Featured Application
subperiosteal custom-made implants can be a safe and effective solution in primary maxillary reconstructions in patients who cannot undergo free bone flap reconstructions. In these difficult cases, this type of custom-made implant can guarantee support to the soft tissues of the middle-third of the face and a solid anchorage for dental prostheses or palatal obturators, thus improving the quality of life of patients.
Abstract
Recent reports on secondary restorations with subperiosteal implants have demonstrated promising results in terms of esthetic and functional restoration. We report the case of a reconstruction of a total maxillectomy with a custom-made subperiosteal implant associated with a temporal muscle flap in a patient who could not undergo bone reconstruction with a free flap. This technique made it possible to restore the division between the oral cavity and the mouth, providing a solid anchorage to the dental prosthesis and correcting an oroantral communication with a small palatal obturator. The subperiosteal implant also granted proper soft tissue support in the middle-third of the face. Subperiosteal implants can be safe and effective even in primary maxillary reconstructions for patients who cannot undergo free bone flap reconstructions. In these difficult cases, this type of implant can provide support to the soft tissues of the middle-third of the face and a solid anchorage for dental prostheses or palatal obturators, thus improving the quality of life for patients.
1. Introduction
The reconstruction of the maxilla after tumor resection is challenging due to the complexity of the anatomy and the functional and esthetic importance of the maxillary region [1,2]. This process may involve a combination of reconstructive surgery, prosthetic devices, and/or implant-supported restorations, depending on the extent of the resection and the patient’s individual needs and goals with the aim of restoring function, esthetics, and quality of life for patients [3,4].
Fibula [5], scapular [6], or iliac crest [7] free flaps currently represent the gold-standard in maxillary reconstruction as they allow the creation of a stable division between the mouth and the nasal and paranasal sinuses, provide adequate projection to the soft tissues of the middle-third of the face, and bring to the recipient site an amount of bone sufficient for the dental implant placement, which can be performed simultaneously with the reconstruction [8]. The loss of dental elements is, in fact, one of the primary causes of reduction in the quality of life of these patients, and implant-prosthetic rehabilitation should, therefore, be considered among the objectives of the treatment [1,9]. Free flaps represent a sophisticated and well-validated technique but are often challenging to perform, especially in elderly patients or those with severe comorbidities [10]. In such cases, less complex reconstructive solutions are generally used. Specifically, temporalis muscle [11] and buccinator myomucosal flaps [12,13] represent local solutions with a lower surgical burden for the patient. However, unlike free flaps, they do not allow for bone reconstruction, making implant-prosthetic rehabilitation very difficult [14].
Subperiosteal implantology was first proposed and developed in Sweden by Gustav Dahal in the early 1940s [15]. However, the technique never gained popularity due to the difficulties in surgery and the poor long-term results caused by the implant’s inadequate fit to the bone [16]. With the advent of endosseous implantology, the technique’s popularity further diminished [17].
In 2017, Mommaerts [18] completely revised the design and manufacturing technique of subperiosteal implants for the rehabilitation of severe maxillary atrophy. Computer-Aided Design and Computer-Aided Manufacturing (CAD/CAM) and laser melting technologies enabled the creation of implants with a perfect fit on the bone, based solely on the preoperative CT scan, eliminating the need for a first surgery to take a bone impression of the jaw [18,19]. Furthermore, applying the concepts of rigid fixation on the maxillary buttresses allowed the surgeon to move the screws away from the surgical wound and provided a firm anchor for the implant to the maxilla, overcoming the problems that reduced the long-term success of first-generation subperiosteal implants [20,21,22].
Few authors have reported the use of subperiosteal implants, either alone or in combination with fibula flaps, for the secondary reconstruction of patients who have undergone maxillectomy [23,24,25,26,27]. In this report, we present the case of a primary reconstruction using a subperiosteal implant and a temporalis muscle flap in a patient undergoing maxillectomy for squamous cell carcinoma.
2. Case Report
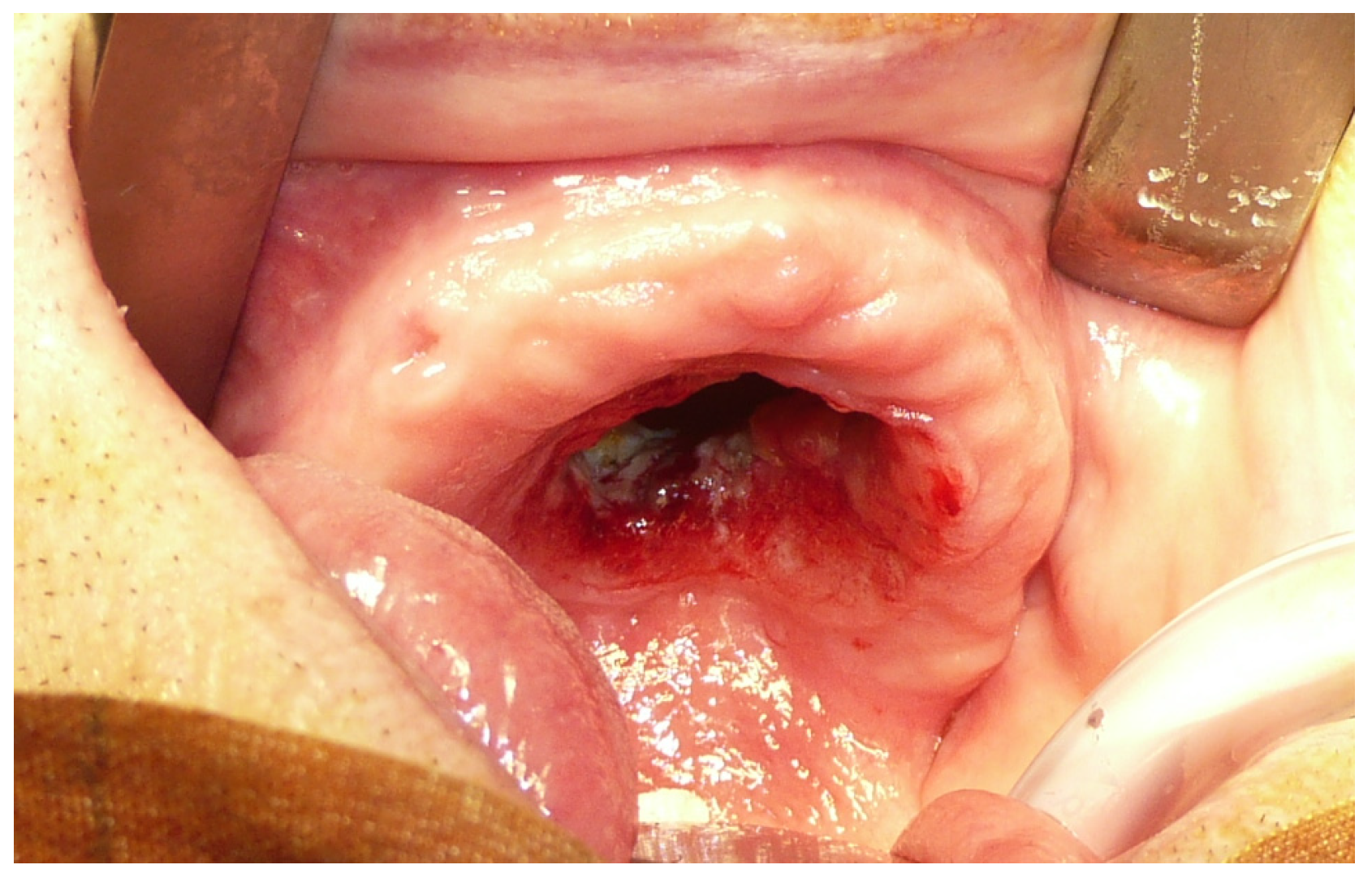
A 90-year-old man was referred to the Maxillofacial Surgery division of the University Hospital of Sassari on the discovery of an ulcerated lesion of the palate, present for approximately two months [Figure 1]. The patient had multiple comorbidities in his medical history: a previous ethmoidectomy for squamous cell carcinoma (22 years earlier), arterial hypertension, a prior cerebral stroke, hypercholesterolemia, and arterial disease of the lower limbs.
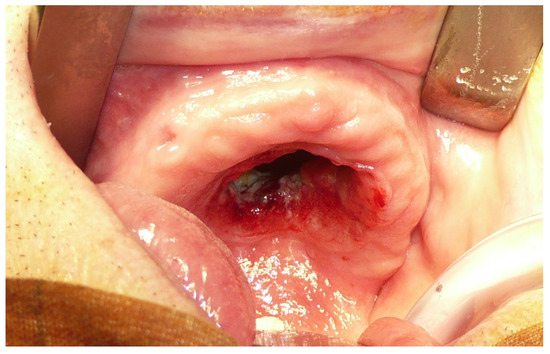
Figure 1.
