Abstract
The synthesis of halide perovskite nanocrystals (PNCs) with mesmerizing photophysical properties has allowed for the fast development of efficient optoelectronic and photovoltaic devices, as well as making them ideal photocatalysts for solar-driven chemical reactions. However, the use of traditional oleic acid/oleylamine with low binding energy and the introduction of some phosphine- and sulfur-based ligands generate the emergence of highly defective PNCs with poor stability, fast quenching of their PL features, and increase in the toxicity of the final perovskite product. In this review, we will show the use of prominent “green” and ecofriendly solvents and capping ligands with the capability to enhance the quality of the PNCs by suppressing structural defects. By introducing promising ecofriendly agents such as biogenic species and ligands extracted from natural sources, it is possible to favor the radiative recombination dynamics into the perovskite, being beneficial to enhance the device performance. Novel passivation alternatives or synthetic routes are highlighted in this contribution, giving a deeper understanding of the control of surface chemistry in PNCs through ligand engineering to prolong the stability of the nanocrystals.
1. Introduction
Metal halide perovskites (MHPs) with ABX3 structure (A = Cs+; methylammonium, MA+; formamidinium, FA+; B = Pb2+; X = Cl−, Br−, I− and combinations) have been established as the most promising semiconductor materials during the last decade, achieving important breakthroughs mainly in photovoltaics and optoelectronics. Here, MHPs have allowed for improving the photoconversion efficiencies of solar cell devices, for instance, prominent perovskite-CIGS monolithic tandem solar cells reaching values closer to 30% [1,2], and the fabrication of efficient light-emitting diodes (LEDs), with external quantum efficiencies (EQEs) >28% [3,4]. However, most of the great advances related to device stability in the above technologies have been limited via iodide-based MHPs, triggered by phase transformation of the photoactive black α-phase to the non-photoactive yellow δ-phase under ambient conditions [5,6,7]. The preparation of nanoconfined MHPs, also known as perovskite nanocrystals (PNCs), has attracted growing interest, allowing the phase and optical stabilization of the photoactive black α-phase in iodide-PNCs under high relative humidity and aerobic conditions [7,8]. Considering that the PNC sizes are in the atomic scale, the quantum confinement effect emerges to provide outstanding features to these materials, such as versatile surface chemistry [9], band gap tunability (varying the composition or particle size) [7,10], admirable tolerance to defects [11,12], improved light harvesting and carrier transport abilities [13], and facile synthesis [14]. These intrinsic characteristics have expanded the use of PNCs in laser [15], photodetectors [16], photo(electro)catalysis [17,18], and scintillators [19], among other interesting applications. More specifically, the carrier transfer ability of the PNCs has been used to prepare PNCs-composites with chalcogenide-based semiconductors as in the case of CsPbBr3 PNCs/TiSe2 nanosheets (NS) [20]. Here, TiSe2 NS mediates the electron mobility from the nanocrystals to the optoelectronic devices, increasing about twice the carrier transfer constant and photocurrent of the heterojunction compared with pristine CsPbBr3 PNCs. This would be convenient for the future fabrication of photoelectrodes for a solar-driven photo(electro)catalysis.
One of the key factors to produce PNCs with high color purity and enhanced PL properties is the use of organic ligands during the material synthesis [8,21]. These molecules anchor to the nanocrystal surface through the covalent interaction with AX and BX2 terminals (more often CsX and PbX2, respectively) [22,23]. This implies that a fraction of ligands can replace A- and X-sites into the PNCs, filling surface defects and decreasing non-radiative carrier traps [23]. Most of the contributions related to the PNCs synthesis have reported the use of oleic acid (OA) and oleylamine (OLA) as the conventional long-chain ligands, producing oleylammonium oleate (OLM) as the determinant species to promote the surface passivation and stabilization of PNCs [24,25,26]. However, (i) the formation of N-oleyloleamide as a byproduct during the OLM formation [7,27] and (ii) the loss of ligands from the nanocrystal surface in the form of oleylammonium halides reduce the fraction of the protective organic layer covering the PNCs [28]. At this point, a highly defective product is generated, which is more prone to suffer a fast self-degradation and experience the progressive quench of the PL properties [14,29]. Additionally, an excess of oleylamine can result in further decomposition of the PNCs, while an excess of oleic acid promotes a phase transition in the perovskite from a cubic to a tetragonal phase. This fact generates an imminent PL quenching [30]. Therefore, some attempts have been made to replace the surface ligands of the CsPbX3 PNCs with organic compounds such as dodecylbenzene sulfonic acid to provide chemical stability to the perovskites, allowing multiple purifications in polar solvents as well as stability against environmental conditions. Unfortunately, the external quantum efficiency in some devices is decreased, attributed to poor conductivity in the photomaterial [31].
To solve these drawbacks, novel synthetic procedures for PNCs growth have been highlighted by the use of capping agents with higher binding capability than conventional OA and OLA, achieving high-quality perovskites with PL quantum yield (PLQY) >90% [14,32,33]. This is the case of prominent capping ligands such as the didodecyldimethylammonium halide family [34], DDAX (X = Cl, Br), and zwitterionic compounds as the case of 3-N,N-(dimethyloctadecylammonio)propanesulfonate [14]. Even DDABr (also known as DDAB) generates partial exfoliation of the PNCs surface, removing part of the [PbBr6]4− octahedra and conducing a decrease in the particle size (strong nanoparticle confinement) [35], this ligand shows a dual role: DDA+ cations also favor a ligand exchange with the native oleylammonium species, while Br− anions fill/replace the halide defect sites, decreasing the density of electron traps [22]. Simultaneously, the above zwitterionic species depict positively and negatively charged functional organic groups which can be anchored to X- anions, and A-site cations, respectively [8]. Therefore, DDAB and zwitterionic ligands hinder the loss of capping agents and the removal of halide domains, promoting the emergence of PNCs with PLQY values up to 100% and longer stability (Figure 1a–c) [14,36], even to harsh polar environments such as alcohols without any type of encapsulation (Figure 1d) [37]. On the other hand, bidentate ligands such as 2,2′-iminodibenzoic acid (IDA) have been studied to restrain the fast α-to-δ phase transformation in CsPbI3 PNCs, through a direct coordination interaction between surface Pb atoms and the double -COOH moiety from the capping agent [38]. In this way, the lattice distortion into the nanocrystal is restricted, enhancing the long-term stability of the photoactive phase in the photomaterial with a PLQY ~95% (Figure 1e). Concerning promising synthetic routes, guanidinium (GA+) cations have been added during the preparation of FAPbBr3 PNCs, achieving a maximum suppression of defect sites into the perovskite to obtain a PLQY ~93% [39]. Even GA+ species depict a bigger size than FA+, going beyond the tolerance factor limit; low GA+ contents ~10 mol% can be incorporated into the nanocrystal due to the entropy stabilization. Here, GA+ can compensate both bulk and surface defect sites by introducing more hydrogen bonds given by the extra-amino group from the cation, interacting with surface Br domains. As a result of the formation of mixed cation FA1−xGAxPbBr3 PNCs with enhanced optical and electronic features, efficient green LEDs with EQEs = 23.4% have been fabricated.
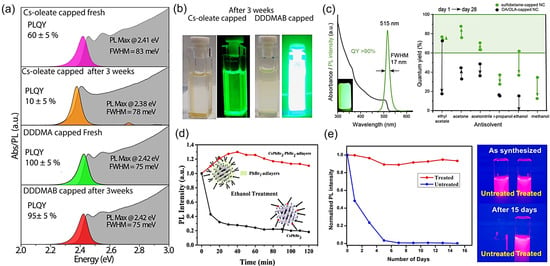
Figure 1.
(a) Typical absorbance and PL features of CsPbBr3 PNCs in absence (purple, orange) and presence of DDAB (green, red, named DDDMAB in this work) before and after three weeks of aging. (b) Photographs of the corresponding colloidal solutions evidence the stable high luminescence behavior of the nanocrystals after the addition of DDAB. Reproduced with permission [36]. Copyright 2019, American Chemical Society. (c) Typical optical features and PLQY of CsPbBr3 PNCs solutions capped with the 3-(N,N-dimethyloctadecylammonio)propanesulfonate and OA/OLA after two purification steps with different solvents at day 1 and after 28 days of aging. Reproduced under the terms of the CC-BY license [14]. Copyright 2018, The Authors. American Chemical Society. (d) Relative PL intensity of the CsPbBr3 PNCs capped with OA/OLA (black line) and DDAB (red line) in ethanol medium after 2 h. Reproduced with permission [37]. Copyright 2019, American Chemical Society. (e) Normalized PL intensity and corresponding photographs of red-emitting CsPbI3 PNCs colloidal solutions without (blue line) and with IDA treatment (red line) at day and after 15 days of aging. Reproduced under the terms of the CC-BY license [38]. Copyright 2018, The Authors. American Chemical Society.
