Featured Application
This optimal preparation protocol of cell-encapsulating alginate capsules would realize a safe and effective cell-based therapy.
Abstract
Cell-based therapy is an excellent therapeutic modality that involves cell transplantation into patients; however, given that most transplanted cells die immediately post-transplantation, the application of this strategy remains limited. Cell encapsulation is a promising technique for prolonging the survival of transplanted cells, although a definitive encapsulation protocol is yet to be established. Herein, we selected sodium alginate as a polymer for cell encapsulation and optimized the structure and function of cell-encapsulating alginate capsules. First, alginate capsules were prepared using various concentrations of sodium alginate and calcium chloride solution. The NanoLuc luciferase (Nluc)-expressing murine mesenchymal stem cell line C3H10T1/2 was used to prepare the alginate capsules, and cell survival was evaluated after transplantation into mice. The structural properties of the alginate capsules were dependent on the preparation conditions. Capsules with adequate hardness were obtained using 1% sodium alginate and 10% calcium chloride solutions. Alginate capsules encapsulating 5 × 103 C3H10T1/2/Nluc cells/10 μL maintained a constant cell number over time under in vitro culture conditions. After transplantation into mice, C3H10T1/2/Nluc cells encapsulated in alginate capsules exhibited a significantly longer survival (≥40 days) than suspended cells. Based on these findings, cell-encapsulating alginate capsules with optimal properties can be used for long-term cell-based therapies.
1. Introduction
Cell-based therapy is an excellent therapeutic modality that involves the transplantation of cells prepared in vitro into patients, and several basic animal studies have reported remarkable therapeutic effects [1,2,3]. In recent years, several types of pluripotent stem cells have been established, including embryonic stem cells and induced pluripotent stem (iPS) cells, and protocols to differentiate these pluripotent stem cells into diverse tissue-specific cells have been developed [4,5], suggesting the possibility of the mass preparation of therapeutic tissue-specific cells. Cellular drugs, such as TEMCELL HS Injection and Kymriah, have been clinically applied in patients with serious diseases and have shown substantial efficacy. It is expected that the growing number of clinical trials on cellular drugs will lead to an expansion of cell-based therapies in the future [6,7]. However, the application of cell-based therapy is hindered by a serious challenge, that is, most transplanted cells die immediately post-transplantation, and the low or short survival of transplanted cells reduces the efficacy of cell-based therapy [8,9]. This cell death is caused by exposure to a severe in vivo environment post-transplantation or the elimination of foreign substances by immune cells [10]. To avoid the elimination of transplanted cells, pre-transplant immunomodulation or immune system deactivation by administering immunosuppressive agents has been investigated and attempted [11,12]. However, immunosuppression-induced side effects owing to the long-term use of immunosuppressive agents could markedly reduce the quality of life of patients [13]. Moreover, the optimization of the administration route and formulation of transplanted cells is also a significant challenge to achieve the distribution and retention of the cells at a proper site in the body [14]. Therefore, developing methods that prolong cell survival or maintain cell function without inducing potential side effects in patients is highly desirable.
Cell encapsulation, in which cells are encapsulated in a biocompatible polymer, physically protects transplanted cells from immune cells in vivo, thereby avoiding rejection post-transplantation [15,16]. Cell encapsulation renders the use of immunosuppressive agents unnecessary and can solve various challenges associated with cell transplantation. In addition, cell encapsulation with various biofunctional materials can facilitate the optimal distribution and retention of the transplanted cells as well as cell viability. Furthermore, capsule-encapsulated cells can survive and continuously release cytokines, which can be used in disease treatment [17]. Cell encapsulation research was initiated in 1964 by Thomas Chang, who proposed the use of ultra-thin polymer membrane microcapsules for the immunoprotection of transplanted cells [18]. Franklin Lim et al. first successfully immobilized pancreatic islets in alginate capsules in 1980 and reported excellent therapeutic effects in a rat model of type 2 diabetes for several weeks post-transplantation [19]. This finding further propagated intensive research on cell encapsulation, and encapsulated cell-based therapies have been explored to address various diseases, including diabetes, hemophilia, cancer, and renal failure [20,21,22]. Based on these superior therapeutic results, the application of encapsulated cells to treat human diseases has been promoted [23,24]. Although cell encapsulation studies have been extensively explored to develop cell-based therapy, advances remain limited in terms of the optimization of preparation protocols [25]. The preparation conditions for cell encapsulation can markedly impact the function of the encapsulated cells [26]; however, no protocol for cell encapsulation has been established, and cell-encapsulating capsules have been prepared under non-standardized conditions. In addition, some previous studies used ambiguous experimental methods, rendering reproducibility impossible. Therefore, the standardization and optimization of cell encapsulation protocols need to be urgently undertaken.
In the present study, we attempted to optimize a cell encapsulation protocol using sodium alginate to achieve long-term cell survival in cell-based therapy. First, the alginate capsule hardness was optimized using varying concentrations of sodium alginate and calcium chloride solutions. The size or cell number of the alginate capsules was optimized by varying the drop volume of the alginate solution or the cell number in the alginate solution. To evaluate cell survival in the alginate capsules, we employed the reporter protein NanoLuc luciferase (Nluc)-expressing murine mesenchymal stem cell line C3H10T1/2, and the relative light units, as measured by the luciferase activity, in the medium were measured to evaluate cell survival. Finally, we evaluated the survival of C3H10T1/2/Nluc cells encapsulated in alginate capsules after intraperitoneal transplantation in mice and examined the hypoglycemic effect of mouse pancreatic β MIN6 cells encapsulated in alginate capsules.
