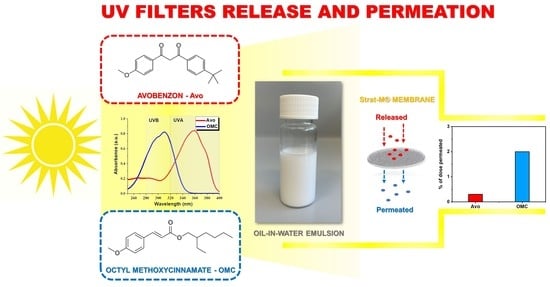
Incorporation of UV Filters into Oil-in-Water Emulsions—Release and Permeability Characteristics
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Emulsions Containing UV Filters
2.2. Physicochemical Characterization of Emulsions
2.2.1. Measurements of pH
2.2.2. Stability Studies
Centrifugation Test
Zeta Potential
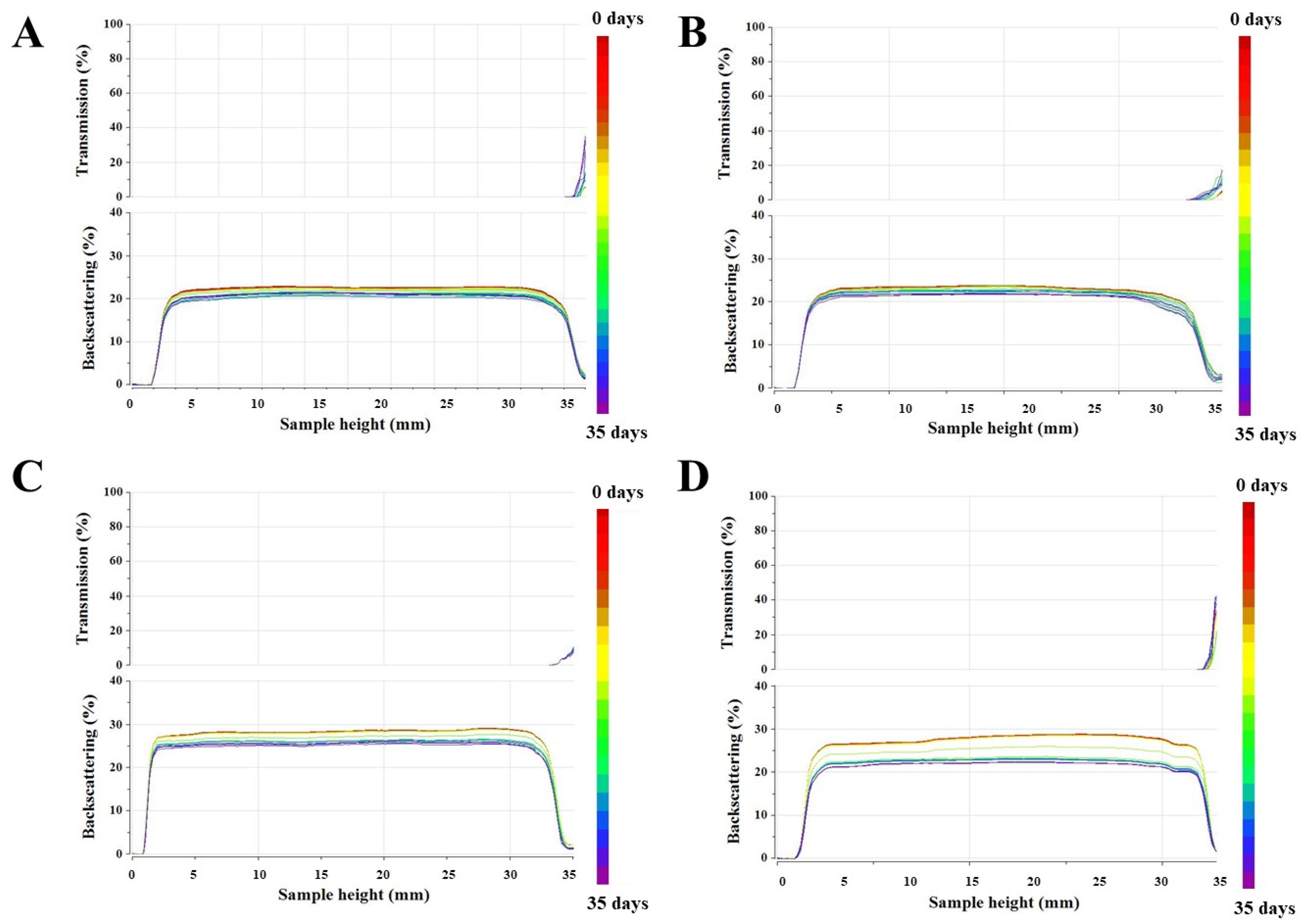
Multiple Light Scattering
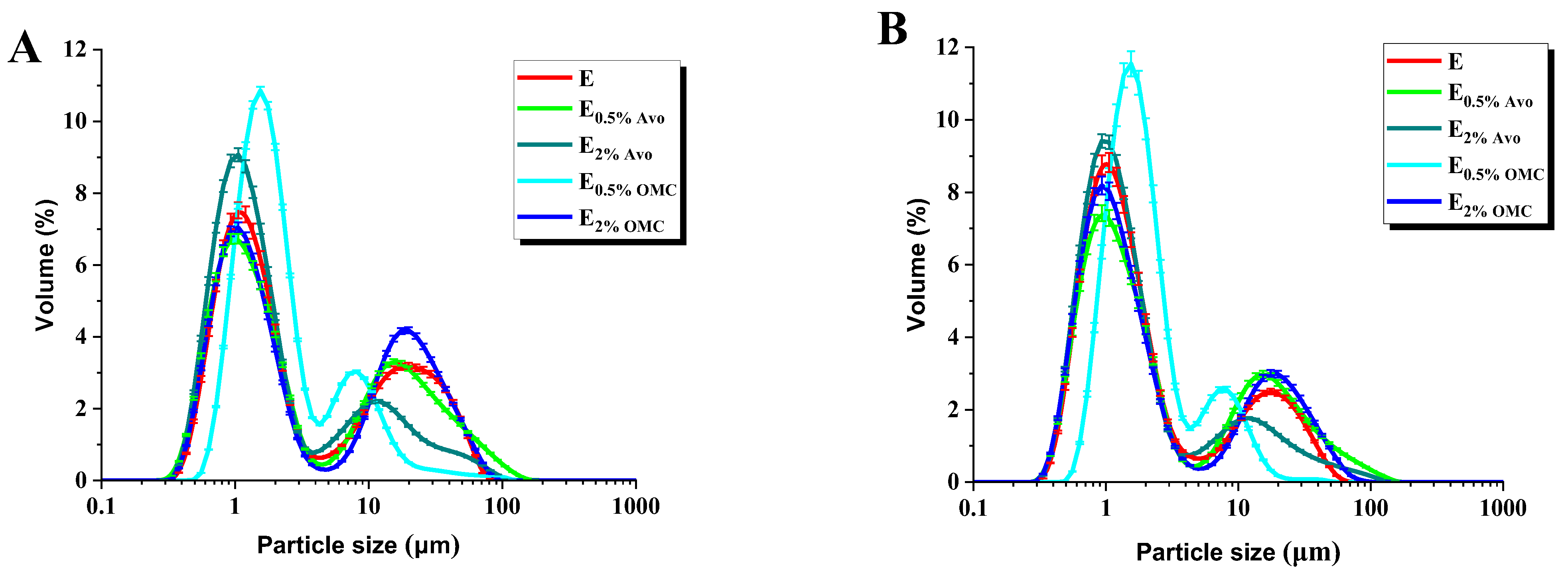
2.2.3. Particle Size Distribution
- d (0.1) (μm)—10% of the particles have a size smaller than the measured value,
- d (0.5) (μm) is the median where 50% of the particles have a size above the measured value and the size of 50% is below the data obtained,
- d (0.9) (μm)—90% of the particles have a size smaller than the results obtained [34].
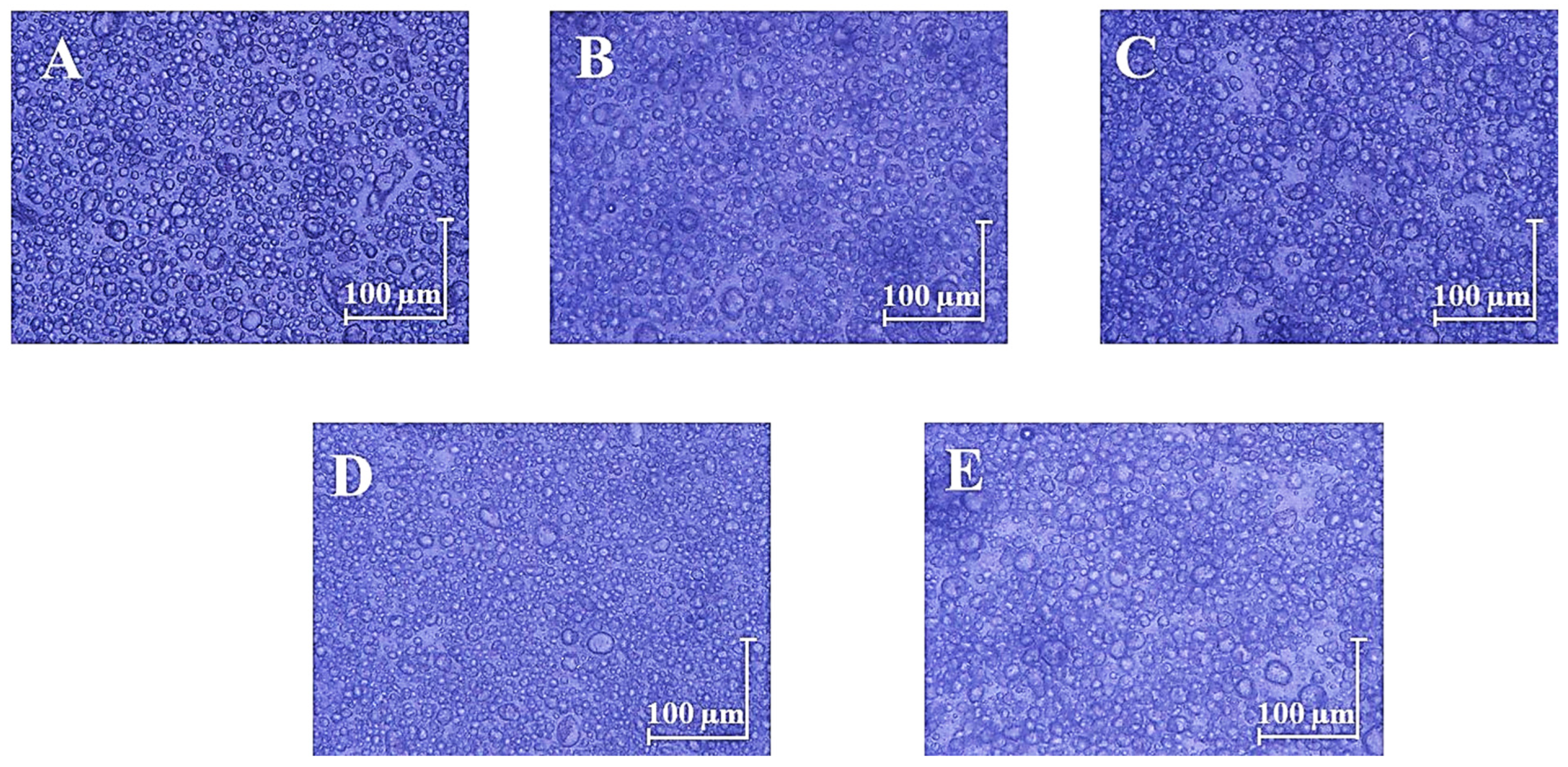
2.2.4. Optical Microscopy
2.3. Release Studies of UV Filters from Emulsions
2.4. Permeation Studies of UV Filters
3. Results and Discussion
3.1. Physicochemical Characterization of Oil-in-Water Emulsions with and without UV Filters
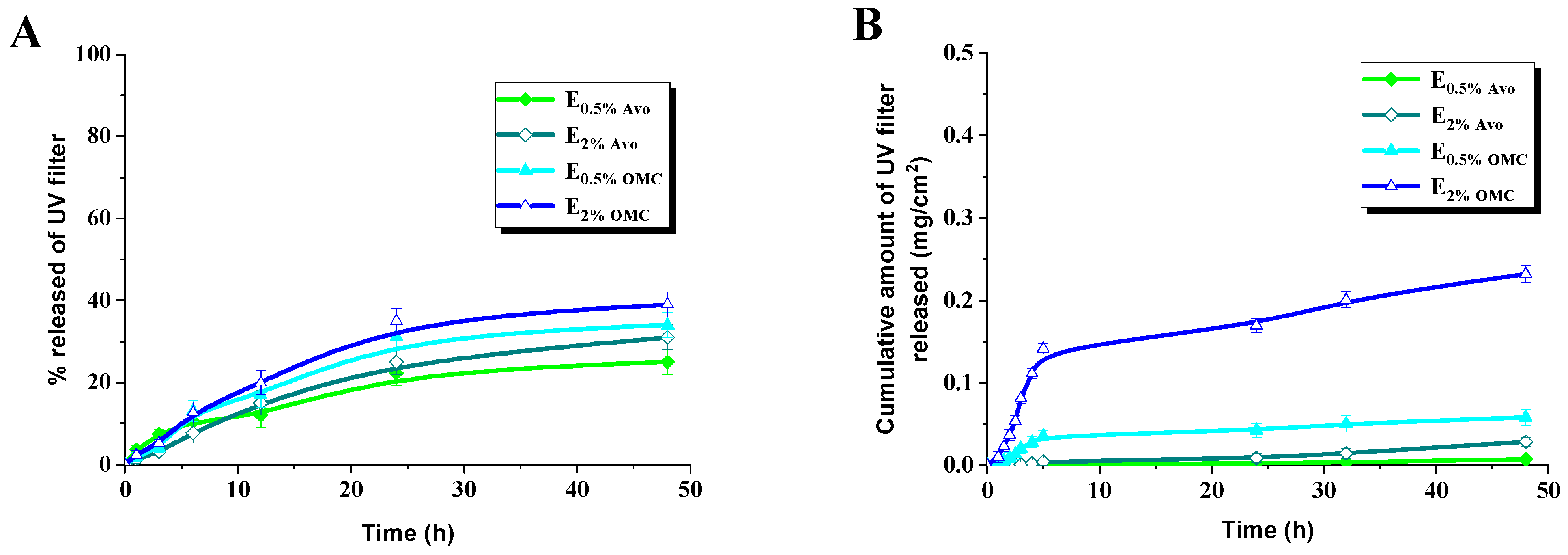
3.2. Release and Permeation of UV Filters from Oil-in-Water Emulsions
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gasparro, F.P.; Mitchnick, M.; Nash, J.F. A Review of Sunscreen Safety and Efficacy. Photochem. Photobiol. 1998, 68, 243–256. [Google Scholar] [CrossRef]
- Souto, E.B.; Jäger, E.; Jäger, A.; Štěpánek, P.; Cano, A.; Viseras, C.; de Melo Barbosa, R.; Chorilli, M.; Zielińska, A.; Severino, P. Lipid Nanomaterials for Targeted Delivery of Dermocosmetic Ingredients: Advances in Photoprotection and Skin Anti-Aging. Nanomaterials 2022, 12, 377. [Google Scholar] [CrossRef] [PubMed]
- Gholap, A.D.; Sayyad, S.F.; Hatvate, N.T.; Dhumal, V.V.; Pardeshi, S.R.; Chavda, V.P.; Vora, L.K. Drug Delivery Strategies for Avobenzone: A Case Study of Photostabilization. Pharmaceutics 2023, 15, 1008. [Google Scholar] [CrossRef] [PubMed]
- Huang, Z.; Ding, A.; Guo, H.; Lu, G.; Huang, X. Construction of Nontoxic Polymeric UV-Absorber with Great Resistance to UV-Photoaging. Sci. Rep. 2016, 6, 25508. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Wang, X.; Zhao, Y.; Xu, Y.; Pan, Y.; Feng, S.; Liu, J.; Huang, X.; Wang, H. Nh3 Plasma Functionalization of UiO-66-NH2 for Highly Enhanced Selective Fluorescence Detection of u (vi) in Water. Anal. Chem. 2022, 94, 10091–10100. [Google Scholar] [CrossRef]
