Y.; Yu, H.Y.; Jeon, H.J.; Chung, T.M. Personalized federated learning with clustering: Non-IID heart rate variability data application. In Proceedings of the 2021 International Conference on Information and Communication Technology Convergence (ICTC), Jeju Island, Republic of Korea, 20–22 October 2021; pp. 1046–1051. [Google Scholar]
- Yoo, J.H.; Jeong, H.; Lee, J.; Chung, T.M. Open problems in medical federated learning. Int. J. Web Inf. Syst. 2022. ahead-of-print. [Google Scholar] [CrossRef]
- Howard, A.G.; Zhu, M.; Chen, B.; Kalenichenko, D.; Wang, W.; Weyand, T.; Andreetto, M.; Adam, H. Mobilenets: Efficient convolutional neural networks for mobile vision applications. arXiv 2017, arXiv:1704.04861. [Google Scholar]
- Yoo, J.H.; Jeong, H.; Lee, J.; Chung, T.M. Federated learning: Issues in medical application. In Proceedings of the International Conference on Future Data and Security Engineering, Virtual Event, 24–26 November 2021; pp. 3–22. [Google Scholar]
- Lord, S.E.; Campbell, A.N.; Brunette, M.F.; Cubillos, L.; Bartels, S.M.; Torrey, W.C.; Olson, A.L.; Chapman, S.H.; Batsis, J.A.; Polsky, D.; et al. Workshop on Implementation Science and Digital Therapeutics for Behavioral Health. JMIR Ment. Health 2021, 8, e17662. [Google Scholar] [CrossRef]
- Ramakrishnan, P.; Yan, K.; Balijepalli, C.; Druyts, E. Changing face of healthcare: Digital therapeutics in the management of diabetes. Curr. Med. Res. Opin. 2021, 37, 2089–2091. [Google Scholar] [CrossRef]
- Jeong, H.; Yoo, J.H.; Song, H. Virtual Agents with Augmented Reality in Digital Healthcare. In Proceedings of the 2022 13th International Conference on Information and Communication Technology Convergence (ICTC), Jeju Island, Republic of Korea, 19–21 October 2022; pp. 2016–2021. [Google Scholar]
- D’Alfonso, S.; Lederman, R.; Bucci, S.; Berry, K. The digital therapeutic alliance and human-computer interaction. JMIR Ment. Health 2020, 7, e21895. [Google Scholar] [CrossRef]
- Tong, F.; Lederman, R.; D’Alfonso, S.; Berry, K.; Bucci, S. Digital Therapeutic Alliance With Fully Automated Mental Health Smartphone Apps: A Narrative Review. Front. Psychiatry 2022, 13, 819623. [Google Scholar] [CrossRef]
- Oinas-Kukkonen, H.; Harjumaa, M. Persuasive systems design: Key issues, process model, and system features. Commun. Assoc. Inf. Syst. 2009, 24, 28. [Google Scholar] [CrossRef]
- Bochicchio, M.A.; Vaira, L.; Mortara, A.; De Maria, R. Which Usability Assessment for Digital Therapeutics and Patient Support Programs? In Proceedings of the 2021 IEEE International Conference on Digital Health (ICDH), Chicago, IL, USA, 5–10 September 2021; pp. 276–282. [Google Scholar]
- Carrera, A.; Zoccarato, F.; Mazzeo, M.; Lettieri, E.; Toletti, G.; Bertoli, S.; Castelnuovo, G.; Fresa, E. What drives patients’ acceptance of digital therapeutics? The interplay between rational and institutional factors. BMC Health Serv. Res. 2023, 23, 145. [Google Scholar] [CrossRef]
- Marangunić, N.; Granić, A. Technology acceptance model: A literature review from 1986 to 2013. Univers. Access Inf. Soc. 2015, 14, 81–95. [Google Scholar] [CrossRef]
- Harvey, P.D. Digital Therapeutics to Enhance Cognition in Major Depression: How Can We Make the Cognitive Gains Translate Into Functional Improvements? Am. J. Psychiatry 2022, 179, 445–447. [Google Scholar] [CrossRef] [PubMed]
- Sharma, L.; Gera, A. A survey of recommendation system: Research challenges. Int. J. Eng. Trends Technol. IJETT 2013, 4, 1989–1992. [Google Scholar]
- Davidson, J.; Liebald, B.; Liu, J.; Nandy, P.; Van Vleet, T.; Gargi, U.; Gupta, S.; He, Y.; Lambert, M.; Livingston, B.; et al. The YouTube video recommendation system. In Proceedings of the Fourth ACM Conference on Recommender Systems, Barcelona, Spain, 26 September 2010; pp. 293–296. [Google Scholar]
- Reddy, S.; Nalluri, S.; Kunisetti, S.; Ashok, S.; Venkatesh, B. Content-based movie recommendation system using genre correlation. In Smart Intelligent Computing and Applications; Springer: Berlin/Heidelberg, Germany, 2019; pp. 391–397. [Google Scholar]
- Da’u, A.; Salim, N. Recommendation system based on deep learning methods: A systematic review and new directions. Artif. Intell. Rev. 2020, 53, 2709–2748. [Google Scholar] [CrossRef]
- Wang, Z.; Yu, X.; Feng, N.; Wang, Z. An improved collaborative movie recommendation system using computational intelligence. J. Vis. Lang. Comput. 2014, 25, 667–675. [Google Scholar] [CrossRef]
- Furtado, F.; Singh, A. Movie recommendation system using machine learning. Int. J. Res. Ind. Eng. 2020, 9, 84–98. [Google Scholar]
- Bhatti, U.A.; Huang, M.; Wu, D.; Zhang, Y.; Mehmood, A.; Han, H. Recommendation system using feature extraction and pattern recognition in clinical care systems. Enterp. Inf. Syst. 2019, 13, 329–351. [Google Scholar] [CrossRef]
- Ihnaini, B.; Khan, M.; Khan, T.A.; Abbas, S.; Daoud, M.S.; Ahmad, M.; Khan, M.A. A smart healthcare recommendation system for multidisciplinary diabetes patients with data fusion based on deep ensemble learning. Comput. Intell. Neurosci. 2021, 2021, 4243700. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).