Treatment of Water Contaminated with Diesel Using Carbon Nanotubes
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Instruments
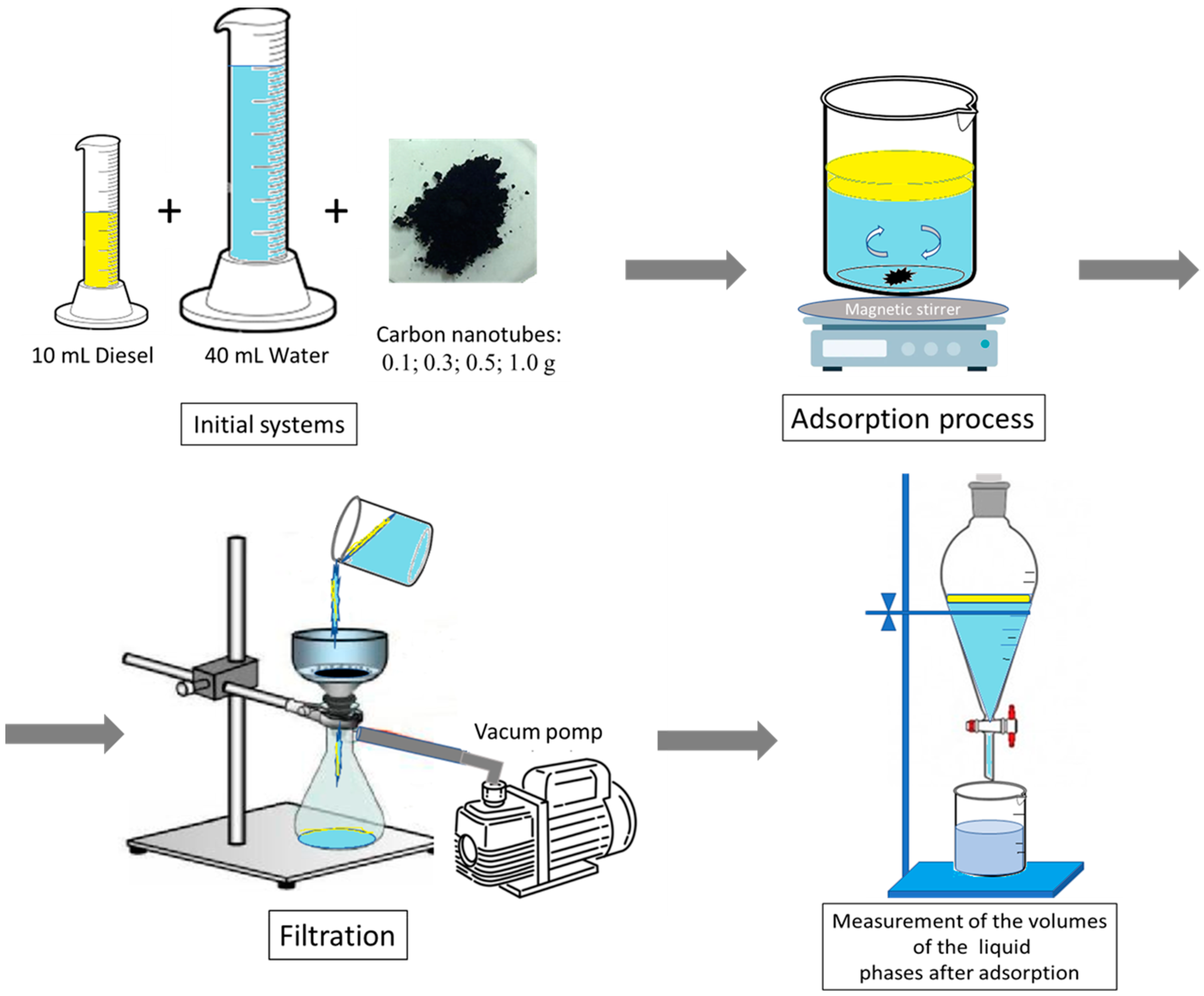
2.3. Adsorption Tests
2.4. Cyclic Adsorption/Desorption Tests
- (1)
- Adsorption: each adsorption test was carried out at room temperature and under stirring for 60 min.
- (2)
- Post-adsorption filtration: after the programmed adsorption time, the carbon nanotubes were separated from the filtration system, the liquid phases obtained (immiscible) were placed in a graduated burette and the quantities of water and diesel were measured residually.
- (3)
- Desorption: it was carried out by proceeding with an extraction with acetone of the gas oil adsorbed by the carbon nanotubes. In particular, the nanotubes recovered in the previous phase were inserted into 30 mL of acetone, stirred for 60 min in a well-sealed container at a temperature of about 40 °C.
- (4)
- Post-desorption filtration: At the end of the programmed extraction time, the CNTs were separated through filtration using a vacuum pump. The liquid phase obtained was placed in a graduated burette, and the volumes were measured.