- Egambaram, O.P.; Kesavan Pillai, S.; Ray, S.S. Materials Science Challenges in Skin UV Protection: A Review. Photochem. Photobiol. 2020, 96, 779–797. [Google Scholar] [CrossRef]
- Zhao, Y.-Y.; Wang, X.-B.; Xu, Q.-K.; Chakir, S.; Xu, Y.-F.; Xu, B.; Hu, Y.-H. Micro-/Nanostructured ZnFe2O4 Hollow Sphere/GO Composite for Structurally Enhanced Photocatalysis Performance. Rare Metals 2023, 42, 813–821. [Google Scholar] [CrossRef]
- Sabzevari, N.; Qiblawi, S.; Norton, S.A.; Fivenson, D. Sunscreens: UV Filters to Protect Us: Part 1: Changing Regulations and Choices for Optimal Sun Protection. Int. J. Womens Dermatol. 2021, 7, 28–44. [Google Scholar] [CrossRef]
- Pantelic, M.N.; Wong, N.; Kwa, M.; Lim, H.W. Ultraviolet Filters in the United States and European Union: A Review of Safety and Implications for the Future of US Sunscreens. J. Am. Acad. Dermatol. 2023, 88, 632–646. [Google Scholar] [CrossRef]
- Klinubol, P.; Asawanonda, P.; Wanichwecharungruang, S.P. Transdermal Penetration of UV Filters. Skin Pharmacol. Physiol. 2008, 21, 23–29. [Google Scholar] [CrossRef]
- Janjua, N.R.; Kongshoj, B.; Andersson, A.; Wulf, H.C. Sunscreens in Human Plasma and Urine after Repeated Whole-body Topical Application. J. Eur. Acad. Dermatol. Venereol. 2008, 22, 456–461. [Google Scholar] [CrossRef]
- Schlumpf, M.; Kypke, K.; Wittassek, M.; Angerer, J.; Mascher, H.; Mascher, D.; Vökt, C.; Birchler, M.; Lichtensteiger, W. Exposure Patterns of UV Filters, Fragrances, Parabens, Phthalates, Organochlor Pesticides, PBDEs, and PCBs in Human Milk: Correlation of UV Filters with Use of Cosmetics. Chemosphere 2010, 81, 1171–1183. [Google Scholar] [CrossRef]
- Kim, S.; Choi, K. Occurrences, Toxicities, and Ecological Risks of Benzophenone-3, a Common Component of Organic Sunscreen Products: A Mini-Review. Environ. Int. 2014, 70, 143–157. [Google Scholar] [CrossRef]
- Gupta, V.K.; Zatz, J.L.; Rerek, M. Percutaneous Absorption of Sunscreens through Micro-Yucatan Pig Skin in Vitro. Pharm. Res. 1999, 16, 1602–1607. [Google Scholar] [CrossRef] [PubMed]
- Arianto, A.; Cella, G.; Bangun, H. Preparation and Evaluation of Sunscreen Nanoemulsions with Synergistic Efficacy on Spf by Combination of Soybean Oil, Avobenzone, and Octyl Methoxycinnamate. Open Access Maced. J. Med. Sci. 2019, 7, 2751. [Google Scholar] [CrossRef] [PubMed]