2. Materials and Methods
2.1. Animals
BALB/c-nu/nu mice (male, 4-week-old) and C57BL/6 mice (male, 4- or 8-week-old) were purchased from Sankyo Labo Service Co., Inc. (Tokyo, Japan) and maintained under specific pathogen-free conditions. The protocols for animal experiments were approved by the Institutional Animal Experimentation Committee of the Tokyo University of Science. All animal experiments were conducted in accordance with the procedures outlined in the National Institutes of Health Guide for the Care and Use of Laboratory Animals. All mice used in the present study were anesthetized during the experiments and euthanized by cervical dislocation under isoflurane anesthesia.
2.2. Materials
Sodium alginate was purchased from MP Biomedicals, LLC. (Tokyo, Japan). Calcium chloride, D-(+)-glucose, NaCl, KCl, NaHCO3, NaH2PO4, Na2HPO4, ethanol, isoflurane, streptozotocin (STZ), penicillin–streptomycin–L-glutamine (PSG) solution, pEBMulti-Hyg plasmid, StemSure Monothioglycerol Solution (MTG), and 1, 1′-dioctadecyl-3, 3, 3′, 3′-tetramethylindocarbocyanine perchlorate (DiIC18(3); DiI) were purchased from FUJIFILM Wako Pure Chemical Corporation (Osaka, Japan). Fetal bovine serum (FBS) and Opti-MEM were purchased from Thermo Fisher Scientific (Waltham, MA, USA). Lipofectamine 3000 and a PureLink HiPure Plasmid Miniprep Kit were purchased from Invitrogen (Carlsbad, CA, USA). Hygromycin B gold, Luria-Bertani (LB) broth base, and LB agar were purchased from InvivoGen Co. (San Diego, CA, USA). KOD-Plus-Neo was purchased from TOYOBO Co., Ltd. (Osaka, Japan). Competent NEB 10-beta Escherichia coli (high-efficiency), SOC medium, EcoRV-HF, and KpnI-HF were purchased from New England Biolabs, Inc. (Ipswich, MA, USA). Nucleospin gels and PCR cleanup reagents were purchased from Takara Bio Co. (Shiga, Japan). O.C.T compound was purchased from Sakura Finetek Japan Co., Ltd. (Tokyo, Japan). Food Plus 75 was purchased from Yamaichi Chemical Industries Co., Ltd. (Tokyo, Japan). A NanoGlo Luciferase Assay System was purchased from Promega (Madison, WI, USA). Heparin sodium salt, 0.5% trypan blue solution, and 2.5 g/L-trypsin /1 mmol/L-EDTA solution were purchased from Nacalai Tesque, Inc. (Kyoto, Japan). Dulbecco’s modified eagle’s medium (DMEM) was purchased from Nissui Pharmaceutical Co., Ltd. (Tokyo, Japan).
2.3. Cell Culture
C3H10T1/2 cells and MIN6 were kindly provided by Dr. Hiroki Kagawa (Department of Cell Biology, Kyoto Pharmaceutical University, Kyoto, Japan) and Professor Junichi Miyazaki (Graduate School of Medicine, Osaka University, Osaka, Japan), respectively. C3H10T1/2 cells were cultured in DMEM supplemented with 15% heat-inactivated FBS and PSG solution at 37 °C in humidified air containing 5% CO2. C3H10T1/2/Nluc cells were cultured in DMEM supplemented with 15% heat-inactivated FBS, 500 μg/mL hygromycin B, and PSG solution at 37 °C in humidified air containing 5% CO2. MIN6 cells were cultured in DMEM supplemented with 9% heat-inactivated FBS, PSG solution, and 0.9% MTG at 37 °C in humidified air containing 5% CO2.
2.4. Plasmid Construction
To construct a plasmid coding the Nluc gene (pEBMulti-Nluc), the fragment of the Nluc gene was amplified by polymerase chain reaction (PCR) using primers (forward:5′-CGGGGTACCATGAACTCCTTCTCCACA-3′, reverse:5′-CCGGATATCTTACGCCAGAATGCGTTC-3′) from the pNL2.3 [secNluc/Hygro] plasmid. Subsequently, the amplified PCR product and pEBMulti-hyg vector were digested using KpnI-HF and EcoRV-HF restriction enzymes overnight at 37 °C; then, the product was isolated by electrophoresis with 1% agarose gel to obtain the specific sequence. This sequence was cleaned using NucleoSpin Gel and PCR Clean-up. The PCR product was then incorporated into the pEBMulti-Hyg vector using Ligation high Ver.2 at 16 °C for 2 h. The constructed pEBMulti-Nluc plasmids were transformed into competent NEB 10-beta E. coli using the heat shock method. The transformed E. coli were then spread onto LB agar plates and incubated for 12 h at 37 °C. E. coli picked from colonies were cultured in LB medium, and plasmids in E. coli were collected using a PureLink HiPure Plasmid Midiprep Kit.
2.5. Establishment of C3H10T1/2/Nluc Cells
NanoLuc luciferase (Nluc)-expressing murine mesenchymal stem cell line C3H10T1/2 was used to quantitatively evaluate the number of living cells by measuring Nluc luciferase activity. Briefly, C3H10T1/2 cells were seeded in a 24-well culture plate and cultured overnight at 37 °C in humidified air containing 5% CO2. C3H10T1/2 cells were transfected with the pEBMulti-Nluc plasmid using Lipofectamine 3000 in Opti-MEM. After 24 h, the medium was replaced, and cells were cultured in normal culture medium for 24 h. These cells were then cultured in a medium containing 500 μg/mL hygromycin B for 5 days, cloned, and incubated until confluency. The Nluc activity in samples could be detected without lysing the cells because the secreted Nluc was used in this study.