3. Results
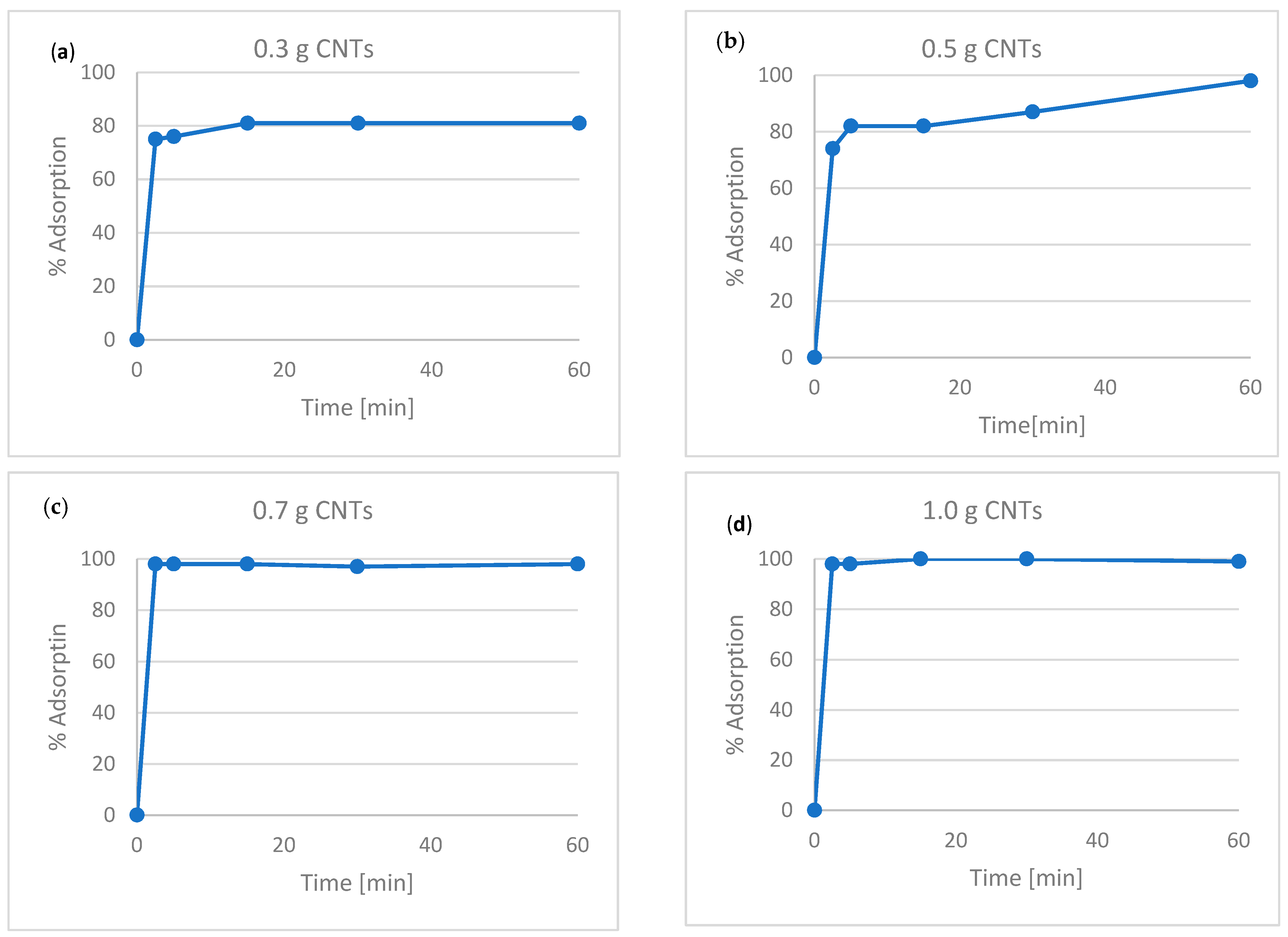
3.1. Adsorption Kinetics
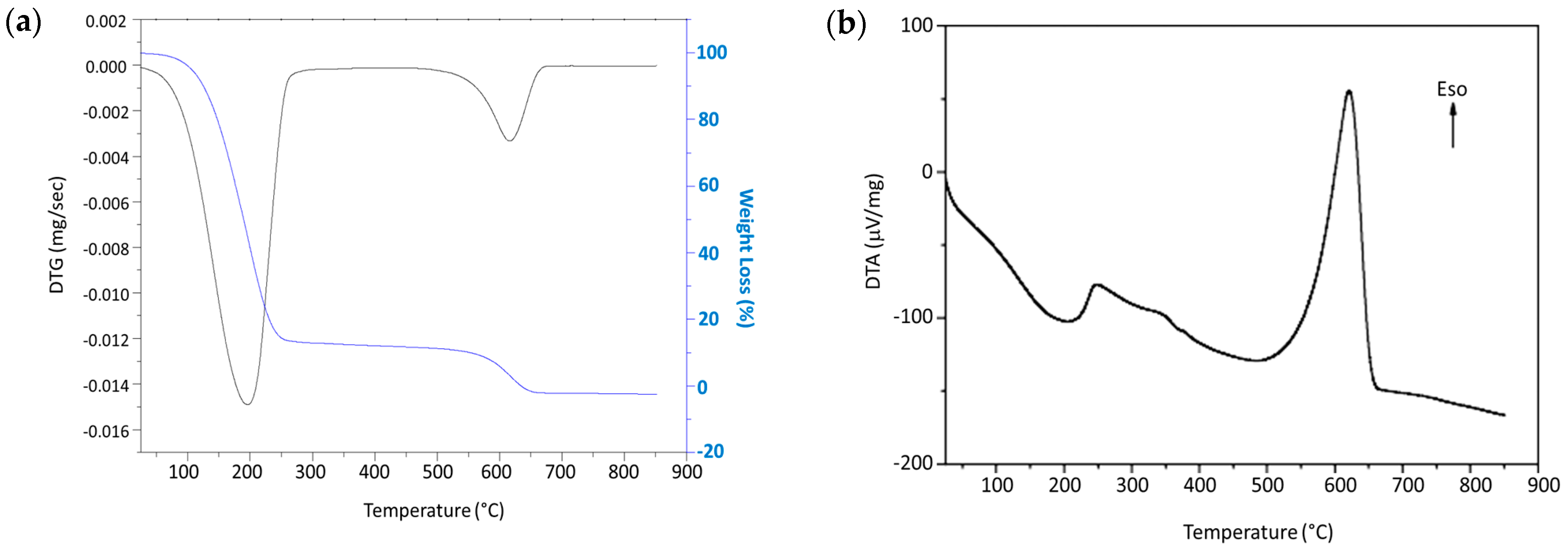
3.2. Thermal Characterization of Post-Adsorption Carbon Nanotubes
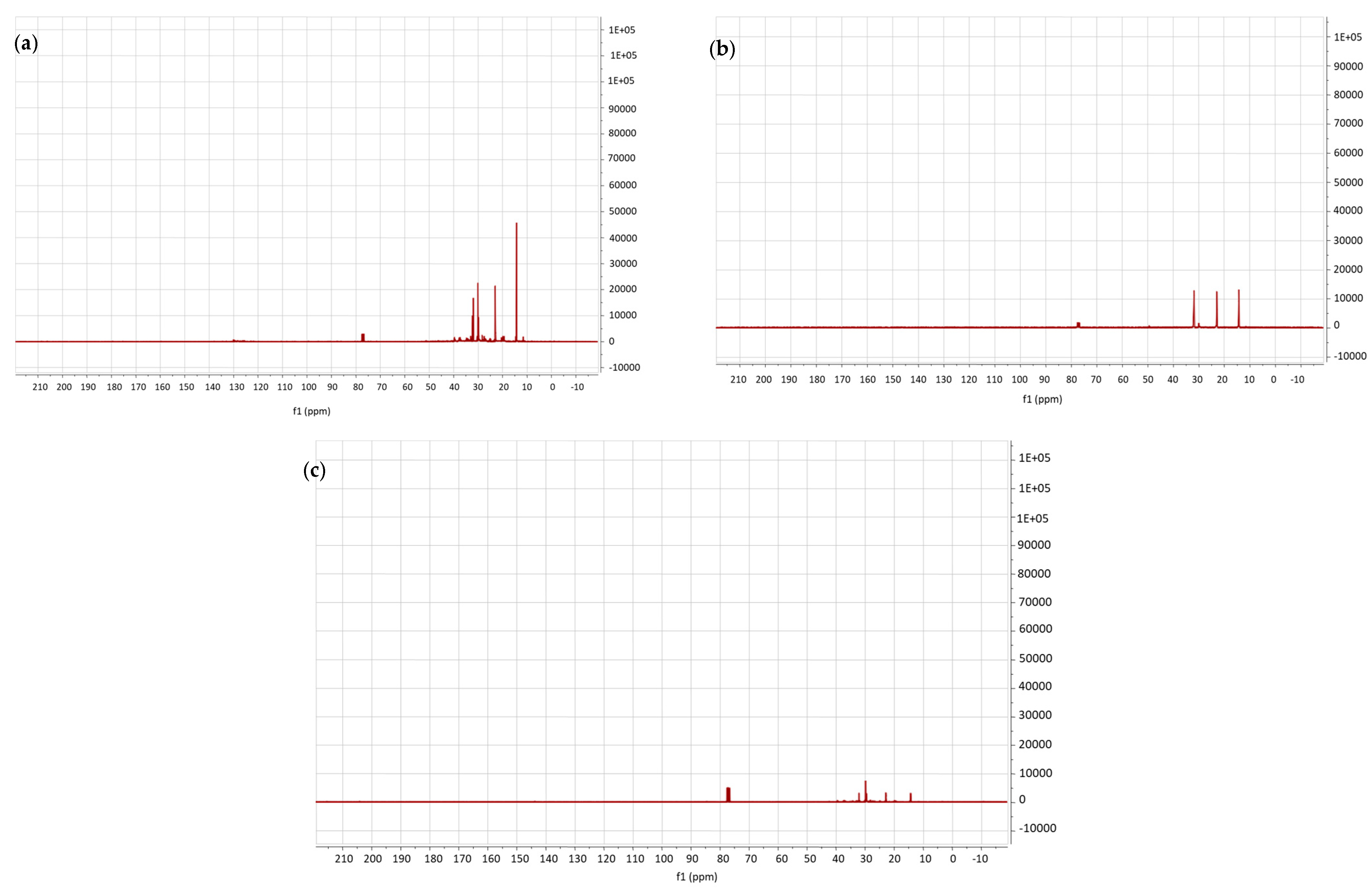
3.3. NMR Characterization
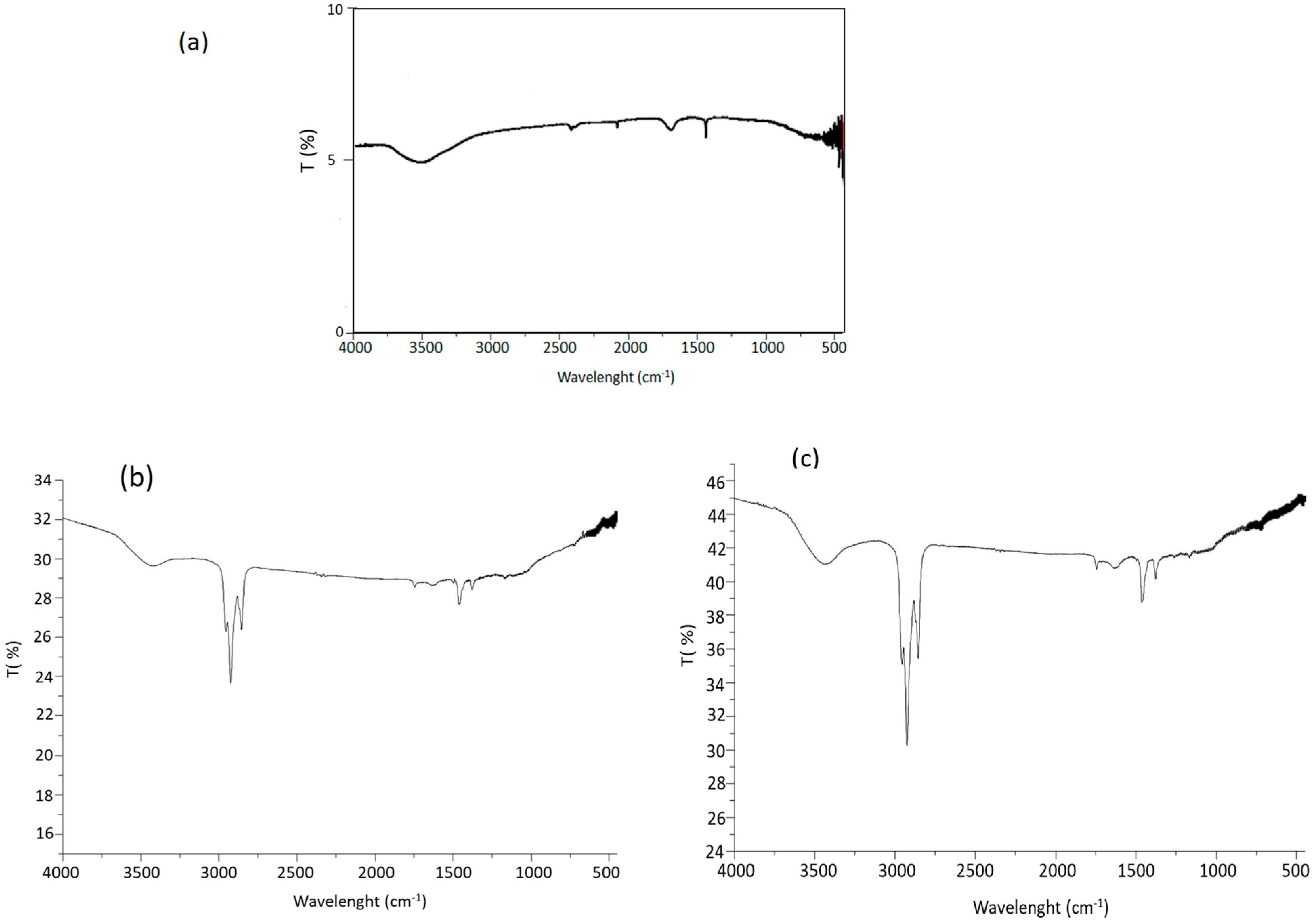
3.4. FT-IR Characterization
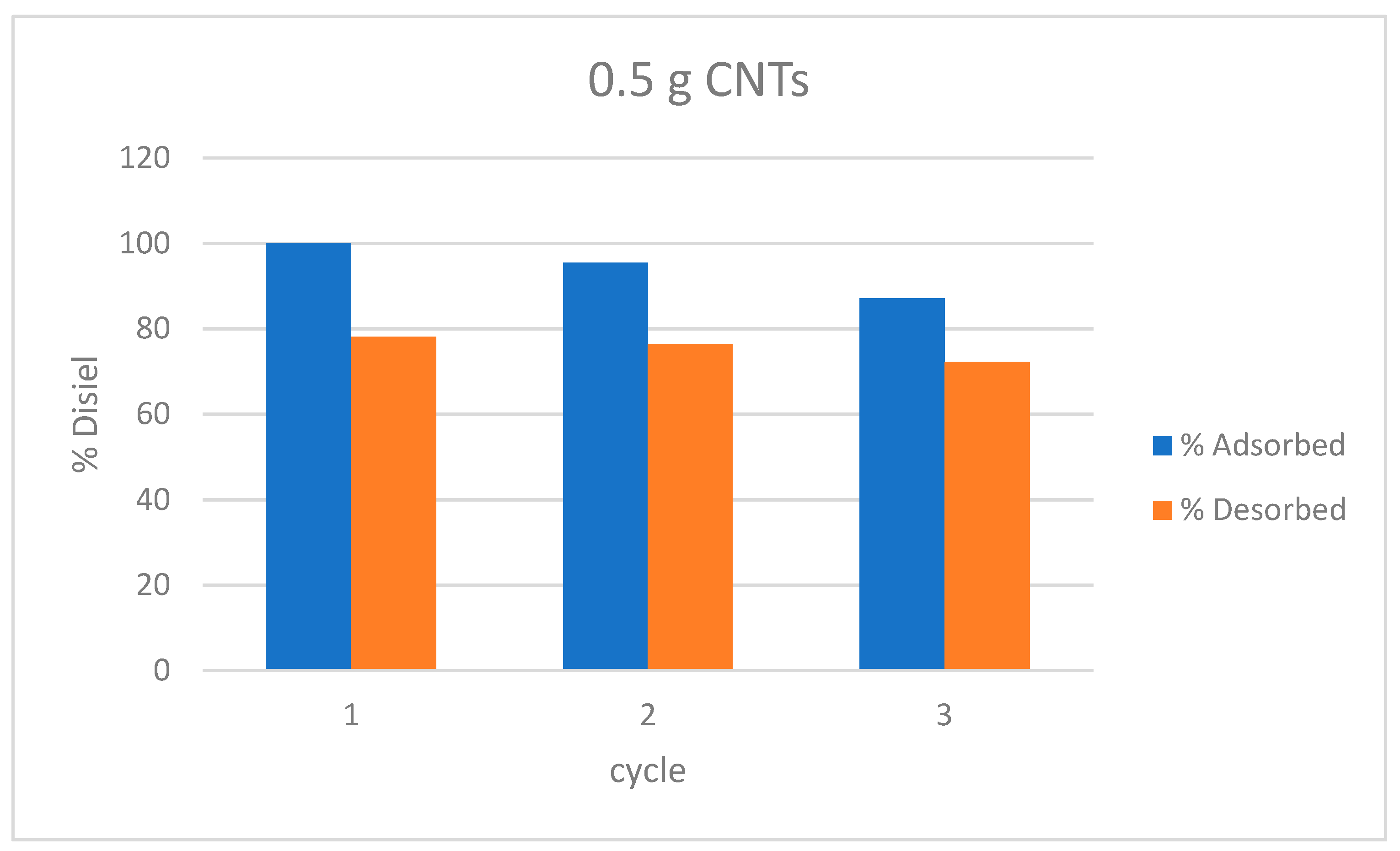
3.5. Adsorption/Desorption Cycles
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Holoubek, I.; Dušek, L.; Sáňka, M.; Hofman, J.; Čupr, P.; Jarkovský, J.; Zbíral, J.; Klánová, J. Soil burdens of persistent organic pollutants—Their levels, fate and risk. Part I. Variation of concentration ranges according to different soil uses and locations. Environ. Pollut. 2009, 157, 3207–3217. [Google Scholar] [CrossRef] [PubMed]
- Grimalt, J.O.; Van Drogge, B.L.; Ribes, A.; Fernández, P.; Appleby, P. Polycyclic aromatic hydrocarbon composition in soils and sediments of high altitude lakes. Environ. Pollut. 2004, 131, 13–24. [Google Scholar] [CrossRef] [PubMed]
- Hilscherová, K.; Dušek, L.; Kubík, V.; Čupr, P.; Hofman, J.; Klánová, J.; Holoubek, I. Redistribution of organic pollutants in river sediments and alluvial soils related to major floods. J. Soils Sediments 2007, 7, 167–177. [Google Scholar] [CrossRef]
- Kunhikrishnan, A.; Bolan, N.S.; Müller, K.; Laurenson, S.; Naidu, R.; Kim, W.-I. The influence of wastewater irrigation on the transformation and bioavailability of heavy metal (loid) s in soil. Adv. Agron. 2012, 115, 215–297. [Google Scholar]
- Karaouzas, I.; Kapetanaki, N.; Mentzafou, A.; Kanellopoulos, T.D.; Skoulikidis, N. Heavy metal contamination status in Greek surface waters: A review with application and evaluation of pollution indices. Chemosphere 2021, 263, 128192. [Google Scholar] [CrossRef]
- Mosquera-Guerra, F.; Trujillo, F.; Parks, D.; Oliveira-da-Costa, M.; Van Damme, P.A.; Echeverría, A.; Franco, N.; Carvajal-Castro, J.D.; Mantilla-Meluk, H.; Marmontel, M. Mercury in populations of river dolphins of the Amazon and Orinoco basins. EcoHealth 2019, 16, 743–758. [Google Scholar] [CrossRef]
- Harrison, J.P.; Sapp, M.; Schratzberger, M.; Osborn, A.M. Interactions Between Microorganisms and Marine Microplastics: A Call for Research. Mar. Technol. Soc. J. 2011, 45, 12–20. [Google Scholar] [CrossRef]
- Mazza, S.; Aiello, D.; Macario, A.; De Luca, P. Vehicular emission: Estimate of air pollutants to guide local political choices. A case study. Environments 2020, 7, 37. [Google Scholar] [CrossRef]