- Shaath, N. Sunscreens: Regulations and Commercial Development; CRC Press: Boca Raton, FL, USA, 2005. [Google Scholar]
- Afonso, S.; Horita, K.; e Silva, J.P.S.; Almeida, I.F.; Amaral, M.H.; Lobão, P.A.; Costa, P.C.; Miranda, M.S.; da Silva, J.C.G.E.; Lobo, J.M.S. Photodegradation of Avobenzone: Stabilization Effect of Antioxidants. J. Photochem. Photobiol. B 2014, 140, 36–40. [Google Scholar] [CrossRef]
- Motley, R.J.; Reynolds, A.J. Photocontact Dermatitis Due to Isopropyl and Butyl Methoxy Dibenzoylmethanes (Eusolex 8020 and Parsol 1789). Contact Dermat. 1989, 21, 109–110. [Google Scholar] [CrossRef] [PubMed]
- Cantrell, A.; McGarvey, D.J. Photochemical Studies of 4-Tert-Butyl-4′-Methoxydibenzoylmethane (BM-DBM). J. Photochem. Photobiol. B 2001, 64, 117–122. [Google Scholar] [CrossRef]
- Ambrogi, V.; Latterini, L.; Marmottini, F.; Pagano, C.; Ricci, M. Mesoporous Silicate MCM-41 as a Particulate Carrier for Octyl Methoxycinnamate: Sunscreen Release and Photostability. J. Pharm. Sci. 2013, 102, 1468–1475. [Google Scholar] [CrossRef]
- Ates, G.; Steinmetz, F.P.; Doktorova, T.Y.; Madden, J.C.; Rogiers, V. Linking Existing in Vitro Dermal Absorption Data to Physicochemical Properties: Contribution to the Design of a Weight-of-Evidence Approach for the Safety Evaluation of Cosmetic Ingredients with Low Dermal Bioavailability. Regul. Toxicol. Pharmacol. 2016, 76, 74–78. [Google Scholar] [CrossRef]
- Montenegro, L.; Turnaturi, R.; Parenti, C.; Pasquinucci, L. In Vitro Evaluation of Sunscreen Safety: Effects of the Vehicle and Repeated Applications on Skin Permeation from Topical Formulations. Pharmaceutics 2018, 10, 27. [Google Scholar] [CrossRef]
- Montenegro, L.; Carbone, C.; Paolino, D.; Drago, R.; Stancampiano, A.H.; Puglisi, G. In Vitro Skin Permeation of Sunscreen Agents from O/W Emulsions. Int. J. Cosmet. Sci. 2008, 30, 57–65. [Google Scholar] [CrossRef]
- de Oliveira, C.A.; Peres, D.D.; Graziola, F.; Chacra, N.A.B.; de Araújo, G.L.B.; Florido, A.C.; Mota, J.; Rosado, C.; Velasco, M.V.R.; Rodrigues, L.M. Cutaneous Biocompatible Rutin-Loaded Gelatin-Based Nanoparticles Increase the SPF of the Association of UVA and UVB Filters. Eur. J. Pharm. Sci. 2016, 81, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Shetty, P.K.; Venuvanka, V.; Jagani, H.V.; Chethan, G.H.; Ligade, V.S.; Musmade, P.B.; Nayak, U.Y.; Reddy, M.S.; Kalthur, G.; Udupa, N. Development and Evaluation of Sunscreen Creams Containing Morin-Encapsulated Nanoparticles for Enhanced UV Radiation Protection and Antioxidant Activity. Int. J. Nanomed. 2015, 10, 6477. [Google Scholar]
