Inactivation of Cercospora lactucae-sativa through Application of Non-Thermal Atmospheric Pressure Gliding Arc, Tesla Coil and Dielectric Barrier Discharge Plasmas
Abstract
1. Introduction
2. Materials and Methods
2.1. Source of the Fungus
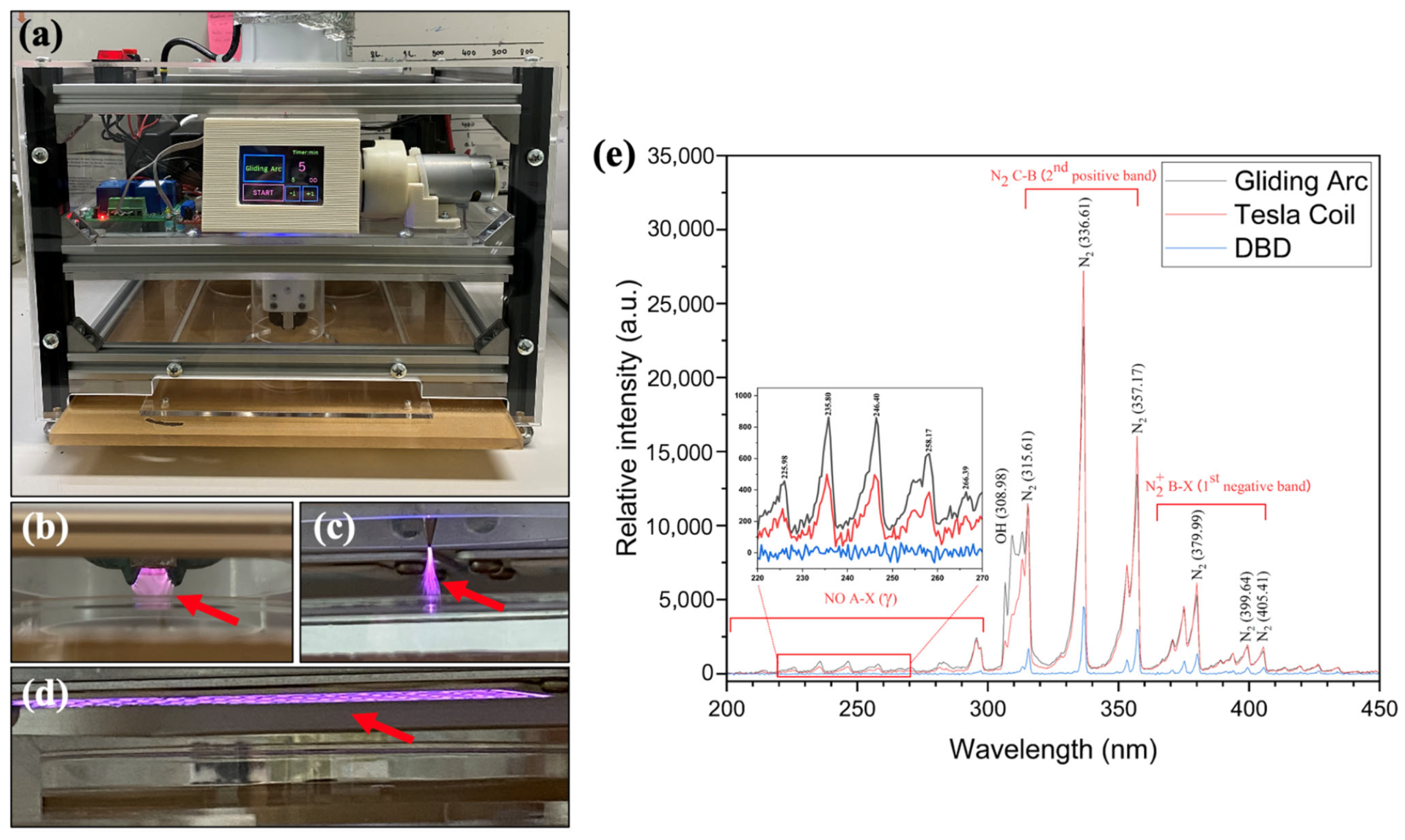
2.2. Plasma Device and Properties
2.3. Inactivation of Mycelial Growth
2.4. Inactivation of Conidial Germination
2.5. Inactivation of Fungal Pathogenesis after Plasma Treatments
2.6. Statistical Analysis
3. Results
3.1. Plasma Device and Properties
3.2. Inactivation of Mycelial Growth
3.3. Inactivation of Conidial Germination
3.4. Inactivation of Fungal Pathogenesis after Plasma Treatments
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Srimai, K.; Akarapisarn, A. Bacillus subtilis LBF02 as Biocontrol Agent against Leaf Spot Diseases Caused by Cercospora lactucae-sativae in Lettuce. J. Agric. Sci. 2014, 6, 151–158. [Google Scholar] [CrossRef]
- Koohakan, P.; Jeanaksorn, T.; Nuntagij, I. Major Diseases of Lettuce Grown by Commercial Nutrient Film Technique in Thailand. Curr. Appl. Sci. Technol. 2008, 8, 56–63. [Google Scholar]
- Liberato, J.R.; Stephens, P.M. Cercospora apii s. Lat. on Lettuce in Australia. Australas. Plant Pathol. 2006, 35, 379–381. [Google Scholar] [CrossRef]
- Thomas, A.; Saravanakumar, D. Effect of Host Extract on Growth and Sporulation of Cercospora lactucae-sativae. Aust. Plant Dis. Notes 2019, 14, 19. [Google Scholar] [CrossRef]
- To-anun, C.; Hidayat, I.; Meeboon, J. Genus Cercospora in Thailand: Taxonomy and Phylogeny (with a Dichotomous Key to Species). Plant Pathol. Quar. 2011, 1, 11–87. [Google Scholar] [CrossRef]
- Lamichhane, J.R.; Durr, C.; Schwanck, A.A.; Robin, M.H.; Sarthou, J.P.; Cellier, V.; Messean, A.; Aubertot, J.N. Integrated Management of Damping-Off Diseases: A Review. Agron. Sustain. Dev. 2017, 37, 10. [Google Scholar] [CrossRef]
- Scholtz, V.; Pazlarova, J.; Souskova, H.; Khun, J.; Julak, J. Nonthermal plasma—A tool for decontamination and disinfection. Biotechnol. Adv. 2015, 33, 1108–1119. [Google Scholar] [CrossRef]
- Zhang, J.J.; Kwon, T.; Kim, S.B.; Jeong, D.K. Plasma Farming: Non-Thermal Dielectric Barrier Discharge Plasma Technology for Improving the Growth of Soybean Sprouts and Chickens. Plasma 2018, 1, 25. [Google Scholar] [CrossRef]
- Misra, N.N.; Tiwari, B.K.; Raghavarao, K.S.M.S.; Cullen, P.J. Nonthermal Plasma Inactivation of Food-Borne Pathogens. Food Eng. Rev. 2011, 3, 159–170. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- López, M.; Calvo, T.; Prieto, M.; Múgica-Vidal, R.; Muro-Fraguas, I.; Alba-Elías, F.; Alvarez-Ordóñez, A. A Review on Non-thermal Atmospheric Plasma for Food Preservation: Mode of Action, Determinants of Effectiveness, and Applications. Front. Microbiol. 2019, 10, 622. [Google Scholar] [CrossRef]
