Transport Properties of Intergrowth Structures Ba5In2Al2ZrO13 and Ba7In6Al2O19
Abstract
1. Introduction
2. Materials and Methods
3. Results and Discussion
3.1. Phase Analysis
3.2. Oxygen-Hydrogen Groups State
3.3. Hydration Behaviour
3.4. Transport Properties
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kavitha, K.; Anuradha, M.A. Review of proton- and oxide-ion-conducting perovskite materials for SOFC applications. Nanomater. Energy 2019, 8, 51–58. [Google Scholar] [CrossRef]
- Sun, C.; Alonso, J.A.; Bian, J. Recent Advances in Perovskite-Type Oxides for Energy Conversion and Storage Applications. Adv. Energy Mater. 2021, 11, 2000459. [Google Scholar] [CrossRef]
- Maiti, T.; Saxena, M.; Roy, P. Double perovskite (Sr2B′B″O6) oxides for high-temperature thermoelectric power generation—A review. J. Mater. Res. 2019, 34, 107–125. [Google Scholar] [CrossRef]
- Žužić, A.; Filipan, V.; Sutlović, I.; Macan, J. Perovskite Oxides for Energy Applications. Tehnički Vjesnik 2022, 29, 1419–1425. [Google Scholar] [CrossRef]
- Kubicek, M.; Bork, A.H.; Rupp, J.L.M. Perovskite oxides—A review on a versatile material class for solar-to-fuel conversion processes. J. Mater. Chem. A 2017, 5, 11983–12000. [Google Scholar] [CrossRef]
- Danilov, N.; Lyagaeva, J.; Vdovin, G.; Medvedev, D. Multifactor performance analysis of reversible solid oxide cells based on proton-conducting electrolytes. Appl. Energy 2019, 237, 924–934. [Google Scholar] [CrossRef]
- He, H.; Yang, Z.; Xu, Y.; Smith, A.T.; Yang, G.; Sun, L. Perovskite oxides as transparent semiconductors: A review. Perovskite oxides as transparent semiconductors: A review. Nano Converg. 2020, 7, 32. [Google Scholar] [CrossRef]
- Tarutin, A.; Kasyanova, A.; Lyagaeva, J.; Vdovin, G.; Medvedev, D. Towards high-performance tubular-type protonic ceramic electrolysis cells with all-Ni-based functional electrodes. J. Energy Chem. 2020, 40, 65–74. [Google Scholar] [CrossRef]
- Mahmoudi, F.; Saravanakumar, K.; Maheskumar, V.; Njaramba, L.K.; Yoon, Y.; Park, C.M. Application of perovskite oxides and their composites for degrading organic pollutants from wastewater using advanced oxidation processes: Review of the recent progress. J. Hazard. Mater. 2022, 436, 129074. [Google Scholar] [CrossRef]
- Colomban, P. Proton conductors and their applications: A tentative historical overview of the early researches. Solid State Ion. 2019, 334, 125–144. [Google Scholar] [CrossRef]
- Vera, C.Y.R.; Hanping Peterson, D.D.; Gibbons, W.T.; Zhou, M.; Ding, D. A mini-review on proton conduction of BaZrO3-based perovskite electrolytes. J. Phys. Energy 2021, 3, 032019. [Google Scholar] [CrossRef]
- Zhang, M.; Jeerh, G.; Zou, P.; Lan, R.; Wang, M.; Wang, H.; Tao, S. Recent development of perovskite oxide-based electrocatalysts and their applications in low to intermediate temperature electrochemical devices. Mater. Today 2021, 49, 351–377. [Google Scholar] [CrossRef]
- Skinner, S.J.; Kilner, J.A. Oxygen ion conductors. Mater. Today 2003, 6, 30–37. [Google Scholar] [CrossRef]