Following this, the other approaches highlighting the use of thiocyanate and tetrafluoroborate salts [8,40] and novel purification methods to remove both undercoordinated Pb and unreacted species [26], have been proposed to produce highly emissive PNCs with improved optical features and color quality. Nevertheless, the washing process of the PNCs using low polar solvents also favors the loss of a fraction of ligands, hindering the control of the particle size and emerging a mixture of bulk and nanoscale materials with low stability [41]. On the other hand, recent literature reports involved in the preparation of PNCs with improved optical performance have established that the post-synthetic treatment of nanocrystals is the best option to compensate for the loss of capping agents and favor the passivation of surface defects [42,43,44]. This ligand protection has been achieved by adding alkylphosphines such as trioctyl- and triphenylphosphine (TOP and TPP, respectively) [45,46,47] or sulfur-based organic compounds such as S-OLA complexes and thionyl chloride [48,49]. Here, P atoms containing lone-pair electrons from TOP and TPP act as Lewis’s base to interact with Lewis’s acid Pb2+ species, promoting electron transfer. In addition, some TOP.PbX2 and TPP.PbX2 adducts are formed to facilitate the complete dissolution of the PbX2 precursor and facilitate the ligand coverage on the final nanocrystal product. Through this modification, high PLQY values can be obtained, with stability in ambient air for up to 1 month. Meanwhile, sulfur functional groups can replace/fill the halide vacancies into the perovskite structure, forming Pb-S bonds. In this context, the PL quenching is mitigated, and the radiative recombination pathway is maximized, providing a PLQY ~86% with stability around 3 months. In addition, it has been shown that thioglycolic and thiolactic acid can decrease the electron trap states near the conduction band [50]. Nevertheless, the use of phosphines and some S-based compounds promote critical health issues, such as a compromised heart and depression of the central nervous system to problems in the circulatory functions. Therefore, it is pivotal to find novel and environmentally safe alternatives to reduce the risk of health issues and eventual ecosystem contamination while preserving similar or better efficiency to prepare high-quality PNCs.
Fortunately, different kinds of nanoparticles (NPs) have been synthesized using ecofriendly environments, among which plant extracts have been the most outstanding reagents due to their easy handling and low cost. In addition, they do not require complex synthesis procedures involving high temperatures [51,52]. For instance, Khan and coworkers [52] have synthesized Ag NPs using Juniperus procera (JP) as a bioreductor and stabilizing agent. This extract is obtained from the fresh aerial parts of the plant through a drying process, grounding step, and refluxing at 80 °C. After the roto evaporation step, a final dark brown extract is reached, being important to synthesize 20 nm spherically shaped JP-Ag NPs. This extract promotes a fast nucleation process of the Ag NPs growth, decreasing the time consumption for material preparation and favoring an adequate coverage of the NPs. This system can be extrapolated to the growth of PNCs, opening the door to novel synthetic routes to conduct in lesser time without disregarding the quality of the final product. In this work, ethyl cellulose (EC), which is found in cosmetic products, has been useful for encapsulating green-emitting CsPbBr3 and yellow-emitting mixed halide CsPbBr1.5I1.5 PNCs, achieving an increase in the PL intensity compared with pristine perovskites [53]. This is an indication that beyond the EC ligand can produce suitable coverage, this molecule also minimizes the density of surface defect sites to generate better device performance.
Accordingly, it is worth noting that although there are several reasons to restrain the commercialization of PNCs and the development of future technologies, upcoming strategies will be proposed to produce lesser toxic nanomaterials to go one step forward to the potential development and scalability. In this review, we emphasize the state-of-the-art use of some non-toxic and ecofriendly solvents and capping ligands with a better surface passivation effect, improving the optical performance of PNCs colloidal solutions for optoelectronic applications. We exhibit some biogenic capping agents, organic ligands obtained from natural sources, and green synthetic approaches to achieve high-quality PNCs. Some implications on the fabrication of optoelectronic devices and a perspective in the preparation of lead-free PNCs are also discussed.
2. Ecofriendly Strategies to Synthesize High-Quality PNCs
2.1. Introduction of Biogenic Organic Ligands
The successful use of PNCs in optoelectronic and photovoltaic technologies has attracted attention from biological systems, where they can be useful for generating luminescent signals, pivotal for bioimaging and optical encoding applications [54]. However, considering the soft nature of the perovskite structure [55,56], these materials are prone to decompose in a biological atmosphere, quenching their PL properties via eventual interaction between the environment and protective organic layers from the nanocrystal’s surface. In this context, Cui and coworkers [57] have developed the encapsulation of CsPbBr3 PNCs with phospholipids (1,2-dioleoyl-sn-glycero-3-phosphocholine, DOPC) through the hydration method. Attending to phospholipids presents a hydrophilic head and a hydrophobic tail, and a micelle-type nanoparticle is formed. This CsPbBr3@DOPC micelle is water resistant, with improved PL emission stable for ~27 days (Figure 2a,a’), and highly resistant to acid/minimum essential medium (MEM), and base aqueous solutions for a time ≥96 h (Figure 2b,c). Considering the biocompatibility of the phospholipid layer, the biofunctionalization of the composite material with folate-conjugated polyethylene glycol is favored. This composite is suitable to be incorporated into HeLa cells with the aim of performing multicolor bioimaging (Inset of Figure 2a). The preparation of this kind of material is one step forward in tumor recognition taking advantage of the optical encoding of PNCs by studying their PL properties. On the other hand, peptides can also be integrated into PNCs, giving potential applications in biomedicine, optical sensing, and imaging. By mixing a peptide based on 12-aminododecanoic acid (12-AA) with MAPbBr3 PNCs [58], a partial ligand exchange is promoted. Here, the carboxylate moiety (-COO−) from 12-AA can link to metallic Pb, decreasing the density of structural defects produced by halide vacancies and the removal of OA. Meanwhile, the ammonium moiety (-NH3+) from the peptide shows a high affinity for Br- species from the perovskite surface, mediating a suitable ligand coverage [59]. This can also be confirmed by the blueshift observed in the PL emission peak from the PNCs in the presence of a higher concentration of 12-AA. This is ascribed to the instant capping effect, which restrains the nanocrystal particle size growth [60].
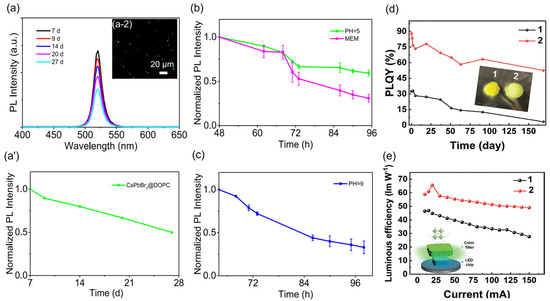
Figure 2.
(a) PL spectra and (a’) corresponding relative intensity of CsPbBr3 PNCs@DOPC micelles immersed in aqueous solutions under 27 days of analysis. Inset of Figure 2a (depicted as a-2) shows the typical PL image of HeLa cells immersed into CsPbBr3@DOPC micelle. Relative PL intensity of the PNCs@DOPC micelles obtained in (b) acid medium/MEM and (c) alkaline medium for a time ≥96 h of analysis. Inset of Figure 1a shows the fluorescence images of the PNCs@DOPC micelles introduced to incubated HeLa cells. Reproduced with permission [57]. Copyright 2019, American Chemical Society. (d) PLQY stability in water for 1-pristine CsPbBr3 and 2-CsPbBr3-Ptd-L-Ser filters monitored for 6 months. Inset of Figure 1d exhibits the appearance of the corresponding PNCs filters. (e) Luminous efficiency in function of the applied current for 1-pristine CsPbBr3 and 2-CsPbBr3-Ptd-L-Ser filters. Inset of Figure 1e depicts the typical green pc-LED configuration using the PNCs color filters. Reproduced under the terms of the CC-BY license [61]. Copyright 2022, The Authors. John Wiley & Sons, Inc.
Another important contribution concerning the use of biogenic ligands has been shown by Duan and coworkers [61], combining an amphiphilic capping agent such as phosphatidyl-L-serine (Ptd-L-Ser) with CsPbBr3 PNCs. This mixture means that the final CsPbBr3-Ptd-L-Ser product reaches an initial PLQY ~97% and long-term stability to storage/heating/water environments for 6 months. Through DFT calculations, Ptd-L-Ser depicts a higher binding energy (507 meV) than OA/OLA (337 meV), resulting in a higher adsorption capability of this phospholipid ligand to the material surface. This modification was supported by the compensation of undercoordinated Pb from the nanocrystals seen by XPS, in which the phospholipid was linked throughout multiple C=O functional moieties. After introducing pristine PNCs and PNCs-Ptd-L-Ser materials into a polystyrene matrix for preparing phosphorus-converted (pc) LEDs, the presence of phospholipid preserves a high PLQY ~80% under air and 56% in water (Figure 2d), compared with the quenched optical features of the pristine nanocrystals. This is an indication of a reduction of carrier traps performed by Ptd-L-Ser. This enhancement allows for fabricating efficient green pc-LEDs with high luminous efficiency of >65 lmW−1 (Figure 2e) and operational stability >700 h and 210 h at 20 mA and 100 mA, respectively. Subsequently, white light-emitting LEDs (WLEDs) were also fabricated by incorporating CsPbBr3-Ptd-L-Ser as a green filter, obtaining a stable white color purity for weeks.