- Liao, X.; Liu, D.; Xiang, Q.; Ahn, J.; Chen, S.; Ye, X.; Ding, T. Inactivation Mechanisms of Non-Thermal Plasma on Microbes: A Review. Food Control 2017, 75, 83–91. [Google Scholar] [CrossRef]
- Conrads, H.; Schmidt, M. Plasma Generation and Plasma Sources. Plasma Sources Sci. Technol. 2000, 9, 441. [Google Scholar] [CrossRef]
- Darvish, F.; Sarkari, N.M.; Khani, M.; Eslami, E.; Shokri, B.; Mohseni, M.; Ebrahimi, M.; Alizadeh, M.; Dee, C.F. Direct Plasma Treatment Approach Based on Non-Thermal Gliding Arc for Surface Modification of Biaxially-Oriented Polypropylene with Post-Exposure Hydrophilicity Improvement and Minus Aging Effects. Appl. Surf. Sci. 2020, 509, 144–815. [Google Scholar] [CrossRef]
- Graves, D.B. Lessons from Tesla for Plasma Medicine. IEEE Trans. Radiat. Plasma Med. Sci. 2018, 2, 594–607. [Google Scholar] [CrossRef]
- Kogelschatz, U. Dielectric-Barrier Discharges: Their History, Discharge Physics, and Industrial Applications. Plasma Chem. Plasma Process. 2002, 23, 1–46. [Google Scholar] [CrossRef]
- Jiang, J.; Lu, Y.; Li, J.; Li, L.; He, X.; Shao, H.; Dong, Y. Effect of Seed Treatment by Cold Plasma on the Resistance of Tomato to Ralstonia solanacearum (bacterial wilt). PLoS ONE 2014, 9, e97753. [Google Scholar] [CrossRef]
- Sawangrat, C.; Phimolsiripol, Y.; Leksakul, K.; Thanapornpoonpong, S.-n.; Sojithamporn, P.; Lavilla, M.; Castagnini, J.M.; Barba, F.J.; Boonyawan, D. Application of Pinhole Plasma Jet Activated Water against Escherichia coli, Colletotrichum gloeosporioides, and Decontamination of Pesticide Residues on Chili (Capsicum annuum L.). Foods 2022, 11, 2859. [Google Scholar] [CrossRef]
- Boonyawan, D.; Lamasai, K.; Umongno, C.; Rattanatabtimtong, S.; Yu, L.D.; Kuensaen, C.; Maitip, J.; Thana, P. Surface Dielectric Barrier Discharge Plasma–Treated Pork Cut Parts: Bactericidal Efficacy and Physiochemical Characteristics. Heliyon 2022, 8, e10915. [Google Scholar] [CrossRef]
- Royintarat, T.; Choi, E.H.; Boonyawan, D.; Seesuriyachan, P.; Wattanutchariya, W. Chemical-Free and Synergistic Interaction of Ultrasound Combined with Plasma-Activated Water (PAW) to Enhance Microbial Inactivation in Chicken Meat and Skin. Sci. Rep. 2020, 10, 1559. [Google Scholar] [CrossRef]
- Kosumsupamala, K.; Thana, P.; Palee, N.; Lamasai, K.; Kuensaen, C.; Ngamjarurojana, A.; Yangkhamman, P.; Boonyawan, D. Air to H2-N2 Pulse Plasma Jet for In-Vitro Plant Tissue Culture Process: Source Characteristics. Plasma Chem. Plasma Process. 2022, 42, 535–559. [Google Scholar] [CrossRef]
- Torres, M.A.; Jones, D.G.; Dangl, J.L. Reactive Oxygen Species Signaling in Response to Pathogens. Plant Physiol. 2006, 141, 373–378. [Google Scholar] [CrossRef]
