Abstract
The negative impact of chemical pesticides on the environment and human health has contributed to the introduction of legal regulations that ensure the reduction in the use of agrochemicals in favor of biological products. The existing review of the literature, including our research, clearly shows that the ideal biocontrol agents are Trichoderma fungi. The production of antibiotics, lytic enzymes degrading the cell walls of plant pathogens, or inducing a defense response in plants are just some of the features supporting the wide use of these microorganisms in sustainable agriculture. It is estimated that currently about 60% of biofungicides used to eliminate fungal pathogens are produced based on Trichoderma sp. strains.
1. Introduction
According to the Food and Agriculture Organization of the United Nations (FAO) [1,2], the world population will exceed 9 billion by 2050. To meet the food needs of a dynamically developing society, food production is expected to increase by at least 70%.
Despite the modernization of agriculture, farmers experience huge losses in the quantity and quality of their crops every year. Each year, 10–15% of the most important crops are lost due to pathogenic factors, of which 70–80% are fungi [3,4]. According to Fisher et al. [5], about 8000 species of fungi and oomycetes are plant pathogens. This group includes Blumeria graminis, Botrytis cinerea, Colletotrichum spp., Fusarium graminearum, Fusarium oxysporum, Magnaporthe oryzae, Melampsora lini, Mycosphaerella graminicola, Passalora fulva, Phakopsora pachyrhizi, Puccinia spp., Rhizoctonia solani, Sclerotinia sclerotiorum, and Ustilago maydis [4,6].
To reduce crop losses, farmers use huge amounts of plant protection products every year. The European Statistical Office (EUROSTAT) [7] indicated that sales of pesticides in 2020 amounted to 346,000 tonnes, of which more than 40% were fungicides aimed at eliminating fungal plant pathogens. Many years of studies have shown that too frequent use of preparations with the same mode of action caused pathogenic microorganisms to develop mechanisms of resistance to synthetic fungicides [8,9,10].
Reports of the toxicity of chemical plant protection products [11,12,13] have led to legislation in The European Union (EU) aimed at protecting the natural ecosystem. ‘Farm to Fork Strategy for a Fair, Healthy, and Environmentally Friendly Food System’ and ‘EU Biodiversity Strategy for 2030, Bringing Nature Back into Our Lives’ are the main strategies of The European Green Deal, which aims to decrease the use of chemical pesticides by 50%, as well as focusing on the development of the organic farming sector based on the principles of Integrated Pest Management (IPM) [14,15,16]. IPM is a system of actions taken to reduce or eliminate factors that are harmful to plants. These methods should be based on the use of biological solutions that are safe for human health and the environment [17,18].
The ideal solution to meet the requirements of the EU programs is the development of biological control agents (BCA). Biological control is defined as the use of selected microorganisms, such as fungi of the genus Trichoderma, which exhibit antagonistic properties to plant pathogens [19].
It is estimated that about 60% of currently available biofungicides contain Trichoderma sp. strains [20,21]. This is due to the ability of these fungi to produce a wide range of secondary metabolites and enzymes with antimicrobial properties, their ability to rapidly colonize the rhizosphere, wide tolerance to environmental factors, and the uncomplicated and quick procedure for their isolation and multiplication. In addition, these microorganisms can induce plant defense mechanisms and increase the efficiency of their physiological processes, and they are therefore used as biofertilizers and biostimulants in crops of various plants [19,22].
Between 2008 and 2011, Sharma et al. [23] observed the effect of T. harzianum on wheat growth in two different agro-climatic zones of India. The results showed that the analyzed strain increased wheat yield in semi-arid zones with sand and wet zones with black soil, by 29% and 36%, respectively. Similarly, Mahato et al. [24] documented that T. viride enhanced wheat grain yield by 36.5%. Li et al. [25] proved that treatment of cucumber seeds with T. asperellum, T. harzianum, and T. pseudokoningii increased their yield by 23.54%, 25.57%, and 33.85%, respectively. A study by Hameen et al. [26] showed that T. koningii enhanced the yield of sugar beet by 14.58 tonnes in 2018/2019 and by 20.00 tonnes in 2019/2020, and Ji et al. [27] reported that a biofertilizer composed of T. asperellum, T. atroviride, T. hamatum, and T. harzianum increased the yield of Chinese cabbage by 37.4% relative to the control.
The objective of this review is to highlight the properties of filamentous fungi of the genus Trichoderma that predispose them to the production of biological plant protection products as well as to approximate the advantages of their use in agriculture, while at the same time outlining the difficulties associated with their production on an industrial scale and the introduction of biopreparations and biofertilizers based on Trichoderma on the market.
This review highlights the practical application of Trichoderma sp., the agricultural use of which aims to reduce or eliminate the use of chemical plant protection products. The most important aspects of this assumption are: (i) the proven phytosanitary efficacy of Trichoderma sp.; (ii) ability to promote the growth and development of selected plants; and (iii) non-toxicity to the environment, humans, and animals.
2. Systematics and Characteristics of Trichoderma Fungi
Nascimento et al. [28] documented that more than 375 species of Trichoderma have been identified and characterized molecularly and morphologically. According to the current MycoBank classification [29], these fungi belong to the domain Eukaryota, kingdom Fungi, subkingdom Dikarya, division Ascomycota, subdivision Pezizomycotina, class Sordariomycetes, subclass Hypocreomycetidae, order Hypocreales, and family Hypocreaceae. Membership of the Ascomycota family is associated with the presence of asexually reproducing individuals (anamorph) and sexually reproducing individuals (teleomorph). The Centre for Agriculture and Bioscience International (CABI) has divided the species belonging to Trichoderma according to their developmental form and classified the teleomorphs as a new genus Hypocrea [30].
The vast majority of Trichoderma species exhibit the ability to reproduce asexually by producing large numbers of single-celled conidia and thick-walled chlamydospores, which act as spores. Dark green (e.g., T. aggressivum, T. asperellum) to grey (e.g., T. erinaceum, T. tomentosum) or brown (e.g., T. melanomagna) conidia [19,31] are produced by multiple branched conidiophores, which are terminated by one or more fialids [32]. Circular conidial zones are made up of loose or collapsed conidiophores [33]. In contrast, Hypocrea species exhibit the ability to reproduce sexually by producing haploid ascospores in the ascus that form the ascocarp (perithecia) [34]. The surface of the colony takes on different colors (most often shades of green, e.g., T. longibrachiatum, T. reesei), is smooth or rough, has a grayish-white or yellowish tinge, and resembles cotton wool (e.g., T. koningiopsis, T. spirale) [35,36] (Figure 1). In addition, the species T. atroviride, T. harzianum, T. koningii, and T. viride can produce volatile 6-pentyl-α-pyrone (6PP), which gives their colonies a characteristic hazelnut and coconut smell [19,31,37].

Figure 1.
Growth of the mycelium of Trichoderma asperellum T3.1a strain (author’s own scheme).
3. Occurrence and Abundance of Trichoderma Fungi in Different Environments
Trichoderma sp. is a genus of filamentous, cosmopolitan fungi commonly found in all soil types and climatic zones [31,33]. They are considered to be saprophytic organisms capable of colonizing and decomposing dead organic matter [32,38]. In addition, they exhibit parasitic properties with respect to other fungi, as well as symbiotic and endophytic abilities toward plants [19,20,34].
A study by Ma et al. [39] showed that the type of ecosystem influences the species diversity of these microorganisms (Table 1). The above-mentioned authors analyzed the mycological condition of soils occurring in forest ecosystems (coniferous forest and coniferous and broadleaf mixed forest) and steppe (desert steppe and temperate steppe) in the northern Xinjiang region. The results showed that the frequency of isolation of Trichoderma sp. fungi from forest soils was twice as high as that of steppe soils and amounted to 79.26% and 36.55%, respectively. In addition, of the 23 identified species, only T. asperellum, T. harzianum, T. oblongisporum, T. paraviridescens, T. rossicum, and T. saturnisporum were present in all ecosystem types (coniferous forest, coniferous and broadleaf mixed forest, desert steppe, and steppe). Błaszczyk et al. [40] compared the species biodiversity of Trichoderma sp. strains obtained from decaying wood in Polish mountain forests. The researchers documented that the highest species diversity was characterized by the Gorce mountain range (Shannon’s biodiversity index—1.71), while the lowest was by the Karkonosze Mountains (Shannon’s biodiversity index—1.08). T. atroviride, T. citrinoviride, T. viride, and T. viridescens were present in all locations, while T. cremeum, T. koningii, and T. longibrachiatum were found only in the Gorce range, T. koningiopsis was found only in the Karkonosze Mountains, and T. gamsii and T. longipile were found only in the Tatra Mountains. Saravanakumar et al. [41] analyzed the mycological condition of wetlands in South Korea and isolated 18 species of Trichoderma sp. The dominant fungi were T. asperellum, T. atroviride, T. aureoviride, T. harzianum, T. koningii, T. koningiopsis, T. velutinum, and T. virens. Cummings et al. [42] recorded 93 isolates of Trichoderma sp. from the roots of tropical plants in northern Borneo. Among them, the most frequent microorganisms were T. asperelloides, T. asperellum, T. harzianum, and T. virens. In contrast, Kamala et al. [43] compared the species biodiversity of Trichoderma sp. isolated from nine sites in India. The above-mentioned authors observed the presence of T. album, T. amazonicum, T. asperellum, T. atroviride, T. aureoviride, T. caribbaeum, T. erinaceum, T. gamsii, T. hamatum, T. harzianum, T. inhamatum, T. koningiopsis, T. longibrachiatum, T. ovalisporum, T. petersen, T. piluliferum, T. spirale, and T. tomentosum. Teleomorphs were also classified as Hypocrea intricate, H. nigricans, H. rufa, and H. virens. Haouhach et al. [44] documented T. afroharzianum, T. atrobrunneum, and T. longibrachiatum from African soils for the first time (Algeria).
Many years of studies have shown that Trichoderma fungi can be isolated from non-anthropogenic natural habitats and cultivated soils. According to Jiang et al. [45], the type of crop is another factor influencing the species diversity of the soil microbiome (Table 1). The studies of the above authors showed that 8 and 9 species of Trichoderma sp. fungi were isolated from soils under wheat and maize cultivation, respectively, and 14 of them were obtained from rice fields. For wheat, the highest frequencies were T. harzianum (35%), T. asperellum (27%), and T. virens (13%). For maize, the most common microorganisms were T. asperellum (29%), T. virens (21%), and T. hamatum (14%). In rice fields, the dominant species were T. hamatum (25%), T. harzianum (22%), T. koningiopsis (18%), and T. virens (18%). Inglis et al. [46] isolated a total of 54 fungi from crop soil samples in Brazil. The researchers documented that T. hamatum and T. koningiopsis were present only in garlic fields, whereas T. afroharzianum, T. asperellum, T. erinaceum, and T. lentiforme were present only in onion fields. In addition, Jiang et al. [45] proved that the species biodiversity of the soil microbiome is related to the season. Their observations showed that during the summer season, the soil was characterized by the greatest diversity of Trichoderma (17 species). During the autumn, only nine species of these microorganisms were isolated from the samples.
Trichoderma sp. can also be isolated from soil not previously intended for cultivation, as proved by Al-Sadi et al. [47] (Table 1). The authors obtained 13 isolates of these fungi from 65 samples of such soil, among which T. asperellum, T. harzianum, T. longibrachiatum, and T. orientalis showed the highest occurrence. Similarly, Du Plessis et al. [48] isolated 19 species of Trichoderma from non-agricultural areas in South Africa. Bohacz and Korniłłowicz-Kowalska [49] obtained T. asperellum, T. citrinoviridae, T. harzianum, and T. lixii from lignocellulosic composts. In turn, McMullin et al. [50] isolated strains of T. atroviride, T. citrinoviride, T. harzianum, and T. koningiopsis from the walls of mold-covered buildings in Canada. Castagnoli et al. [51] obtained T. atroviride, T. citrinoviride, T. paraviridescens, and T. trixiae from settled dust, mineral wool, and exhaust air filters found in buildings in different Finnish cities.

Table 1.
Occurrence of Trichoderma fungi in different environments.
Table 1.
Occurrence of Trichoderma fungi in different environments.
| Types of Environments | Species of Trichoderma | Reference |
|---|---|---|
| desert soil | T. afroharzianum, T. atrobrunneum, T. longibrachiatum | [44] |
| forest soil | T. afroharzianum, T. asperellum, T. atroviride, T. citrinoviride, T. fertile, T. harzianum, T. longibrachiatum, T. oblongisporum, T. pararogersonii, T. paraviridescens, T. pleurotum, T. piluliferum, T. polysporum, T. rossicum, T. saturnisporum, T. viridescens | [39] |
| steppe soil | T. afroharzianum, T. asperellum, T. atroviride, T. brevicompactum, T. caerulescens, T. gamsii, T. ghanense, T. hamatum, T. harzianum, T. koningii, T. longibrachiatum, T. oblongisporum, T. paraviridescens, T. pleurotum, T. polysporum, T. rossicum, T. saturnisporum, T. semiorbis, T. viridescens | [39] |
| wetland soil | T. asperellum, T. atroviride, T. aureoviride, T. harzianum, T. koningii, T. koningiopsis, T. velutinum, T. virens | [41] |
| soil from garlic crops | T. asperelloides, T. azevedoi, T. hamatum, T. koningiopsis, T. longibrachiatum, T. peberdyi | [46] |
| soil from maize crops | T. asperellum, T. brevicompactum, T. fertile, T. hamatum, T. harzianum, T. koningiopsis, T. longibrachiatum, T. pleuroticola, T. virens | [45] |
| soil from onion crops | T. afroharzianum, T. asperelloides, T. asperellum, T. azevedoi, T. erinaceum, T. lentiforme, T. longibrachiatum, T. peberdyi | [46] |
| soil from rice crops | T. asperellum, T. atroviride, T. brevicompactum, T. capillare, T. erinaceum, T. fertile, T. hamatum, T. harzianum, T. koningiopsis, T. longibrachiatum, T. polysporum, T. saturnisporum, T. spirale, T. velutinum, T. virens | [45] |
| soil from wheat crops | T. asperellum, T. atroviride, T. brevicompactum, T. erinaceum, T. hamatum, T. harzianum, T. koningiopsis, T. virens | [45] |
| root of plant | T. afroharzianum, T. asperelloides, T. asperellum, T. guizhouense, T. harzianum, T. reesei, T. strigosum, T. virens | [42] |
| bark of tree | T. atroviride, T. erinaceum, T. harzianum, T. hebeiensis, T. longibrachiatum, T. parareesei, T. reesei | [52] |
| decaying woods | T. atroviride, T. citrinoviride, T. cremeum, T. gamsii, T. harzianum, T. koningii, T. koningiopsis, T. longibrachiatum, T. longipile, T. paraviridescens, T. trixiae, T. viride, T. viridescens | [40] |
| lignocellulosic compost | T. asperellum, T. citrinoviridae, T. harzianum, T. lixii | [49] |
4. Application of Trichoderma Fungi in the Control of Fungal Plant Pathogens
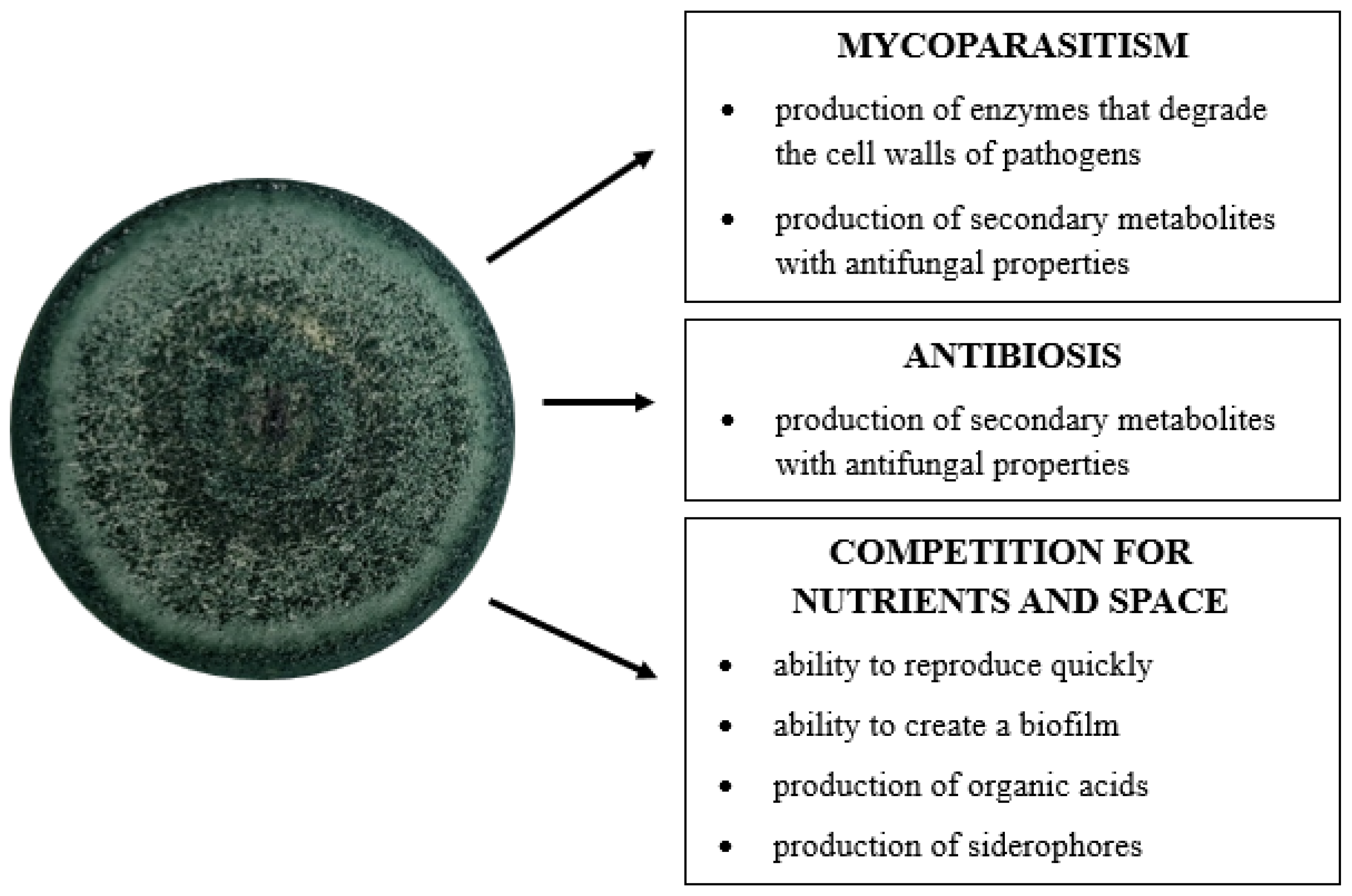
The main mechanisms responsible for the antifungal properties of Trichoderma sp. include mycoparasitism, antibiotics, and competition with plant pathogens for nutrients and space [21,22,32,53,54,55]. According to Błaszczyk et al. [31], the combination of these strategies provide fungi with a very high level of antagonism against pathogenic microorganisms (Figure 2).