On the other hand, Zhou and coworkers [62] have incorporated a phospholipid based on creatine phosphate (CP) molecules to prepare wide-bandgap A-site mixed Eu3+ doped CsxK1-xPbCl3 PNCs, with efficient energy transfer from the photoexcited carriers to the lanthanide species. Through the direct binding between undercoordinated Pb and Lewis-based C=O and P=O functional groups from CP, the intrinsic defect sites are repaired, improving the color quality and the radiative recombination mechanism. In this context, a PLQY ~84% and 61% have been estimated for the PNCs-based solutions and films, respectively, with a stable optical performance of 84 days until to decrease the PL intensity of the perovskite ~70% from the initial value. Attending to the energy transfer from the PNCs host and Eu3+ species and the enhanced intrinsic features of the nanocrystals in the presence of CP, efficient single-component WLEDs were fabricated with EQE ~5.4% and an operational lifetime of 220 s at 6 V.
2.2. Capping Ligands from Natural Sources
In line with employing green chemistry in the preparation and stabilization of colloidal PNCs, it is also remarkable that some of the capping agents can be obtained from natural sources such as seeds, fruits, and vegetables, among others. This is the case of the introduction of soy lecithin during the synthesis of CsPbBr3 PNCs, studied first by Kovalenko and coworkers [63]. This interesting ligand is a zwitterionic phospholipid presenting a mixture of saturated and unsaturated long hydrocarbon chains with some functional ramifications, such as palmityl, stearyl, oleyl, linolyl, and linolelyl chains. Unlike colloidal solutions based on OA/OLA, where high PNCs concentrations cause material aggregation, lecithin-covered PNCs preserve a fully dispersed state even at ultrahigh or ultralow nanocrystal concentrations. This feature keeps the PL emission peak positions and PLQY ~80% (Figure 3a,b) unaltered, indicating a good PNCs size homogeneity without any sign of aggregation, with a low density of surface defects to improve the radiative recombination pathway. Additionally, diluted colloidal PNCs can resist several purification repetitions with non-polar and polar solvents as in the case of acetone, acetonitrile, and isopropanol, providing a stable PLQY close to 100%. The benefits of this natural capping agent have been taken advantage of by Bakr and coworkers [64], synthesizing red-emitting CsPbI3 PNCs with long-term stability for around 6 months under open-air and room conditions and maintaining a PLQY of up to 100% for the solution, and 60% in films (Figure 3c). Lecithin is anchored to the PNCs surface through oxygen incorporation, generating Cs-O and Pb-O bonds [14]. Here, it does not exist any hydrogen bonding interaction, which is exhibited by the use of an OA/OLA combination to promote surface lattice strain [65,66]. In the absence of hydrogen bondings, the possibility of reaching a crystalline phase transformation, for instance, α-to-δ phase transition is avoided, which is pivotal to obtaining a stabilized photoactive phase for optoelectronic applications. At this stage, the authors have fabricated highly efficient bright red-LEDs with an electroluminescence peak position of ~634 nm at 3.5 V initial voltage (Figure 3d), a luminance of 1391 cdm−2, EQE ~7.1%, and an operational lifetime of 33 min. This performance is a breakthrough in Rec. 2020 coordinates for bright-red emissions. Here, the linked lecithin molecules fill iodide vacancies from the nanocrystal surface, suppressing non-radiative carrier traps, which promotes better carrier injection and mobility into the device.

Figure 3.
(a) Photographs of the lecithin–modified CsPbBr3 PNCs by varying the concentration of the nanocrystal product under visible (up) and UV illumination (down). (b) UV-vis absorption (black and grey lines)/PL emission features (green line) of CsPbBr3 at ultradiluted concentration 4 × 10 −5 mg mL−1. Reproduced under the terms of the CC-BY license [63]. Copyright 2019, The Authors. American Chemical Society. (c) PLQY of colloidal solutions and films and (d) EQE of red-LEDs devices for pristine (OA+OLA) and lecithin-modified CsPbI3 PNCs. Reproduced with permission [64]. Copyright 2022, American Chemical Society. (e) PLQY of fresh α-TCP-modified PNCs (X-T@P1) by varying the α-TCP concentration (X). (f) PL emission of α-TCP-modified CsPbX3-acrylate composites without (short dot) and with (solid line) α-TCP. Reproduced under the terms of the CC-BY license [67]. Copyright 2022, The Authors. John Wiley & Sons, Inc.
With the aim to hinder the α-to-δ phase transformation into CsPbI3 PNCs and generate high-quality perovskite inks for 3D printing applications, a previous contribution has investigated the use of α-tocopherol (α-TCP) for the first time [67]. This molecule is the active component of the vitamin E. By performing a post-synthetic treatment of the nanocrystals by adding diverse concentrations of α-TCP, the intrinsic properties of perovskite can be stabilized for more than 2 months under room conditions and ambient air, reaching PLQY values up to 100% (Figure 3e). The addition of α-TCP also restores the PL properties of aged PNC colloidal solutions, also keeping their structural integrity for 60 days. Through the phenolate moiety contained in the α-TCP structure, halide vacancies are filled, inducing an effective ligand coverage against O2 and humidity. Hence, a suitable α-TCP content produces an enhancement of the radiative recombination dynamics into the nanocrystal, increasing the PLQY. In contrast, an excess of vitamins can affect the surface stoichiometry of the perovskite, mediating the quenching of its PL properties. By achieving PNCs inks with suitable optical performance, these materials have been mixed with acrylate monomers to fabricate a highly emissive α-TCP-modified PNCs-acrylate 3D solid stable for 4 months, with a PLQY ~92%. Then, the luminescence of the 3D solids was tuned by varying the halide composition into the nanocrystal (blue and green emission) giving a higher PL intensity than the absence of the α-TCP (Figure 3f).
On the other hand, an attractive alternative to produce ecofriendly PNC products can come from the use of plant extracts that would be the most sustainable and environmentally friendly route towards the future commercialization of this kind of nanomaterials. In this case, Siddiqui and coworkers [68] have introduced Salvadora persica L. (S. persica L.) roots (miswak) extract as a main reducing agent in the bioreduction of graphene oxide (GRO). This extract used as a capping ligand hinders the material agglomeration, making GRO sheets more efficiently transformed in their reduced form compared with the chemically reduced GRO. In addition, this surfactant provides good dispersibility of the material in polar media such as water. Accordingly, if this type of plant extract can functionalize the nanomaterial surface by introducing main organic groups such as carboxylates and amine moieties, suitable surface passivation of PNCs can be expected, showing long-term stability features even in polar solvents.
At this point, it is also relevant to highlight that different characterization techniques are pivotal to determining the success of the surface stabilization of PNCs. Furthermore, we can deduce the effectiveness of the diverse types of capping ligands and how their chemical nature defines the intrinsic properties of the PNCs. A spectroscopic tool as the PLQY measurement is the first and initial step to evaluate the quality of the nanocrystals, accompanied by steady-state PL measurements. Both cases are useful for observing the stability of the PNCs over time and analyzing the suitability of the surface coverage offered by the capping agent. Then, the combination of FTIR, XPS, and NMR allows the revealing of the chemical composition and the main organic functional groups anchored to the nanocrystal surface [8]. Here, the grade of passivation offered by the ligands can be determined by the function of the fraction of atoms linked to the surface, coming from the capping agent and the initial/final composition of the perovskite host, see ref. [67] for details.
Although no LED devices were fabricated using X-T@P1 materials, we show in Scheme 1 the complete description of the steps (from I to IV) during the combination between the vitamin E and the CsPbI3 PNCs to prepare colloidal inks or highly emitting 3D solids. This preparation can also be extended to other halide perovskite materials for future contributions to novel strategies for PNC processing and robust optoelectronic technologies.
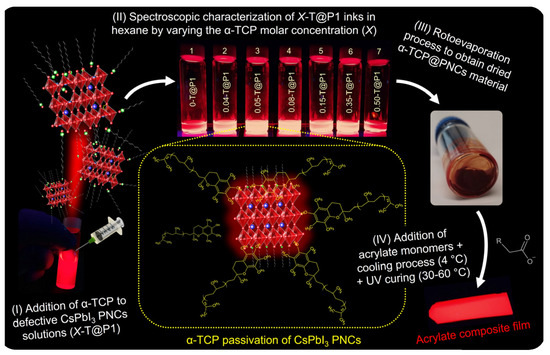
Scheme 1.
Sequential preparation of X-T@P1 colloidal inks and 3D solid composites.