- Cruywagen, E.M.; Slippers, B.; Roux, J.; Wingfield, M.J. Phylogenetic Species Recognition and Hybridization in Lasiodiplodia: A Case Study on Species from Baobabs. Fungal biol. 2017, 121, 420–436. [Google Scholar] [CrossRef]
- Tarabova, B.; Lukes, P.; Janda, M.; Hensel, K.; Sikurova, L.; Machala, Z. Specificity of Detection Methods of Nitrites and Ozone in Aqueous Solutions Activated by Air Plasma. Plasma Process. Polym. 2018, 15, 1800030. [Google Scholar] [CrossRef]
- Kumar, V.; Tyagi, D. Antifungal Activity of Different Extracts of Bergeniastracheyi. Int. J. Curr. Microbiol. Appl. Sci. 2013, 2, 69–78. [Google Scholar]
- Chakraborty, M.; Mahmud, N.U.; Muzahid, A.N.M.; Rabby, S.M.F.; Islam, T. Oligomycins Inhibit Magnaporthe oryzae Triticum and Suppress Wheat Blast Disease. PLoS ONE 2020, 15, e0233665. [Google Scholar] [CrossRef]
- Promwe, A.; Intana, W. Trichoderma asperellum (NST-009): A Potential Native Antagonistic Fungus to Control Cercospora Leaf Spot and Promote the Growth of ‘Green Oak’ Lettuce (Lactuca sativa L.) Cultivated in the Commercial NFT Hydroponic System. Plant Prot. Sci. 2022, 58, 139–149. [Google Scholar] [CrossRef]
- Kumar, R.; Mazakova, J.; Ali, A.; Sur, V.P.; Sen, M.K.; Bolton, M.D.; Manasova, M.; Rysanek, P.; Zouhar, M. Characterization of the Molecular Mechanisms of Resistance Against DMI Fungicides in Cercospora beticola Populations from the Czech Republic. J. Fungi 2021, 7, 1062. [Google Scholar] [CrossRef]
- Dharini, M.; Jaspin, S.; Mahendran, R. Cold Plasma Reactive Species: Generation, Properties, and Interaction with Food Biomolecules. Food Chem. 2023, 45, 134746. [Google Scholar] [CrossRef]
- Moisan, M.; Barbeau, J.; Crevier, M.C.; Pelletier, J.; Philip, N.; Saoudi, B. Plasma Sterilization. Methods and Mechanisms. Pure Appl. Chem. 2022, 74, 349–358. [Google Scholar] [CrossRef]
- Polcic, P.; Machala, Z. Effects of Non-Thermal Plasma on Yeast Saccharomyces cerevisiae. Int. J. Mol. Sci. 2021, 22, 2247. [Google Scholar] [CrossRef]
- Volkov, A.G.; Xu, K.G.; Kolobov, V.I. Plasma-Generated Reactive Oxygen and Nitrogen Species Can Lead to Closure, Locking and Constriction of the Dionaea muscipula Ellis trap. J. R. Soc. Interface 2019, 16, 20180713. [Google Scholar] [CrossRef]
- Laroussi, M. Nonthermal Decontamination of Biological Media by Atmospheric pressure Plasmas: Review, Analysis, and Prospects. IEEE Trans. Plasma Sci. 2002, 30, 1409–1415. [Google Scholar] [CrossRef]
- Supakitthanakorn, S.; Ruangwong, O.-U.; Sawangrat, C.; Srisuwan, W.; Boonyawan, D. Potential of Nonthermal Atmospheric pressure Dielectric Barrier Discharge Plasma for Inhibition of Athelia Rolfsii Causing Southern Blight Disease in Lettuce. Agriculture 2023, 13, 167. [Google Scholar] [CrossRef]