2.6. Preparation of Alginate Capsules
Phosphate-buffered saline (PBS) solutions containing 0.5% trypan blue or suspended C3H10T1/2 or C3H10T1/2/Nluc cells at concentrations of 1 × 104, 1 × 105, 5 × 105, 1 × 106, or 1 × 107 cells/mL were prepared. Subsequently, samples of 1 mg (0.1%), 2.5 mg (0.25%), 5 mg (0.5%), 10 mg (1%), or 20 mg (2%) of sodium alginate were added to the solutions. Then, samples of 2.5, 5, 10, 25, or 50 μL of each solution were collected using a micropipette, dropped into 1, 2.5, 5, 10, or 25% calcium chloride solution, and incubated for 10 s, 30 s, 1 min, or 5 min at 15–25 °C. To label the C3H10T1/2 cells with DiI, the C3H10T1/2 cells were cultured in a DMEM medium containing 0.01 mg/mL DiI for 30 min at 37 °C under humidified air containing 5% CO2. Thereafter, the culture supernatant was removed, and cells were washed twice with PBS. DiI-labeled C3H10T1/2 cells encapsulated in alginate capsules were prepared using 1% sodium alginate and 10% calcium chloride solutions. The prepared alginate capsules were observed using a BZ-9000 Fluorescence Microscope (BIOREVO, Keyence Corporation, Osaka, Japan), and bright-field and fluorescence images were acquired.
2.7. Evaluation of Nluc Secretion of Encapsulated C3H10T1/2/Nluc Cells
Encapsulated C3H10T1/2/Nluc cells in alginate capsules were seeded in 12- or 24-well culture plates with 1 capsule/well and cultured overnight at 37 °C under humidified air containing 5% CO2. The medium was changed every one or two days, and then 5 μL of the Nano-Glo assay reagent was mixed with 20 μL of the culture supernatant. The relative light units were measured as the luciferase activity using a microplate reader (EnVision, PerkinElmer Japan, Kanagawa, Japan).
2.8. Evaluation of C3H10T1/2/Nluc Cell Survival after Transplantation into Mice
Ten alginate capsules encapsulating C3H10T1/2/Nluc cells were transplanted intraperitoneally into BALB/c-nu/nu mice. As a control, 5 × 104 suspended C3H10T1/2/Nluc cells were transplanted intraperitoneally. The cell samples were maintained in serum-free DMEM on ice until transplantation. The capsules were transplanted via laparotomy, and the suspended cells were injected using a 26G needle and syringe. After transplantation, blood was collected by puncturing the facial vein of mice using an animal lancet (Bio Research Center, Tokyo, Japan), and the obtained blood sample was centrifuged at 12,000× g for 5 min at 4 °C. Subsequently, the supernatant was used as a plasma sample. The obtained plasma sample (20 μL) was mixed with 5 μL of the Nano-Glo assay reagent, and the relative light units were measured using an EnVision microplate reader (PerkinElmer, Chiba, Japan).
2.9. Evaluation of the Hypoglycemic Effect of MIN6 Cell-Encapsulated Capsules in Diabetic Mice
STZ was dissolved in a physiological saline solution at a concentration of 10 mg/mL and left on ice for at least 30 min to equilibrate the α and β anomers. C57BL/6 mice were intraperitoneally administered with 15–200 mg/kg STZ, and their blood glucose levels were measured daily using an Accu-Chek Aviva device (Roche Diagnostics K.K., Tokyo, Japan). To measure blood glucose levels, blood samples were diluted twice with an equal volume of physiological saline. Mice exhibiting blood glucose levels of 350 mg/dL were deemed to be diabetic mice. Fifteen alginate capsules encapsulating MIN6 cells at a density of 5 × 104 cells/capsule were intraperitoneally transplanted into STZ-induced diabetic mice. As a control, 7.5 × 105 suspended MIN6 cells were transplanted intraperitoneally. The cell samples were administered as described above. Blood was collected from the mice, and blood glucose levels were measured using an Accu-Chek Aviva device after a two-fold dilution with physiological saline solution.
2.10. Statistical Analysis
Statistical differences were assessed using one-way analysis of variance (ANOVA), followed by either Dunnett’s test for multiple comparisons or Student’s t-test (equal variance, one-sided testing) for the two groups. A p-value of <0.05 was considered statistically significant.
3. Results
3.1. Optimization of the Preparation Conditions for Alginate Capsules
On dissolving sodium alginate in PBS containing trypan blue, the upper limit of the sodium alginate concentration was 2%. To prepare an alginate capsule, 10 μL of 0.1–2% sodium alginate solution containing trypan blue was immersed in 1, 2.5, 5, or 10% calcium chloride solution using a micropipette for 10 s (Figure 1a). In the 0.1 and 0.25% alginate solutions, the capsules were poorly formed and disintegrated irrespective of the calcium chloride concentration. Considering the 0.5% alginate solution, capsule formation occurred when the calcium chloride solution was ≥5%. Conversely, spherical capsules were formed with the 1 or 2% alginate solutions irrespective of the calcium chloride concentration. However, the capsules obtained with the 0.5% alginate solution and 5% calcium chloride solution or with the 1% alginate solution and 1–2.5% calcium chloride solution were fragile under external forces. Therefore, a combination of the 1% alginate solution and 10% calcium chloride solutions, the minimum concentration required to prepare capsules capable of maintaining a spherical shape, was selected as the optimal preparation condition for the alginate capsules. DiI-labeled C3H10T1/2 cell-encapsulating alginate capsules were prepared using these optimal conditions (1% alginate solution and 10% calcium chloride solutions). Figure 1b presents the bright-field and fluorescence images of prepared capsules, revealing that DiI-labeled C3H10T1/2 cells were encapsulated in the alginate capsules.
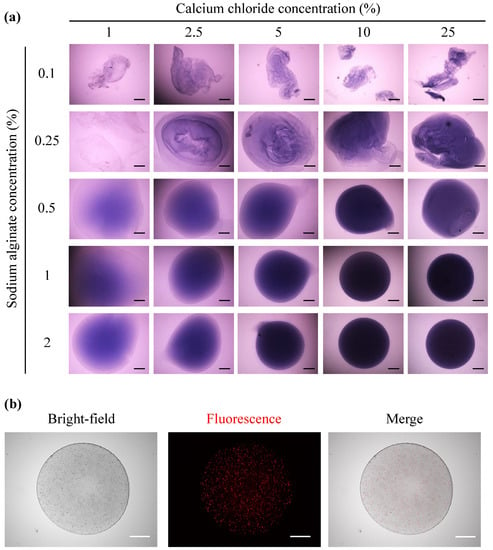
Figure 1.