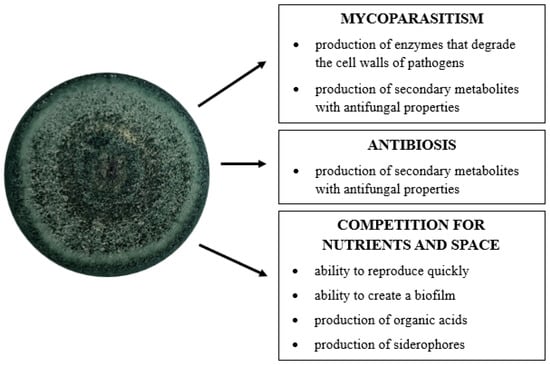
Figure 2.
Direct mechanisms of Trichoderma fungi in the control of fungal plant pathogens (author’s own scheme).
4.1. Antibiosis
Antibiosis belongs to a particular type of interaction between microorganisms, in which the antagonist produces various metabolites that interfere with the proper functioning of pathogens [21,31]. Secondary Metabolites (SM) are antibiotics and toxic substances that limit the growth, development, and colonization of pathogens [56,57] by producing reactive oxygen forms (ROS), deactivating the proteins and enzymes responsible for basic metabolic processes and biosynthesis of the structural elements of cells, and also the formation of channels in the cell membrane, leading to cytoplasm leakage and death of the microorganism [58,59,60,61,62,63].
Trichoderma sp. produces a wide range of antifungal compounds (Table 2). It is estimated that these fungi produce about 1000 different metabolites [59]. SM are the natural low-molecular-weight biosynthesis products of primary metabolites produced in specialized pathways [60,62]. These compounds are not necessary for the proper growth and functioning of the organism but support its defense and signaling [63]. Khan et al. [61] distinguished 11 groups of secondary metabolites produced by fungi of the genus Trichoderma during contact with the pathogen, which include anthraquinones, azaphilones, butenolides, epipolythiodioxopiperazines, koninginins, lactones, peptaibols, pyridones, pyrones, steroids, and trichothecenes.

Table 2.
Secondary metabolites with antifungal properties produced by Trichoderma sp.
Complex in vivo and in vitro studies show that secondary metabolites with antifungal properties produced by Trichoderma sp. have a high potential for use in plant production. Alfaro-Vargas et al. [65] reported that trichotoxin extract obtained from T. asperellum reduced the growth of C. gloeosporioides (growth inhibition − 92.2%), B. cinerea (growth inhibition − 74.29%), A. alternata (growth inhibition − 58.4%), and F. oxysporum (growth inhibition − 36.2%). Moreover, the incidence of A. alternata disease in tomatoes was found to be 92.5% under in vivo control conditions. In turn, treating seeds with trichotoxin extract reduced the incidence of infections to 0%. Similarly, Cai et al. [76] demonstrated that in a potting experiment, a harzianolide extract obtained from T. harzianum stimulated tomato seed growth and also induced systemic resistance in the plant to infection with S. sclerotiorum. Application of the extract increased the growth and weight of plants and reduced lesion size by 28.5% (metabolite concentration − 1 ppm) and 30.7% (metabolite concentration − 0.1 ppm) compared to the control.
4.2. Mycoparasitism
According to many researchers, the most effective way to inhibit the development of fungal plant pathogens is the process of mycoparasitism [54,63,86]. The mycoparasite recognizes and surrounds another fungus, produces appressorium, and develops on the host surface. Then, with the help of lytic enzymes and toxic compounds, the antagonist degrades the cell wall of the pathogen and absorbs nutrients [20,31,32,55]. Depending on the method of obtaining food, the mycoparasite may take the form of a biotroph, which extracts the necessary substances from the living tissues of the host, or a necrotroph, which first attacks, kills the cells of the pathogen, and then takes nutrients from dead tissues [86,87]. According to Tyśkiewicz et al. [32], fungi from the genus Trichoderma are most often classified as necrotrophic mycoparasites.
Mycoparasitic activity involves a multidirectional mode of action, consisting of the production by the antagonist of proteins responsible for transporting nutrients released from host tissues, substances toxic to pathogens (Table 1), as well as Cell Wall-Degrading Enzymes (CWDEs) [87]. Phytopathogenic cell walls are composed of 90% polysaccharides, such as chitin, glucan, chitosan, mannan, and galactomannan, as well as glycoproteins and proteins. Therefore, the main extracellular enzymes responsible for mycoparasitism include chitinases and β-glucanases, which directly degrade host cell walls [21,32]. In addition, these microorganisms produce proteases which, on the one hand, control the activity, stability, and secretion of extracellular enzymes by Trichoderma sp. [31], while on the other hand, proteases interfere with enzymes and the integrity of cell membranes of fungal pathogens [86,88].
This mechanism shows the great potential of Trichoderma sp. for use in plant production. Hirpara et al. [89] demonstrated that among 11 Trichoderma isolates, the strain T. virens NBAII inhibited the growth of S. rolfsii mycelium to the greatest extent in biculture (growth inhibition-76.37% on day 6, 87.91% on day 12). Moreover, it was observed that this isolate exhibited the highest chitinase and β-1,3-glucanase activities of 2.13 μm/mg and 2.67 mM/mg, respectively. Similarly, El Komy et al. [90] reported that under in vitro conditions, T. asperellum significantly reduced the development of F. oxysporum (percent of inhibition ranging from 68% to 71%). It was also found that this strain showed the highest chitinase and β-1,3-glucanase activities of 8.7–10.3 pmol/s/mL and 1.4–1.98 nmol/s/mL, respectively. In turn, Saravanakumar et al. [91] found that T. asperellum, which showed the highest activity of CDWEs enzymes among the tested strains (chitinase-87.5%, protease-52.9%, cellulase-84.8%, β-1,3-glucanase-60.5%), greatly inhibited the development of F. oxysporum, both in vitro (inhibition rate-99%) and in a greenhouse experiment with cucumber (disease reduction-71.67%). Moreover, long-term studies by many researchers showed that high antagonism of Trichoderma sp. resulting from the increased production of CDWE enzymes limits the growth of the following pathogens: Corynespora cassiicola, Curvularia aeria [92,93], Sclerotinia sclerotiorum [94], Sclerotium rolfsii [89], Aspergillus niger, Fusarium oxysporum [95,96], Colletotrichum gloeosporioides, Botrytis cinerea [97,98], Aspergillus flavus, Aspergillus fumigatus, Candida albicans, Mucor circinelloides, Rhizoctonia solani [99,100,101], Fusarium graminearum [68], Fusarium fujikuroi, Fusarium tricinctum, and Fusarium cantenulatum [102].
4.3. Competition for Nutrients and Space
Fungi of the genus Trichoderma can quickly grow and colonize culture media as well as the rhizosphere and spermosphere, e.g., of soybean, maize, tomato, and lettuce, which also leads to the limitation or complete inhibition of the development of plant pathogens [103,104,105,106]. In the soil environment, this process is closely related to the ability of microorganisms to form a biofilm. Trichoderma sp. secrete extracellular polymeric substances, which leads to the formation of extensive aggregates based on soil-fungal exudate cells on the surface of roots or soil [87].
A study by Sempere Ferre and Santamarina [107] showed that T. harzianum competed for space and nutrients with F. culmorum. Based on a macroscopic analysis of Petri dishes on which the antagonist and pathogen biculture was formed, it was found that Trichoderma sp. colonized the available surface much faster than Fusarium at 15 °C and 25 °C. Similarly, Mokhtar and Dehimat [108] observed that T. harzianum inhibited the growth of A. alternata, A. infectoria, and F. acuminatum. The authors documented that the growth rate of the antagonist mycelia in biculture was 3.4–4 cm/day, while for the pathogens, the growth rate was in the range of 0.8–1.2 cm/day. Imran et al. [109] demonstrated that Trichoderma fungi inhibited the development of A. solani under in vitro conditions. The results of the analyses indicated that the colony diameter of the pathogen in the control sample was 82.66 mm, while in the bicultures, the diameters of A. solani were 30.6 mm, 27.3 mm, and 23.3 mm for T. atroviride, T. harzianum, and T. longibrachiatum, respectively. Sudantha and Suwardji [110] proved that T. hamatum, T. harzianum, T. koningii, T. longibrachiatum, T. piluliferum, and T. viride reduced the growth of F. oxysporum on a PDA medium by an average of 64.8–77.2%. In turn, Modrzewska et al. [111] reported that T. atroviride, T. viride, and T. viridescens inhibited the development of F. cerealis and F. culmorum by 24–66%. Trichoderma species colonized 75–100% of the medium surface.
According to Rajesh et al. [112] and Kumar and Ashraf [113], the most common cause of the death of pathogenic microorganisms is competition for nutrients that are present in limited amounts in the medium. Compared to other fungi, Trichoderma sp. has a much greater capacity to absorb nutrients from the substrate and from the soil than other microorganisms [63,114]. This feature is related to the production of a wide range of organic acids (gluconic acid, citric acid, fumaric acid) by these fungi, which lower the pH of the soil and thus increase the solubility and absorption of the minerals present in the ground, including phosphates [32,55]. Acidification of the substrate disrupts the equilibrium, leading to the elimination of microorganisms that are unable to survive in the new environmental conditions. Trichoderma sp. also gains an advantage over pathogens in nutrient extraction, owing to its high ability to absorb energy from many different types of sugars that are a part of root secretions. This energy, stored in the form of ATP, allows these fungi to efficiently and quickly extract nutrients from the soil [21,112]. According to studies by Srivastava et al. [115], the production of siderophores by Trichoderma sp. depends on the pH of the substrate. The authors tested three pH ranges (4.5–6.5) in in vitro tests and observed the strongest production of siderophores in the medium with the lowest pH (4.5). In contrast, Dutt et al. [116] found that the optimal pH for siderophore production is maintained at the neutral level.
One of the microelements necessary for the growth and proper functioning of many microorganisms are iron ions [87]. In alkaline soils and under aerobic conditions, these ions form insoluble and difficult-to-absorb iron oxides. Fungi of the genius Trichoderma have developed a mechanism responsible for the biosynthesis of substances, which allows them to extract this element from the soil much faster than other microbes. Siderophores are low-molecular compounds that chelate irons by forming a complex with them. Once the ferric-siderophore complex is formed, the Fe3+ cations are converted to soluble Fe2+, which are recognized by the membrane receptors of Trichoderma sp. and then taken up by them [55,113]. In addition to iron, siderophores also chelate and convert manganese, nickel, molybdenum, and cobalt ions into soluble forms [117,118]. The mechanism by which Trichoderma makes Fe available to plants is still under investigation. However, there are studies confirming that the levels of iron in cucumber, soybean, or in wheat after treatment with the Trichoderma sp. strain were significantly higher than those in unvaccinated controls [119,120,121].
Ghosh et al. [122] documented that T. asperellum, T. harzianum, T. longibrachiatum, and T. viride showed the ability to produce ferric ion chelators belonging to the carboxylate and hydroxamate groups. Chen et al. [123] proved that T. atroviride, T. koningii, and T. paraviridescens produced siderophores of the catecholate type, whereas T. virens, T. atrobrunneum, T. citrinoviride, T. crissum, T. guizhouense, T. hamatum, T. simmonsii, and T. tomentosum produced compounds belonging to hydroxamate group. In addition, T. asperellum showed the ability to produce both types of ferric chelators.
5. Application of Trichoderma Fungi in Promoting Plant Growth
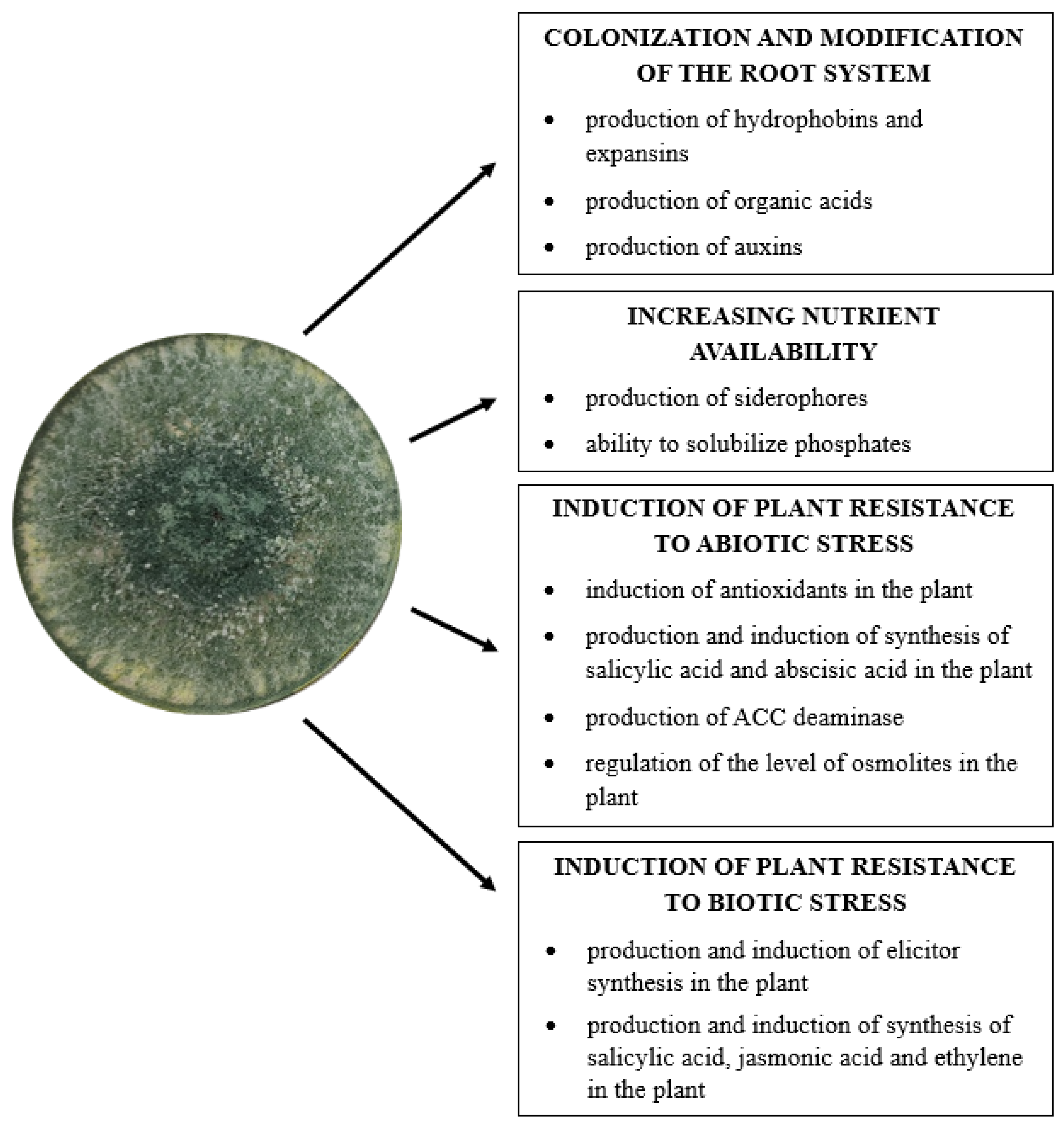
Many secondary metabolites and volatile substances produced by Trichoderma sp. exhibit, in addition to antifungal properties, the ability to promote plant growth. These compounds are responsible for activating signaling pathways leading to specific reactions in the plant which include the induction of the defense system, the production of substances that reduce stress caused by environmental factors, the synthesis of phytohormones, and other substances that stimulate plant growth and development. In addition, these microorganisms convert hard-to-reach nutrients present in the soil into soluble compounds, which are then taken up and used by plants [21,22,34,55,124,125] (Figure 3).
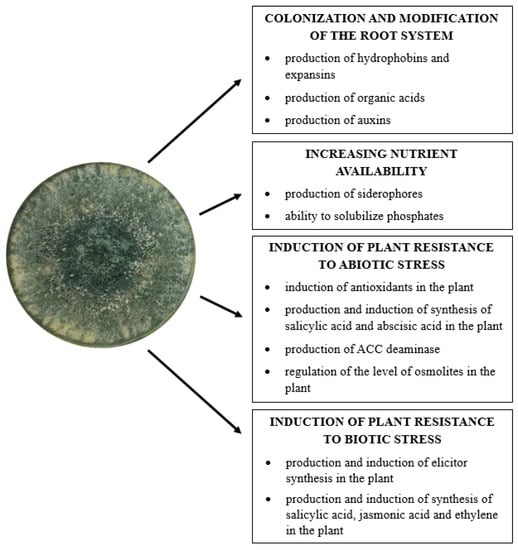
Figure 3.