- Schwarze, P.E.; Ovreik, J.; Lag, M.; Refsnes, M.; Nafstad, P.; Hetland, R.B.; Dybing, E. Particulate matter properties and health effects. Consistency of epidemiological and toxicological studies. Hum. Exp. Toxicol. 2006, 25, 559–579. [Google Scholar] [CrossRef]
- Bertazzi, P.A. Air pollution risks to human health. Intern. Emerg. Med. 2013, 8, S31–S38. [Google Scholar]
- Čelechovská, O.; Malota, L.; Zima, S. Entry of heavymetals into food chains: A 20-year comparison study in Northern Moravia (Czech Republic). Acta Vet. Brno 2008, 77, 645–652. [Google Scholar] [CrossRef]
- Kumar, S.; Prasad, S.; Yadav, K.K.; Shrivastava, M.; Gupta, N.; Nagar, S.; Bach, Q.V.; Kamyab, H.; Khan, S.A.; Yadav, S.; et al. Hazardous heavy metals contamination of vegetables and food chain: Role of sustainable remediation approaches—A review. Environ. Res. 2019, 179, 108792. [Google Scholar] [CrossRef]
- Kaldor, J.; Harris, J.A.; Glazer, E.; Glaser, S.; Neutra, R.; Mayberry, R.; Nelson, V.; Robinson, L.; Reed, D. Statistical association between cancer incidence and major-cause mortality, and estimated residential exposure to air emissions from petroleum and chemical plants. Environ. Health Perspect. 1984, 54, 319–332. [Google Scholar] [CrossRef]
- Duruibe, J.O.; Jude, N.E.; Ogwuegbu, M.O.C. Heavy Metal Pollution and Human Biotoxic Effects. Int. J. Phys. Sci. 2007, 2, 112–118. [Google Scholar]
- De Luca, P.; Roberto, B.; Vuono, D.; Siciliano, C.; B.Nagy, J. Preparation and optimization of natural glues based on larice pine resin. IOP Conf. Ser. Mater. Sci. Eng. 2018, 374, 012071. [Google Scholar] [CrossRef]
- Kymalainen, H.R.; Sjoberg, A.M. Flax and hemp fibres as raw materials for thermal insulations. Build. Environ. 2008, 43, 1261–1269. [Google Scholar] [CrossRef]
- Buonocore, G.; De Luca, P. Preparation and Characterization of Insulating Panels from Recycled Polylaminate (Tetra Pak) materials. Sustainability 2022, 14, 6858. [Google Scholar] [CrossRef]
- Bakatovich, A.; Davydenko, N.; Gaspar, F. Thermal insulating plates produced on the basis of vegetable agricultural waste. Energy Build. 2018, 180, 72–82. [Google Scholar] [CrossRef]
- Candamano, S.; Crea, F.; Coppola, L.; De Luca, P.; Coffetti, D. Influence of acrylic latex and pre-treated hemp fibers on cement based mortar properties. Constr. Build. Mater. 2021, 273, 121720. [Google Scholar] [CrossRef]
- Chen, Y.; Zhai, B.; Liang, Y. Enhanced degradation performance of organic dyes removal by semiconductor/MOF/graphene oxide composites under visible light irradiation. Diam. Relat. Mater. 2019, 98, 107508. [Google Scholar] [CrossRef]