Pre-operative view of the maxillary tumor.
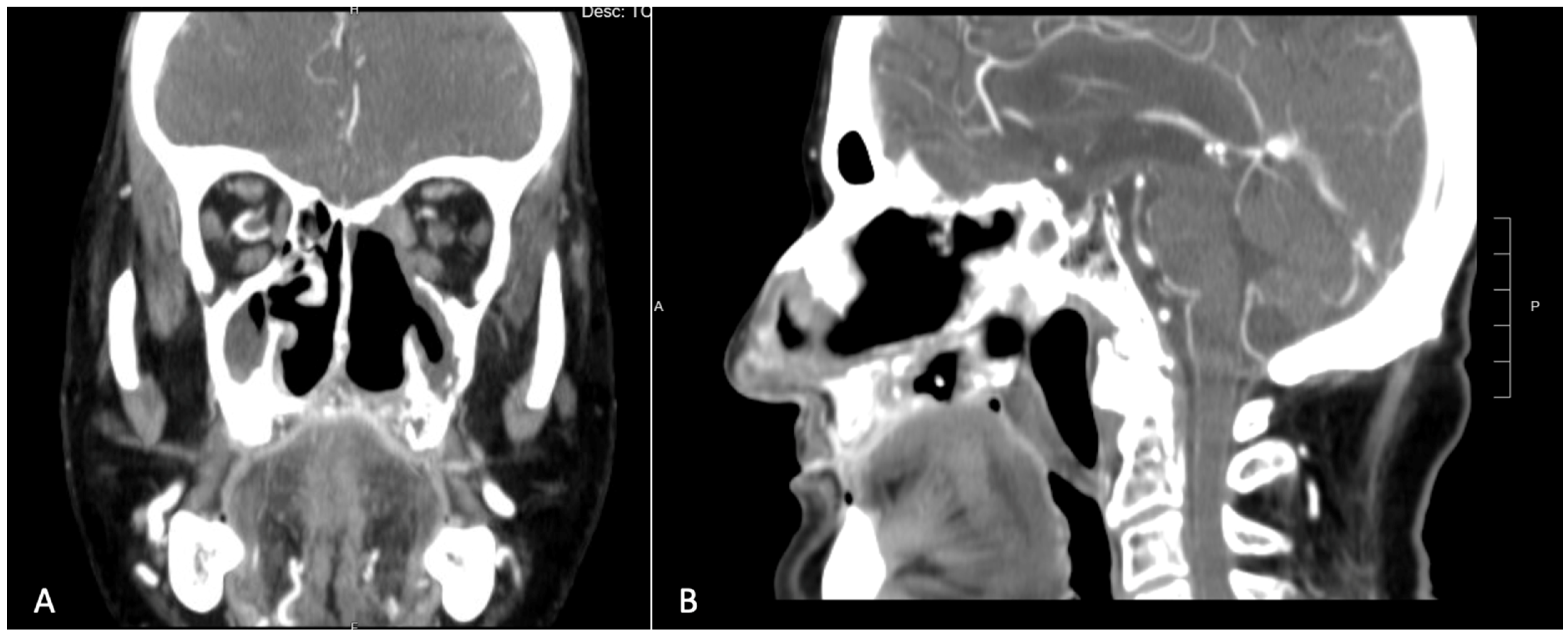
An incisional biopsy of the lesion confirmed the diagnosis of squamous cell carcinoma. The patient then underwent a contrast-enhanced CT scan, which revealed a large area of osteolysis involving the left maxilla, extending beyond the midline and encompassing the entire pre-maxilla [Figure 2]. Neck scans showed no suspicious lymphadenopathy.
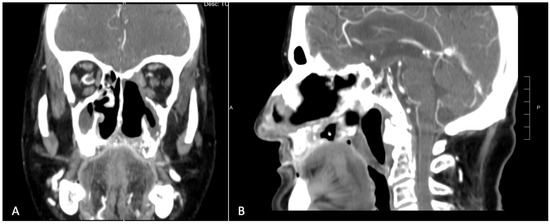
Figure 2.
Pre-operative CT scan showing a large area of osteolysis involving the left maxilla which exceeded the midline and the entire premaxilla. (A) Coronal view. (B) Sagittal view.
The patient was then considered a candidate for total maxillectomy. Given his comorbidities, age, and general state of health, the medical team ruled out the possibility of a free flap, opting instead for a local solution: a temporalis muscle flap. The decision to use a temporalis muscle flap over a buccinator myomucosal flap was guided by several factors. First, given the size of the defect, it would have been necessary to harvest and transpose two myomucosal flaps. The donor site morbidity of cheek flaps is acceptable only if the patient is cooperative. Intensive post-operative exercises and scar manipulation are fundamental to preventing scar retraction on the cheek [28]. This patient did not guarantee such cooperation.
Additionally, the temporalis flap tends to undergo rapid re-epithelization, forming scar tissue that is very similar to the keratinized gingiva, making it easier to prosthetize. On the contrary, myomucosal flaps retain a certain bulk, and it would still have been necessary to proceed secondarily to the section of the pedicle to allow for the accommodation of the prosthesis. Thus, considering the overall patient scenario and anticipated outcomes, the temporalis muscle flap was deemed the most suitable option.
To provide support to the soft tissues of the middle-third of the face and to enhance the prospects for implant-prosthetic rehabilitation, it was also decided to position a custom-made maxillary subperiosteal implant.
2.1. Subperiosteal Implant Planning and Production
The planning was based on thin-slice CT acquisitions performed for patient staging. The DICOM files were sent to the company responsible for producing the implant (Sintac Biomedical Engineering, Trento, Italy). The DICOM files were reprocessed into a 3D format using Mimics software (Materialise, Louvain, Belgium). During this phase, the quality of the DICOM images was checked to ensure that there were no artifacts that could affect the accuracy of the 3D model. The files were then exported in the STL format for processing with Geomagic Freeform software (3D Systems inc., Rock Hill, SC, USA). Once the 3D model had been created, a meeting with the surgeons was scheduled to establish the extent of the bone resection, the shape and position of the implant and prosthetic abutments, and the position of the osteosynthesis screws.
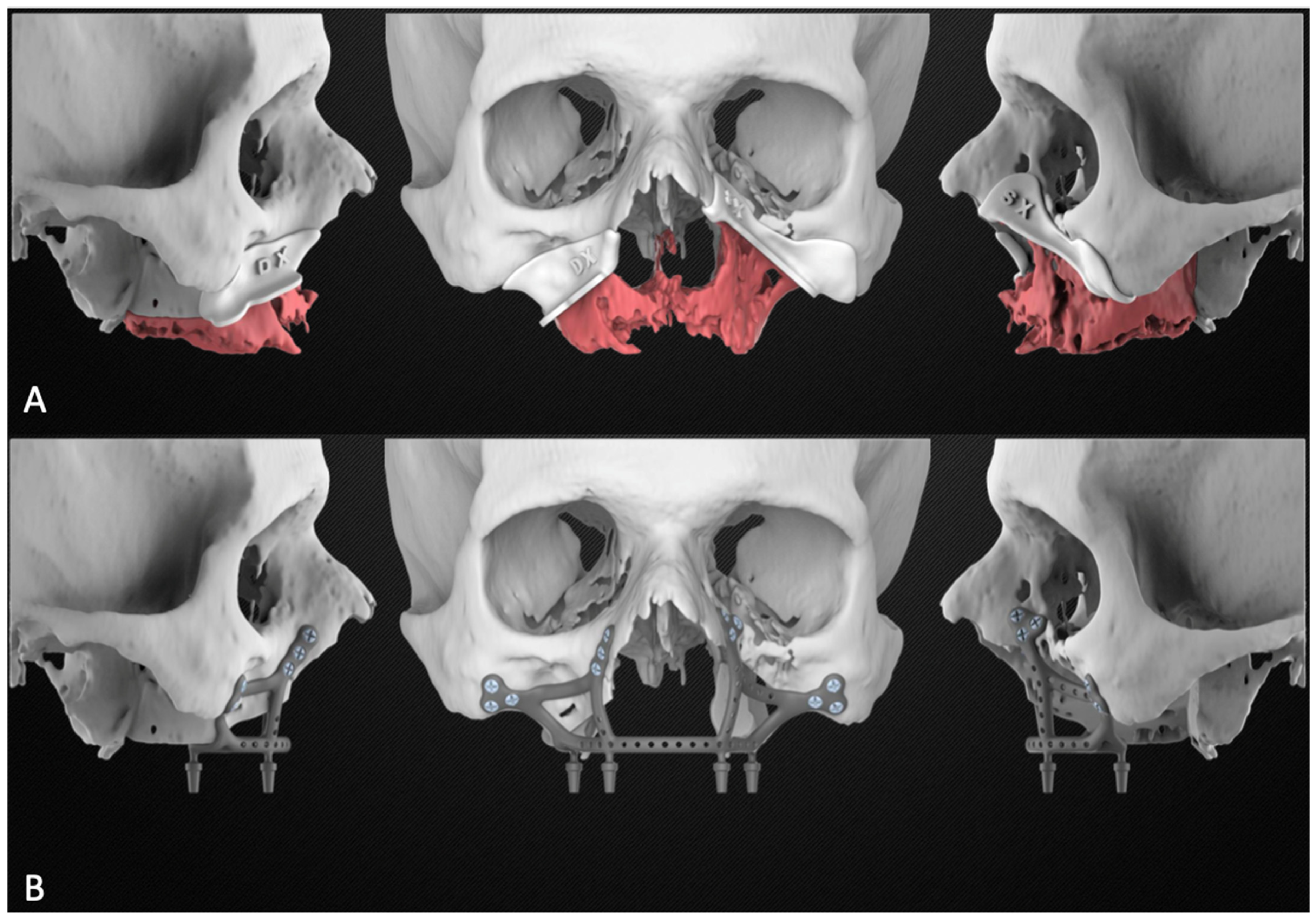
Based on the plan, the engineer then proceeded to design the subperiosteal implant and the bone resection templates. The planning report was finally sent to the surgeons for approval [Figure 3].