Direct mechanisms of Trichoderma fungi in promoting plant growth (author’s own scheme).
5.1. Colonization and Modification of the Root System of Plants
Trichoderma sp. shows high resistance to phenols, phytoalexins, terpenoids, aglycones, and flavonoids produced by plants at the time of infection by foreign microorganisms. This ability allows these fungi to quickly colonize and modify the root system of plants [31,32]. In turn, the expansion of the root system contributes to increasing its colonization by beneficial soil microorganisms and the easier uptake of minerals by plants from the ground [126,127,128].
Colonization of the root by Trichoderma sp. is preceded by the production of chemical signaling substances by the plant [21,129]. This process leads to the adsorption of these microbes on the surface of underground plant structures and the formation of mycelium. These microorganisms then penetrate the tissues of the plants and then become established in the plant tissues [87]. Fungi of the genus Trichoderma are capable of ectomycorisis, in which flakes fill the intercellular spaces of the cortex of the primary roots [58,125]. Species of these microorganisms that exhibit mycorrhizal potential include T. album, T. aureoviride, T. fertile, T. glaucum, T. hamatum, T. harzianum, T. koningii, T. lignorum, T. longipilis, T. nunbergii, T. polysporum, T. pseudokoningii, T. pubescens, T. strictipilis, T. strigosum, T. verruculosa, T. virens, and T. viride [130].
Trichoderma sp., like other mycorrhizal species, produce cysteine-rich hydrophobins which are responsible for host recognition and fungal adhesion to hydrophobic root fragments [32]. Root penetration is facilitated by cellulose-binding expansins produced by these microorganisms, which disrupt the structures of plant cell walls by creating channels in them, which additionally enable plants to absorb more minerals from the substrate [21,55,79,131].
Meng et al. [132] observed that TgSWO protein extracted from T. guizhouense, like expansins, promoted cucumber growth by modifying its root system. The protein loosened and expanded the cell walls of the roots, thus outcompeting pathogenic fungi and bacteria for nutrients, an enhancement in the number of roots, stem diameter, root fresh weight, and shoot fresh weight of cucumber. In turn, Zhang et al. [133] proved that the root colonization process by T. harzianum was facilitated by the citric acid, malic acid, and oxalic acid produced by this strain. Increased adhesion of these fungi to the surface of cucumber roots caused a significantly enhanced shoot height, and shoot and root dry weights by 63.7%, 55.1%, and 36.1%, respectively, compared to the control. Samolski et al. [134] documented that T. harzianum also produced a cysteine-rich protein which, by inducing root hair growth, increased the available absorption area in the root system of cucumber. This change facilitated a greater uptake and translocation of carbon, lithium, manganese, and potassium in the plant, thus leading to an enhancement in crop biomass.
According to López-Bucio et al. [34], and Qin and Huang [135], the main phytohormones responsible for modifying the root system of plants are auxins, particularly indole-3-acetic acid (IAA). A low level of IAA in the plant induces primary growth and lengthening of existing roots, while disturbed transport leading to a higher concentration of auxins stimulates the formation of new native and lateral roots [32].
According to Joo and Husein [136], one of the mechanisms of Trichoderma responsible for promoting plant growth is the ability of the fungi to solubilize inaccessible forms of phosphorus and the production of phytohormones and phytoregulators by these microorganisms. Therefore, the researchers analyzed the ability of 10 Trichoderma strains to synthesize siderophores and auxins. The results of the studies showed that the species T. asperellum, T. atroviride, T. harzianum, T. koningii, T. longibrachiatum, T. reesei, and T. virens produced indole-3-acetic acid in the range of 10.94–59.10 µg/mL, making them potential candidates for biological plant growth promoters. Kumar et al. [137] observed that the increase in maize root length was correlated with the production of auxins by fungi of the genus Trichoderma. Among the analyzed isolates, T. harzianum displayed the highest IAA production at 36.4 µg/mL, which then accounted for the largest average enhancement in plant root length. Similar observations were made by Zhang et al. [138], who found that auxin production and root colonization were the mechanisms responsible for stimulating cucumber growth. The results of the studies showed that T. harzianum T-E5, which produced auxins at a level of 14.2 mg/L, increased the dry weight and vigor of the roots by 0.10 g and 0.26 mg/g/h, respectively, compared to the control. In contrast, T. harzianum strain SQR-T037, which produced indole-3-acetic acid at a level of 10.9 mg/L, enhanced the dry weight and vigor of the roots by 0.06 g and 0.16 mg/g/h, respectively, compared to the control. Therefore, IAA production was positively correlated with root growth parameters.
5.2. Increasing the Availability of Nutrients
The ability of Trichoderma to produce siderophores under conditions of poor iron availability is not only a mechanism of competition for nutrients with other microorganisms but is also responsible for stimulating plant growth [21,62]. The formation of soluble forms of iron enables plants to absorb Fe2+ ions through the root system and then use them for proper growth and development [32,34]. This element also acts as a cofactor necessary for the activity of enzymes such as cytochrome P450, catalases, and peroxidases which protect the plant from the toxic effects of hydrogen peroxide [139].
Joo and Hussein [136] documented that species T. asperellum, T. atroviride, T. harzianum, T. koningii, T. longibrachiatum, T. reesei, and T. virens produced siderophores in the range of 20.69–91.68%. An example of a compound that, by chelating iron ions, exhibits the ability to promote plant growth is harzianic acid (HA). Vinale et al. [140] analyzed the effect of HA produced by T. harzianum on tomato growth. The results showed that treatment of plants with harzianic acid induced seed germination, shoot, and root length by 76% and 66%, respectively, compared to the control. In addition, it was found that despite the low concentration of iron in the environment, the use of siderophores increased the concentration of this element in tomatoes. Zhao et al. [121] proved that a purified siderophore eluent produced by T. asperellum affected the growth of cucumber seedlings. The treatment of plants with the mixture induced increases in root and leaf length and fresh weight by 57.4%, 23.3%, and 33.7%, respectively. It was also found that inoculation of cucumber plants with the tested strain enhanced the Fe2+ ion concentration in leaves by 19.5% compared to the control.
The basic nutrient necessary for the proper functioning of plants is nitrogen. This element is a structural element of amino acids, proteins, and nucleic acids, as well as enzymes and chlorophyll, which are necessary for photosynthesis [141]. According to Fathi [142], properly conducted photosynthesis leads to an increase in the yield and dry weight of cultivated plants. In addition, an appropriate level of nitrogen reduces the aging process and chlorosis of the leaves, which has a positive effect on the morphological characteristics of the plants [141].
Fiorentino et al. [143] showed that T. virens enhanced nitrogen uptake for both lettuce and rocket by 51% and 59%, respectively. Tanwar et al. [144] reported that compared to the control, soil treatment with T. viride resulted in an increase in nitrogen content of 1.8% in shoots and 1.28% in broccoli roots. Similarly, Singh et al. [145] demonstrated that inoculation of tomato seeds with two strains of T. harzianum enhanced the ability of the plants to extract nitrogen ions from the soil. In the control sample, the uptake was 1.31%, while the use of Trichoderma isolates increased nitrogen uptake to 2.27%.
The main macroelement necessary for proper plant development is phosphorus, which is a component of enzymes, cofactors, and biomolecules [34,58]. In addition, this element participates in the process of photosynthesis, fruit ripening, and nitrogen binding. Plants are only capable of absorbing phosphorus in the form of soluble anions H2PO4− and HPO42−, which have an average soil content of only 0.1%. The remainder of P is in the form of insoluble salts of iron, aluminum, and calcium or specifically adsorbed on clay colloids. Trichoderma sp. has developed mechanisms of phosphate solubilization and mineralization, responsible for converting unavailable forms into orthophosphate ions, which can then be absorbed by plants [146,147,148].
Joo and Hussein [136] found that species T. asperellum, T. atroviride, T. harzianum, T. koningii, T. longibrachiatum, T. reesei, and T. virens solubilized phosphorus in the range of 2.1–152.2 μg/mL. Fiorentino et al. [143] documented that isolates of T. harzianum and T. virens increased the phosphate concentration in lettuce leaves by 1.2 and 1.9 g/kg dry weight, respectively. In turn, Bononi et al. [149] reported that two strains of Trichoderma sp. isolated from the Amazon rainforests showed the ability to dissolve calcium phosphate at 82.6% and 90.3%. Moreover, these isolates produced organic acids such as lactic acid, fumaric acid, citric acid, isocitric acid, and phytic acid during solubilization. The treatment of soybeans with the analyzed strains increased the dry weight of leaves and roots and plant growth. The phosphorus absorption efficiency of the tested soybeans was in the range of 111.2–156.1% and 81.7–140.6%, respectively. Promwee et al. [150] also reported that the production of organic acids is one of the mechanisms responsible for the release of phosphorus ions, which appear to be inaccessible to plants in most soils. According to Bader et al. [151], indole-3-acetic acid production and phosphate solubilization by T. harzianum significantly stimulated the growth of tomato seedlings. The analyzed isolates produced auxins at the level of 13.38–21.14 μg/mL and dissolved calcium phosphate at the level of 215.80–288.18 μg/mL. Plants treated with these strains increased leaf area, steam length, and fresh and dry weight of shoots and roots by 87.3–140.9%, 22.7–42.1%, 226.4–332%, 53.91–135.6%, 50–196.1%, and 166–400%, respectively, compared to the control. Trichoderma fungi also increase the availability and uptake of magnesium ions, which are the main components of mRNA and chlorophyll synthesis enzymes [21], and potassium ions, which induce the activity of enzymes involved in respiration and photosynthesis, and regulate the osmotic potential of plants cells. The treatment of plants with these microorganisms also leads to an enhancement in their organisms of calcium ions, which participate in the signaling and metabolic processes of cells and the formation of the mitotic spindle [141]. In addition, Trichoderma sp. has the ability to increase the availability of micronutrients, such as copper, zinc, and manganese ions, which affect crop size and plant nutrition [58,79,148]. These observations are confirmed by the studies of Illescas et al. [152], which show that T. asperellum increased manganese concentration in wheat grains by 7.01 mg per kg and copper concentration by 0.51 mg per kg of grain. Azarmi et al. [153] reported that the treatment of soil and tomato seeds with T. harzianum significantly increased the concentrations of calcium, magnesium, potassium, and phosphorus in the roots and shoots of the analyzed plants. Similarly, Fiorentino et al. [143] noted that isolates of T. harzianum and T. virens enhanced potassium and calcium concentrations in lettuce leaves by 7.9 and 11.6 g/kg dry weight and 1.2 and 1.7 g/kg dry weight, respectively, compared to the control.
5.3. Induction of Plant Resistance to Biotic Stress
The induction of plant systemic resistance to stress by Trichoderma sp. is a complex process based on many related chemical reactions. When a potential pathogen is detected, these microorganisms send signals to the plants, leading to an increase in the number of active enzymes and defense metabolites produced by these organisms [21,63,87], such as xylanase, chitinase, lipoxygenase, phenyl ammonia-lyase, peroxidase, polyphenol oxidase, terpenoid, pathogenesis-related proteins (PR proteins), phytoalexins (coumarin, lubimin, phytotuberol, resveratrol, rishitin, solevetivone), and antioxidants (abscisic acid (ABA), glutathione) [124]. The accumulation and increase in the concentrations of these elicitors are associated with the activation of the immune system in the plant, which includes Induced Systemic Resistance (IRS) directed at necrotrophic pathogens and Systemic Acquired Resistance (SAR) directed at biotrophic and hemibiotrophic pathogens [32,113]. The IRS response is associated with the synthesis of jasmonic acid (JA) and ethylene (ET), which are responsible for chlorosis, rapid aging, and the death of infected tissue. In contrast, the SAR response is associated with the synthesis of PR proteins and salicylic acid (SA), which limit the spread of pathogens in the plant by inducing programmed death of the infected cell [55,58,154,155,156] (Table 3).
Mayo et al. [157] documented that T. harzianum, when used to infect bean, in response to the threat of R. solani, synthesized greater amounts of ergosterol and squalene, which then activated the bean’s defense mechanisms and limited pathogen growth. Maintaining adequate levels of ergosterol and squalene ensures the stability and integrity of plant cell membranes, which are barriers against attack by pests. Nawrocka et al. [158] proved that the treatment of cucumber with T. atroviride induced the accumulation of volatile organic compounds and salicylic acid derivatives, which stimulated its immune system against R. solani. The inoculation of plants with the analyzed isolate resulted in enhanced activity of defense enzymes (guaiacol peroxidase, phenylalanine ammonia lyase, polyphenol oxidase, syringaldazine peroxidase), accumulation of phenolic and hydrogen peroxide, as well as decreased lipid peroxidation. In addition, T. atroviride increased the deposition of lignin and callose in cucumber cells, thus protecting its vascular system and assimilation cells from pathogen action. Treatment with the test strain significantly enhanced the fresh weight of the shoot and dry weight of roots by 55% and 77%, respectively, compared to the control. In contrast, Yuan et al. [159] reported that in response to the threat of B. cinerea, T. longibrachiatum activated signaling pathways associated with salicylic acid, jasmonic acid, and ethylene in cucumber. The treatment of plants with the analyzed strain caused a significant increase in the concentration of phytohormones SA, JA, and ET, thus leading to the induction of resistance, synthesis of defense metabolites, and inhibition of the pathogen growth by 49.62% on the fourth day. In addition, T. longibrachiatum enhanced root length, plant height, and fresh weight of cucumber by 48.68%, 10.82%, and 27.44%, respectively. Similarly, Wang et al. [160] found that T. asperellum activated the expression of genes associated with the synthesis of jasmonic acid and ethylene, thereby inducing IRS and SAR resistance against B. cinerea in tomato and reducing the disease incidence by 38%. Root length and seed germination rate were also increased by 37.86% and 5.55%, respectively.

Table 3.
Mechanisms of Trichoderma fungi in inducing plant resistance to biotic stress.
Table 3.
Mechanisms of Trichoderma fungi in inducing plant resistance to biotic stress.
| Types of Pathogens | Species of Trichoderma | Crops | Mechanisms of Action | Reference |
|---|---|---|---|---|
| Rhizoctonia solani | Trichoderma harzianum | bean |
| [157] |
| Rhizoctonia solani | Trichoderma atroviride | cucumber |
| [158] |
| Botrytis cinerea | Trichoderma longibrachiatum | cucumber |
| [159] |
| Botrytis cinerea | Trichoderma asperellum | tomato |
| [160] |
| Colletotrichum graminicola | Trichoderma asperellum | sorghum |
| [161] |
| Colletotrichum truncatum | Trichoderma asperellum | chilli |
| [162] |
5.4. Induction of Plant Resistance to Abiotic Stress
Urban et al. [163] define plant tolerance to abiotic stress as the ability to maintain crop yields at an appropriate level despite extreme environmental conditions. According to Tyśkiewicz et al. [32] and López-Bucio et al. [34], the factors that most affect the quantity and quality of crops are temperature variability, inadequate level of soil salinity, drought and lack of rainfall, and the presence of toxic heavy metals in the soil. According to Zaidi et al. [164], stressful environmental conditions: reduce the efficiency of photosynthesis, inhibit chlorophyll biosynthesis and seed germination; interfere with the proper functioning of hormonal and water management, the conductivity of the stomata, uptake, and transport of nutrients in the plant; and induce the formation of reactive oxygen forms (ROS), which destroy plant biomolecules (lipid, protein, nucleic acids) [20]. These changes lead to damage to individual cells and tissues, inhibition of the growth of roots and shoots, premature aging, chlorosis, necrosis, and ultimately the death of the entire organism [165,166]. In addition, stress interferes with the uptake and accumulation of carbon, which is necessary for the synthesis of cellular structures and defense molecules [163,167].
It has been shown that Trichoderma sp. fungi can reduce the effects of abiotic stress in plants, mainly by regulating the activity of their enzymatic and non-enzymatic antioxidants, production, and regulation of phytohormones and osmolites (Table 4).

Table 4.
Mechanisms of Trichoderma fungi in inducing plant resistance to abiotic stress.
The main mechanism used by Trichoderma fungi to increase plant tolerance to stressful environmental conditions is the induction of the activity of plant antioxidant enzymes such as ascorbate peroxidase (APX), catalase (CAT), glutathione peroxidase (GPX), glutathione reductase (GR), glutathione s-transferase (GST), phenylalanine ammonia-lyase (PAL), peroxidase (POD), polyphenol oxidase (PPO), superoxide dismutase (SOD), monodehydroascorbate reductase (MDHAR), and dehydroascorbate reductase (DHAR). In addition, Trichoderma sp. fungi activate non-enzymatic plant antioxidants such as proline, ascorbic acid, betaine, and glutathione. These compounds are responsible for the elimination of reactive oxygen forms (ROS), maintenance of the proper structure of proteins and cell membranes, and regulation of osmotic and metabolic homeostasis of plants [176,177,178].