- De Luca, P.; De Luca, P.; Candamano, S.; Macario, A.; Crea, F.; B.Nagy, J. Preparation and characterization of plasters with photodegradative action. Buildings 2018, 8, 122. [Google Scholar] [CrossRef]
- Singh, P.; Borthakur, A. A review on biodegradation and photocatalytic degradation of organic pollutants: A bibliometric and comparative analysis. J. Clean. Prod. 2018, 196, 1669–1680. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; B.Nagy, J. Activated ceramic materials with deposition of photocatalytic titano-silicate micro-crystal. Sustain. Chem. 2011, 154, 155–165. [Google Scholar]
- Mamaghani, A.H.; Haghighat, F.; Lee, C.-S. Photocatalytic oxidation technology for indoor environment air purification: The state-of-the-art. Appl. Catal. B Environ. 2017, 203, 247–269. [Google Scholar] [CrossRef]
- De Luca, P.; Foglia, P.; Siciliano, C.; B.Nagy, J.; Macario, A. Water contaminated by industrial textile dye: Study on decolorization process. Environments 2019, 6, 101. [Google Scholar] [CrossRef]
- Derylo-Marczewska, A.; Blachnio, M.; Marczewski, A.W.; Seczkowska, M.; Tarasiuk, B. Phenoxyacid pesticide adsorption on activated carbon–Equilibrium and kinetics. Chemosphere 2019, 214, 349–360. [Google Scholar] [CrossRef]
- De Luca, P.; Mastroianni, C.; B.Nagy, J. Synthesis of self-bonded pellets of ETS-4 phase by new methodology of preparation. IOP Conf. Ser. Mater. Sci. Eng. 2018, 347, 012003. [Google Scholar] [CrossRef]
- Zango, Z.U.; Sambudi, N.S.; Jumbri, K.; Ramli, A.; Hana, N.; Abu, H.; Saad, B.; Nur, M.; Rozaini, H.; Isiyaka, H.A.; et al. An Overview and Evaluation of Highly Porous Adsorbent Materials for Polycyclic Aromatic Hydrocarbons and Phenols Removal from Wastewater. Water 2020, 12, 2921. [Google Scholar] [CrossRef]
- Liu, Y.; Xiao, T.; Baveye, P.C.; Zhu, J.; Ning, Z.; Li, H. Potential health risk in areas with high naturally-occurring cadmium background in southwestern China. Ecotoxicol. Environ. Saf. 2015, 112, 122–131. [Google Scholar] [CrossRef]
- Kelly, J.; Thornton, I.; Simpson, P.R. Urban Geochemistry: A study of the influence of anthropogenic activity on the heavy metal content of soils in traditionally industrial and non-industrial areas of Britain. Appl. Geochem. 1996, 11, 363–370. [Google Scholar] [CrossRef]
- Laidlaw, M.A.S.; Filippelli, G.M.; Brown, S.; Paz-Ferreiro, J.; Reichman, S.M.; Netherway, P.; Truskewycz, A.; Ball, A.S.; Mielke, H.W. Case studies and evidence-based approaches to addressing urban soil lead contamination. Appl. Geochem. 2017, 83, 14–30. [Google Scholar] [CrossRef]