Preparation of alginate capsules. (a) Alginate capsules were prepared by dropping 0.1, 0.25, 0.5, or 1% alginate solution (10 μL) into 1, 2.5, 5, 10, or 25% calcium chloride solution (2 mL). A 0.5% trypan blue solution was added to the sodium alginate solution at a ratio of 10%. (b) Bright-field and fluorescence images of an alginate capsule encapsulating 5 × 103 DiI-labeled C3H10T1/2 cells, prepared with 1% alginate solution and 10% calcium chloride solution. The scale bars indicate 500 μm.
3.2. Effect of the Capsule Size on the Viability of Encapsulated Cells
Next, we evaluated the effect of the capsule size on the viability of the encapsulated cells. C3H10T1/2/Nluc cells were suspended in PBS at 5 × 105 cells/mL, and sodium alginate was added to prepare a 1% alginate solution. The cell-suspended alginate solution was collected using a micropipette, and 2.5, 5, 10, 25, or 50 μL of the collected solution was dropped into 10% calcium chloride solution and immersed for 10 s to prepare the alginate capsules. Capsules of different sizes were obtained (Figure 2a). C3H10T1/2/Nluc cell-encapsulated alginate capsules of different sizes were cultured at 37 °C in humidified air containing 5% CO2, and the relative light units in the culture supernatant were measured every two days (Figure 2b). The relative light units of the 50 μL alginate capsules gradually decreased from day 2, and the relative light units on day 12 was one quarter of that observed on day 2. Conversely, the relative light units of the 25, 10, and 5 μL alginate capsules were almost constant from days 2 to 12. The relative light units of the 2.5 μL alginate capsules gradually decreased from days 2 to 12. Based on these results, the suitable volume range for the encapsulated C3H10T1/2/Nluc cells was 5–25 μL. In subsequent studies, 10 μL of alginate solution was used owing to the ease of preparing the alginate capsules.
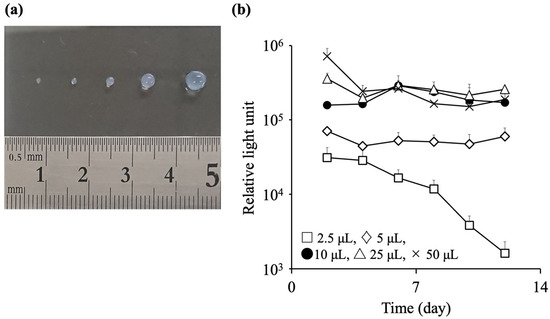
Figure 2.
Effect of the capsule size on the viability of encapsulated cells. Sodium alginate (10 mg) was dissolved in 1 mL phosphate-buffered saline (PBS) containing C3H10T1/2/Nluc cells at a density of 5 × 105 cells/mL. The alginate solution was collected using a micropipette, and 2.5, 5, 10, 25, or 50 μL of the collected solution was immersed in 10% calcium chloride solution for 10 s to prepare alginate capsules. (a) Typical image of capsules prepared using 2.5, 5, 10, 25, and 50 μL alginate solution (from left to right). (b) Relative light units in the culture supernatant of C3H10T1/2/Nluc cell-containing alginate capsules with different sizes. Results are expressed as the mean ± standard deviation (SD) (n = 3–4).
3.3. Effect of Calcium Chloride on the Survival Rate of Encapsulated Cells
We evaluated the effect of the calcium chloride solution on the viability of the encapsulated cells. C3H10T1/2/Nluc cells were suspended in PBS at 5 × 105 cells/mL, and sodium alginate was added to prepare a 1% alginate solution. The cell-suspended alginate solution was collected using a micropipette, and 10 μL of the solution was dropped into 5 or 10% mL of calcium chloride solution, followed by immersion for 10 s to prepare the alginate capsules (Figure 3a). C3H10T1/2/Nluc cell-encapsulated alginate capsules were cultured at 37 °C in humidified air containing 5% CO2, and the relative light units in the culture supernatant were measured every two days. The encapsulated C3H10T1/2/Nluc cells exhibited a constant level of relative light units in both the 5 and 10% calcium chloride solutions, with no significant differences being observed between the two groups. Next, 10 μL of the cell-suspended alginate solution was dropped into 10% calcium chloride solution and immersed for 10 s, 30 s, 1 min, or 5 min to prepare the alginate capsules; the relative light units in the culture supernatant were measured after 48 h (Figure 3b). We observed that the luciferase activity decreased depending on the immersion time in the calcium chloride solution. Therefore, we selected the 10% calcium chloride for encapsulation and 10 s as the immersion time, which is the shortest time required for gelating alginate with minimal cell damage.
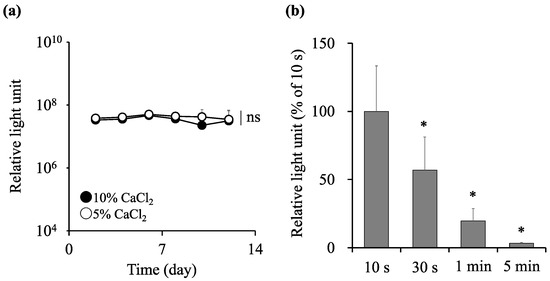
Figure 3.
Effect of calcium chloride on the survival of encapsulated cells. Sodium alginate (10 mg) was dissolved in 1 mL phosphate-buffered saline (PBS) containing C3H10T1/2/Nluc cells at a density of 5 × 105 cells/mL. The alginate solution was immersed in 5 or 10% calcium chloride solution for 10 s, 30 s, 1 min, or 5 min to prepare the alginate capsules. The cell-encapsulated alginate capsules were cultured, and the relative light units in the culture supernatant was measured (a) every two days or (b) at 48 h. Results are expressed as the mean ± standard deviation (SD) (n = 4–6). The level of significance was measured using Student’s t-test (a) or ANOVA following Dunnett’s test compared to 10 s group (b) (ns: not significant, * p < 0.05).