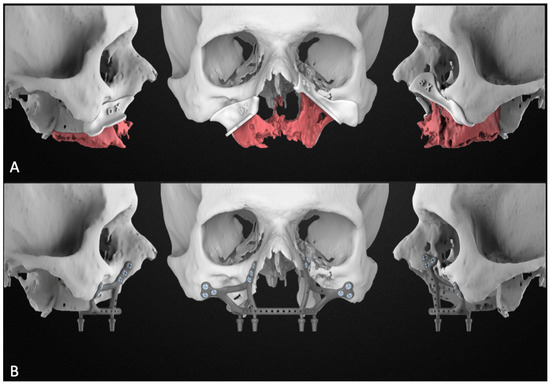
Figure 3.
Planning report including the maxillary resection with the cutting guides (A) and the subperiosteal implant design (B).
The implant was then produced using direct metal laser sintering technology with the titanium superalloy Ti6Al4V. Elements in polyether ether ketone (e.g., cutting guides and anatomical models of the jaw) were produced using selective 3D printing technology. Once the sintering cycle had been completed, the titanium implant and elements underwent heat treatment to increase fracture toughness, ductility at room temperature, dimensional and thermal stability, and creep resistance. Finally, the supports were removed, the titanium components were sandblasted, and the implant was sterilized. The entire process took nine days.
2.2. Surgical Procedure
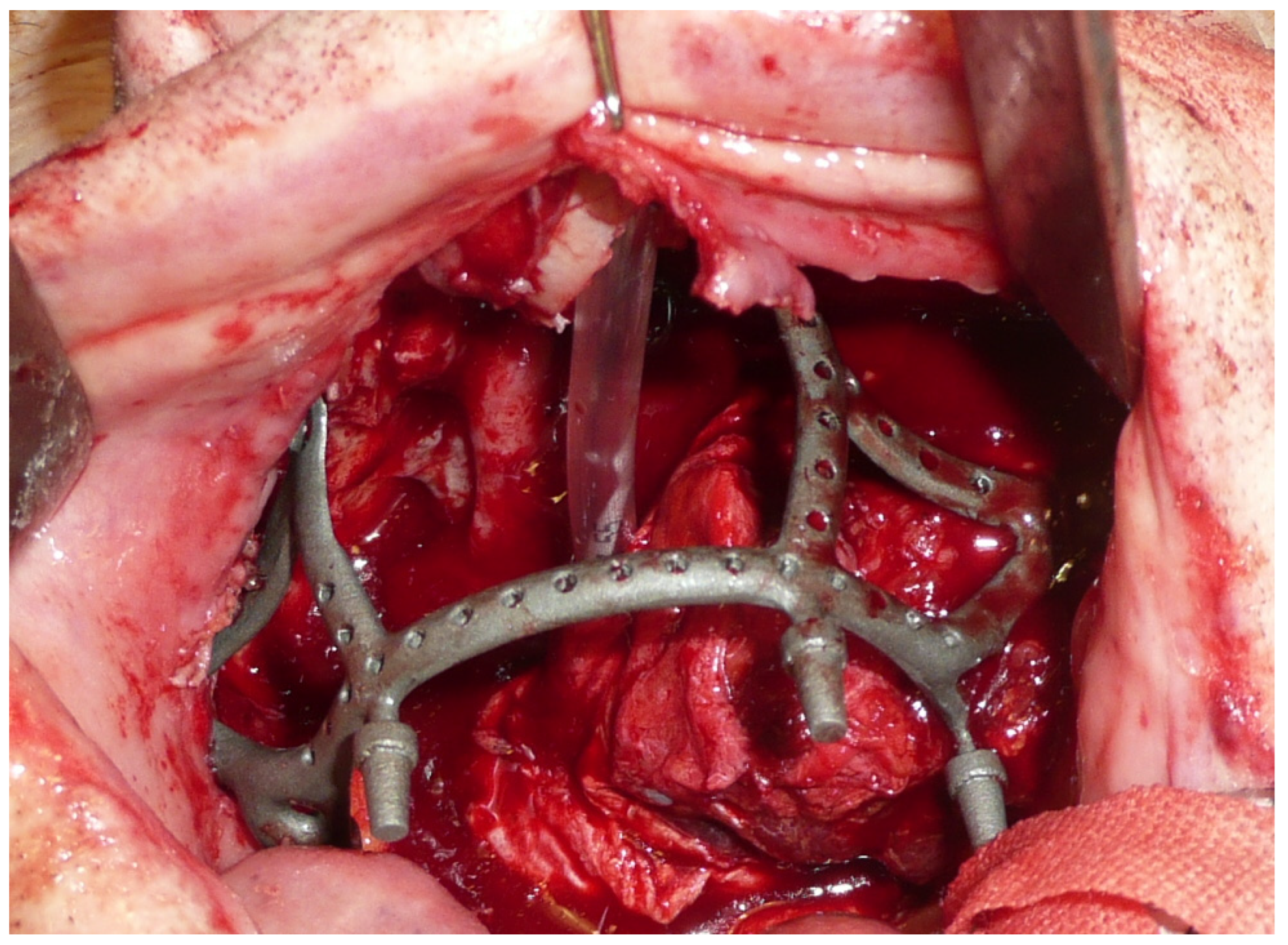
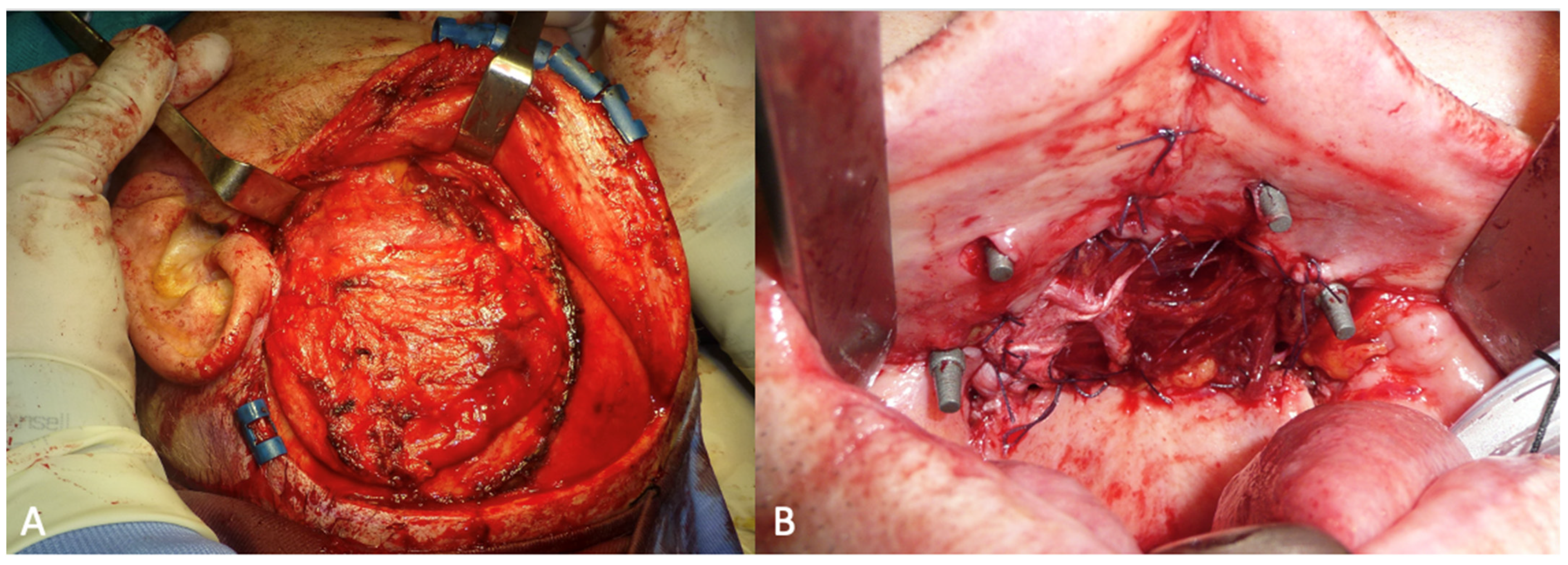
The surgery was performed under general anesthesia with orotracheal intubation. Through a circumvestibular mucosal incision with releases on the tuber maxillae bilaterally, the maxilla was completely skeletonized up to the zygomatic knobs laterally and the apical portion of the nasomaxillary pillars medially. The cutting guides were then positioned, and the bone osteotomy was performed. After detaching the pterygoid plates, the maxilla was mobilized, and the resection was completed by incising the mucosa at the boundary between the hard and soft palate. The subperiosteal implant was then placed and fixed with 2.0 osteosynthesis screws (Stryker, Kalamazoo, MI, USA) on the nasomaxillary pillars and zygomatic knobs [Figure 4].