- Adhikari, K.; Pal, S. Assessment of Pollution Potential of Soil and Groundwater in a Non-Engineered MSW Landfill Site. Intern. J. Environ. Sci. Dev. 2016, 7, 207–210. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Giustra, M.G.; Vagliasindi, F.G.A. Influence of soil texture on contaminant adsorption capacity and removal efficiency in ex situ remediation of diesel-polluted soil by thermal desorption. Chem. Ecol. 2011, 27, 119–130. [Google Scholar] [CrossRef]
- Falciglia, P.P.; Giustra, M.G.; Vagliasindi, F.G.A. Low temperature thermal desorption of diesel-polluted soil: Influence of temperature and soil texture on contaminant removal kinetics. J. Hazard. Mater. 2011, 185, 392–400. [Google Scholar] [CrossRef]
- Fernández, M.D.; Pro, J.; Alonso, C.; Aragonese, P.; Tarazona, J.V. Terrestrial microcosms in a feasibility study on the remediation of diesel-contaminated soils. Ecotoxicol. Environ. Saf. 2011, 74, 2133–2140. [Google Scholar] [CrossRef]
- Hewelke, E.; Szatyłowicz, J.; Hewelke, P.; Gnatowski, T.; Aghalarov, R. The Impact of Diesel Oil Pollution on the Hydrophobicity and CO2 Efflux of Forest Soils. Water Air Soil Pollut. 2018, 229, 51. [Google Scholar] [CrossRef]
- García-Borboroglu, P.; Boersma, D.; Reyes, L.M.; Skewgar, E. Petroleum pollution and penguins: Marine conservation tools to reduce the problem. In Marine Pollution: New Research; Hofer, T.N., Ed.; Nova Science Publishers Inc.: New York, NY, USA, 2008; pp. 339–356. [Google Scholar]
- Brown, D.M.; Bonte, M.; Gill, R.; Dawick, J.; Boogaard, P.J. Heavy hydrocarbon fate and transport in the environment. Q. J. Eng. Geol. Hydrogeol. 2017, 50, 333–346. [Google Scholar] [CrossRef]
- Susarla, S.; Medina, V.F.; McCutcheon, S.C. Phytoremediation: An ecological solution to organic chemical contamination. Ecol. Eng. 2002, 18, 647–658. [Google Scholar] [CrossRef]
- Abdulwahab Al-Baldawi, I.; Rozaimah, S.; Abdullah, S.; Anuar, N.; Suja, F.; Mushrifah, I. Phytodegradation of total petroleum hydrocarbon (TPH) in diesel-contaminated water using Scirpus grossus. Ecol. Eng. 2015, 74, 463–473. [Google Scholar] [CrossRef]
- Kozhukhova, E.; Litvinova, M.; Makarevich, E.; Malaeva, A. Biodegradation of petroleum hydrocarbons by bioflocculant-producing microorganisms of the aquatic ecosystems in the Arctic region. IOP Conf. Ser. Earth Environ. Sci. 2020, 539, 012192. [Google Scholar] [CrossRef]
- Nath, S.; Deb, B.; Sharma, I. Isolation and characterization of cadmium and lead resistant bacteria. Glob. Adv. Res. J. Microbiol. 2012, 1, 194–198. [Google Scholar]
- Patel, V.; Shah, K. Petroleum hydrocarbon pollution and its biodegradation. Int. J. ChemTech Appl. 2014, 2, 63–80. [Google Scholar]