- Daneluti, A.L.M.; Neto, F.M.; Ruscinc, N.; Lopes, I.; Velasco, M.V.R.; Matos, J.D.R.; Baby, A.R.; Kalia, Y.N. Using Ordered Mesoporous Silica SBA-15 to Limit Cutaneous Penetration and Transdermal Permeation of Organic UV Filters. Int. J. Pharm. 2019, 570, 118633. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-C.; Lin, C.-F.; Alalaiwe, A.; Wang, P.-W.; Fang, Y.-P.; Fang, J.-Y. UV Filter Entrapment in Mesoporous Silica Hydrogel for Skin Protection against UVA with Minimization of Percutaneous Absorption. Eur. J. Pharm. Sci. 2018, 122, 185–194. [Google Scholar] [CrossRef] [PubMed]
- Blasi, P.; Schoubben, A.; Giovagnoli, S.; Rossi, C.; Ricci, M. The Real Value of Novel Particulate Carriers for Sunscreen Formulation. Expert Rev. Dermatol. 2011, 6, 509–517. [Google Scholar] [CrossRef]
- Palm, M.D.; O’Donoghue, M.N. Update on Photoprotection. Dermatol. Ther. 2007, 20, 360–376. [Google Scholar] [CrossRef]
- Jiménez, M.M.; Pelletier, J.; Bobin, M.F.; Martini, M.C. Influence of Encapsulation on the in Vitro Percutaneous Absorption of Octyl Methoxycinnamate. Int. J. Pharm. 2004, 272, 45–55. [Google Scholar] [CrossRef]
- Yener, G.; Incegül, T.; Yener, N. Importance of Using Solid Lipid Microspheres as Carriers for UV Filters on the Example Octyl Methoxy Cinnamate. Int. J. Pharm. 2003, 258, 203–207. [Google Scholar] [CrossRef]
- Scalia, S.; Tursilli, R.; Bianchi, A.; Nostro, P.L.; Bocci, E.; Ridi, F.; Baglioni, P. Incorporation of the Sunscreen Agent, Octyl Methoxycinnamate in a Cellulosic Fabric Grafted with β-Cyclodextrin. Int. J. Pharm. 2006, 308, 155–159. [Google Scholar] [CrossRef] [PubMed]
- Andreani, T.; Dias-Ferreira, J.; Fangueiro, J.F.; Souza, A.L.R.; Kiill, C.P.; Gremião, M.P.D.; García, M.L.; Silva, A.M.; Souto, E.B. Formulating Octyl Methoxycinnamate in Hybrid Lipid-Silica Nanoparticles: An Innovative Approach for UV Skin Protection. Heliyon 2020, 6, e03831. [Google Scholar] [CrossRef]