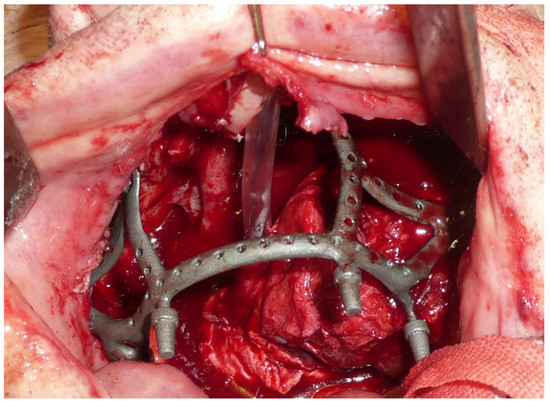
Figure 4.
Intraoperative view of the implant placed in the recipient site.
The temporalis muscle flap was harvested through a hemi-coronal, arciform cutaneous access extending from the root of the helix to the left temporoparietal region. During the access, the superficial temporal vessels were preserved to allow the transposition of the temporal fascia as well as the muscle. The skin flap was completely elevated to expose the underlying muscle, which was subsequently incised and elevated by carefully detaching it from the underlying bone and preserving the deep temporal vessels. [Figure 5A] Once the harvesting was completed, the flap was transposed into the oral cavity through a tunnel created beneath the zygomatic arch. The flap reconstructed the hard palate and was sutured to the residual mucosa of the fornix [Figure 5B].
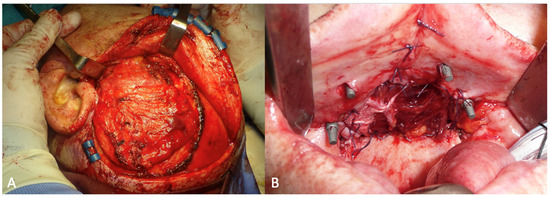
Figure 5.
(A) Intraoperative view of the temporalis muscle flap. (B) The post-ablative defect closed with the temporal muscle flap.
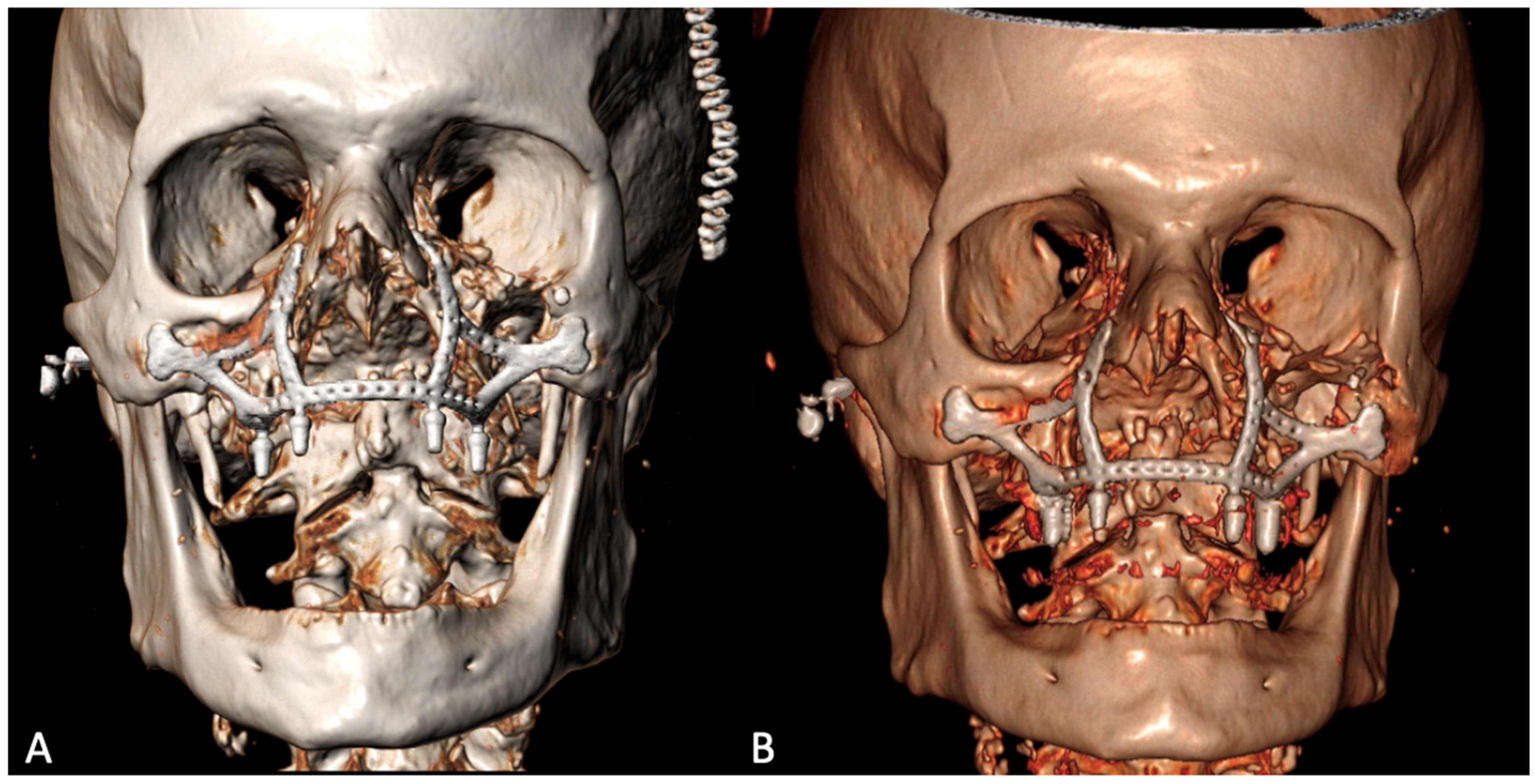
The histological examination confirmed the diagnosis of squamous cell carcinoma with bone infiltration and clear resection margins. The patient was a candidate for adjuvant radiotherapy, which, however, was not performed due to his advanced age and poor compliance. The post-operative 3D CT showed the correct positioning of the implant, adhering to the virtual planning, and its stability over time [Figure 6].