- Dave, D.; Ghaly, A.E. Remediation technologies for marine oil spills: A critical review and comparative analysis. Am. J. Environ. Sci. 2011, 7, 423–440. [Google Scholar] [CrossRef]
- Karpenko, O.; Lubenets, V.; Karpenko, E.; Novikov, V. Chemical oxidants for remediation of contaminated soil and water. A review. Chem. Chem. Technol. 2008, 3, 41–45. [Google Scholar] [CrossRef]
- Kapsalis, K.; Kavvalou, M.; Damikouka, I.; Cavoura, O. Investigation of petroleum hydrocarbon pollution along the coastline of South Attica, Greece, after the sinking of the Agia Zoni ΙΙ oil tanker. SN Appl. Sci. 2021, 3, 48. [Google Scholar] [CrossRef]
- Simate, G.S.; Maledi, N.; Ochieng, A.; Ndlovu, S.; Walubita, L.F. Coal-based adsorbents for water and wastewater treatment. J. Environ. Chem. Eng. 2016, 4, 2291–2312. [Google Scholar] [CrossRef]
- Zhang, S.; Shao, T.; Kose, H.S.; Karanfil, T. Adsorption of aromatic compounds by carbonaceous adsorbents: A comparative study on granular activated carbon, activated carbon fiber, and carbon nanotubes. Environ. Sci. Technol. 2010, 44, 6377–6383. [Google Scholar] [CrossRef]
- De Luca, P.; Bernaudo, I.; Elliani, R.; Tagarelli, A.; B.Nagy, J.; Macario, A. Industrial Waste Treatment by ETS-10 Ion Exchanger Material. Materials 2018, 11, 2316. [Google Scholar] [CrossRef]
- De Luca, P.; Poulsen, T.G.; Salituro, A.; Madaràsz, D.; B.Nagy, J. Evaluation and comparison of the ammonia adsorption capacity of titanosilicates ETS-4 and ETS-10 and aluminotitanosilicates ETAS-4 and ETAS-10. J. Therm. Anal. Calorim. 2015, 122, 1257–1267. [Google Scholar] [CrossRef]
- De Luca, P.; Candamano, S. Carbon nanotubes and Engelhard titanium silicates as eco-friendly adsorbent materials: A short review. J. Phys. Conf. Ser. 2021, 1960, 012005. [Google Scholar] [CrossRef]
- Das, S.K.; Ghosh, G.K.; Avasthe, R.K.; Sinha, K. Compositional heterogeneity of different biochar: Effect of pyrolysis temperature and feedstocks. J. Environ. Manag. 2021, 27, 111501. [Google Scholar] [CrossRef]
- Tian, R.; Li, C.; Xie, S.; You, F.; Cao, Z.; Xu, Z.; Yu, G.; Wang, Y. Preparation of biochar via pyrolysis at laboratory and pilot scales to remove antibiotics and immobilize heavy metals in livestock feces. J. Soils Sediments 2019, 19, 2891–2902. [Google Scholar] [CrossRef]
- Rinaudo, M. Chitin and chitosan: Properties and applications. Prog. Polym. Sci. 2006, 31, 603–632. [Google Scholar] [CrossRef]
- Tian, X.; Song, Y.; Shen, Z.; Zhou, Y.; Wang, K.; Jin, X.; Han, Z.; Liu, T. A comprehensive review on toxic petrochemical wastewater pretreatment and advanced treatment. J. Clean. Prod. 2020, 245, 118692. [Google Scholar] [CrossRef]