2.3. Synthetic Procedures for PNCs Preparation via Green Solvents
The conventional synthesis procedure involving the use of organic ligands and solvents has been established to produce PNCs with different morphologies and sizes, tunable optical features, and the possibility to inhibit halide migration into the perovskite lattice itself [69,70]. However, in addition to the harmful features of these compounds on people’s health, some of them show long carbon chain structures that can hinder the carrier’s transport into a device, decreasing its final performance [71,72]. On the other hand, the use of bulky organic ligands makes that the PNCs growth is conducted in organic media (for instance, 1-octadecene or analogous compounds) [73,74,75], avoiding the use of more economically and ecofriendly polar/ionic systems where the structural integrity of PNCs is rapidly deteriorated. To face these drawbacks, Manna and coworkers [76,77] have developed a solvent-free synthetic method where conventional precursors such as CsBr and PbBr2 were combined with stoichiometric amounts of molten salts NaNO3, KNO3, and KBr at high temperatures ~350 °C, under ambient conditions. Furthermore, a mesoporous silica layer (m-SiO2) with thermal, chemically stable, and nontoxic characteristics was added to prepare PNCs@m-SiO2 composites, promoting the perovskites nanoconfinement and preserving their optical properties in polar environments. Here, PNCs@m-SiO2 materials delivered PLQY ~89 ± 10%, keeping their PL properties for a time up to 3 h at 180 °C, ~28 days in saline water at 90 °C and 1 month in both water and aqua regia systems (Figure 4a–c). At this stage, ionic species from the molten salts such as K+ and Br− favor an efficient defect passivation of CsPbBr3 PNCs surface by filling A- and X-sites [78,79]. The above-mentioned properties make these PNCs@m-SiO2 composites suitable for green phosphors for the down-conversion process into liquid crystal displays.
On the other hand, Zhang and coworkers have proposed for the first time the use of natural deep eutectic solvents (NADESs) during the CsPbBr3 PNCs synthesis via ligand-assisted reprecipitation (LARP) approach [80]. These NADESs are used as solvents for the mixture reaction, and simultaneously as capping agents stabilizing the final PNCs product. Interestingly, NADESs contain a hydrogen bond donor (HBD) and a hydrogen bond acceptor (HBA) and present some advantages, such as facile preparation, low cost, and biodegradable [81]. By mixing two kinds of HBA such as DL-menthol and thymol, with eight different HBD species such as L-lactic acid, n-octanoic acid, decanoic acid, lauric acid, ethanol, n-butanol, n-hexanol, and n-octanol, several NADESs solvents were obtained. The authors realized that the green solvent composed of thymol-lactic acid and thymol-n-octanol were pivotal to dissolving CsBr and PbBr2, respectively, while a NADES based on thymol-decanoic acid was used as an antisolvent. By combining this NADES with the corresponding perovskite precursors, high-quality PNCs were obtained with PLQY ∼97%, narrow full width at half-maximum ∼19 nm, and long-term stability of the PL properties for 70 days. Modified PNCs are employed as green phosphorous in pc-LEDs, with a maximum power efficiency of ~55.61 LmW−1 at 20 mA. The explanation of the improvement in the material stability arises from the co-existence of -COOH/-OH moieties from carboxylic acid/alcohol, which bind to Pb2+, reducing the likelihood of having surface defects.
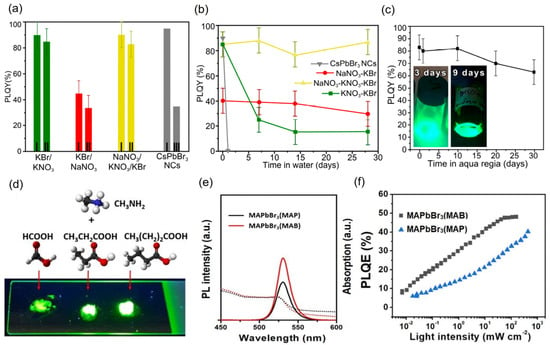
Figure 4.
(a) PLQY of pristine CsPbBr3 PNCs (grey) and PNCs/m-SiO2 composites in the presence of molten salts combinations: KNO3–KBr (green), NaNO3–KBr (red), and KNO3–NaNO3–KBr (yellow) (I) in the absence and after annealing at 180 °C, at different times: (III) 2 h and (II) 3 h. (b) PLQY stability of pristine PNCs and PNCs/m–SiO2 composites in water for ~28 days of analysis. (c) PLQY of the composite in the presence of KNO3–NaNO3-KBr combination after 1 month of analysis. The inset of Figure 3c shows the green emission feature of the composite after 3 and 9 days immersed in aqua regia. Reproduced under the terms of the CC-BY license [76]. Copyright 2021, The Authors. American Chemical Society. (d) Photograph of the green emitting MAPbBr3 PNCs prepared in the presence of MAF, MAP, and MAB, (from the left to right, respectively). (e) optical features and (f) PLQY in function of the light intensity for PNCs synthesized via MAP and MAB. Reproduced with permission [82]. Copyright 2020, Royal Society of Chemistry.
Furthermore, Wang and coworkers [82] have shown a novel method for PNCs synthesis via green ionic liquids based on protic methylammonium carboxylates, obtained using the Brønsted acid-base reaction between methylamine and three different carboxylic acids: formic acid, propionic acid, and butyric acid. In a facile procedure, the PbBr2 precursor is combined with the above ionic liquids (ILs), reacting instantaneously under room conditions. Subsequently, a yellow powder with green luminescence MAPbBr3 PNCs under UV light is obtained (Figure 4d). At this stage, perovskite samples with different morphologies show a higher PL emission intensity by increasing the long-carbon chain of the carboxylic acid used for ILs preparation (Figure 4e). In this way, MAPbBr3 PNCs synthesized in the presence of methylammonium butylate (MAB) exhibit the highest PL intensity than those generated with methylammonium formate (MAF) and methylammonium propionate (MAP). This indicates that butylate anions can prove a better ligand coverage on the nanocrystal surface. Then, by considering the discrepancy of ionicity between the three ILs, following the trend MAF > MAP > MAB, higher ionicity promotes a faster material aggregation. Meanwhile, a lower ionicity promotes a smaller particle size. This is the explanation for observing an enhanced radiative recombination process in MAB, a consequence of the effective suppression of non-radiative trap sites. Even though the maximum PLQY of the samples was ~50% by increasing the light intensity for excitation (Figure 4f), we propose that the incorporation of multiple A-site cations such as Cs+, FA+ [83], and those to prepare 2D perovskites as the case of guanidinium [39]. These cations can favor better surface passivation and extend the scope of improvement to use this PNCs growth strategy.
As seen in Table 1, most of the combinations employing the green solvents and capping ligands have been applied to Br-PNCs, due to this material being lesser prone to lose their PL features compared with more sensitive PNCs as the case of iodide-based ones. However, the addition of capping ligands from natural sources makes the stability and optical performance (described for the PLQY) of I-PNCs are improved, even showing long-term stability under room conditions for up to 6 months, providing highly emissive 3D solid composites and allowing the fabrication of efficient LED devices. This option was adequate by studying the most important organic functional groups linked to the PNCs surface and understanding the type of bond formed with the different A-site, B-site cations, and X-site anions available for the interaction. In this way, if an exhaustive study can be applied on how the organic functionalities of the different green solvents/capping agents can favor a ligand surface passivation and hinder the emergence of defect sites into the nanocrystals (in the function of the reagent concentration, analysis of synthetic factors such as temperature, ligand exchange processes, and provide a suitable stoichiometry of the initial precursors) could facilitate the choice and suitability of the combination between the green reagent and a specific type of halide PNCs.

Table 1.
Analysis of the PLQY and stability of the actual state-of-the-art PNCs in function type of green reagent used for material preparation. Applications have also been considered depending on the PL properties of the resultant PNCs product.
3. Concluding Remarks
In this review, we have shown the most promising alternatives to use green and ecofriendly solvents/ligands to synthesize PNCs with improved PL properties and stability. Although the use of traditional capping agents such as OA/OLA and some organic molecules with inherent toxicity has indicated the way to elucidate the surface chemistry of PNCs, it is also clear that the introduction of non-toxic, low-cost prominent capping agents and novel synthetic routes are pivotal to go one step forward to the future commercialization and development of nanoscale-based technologies. Therefore, the use of biogenic molecules ensures the total encapsulation of the PNCs, hindering the accessibility of polar molecules as water or direct interaction with biological systems, improving the optical performance of the perovskite by conducting ligand exchange processes. On the other hand, the use of capping ligands extracted from natural sources guarantees the filling/replacement of defect sites in the nanocrystal surface, favoring the preparation of ultradiluted colloidal solutions with the high optical response, mitigating the fast crystalline phase transformations from sensitive perovskites (for instance iodide-PNCs) and favor the preparation of chemical formulations to fabricate emissive 3D printed composites. Meanwhile, the modified synthetic methods make use of the chemical nature of the perovskite precursors to choose suitable green solvents for the mixture reaction, considering that these solvents also act as ligands to stabilize the final nanocrystal product. By controlling the stoichiometry between the perovskite precursor and solvents, fewer defective PNCs with PLQY close to 100% and high color purity can be obtained. From our experience, the use of natural capping agents would be the most rentable and adequate approach to obtain more “green” PNCs, which can be added in situ or during a post-synthetic treatment, restoring efficiently the damaged nanocrystal structure and optical features. In this context, new perspectives can be focused on how the main functional groups contained in the green reagents can favor the ligand passivation of the PNCs, understanding the type of interaction between the external compound and the perovskite surface. This knowledge would be valuable to choose an adequate chemical environment and conduct an effective combination between the ecofriendly species and a specific type of halide perovskite.