- Goscianska, J.; Olejnik, A.; Nowak, I.; Marciniak, M.; Pietrzak, R. Ordered Mesoporous Silica Modified with Lanthanum for Ibuprofen Loading and Release Behaviour. Eur. J. Pharm. Biopharm. 2015, 94, 550–558. [Google Scholar] [CrossRef] [PubMed]
- Filippa, L.; Trento, A.; Álvarez, A.M. Sauter Mean Diameter Determination for the Fine Fraction of Suspended Sediments Using a LISST-25X Diffractometer. Measurement 2012, 45, 364–368. [Google Scholar] [CrossRef]
- Olejnik, A.; Panek, R.; Madej, J.; Franus, W.; Goscianska, J. Low-Cost Zeolitic Carriers for Delivery of Hydroxychloroquine Immunomodulatory Agent with Antiviral Activity. Micropor. Mesopor. Mater. 2022, 346, 112315. [Google Scholar] [CrossRef]
- Smaoui, S.; Hlima, H.B.; Chobba, I.B.; Kadri, A. Development and Stability Studies of Sunscreen Cream Formulations Containing Three Photo-Protective Filters. Arab. J. Chem. 2017, 10, S1216–S1222. [Google Scholar] [CrossRef]
- Schmid-Wendtner, M.-H.; Korting, H.C. The PH of the Skin Surface and Its Impact on the Barrier Function. Skin Pharmacol. Physiol. 2006, 19, 296–302. [Google Scholar] [CrossRef]
- Available online: https://nyscc.org/blog/sunscreen-formulations-emphasis-on-inorganic-sunscreens/ (accessed on 19 May 2023).
- Das, P.; Das, M.K. Production and Physicochemical Characterization of Nanocosmeceuticals. In Nanocosmeceuticals; Elsevier: Amsterdam, The Netherlands, 2022; pp. 95–138. [Google Scholar]
- Olejnik, A.; Kapuscinska, A.; Schroeder, G.; Nowak, I. Physico-Chemical Characterization of Formulations Containing Endomorphin-2 Derivatives. Amino Acids 2017, 49, 1719–1731. [Google Scholar] [CrossRef]
- Trujillo-Cayado, L.A.; Ramírez, P.; Alfaro, M.C.; Ruíz, M.; Muñoz, J. Adsorption at the Biocompatible α-Pinene–Water Interface and Emulsifying Properties of Two Eco-Friendly Surfactants. Colloids Surf. B Biointerfaces 2014, 122, 623–629. [Google Scholar] [CrossRef]
- Celia, C.; Trapasso, E.; Cosco, D.; Paolino, D.; Fresta, M. Turbiscan Lab® Expert Analysis of the Stability of Ethosomes® and Ultradeformable Liposomes Containing a Bilayer Fluidizing Agent. Colloids Surf. B Biointerfaces 2009, 72, 155–160. [Google Scholar] [CrossRef]
- Mengual, O.; Meunier, G.; Cayré, I.; Puech, K.; Snabre, P. TURBISCAN MA 2000: Multiple Light Scattering Measurement for Concentrated Emulsion and Suspension Instability Analysis. Talanta 1999, 50, 445–456. [Google Scholar] [CrossRef] [PubMed]