Another mechanism used by Trichoderma fungi to increase the sensitivity of plants to stressful environmental conditions is the induction of production and control of the level of salicylic acid (SA) levels in the plant. According to Urban et al. [163], SA regulates antioxidant metabolism, thereby inducing an antioxidant response and modifying the level of photosynthesis efficiency. Li et al. [179] also indicate that the acid improves the ratios of K+ to Na+ and reduces lipid peroxidation. In contrast, Miura and Tada [180] report that increasing the salicylic acid content of the plant causes the accumulation of reactive forms of oxygen, hydrogen peroxide, and calcium ions, which lead to the closing of the stomata in situations of water deficits and drought. Another phytohormone regulated by Trichoderma sp. that controls the conductivity of the stomata, the water management of plants and the hydraulic conductivity of the roots, is abscisic acid (ABA). The high concentration of ABA causes the closing of the stomata, thus protecting the plant from the loss of a large amount of water [181].
Many analyses show that one of the mechanisms responsible for increasing plant tolerance to stressful environmental conditions is the production of ACC deaminase (ACCD) by Trichoderma sp., which reduces the concentration of ACC acid in plant tissues. In turn, this acid is a precursor of ethylene (ET), the accumulation of which accelerates the ripening process. Therefore, the production of ACCD enzymes delays the aging of plants [135,182,183].
5.5. Regulation of Growth, Physiological and Biochemical Processes of Plants
Fungi of the genus Trichoderma regulate the hormonal, water, and physiological processes of plants, such as gas exchange, photosynthesis, transpiration, or stomatal conductance. In addition, they modify their morphological characteristics [34], thus leading to increased yields and crop biomass [21,55,58].
Doni et al. [184] documented that Trichoderma sp. had a positive effect on the physiology and morphology of rice. The results of the studies of the above-mentioned authors showed that inoculation of plants with the microorganisms enhanced the stomatal conductance, thus leading to an increase in the concentration of carbon dioxide and water in rice. In turn, the increase in internal CO2 levels and H2O use efficiency resulted in enhancing the net photosynthetic rate to 8.66 µmol CO2/m2s. In addition, the treatment of seeds with Trichoderma isolates contributed to significant plant height, length, and fresh weight of roots and the number of leaves and tiller of rice. Mahato et al. [24] proved that T. viride induced wheat height, root weight, leaf length, and the number of grains, thus leading to a 36.5% increase in grain yield. In turn, Carillo et al. [185] reported that T. harzianum enhanced the nutritional and functional quality and yield of plum tomato crops. Treatment of plant roots with analyzed microorganisms increased the number and weight of fruits. There was also a 40.1% enhancement in tomato yield and an increase in lycopene, asparagine, gamma-aminobutyric acid, and monoethyl amine in plants by 49%, 37%, 87%, and 102%, respectively, compared to the control. Similarly, analyses by Lombardi et al. [186] showed that two strains of T. harzianum increased total strawberry yield by 35% and 38% and the number of fruits by 17% and 39%, respectively. In contrast, the T. virens induced root length and fresh and dry weight by 11%, 17%, and 21%, respectively. In addition, the treatment of plants with these fungi had a positive effect on the accumulation of anthocyanins in fruits and the regulation of proteins associated with metabolism, nutrient uptake, and plant stress response. By carrying out further studies, the above-mentioned authors found that the physiological and morphological changes occurring in strawberries are probably the result of the action of harzianic acid, 6-pentyl-α-pyrone, and hydrophobins produced by Trichoderma sp. [187]. Similarly, Marra et al. [188] showed that harzianic acid, 6-pentyl-α-pyrone, and hydrophobins induced soybean growth. Treatment of plants with metabolites significantly increased stem length, fresh and dry weight of soybean, and the fatty acid concentration in seeds by 17%. The ability of plants to absorb sodium, aluminum, and lithium was also enhanced by 58%, 89%, and 150%, respectively.
6. Impact of Environmental Conditions on Growth, Spores, and Phytosanitary Properties of Trichoderma Fungi
6.1. Temperature
Most fungi of the genus Trichoderma belong to mesophilic microorganisms for which the optimal temperature of growth and sporulation is between 25 °C and 35 °C [189,190]. However, some species have different temperature requirements and are isolated from the soil in colder or warmer regions of the world. According to Sharma et al. [191], T. harzianum, T. koningii, and T. viride were highly adapted to growth in a wide temperature range from 4 °C to 42 °C. Kamo et al. [192] demonstrated that T. polysporum developed at temperatures from 0 °C to 28 °C. Similarly, Zhang et al. [193] reported that the above-mentioned species grew within the range of 6 °C to 15 °C. The research of Montoya-Gonzalez et al. [194] and Poosapati et al. [195] proved that T. asperellum isolated from desert soil developed and spored properly at temperatures up to 50 °C and 52 °C.
The study by Andres et al. [196] showed that T. atroviride, T. harzianum, and T. sulpureum limited the growth of R. solani at 25 °C to a greater extent than at 15 °C. Similarly, Petrisor et al. [190] reported that the rates of inhibition of R. solani by T. pseudokoningii and T. viride were significantly higher at 30 °C than 25 °C. Mukherjee and Raghu [197] documented that Trichoderma sp. isolates limited the development of S. rolfsii mycelium at 25 °C and 30 °C. In contrast, at higher incubation temperatures (33 °C–37 °C), the pathogen inhibited the growth of the tested antagonists. Malathi and Doraisamy [198] also proved that with an increasing temperature (25 °C–35 °C), the antagonistic activity of T. hamatum, T. harzianum, T. koningii, T. longibrachiatum, and T. viride against M. phaseolina decreases. Di Leilo et al. [199] analyzed the effect of T. afroharzianum and T. atroviride on the induction of expression of genes involved in tomato defense responses. The above-mentioned authors reported that at 20 °C, T. atroviride significantly increased the activity of nine genes responsible for plant defense against pathogens, while at 25 °C, only three genes were affected. In contrast, T. afroharzianum significantly induced the expression of two and nine genes at 20 °C and 25 °C, respectively.
6.2. PH
Ali et al. [200] found that the optimal pH for Trichoderma sp. development was 6. In addition, the results showed that in an alkaline medium with pH 8, the mycelium is reduced and less developed than in an acidic environment with pH 4. According to Adnan et al. [19] as the acidity of the medium increases, the ability of the microorganisms to germinate increases, while alkaline conditions limit the growth of Trichoderma sp. Singh et al. [201] documented that the Trichoderma sp. were highly adapted to growing in a medium with a pH value between 5.5 and 7.5. In contrast, minimal quantities of their mycelium were obtained at pH 4 and 4.5. Zehra et al. [189] reported that the optimal pH for the development of T. asperellum, T. hamatum, T. harzianum, and T. viride ranged from 4.6 to 7.6. According to Sharma et al. [191], T. harzianum, T. koningii, and T. viride grew not only in a wide temperature range but also in a wide pH range from 3 to 13. However, the greatest increase in mycelium was observed at pH 5.5.
A study by Abeyratne and Deshappriya [202] showed that the pH of a medium also affected the antagonistic properties of Trichoderma sp. against plant pathogens. According to the above-mentioned authors, the analyzed strains limited the development of Fusarium sp. in the pH range from 4 to 7, with the highest inhibition rate occurring in a medium with a pH of 6 and amounting to 56.79%. Andres et al. [196] documented that T. atroviride, T. harzianum, and T. sulpureum limited the growth of R. solani at pH 5.5 to a greater extent than at pH 7 and 8.5. Similarly, Petrisor et al. [190] reported that the rates of inhibition of R. solani by T. pseudokoningii and T. viride were significantly higher at pH 4.5 than 8.5.
6.3. Salinity
Adnan et al. [19] noted that 5% NaCl inhibited the growth of T. hamatum, T. polysporum, and T. saturisporum by more than 96%. In contrast, a 5% saline solution had no effect on the development of mycelium of T. harzianum, T. lwningii, and T. viride. According to Ikram et al. [203], T. reesei resisted NaCl concentrations below 200 mM, whereas at 250 mM, there was a 50% reduction in dry and fresh mycelium mass. Zehra et al. [189] documented that T. asperellum, T. hamatum, T. harzianum, and T. viride showed high tolerance to the presence of sodium chloride in a medium. The above-mentioned authors reported that a concentration of 600 µM reduced sporulation but did not decrease mycelium, while a solution of 1000 µM NaCl completely inhibited mycelium growth.
Zhang et al. [204] proved that the salinity level of the medium affected the ability of T. atroviride to become simultaneously mycoparasitic as well as stimulate the growth of cucumber plants. According to Zhang et al. [204], the non-volatile metabolites produced in vitro by Trichoderma sp. more strongly limited the growth of F. oxysporum mycelium after the use of the medium in which the concentration of NaCl was 100 mM than when it was 0 mM. Volatile metabolites inhibited pathogen development by 50% and 58%, respectively, in conditions of the absence of salt in the medium and under salt stress. The results of observations in the greenhouse showed that the disease index of cucumber root rot was 43.125% at 0 mM sodium chloride concentration and 55% at 100 mM sodium chloride concentration. The researchers also analyzed the effect of Trichoderma sp. on cucumber growth at 100 mM and 200 mM NaCl medium. The fungi increased the length, fresh and dry root and shoot mass, and chlorophyll a and b concentration in cucumber plants at 100 mM NaCl medium to a greater extent than at 200 mM. The enhancement in total chlorophyll content was 13.93% and 19.68%, respectively, for 100 mM and 200 mM salt concentrations. Similarly, Boamah et al. [205] documented that as the NaCl content of the medium increased, the ability of T. longibrachiatum to limit the growth of F. pseudograminearum improved. The average rate of pathogen inhibition was 33.86% for the control, 36.32% for 50 mM NaCl, 44.59% for 100 mM NaCl, and 46.62% for 150 mM NaCl. In addition, it was reported that saline concentration influenced the growth parameters of wheat in different ways. The greatest enhancement in fresh and dry root weight and relative water content occurred at 150 mM sodium chloride concentration. Zhang et al. [206] found that T. longibrachiatum increased root length and wheat height by 4.16 cm and 4.20 cm, respectively, and chlorophyll content by 0.53 mg/g compared to salt stress conditions. The authors reported that the treatment of seeds with the analyzed fungi resulted in the enhancement of fresh and dry root and shoot weight, proline content, soluble sugars and proteins, and expression of genes encoding antioxidant enzymes in wheat. In contrast, a study by Kumar et al. [137] showed that the biomass, root length, and shoot length of maize inoculated with T. asperellum, T. aureoviride, T. harzianum, and T. koningiopsis decreased as the medium salinity increased. In addition, the average chlorophyll concentration decreased from 2.75 mg/g to 1.02 mg/g, with NaCl concentrations of 1.67 dS/m and 52 dS/m, respectively.
According to Ahmad et al. [165] too high a content of NaCl salt in the soil reduces its microbiological activity, porosity, and aeration and interferes with the conduction of minerals and water. Salinity stress induces ionic and osmotic stress in the plant, which leads to the production of reactive oxygen forms (ROS), destroying biomolecules [20] and lipid peroxidation, thus damaging the cytoplasmic membranes of plant cells. Long-term accumulation of a large amount of salt reduces the activity of enzymes responsible for chlorophyll biosynthesis. Oxidation of pigments present in chloroplasts leads to a decrease in chlorophyll content and impaired photosynthesis. In addition, gas exchange, growth of roots and shoots, and growth of fresh and dry weight of plants are inhibited [207,208]. According to Kumar et al. [137], Zhang et al. [204], Boamah et al. [205], and Zhang et al. [206], the Trichoderma fungi analyzed by them induced resistance to salinity stress in plants, mainly by increasing the activities of enzymatic (POD, CAT, SOD) and non-enzymatic (proline) plant antioxidants, which eliminated reactive oxygen forms. In addition, these microorganisms enhanced the length of the roots, thus allowing plants to absorb many more nutrients and water. Kumar et al. [137] reported that the increase in the surface area of the root system may be a consequence of the production by Trichoderma sp. of exogenous indole-3-acetic acid (IAA). In turn, Boamah et al. [205] found that analyzed microorganisms produced salicylic acid (SA), which induces antioxidant enzyme activity and reduces the accumulation of MDA, a byproduct of lipid peroxidation and a marker of plant stress tolerance. In contrast, Zhang et al. [206] documented that the enhancement in chlorophyll concentration could be associated with an increase in the leaf area of plants inoculated with T. longibrachiatum.
7. Fungicides and Plant Growth Stimulants Based on Fungi of the Genus Trichoderma
7.1. Market of Available Fungicides and Plant Growth Stimulants Based on Fungi of the Genus Trichoderma
Biopesticides, that is, preparations based on microorganisms or plant extracts used to control undesirable organisms, account for only 1.3% of all plant protection products produced. Current reports indicate that the global market for bioproducts is worth around EUR 1.6 billion, while the European market is worth around EUR 550 million [209]. According to the International Association of Producers of Biocontrols, global biopesticide production will increase by 14.7% by 2050, of which biofungicide production will increase by 16.1% [210].
According to Meher et al. [211], 41% of the bioproducts available on the market are made from microorganisms, of which 74% are bacteria and 10% are fungi. Due to their antagonistic properties, Trichoderma fungi are currently the most common and widely used microorganisms in biological plant protection. It is estimated that about 50–60% of commercial biofungicides are produced from these microorganisms. Analyses carried out by Tyśkiewicz et al. [32] show that currently there are about 77 biopesticides on the world market, produced from single or several Trichoderma strains, which eliminate more than 100 different fungal plant pathogens [212].
Recent data from the United States Environmental Protection Agency (EPA) [213] show that biofungicides based on strains of T. asperellum, T. atroviride, T. gamsii, T. hamatum, T. harzianum, T. polysporum, T. virens, and T. viride are produced in the USA. Bioproducts available in Brazil include T. afroharzianum, T. asperellum, and T. harzianum [32]. In Australia, authorized biopesticides are produced from T. atroviride and T. harzianum [214,215], and in India, they are produced from T. harzianum and T. viride [216]. Current data from the Ministry of Agriculture and Rural Affairs of the People’s Republic of China (MARA) [217] show that China produces biofungicides based on T. harzianum and a group of fungi generally known as Trichoderma sp. According to the recent regulations of the European Commission, bioproducts based on T. afroharzianum, T. asperellum, T. atrobrunneum, T. atroviride, T. gamsii, and T. harzianum are currently used in the European Union (Table 5).

Table 5.
Examples of Trichoderma sp. biofungicides authorized for use in the European Union [218,219,220].
7.2. Advantages of Using Plant Protection Products Based on Fungi of the Genus Trichoderma
Biopesticides based on Trichoderma sp. fungi and their secondary metabolites show very high specificity and selectivity, which means that they reduce strictly defined populations of pathogens and pests, and they do not eliminate crops and beneficial organisms [221]. They are non-toxic and non-pathogenic [222] to pollinators, farmers, and people living close to fields where these microorganisms are applied [223,224,225].
Compared to synthetic pesticides, biological agents are fully biodegradable, rapidly decompose, and do not accumulate in ecosystems [221,225,226]. According to Pertot et al. [224], the relatively short half-life of the microorganisms and the harmless byproducts mean that microbial biopesticides have limited or no requirements in terms of time to harvest and can be used just before harvest.
The complex and diverse indirect and direct mechanisms of action of Trichoderma sp. in combating a wide range of fungal plant pathogens (antibiosis, mycoparasitism, competition for space and nutrients) and stimulating plant growth (modification of the root system, increasing nutrient availability, induction of plant resistance to biotic and abiotic stress) mean that crop pests have not developed resistance to commercially available biopreparations [224]. Therefore, Trichoderma sp.-based agents can be used to eliminate pathogens that have previously become resistant to chemical pesticides [209].
The easy process of isolation of Trichoderma sp. fungi and the possibility of their rapid multiplication on basic microbiological substrates, as well as waste raw materials (e.g., sugar molasses) in large industrial bioreactors, translates into low production costs of biological plant protection products [209]. In turn, the wide tolerance to environmental factors and the prevalence of these microorganisms make preparations based on these fungi suitable for use in any ecosystem and climatic zone in the world [222].
Due to their low toxicity and the natural origin of microorganisms and their secondary metabolites, biopesticides are classified as ‘low-risk active substances’ which have a longer authorization period [223]. In addition, microbiological bioproducts may be used in developing organic farming and in sensitive areas where the use of chemical pesticides has been banned [209,222,224].
7.3. Problems of Introducing Biological Plant Protection Products Based on Trichoderma sp. to the Commercial Market
Despite continuous research, many plant protection products based on biological agents are not authorized for commercialization on a global scale. A huge barrier in the study of potential biopesticides is the precise understanding and determination of their modes of action, i.e., their interactions in dynamic systems, composed of antagonistic organisms, pathogens, and plants [22,32,87]. Technical limitations in the development of biological plant protection products are very often the result of difficulties in transferring the results obtained in laboratory conditions to field conditions. Many microorganisms show high efficacy under controlled in vitro conditions but do not meet expectations under in vivo conditions [87,209].
The formula of biopreparation must be effective, practical to use, and economically and environmentally viable. The challenge of introducing bioproducts based on Trichoderma fungi to the commercial market may be to verify and comply with the requirements related to their stability because they contain biotic agents whose activity and effectiveness may change due to environmental factors [224,227].
In addition to climatic conditions, interactions with autochthonous microorganisms present in an environment may adversely affect the efficacy of microorganisms included in biopreparations [224]. According to Kubiak et al. [209], the main environmental factors affecting the activity and survival of beneficial microorganisms, including their sporulation processes, include temperature, humidity, acidification of the substrate, and availability of nutrients. The main challenge here is to select microbes that will show high tolerance to changing climatic conditions [87] and resistance to chemical pesticides. Moreover, it turns out that even with optimal growth and development conditions, a significant proportion of the beneficial microorganisms contained in biopreparations can partially eliminate only one species of pathogens [222].