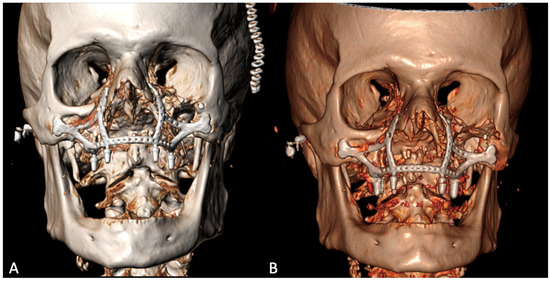
Figure 6.
Post-operative 3D CT scan 9 days (A) and 6 months (B) after the surgery.
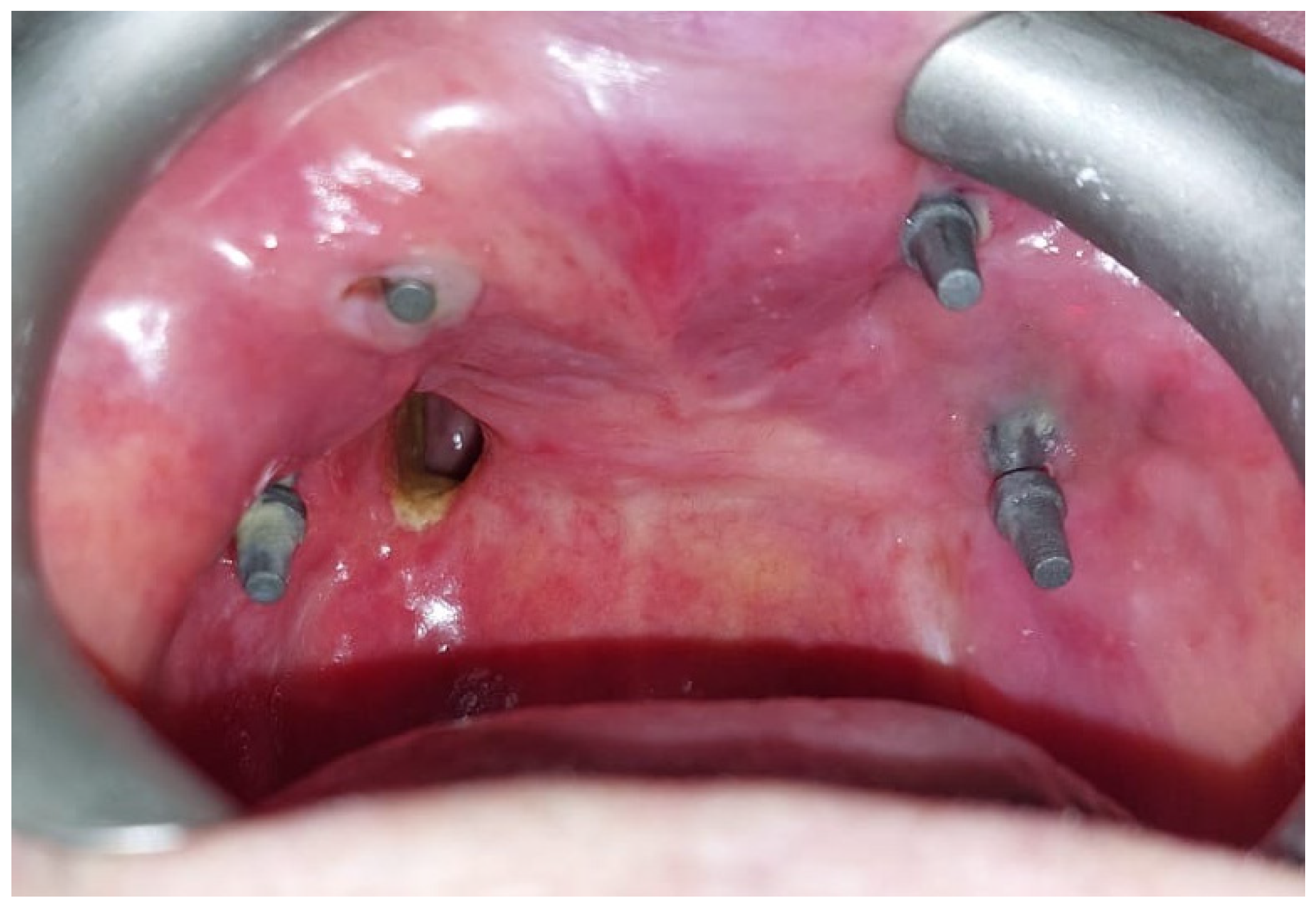
The post-operative course was complicated by a wound dehiscence on the right side with oroantral fistula formation and a passage of liquids and food into the nose [Figure 7]. The dehiscence of the surgical wound and the consequent formation of the oroantral fistula was due to the necrosis of the temporal fascia, transposed to the recipient site together with the muscle. This area of the post-ablative defect had in fact been reconstructed only with fascia and not with muscle [Figure 5A]. The patient was discharged on the tenth post-operative day.
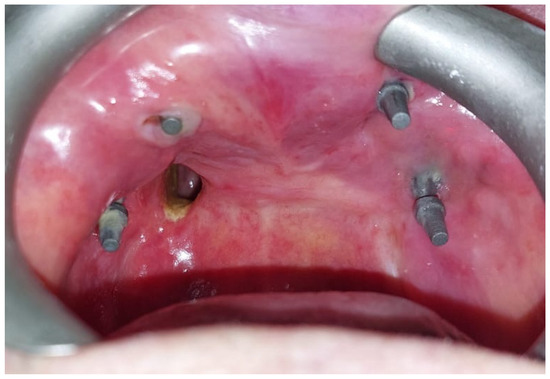
Figure 7.
Follow-up at 2 months showing oroantral fistula on the right side and complete re-epithelialization of the temporalis muscle.
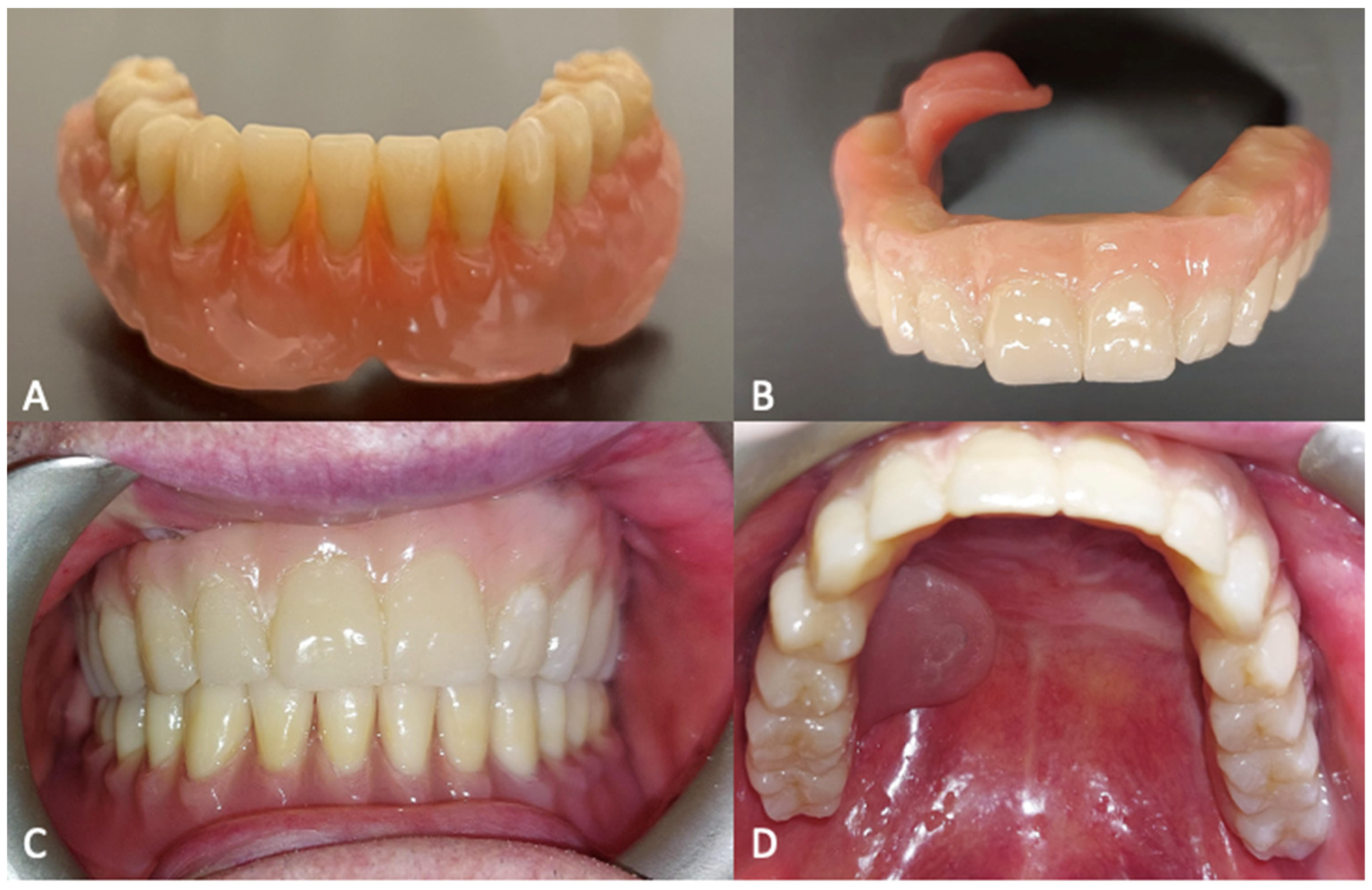
After the complete healing of the surgical wounds, 60 days after surgery, the rehabilitation was completed with the placement of the prosthesis [Figure 8].