- Carbone, C.; Musumeci, T.; Lauro, M.R.; Puglisi, G. Eco-Friendly Aqueous Core Surface-Modified Nanocapsules. Colloids Surf. B Biointerfaces 2015, 125, 190–196. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Tan, G. Lubricating Properties of Oil-in-Water Emulsion with Low Oil Concentration: Competitive Wetting Effect. Sci. China Technol. Sci. 2013, 56, 369–375. [Google Scholar] [CrossRef]
- Hashim, A.A.-J.; Rajab, N.A.; Tekie, F.S.M.; Dinarvand, R.; Akrami, M. Investigations Factors Affecting Formulation of Anastrozole as Nanostructured Lipid Carrier. Int. J. Pharm. Res. 2020, 12, 937–945. [Google Scholar]
- André, V.; Willenbacher, N.; Debus, H.; Börger, L.; Fernandez, P.; Frechen, T.; Rieger, J. Prediction of Emulsion Stability: Facts and Myth. Cosmet. Toilet. Manuf. Worldw. 2003, 102, 220–231. [Google Scholar]
- Wester, R.C.; Maibach, H.I. Cutaneous Pharmacokinetics: 10 Steps to Percutaneous Absorption. Drug Metab. Rev. 1983, 14, 169–205. [Google Scholar] [CrossRef]
- Németh, Z.; Pirger, Z.; Fodor, I.; Óvári, M.; Komáromy, A. Analytical Methods for Investigating the Presence, Photoisomerisation-, and Degradation Kinetics of the UV-A Filter Avobenzone under Aqueous Conditions to Ensure a More Realistic Environmental Measurement. J. Photochem. Photobiol. A Chem. 2023, 439, 114621. [Google Scholar] [CrossRef]
- Miranda, M.S.; Pinto da Silva, L.; Esteves da Silva, J.C.G. UV Filter 2-ethylhexyl 4-methoxycinnamate: A Structure, Energetic and UV–Vis Spectral Analysis Based on Density Functional Theory. J. Phys. Org. Chem. 2014, 27, 47–56. [Google Scholar] [CrossRef]
- Available online: https://cdn.caymanchem.com/cdn/insert/23836.pdf (accessed on 25 May 2023).
- Montenegro, L.; Puglisi, G. Evaluation of Sunscreen Safety by In Vitro Skin Permeation Studies: Effects of Vehicle Composition. Die Pharm. Int. J. Pharm. Sci. 2013, 68, 34–40. [Google Scholar]
- Montenegro, L.; Paolino, D.; Puglisi, G. Effects of Silicone Emulsifiers on In Vitro Skin Permeation of Sunscreens from Cosmetic Emulsions. J. Cosmet. Sci. 2004, 55, 509–518. [Google Scholar]
- Li, C.-C.; Lin, L.-H.; Lee, H.-T.; Tsai, J.-R. Avobenzone Encapsulated in Modified Dextrin for Improved UV Protection and Reduced Skin Penetration. Chem. Pap. 2016, 70, 840–847. [Google Scholar] [CrossRef]