The recognition of the PNCs-green reagent type interaction could be the main factor for avoiding the structural reorganization into the 2D lead-free perovskites (for instance, Sn-based materials), where the PL properties are dictated by the self-trapped exciton (STE) phenomenon [84,85]. Attending to the fact that the colloidal hot-injection method is usually employed to prepare this kind of perovskites, the OA ligand defines the directional 2D perovskite growth, providing a suitable interlayer spacing between the [SnX6]4− octahedra layers in co-existence with the bulky A-site cation producing this separation. Ref. [86] In this context, a perovskite product with a band-to-band (b-b) transition feature is obtained. However, the washing process or the addition of non-polar solvents to prepare the perovskite dispersions promote the expulsion of OA from the 2D structure, causing a rearrangement in the stacking distance and favoring the formation of a defective white perovskite material with STE-type emission. Thus, the incorporation of natural ligands during or after the synthesis of 2D perovskites can fix the absence of native ligands essential to preserve the initial 2D perovskite with the b-b feature, being a great advance in the stabilization of the photophysical properties of Sn-based perovskites. In this way, a general synthetic route could be standardized for Pb- and lead-free perovskites adjusting the requirements for the established and upcoming technologies.
Author Contributions
Conceptualization, writing–original draft preparation, writing–review and editing, H.E.S.-G. and A.F.G.-R. Supervision, A.F.G.-R. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
H.E. Sánchez-Godoy acknowledges CONACYT for the Postdoctoral Fellowship at Instituto de Investigación en Metalurgia y Materiales, Universidad Michoacana de San Nicolás de Hidalgo. A.F. Gualdrón-Reyes acknowledges Instituto de Ciencias Químicas (ICQ) from Universidad Austral de Chile for financial support. We are grateful to Samrat Das Adhikari for reviewing the English editing in the manuscript.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Al-Ashouri, A.; Köhnen, E.; Li, B.; Magomedov, A.; Hempel, H.; Caprioglio, P.; Márquez, J.A.; Morales Vilches, A.B.; Kasparavicius, E.; Smith, J.A.; et al. Monolithic perovskite/silicon tandem solar cell with >29% efficiency by enhanced hole extraction. Science 2020, 370, 1300–1309. [Google Scholar] [CrossRef]
- Shrivastav, N.; Kashyap, S.; Madan, J.; Al-Mousoi, A.K.; Mohammed, M.K.A.; Hossain, M.K.; Pandey, R.; Ramanujam, J. Perovskite-CIGS Monolithic Tandem Solar Cells with 29.7% Efficiency: A Numerical Study. Energy Fuels 2023, 37, 3083–3090. [Google Scholar] [CrossRef]
- Kim, J.S.; Heo, J.-M.; Park, G.-S.; Woo, S.-J.; Cho, C.; Yun, H.J.; Kim, D.-H.; Park, J.; Lee, S.-C.; Park, S.-H.; et al. Ultra-bright, efficient and stable perovskite light-emitting diodes. Nature 2022, 611, 688–694. [Google Scholar] [CrossRef]
- Liu, Z.; Qiu, W.; Peng, X.; Sun, G.; Liu, X.; Liu, D.; Li, Z.; He, F.; Shen, C.; Gu, Q.; et al. Perovskite Light-Emitting Diodes with EQE Exceeding 28% through a Synergetic Dual-Additive Strategy for Defect Passivation and Nanostructure Regulation. Adv. Mater. 2021, 33, 2103268. [Google Scholar] [CrossRef]
- Masi, S.; Gualdrón-Reyes, A.F.; Mora-Seró, I. Stabilization of Black Perovskite Phase in FAPbI3 and CsPbI3. ACS Energy Lett. 2020, 5, 1974–1985. [Google Scholar] [CrossRef]
- Erazo, E.A.; Sánchez-Godoy, H.E.; Gualdrón-Reyes, A.F.; Masi, S.; Mora-Seró, I. Photo-Induced Black Phase Stabilization of CsPbI3 QDs Films. Nanomaterials 2020, 10, 1586. [Google Scholar] [CrossRef] [PubMed]
- Hassanabadi, E.; Latifi, M.; Gualdrón-Reyes, A.F.; Masi, S.; Joon, S.Y.; Poyatos, M.; Julián-López, B.; Mora Seró, I. Ligand & Band Gap Engineering: Tailoring the Protocol Synthesis for Achieving High-Quality CsPbI3 Quantum Dots. Nanoscale 2020, 12, 14194–14203. [Google Scholar] [CrossRef]
- Dey, A.; Ye, J.; De, A.; Debroye, E.; Ha, S.K.; Bladt, E.; Kshirsagar, A.S.; Wang, Z.; Yin, J.; Wang, Y.; et al. State of the Art and Prospects for Halide Perovskite Nanocrystals. ACS Nano 2021, 15, 10775–10981. [Google Scholar] [CrossRef]
- Pradhan, N. Journey of Making Cesium Lead Halide Perovskite Nanocrystals: What’s Next. J. Phys. Chem. Lett. 2019, 10, 5847–5855. [Google Scholar] [CrossRef] [PubMed]
- Scharf, E.; Krieg, F.; Elimelech, O.; Oded, M.; Levi, A.; Dirin, D.N.; Kovalenko, M.V.; Banin, U. Ligands Mediate Anion Exchange between Colloidal Lead-Halide Perovskite Nanocrystals. Nano Lett. 2022, 22, 4340–4346. [Google Scholar] [CrossRef]
- Yao, J.-S.; Ge, J.; Wang, K.-H.; Zhang, G.; Zhu, B.-S.; Chen, C.; Zhang, Q.; Luo, Y.; Yu, S.-H.; Yao, H.-B. Few-Nanometer-Sized α-CsPbI3 Quantum Dots Enabled by Strontium Substitution and Iodide Passivation for Efficient Red-Light Emitting Diodes. J. Am. Chem. Soc. 2019, 141, 2069–2079. [Google Scholar] [CrossRef]
- Zheng, X.; Hou, Y.; Sun, H.-T.; Mohammed, O.F.; Sargent, E.H.; Bakr, O.M. Reducing Defects in Halide Perovskite Nanocrystals for Light-Emitting Applications. J. Phys. Chem. Lett. 2019, 10, 2629–2640. [Google Scholar] [CrossRef]
- Lee, C.; Shin, Y.; Villanueva-Antoli, A.; Das Adhikari, S.; Rodríguez-Pereira, J.; Macak, J.M.; Mesa, C.; Yoon, S.J.; Gualdrón-Reyes, A.F.; Mora Seró, I. Efficient and Stable Blue- and Red-Emitting Perovskite Nanocrystals through Defect Engineering: PbX2 Purification. Chem. Mater. 2021, 33, 8745–8757. [Google Scholar] [CrossRef]
- Krieg, F.; Ochsenbein, S.T.; Yakunin, S.; ten Brinck, S.; Aellen, P.; Süess, A.; Clerc, B.; Guggisberg, D.; Nazarenko, O.; Shynkarenko, Y.; et al. Colloidal CsPbX3 (X = Cl, Br, I) Nanocrystals 2.0: Zwitterionic Capping Ligands for Improved Durability and Stability. ACS Energy Lett. 2018, 3, 641–646. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Chen, Q.; Zhang, C.; Wang, R.; Wu, H.; Zhang, X.; Xing, G.; Yu, W.W.; Wang, X.; Zhang, Y.; et al. Two-Photon-Pumped Perovskite Semiconductor Nanocrystal Lasers. J. Am. Chem. Soc. 2016, 138, 3761–3768. [Google Scholar] [CrossRef]
- Liu, Y.; Gong, W.; Xiang, F.; Li, Y.; Guo, H.; Hao, F.; Niu, X. High-Performance Self-Powered Photodetectors with Space-Confined Hybrid Lead Halide Perovskite Nanocrystals. Adv. Opt. Mater. 2022, 11, 2202215. [Google Scholar] [CrossRef]