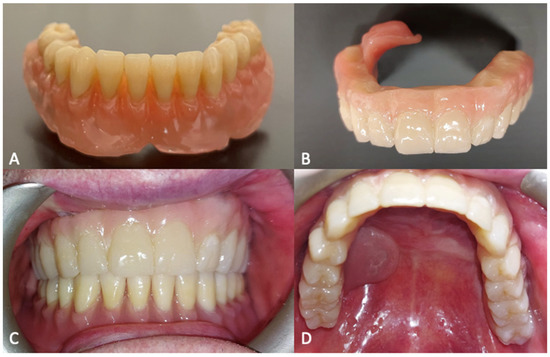
Figure 8.
(A) Lower prosthesis. (B) Upper prosthesis. (C) Intraoral view 6 months after the surgery. (D) Oroantral fistula closure with palatal obturator included in the prosthesis.
The oroantral fistula was closed with a palatal obturator included within the prosthesis. The subperiosteal implant provided satisfactory soft tissue support and a proper projection of the middle-third of the face [Figure 9]. No complications occurred in the first six months of follow-up.
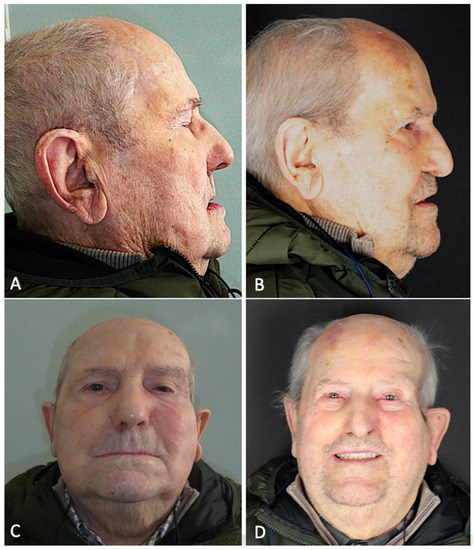
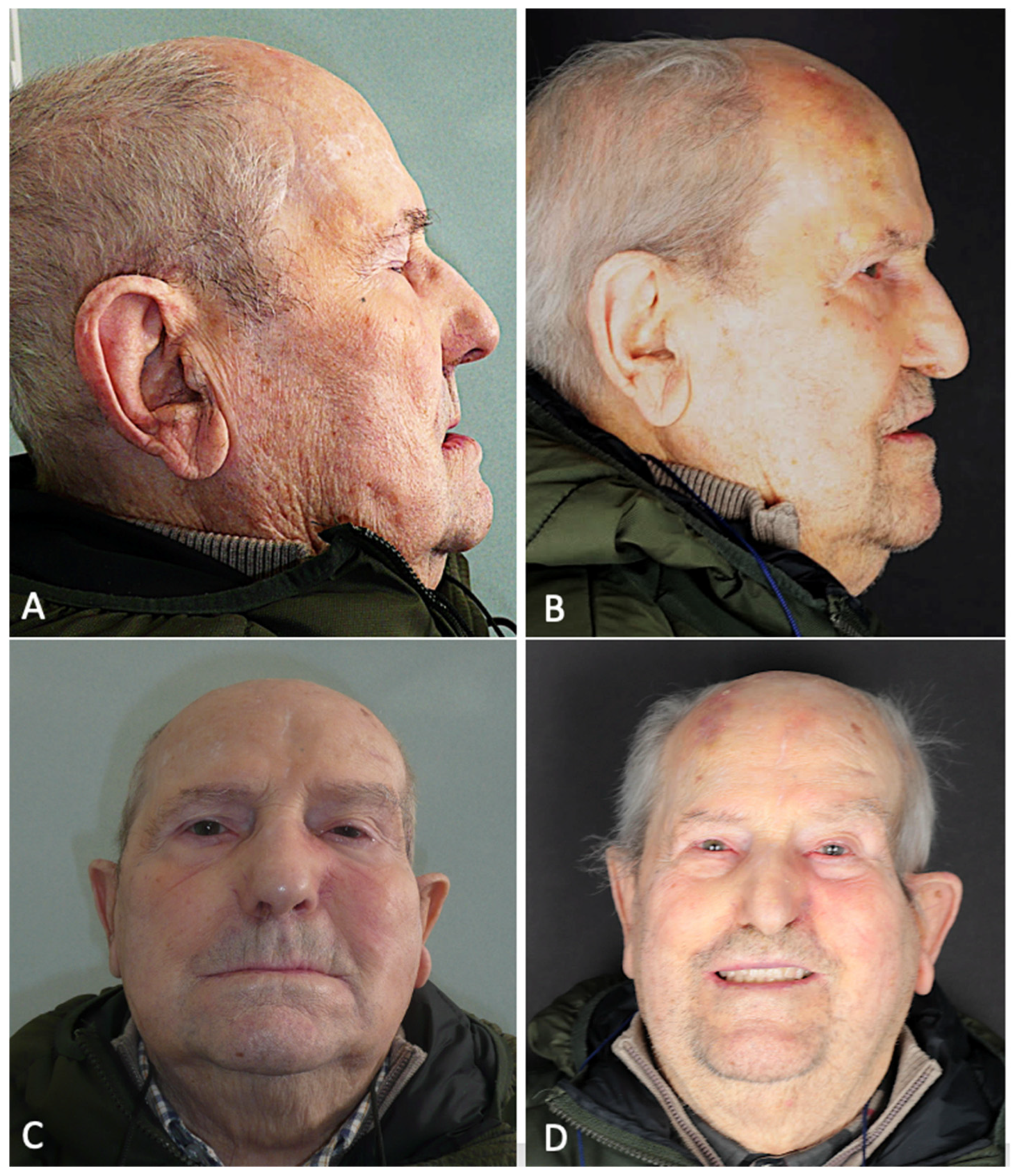
Figure 9.
(A) Pre-operative lateral profile. (B) Post-operative lateral profile at 6 months follow-up. (C) Pre-operative frontal view. (D) Post-operative frontal view after prosthetic rehabilitation at 6 months follow-up.