- Tampucci, S.; Burgalassi, S.; Chetoni, P.; Monti, D. Cutaneous Permeation and Penetration of Sunscreens: Formulation Strategies and in Vitro Methods. Cosmetics 2017, 5, 1. [Google Scholar] [CrossRef]
- Fosca, M.; Rau, J.V.; Uskoković, V. Factors Influencing the Drug Release from Calcium Phosphate Cements. Bioact. Mater. 2022, 7, 341–363. [Google Scholar] [CrossRef] [PubMed]
- Arce, F.J.; Asano, N.; See, G.L.; Itakura, S.; Todo, H.; Sugibayashi, K. Usefulness of Artificial Membrane, Strat-M®, in the Assessment of Drug Permeation from Complex Vehicles in Finite Dose Conditions. Pharmaceutics 2020, 12, 173. [Google Scholar] [CrossRef]
- Haq, A.; Goodyear, B.; Ameen, D.; Joshi, V.; Michniak-Kohn, B. Strat-M® Synthetic Membrane: Permeability Comparison to Human Cadaver Skin. Int. J. Pharm. 2018, 547, 432–437. [Google Scholar] [CrossRef] [PubMed]
- Uchida, T.; Kadhum, W.R.; Kanai, S.; Todo, H.; Oshizaka, T.; Sugibayashi, K. Prediction of Skin Permeation by Chemical Compounds Using the Artificial Membrane, Strat-MTM. Eur. J. Pharm. Sci. 2015, 67, 113–118. [Google Scholar] [CrossRef] [PubMed]


| UV Filter | Structure | State of Matter | MW (g/mol) | Area (Å2) | Volume (Å3) | Topological Polar Surface Area (Å2) | Log Kow 1 | MP (°C) |
|---|---|---|---|---|---|---|---|---|
| Avo |  | solid | 310 | 360.12 | 342.12 | 43.4 | 4.51 | 83.5 |
| OMC | 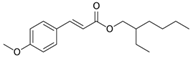 | liquid | 290 | 367.44 | 334.87 | 35.5 | 6.10 | −68.3 |
| Ingredients | E (% w/w) | E0.5%Avo (% w/w) | E2%Avo (% w/w) | E0.5%OMC (% w/w) | E2%OMC (% w/w) |
|---|---|---|---|---|---|
| Water | 69.3 | 68.8 | 67.3 | 68.8 | 67.3 |
| Glycerin | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Xanthan gum | 0.3 | 0.3 | 0.3 | 0.3 | 0.3 |
| Glycerol stearate | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Polyglyceryl-3 metylglucose distearate | 2.0 | 2.0 | 2.0 | 2.0 | 2.0 |
| Cetyl alcohol | 3.0 | 3.0 | 3.0 | 3.0 | 3.0 |
| Shea butter | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Vegetable oil | 7.5 | 7.5 | 7.5 | 7.5 | 7.5 |
| Avo/OMC | - | 0.5 | 2.0 | 0.5 | 2.0 |
| Phenoxyethanol | 0.9 | 0.9 | 0.9 | 0.9 | 0.9 |
| Sample | pH | Zeta Potential (mV) | Refractive Index |
|---|---|---|---|
| E | 6.02 ± 0.04 | −79.0 ± 1.3 | 1.3590 |
| E0.5%Avo | 6.04 ± 0.06 | −75.1 ± 1.9 | 1.3543 |
| E2%Avo | 6.06 ± 0.08 | −73.0 ± 1.5 | 1.3555 |
| E0.5%OMC | 6.09 ± 0.07 | −57.1 ± 2.0 | 1.3580 |