- Lotfy, K.; Al-Qahtani, S.; Al-Harbi, N.; El-Absy, K.; Shulaybi, F.B.; Alali, S.; Mashtoly, T. Decontamination Potential of Date Palm Fruit via Non-Thermal Plasma Technique. Sci. Rep. 2022, 12, 17323. [Google Scholar] [CrossRef]
- Na, Y.H.; Park, G.; Choi, E.H.; Ehm, H.S. Effects of the Physical Parameters of a Microwave Plasma Jet on the Inactivation of Fungal Spores. Thin Solid Films 2013, 547, 125–131. [Google Scholar] [CrossRef]
- Ebrahimi, E.; Hosseini, S.I.; Samadlouie, H.R.; Mohammadhosseini, B.; Cullen, P.J. Surface Barrier Discharge Remote Plasma Inactivation of Aspergillus niger ATCC 10864 Spores for Packaged Crocus sativus. Food Bioprocess Technol. 2023. [Google Scholar] [CrossRef]
- Holubova, L.; Kyzek, S.; Durovcova, I.; Fabova, J.; Horvathova, E.; Sevcovicova, A.; Galova, E. Non-Thermal Plasma—A New Green Priming Agent for Plants? Int. J. Mol. Sci. 2020, 21, 9466. [Google Scholar] [CrossRef]


| Treatment | Mean of Percent Inhibition * ± SD | |||
|---|---|---|---|---|
| 5 min | 10 min | 15 min | 20 min | |
| Non-treated control | 0.00 ± 0.00 b ** | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 0.00 ± 0.00 d |
| Gliding arc (GA) | 0.00 ± 0.00 b | 33.70 ± 0.64 b | 40.74 ± 1.28 b | 45.19 ± 0.64 b |
| Tesla coil (TC) | 0.00 ± 0.00 b | 0.00 ± 0.00 c | 0.00 ± 0.00 c | 13.33 ± 0.11 c |
| Dielectric barrier discharge (DBD) | 27.41 ± 1.70 a | 49.26 ± 1.28 a | 72.59 ± 1.70 a | 93.33 ± 1.11 a |
| CV (%) | 12.37 | 3.42 | 3.75 | 2.24 |
| p-value | 0.69 | 0.58 | 0.87 | 0.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Supakitthanakorn, S.; Ruangwong, O.-U.; Boonyawan, D. Inactivation of Cercospora lactucae-sativa through Application of Non-Thermal Atmospheric Pressure Gliding Arc, Tesla Coil and Dielectric Barrier Discharge Plasmas. Appl. Sci. 2023, 13, 6643. https://doi.org/10.3390/app13116643
Supakitthanakorn S, Ruangwong O-U, Boonyawan D. Inactivation of Cercospora lactucae-sativa through Application of Non-Thermal Atmospheric Pressure Gliding Arc, Tesla Coil and Dielectric Barrier Discharge Plasmas. Applied Sciences. 2023; 13(11):6643. https://doi.org/10.3390/app13116643
Chicago/Turabian StyleSupakitthanakorn, Salit, On-Uma Ruangwong, and Dheerawan Boonyawan. 2023. "Inactivation of Cercospora lactucae-sativa through Application of Non-Thermal Atmospheric Pressure Gliding Arc, Tesla Coil and Dielectric Barrier Discharge Plasmas" Applied Sciences 13, no. 11: 6643. https://doi.org/10.3390/app13116643
APA StyleSupakitthanakorn, S., Ruangwong, O.-U., & Boonyawan, D. (2023). Inactivation of Cercospora lactucae-sativa through Application of Non-Thermal Atmospheric Pressure Gliding Arc, Tesla Coil and Dielectric Barrier Discharge Plasmas. Applied Sciences, 13(11), 6643. https://doi.org/10.3390/app13116643