- Fernández-Climent, R.; Gualdrón-Reyes, A.F.; García-Tecedor, M.; Mesa, C.A.; Cárdenas-Morcoso, D.; Montañes, L.; Barea, E.M.; Mas-Marzá, E.; Julián-López, B.; Mora-Seró, I.; et al. Switchable All Inorganic Halide Perovskite Nanocrystalline Photoelectrodes for Solar-Driven Organic Transformations. Sol. RRL 2021, 6, 2100723. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Rodríguez-Pereira, J.; Amado-González, E.; Rueda-P, J.; Ospina, R.; Masi, S.; Yoon, S.J.; Tirado, J.; Jaramillo, F.; Agouram, S.; et al. Unravelling the Photocatalytic Behavior of All-Inorganic Mixed Halide Perovskites: The Role of Surface Chemical States. ACS Appl. Mater. Interfaces 2020, 12, 914–924. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.-X.; Wang, X.; Yin, J.; Gutiérrez-Arzaluz, L.; He, T.; Chen, C.; Han, Y.; Zhang, Y.; Bakr, O.M.; Eddaoudi, M.; et al. Perovskite-Nanosheet Sensitizer for Highly Efficient Organic X-ray Imaging Scintillator. ACS Energy Lett. 2021, 7, 10–16. [Google Scholar] [CrossRef]
- Kumar, A.; Swami, S.K.; Sharma, R.; Yadav, S.; Singh, V.N.; Schneider, J.J.; Sinha, O.P.; Srivastava, R. A study on structural, optical, and electrical characteristics of perovskite CsPbBr3 QD/2D-TiSe2 nanosheet based nanocomposites for optoelectronic applications. Dalton Trans. 2022, 51, 4104–4112. [Google Scholar] [CrossRef] [PubMed]
- Zaccaria, F.; Zhang, B.; Goldoni, L.; Imran, M.; Zito, J.; van Beek, B.; Lauciello, S.; De Trizio, L.; Manna, L.; Infante, I. The Reactivity of CsPbBr3 Nanocrystals toward Acid/Base Ligands. ACS Nano 2022, 16, 1444–1455. [Google Scholar] [CrossRef]
- Bodnarchuk, M.I.; Boehme, S.C.; ten Brinck, S.; Bernasconi, C.; Shynkarenko, Y.; Krieg, F.; Widmer, R.; Aeschlimann, B.; Günther, D.; Kovalenko, M.V.; et al. Rationalizing and Controlling the Surface Structure and Electronic Passivation of Cesium Lead Halide Nanocrystals. ACS Energy Lett. 2018, 4, 63–74. [Google Scholar] [CrossRef]
- Wang, X.-D.; Huang, Y.-H.; Liao, J.-F.; Wei, Z.-F.; Li, W.-G.; Xu, Y.-F.; Chen, H.-Y.; Kuang, D.-B. Surface passivated halide perovskite single-crystal for efficient photoelectrochemical synthesis of dimethoxydihydrofuran. Nat. Commun. 2021, 12, 1202. [Google Scholar] [CrossRef]
- Almeida, G.; Goldoni, L.; Akkerman, Q.; Dang, Z.; Khan, A.H.; Marras, S.; Moreels, I.; Manna, L. Role of Acid–Base Equilibria in the Size, Shape, and Phase Control of Cesium Lead Bromide Nanocrystals. ACS Nano 2018, 12, 1704–1711. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Macias-Pinilla, D.F.; Masi, S.; Echeverría-Arrondo, C.; Agouram, S.; Muñoz-Sanjosé, V.; Rodríguez-Pereira, J.; Macak, J.M.; Mora-Seró, I. Engineering Sr-doping for enabling long-term stable FAPb1−xSrxI3 quantum dots with 100% photoluminescence quantum yield. J. Mater. Chem. C 2021, 9, 1555–1566. [Google Scholar] [CrossRef]
- Zhang, Y.; Siegler, T.D.; Thomas, C.J.; Abney, M.K.; Shah, T.; De Gorostiza, A.; Greene, R.M.; Korgel, B.A. A “Tips and Tricks” Practical Guide to the Synthesis of Metal Halide Perovskite Nanocrystals. Chem. Mater. 2020, 32, 5410–5423. [Google Scholar] [CrossRef]
- Grisorio, R.; Di Clemente, M.E.; Fanizza, E.; Allegretta, I.; Altamura, D.; Striccoli, M.; Terzano, R.; Giannini, C.; Irimia-Vladu, M.; Suranna, G.P. Exploring the surface chemistry of cesium lead halide perovskite nanocrystals. Nanoscale 2019, 11, 986–999. [Google Scholar] [CrossRef]
- Gualdrón-Reyes, A.F.; Masi, S.; Mora-Seró, I. Progress in halide-perovskite nanocrystals with near-unity photoluminescence quantum yield. Trends Chem. 2021, 3, 499–511. [Google Scholar] [CrossRef]
- ten Brinck, S.; Zaccaria, F.; Infante, I. Defects in Lead Halide Perovskite Nanocrystals: Analogies and (Many) Differences with the Bulk. ACS Energy Lett. 2019, 4, 2739–2747. [Google Scholar] [CrossRef]
- Ding, H.; Jiang, H.; Wang, X. How organic ligands affect the phase transition and fluorescent stability of perovskite nanocrystals. J. Mater. Chem. C 2020, 8, 8999–9004. [Google Scholar] [CrossRef]
- Yang, D.; Li, X.; Zhou, W.; Zhang, S.; Meng, C.; Wu, Y.; Wang, Y.; Zeng, H. CsPbBr3 Quantum Dots 2.0: Benzenesulfonic Acid Equivalent Ligand Awakens Complete Purification. Adv. Mater. 2019, 31, 1900767. [Google Scholar] [CrossRef] [PubMed]
- Grisorio, R.; Fasulo, F.; Muñoz-García, A.B.; Pavone, M.; Conelli, D.; Fanizza, E.; Striccoli, M.; Allegretta, I.; Terzano, R.; Margiotta, N.; et al. In Situ Formation of Zwitterionic Ligands: Changing the Passivation Paradigms of CsPbBr3 Nanocrystals. Nano Lett. 2022, 22, 4437–4444. [Google Scholar] [CrossRef]
- Wang, Q.; Zheng, X.; Deng, Y.; Zhao, J.; Chen, Z.; Huang, J. Stabilizing the α-Phase of CsPbI3 Perovskite by Sulfobetaine Zwitterions in One-Step Spin-Coating Films. Joule 2017, 1, 371–382. [Google Scholar] [CrossRef]
- Shynkarenko, Y.; Bodnarchuk, M.I.; Bernasconi, C.; Berezovska, Y.; Verteletskyi, V.; Ochsenbein, S.T.; Kovalenko, M.V. Direct Synthesis of Quaternary Alkylammonium-Capped Perovskite Nanocrystals for Efficient Blue and Green Light-Emitting Diodes. ACS Energy Lett. 2019, 4, 2703–2711. [Google Scholar] [CrossRef] [PubMed]
- Balakrishnan, S.K.; Kamat, P.V. Ligand Assisted Transformation of Cubic CsPbBr3 Nanocrystals into Two-Dimensional CsPb2Br5 Nanosheets. Chem. Mater. 2017, 30, 74–78. [Google Scholar] [CrossRef]
- Imran, M.; Ijaz, P.; Goldoni, L.; Maggioni, D.; Petralanda, U.; Prato, M.; Almeida, G.; Infante, I.; Manna, L. Simultaneous Cationic and Anionic Ligand Exchange For Colloidally Stable CsPbBr3 Nanocrystals. ACS Energy Lett. 2019, 4, 819–824. [Google Scholar] [CrossRef]
- Ruan, L.J.; Tang, B.; Ma, Y. Improving the Stability of CsPbBr3 Nanocrystals in Ethanol by Capping with PbBr2-Adlayers. J. Phys. Chem. C 2019, 123, 11959–11967. [Google Scholar] [CrossRef]
- Pan, J.; Shang, Y.; Yin, J.; De Bastiani, M.; Peng, W.; Dursun, I.; Sinatra, L.; El-Zohry, A.M.; Hedhili, M.N.; Emwas, A.-H.; et al. Bidentate Ligand-Passivated CsPbI3 Perovskite Nanocrystals for Stable Near-Unity Photoluminescence Quantum Yield and Efficient Red Light-Emitting Diodes. J. Am. Chem. Soc. 2017, 140, 562–565. [Google Scholar] [CrossRef]
- Kim, Y.-H.; Kim, S.; Kakekhani, A.; Park, J.; Park, J.; Lee, Y.-H.; Xu, H.; Nagane, S.; Wexler, R.B.; Kim, D.-H.; et al. Comprehensive defect suppression in perovskite nanocrystals for high-efficiency light-emitting diodes. Nat. Photonics 2021, 15, 148–155. [Google Scholar] [CrossRef]
- Shamsi, J.; Urban, A.S.; Imran, M.; De Trizio, L.; Manna, L. Metal Halide Perovskite Nanocrystals: Synthesis, Post-Synthesis Modifications, and Their Optical Properties. Chem. Rev. 2019, 119, 3296–3348. [Google Scholar] [CrossRef]