- Mohan, D.; Singh, K.P. Single- and multi-component adsorption of cadmium and zinc using activated carbon derived from bagasse—An agricultural waste. Water Res. 2002, 36, 2304–2318. [Google Scholar] [CrossRef]
- Apul, O.G.; Karanfil, T. Adsorption of synthetic organic contaminants by carbon nanotubes: A critical review. Water Res. 2015, 68, 34–55. [Google Scholar] [CrossRef]
- Chen, W.; Duan, L.; Zhu, D. Adsorption of polar and nonpolar organic chemicals to carbon nanotubes. Environ. Sci. Technol. 2007, 41, 8295–8300. [Google Scholar] [CrossRef]
- Cho, H.H.; Smith, B.A.; Wnuk, J.D.; Fairbrother, D.H.; Ball, W.P. Influence of surface oxides on the adsorption of naphthalene onto multiwalled carbon nanotubes. Environ. Sci. Technol. 2008, 42, 2899–2905. [Google Scholar] [CrossRef]
- Fard, A.K.; Rhadfi, T.; Mckay, G.; Al-marri, M.; Abdala, A.; Hilal, N.; Hussein, M.A. Enhancing oil removal from water using ferric oxide nanoparticles doped carbon nanotubes adsorbents. Chem. Eng. J. 2016, 293, 90–101. [Google Scholar] [CrossRef]
- Policicchio, A.; Vuono, D.; Rugiero, T.; De Luca, P.; B.Nagy, J. Study of MWCNTs adsorption perfomances in gas processes. J. CO2 Util. 2015, 10, 30–39. [Google Scholar] [CrossRef]
- De Benedetto, C.; Macario, A.; Siciliano, C.; B.Nagy, J.; De Luca, P. Adsorption of reactive blue 116 dye and reactive yellow 81 dye from aqueous solutions by multi-walled carbon nanotubes. Materials 2020, 13, 2757. [Google Scholar] [CrossRef] [PubMed]
- Iijima, S.; Ichihashi, T. Single-shell carbon nanotubes of 1-nm diameter. Nature 1993, 363, 603. [Google Scholar] [CrossRef]
- Zhang, Y.; Shi, Z.; Gu, Z.; Iijima, S. Structure modification of single-wall carbon nanotubes. Carbon 2000, 38, 2055–2059. [Google Scholar] [CrossRef]
- Iijima, S. Helical microtubules of graphitic carbon. Nature 1991, 354, 56. [Google Scholar] [CrossRef]
- Popov, V.N. Carbon nanotubes: Properties and application. Mater. Sci. Eng. 2004, 43, 61–102. [Google Scholar] [CrossRef]
- Baughman, R.H.; Zakhidov, A.A.; de Heer, W.A. Carbon nanotubes-the route toward applications. Science 2002, 297, 787–792. [Google Scholar] [CrossRef]
- Lico, D.; Vuono, D.; Siciliano, C.; B.Nagy, J.; De Luca, P. Removal on unleaded gasoline from water by multi-walled carbon nanotubes. J. Environ. Manag. 2019, 237, 636–643. [Google Scholar] [CrossRef]
- Gotovac, S.; Yang, C.M.; Hattori, Y.; Takahashi, K.; Kanoh, H.; Kaneko, K. Adsorption of polyaromatic hydrocarbons on single wall carbon nanotubes of different functionalities and diameters. J. Colloid Interf. Sci. 2007, 314, 18–24. [Google Scholar] [CrossRef]