3.4. Effect of Cell Density on the Survival of Encapsulated Cells
Given that the density of cells encapsulated in the alginate capsule can markedly impact cell viability [27], we next examined the viability of the encapsulated cells. C3H10T1/2/Nluc cells were encapsulated at densities of 1 × 104, 1 × 105, 5 × 105, 1 × 106, or 1 × 107 cells/mL, and the relative light units in the culture supernatant were measured (Figure 4). The relative light units gradually decreased in the groups exhibiting cell densities of 1 × 106 cells/mL or higher, whereas they were almost constant for 14 days or more in the groups with a density of 5 × 105 cells/mL or lower. Therefore, we selected 5 × 105 cells/mL as the cell density, which was the highest cell density that maintained a constant cell viability.
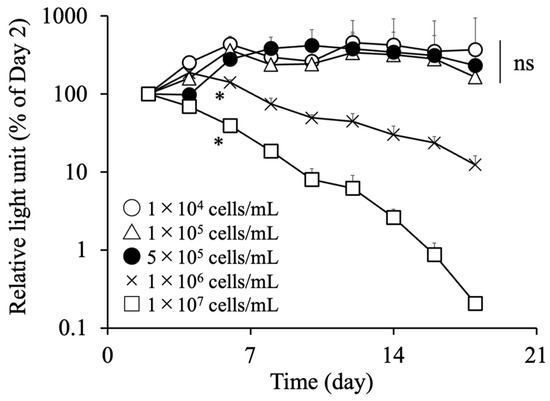
Figure 4.
Effect of the cell density on the survival of encapsulated cells. Sodium alginate (10 mg) was dissolved in 1 mL phosphate-buffered saline (PBS) containing C3H10T1/2/Nluc cells at a density of 1 × 104, 1 × 105, 5 × 105, 1 × 106, or 1 × 107 cells/mL. Each 10 μL alginate solution was immersed in 10% calcium chloride solution for 10 s to prepare alginate capsules. The cell-encapsulated alginate capsules were cultured, and the relative light units in the culture supernatant were measured every two days. Results are expressed as the mean ± standard deviation (SD) (n = 4). The level of significance was measured using ANOVA following Dunnett’s test compared to the 1 × 104 cells/mL group (* p < 0.05).
3.5. Survival and Therapeutic Effect of Encapsulated Cells after Transplantation into Mice
The preparation conditions of the cell-encapsulating alginate capsules were optimized based on the results of Figure 1, Figure 2, Figure 3 and Figure 4. Specifically, a 1% alginate solution was prepared by dissolving 10 mg of sodium alginate in 1 mL of cell suspension at a density of 5 × 105 cells/mL. Then, 10 μL of the 1% alginate solution was dropped into a 10% calcium chloride solution and gelled by immersing for 10 s. The survival rate and therapeutic effect of the encapsulated cells prepared by the optimized conditions were evaluated after transplantation into mice. Ten alginate capsules containing C3H10T1/2/Nluc cells at a density of 5 × 103 cells/capsule were intraperitoneally transplanted into BALB/c-nu/nu mice. As a control, 5 × 104 suspended C3H10T1/2/Nluc cells were intraperitoneally transplanted into BALB/c-nu/nu mice. The capsule group showed an almost constant level of relative light units in the blood for 40 days, whereas the cell suspension group showed a remarkable decrease in the level of relative light units from day 4 post-transplantation (Figure 5a). Accordingly, it can be suggested that the cell encapsulation in the alginate capsules prolonged cell survival post-transplantation. Furthermore, we evaluated the usefulness of the cell-encapsulated alginate capsules using insulin-secreting MIN6 cells. Fifteen alginate capsules containing 5 × 104 MIN6 cells (7.5 × 105 cells in total) were intraperitoneally transplanted into STZ-induced diabetic mice. To establish a control group, 7.5 × 105 suspended MIN6 cells were intraperitoneally transplanted into mice. The suspension group had lower blood glucose levels than the sham-treated group, but no significant decrease was observed immediately after transplantation (Figure 5b). Conversely, the blood glucose levels in the capsule group were significantly decreased from day 1 post-transplantation. Accordingly, encapsulating the cells in alginate capsules improved the therapeutic effect of the insulin-secreting MIN6 cells.
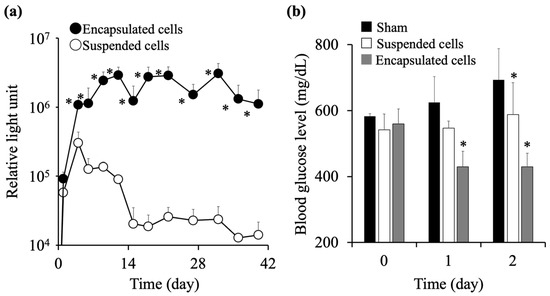
Figure 5.
Survival and therapeutic effect of encapsulated cells after transplantation into mice. (a) Ten alginate capsules containing C3H10T1/2/Nluc cells at 5 × 103 cells/capsule were intraperitoneally transplanted in BALB/c-nu/nu mice. In addition, 5 × 104 suspended C3H10T1/2/Nluc cells were intraperitoneally transplanted. Relative light units in blood were measured. Results are expressed as the mean ± standard deviation (SD) (n = 4–6). The level of significance was measured using Student’s t-test compared to the suspended cell group (* p < 0.05). (b) Fifteen alginate capsules containing 5 × 104 MIN6 cells were intraperitoneally transplanted into C57BL/6 diabetic mice. Suspended MIN6 cells were intraperitoneally transplanted at a level of 7.5 × 105 cells. Blood glucose levels were measured. Results are expressed as the mean ± SD (n = 4–9). The level of significance was measured using ANOVA following Dunnett’s test compared to the sham-treated group (* p < 0.05).