3. Discussion
Since 2017, subperiosteal implants have been gaining renewed popularity for the rehabilitation of maxillary atrophies [18,19,20,21,22,29,30,31]. This increased attention has been based on the possibilities introduced by CAD/CAM technology, which allows for the obtainment of implants that precisely fit the bone surfaces. Moreover, the introduction of the technique of rigid fixation on the maxilla’s resistance pillars has enabled a balanced distribution of the masticatory forces on the osteosynthesis screws, making the contribution to the stability provided by the alveolar bone less important [19,21,32]. This provides the rationale for the use of subperiosteal implants in patients undergoing maxillary resection without reconstructing the crestal bone lining. Vosselman et al. [23] and Kondaka et al. [26] have previously reported two cases of the secondary reconstruction of the maxilla with subperiosteal implants. These two patients had wide oroantral communications and the subperiosteal implant, positioned without being covered with local or distant flaps, had the dual function of rehabilitating the patient from a prosthetic point of view, and provided support to the palatal obturator. Cebrian-Carretero et al. [25] reported their experience with four patients, primarily reconstructed with a free fibula flap and subsequently rehabilitated with custom-made subperiosteal implants. The largest series of secondary reconstructions was published by Korn et al. [27] and includes 19 patients who had undergone maxillectomy and reconstruction with microvascular flaps which had subsequently failed. According to Korn et al., middle-third reconstructions after cancer resection should always include a fixed prosthetic rehabilitation and correct soft tissue support, which both enhance definitely the quality of life of these patients. In this sense, subperiosteal customized implants, in combination with soft tissue flaps to reconstruct the oronasal layer, could speed up the reconstructive process, in comparison with free bone flaps in combination with conventional endosseous implants [24,27]. The surgical time, donor site morbidity, osseointegration time, and ease of reconstruction have, in fact, an incomparably smaller effect. This case report demonstrates how in selected cases, when even fascio-cutaneous free flaps are not feasible and it is necessary to fall back on local reconstructive solutions, the simultaneous adoption of a subperiosteal CAD/CAM titanium implant with a dental connection can provide the patient with the possibility of an implant-prosthetic rehabilitation prosthetic and, at the same time, adequate support for the soft tissues of the middle-third of the face. Additionally, implant connections can provide solid support for palatal obturators where oroantral communications are present. In this way, it is possible to reduce considerably the size of the obturator, making it more comfortable, and guaranteeing, at the same time, a much more watertight closure of the oroantral communication compared to that provided by mobile devices [33,34]. This is very important as maxillectomies can negatively impact the patient’s quality of life in several ways. Difficulty in chewing and swallowing, speech impairment, and changes in facial appearance can lead to psychological distress and social isolation. Furthermore, the formation of an oroantral fistula, which allows the passage of food from the mouth to the nose, can cause further discomfort and embarrassment [35,36].
In this report, the possibility of performing a primary reconstruction with subperiosteal implants and local flaps is described in patients who cannot undergo reconstructions with bone flaps. In the design of the implant, some critical points must be considered. First, the bone resection must be carefully planned through an assessment of pre-operative investigations, ensuring radical excision of the tumor. If this does not happen, the extent of the bone resection may be necessary during the operation, rendering the cutting templates useless, and risking the loss of the implant’s anchoring points. For this reason, it is essential to maintain a safe distance between the most inferior screw holes and the planned osteotomy margin to allow for a correct rigid fixation, even if it is necessary to widen the demolition. Secondly, the arms of the implant are the site of maximum stress during chewing and, therefore, need to be particularly thick and reinforced [32]. In accordance with the results reported for maxillary reconstructions with load-bearing plates [37], the subperiosteal implant was designed with 2.8 mm thick arms. Thirdly, when planning the location and orientation of the screw holes, consideration should be given to which surgical approach will be performed and at what angle it will be possible to insert the screws. This is especially important for the screws on the zygomatic knob which would be impossible to insert if the holes were placed on the lateral aspect of the cheekbone and not on the anterior aspect [Figure 3]. In this position it is also feasible to exploit the major axis of the cheekbone and to insert longer screws, which ensure better stability of the implant [18,21].
Most importantly, this report demonstrates, for the first time, that it is not mandatory to delay the subperiosteal implant with dental connections to the performance of secondary revision surgery, as recommended by other authors [27]. By speeding up the manufacturing process, the implant can be ready for the first oncological surgery and inserted in combination with free or local flap reconstructions. Furthermore, the presence of the implant does not represent a contraindication to post-operative radiation therapy, in accordance with the use of titanium plates in maxillary reconstruction.
The limitations of this study are primarily related to its anecdotal nature. Cohort studies with larger patient series will be necessary to evaluate the outcomes and potential complications of this procedure. Secondly, although the medium-term results of this case are satisfactory, the patient will need to be monitored over time to evaluate the reliability of this type of reconstruction in the long run.
4. Conclusions
In conclusion, a larger patient series and randomized trials are needed to evaluate the short- and long-term outcomes and the safety of maxillary reconstructions with customized subperiosteal titanium maxillary implants. Studies on larger case studies are currently underway in our center. Recent reports on secondary restorations have demonstrated promising results in terms of esthetic and functional restoration. Based on our experience, we believe that subperiosteal implants can be safe and effective, even in primary maxillary reconstructions, for patients who cannot undergo free bone flap reconstructions. In these difficult cases, this type of implant can provide support to the soft tissues of the middle-third of the face and a solid anchorage for dental prostheses or palatal obturators, thus improving the quality of life for the patients. In our opinion, for younger patients or those without severe comorbidities, free bone flaps remain the first choice as the long-term results are well established [1,2,3,4,5,6,7,8] and a substantial amount of tissue can be brought to the donor site for optimal support to the middle-third of the face. In these cases, the production of a subperiosteal implant, instead of standard osteosynthesis plates, can still be incorporated into the digital workflow of the virtual planning of custom-made bone reconstructions [38]. This approach enables precise, patient-specific surgical planning, and outcomes, maximizing the benefits of the bone flap reconstruction technique allowing for a quicker and easier dental restoration [39].
Author Contributions
Conceptualization, G.D.R., D.S. and L.A.V.; data collection A.B., D.M. and F.M.; revision of the literature M.T.R., G.S. and P.P.; writing—original draft preparation, L.A.V. and J.R.L.; writing—review and editing, all the authors. All authors have read and agreed to the published version of the manuscript.
Funding
This research has received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Written informed consent has been obtained from the patient for the publication this paper.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author, L.A.V.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Lee, Z.H.; Cripps, C.; Rodriguez, E.D. Current concepts in maxillary reconstruction. Plast. Reconstr. Surg. 2022, 150, 168e–175e. [Google Scholar] [CrossRef] [PubMed]
- Lennox, N.D.; Kim, D.D. Maxillary reconstruction. Oral Maxillofac. Surg. Clin. N. Am. 2013, 25, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Peng, X.; Mao, C.; Yu, G.Y.; Guo, C.B.; Huang, M.X.; Zhang, Y. Maxillary reconstruction with the free fibula flap. Plast. Reconstr. Surg. 2005, 115, 1562–1569. [Google Scholar] [CrossRef]