- Li, Y.H.; Zhao, Y.M.; Hu, W.B.; Ahmad, I.; Zhu, Y.Q.; Peng, X.J.; Luan, Z.K. Carbon nanotubes-the promising adsorbent in wastewater treatment. J. Phys. Conf. Ser. 2007, 61, 698. [Google Scholar] [CrossRef]
- Yang, K.; Zhu, L.; Xing, B. Adsorption of Polycyclic Aromatic Hydrocarbons by Carbon Nanomaterials. Environ. Sci. Technol. 2006, 40, 1855–1861. [Google Scholar] [CrossRef]
- Sajid, M.; Asif, M.; Baig, N.; Kabeer, M.; Ihsanullah, I.; Mohammad, A.W. Carbon nanotubes-based adsorbents: Properties, functionalization, interaction mechanisms, and applications in water purification. J. Water Process Eng. 2022, 47, 102815. [Google Scholar] [CrossRef]
- Sarıkoç, S. Fuels of the Diesel-Gasoline Engines and Their Properties. In Diesel and Gasoline Engines; Viskup, R., Ed.; Intechopen: London, UK, 2020. [Google Scholar] [CrossRef]
- De Luca, P.; Chiodo, A.; Macario, A.; Siciliano, C.; B.Nagy, J. Semi-continuous adsorption processes with multi-walled carbon nanotubes for the treatment of water contaminated by an organic textile dye. Appl. Sci. 2021, 11, 1687. [Google Scholar] [CrossRef]
- De Luca, P.; Macario, A.; Siciliano, C.; B.Nagy, J. Recovery of Biophenols from Olive Vegetation Waters by Carbon Nanotubes. Materials 2022, 15, 2893. [Google Scholar] [CrossRef]
- De Luca, P.; Siciliano, C.; Macario, A.; B.Nagy, J. The role of carbon nanotube pretreatments in the adsorption of benzoic acid. Materials 2021, 14, 2118. [Google Scholar] [CrossRef] [PubMed]

| Experimental Parameters | |
|---|---|
| Water [mL] | 40 |
| Diesel [mL] | 10 |
| Rotation speed [rpm] | 350 |
| Carbon Nanotubes [g] | 0.3; 0.5; 0.7; 1 |
| Adsorption time [min] | 2.5; 5; 15; 30; 45; 60; 120 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
De Luca, P.; Siciliano, C.; B.Nagy, J.; Macario, A. Treatment of Water Contaminated with Diesel Using Carbon Nanotubes. Appl. Sci. 2023, 13, 6226. https://doi.org/10.3390/app13106226
De Luca P, Siciliano C, B.Nagy J, Macario A. Treatment of Water Contaminated with Diesel Using Carbon Nanotubes. Applied Sciences. 2023; 13(10):6226. https://doi.org/10.3390/app13106226
Chicago/Turabian StyleDe Luca, Pierantonio, Carlo Siciliano, Janos B.Nagy, and Anastasia Macario. 2023. "Treatment of Water Contaminated with Diesel Using Carbon Nanotubes" Applied Sciences 13, no. 10: 6226. https://doi.org/10.3390/app13106226
APA StyleDe Luca, P., Siciliano, C., B.Nagy, J., & Macario, A. (2023). Treatment of Water Contaminated with Diesel Using Carbon Nanotubes. Applied Sciences, 13(10), 6226. https://doi.org/10.3390/app13106226