4. Discussion
Cell-based therapy has been recognized as a next-generation therapeutic method because of the excellent therapeutic effects it delivers, but it has serious issues in therapeutic application including low cell survival after transplantation, adverse effects, high cost, and heterogeneous quality [1,28]. Recent studies have successfully addressed these issues by designing or functionalizing cells. The cell sheet technique is one of the well-known cell functionalization methods, which has been applied to some cellular drugs used in clinical applications. In addition, a three-dimensional cell sheet construction method and a vascular network structure formation method have been developed and have improved the therapeutic usefulness of the cell sheets [29]. Second, a three-dimensional cell culture such as cell spheroids is also useful. As with the cell sheet method, this method can realize a strong intercellular interaction in the cell aggregates, causing the improvement of cellular function and cell survival after transplantation [30]. Third, gene transferring to cells has dramatically changed cell-based therapy, and a typical example is chimeric antigen receptor (CAR) T cells therapy [31]. Since the expression of functional proteins in living cells by gene transferring can overcome several issues in cell-based therapy, genetically modified cellular drugs will be increasingly developed. In addition, cell capsulation, hydrogel, cell surface modification, and mitochondria transferring have shown their usefulness in cell-based therapy. These methods are essential to maximize the therapeutic effects of cell-based therapy with safety, and functionalized or designed cellular drugs will be developed by using these methods.
Cell encapsulation is an excellent method for cell-based therapy, and various materials have been employed for encapsulation [32]. Although agarose, hyaluronic acid, gelatin, fibrin, and other materials have been used for encapsulation, they differ in terms of their origin, mode of gelation, and biodegradability [33]. Among these, sodium alginate is the most commonly used owing to its availability, low cost, and ease of fabrication. Hydrogels consisting of collagen, chitosan, or agarose are frequently used; however, achieving optimal cell internalization with these agents can be challenging, thus restricting their application [34]. Recently, chemically modified alginic acid has been developed and was found to inhibit capsule fibrosis after transplantation. These alginates, which are strong polyanions, neither adhere to cells nor activate an immune response, while cationic materials tend to induce inflammatory responses [35]. Alginate gels are obtained when sodium alginate solution is slowly dropped into a calcium chloride solution in physiological conditions. This mild condition required to form these gels as well as its high biocompatibility are some reasons for its superiority as a material for cell capsulation. Further development of cell encapsulation using alginates that can be applied to cell-based therapies is expected. However, calcium chloride, which has been commonly used as a crosslinker, forms alginate gel very rapidly, leading to an increased non-uniformity, permeability, and instability of the structure [34]. Therefore, the preparation protocol of these techniques should be optimized.
During the alginate capsule preparation in this study, differences in the concentrations of each solution significantly impacted the morphology and strength of the prepared alginate capsules (Figure 1). On altering the concentration of the alginate solution and maintaining a fixed concentration of the calcium chloride solution, the capsules failed to retain their shape with decreasing the concentration of the alginate solution. However, on altering the concentration of the calcium chloride solution while maintaining the concentration of the alginate solution, the changes in their morphology were relatively small. Accordingly, the concentration of the alginate solution had a greater impact on the physical properties of the capsules than the concentration of the calcium chloride solution during the preparation of the alginate capsules. These results were in good agreement with previously reported results [36].
Data regarding the effect of capsule size on the viability of encapsulated cells remain limited. Herein, we demonstrated that the viability of cells encapsulated in large alginate capsules (50 μL) decreased in a long-term culture (Figure 2b). When the capsules become larger, the efficiency of substance exchange across the capsule decreases [37], suggesting that the viability of cells in the center of the capsules may decrease. Herein, the viability of the cells encapsulated in small alginate capsules (2.5 μL) was reduced, which could be attributed to the infiltration of the calcium chloride solution into the capsule during immersion, thus damaging the encapsulated cells. The cell damage can be explained by the cytotoxic effect of the 10 mM calcium chloride solution toward the encapsulated cells owing to its high ion concentration. Given that the relative light units decreased depending on the immersion time, the reaction time of calcium chloride with sodium alginate on the capsule surface was critical for the cell viability within the capsule.
The cell density in alginate capsules has been shown to markedly impact cell viability [38]. Consistently, we revealed the effect of the cell density on cell viability in the capsules (Figure 4). The air exchange, liquid exchange, and nutrients required for cells in alginate capsules increase at a high cell density. In contrast, the surface areas of the capsules remain constant. Therefore, it is possible that cells in the center of the capsule experience a shortage of required substances and may die during long-term culture. Based on these results, we optimized the protocol for alginate capsule preparation with respect to the hardness, size, and cell density of the alginate capsule and the immersion time in calcium chloride solution.
To evaluate the usefulness of the optimized preparation protocol for alginate capsules, we transplanted alginate capsules containing C3H10T1/2/Nluc or MIN6 cells into mice and demonstrated the long-term survival of the cells and a high cellular function post-transplantation (Figure 5). However, 15 capsules encapsulating MIN6 cells were transplanted for the evaluation of hypoglycemic effects, suggesting that a large number of capsules are required to exert therapeutic efficacy in clinical studies. Further investigations regarding the number of capsules for transplantation are needed for clinical studies. Considering these results, an optimal preparation protocol for alginate capsules is mandatory for effective cell-based therapy using encapsulated cells. The protocol optimized in this study is scalable because it is based on the external gelation method that has advantages for large-scale applications [39], although further studies on the preparation conditions such as the injection speed are required. In addition, it provides superior cost-effectiveness in that it does not require special equipment and materials. The clinical application of alginate capsules in cell-based therapy can be expected in the future.