The non-uniform registration process for bioproducts is also a challenge. Each country has its organizations controlling the commercialization of the resulting plant protection products, and therefore, there is a lack of universal procedures for the production and introduction of biopesticides to the global market [209]. Despite the limitations resulting from the use of biological plant protection products, it seems possible to find optimal solutions that ensure the economic viability of agricultural production and, at the same time, reverse the degradation of soil ecosystems. The scientific studies presented in this study prove that the use of bioproducts based on Trichoderma sp. fully complies with the principles of integrated plant protection.
8. Conclusions
Analyses of the toxicity of chemical preparations and the development of resistance of plant pathogens to synthetic pesticides have led to the introduction of legislation that restricts or prohibits their use in an increasing number of countries. According to the researchers, the ideal solution that can minimize the risk of crop loss is to place a significantly larger number of biopesticides on the market. Therefore, scientists around the world are conducting extensive analyses to obtain microorganisms and secondary metabolites with antimicrobial properties that will additionally stimulate crop growth. Many years of studies have shown that fungi of the genus Trichoderma, in particular, the species T. asperellum, T. atroviride, T. harzianum, T. koningii, T. longibrachiatum, T. virens, and T. viride, are the ideal biocontrol agents with the desired characteristics.
Unfortunately, despite many reports of the beneficial effects of these fungi on plant growth and development, the exact mode of action of these antagonists is still unknown. Therefore, the greatest challenge in the study of potential biopesticides is to establish the interactions in the highly dynamic Trichoderma–pathogen, Trichoderma–plant, and Trichoderma–plant–pathogen systems. To this end, rapid and effective methods should be developed to test the antagonistic and phytosanitary properties of these fungi, as well as their toxicity to humans and the environment. In addition, a huge technological barrier is the transfer of results obtained under laboratory conditions to field conditions. Nevertheless, it seems that the greatest challenge in the development of biological plant protection products is to increase public awareness of the effectiveness and safety of bioproducts based on Trichoderma sp.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
No new data were created or analyzed in this study. Data sharing is not applicable to this article.
Conflicts of Interest
The authors declare no conflict of interest.
References
- FAO. How to feed the World in 2050. In Proceedings of the Expert Meeting on How to Feed the World in 2050, Rome, Italy, 12–13 October 2009. [Google Scholar]
- FAO. The Future of Food and Agriculture—Alternative Pathways to 2050; Summary Version; FAO: Rome, Italy, 2018; p. 60. ISBN 9789251309896. [Google Scholar]
- Peng, Y.; Li, S.J.; Yan, J.; Tang, Y.; Cheng, J.P.; Gao, A.J.; Yao, X.; Ruan, J.J.; Xu, B.L. Research Progress on Phytopathogenic Fungi and Their Role as Biocontrol Agents. Front. Microbiol. 2021, 12, 670135. [Google Scholar] [CrossRef] [PubMed]
- Doehlemann, G.; Ökmen, B.; Zhu, W.; Sharon, A. Plant pathogenic fungi. Microbiol Spectr. 2017, 5, 5.1.14. [Google Scholar] [CrossRef]
- Fisher, M.C.; Gurr, S.J.; Cuomo, C.A.; Blehert, D.S.; Jin, H.; Stukenbrock, E.H.; Stajich, J.E.; Kahmann, R.; Boone, C.; Denning, D.W.; et al. Threats posed by the fungal kingdom to humans, wildlife, and agriculture. mBio 2020, 11, e00449-20. [Google Scholar] [CrossRef] [PubMed]
- Dean, R.; Van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology: Top 10 fungal pathogens. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef] [PubMed]
- EUROSTAT (European Statistical Office). Available online: https://ec.europa.eu/eurostat (accessed on 10 April 2023).
- Hollomon, D.W. Fungicide resistance: Facing the challenge—A review. Plant. Prot. Sci. 2015, 51, 170–176. [Google Scholar] [CrossRef]
- Lucas, J.A.; Hawkins, N.J.; Fraaije, B.A. The Evolution of Fungicide Resistance. In Advances in Applied Microbiology; Elsevier: Amsterdam, The Netherlands, 2015; Volume 90, pp. 29–92. [Google Scholar] [CrossRef]
- Ishii, H.; Hollomon, D.W. Fungicide Resistance in Plant Pathogens: Principles and a Guide to Practical Management; Springer: Tokyo, Japan, 2015. [Google Scholar] [CrossRef]
- Shefali; Kumar, R.; Sankhla, M.S.; Kumar, R.; Sonone, S.S. Impact of Pesticide Toxicity in Aquatic Environment. Biointerface Res. Appl. Chem. 2020, 11, 10131–10140. [Google Scholar] [CrossRef]
- Meena, R.S.; Kumar, S.; Datta, R.; Lal, R.; Vijayakumar, V.; Brtnicky, M.; Sharma, M.P.; Yadav, G.S.; Jhariya, M.K.; Jangir, C.K.; et al. Impact of Agrochemicals on Soil Microbiota and Management: A Review. Land 2020, 9, 34. [Google Scholar] [CrossRef]
- Gupta, P.K. Toxicity of Fungicides. In Veterinary Toxicology; Gupta, R.C., Ed.; Academic Press: Cambridge, MA, USA, 2018; pp. 569–580. [Google Scholar] [CrossRef]
- European Commission. Communication from the Commmission to the European Parliament, the European Council, the Council, the European Economic and Social Commmittee and the Commmittee of the Regions, the European Green Deal; European Commission: Brussels, Belgium, 2019. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=COM%3A2019%3A640%3AFIN (accessed on 10 April 2023).
- European Commission. Communication from the Commission to the European Parliament, the Council, the European Economic and Social Committee and the Committee of the Regions, EU Biodiversity Strategy for 2030, Bringing Nature Back into Our Lives; European Commission: Brussels, Belgium, 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX:52020DC0380 (accessed on 10 April 2023).
- European Commission. Communication from the Commmission to the European Parliament, the European Council, the Council, the European Economic and Social Commmittee and the Commmittee of the Regions, Farm to Fork Strategy for a Fair, Healthy and Environmentally-Friendly Food System; European Commission: Brussels, Belgium, 2020. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=CELEX%3A52020DC0381 (accessed on 10 April 2023).
- European Union. Regulation (EC) No 1107/2009 of the European Parliament and of the Council of 21 October 2009 Concerning the Placing of Plant Protection Products on the Market and Repealing Council Directives 79/117/EEC and91/414/EEC. Official Journal of the European Union. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/TXT/?uri=celex%3A32009R1107 (accessed on 10 April 2023).
- European Union. Directive 2009/128/EC of the European Parliament and of the Council of 21 October 2009 Establishing a Framework for Community Action to Achieve the Sustainable Use of Pesticides, Text with EEA Relevance. Official Journal of the European Union. 2009. Available online: https://eur-lex.europa.eu/legal-content/EN/ALL/?uri=celex%3A32009L0128 (accessed on 10 April 2023).
- Adnan, M.; Islam, W.; Shabbir, A.; Khan, K.A.; Ghramh, H.A.; Huang, Z.; Lu, G.D. Plant defense against fungal pathogens by antagonistic fungi with Trichoderma in focus. Microb. Pathog. 2019, 129, 7–18. [Google Scholar] [CrossRef]
- Japanis, F.G.; Vetaryan, S.; Raja, N.K.K.; Mokhtar, M.A.A.; Mohd Fishal, E.M. The Impact of Trichoderma spp. on Agriculture and Their Identification. MAB 2022, 51, 1–15. [Google Scholar] [CrossRef]
- Sood, M.; Kapoor, D.; Kumar, V.; Sheteiwy, M.S.; Ramakrishnan, M.; Landi, M.; Araniti, F.; Sharma, A. Trichoderma: The “secrets” of a multitalented biocontrol agent. Plants 2020, 9, 762. [Google Scholar] [CrossRef]
- Manzar, N.; Kashyap, A.S.; Goutam, R.S.; Rajawat, M.V.S.; Sharma, P.K.; Sharma, S.K.; Singh, H.V. Trichoderma: Advent of Versatile Biocontrol Agent, Its Secrets and Insights into Mechanism of Biocontrol Potential. Sustainability 2022, 14, 12786. [Google Scholar] [CrossRef]
- Sharma, P.; Nath Patel, A.; Kumar Saini, M.; Deep, S. Field demonstration of Trichoderma harzianum as a plant growth promoter in wheat (Triticum aestivum L.). J. Agric. Sci. 2012, 4, 65–73. [Google Scholar] [CrossRef]
- Mahato, S.; Bhuju, S.; Shrestha, J. Effect of Trichoderma viride as biofertilizer on growth and yield of wheat. Malays. J. Sustain. Agric. 2018, 2, 1–5. [Google Scholar] [CrossRef]
- Li, M.; Ma, G.; Lian, H.; SU, X.; Tian, Y.; Huang, W.; Mie, J.; Jiang, X. The effects of Trichoderma on preventing cucumber Fusarium wilt and regulating cucumber physiology. J. Integr. Agric. 2019, 18, 607–617. [Google Scholar] [CrossRef]
- Hamden, S.; Shalaby, M.E.; Elkady, E.M.; Elfahar, S.A.; El-Emary, S.A. Potential Impacts of Eminent Fungicide, Certain Bacterial and Fungal Antagonists for Controlling Cercospora Leaf Spot Disease in Sugar Beet. J. Sustain. Agric. Sci. 2023, 49, 1–12. [Google Scholar] [CrossRef]
- Ji, S.; Liu, Z.; Liu, B.; Wang, Y.; Wang, J. The effect of Trichoderma biofertilizer on the quality of flowering Chinese cabbage and the soil environment. Sci. Hortic. 2020, 262, 109069. [Google Scholar] [CrossRef]
- Nascimento, V.C.; Rodrigues-Santos, K.C.; Carvalho-Alencar, K.L.; Castro, M.B.; Kruger, R.H.; Lopes, F.A.C. Trichoderma: Biological control efficiency and perspectives for the Brazilian Midwest states and Tocantins. Braz. J. Biol. 2022, 82, e260161. [Google Scholar] [CrossRef]
- MycoBank Database. Available online: http://www.MycoBank.org (accessed on 10 April 2023).
- CABI Databases. Available online: https://www.speciesfungorum.org/Names/fundic.asp (accessed on 10 April 2023).
- Błaszczyk, L.; Siwulski, M.; Sobieralski, K.; Lisiecka, J.; Jędryczka, M. Trichoderma spp.—Application and prospects for use in organic farming and industry. J. Plant Prot. Res. 2014, 54, 309–317. [Google Scholar] [CrossRef]
- Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Jaroszuk-Ściseł, J. Trichoderma: The Current Status of Its Application in Agriculture for the Biocontrol of Fungal Phytopathogens and Stimulation of Plant Growth. Int. J. Mol. Sci. 2022, 23, 2329. [Google Scholar] [CrossRef]
- Zin, N.A.; Badaluddin, N.A. Biological functions of Trichoderma spp. for agriculture applications. Ann. Agric. Sci. 2020, 65, 168–178. [Google Scholar] [CrossRef]
- López-Bucio, J.; Pelagio-Flores, R.; Herrera-Estrella, A. Trichoderma as biostimulant: Exploiting the multilevel properties of a plant beneficial fungus. Sci. Hortic. 2015, 196, 109–123. [Google Scholar] [CrossRef]
- Siddiquee, S. Morphology-based characterization of Trichoderma species. In Practical Handbook of the Biology and Molecular Diversity of Trichoderma Species from Tropical Regions; Springer: Cham, Switzerland, 2017; pp. 41–73. [Google Scholar] [CrossRef]
- Kumar, G.; Singh, A.; Pandey, S.; Singh, J.; Chauhan, S.S.; Srivastava, M. Morphomolecular Identification of Trichoderma sp. and their Mycoparasitic Activity Against Soil Borne Pathogens. Int. J. Bio-Resour. Stress Manag. 2021, 11, 613–627. [Google Scholar] [CrossRef]
- Fadel, H.H.M.; Mahmoud, M.G.; Asker, M.M.S.; Lotfy, S.N. Characterization and evaluation of coconut aroma produced by Trichoderma viride EMCC-107 in solid state fermentation on sugarcane bagasse. Electron. J. Biotechnol. 2015, 18, 5–9. [Google Scholar] [CrossRef]
- Sharma, S.; Kour, D.; Rana, K.L.; Dhiman, A.; Thakur, S.; Thakur, P.; Thakur, S.; Thakur, N.; Sudheer, S.; Yadav, N.; et al. Trichoderma: Biodiversity, ecological significances, and industrial applications. In Recent Advancement in White Biotechnology through Fungi; Springer: Berlin/Heidelberg, Germany, 2019; pp. 85–120. [Google Scholar] [CrossRef]
- Ma, J.; Tsegaye, E.; Li, M.; Wu, B.; Jiang, X. Biodiversity of Trichoderma from grassland and forest ecosystems in Northern Xinjiang, China. 3 Biotech 2020, 10, 362. [Google Scholar] [CrossRef] [PubMed]
- Błaszczyk, L.; Strakowska, J.; Chełkowski, J.; Gąbka-Buszek, A.; Kaczmarek, J. Trichoderma species occurring on wood with decay symptoms in mountain forests in Central Europe: Genetic and enzymatic characterization. J. Appl. Genet. 2016, 57, 397–407. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Wang, M.H. Isolation and molecular identification of Trichoderma species from wetland soil and their antagonistic activity against phytopathogens. Physiol. Mol. Plant Pathol. 2020, 109, 101458. [Google Scholar] [CrossRef]
- Cummings, N.; Ambrose, A.; Braithwaite, M.; Bissett, J.; Roslan, H.A.; Abdullah, J.; Stewart, A.; Agbayani, F.V.; Steyaert, J.; Hill, R.A. Diversity of root-endophytic Trichoderma from Malaysian Borneo. Mycol. Progr. 2016, 15, 50. [Google Scholar] [CrossRef]
- Kamala, T.; Devi, S.I.; Sharma, K.C.; Kennedy, K. Phylogeny and taxonomical investigation of Trichoderma spp. from Indian region of Indo-Burma biodiversity hot spot region with special reference to Manipur. BioMed Res. Int. 2015, 2015, 285261. [Google Scholar] [CrossRef]
- Haouhach, S.; Karkachi, N.; Oguiba, B.; Sidaoui, A.; Chamorro, I.; Kihal, M.; Monte, E. Three New Reports of Trichoderma in Algeria: T. atrobrunneum (South), T. longibrachiatum (South), and T. afroharzianum (Northwest). Microorganisms 2020, 8, 1455. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, J.L.; Chen, J.; Mao, L.J.; Feng, X.X.; Zhang, C.L.; Lin, F.C. Trichoderma biodiversity of agricultural fields in east China reveals a gradient distribution of species. PLoS ONE 2016, 11, e0160613. [Google Scholar] [CrossRef]
- Inglis, P.W.; Mello, S.C.M.; Martins, I.; Silva, J.B.T.; Macedo, K.; Sifuentes, D.N.; Valadares-Inglis, M.C. Trichoderma from Brazilian garlic and onion crop soils and description of two new species: Trichoderma azevedoi and Trichoderma peberdyi. PLoS ONE 2020, 15, e0228485. [Google Scholar] [CrossRef] [PubMed]