- Kazes, M.; Udayabhaskararao, T.; Dey, S.; Oron, D. Effect of Surface Ligands in Perovskite Nanocrystals: Extending in and Reaching out. Acc. Chem. Res. 2021, 54, 1409–1418. [Google Scholar] [CrossRef]
- Huang, Y.; Luan, W.; Liu, M.; Turyanska, L. DDAB-assisted synthesis of iodine-rich CsPbI3 perovskite nanocrystals with improved stability in multiple environments. J. Mater. Chem. C 2020, 8, 2381–2387. [Google Scholar] [CrossRef]
- Park, J.; Park, S.; Cho, S.; Kim, Y.; Kim, H.; Jeong, S.; Woo, J.Y. Air-stable cesium lead bromide perovskite nanocrystals via post-synthetic treatment with oleylammonium bromides. New J. Chem. 2022, 46, 19514–19522. [Google Scholar] [CrossRef]
- Dutt, V.G.V.; Akhil, S.; Singh, R.; Palabathuni, M.; Mishra, N. Year-Long Stability and Near-Unity Photoluminescence Quantum Yield of CsPbBr3 Perovskite Nanocrystals by Benzoic Acid Post-treatment. J. Phys. Chem. C 2022, 126, 9502–9508. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Ding, C.; Kobayashi, S.; Izuishi, T.; Nakazawa, N.; Toyoda, T.; Ohta, T.; Hayase, S.; Minemoto, T.; et al. Highly Luminescent Phase-Stable CsPbI3 Perovskite Quantum Dots Achieving Near 100% Absolute Photoluminescence Quantum Yield. ACS Nano 2017, 11, 10373–10383. [Google Scholar] [CrossRef] [PubMed]
- Peng, K.-H.; Yang, S.-H.; Wu, Z.-Y.; Hsu, H.-C. Synthesis of Red Cesium Lead Bromoiodide Nanocrystals Chelating Phenylated Phosphine Ligands with Enhanced Stability. ACS Omega 2021, 6, 10437–10446. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Sui, N.; Bai, X.; Zhang, Y.; Rice, Q.; Seo, F.J.; Zhang, Q.; Colvin, V.L.; Yu, W.W. Emission Recovery and Stability Enhancement of Inorganic Perovskite Quantum Dots. J. Phys. Chem. Lett. 2018, 9, 4166–4173. [Google Scholar] [CrossRef] [PubMed]
- Huynh, K.A.; Bae, S.-R.; Nguyen, T.V.; Do, H.H.; Heo, D.Y.; Park, J.; Lee, T.-W.; Le, Q.V.; Ahn, S.H.; Kim, S.Y. Ligand-Assisted Sulfide Surface Treatment of CsPbI3 Perovskite Quantum Dots to Increase Photoluminescence and Recovery. ACS Photonics 2021, 8, 1979–1987. [Google Scholar] [CrossRef]
- Zhang, B.-B.; Yuan, S.; Ma, J.-P.; Zhou, Y.; Hou, J.; Chen, X.; Zheng, W.; Shen, H.; Wang, X.-C.; Sun, B.; et al. General Mild Reaction Creates Highly Luminescent Organic-Ligand-Lacking Halide Perovskite Nanocrystals for Efficient Light-Emitting Diodes. J. Am. Chem. Soc. 2019, 141, 15423–15432. [Google Scholar] [CrossRef]
- Goswami, P.N.; Mandal, D.; Rath, A.K. The role of surface ligands in determining the electronic properties of quantum dot solids and their impact on photovoltaic figure of merits. Nanoscale 2018, 10, 1072–1080. [Google Scholar] [CrossRef]
- Chinni, S.V.; Gopinath, S.C.B.; Anbu, P.; Fuloria, N.K.; Fuloria, S.; Mariappan, P.; Krusnamurthy, K.; Veeranjaneya Reddy, L.; Ramachawolran, G.; Sreeramanan, S.; et al. Characterization and Antibacterial Response of Silver Nanoparticles Biosynthesized Using an Ethanolic Extract of Coccinia indica Leaves. Crystals 2021, 11, 97. [Google Scholar] [CrossRef]
- Khan, M.; Karuppiah, P.; Alkhathlan, H.Z.; Kuniyil, M.; Khan, M.; Adil, S.F.; Shaik, M.R. Green Synthesis of Silver Nanoparticles Using Juniperus procera Extract: Their Characterization, and Biological Activity. Crystals 2022, 12, 420. [Google Scholar] [CrossRef]
- Kumar, A.; Swami, S.K.; Singh, V.N.; Gupta, B.K.; Sinha, O.P.; Srivastava, R. Ethylcellulose-Encapsulated Inorganic Lead Halide Perovskite Nanoparticles for Printing and Optoelectronic Applications. Part. Part. Syst. Charact. 2022, 39, 2100250. [Google Scholar] [CrossRef]
- Gomez, L.; de Weerd, C.; Hueso, J.L.; Gregorkiewicz, T. Color-stable water-dispersed cesium lead halide perovskite nanocrystals. Nanoscale 2017, 9, 631–636. [Google Scholar] [CrossRef]
- Murali, B.; Dey, S.; Abdelhady, A.L.; Peng, W.; Alarousu, E.; Kirmani, A.R.; Cho, N.; Sarmah, S.P.; Parida, M.R.; Saidaminov, M.I.; et al. Surface Restructuring of Hybrid Perovskite Crystals. ACS Energy Lett. 2016, 1, 1119–1126. [Google Scholar] [CrossRef]
- Huang, H.; Pradhan, B.; Hofkens, J.; Roeffaers, M.B.J.; Steele, J.A. Solar-Driven Metal Halide Perovskite Photocatalysis: Design, Stability, and Performance. ACS Energy Lett. 2020, 5, 1107–1123. [Google Scholar] [CrossRef]
- Yang, Z.; Xu, J.; Zong, S.; Xu, S.; Zhu, D.; Zhang, Y.; Chen, C.; Wang, C.; Wang, Z.; Cui, Y. Lead Halide Perovskite Nanocrystals–Phospholipid Micelles and Their Biological Applications: Multiplex Cellular Imaging and in Vitro Tumor Targeting. ACS Appl. Mater. Interfaces 2019, 11, 47671–47679. [Google Scholar] [CrossRef] [PubMed]
- Luo, B.; Naghadeh, S.B.; Allen, A.L.; Li, X.; Zhang, J.Z. Peptide-Passivated Lead Halide Perovskite Nanocrystals Based on Synergistic Effect between Amino and Carboxylic Functional Groups. Adv. Funct. Mater. 2017, 27, 1604018. [Google Scholar] [CrossRef]
- Zhao, J.; Cao, S.; Li, Z.; Ma, N. Amino Acid-Mediated Synthesis of CsPbBr3 Perovskite Nanoplatelets with Tunable Thickness and Optical Properties. Chem. Mater. 2018, 30, 6737–6743. [Google Scholar] [CrossRef]
- Dutta, A.; Dutta, S.K.; Das Adhikari, S.; Pradhan, N. Tuning the Size of CsPbBr3 Nanocrystals: All at One Constant Temperature. ACS Energy Lett. 2018, 3, 329–334. [Google Scholar] [CrossRef]
- Duan, Y.; Chordiya, K.; Kahaly, M.U.; Oropeza, F.E.; de la Peña O’Shea, V.A.; Wang, D.Y.; Costa, R.D. Rational Amphiphilic Ligand Engineering Enables Enhanced Stability and Efficiency of CsPbBr3 Nanocrystals Based Light Emitting Diodes. Adv. Opt. Mater. 2022, 10, 2201176. [Google Scholar] [CrossRef]
- Sun, R.; Zhou, D.; Ding, Y.; Wang, Y.; Wang, Y.; Zhuang, X.; Liu, S.; Ding, N.; Wang, T.; Xu, W.; et al. Efficient single-component white light emitting diodes enabled by lanthanide ions doped lead halide perovskites via controlling Förster energy transfer and specific defect clearance. Light Sci. Appl. 2022, 11, 340. [Google Scholar] [CrossRef]
- Krieg, F.; Ong, Q.K.; Burian, M.; Rainò, G.; Naumenko, D.; Amenitsch, H.; Süess, A.; Grotevent, M.J.; Krumeich, F.; Bodnarchuk, M.I.; et al. Stable Ultraconcentrated and Ultradilute Colloids of CsPbX3 (X = Cl, Br) Nanocrystals Using Natural Lecithin as a Capping Ligand. J. Am. Chem. Soc. 2019, 141, 19839–19849. [Google Scholar] [CrossRef] [PubMed]
- Mir, W.J.; Alamoudi, A.; Yin, J.; Yorov, K.E.; Maity, P.; Naphade, R.; Shao, B.; Wang, J.; Lintangpradipto, M.N.; Nematulloev, S.; et al. Lecithin Capping Ligands Enable Ultrastable Perovskite-Phase CsPbI3 Quantum Dots for Rec. 2020 Bright-Red Light-Emitting Diodes. J. Am. Chem. Soc. 2022, 144, 13302–13310. [Google Scholar] [CrossRef]