| E2%OMC | 6.11 ± 0.10 | −54.2 ± 1.8 | 1.3567 |
| Emulsion | E | E0.5%Avo | E2%Avo | E0.5%OMC | E2%OMC |
|---|---|---|---|---|---|
| D [3,2] | 1.570 ± 0.008 μm | 1.620 ± 0.014 μm | 1.300 ± 0.001 μm | 1.790 ± 0.001 μm | 1.640 ± 0.016 μm |
| d(0.1) | 0.671 ± 0.002 μm | 0.716 ± 0.001 μm | 0.652 ± 0.002 μm | 0.983 ± 0.001μm | 0.696 ± 0.026 μm |
| d(0.5) | 1.850 ± 0.011 μm | 1.780 ± 0.023 μm | 1.370 ± 0.006 μm | 1.850 ± 0.001 μm | 1.890 ± 0.203 μm |
| d(0.9) | 33.000 ± 0.153 μm | 30.300 ± 1.535 μm | 16.500 ± 1.170 μm | 9.340 ± 0.045 μm | 31.300 ± 1.077 μm |
| Ultrasound treatment | |||||
| D [3,2] | 1.400 ± 0.088 μm | 1.350 ± 0.083 μm | 1.190 ± 0.082 μm | 1.660 ± 0.016 μm | 1.310 ± 0.108μm |
| d(0.1) | 0.630 ± 0.021 μm | 0.641 ± 0.026 μm | 0.614 ± 0.019 μm | 0.946 ± 0.007 μm | 0.617 ± 0.036 μm |
| d(0.5) | 1.570 ± 0.145 μm | 1.370 ± 0.097 μm | 1.250 ± 0.076 μm | 1.730 ± 0.024 μm | 1.400 ± 0.206 μm |
| d(0.9) | 26.400 ± 2.533 μm | 19.400 ± 2.146 μm | 14.300 ± 1.543 μm | 7.280 ± 0.446 μm | 24.200 ± 1.720 μm |
| Sample | Zero Order Model | First Order Model | Higuchi Model | Hixson–Crowell Model | Korsmeyer–Peppas Model | |
|---|---|---|---|---|---|---|
| Correlation Co-Efficient (R2) | n | |||||
| E0.5% Avo | 0.889 | 0.898 | 0.949 | 0.895 | 0.964 | 0.575 |
| E2%Avo | 0.968 | 0.966 | 0.907 | 0.961 | 0.970 | 0.824 |
| E0.5%OMC | 0.880 | 0.878 | 0.877 | 0.879 | 0.931 | 0.989 |
| E2%OMC | 0.959 | 0.962 | 0.931 | 0.961 | 0.968 | 0.978 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olejnik, A.; Goscianska, J. Incorporation of UV Filters into Oil-in-Water Emulsions—Release and Permeability Characteristics. Appl. Sci. 2023, 13, 7674. https://doi.org/10.3390/app13137674
Olejnik A, Goscianska J. Incorporation of UV Filters into Oil-in-Water Emulsions—Release and Permeability Characteristics. Applied Sciences. 2023; 13(13):7674. https://doi.org/10.3390/app13137674
Chicago/Turabian StyleOlejnik, Anna, and Joanna Goscianska. 2023. "Incorporation of UV Filters into Oil-in-Water Emulsions—Release and Permeability Characteristics" Applied Sciences 13, no. 13: 7674. https://doi.org/10.3390/app13137674
APA StyleOlejnik, A., & Goscianska, J. (2023). Incorporation of UV Filters into Oil-in-Water Emulsions—Release and Permeability Characteristics. Applied Sciences, 13(13), 7674. https://doi.org/10.3390/app13137674