- Hanasono, M.M.; Silva, A.K.; Yu, P.; Skoracki, R.J. A comprehensive algorithm for oncologic maxillary reconstruction. Plat. Reconstr. Surg. 2013, 131, 47–60. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.B.; Wang, Y.; Liu, X.J.; Mao, C.; Guo, C.B.; Yu, G.Y.; Peng, X. Reconstruction of maxillary defects with free fibula flap assisted by computer techniques. J. Craniomaxillofac. Surg. 2015, 43, 630–636. [Google Scholar] [CrossRef] [PubMed]
- Ferri, A.; Perlangeli, G.; Bianchi, B.; Zito, F.; Sesenna, E.; Ferrari, S. Maxillary recontruction with scapular tip chimeric free flap. Microsurgery 2021, 41, 207–215. [Google Scholar] [CrossRef]
- Bianchi, B.; Ferri, A.; Ferrari, S.; Copelli, C.; Boni, P.; Sesenna, E. Iliac crest free flap for maxillary recontruction. Microsurgery 2010, 68, 2706–2713. [Google Scholar]
- Patel, S.Y.; Kim, D.D.; Ghali, G.E. Maxillofacial reconstruction using vascularized fibula free flaps and endosseous implants. Oral Maxillofac. Surg. Clin. N. Am. 2019, 31, 259–284. [Google Scholar] [CrossRef] [PubMed]
- Terada, N.; Numata, T.; Kobayashi, N.; Gorai, S.; Tadashi, K.; Ono, K.I.; Konno, A. State of mastication affects quality of life in patients with maxillary sinus cancer. Laryngoscope 2003, 113, 729–736. [Google Scholar] [CrossRef]
- Onoda, S.; Kinioshita, M.; Ariypshi, Y. Investigation of free-flap transfer reconstruction in elderly patients and oral intake function. J. Craniofac. Surg. 2020, 31, e679–e681. [Google Scholar] [CrossRef]
- di Spilimbergo, S.S.; Nordera, P.; Mardini, S.; Castiglione, G.; Chim, H.; Pinna, V.; Brunello, M.; Cusino, C.; Roberto, S.; Baciliero, U. Pedicled temporalis muscle flap for craniofacial reconstruction: A 35-year clinical experience with 366 flaps. Plast. Reconstr. Surg. 2017, 139, 468e–476e. [Google Scholar] [CrossRef] [PubMed]
- Massarelli, O.; Vaira, L.A.; Biglio, A.; Gobbi, R.; Piombino, P.; De Riu, G. Rational and simplified nomenclature for buccinator myomucosal flaps. Oral Maxillofac. Surg. 2017, 21, 453–459. [Google Scholar] [CrossRef]
- Massarelli, O.; Baj, A.; Gobbi, R.; Soma, D.; Marelli, S.; De Riu, G.; Tullio, A.; Giannì, A.B. Cheek mucosa: A versatile donor site of myomucosal flaps. Technical and functional considerations. Head Neck 2013, 35, 109–117. [Google Scholar] [CrossRef] [PubMed]
- Gabrysz-Forget, F.; Tabet, P.; Rahal, A.; Bissada, E.; Christopoulos, A.; Ayad, T. Free versus pedicled flaps for reconstruction of head and neck cancer defects: A systematic review. J. Otolaryngol. Head Neck Surg. 2019, 14, 13. [Google Scholar] [CrossRef] [PubMed]
- Dahl, G. Om mojligheten for inplantation i kaken av metallskelett som bas eller retention for fasta eller avtagbara proteser. Odontol. Tidskr. 1943, 51, 440–449. [Google Scholar]
- Schou, S.; Pallesen, L.; Hjorting-Hansen, E.; Pedersen, C.S.; Fibaek, B. A 41-year history of a mandibular subperiosteal implant. Clin. Oral Impl. Res. 2000, 11, 171–178. [Google Scholar] [CrossRef]
- Buser, D.; Sennerby, L.; De Bruyn, H. Modern implant dentistry based on osseointegration: 50 years of progress, current trends and open questions. Periodontology 2000 2017, 73, 7–21. [Google Scholar] [CrossRef]
- Mommaerts, M.Y. Additively manufactured sub-periosteal jaw implants. Int. J. Oral Maxillofac. Surg. 2017, 46, 938–940. [Google Scholar] [CrossRef]
- Bai, L.; Zheng, L.; Ji, P.; Wan, H.; Zhou, N.; Liu, R.; Wang, C. Additively manufactured lattice-like subperiosteal implants for rehabilitation of the severely atrophic ridge. ACS Biomater. Sci. Eng. 2022, 8, 912–920. [Google Scholar] [CrossRef]
- Van den Borre, C.; Rinaldi, M.; De Neef, B.; Loomans, N.A.J.; Nout, E.; Van Doorne, L.; Naert, I.; Politis, C.; Schouten, H.; Klomp, G.; et al. Patient- and clinician-reported outcomes for the additively manufactured sub-periosteal jaw implant (AMSJI) in the maxilla: A prospective multicentre one-year follow-up study. Int. J. Oral Maxillofac. Surg. 2022, 51, 243–250. [Google Scholar] [CrossRef]
- Mommaerts, M.Y. Evolutionary steps in the design and biofunctionalization of the additively manufactured sub-periosteal jaw implant “AMSJI” for the maxilla. Int. J. Oral Maxillofac. Surg. 2019, 48, 108–114. [Google Scholar] [CrossRef] [PubMed]
- Van den Borre, C.; Rinaldi, M.; De Neef, B.; Loomans, N.A.J.; Nout, E.; Van Doorne, L.; Naert, I.; Politis, C.; Schouten, H.; Klomp, G.; et al. Radiographic evaluation of bone remodeling after additively manufactured subperiosteal jaw implantation (AMSJI) in the maxilla: A one-year follow-up study. J. Clin. Med. 2021, 10, 3542. [Google Scholar] [CrossRef] [PubMed]
- Vosselman, N.; Marema, B.J.; Schepman, K.P.; Raghoebar, G.M. Patient-specific sub-periosteal zygoma implant for prothetic rehabilitation of large maxillary defects after oncological resection. Int. J. Oral Maxillofac. Surg. 2019, 48, 115–117. [Google Scholar] [CrossRef] [PubMed]
- Garrido-Martinez, P.; Quispe-Lopez, N.; Montesdeoca-Garcia, N.; Esparza-Gomez, G.; Cebrian-Carretero, J.L. Maxillary reconstruction with subperiosteal implants in a cancer patient: A one-year follow-up. J. Clin. Exp. Dent. 2022, 14, e293–e297. [Google Scholar] [CrossRef]
- Cebrian-Carretero, J.L.; Del Castillo Pardo de Vera, J.L.; Montesdeoca Garcia, N.; Garrido Martinez, P.; Pampin Martinez, M.M.; Aragon Nino, I.; Navarro Cuéllar, I.; Navarro Cuéllar, C. Virtual surgical planning and customized subperiosteal titanium maxillary implant (CSTMI) for three dimensional recontruction and dental implants of maxillary defects after oncological resection: Case series. J. Clin. Med. 2022, 11, 4594. [Google Scholar] [CrossRef]
- Kondaka, S.; Dal Singh, V.; Vadlamudi, C.; Rao Bathala, L. Prosthetic rehabilitation of untailored defects using patient-specific implants. Dent. Res. J. 2022, 19, 83. [Google Scholar]
- Korn, P.; Gellrich, N.C.; Jehn, P.; Spalthoff, S.; Rahlf, B. A new strategy for patient-specific implant-borne dental rehabilitation in patients with extended maxillary defects. Front. Oncol. 2021, 11, 718872. [Google Scholar] [CrossRef]
- Massarelli, O.; Vaira, L.A.; Gobbi, R.; Biglio, A.; Dell’aversana Orabona, G.; De Riu, G. Soft palate functional reconstruction with buccinator myomucosal island flaps. Int. J. Oral Maxillofac. Surg. 2018, 47, 316–323. [Google Scholar] [CrossRef]
- Dimitroulis, G.; Gupta, B.; Wilson, I.; Hart, C. The atrophic edentulous alveolus. A preliminary study on a new generation of subperiosteal implants. Oral Maxillofac. Surg. 2023, 27, 69–78. [Google Scholar] [CrossRef]
- Mangano, C.; Bianchi, A.; Mangano, F.G.; Dana, J.; Colombo, M.; Solop, I.; Admakin, O. Custom-made 3D printed subperiosteal titanium implants for the prosthetic restoration of the atrophic posterior mandible of elderly patients: A case series. 3D Print Med. 2020, 6, 1. [Google Scholar] [CrossRef]
- Cerea, M.; Dolcini, G.A. Custom-made direct metal laser sintering titanium subperiosteal implants: A retrospective clinical study on 70 patients. Biomed. Res. Int. 2018, 2018, 5420391. [Google Scholar] [CrossRef] [PubMed]
- De Moor, E.; Huys, S.E.F.; van Lenthe, G.H.; Mommaerts, M.Y.; Vander Sloten, J. Mechanical evaluation of a patient-specific additively manufactured subperiosteal jaw implant (AMSJI) using finite-element analysis. Int. J. Oral Maxillofac. Surg. 2022, 51, 405–411. [Google Scholar] [CrossRef] [PubMed]
- Alfaraj, A.; Su, F.Y.; Lin, W.S. CAD-CAM hollow obturator prosthesis: A technical report. J. Prosthodont. 2022, 31, 635–638. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, B.L.; Pogrel, M.A.; Young, C.W.; Sharma, A. Reconstruction of extensive maxillary defects using zygomaticus implants. J. Oral Maxillofac. Surg. 2004, 62, 82–89. [Google Scholar] [CrossRef] [PubMed]
- Rogers, S.N.; Lowe, D.; McNally, D.; Brown, J.S.; Vaughan, E.D. Health-related quality of life after maxillectomy: A comparison between prosthetic obturation and free flap. J. Oral Maxillofac. Surg. 2003, 61, 174–181. [Google Scholar] [CrossRef]
- Kreeft, A.M.; Krap, M.; Wismeiker, D.; Speksnijder, C.M.; Smeele, L.E.; Bosch, S.D.; Muijen, M.S.A.; Balm, A.J.M. Oral function after maxillectomy and reconstruction with an obturator. Int. J. Oral Maxillofac. Surg. 2012, 41, 1387–1392. [Google Scholar] [CrossRef]
- Bujtàr, P.; Simonovics, J.; Varadi, K.; Sandor, G.K.B.; Avery, C.M.E. The biomechanical aspects of reconstruction for segmental defects of the mandible: A finite element study to assess the optimization of plate and screw factors. J. Craniomaxillofac. Surg. 2014, 42, 855–862. [Google Scholar] [CrossRef]
- Cho, M.J.; Hanasono, M.M. Virtual surgical planning in free tissue transfer for orbito-maxillary reconstruction. Semin. Plast. Surg. 2022, 36, 183–191. [Google Scholar] [CrossRef]
- Rosen, E.B.; Allen, R.J., Jr.; Nelson, J.; Matros, E. Inset guide for the osteocutaneous fibula flap with endosseous implants in oncologic jaw reconstruction. Plast. Reconstr. Surg. Glob. Open 2019, 7, e2475. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).