5. Conclusions
Herein, we established an optimal protocol for preparing alginate capsules for cell-based therapies. C3H10T1/2/Nluc cells encapsulated in alginate capsules prepared using the optimal protocol could survive for a prolonged period post-transplantation in mice, and MIN6 cells encapsulated in alginate capsules afforded excellent hypoglycemic effects in diabetic mice. These results indicate that cell-encapsulated alginate capsules prepared using the optimal protocol could be valuable for long-term cell-based therapy.
Author Contributions
Conceptualization, R.S. and K.K.; methodology, R.S. and K.K.; validation, R.S., K.K. and M.N.; formal analysis, R.S., K.K. and M.N.; investigation, R.S., K.K., K.T., Y.M. and S.O.; data curation, R.S., K.K. and M.N.; writing—original draft preparation, R.S.; writing—review and editing, K.K., S.O., S.I. and M.N.; visualization, R.S. and K.K.; supervision, M.N.; project administration, K.K.; funding acquisition, K.K. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by a Grant-in-Aid for Scientific Research (C) (grant number 20K12653) and Fostering Joint International Research (B) (grant number 21KK0200) from the Japan Society for the Promotion of Science (JSPS) and an Adaptable and Seamless Technology Transfer Program through a Target-driven R&D grant (grant number JPMJTM20LJ) from the Japan Science and Technology Agency.
Institutional Review Board Statement
The protocols for animal experimentation were approved by the Animal Experimentation Committee of the Tokyo University of Science. Title of approved project: Preparation of pathological animal models and evaluation of cellular functions by cell transplantation into rodents; approval number, Y22016; date of approval, 9 May 2022.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
We wish to thank Mari Tusjimura (Laboratory of Biopharmaceutics, Faculty of Pharmaceutical Sciences, Tokyo University of Science, Noda, Chiba, Japan) for their kind support in certain experiments.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Bashor, C.J.; Hilton, I.B.; Bandukwala, H.; Smith, D.M.; Veiseh, O. Engineering the next generation of cell-based therapeutics. Nat. Rev. Drug Discov. 2022, 21, 655–675. [Google Scholar] [CrossRef] [PubMed]
- Passier, R.; Laake, L.W.; Mummery, C.L. Stem-cell-based therapy and lessons from the heart. Nature 2008, 453, 322–329. [Google Scholar] [CrossRef] [PubMed]
- Heathman, T.R.J.; Nienow, A.W.; McCall, M.J.; Coopman, K.; Kara, B.; Hewitt, C.J. The translation of cell-based therapies: Clinical landscape and manufacturing challenges. Regen. Med. 2015, 10, 49–64. [Google Scholar] [CrossRef] [PubMed]
- Lyra-Leite, D.M.; Gutiérrez-Gutiérrez, O.; Wang, M.; Zhou, Y.; Cyganek, L.; Burridge, P.W. A review of protocols for human iPSC culture, cardiac differentiation, subtype-specification, maturation, and direct reprogramming. STAR Protoc. 2022, 3, 101560. [Google Scholar] [CrossRef]
- Rore, H.; Owen, N.; Piña-Aguilar, R.E.; Docherty, K.; Sekido, R. Testicular somatic cell-like cells derived from embryonic stem cells induce differentiation of epiblasts into germ cells. Commun. Biol. 2021, 4, 802. [Google Scholar] [CrossRef]
- Aly, R.M. Current state of stem cell-based therapies: An overview. Stem Cell Investig. 2020, 7, 8. [Google Scholar] [CrossRef]
- Lv, Z.-Y.; Li, Y.; Liu, J. Progress in clinical trials of stem cell therapy for cerebral palsy. Neural Regen. Res. 2021, 16, 1377–1382. [Google Scholar]
- Liew, L.C.; Ho, B.X.; Soh, B.-S. Mending a broken heart: Current strategies and limitations of cell-based therapy. Stem Cell Res. Ther. 2020, 11, 138. [Google Scholar] [CrossRef]
- Li, L.; Chen, X.; Wang, W.E.; Zeng, C. How to improve the survival of transplanted mesenchymal stem cell in ischemic heart? Stem Cells Int. 2016, 2016, 9682757. [Google Scholar] [CrossRef]
- Xue, X.; Liu, Y.; Zhang, J.; Liu, T.; Yang, Z.; Wang, H. Bcl-xL Genetic modification enhanced the therapeutic efficacy of mesenchymal stem cell transplantation in the treatment of heart infarction. Stem Cells Int. 2015, 2015, 176409. [Google Scholar] [CrossRef]
- Parlakpinar, H.; Gunata, M. Transplantation and immunosuppression: A review of novel transplant-related immunosuppressant drugs. Immunopharmacol. Immunotoxicol. 2021, 43, 651–665. [Google Scholar] [CrossRef] [PubMed]
- Allison, T.L. Immunosuppressive therapy in transplantation. Nurs. Clin. N. Am. 2016, 51, 107–120. [Google Scholar] [CrossRef] [PubMed]
- Schonder, K.S.; Mazariegos, G.V.; Weber, R.J. Adverse effects of immunosuppression in pediatric solid organ transplantation. Paediatr. Drugs 2010, 12, 35–49. [Google Scholar] [CrossRef] [PubMed]
- Caplan, H.; Olson, S.D.; Kumar, A.; George, M.; Prabhakara, K.S.; Wenzel, P.; Bedi, S.; Toledano-Furman, N.E.; Triolo, F.; Kamhieh-Milz, J.; et al. Mesenchymal Stromal Cell Therapeutic Delivery: Translational Challenges to Clinical Application. Front. Immunol. 2019, 10, 1645. [Google Scholar] [CrossRef]