- Malavasi, L.; Fisher, C.A.; Islam, M.S. Oxide-ion and proton conducting electrolyte materials for clean energy applications: Structural and mechanistic features. Chem. Soc. Rev. 2010, 39, 4370–4387. [Google Scholar] [CrossRef]
- Kharton, V.V. Solid State Electrochemistry I: Fundamentals, Materials and Their Applications; Wiley-VCH: Weinheim, Germany, 2009. [Google Scholar]
- Meng, Y.; Gao, J.; Zhao, Z.; Amoroso, J.; Tong, J.; Brinkman, K.S. Review: Recent progress in low–temperature proton–conducting ceramics. J. Mater. Sci. 2019, 54, 9291–9312. [Google Scholar] [CrossRef]
- Cao, J.; Ji, Y.; Shao, Z. Perovskites for protonic ceramic fuel cells: A review. Energy Environ. Sci. 2022, 15, 2200–2232. [Google Scholar] [CrossRef]
- Coors, W.G. Protonic ceramic fuel cells for high-efficiency operation with methane. J. Power Sources 2003, 118, 150–156. [Google Scholar] [CrossRef]
- Medvedev, D.A.; Lyagaeva, J.G.; Gorbova, E.V.; Demin, A.K.; Tsiakaras, P. Advanced materials for SOFC application: Strategies for the development of highly conductive and stable solid oxide proton electrolytes. Prog. Mater. Sci. 2016, 75, 38–79. [Google Scholar] [CrossRef]
- Medvedev, D.A. Current drawbacks of proton–conducting ceramic materials: How to overcome them for real electrochemical purposes. Curr. Opin. Green Sustain. Chem. 2021, 32, 100549. [Google Scholar] [CrossRef]
- Kochetova, N.; Animitsa, I.; Medvedev, D.; Demin, A.; Tsiakaras, P. Recent activity in the development of proton-conducting oxides for high-temperature applications. RSC Adv. 2016, 6, 73222–73268. [Google Scholar] [CrossRef]
- Kasyanova, A.V.; Radenko, A.O.; Lyagaeva, Y.G.; Medvedev, D.A. Lanthanum-Containing Proton-Conducting Electrolytes with Perovskite Structures. Membr. Membr. Technol. 2021, 3, 73–97. [Google Scholar] [CrossRef]
- Haugsrud, R.; Norby, T. High-temperature proton conductivity in acceptor-substituted rare-earth ortho-tantalates, LnTaO4. J. Am. Ceram. Soc. 2007, 90, 1116–1121. [Google Scholar] [CrossRef]
- Fjeld, H.; Kepaptsoglou, D.M.; Haugsrud, R.; Norby, T. Charge carriers in grain boundaries of 0.5% Sr-doped LaNbO4. Solid State Ion. 2010, 181, 104–109. [Google Scholar] [CrossRef]
- Magraso, A.; Fontaine, M.L.; Larring, Y.; Bredesen, R.; Syvertsen, G.E.; Lein, H.L.; Grande, T.; Huse, M.; Strandbakke, R.; Haugsrud, R. Development of proton conducting SOFCs based on LaNbO4 electrolyte—Status in Norway. Fuel Cells 2011, 11, 17–25. [Google Scholar] [CrossRef]
- Vigen, C.K.; Haugsrud, R. Proton Conductivity in Solid Solution 0.7(CaWO4)–0.3(La0.99Ca0.01NbO4) and Ca(1− x)LaxW(1− y)TayO4. J. Am. Ceram. Soc. 2013, 96, 3812–3820. [Google Scholar] [CrossRef]
- Bi, L.; Fabbri, E.; Traversa, E. Solid oxide fuel cells with proton–conducting La0.99Ca0.01NbO4 electrolyte. Electrochim. Acta 2018, 260, 748–754. [Google Scholar] [CrossRef]
- Hakimova, L.; Kasyanova, A.; Farlenkov, A.; Lyagaeva, J.; Medvedev, D.; Demin, A.; Tsiakaras, P. Effect of isovalent substitution of La3+ in Ca–doped LaNbO4 on the thermal and electrical properties. Ceram. Int. 2019, 45, 209–215. [Google Scholar] [CrossRef]