- Zhao, Q.; Hazarika, A.; Schelhas, L.T.; Liu, J.; Gaulding, E.A.; Li, G.; Zhang, M.; Toney, M.F.; Sercel, P.C.; Luther, J.M. Size-Dependent Lattice Structure and Confinement Properties in CsPbI3 Perovskite Nanocrystals: Negative Surface Energy for Stabilization. ACS Energy Lett. 2019, 5, 238–247. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, H.; Piotrowski, M.; Han, X.; Ge, Z.; Dong, L.; Wang, C.; Pinisetty, S.K.; Balguri, P.K.; Bandela, A.K.; et al. Cesium Lead Iodide Perovskites: Optically Active Crystal Phase Stability to Surface Engineering. Micromachines 2022, 13, 1318. [Google Scholar] [CrossRef] [PubMed]
- Recalde, I.; Gualdrón-Reyes, A.F.; Echeverría-Arrondo, C.; Villanueva-Antolí, A.; Simancas, J.; Rodriguez-Pereira, J.; Zanatta, M.; Mora-Seró, I.; Sans, V. Vitamins as Active Agents for Highly Emissive and Stable Nanostructured Halide Perovskite Inks and 3D Composites Fabricated by Additive Manufacturing. Adv. Funct. Mater. 2022, 33, 2210802. [Google Scholar] [CrossRef]
- Khan, M.; Al-Marri, A.H.; Khan, M.; Shaik, M.R.; Mohri, N.; Adil, S.F.; Kuniyil, M.; Alkhathlan, H.Z.; Al-Warthan, A.; Tremel, W.; et al. Green Approach for the Effective Reduction of Graphene Oxide Using Salvadora persica L. Root (Miswak) Extract. Nanoscale Res. Lett. 2015, 10, 281. [Google Scholar] [CrossRef]
- Sun, W.; Yun, R.; Liu, Y.; Zhang, X.; Yuan, M.; Zhang, L.; Li, X. Ligands in Lead Halide Perovskite Nanocrystals: From Synthesis to Optoelectronic Applications. Small 2022, 19, 2205950. [Google Scholar] [CrossRef]
- Hassan, Y.; Park, J.H.; Crawford, M.L.; Sadhanala, A.; Lee, J.; Sadighian, J.C.; Mosconi, E.; Shivanna, R.; Radicchi, E.; Jeong, M.; et al. Ligand-engineered bandgap stability in mixed-halide perovskite LEDs. Nature 2021, 591, 72–77. [Google Scholar] [CrossRef]
- Kuan, C.-H.; Yang, S.-H. Surface ligand engineering of perovskite nanocrystals with a conjugated sulfonate ligand for light-emitting applications. Mater. Adv. 2022, 3, 7824–7832. [Google Scholar] [CrossRef]
- Swarnkar, A.; Marshall, A.R.; Sanehira, E.M.; Chernomordik, B.D.; Moore, D.T.; Christians, J.A.; Chakrabarti, T.; Luther, J.M. Quantum dot-induced phase stabilization of α-CsPbI3 perovskite for high-efficiency photovoltaics. Science 2016, 354, 92–95. [Google Scholar] [CrossRef]
- Baranov, D.; Caputo, G.; Goldoni, L.; Dang, Z.; Scarfiello, R.; De Trizio, L.; Portone, A.; Fabbri, F.; Camposeo, A.; Pisignano, D.; et al. Transforming colloidal Cs4PbBr6 nanocrystals with poly(maleic anhydride-alt-1-octadecene) into stable CsPbBr3 perovskite emitters through intermediate heterostructures. Chem. Sci. 2020, 11, 3986–3995. [Google Scholar] [CrossRef] [PubMed]
- Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Krieg, F.; Caputo, R.; Hendon, C.H.; Yang, R.X.; Walsh, A.; Kovalenko, M.V. Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, and I): Novel Optoelectronic Materials Showing Bright Emission with Wide Color Gamut. Nano Lett. 2015, 15, 3692–3696. [Google Scholar] [CrossRef]
- Nedelcu, G.; Protesescu, L.; Yakunin, S.; Bodnarchuk, M.I.; Grotevent, M.J.; Kovalenko, M.V. Fast Anion-Exchange in Highly Luminescent Nanocrystals of Cesium Lead Halide Perovskites (CsPbX3, X = Cl, Br, I). Nano Lett. 2015, 15, 5635–5640. [Google Scholar] [CrossRef] [PubMed]
- An, M.N.; Park, S.; Brescia, R.; Lutfullin, M.; Sinatra, L.; Bakr, O.M.; De Trizio, L.; Manna, L. Low-Temperature Molten Salts Synthesis: CsPbBr3 Nanocrystals with High Photoluminescence Emission Buried in Mesoporous SiO2. ACS Energy Lett. 2021, 6, 900–907. [Google Scholar] [CrossRef]
- Liu, Z.; Sinatra, L.; Lutfullin, M.; Ivanov, Y.P.; Divitini, G.; De Trizio, L.; Manna, L. One Hundred-Nanometer-Sized CsPbBr3/m-SiO2 Composites Prepared via Molten-Salts Synthesis are Optimal Green Phosphors for LCD Display Devices. Adv. Energy Mater. 2022, 12, 2201948. [Google Scholar] [CrossRef]
- Abdi-Jalebi, M.; Andaji-Garmaroudi, Z.; Cacovich, S.; Stavrakas, C.; Philippe, B.; Richter, J.M.; Alsari, M.; Booker, E.P.; Hutter, E.M.; Pearson, A.J.; et al. Maximizing and stabilizing luminescence from halide perovskites with potassium passivation. Nature 2018, 555, 497–501. [Google Scholar] [CrossRef]
- Yang, J.-N.; Song, Y.; Yao, J.-S.; Wang, K.-H.; Wang, J.-J.; Zhu, B.-S.; Yao, M.-M.; Rahman, S.U.; Lan, Y.-F.; Fan, F.-J.; et al. Potassium Bromide Surface Passivation on CsPbI3-xBrx Nanocrystals for Efficient and Stable Pure Red Perovskite Light-Emitting Diodes. J. Am. Chem. Soc. 2020, 142, 2956–2967. [Google Scholar] [CrossRef]
- Lu, H.; Tan, X.; Huang, G.; Wu, S.; Zhou, Y.; Zhang, J.; Zheng, Q.; Chen, T.; Li, F.; Cai, Z.; et al. Green synthesis of highly stable CsPbBr3 perovskite nanocrystals using natural deep eutectic solvents as solvents and surface ligands. Nanoscale 2022, 14, 17222–17229. [Google Scholar] [CrossRef]
- Fan, Y.; Wu, H.; Cai, D.; Yang, T.; Yang, L. Effective extraction of harmine by menthol/anise alcohol-based natural deep eutectic solvents. Sep. Purif. Technol. 2020, 250, 117211. [Google Scholar] [CrossRef]
- Hoang, M.T.; Pham, N.D.; Yang, Y.; Tiong, V.T.; Zhang, C.; Gui, K.; Chen, H.; Chang, J.; Wang, J.; Golberg, D.; et al. A facile, environmentally friendly synthesis of strong photo-emissive methylammonium lead bromide perovskite nanocrystals enabled by ionic liquids. Green Chem. 2020, 22, 3433–3440. [Google Scholar] [CrossRef]
- Hao, M.; Bai, Y.; Zeiske, S.; Ren, L.; Liu, J.; Yuan, Y.; Zarrabi, N.; Cheng, N.; Ghasemi, M.; Chen, P.; et al. Ligand-assisted cation-exchange engineering for high-efficiency colloidal Cs1−xFAxPbI3 quantum dot solar cells with reduced phase segregation. Nat. Energy 2020, 5, 79–88. [Google Scholar] [CrossRef]
- Zhang, X.; Wang, C.; Zhang, Y.; Zhang, X.; Wang, S.; Lu, M.; Cui, H.; Kershaw, S.V.; Yu, W.W.; Rogach, A.L. Bright Orange Electroluminescence from Lead-Free Two-Dimensional Perovskites. ACS Energy Lett. 2018, 4, 242–248. [Google Scholar] [CrossRef]
- Yin, J.; Brédas, J.-L.; Bakr, O.M.; Mohammed, O.F. Boosting Self-Trapped Emissions in Zero-Dimensional Perovskite Heterostructures. Chem. Mater. 2020, 32, 5036–5043. [Google Scholar] [CrossRef]
- Ghimire, S.; Oldenburg, K.; Bartling, S.; Lesyuk, R.; Klinke, C. Structural Reconstruction in Lead-Free Two-Dimensional Tin Iodide Perovskites Leading to High Quantum Yield Emission. ACS Energy Lett. 2022, 7, 975–983. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).