- Al-Sadi, A.M.; Al-Oweisi, F.A.; Edwards, S.G.; Al-Nadabi, H.; Al-Fahdi, A.M. Genetic analysis reveals diversity and genetic relationship among Trichoderma isolates from potting media, cultivated soil and uncultivated soil. BMC Microbiol. 2015, 15, 147. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, I.L.; Druzhinina, I.S.; Atanasova, L.; Yarden, O.; Jacobs, K. The diversity of Trichoderma species from soil in South Africa, with five new additions. Mycologia 2018, 110, 559–583. [Google Scholar] [CrossRef] [PubMed]
- Bohacz, J.; Korniłłowicz-Kowalska, T. Modification of post-industrial lignin by fungal strains of the genus Trichoderma isolated from different composting stages. J. Environ. Manag. 2020, 266, 110573. [Google Scholar] [CrossRef] [PubMed]
- McMullin, D.R.; Renaud, J.B.; Barasubiye, T.; Sumarah, M.W.; Miller, J.D. Metabolites of Trichoderma species isolated from damp building materials. Can. J. Microbiol. 2017, 63, 621–632. [Google Scholar] [CrossRef] [PubMed]
- Castagnoli, E.; Marik, T.; Mikkola, R.; Kredics, L.; Andersson, M.A.; Salonen, H.; Kurnitski, J. Indoor Trichoderma strains emitting peptaibols in guttation droplets. J. Appl. Microbiol. 2018, 125, 1408–1422. [Google Scholar] [CrossRef] [PubMed]
- Swain, H.; Adak, T.; Mukherjee, A.K.; Sarangi, S.; Samal, P.; Khandual, A.; Jena, R.; Bhattacharyya, P.; Naik, S.K.; Mehetre, S.T.; et al. Seed biopriming with Trichoderma strains isolated from tree bark improves plant growth, antioxidative defense system in rice and enhance straw degradation capacity. Front. Microbiol. 2021, 12, 633881. [Google Scholar] [CrossRef] [PubMed]
- Thapa, S.; Sotang, N.; Limbu, A.K.; Joshi, A. Impact of Trichoderma sp. in Agriculture: A Mini-Review. J. Biol. Today’s World 2020, 9, 227. [Google Scholar]
- Mukhopadhyay, R.; Kumar, D. Trichoderma: A beneficial antifungal agent and insights into its mechanisms of biocontrol potential. Egypt. J. Biol. Pest Control 2020, 30, 133. [Google Scholar] [CrossRef]
- Kumar, N.; Khurana, S.M.P. Trichoderma-plant-pathogen interactions for benefit of agriculture and environment. In Biocontrol Agents and Secondary Metabolites; Elsevier: Sawston, UK, 2021; pp. 41–63. [Google Scholar] [CrossRef]
- Kaddes, A.; Fauconnier, M.L.; Sassi, K.; Nasraoui, B.; Jijakli, M.H. Endophytic Fungal Volatile Compounds as Solution for Sustainable Agriculture. Molecules 2019, 24, 1065. [Google Scholar] [CrossRef] [PubMed]
- Chandrasekaran, M.; Paramasivan, M.; Sahayarayan, J.J. Microbial Volatile Organic Compounds: An Alternative for Chemical Fertilizers in Sustainable Agriculture Development. Microorganisms 2022, 11, 42. [Google Scholar] [CrossRef] [PubMed]
- Monfil, V.O.; Casas-Flores, S. Molecular Mechanisms of Biocontrol in Trichoderma spp. and Their Applications in Agriculture. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Chapter 32; pp. 429–453. [Google Scholar] [CrossRef]
- Zeilinger, S.; Gruber, S.; Bansal, R.; Mukherjee, P.K. Secondary Metabolism in Trichoderma–Chemistry Meets Genomics. Fungal Biol. Rev. 2016, 30, 74–90. [Google Scholar] [CrossRef]
- Al-Ani, L.K.T. Bioactive secondary metabolites of Trichoderma spp. for efficient management of phytopathogens. In Secondary Metabolites of Plant Growth Promoting Rhizomicroorganisms; Singh, H.B., Keswani, C., Reddy, M.S., Sansinenea, E., Garcia-Estrada, C., Eds.; Springer: Singapore, 2019; pp. 125–143. [Google Scholar] [CrossRef]
- Khan, R.A.A.; Najeeb, S.; Hussain, S.; Xie, B.; Li, Y. Bioactive secondary metabolites from Trichoderma spp. against phytopathogenic fungi. Microorganisms 2020, 8, 817. [Google Scholar] [CrossRef]
- Vinale, F.; Sivasithamparam, K. Beneficial effects of Trichoderma secondary metabolites on crops. Phytother. Res. 2020, 34, 2835–2842. [Google Scholar] [CrossRef] [PubMed]
- Kolli, S.K.; Adusumilli, N. Trichoderma—Its paramount role in agriculture. In New and Future Developments in Microbial Biotechnology and Bioengineering; Singh, J., Gehlot, P., Eds.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 69–83. [Google Scholar] [CrossRef]
- Malmierca, M.G.; Barua, J.; McCormick, S.P.; Izquierdo-Bueno, I.; Cardoza, R.E.; Alexander, N.J.; Hermosa, R.; Collado, I.G.; Monte, E.; Gutierrez, S. Novel aspinolide production by Trichoderma arundinaceum with a potential role in Botrytis cinerea antagonistic activity and plant defense priming. Environ. Microbiol. 2014, 17, 1103–1118. [Google Scholar] [CrossRef]
- Alfaro-Vargas, P.; Bastos-Salas, A.; Munoz-Arrieta, R.; Pereira-Reyes, R.; Redondo-Solano, M.; Fernandez, J.; Mora-Villalobos, A.; Lopez-Gomez, J.P. Peptaibol Production and Characterization from Trichoderma asperellum and Their Action as Biofungicide. J. Fungi 2022, 8, 1037. [Google Scholar] [CrossRef]
- Kamaruzzaman, M.; Islam, M.S.; Mahmud, S.; Polash, S.A.; Sultana, R.; Hasan, M.A.; Wang, C.; Jiang, C. In vitro and in silico approach of fungal growth inhibition by Trichoderma asperellum HbGT6-07 derived volatile organic compounds. Arab. J. Chem. 2021, 14, 103290. [Google Scholar] [CrossRef]
- Wonglom, P.; Ito, S.I.; Sunpapao, A. Volatile Organic Compounds Emitted from Endophytic Fungus Trichoderma asperellum T1 Mediate Antifungal Activity, Defense Response and Promote Plant Growth in Lettuce (Lactuca sativa). Fungal Ecol. 2020, 43, 100867. [Google Scholar] [CrossRef]
- Li, Y.; Sun, R.; Yu, J.; Saravanakumar, K.; Chen, J. Antagonistic and biocontrol potential of Trichoderma asperellum ZJSX5003 against the maize stalk rot pathogen Fusarium graminearum. Indian J. Microbiol. 2016, 56, 318–327. [Google Scholar] [CrossRef]
- Degani, O.; Khatib, S.; Becher, P.; Gordani, A.; Harris, R. Trichoderma asperellum Secreted 6-Pentyl-α-Pyrone to Control Magnaporthiopsis maydis, the Maize Late Wilt Disease Agent. Biology 2021, 10, 897. [Google Scholar] [CrossRef]
- Srinivasa, N.; Sriram, S.; Singh, C.; Shivashankar, K.S. Secondary Metabolites Approach to study the bio-efficacy of Trichoderma asperellum isolates in India. Int. J. Curr. Microbiol. Appl. Sci. 2017, 6, 1105–1123. [Google Scholar] [CrossRef][Green Version]
- Ruangwong, O.U.; Wonglom, P.; Suwannarach, N.; Kumla, J.; Thaochan, N.; Chomnunti, P.; Pitija, K.; Sunpapao, A. Volatile Organic Compound from Trichoderma asperelloides TSU1: Impact on Plant Pathogenic Fungi. J. Fungi 2021, 7, 187. [Google Scholar] [CrossRef] [PubMed]
- Elsherbiny, E.A.; Amin, B.H.; Aleem, B.; Kingsley, K.L.; Bennett, J.W. Trichoderma volatile organic compounds as a biofumigation tool against late blight pathogen Phytophthora infestans in postharvest potato tubers. J. Agric. Food Chem. 2020, 68, 8163–8171. [Google Scholar] [CrossRef] [PubMed]
- Shentu, X.; Zhan, X.; Ma, Z.; Yu, X.; Zhang, C. Antifungal activity of metabolites of the endophytic fungus Trichoderma brevicompactum from garlic. Braz. J. Microbiol. 2014, 45, 248–254. [Google Scholar] [CrossRef] [PubMed]
- Vinale, F.; Strakowska, J.; Mazzei, P.; Piccolo, A.; Marra, R.; Lombardi, N.; Manganiello, G.; Pascale, A.; Woo, S.L.; Lorito, M. Cremenolide, a new antifungal, 10-member lactone from Trichoderma cremeum with plant growth promotion activity. Nat. Prod. Res. 2016, 30, 2575–2581. [Google Scholar] [CrossRef]
- Jeerapong, C.; Phupong, W.; Bangrak, P.; Intana, W.; Tuchinda, P. Trichoharzianol, a new antifungal from Trichoderma harzianum F031. J. Agric. Food Chem. 2015, 63, 3704–3708. [Google Scholar] [CrossRef]
- Cai, F.; Yu, G.; Wang, P.; Wei, Z.; Fu, L.; Shen, Q.; Chen, W. Harzianolide, a Novel Plant Growth Regulator and Systemic Resistance Elicitor from Trichoderma harzianum. Plant. Physiol. Biochem. 2013, 73, 106–113. [Google Scholar] [CrossRef]
- Li, N.; Alfiky, A.; Wang, W.; Islam, M.; Nourollahi, K.; Liu, X.; Kang, S. Volatile Compound-Mediated Recognition and Inhibition Between Trichoderma Biocontrol Agents and Fusarium oxysporum. Front. Microbiol. 2018, 9, 2614. [Google Scholar] [CrossRef]
- Ahluwalia, V.; Kumar, J.; Rana, V.S.; Sati, O.P.; Walia, S. Comparative evaluation of two Trichoderma harzianum strains for major secondary metabolite production and antifungal activity. Nat. Prod. Res. 2015, 29, 914–920. [Google Scholar] [CrossRef]
- Contreras-Cornejo, X.A.; Macias-Rodriguez, L.; del-Val, E.; Larsen, J. Ecological functions of Trichoderma spp. and their secondary metabolites in the rhizosphere: Interactions with plants. FEMS Microbiol. Ecol. 2016, 92, fiw036. [Google Scholar] [CrossRef]
- You, J.; Li, G.; Li, C.; Zhu, L.; Yang, H.; Song, R.; Gu, W. Biological Control and Plant Growth Promotion by Volatile Organic Compounds of Trichoderma koningiopsis T-51. J. Fungi 2022, 8, 131. [Google Scholar] [CrossRef]
- Hu, M.; Li, Q.L.; Yang, Y.B.; Liu, K.; Miao, C.P.; Zhao, L.X.; Ding, Z.T. Koninginins R-S from the endophytic fungus Trichoderma koningiopsis. Nat. Prod. Res. 2017, 31, 835–839. [Google Scholar] [CrossRef] [PubMed]
- Sahar, L.; Doustmorad, Z. Antiproliferative and antimicrobial activities of secondary metabolites and phylogenetic study of endophytic Trichoderma species from Vinca plants. Front. Microbiol. 2018, 9, 1484. [Google Scholar] [CrossRef]
- Rajani, P.; Rajasekaran, C.; Vasanthakumari, M.M.; Olsson, S.B.; Ravikanth, G.; Shaanker, R.U. Inhibition of plant pathogenic fungi by endophytic Trichoderma spp. through mycoparasitism and volatile organic compounds. Microbiol. Res. 2021, 242, 126595. [Google Scholar] [CrossRef] [PubMed]
- Shi, M.; Chen, L.; Wang, X.W.; Zhang, T.; Zhao, P.B.; Song, X.Y.; Sun, C.Y.; Chen, X.L.; Zhou, B.C.; Zhang, Y.Z. Antimicrobial peptaibols from Trichoderma pseudokoningii induce programmed cell death in plant fungal pathogens. Microbiology 2012, 158, 166–175. [Google Scholar] [CrossRef] [PubMed]
- Hua, L.; Zeng, H.; He, L.; Jiang, Q.; Ye, P.; Liu, Y.; Sun, X.; Zhang, M. Gliotoxin Is an Important Secondary Metabolite Involved in Suppression of Sclerotium Rolfsii of Trichoderma virens T23. Phytopathology 2021, 111, 1720–1725. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Mendoza-Mendoza, A.; Zeilinger, S.; Horwitz, B.A. Mycoparasitism as a mechanism of Trichoderma-mediated suppression of plant diseases. Fungal Biol. Rev. 2022, 39, 15–33. [Google Scholar] [CrossRef]
- Lahlali, R.; Ezrari, S.; Radouane, N.; Kenfaoui, J.; Esmaeel, Q.; El Hamss, H.; Belabess, Z.; Barka, E.A. Biological Control of Plant Pathogens: A Global Perspective. Microorganisms 2022, 10, 596. [Google Scholar] [CrossRef]
- Abbas, A.; Mubeen, M.; Zheng, H.; Sohail, M.A.; Shakeel, Q.; Solanki, M.K.; Iftikhar, Y.; Sharma, S.; Kashyap, B.K.; Hussain, S.; et al. Trichoderma spp. Genes Involved in the Biocontrol Activity Against Rhizoctonia solani. Front. Microbiol. 2022, 13, 884469. [Google Scholar] [CrossRef]
- Hirpara, D.G.; Gajera, H.P.; Hirpara, H.Z.; Golakiya, B.A. Antipathy of Trichoderma against Sclerotium rolfsii Sacc.: Evaluation of Cell Wall-Degrading Enzymatic Activities and Molecular Diversity Analysis of Antagonists. Microb. Physiol. 2017, 27, 22–28. [Google Scholar] [CrossRef]
- El_Komy, M.H.; Saleh, A.A.; Eranthodi, A.; Molan, Y.Y. Characterization of novel Trichoderma asperellum isolates to select effective biocontrol agents against tomato Fusarium wilt. Plant Pathol. J. 2015, 31, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Yu, C.; Dou, K.; Wang, M.; Li, Y.; Chen, J. Synergistic effect of Trichoderma-derived antifungal metabolites and cell wall degrading enzymes on enhanced biocontrol of Fusarium oxysporum f. sp cucumerinum. Biol. Control 2016, 94, 37–46. [Google Scholar] [CrossRef]
- Baiyee, B.; Ito, S.I.; Sunpapao, A. Trichoderma asperellum T1 mediated antifungal activity and induced defense response against leaf spot fungi in lettuce (Lactuca sativa L.). Physiol. Mol. Plant Pathol. 2019, 106, 96–101. [Google Scholar] [CrossRef]
- Baiyee, B.; Pornsuriya, C.; Ito, S.; Sunapapo, A. Trichoderma spirale T76-1 displays biocontrol activity on lettuce (Lactuca sativa L.) caused by Corynespora cassiicola or Curvularia aeria. Biol. Control. 2019, 129, 195–200. [Google Scholar] [CrossRef]
- Troian, R.F.; Steindorff, A.S.; Ramada, M.H.S.; Arruda, W.; Ulhoa, C.J. Mycoparasitism studies of Trichoderma harzianum against Sclerotinia sclerotiorum: Evaluation of antagonism and expression of cell wall-degrading enzymes genes. Biotechnol. Lett. 2014, 36, 2095–2101. [Google Scholar] [CrossRef]
- Khatri, D.K.; Tiwari, D.N.; Bariya, H.S. Chitinolytic efficacy and secretion of cell wall-degrading enzymes from Trichoderma spp. in response to phytopathological fungi. J. App. Biol. Biotech. 2017, 5, 1–8. [Google Scholar] [CrossRef]
- Khare, E.; Kumar, S.; Kim, K. Role of peptaibols and lytic enzymes of Trichoderma cerinum Gur1 in biocontrol of Fusarium oxysporum and chickpea wilt. Environ. Sustain. 2018, 1, 39–47. [Google Scholar] [CrossRef]
- Aoki, Y.; Haga, S.; Suzuki, S. Direct antagonistic activity of chitinase produced by Trichoderma sp. SANA20 as biological control agent for gray mould caused by Botrytis cinerea. Cogent Biol. 2020, 6, 1747903. [Google Scholar] [CrossRef]
- Xue, M.; Wang, R.; Zhang, C.; Wang, W.; Zhang, F.; Chen, D.; Ren, S.; Manman, Z.; Hou, J.; Liu, T. Screening and Identification of Trichoderma Strains isolated from Natural Habitats in China with Potential Agricultural Applications. BioMed Res. Int. 2021, 2021, 7913950. [Google Scholar] [CrossRef]
- Deng, J.J.; Huang, W.Q.; Li, Z.W.; Lu, D.L.; Zhang, Y.; Luo, X.C. Biocontrol activity of recombinant aspartic protease from Trichoderma harzianum against pathogenic fungi. Enzyme Microb. Technol. 2018, 112, 35–42. [Google Scholar] [CrossRef]
- Ghasemi, S.; Safaie, N.; Shahbazi, S.; Shams-Bakhsh, M.; Askari, H. Enhancement of Lytic Enzymes Activity and Antagonistic Traits of Trichoderma harzianum Using γ-Radiation Induced Mutation. J. Agric. Sci. Technol. 2019, 21, 1035–1048. Available online: http://jast.modares.ac.ir/article-23-15277-en.html (accessed on 10 April 2023).