- Ashimova, A.; Yegorov, S.; Negmetzhanov, B.; Hortelano, G. Cell encapsulation within alginate microcapsules: Immunological challenges and outlook. Front. Bioeng. Biotechnol. 2019, 7, 380. [Google Scholar] [CrossRef]
- Nafea, E.H.; Marson, A.; P-Warren, L.A.; Martens, P.J. Immunoisolating semi-permeable membranes for cell encapsulation: Focus on hydrogels. J. Control. Release 2011, 154, 110–122. [Google Scholar] [CrossRef]
- Schwenter, F.; Zarei, S.; Luy, P.; Padrun, V.; Bouche, N.; Lee, J.S.; Mulligan, R.C.; Morel, P.; Mach, N. Cell encapsulation technology as a novel strategy for human anti-tumor immunotherapy. Cancer Gene Ther. 2011, 18, 553–562. [Google Scholar] [CrossRef]
- Chang, T.M. Semipermeable Microcapsules. Science 1964, 146, 524–525. [Google Scholar] [CrossRef]
- Lim, F.; Sun, A.M. Microencapsulated islets as bioartificial endocrine pancreas. Science 1980, 210, 908–910. [Google Scholar] [CrossRef]
- Basta, G.; Montanucci, P.; Calafiore, R. Microencapsulation of cells and molecular therapy of type 1 diabetes mellitus: The actual state and future perspectives between promise and progress. J. Diabetes Investig. 2021, 12, 301–309. [Google Scholar] [CrossRef]
- Chen, Z.; Hu, Q.; Gu, Z. Leveraging engineering of cells for drug delivery. Acc. Chem. Res. 2018, 51, 661–677. [Google Scholar] [CrossRef] [PubMed]
- Shah, K. Encapsulated stem cells for cancer therapy. Biomatter 2013, 3, e24278. [Google Scholar] [CrossRef] [PubMed]
- Galvez-Martín, P.; Martin, J.M.; Ruiz, A.M.; Clares, B. Encapsulation in cell therapy: Methodologies, materials, and clinical applications. Curr. Pharm. Biotechnol. 2017, 18, 365–377. [Google Scholar] [CrossRef] [PubMed]
- Santos-Vizcaino, E.; Orive, G.; Pedraz, J.L.; Hernandez, R.M. Clinical applications of cell encapsulation technology. Methods Mol. Biol. 2020, 2100, 473–491. [Google Scholar]
- Correia, C.R.; Ghasemzadeh-Hasankolaei, M.; Mano, J.F. Cell encapsulation in liquified compartments: Protocol optimization and challenges. PLoS ONE 2019, 14, e0218045. [Google Scholar] [CrossRef] [PubMed]
- Uludag, H.; Vos, P.D.; Tresco, P.A. Technology of mammalian cell encapsulation. Adv. Drug Deliv. Rev. 2000, 42, 29–64. [Google Scholar] [CrossRef] [PubMed]
- Song, H.; Yu, W.; Liu, X.; Ma, X. Improved probiotic viability in stress environments with post-culture of alginate-chitosan microencapsulated low density cells. Carbohydr. Polym. 2014, 108, 10–16. [Google Scholar] [CrossRef]
- Kusamori, K. Development of advanced cell-based therapy by regulating cell-cell interactions. Biol. Pharm. Bull. 2021, 44, 1029–1036. [Google Scholar] [CrossRef]
- Li, M.; Ma, J.; Gao, Y.; Yang, L. Cell sheet technology: A promising strategy in regenerative medicine. Cytotherapy 2019, 21, 3–16. [Google Scholar] [CrossRef]
- Shimazawa, Y.; Kusamori, K.; Tsujimura, M.; Shimomura, A.; Takasaki, R.; Takayama, Y.; Shimizu, K.; Konishi, S.; Nishikawa, M. Intravenous injection of mesenchymal stem cell spheroids improves the pulmonary delivery and prolongs in vivo survival. Biotechnol. J. 2022, 17, e2100137. [Google Scholar] [CrossRef]
- Cappell, K.M.; Kochenderfer, J.N. Long-term outcomes following CAR T cell therapy: What we know so far. Nat. Rev. Clin. Oncol. 2023, 20, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Kharbikar, N.; Mohindra, P.; Desai, T.A. Biomaterials to enhance stem cell transplantation. Cell Stem Cell 2022, 29, 692–721. [Google Scholar] [CrossRef] [PubMed]
- Kang, A.; Park, J.; Ju, J.; Jeong, G.S.; Lee, S.-H. Cell encapsulation via microtechnologies. Biomaterials 2014, 35, 2651–2663. [Google Scholar] [CrossRef]
- Santos, E.; Zarate, J.; Orive, G.; Hernández, R.M.; Pedraz, J.L. Biomaterials in cell microencapsulation. Adv. Exp. Med. Biol. 2010, 670, 5–21. [Google Scholar]
- Bhatia, S.R.; Khattak, S.F.; Roberts, S.C. Polyelectrolytes for cell encapsulation. Curr. Opin. Colloid Interface Sci. 2005, 10, 45–51. [Google Scholar] [CrossRef]
- Dhamecha, D.; Movsas, R.; Sano, U.; Menon, J.U. Applications of alginate microspheres in therapeutics delivery and cell culture: Past, present and future. Int. J. Pharm. 2019, 569, 118627. [Google Scholar] [CrossRef]
- Sakai, S.; Mu, C.; Kawabata, K.; Hashimoto, I.; Kawakami, K. Biocompatibility of subsieve-size capsules versus conventional-size microcapsules. J. Biomed. Mater. Res. A 2006, 78, 394–398. [Google Scholar] [CrossRef]
- Westman, J.O.; Ylitervo, P.; Franzén, C.J.; Taherzadeh, M.J. Effects of encapsulation of microorganisms on product formation during microbial fermentations. Appl. Microbiol. Biotechnol. 2012, 96, 1441–1454. [Google Scholar] [CrossRef]
- Swiokloa, S.; Dingb, P.; Pacekb, A.W.; Connona, C.J. Process parameters for the high-scale production of alginate-encapsulated stem cells for storage and distribution throughout the cell therapy supply chain. Process Biochem. 2017, 59, 289–296. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).