- Ghasemi, S.; Safaie, N.; Shahbazi, S.; Shams-Bakhsh, M.; Askari, H. The Role of Cell Wall Degrading Enzymes in Antagonistic Traits of Trichoderma virens Against Rhizoctonia solani. Iran. J. Biotechnol. 2020, 18, e2333. [Google Scholar] [CrossRef] [PubMed]
- Win, T.T.; Bo, B.; Malec, P.; Khan, S.; Fu, P. Newly isolated strain of Trichoderma asperellum from disease suppressive soil is a potential bio-control agent to suppress Fusarium soil borne fungal phytopathogens. J. Plant Pathol. 2021, 103, 549–561. [Google Scholar] [CrossRef]
- John, R.P.; Tyagi, R.D.; Prévost, D.; Brar, S.K.; Pouleur, S.; Surampalli, R.Y. Mycoparasitic Trichoderma Viride as a Biocontrol Agent against Fusarium Oxysporum f. Sp. Adzuki and Pythium Arrhenomanes and as a Growth Promoter of Soybean. Crop Prot. 2010, 29, 1452–1459. [Google Scholar] [CrossRef]
- Elshahawy, I.E.; El-Mohamedy, R.S. Biological control of Pythium damping-off and root-rot diseases of tomato using Trichoderma isolates employed alone or in combination. J. Plant Pathol. 2019, 101, 597–608. [Google Scholar] [CrossRef]
- Moreira, V.D.A.; Oliveira, C.E.D.S.; Jalal, A.; Gato, I.M.B.; Oliveira, T.J.S.S.; Boleta, G.H.M.; Giolo, V.M.; Vitória, L.S.; Tamburi, K.V.; Filho, M.C.M.T. Inoculation with Trichoderma harzianum and Azospirillum brasilense increases nutrition and yield of hydroponic lettuce. Arch. Microbiol. 2022, 204, 440. [Google Scholar] [CrossRef]
- Gaderer, R.; Lamdan, N.L.; Frischmann, A.; Sulyok, M.; Krska, R.; Horwitz, B.A.; Seidl-Seiboth, V. Sm2, a paralog of the Trichoderma cerato-platanin elicitor Sm1, is also highly important for plant protection conferred by the fungal-root interaction of Trichoderma with maize. BMC Microbiol. 2015, 15, 2. [Google Scholar] [CrossRef]
- Sempere Ferre, F.; Santamarina, M.P. Efficacy of Trichoderma harzianum in suppression of Fusarium culmorum. Ann. Microbiol. 2010, 60, 335–340. [Google Scholar] [CrossRef]
- Mokhtar, H.; Dehimat, A. Contribution in isolation and identification of some pathogenic fungi from wheat seeds, and evaluation of antagonistic capability of Trichoderma harzianum against those isolated fungi in vitro. Agri. Biol. J. N. Am. 2013, 4, 145–154. [Google Scholar] [CrossRef]
- Imran, M.; Abo-Elyousr, K.A.M.; Mousa, M.A.; Saad, M.M. Screening and biocontrol evaluation of indigenous native Trichoderma spp. against early blight disease and their field assessment to alleviate natural infection. Egypt. J. Biol. Pest Control 2022, 32, 40. [Google Scholar] [CrossRef]
- Sudantha, I.M.; Suwardji, S. Biodiversity of Trichoderma antagonist saprophytic fungi and its use for biocontrol of Fusarium wilt disease on shallots at Lombok Island, West Nusa Tenggara, Indonesia. IOP Conf. Ser. Earth Environ. Sci. 2021, 886, 012123. [Google Scholar] [CrossRef]
- Modrzewska, M.; Błaszczyk, L.; Stepien, Ł.; Urbaniak, M.; Waskiewicz, A.; Yoshinari, T.; Bryła, M. Trichoderma versus Fusarium Inhibition of Pathogen Growth and Mycotoxin Biosynthesis. Molecules 2022, 27, 8146. [Google Scholar] [CrossRef] [PubMed]
- Rajesh, R.W.; Rahul, M.S.; Ambalal, N.S. Trichoderma: A significant fungus for agriculture and environment. Afr. J. Agric. Res. 2016, 11, 1952–1965. [Google Scholar] [CrossRef]
- Kumar, M.; Ashraf, S. Role of Trichoderma spp. as a biocontrol agent of fungal plant pathogens. In Probiotics and Plant Health; Kumar, V., Kumar, M., Sharma, S., Prasad, R., Eds.; Springer: Singapore, 2017; pp. 497–506. [Google Scholar] [CrossRef]
- Oszust, K.; Cybulska, J.; Frąc, M. How Do Trichoderma Genus Fungi Win a Nutritional Competition Battle against Soft Fruit Pathogens? A Report on Niche Overlap Nutritional Potentiates. Int. J. Mol. Sci. 2020, 21, 4235. [Google Scholar] [CrossRef] [PubMed]
- Srivastava, M.P.; Tiwari, R.; Sharma, N. Effect of different cultural variables on siderophores produced by Trichoderma spp. Int. J. Adv. Res. 2013, 1, 1–6. [Google Scholar]
- Dutta, S.; Kundu, A.; Chakraborty, M.R.; Ojha, S.; Chakrabarti, J.; Chatterjee, N.C. Production and optimization of Fe(III) specific ligand, the siderophore of soil inhabiting and wood rotting fungi as deterrent to plant pathogens. Acta Phytopathol. Entomol. Hung. 2006, 41, 237–248. [Google Scholar] [CrossRef]
- Ahmed, E.; Holmstrom, S.J.M. Siderophores in environmental research: Roles and applications. Microb. Biotechnol. 2014, 7, 196–208. [Google Scholar] [CrossRef]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator-siderophore: A review. Microbiol. Res. 2018, 212–213, 103–111. [Google Scholar] [CrossRef]
- De Santiago, A.; Quintero, J.M.; Avilés, M.; Delgado, A. Effect of Trichoderma asperellum strain T34 on iron, copper, manganese, and zinc uptake by wheat grown on a calcareous medium. Plant Soil 2011, 342, 97–104. [Google Scholar] [CrossRef]
- Kabir, A.H.; Rahman, M.A.; Rahman, M.M.; Brailey-Jones, P.; Lee, K.W.; Bennetzen, J.L. Mechanistic assessment of tolerance to iron deficiency mediated by Trichoderma harzianum in soybean roots. J. Appl. Microbiol. 2022, 133, 2760–2778. [Google Scholar] [CrossRef]
- Zhao, L.; Wang, F.; Zhang, Y.; Zhang, J. Involvement of Trichoderma asperellum strain T6 in regulating iron acquisition in plant. J. Basic Microbiol. 2014, 54, S115–S124. [Google Scholar] [CrossRef] [PubMed]
- Ghosh, S.K.; Banerjee, S.; Sengupta, C. Siderophore production by antagonistic fungi. J. Biopestic. 2017, 10, 105–112. [Google Scholar] [CrossRef]
- Chen, L.; Bóka, B.; Kedves, O.; Nagy, V.D.; Szucs, A.; Champramary, S.; Roszik, R.; Patocskai, Z.; Munsterkotter, M.; Huynh, T.; et al. Towards the biological control of devastating forest pathogens from the genus Armillaria. Forests 2019, 10, 1013. [Google Scholar] [CrossRef]
- Singh, R.; Anbazhagan, P.; Viswanath, H.S.; Tomer, A. Trichoderma Species: A Blessing for Crop Production. In Trichoderma: Agricultural Applications and Beyond; Manoharachary, C., Ed.; Springer: Cham, Switzerland, 2020; Volume 61, pp. 127–158. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Doni, F.; Mispan, M.S.; Saiman, M.Z.; Yusuf, Y.M.; Oke, M.A.; Suhaimi, N.S.M. Harnessing Trichoderma in Agriculture for Productivity and Sustainability. Agronomy 2021, 11, 2559. [Google Scholar] [CrossRef]
- Nieto-Jacobo, M.F.; Steyaert, J.M.; Salazar-Badillo, F.B.; Nguyen, D.V.; Rostas, M.; Braithwaite, M.; De Souza, J.T.; Jimenez-Bremont, J.F.; Ohkura, M.; Stewart, A.; et al. Environmental growth conditions of Trichoderma spp. affects indole acetic acid derivatives, volatile organic compounds, and plant growth promotion. Front. Plant Sci. 2017, 8, 102. [Google Scholar] [CrossRef]
- Lombardi, N.; Vitale, S.; Turra, D.; Reverberi, M.; Fanelli, C.; Vinale, F.; Marra, R.; Ruocco, M.; Pascale, A.; D’Errico, G.; et al. Root exudates of stressed plants stimulate and attract Trichoderma soil fungi. Mol. Plant Microbe Interact. 2018, 31, 982–994. [Google Scholar] [CrossRef]
- Verma, S.K.; Sahu, P.K.; Kumar, K.; Pal, G.; Gond, S.K.; Kharwar, R.N.; White, J.F. Endophyte roles in nutrient acquisition, root system architecture development and oxidative stress tolerance. J. Appl. Microbiol. 2021, 131, 2161–2177. [Google Scholar] [CrossRef]
- Jacoby, R.; Peukert, M.; Succurro, A.; Koprivova, A.; Kopriva, S. The role of soil microorganisms in plant mineral nutrition—Current knowledge and future directions. Front. Plant Sci. 2017, 8, 1167. [Google Scholar] [CrossRef]
- Grzywacz, A. Gatunkowa różnorodność biologiczna grzybów terenów leśnych. Stud. I Mater. Cent. Edukac. Przyr.-Leśnej 2015, 44, 239–253. [Google Scholar]
- Ramirez-Valdespino, C.A.; Casas-Flores, S.; Olmedo-Monfil, V. Trichoderma as a Model to Study Effector-like Molecules. Front. Microbiol. 2019, 10, 1030. [Google Scholar] [CrossRef]
- Meng, X.; Miao, Y.; Liu, Q.; Ma, L.; Guo, K.; Liu, D.; Ran, W.; Shen, Q. TgSWO from Trichoderma guizhouense NJAU4742 promotes growth in cucumber plants by modifying the root morphology and the cell wall architecture. Microb. Cell Factories 2019, 18, 148. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Meng, X.; Yang, X.; Ran, W.; Shen, Q. Quantification and role of organic acids in cucumber root exudates in Trichoderma harzianum T-E5 colonization. Plant Physiol. Biochem. 2014, 83, 250–257. [Google Scholar] [CrossRef] [PubMed]
- Samolski, I.; Rincon, A.M.; Pinzon, L.M.; Viterbo, A.; Monte, E. The qid74 gene from Trichoderma harzianum has a role in root architecture and plant biofertilization. Microbiology 2012, 158, 129–138. [Google Scholar] [CrossRef] [PubMed]
- Qin, H.; Huang, R. Auxin Controlled by Ethylene Steers Root Development. Int. J. Mol. Sci. 2018, 19, 3656. [Google Scholar] [CrossRef] [PubMed]
- Joo, J.H.; Hussein, K.A. Biological Control and Plant Growth Promotion Properties of Volatile Organic Compound-Producing Antagonistic Trichoderma spp. Front. Plant Sci. 2022, 13, 897668. [Google Scholar] [CrossRef]
- Kumar, K.; Manigundan, K.; Amaresan, N. Influence of salt tolerant Trichoderma spp. on growth of maize (Zea mays) under different salinity conditions. J. Basic Microbiol. 2017, 57, 141–150. [Google Scholar] [CrossRef]
- Zhang, F.; Yuan, J.; Yang, X.; Cui, Y.; Chen, L.; Ran, W.; Shen, Q. Putative Trichoderma harzianum mutant promotes cucumber growth by enhanced production of indole acetic acid and plant colonization. Plant Soil 2013, 368, 433–444. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Hurley, J.F.; Taylor, J.T.; Puckhaber, L.; Lehner, S.; Druzhinina, I.; Schumacher, R.; Kenerley, C.M. Ferricrocin, the intracellular siderophore of Trichoderma virens, is involved in growth, conidiation, gliotoxin biosynthesis and induction of systemic resistance in maize. Biochem. Biophys. Res. Commun. 2018, 505, 606–611. [Google Scholar] [CrossRef]
- Vinale, F.; Nigro, M.; Sivasithamparam, K.; Flematti, G.; Ghisalberti, E.L.; Ruocco, M.; Varlese, R.; Marra, R.; Lanzuise, S.; Eid, A.; et al. Harzianic acid: A novel siderophore from Trichoderma harzianum. FEMS Microbiol. Lett. 2013, 347, 123–129. [Google Scholar] [CrossRef]
- Shrivastav, P.; Prasad, M.; Singh, T.B.; Yadav, A.; Goyal, D.; Ali, A.; Dantu, P.K. Role of Nutrients in Plant Growth and Development. In Contaminants in Agriculture; Naeem, M., Ansari, A., Gill, S., Eds.; Springer: Cham, Switzerland, 2002; pp. 43–59. [Google Scholar] [CrossRef]
- Fathi, A. Role of nitrogen (N) in plant growth, photosynthesis pigments, and N use efficiency: A review. Agrisost 2022, 28, 1–8. [Google Scholar] [CrossRef]
- Fiorentino, N.; Ventorino, V.; Woo, S.L.; Pepe, O.; De Rosa, A.; Gioia, L.; Romano, I.; Lombardi, N.; Napolitano, M.; Colla, G.; et al. Trichoderma-based biostimulants modulate rhizosphere microbial populations and improve N uptake efficiency, yield, and nutritional quality of leafy vegetables. Front. Plant Sci. 2018, 9, 743. [Google Scholar] [CrossRef] [PubMed]
- Tanwar, A.; Aggarwal, A.; Kaushish, S.; Chauhan, S. Interactive effect of AM fungi with Trichoderma viride and Pseudomonas fluorescens on growth and yield of broccoli. Plant Prot. Sci. 2013, 49, 137–145. [Google Scholar] [CrossRef]
- Singh, S.P.; Singh, H.B.; Singh, D.K.; Rakshit, A. Trichoderma-mediated enhancement of nutrient uptake and reduction in incidence of Rhizoctonia solani in tomato. Egypt J. Biol. 2014, 16, 29–38. [Google Scholar] [CrossRef]
- Kapri, A.; Tewari, L. Phosphate solubilization potential and phosphatase activity of rhizospheric Trichoderma spp. Braz. J. Microbiol. 2010, 41, 787–795. [Google Scholar] [CrossRef]
- Saravanakumar, K.; Shanmuga Arasu, V.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Timofeeva, A.; Galyamova, M.; Sedykh, S. Prospects for Using Phosphate-Solubilizing Microorganisms as Natural Fertilizers in Agriculture. Plants 2022, 11, 2119. [Google Scholar] [CrossRef]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 2858. [Google Scholar] [CrossRef]
- Promwee, A.; Issarakraisila, M.; Intana, W.; Chamswarng, C.; Yenjit, P. Phosphate solubilization and growth promotion of rubber tree (Hevea brasiliensis Muell. Arg.) by Trichoderma strains. J. Agric. Sci. 2014, 6, 8. [Google Scholar] [CrossRef]
- Bader, A.N.; Salerno, G.L.; Covacevich, F.; Consolo, V.F. Native Trichoderma harzianum strains from Argentina produce indole-3-acetic acid and phosphorus solubilization, promote growth and control wilt disease on tomato (Solanum lycopersicum L.). J. King Saud Univ. Sci. 2020, 32, 867–873. [Google Scholar] [CrossRef]
- Illescas, M.; Moran-Diez, M.E.; Martinez de Alba, A.E.; Hermosa, R.; Monte, E. Effect of Trichoderma asperellum on Wheat Plants’ Biochemical and Molecular Responses, and Yield under Different Water Stress Conditions. Int. J. Mol. Sci. 2022, 23, 6782. [Google Scholar] [CrossRef]
- Azarmi, R.; Hajieghrari, B.; Giglou, A. Effect of Trichoderma isolates on tomato seedling growth response and nutrient uptake. Afr. J. Biotechnol. 2011, 10, 5850–5855. [Google Scholar] [CrossRef]
- Yang, Y.; Ahammed, G.; Wu, C.; Fan, S.; Zhou, Y. Crosstalk among jasmonate, salicylate and ethylene signaling pathways in plant disease and immune responses. Curr. Protein Pept. Sci. 2015, 16, 450–461. [Google Scholar] [CrossRef] [PubMed]
- Ding, P.; Ding, Y. Stories of salicylic acid: A plant defense hormone. Trends Plant Sci. 2020, 25, 549–565. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Mostafa, S.; Zeng, W.; Jin, B. Function and Mechanism of Jasmonic Acid in Plant Responses to Abiotic and Biotic Stresses. Int. J. Mol. Sci. 2021, 22, 8568. [Google Scholar] [CrossRef] [PubMed]
- Mayo, S.; Gutierrez, S.; Malmierca, M.G.; Lorenzana, A.; Campelo, M.P.; Hermosa, R.; Casquero, P.A. Influence of Rhizoctonia solani and Trichoderma spp. in growth of bean (Phaseolus vulgaris L.) and in the induction of plant defense-related genes. Front. Plant Sci. 2015, 6, 685. [Google Scholar] [CrossRef]
- Nawrocka, J.; Małolepsza, U.; Szymczak, K.; Szczech, M. Involvement of metabolic components, volatile compounds, PR proteins and mechanical strengthening in multilayer protection of cucumber plants against Rhizoctonia solani activated by Trichoderma atroviride TRS25. Protoplasma 2018, 255, 359–373. [Google Scholar] [CrossRef]
- Yuan, M.; Huang, Y.; Ge, W.; Jia, Z.; Song, S.; Zhang, L.; Huang, Y. Involvement of jasmonic acid, ethylene and salicylic acid signaling pathways behind the systemic resistance induced by Trichoderma longibrachiatum H9 in cucumber. BMC Genom. 2019, 20, 144. [Google Scholar] [CrossRef]
- Wang, R.; Chen, D.; Khan, R.A.A.; Cui, J.; Hou, J.; Liu, T. A novel Trichoderma asperellum strain DQ-1 promotes tomato growth and induces resistance to gray mold caused by Botrytis cinerea. FEMS Microbiol. Lett. 2021, 368, fnab140. [Google Scholar] [CrossRef]
- Manzar, N.; Singh, Y.; Kashyap, A.S.; Sahu, P.K.; Rajawat, M.V.S.; Bhowmik, A.; Sharma, P.K.; Saxena, A.K. Biocontrol potential of native Trichoderma spp. against anthracnose of great millet (Sorghum bicolour L.) from Tarai and hill regions of India. Biol. Control 2020, 152, 104474. [Google Scholar] [CrossRef]
- Yadav, M.; Dubey, M.K.; Upadhyay, R.S. Systemic Resistance in Chilli Pepper against Anthracnose (Caused by Colletotrichum truncatum) Induced by Trichoderma harzianum, Trichoderma asperellum and Paenibacillus dendritiformis. J. Fungi 2021, 7, 307. [Google Scholar] [CrossRef]
- Urban, L.; Lauri, F.; Ben Hdech, D.; Aarrouf, J. Prospects for Increasing the Efficacy of Plant Resistance Inducers Stimulating Salicylic Acid. Agronomy 2022, 12, 3151. [Google Scholar] [CrossRef]
- Zaidi, N.W.; Dar, M.H.; Singh, S.; Singh, U.S. Trichoderma species as abiotic stress relievers in plants. In Biotechnology and Biology of Trichoderma; Gupta, V.K., Schmoll, M., Herrera-Estrella, A., Upadhyay, R.S., Druzhinina, I., Tuohy, M.G., Eds.; Elsevier: Amsterdam, The Netherlands, 2014; Volume 38, pp. 515–525. [Google Scholar] [CrossRef]
- Ahmad, P.; Hashem, A.; Abd-Allah, E.F.; Alqarawi, A.A.; John, R.; Egamberdieva, D.; Gucel, S. Role of Trichoderma harzianum in mitigating NaCl stress in Indian mustard (Brassica juncea L.) through antioxidative defense system. Front. Plant Sci. 2015, 6, 868. [Google Scholar] [CrossRef] [PubMed]
- Wungrampha, S.; Joshi, R.; Singla-Pareek, S.; Pareek, A. Photosynthesis and salinity: Are these mutually exclusive? Photosynthetica 2018, 56, 366–381. [Google Scholar] [CrossRef]
- Lata, R.; Chowdhury, S.; Gond, S.K.; White, J.F., Jr. Induction of abiotic stress tolerance in plants by endophytic microbes. Lett. Appl. Microbiol. 2018, 66, 268–276. [Google Scholar] [CrossRef]
- Tripathi, R.; Keswani, C.; Tewari, R. Trichoderma koningii enhances tolerance against thermal stress by regulating ROS metabolism in tomato (Solanum lycopersicum L.) plants. J. Plant Interact. 2021, 16, 116–126. [Google Scholar] [CrossRef]
- Ghorbanpour, A.; Salimi, A.; Ghanbary, M.A.T.; Pirdashti, H.; Dehestani, A. The effect of Trichoderma harzianum in mitigating low temperature stress in tomato (Solanum lycopersicum L.) plants. Sci. Hortic. 2018, 230, 134–141. [Google Scholar] [CrossRef]
- Boamah, S.; Zhang, S.; Xu, B.; Li, T.; Calderón-Urrea, A.; John Tiika, R. Trichoderma longibrachiatum TG1 increases endogenous salicylic acid content and antioxidants activity in wheat seedlings under salinity stress. PeerJ 2022, 10, e12923. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, Y. Effects of phosphate solubilization and phytohormone production of Trichoderma asperellum Q1 on promoting cucumber growth under salt stress. J. Integr. Agr. 2015, 14, 1588–1597. [Google Scholar] [CrossRef]
- Pandey, V.; Ansari, M.W.; Tula, S.; Yadav, S.; Sahoo, R.K.; Shukla, N.; Bains, G.; Badal, S.; Chandra, S.; Gaur, A.K.; et al. Dose-Dependent Response of Trichoderma harzianum in Improving Drought Tolerance in Rice Genotypes. Planta 2016, 243, 1251–1264. [Google Scholar] [CrossRef]
- Mona, S.A.; Hashem, A.; Abd Allah, E.F.; Alqarawi, A.A.; Soliman, D.W.K.; Wirth, S.; Egamberdieva, D. Increased resistance of drought by Trichoderma harzianum fungal treatment correlates with increased secondary metabolites and proline content. J. Integr. Agric. 2017, 16, 1751–1757. [Google Scholar] [CrossRef]
- Devi, S.S.; Sreenivasulu, Y.; Rao, K.V.B. Protective Role of Trichoderma logibrachiatum (WT2) on Lead Induced Oxidative Stress in Helianthus annus L. Indian J. Exp. Biol. 2017, 55, 235–241. [Google Scholar]
- Vargas, J.T.; Rodriguez-Monroy, M.; Meyer, M.L.; Montes-Belmont, R.; Sepulveda-Jimenez, G. Trichoderma asperellum ameliorates phytotoxic effects of copper in onion (Allium cepa L.). Environ. Exp. Bot. 2017, 136, 85–93. [Google Scholar] [CrossRef]
- Fu, J.; Liu, Z.; Li, Z.; Wang, Y.; Yang, K. Alleviation of the effects of saline-alkaline stress on maize seedlings by regulation of active oxygen metabolism by Trichoderma asperellum. PLoS ONE 2017, 12, e0179617. [Google Scholar] [CrossRef]
- Zhang, S.; Xu, B.; Gan, Y. Seed Treatment with Trichoderma Longibrachiatum T6 Promotes Wheat Seedling Growth under Nacl Stress through Activating the Enzymatic and Nonenzymatic Antioxidant Defense Systems. Int. J. Mol. Sci. 2019, 20, 3729. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Wang, Y.; Liu, C.; Chen, F.; Ge, H.; Tian, F.; Yang, T.; Ma, K.; Zhang, Y. Trichoderma harzianum mitigates salt stress in cucumber via multiple responses. Ecotoxicol. Environ. Saf. 2019, 170, 436–445. [Google Scholar] [CrossRef]
- Li, G.; Peng, X.; Wei, L.; Kang, G. Salicylic acid increases the contents of glutathione and ascorbate and temporally regulates the related gene expression in salt-stressed wheat seedlings. Gene 2013, 529, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Miura, K.; Tada, Y. Regulation of water, salinity, and cold stress responses by salicylic acid. Front. Plant Sci. 2014, 5, 4. [Google Scholar] [CrossRef] [PubMed]
- Saradadevi, R.; Palta, J.A.; Siddique, K.H.M. ABA-mediated stomatal response in regulating water use during the development of terminal drought in wheat. Front. Plant Sci. 2017, 8, 1251. [Google Scholar] [CrossRef]
- Hu, Y.; Vandenbussche, F.; Van dDer Straeten, D. Regulation of seedling growth by ethylene and the ethylene-auxin crosstalk. Planta 2017, 245, 467–489. [Google Scholar] [CrossRef]
- Illescas, M.; Pedrero-Mendez, A.; Pitorini-Bovolini, M.; Hermosa, R.; Monte, E. Phytohormone production profiles in Trichoderma species and their relationship to wheat plant responses to water stress. Pathogens 2021, 10, 991. [Google Scholar] [CrossRef]
- Doni, F.; Isahak, A.; Zain, C.R.C.M.; Yusoff, W.M.W. Physiological and growth response of rice plants (Oryza sativa L.) to Trichoderma spp. inoculants. AMB Express 2014, 4, 45. [Google Scholar] [CrossRef] [PubMed]
- Carillo, P.; Woo, S.L.; Comite, E.; El-Nakhel, C.; Rouphael, Y.; Fusco, G.M.; Borzacchiello, A.; Lanzuise, S.; Vinale, F. Application of Trichoderma harzianum, 6-Pentyl-α-Pyrone and Plant Biopolymer Formulations Modulate Plant Metabolism and Fruit Quality of Plum Tomatoes. Plants 2020, 9, 771. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Caira, S.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Salzano, A.M.; Lorito, M.; Woo, S.L. Trichoderma Applications on Strawberry Plants Modulate the Physiological Processes Positively Affecting Fruit Production and Quality. Front. Microbiol. 2020, 11, 1364. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, N.; Salzano, A.M.; Troise, A.D.; Scaloni, A.; Vitaglione, P.; Vinale, F.; Marra, R.; Caira, S.; Lorito, M.; d’Errico, G.; et al. Effect of Trichoderma Bioactive Metabolite Treatments on the Production, Quality, and Protein Profile of Strawberry Fruits. J. Agric. Food Chem. 2020, 68, 7246–7258. [Google Scholar] [CrossRef] [PubMed]
- Marra, R.; Lombardi, N.; d’Errico, G.; Troisi, J.; Scala, G.; Vinale, F.; Woo, S.L.; Bonanomi, G.; Lorito, M. Application of Trichoderma Strains and Metabolites Enhances Soybean Productivity and Nutrient Content. J. Agric. Food Chem. 2019, 67, 1814–1822. [Google Scholar] [CrossRef]
- Zehra, A.; Dubey, M.K.; Meena, M.; Upadhyay, R.S. Effect of different environmental conditions on growth and sporulation of some Trichoderma species. J. Environ. Biol. 2017, 38, 197–203. [Google Scholar] [CrossRef]
- Petrisor, C.; Paica, A.; Constantinescu, F. Influence of abiotic factors on in vitro growth of Trichoderma strains. Proc. Rom. Acad. Ser. B 2016, 18, 11–14. [Google Scholar]
- Sharma, P.K.; Gothalwal, R.; Tiwari, R.K.S. Isolation of cold tolerant antifungal strains of Trichoderma sp. from Northern Hilly Zones of Chhattisgarh. Int. J. Plant Prot. 2013, 6, 236–240. [Google Scholar]
- Kamo, M.; Tojo, M.; Yamazaki, Y.; Itabashi, T.; Takeda, H.; Wakana, D.; Hosoe, T. Isolation of growth inhibitors of the snow rot pathogen Pythium iwayamai from an arctic strain of Trichoderma polysporum. J. Antibiot. 2016, 69, 451–455. [Google Scholar] [CrossRef]
- Zhang, T.; Chaturvedi, V.; Chaturvedi, S. Novel Trichoderma polysporum Strain for the Biocontrol of Pseudogymnoascus destructans, the Fungal Etiologic Agent of Bat White Nose Syndrome. PLoS ONE 2015, 10, e0141316. [Google Scholar] [CrossRef]
- Montoya-Gonzalez, A.H.; Quijano-Vicente, G.; Morales-Maza, A.; Ortiz-Uribe, N.; Hernandez-Martinez, R. Isolation of Trichoderma spp. from Desert Soil, Biocontrol Potential Evaluation and Liquid Culture Production of Conidia Using Agricultural Fertilizers. J. Fertil. Pestic. 2016, 7, 163. [Google Scholar] [CrossRef]
- Poosapati, S.; Ravulapalli, P.D.; Tippirishetty, N.; Vishwanathaswamy, D.K.; Chunduri, S. Selection of high temperature and salinity tolerant Trichoderma isolates with antagonistic activity against Sclerotium rolfsii. SpringerPlus 2014, 3, 641. [Google Scholar] [CrossRef]
- Andres, P.A.; Alejandra, P.M.; Benedicto, M.C.; Nahuel, R.I.; Clara, B.M. A Comparative Study of Different Strains of Trichoderma under Different Conditions of Temperature and pH for the Control of Rhizoctonia solani. Agric. Sci. 2022, 13, 702–714. [Google Scholar] [CrossRef]
- Mukherjee, P.K.; Raghu, K. Effect of temperature on antagonistic and biocontrol potential of shape Trichoderma sp. on Sclerotium rolfsii. Mycopathologia 1997, 139, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Malathi, P.; Doraisamy, S. Effect of temperature on growth and antagonistic activity of Trichoderma spp. against Macrophomina phaseolina. J. Biol. Control 2003, 17, 153–159. [Google Scholar]
- Di Lelio, I.; Coppola, M.; Comite, E.; Molisso, D.; Lorito, M.; Woo, S.L.; Pennacchio, F.; Rao, R.; Digilio, M.C. Temperature Differentially Influences the Capacity of Trichoderma Species to Induce Plant Defense Responses in Tomato Against Insect Pests. Front. Plant Sci. 2021, 12, 678830. [Google Scholar] [CrossRef]
- Ali, H.Z.; Aboud, H.M.; Dheyab, N.S.; Musa, N.K.; Gasam, F.H. Effects of pH and ECW on Growth and Sporulation of Indigenous Trichoderma spp. Int. J. Phytopathol. 2015, 4, 15–20. [Google Scholar] [CrossRef]
- Singh, A.; Shahid, M.; Srivastava, M.; Pandey, S.; Sharma, A.; Kumar, V. Optimal physical parameters for growth of Trichoderma species at varying pH, temperature and agitation. Virol. Mycol. 2014, 3, 127. [Google Scholar] [CrossRef]
- Abeyratne, G.D.D.; Deshappriya, N. The effect of pH on the biological control activities of a Trichoderma sp. against Fusarium sp. isolated from the commercial onion fields in Sri Lanka. Trop. Plant Res. 2018, 5, 121–128. [Google Scholar] [CrossRef]
- Ikram, M.; Ali, N.; Jan, G.; Iqbal, A.; Hamayun, M.; Jan, F.G.; Hussain, A.; Lee, I.J. Trichoderma reesei improved the nutrition status of wheat crop under salt stress. J. Plant Interact. 2019, 14, 590–602. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, W.; Hu, Y.; Peng, Z.; Ren, S.; Xue, M.; Liu, Z.; Hou, J.; Xing, M.; Liu, T. A novel salt-tolerant strain Trichoderma atroviride HN082102.1 isolated from marine habitat alleviates salt stress and diminishes cucumber root rot caused by Fusarium oxysporum. BMC Microbiol. 2022, 22, 67. [Google Scholar] [CrossRef] [PubMed]
- Boamah, S.; Zhang, S.; Xu, B.; Li, T.; Calderón-Urrea, A. Trichoderma longibrachiatum (TG1) Enhances Wheat Seedlings Tolerance to Salt Stress and Resistance to Fusarium Pseudograminearum. Front. Plant Sci. 2021, 12, 741231. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S.; Gan, Y.; Xu, B. Application of plant-growth-promoting fungi Trichoderma longibrachiatum T6 enhances tolerance of wheat to salt stress through improvement of antioxidative defense system and gene expression. Front. Plant Sci. 2016, 7, 1405. [Google Scholar] [CrossRef]
- Parihar, P.; Singh, S.; Singh, R.; Singh, V.P.; Prasad, S.M. Effect of salinity stress on plants and its tolerance strategies: A review. Environ. Sci. Pollut. Res. 2015, 22, 4056–4075. [Google Scholar] [CrossRef] [PubMed]
- Mushtaq, Z.; Faizan, S.; Gulzar, B. Salt stress, Its impacts on plants and the strategies plants are employing against it: A review. J. Appl. Biol. Biotechnol. 2020, 8, 81–91. [Google Scholar]
- Kubiak, A.; Wolna-Maruwka, A.; Niewiadomska, A.; Pilarska, A.A. The Problem of Weed Infestation of Agricultural Plantations vs. the Assumptions of the European Biodiversity Strategy. Agronomy 2022, 12, 1808. [Google Scholar] [CrossRef]
- Khakimov, A.A.; Omonlikov, A.U.; Utaganov, S.B.U. Current status and prospects of the use of biofungicides against plant diseases. GSC Biol. Pharm. Sci. 2020, 13, 119–126. [Google Scholar] [CrossRef]
- Meher, J.; Rajput, R.S.; Bajpai, R.; Teli, B.; Sarma, B.K. Trichoderma: A Globally Dominant Commercial Biofungicide. In Trichoderma: Agricultural Applications and Beyond; Manoharachary, C., Ed.; Springer: Cham, Switzerland, 2020; Volume 61, pp. 195–208. [Google Scholar] [CrossRef]
- Thambugala, K.M.; Daranagama, D.A.; Phillips, A.J.L.; Kannangara, S.D.; Promputtha, I. Fungi vs. fungi in biocontrol: An overview of fungal antagonists applied against fungal plant pathogens. Front. Cell. Infect. Microbiol. 2020, 10, 604923. [Google Scholar] [CrossRef]
- United States Environmental Protection Agency. Biopesticide Active Ingredients. Available online: https://www.epa.gov/ingredients-used-pesticide-products/biopesticide-active-ingredients (accessed on 10 April 2023).
- Australian Pesticides and Veterinary Medicines Authority. Public Chemical Registration Information System Search. Available online: https://services.apvma.gov.au/web/guest/pubcris (accessed on 10 April 2023).
- Agrimm Technology—Effective, Safe and Environmentally Friendly Biological Crop Protection. Available online: https://agrimm.co.nz/ (accessed on 10 April 2023).
- CABI BioProtection Portal. Available online: https://bioprotectionportal.com/india (accessed on 10 April 2023).
- China MoARA Drafts Active Ingredient List to Facilitate Biopesticide Registration. Available online: https://agrochemical.chemlinked.com/news/china-moara-drafts-active-ingredient-list-facilitate-biopesticide-registration?referer=chemycal (accessed on 10 April 2023).
- Ministerstwo Rolnictwa i Rozwoju Wsi. Wyszukiwarka Środków Ochrony Roślin–Zastosowanie. Available online: https://www.gov.pl/web/rolnictwo/wyszukiwarka-srodkow-ochrony-roslin---zastosowanie (accessed on 10 April 2023).
- EU Pesticides Database. Available online: https://ec.europa.eu/food/plant/pesticides/eu-pesticides-database/start/screen/active-substances (accessed on 10 April 2023).
- BPDB: Bio-Pesticides DataBase. Available online: https://sitem.herts.ac.uk/aeru/bpdb/atoz.htm (accessed on 10 April 2023).
- Kumar, J.; Ramlal, A.; Mallick, D.; Mishra, V. An Overview of Some Biopesticides and Their Importance in Plant Protection for Commercial Acceptance. Plants 2021, 10, 1185. [Google Scholar] [CrossRef]
- Muhammad, M.; Wahab, R.A.; Huyop, F.; Rusli, M.H.; Yaacob, S.N.S.; Teo, H.L. An overview of the potential role of microbial metabolites as greener fungicides for future sustainable plant diseases management. J. Crop Prot. 2022, 11, 1–27. [Google Scholar]
- Czaja, K.; Goralczyk, K.; Struciński, P.; Hernik, A.; Korcz, W.; Minorczyk, M.; Łyczewska, M.; Ludwicki, J.K. Biopesticides—Towards increased consumer safety in the European Union. Pest Manag. Sci. 2015, 71, 3–6. [Google Scholar] [CrossRef] [PubMed]
- Pertot, I.; Puopolo, G.; Giovannini, O.; Angeli, D.; Sicher, C.; Perazzolli, M. Advantages and limitations involved in the use of microbial biofungicides for the control of root and foliar phytopathogens of fruit crops. Italus Hortus 2016, 23, 3–12. [Google Scholar]
- Abbey, L.; Abbey, J.; Leke-Aladekoba, A.; Iheshiulo, E.M.A.; Ijenyo, M. Biopesticides and Biofertilizers: Types, Production, Benefits, and Utilization. In Byproducts from Agriculture and Fisheries: Adding Value for Food, Feed, Pharma, and Fuels; Simpson, B.K., Aryee, A.N.A., Toldra, F., Eds.; John Wiley & Sons Ltd.: Hoboken, NJ, USA, 2020; pp. 479–500. [Google Scholar] [CrossRef]
- Kumar, S.; Singh, A. Biopesticides: Present status and the future prospects. J. Fertil. Pestic. 2015, 6, e129. [Google Scholar] [CrossRef]
- Palmieri, D.; Ianiri, G.; Del Grosso, C.; Barone, G.; De Curtis, F.; Castoria, R.; Lima, G. Advances and Perspectives in the Use of Biocontrol Agents against Fungal Plant Diseases. Horticulturae 2022, 8, 577. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).