- Afif, A.; Zaini, J.; Rahman, S.M.H.; Eriksson, S.; Islam, M.A.; Azad, A.K. Scheelite type Sr1−xBaxWO4 (x = 0.1, 0.2, 0.3) for possible application in Solid Oxide Fuel Cell electrolytes. Sci. Rep. 2019, 9, 9173. [Google Scholar] [CrossRef] [PubMed]
- Omata, T.; Ikeda, K.; Tokashiki, R.; Otsuka-Yao-Matsuo, S. Proton solubility for La2Zr2O7 with a pyrochlore structure doped with a series of alkaline–earth ions. Solid State Ion. 2004, 167, 389–397. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Pigalskiy, K.S.; Belov, D.A.; Lyskov, N.V.; Kharitonova, E.P.; Kolbanev, I.V.; Eremeev, N.F. Proton and oxygen ion conductivity in the pyrochlore/fluorite family of Ln2− xCaxScMO7− δ (Ln = La, Sm, Ho, Yb; M = Nb, Ta; x = 0, 0.05, 0.1) niobates and tantalates. Dalton Trans. 2018, 47, 2376–2392. [Google Scholar] [CrossRef] [PubMed]
- Shlyakhtina, A.V.; Lyskov, N.V.; Shchegolikhin, A.N.; Chernyak, S.A.; Knotko, A.V.; Kolbanev, I.V.; Shcherbakova, L.G. Structure evolution, ionic and proton conductivity of solid solutions based on Nd2Hf2O7. Ceram. Intern. 2020, 46, 17383–17391. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Abrantes, J.C.C.; Gomes, E.; Lyskov, N.V.; Konysheva, E.Y.; Chernyak, S.A.; Kharitonova, E.P.; Karyagina, O.K.; Kolbanev, I.V.; Shcherbakova, L.G. Evolution of oxygen–ion and proton conductivity in Ca doped Ln2Zr2O7 (Ln = Sm, Gd), located near pyrochlore-fluorite phase boundary. Materials 2019, 12, 2452. [Google Scholar] [CrossRef] [PubMed]
- Shlyakhtina, A.V.; Lyskov, N.V.; Konysheva, E.Y.; Chernyak, S.A.; Kolbanev, I.V.; Vorobieva, G.A.; Shcherbakova, L.G. Gas–tight proton–conducting Nd2−xCaxZr2O7−δ (x = 0, 0.05) ceramics. J. Solid State Electrochem. 2020, 24, 1475–1486. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Lyskov, N.V.; Nikiforova, G.E.; Kasyanova, A.V.; Vorobieva, G.A.; Kolbanev, I.V.; Stolbov, D.N.; Medvedev, D.A. Proton Conductivity of La2(Hf2−xLax)O7−x/2 “Stuffed” Pyrochlores. Appl. Sci. 2022, 12, 4342. [Google Scholar] [CrossRef]
- Tarasova, N.; Animitsa, I. Materials AIILnInO4 with Ruddlesden–Popper Structure for Electrochemical Applications: Relationship between Ion (Oxygen–Ion, Proton) Conductivity, Water Uptake, and Structural Changes. Materials 2022, 15, 114. [Google Scholar] [CrossRef] [PubMed]
- Nirala, G.; Yadav, D.; Upadhyay, S. Ruddlesden–Popper phase A2BO4 oxides: Recent studies on structure, electrical, dielectric, and optical properties. J. Adv. Ceram. 2020, 9, 29–148. [Google Scholar] [CrossRef]
- Zhou, Y.; Shiraiwa, M.; Nagao, M.; Fujii, K.; Tanaka, I.; Yashima, M.; Baque, L.; Basbus, J.F.; Mogni, L.V.; Skinner, S.J. Protonic Conduction in the BaNdInO4 Structure Achieved by Acceptor Doping. Chem. Mater. 2021, 33, 2139–2146. [Google Scholar] [CrossRef]
- León-Reina, L.; Porras-Vázquez, J.M.; Losilla, E.R.; Aranda, M.A. Phase transition and mixed oxide-proton conductivity in germanium oxy-apatites. J. Solid State Chem. 2007, 180, 1250–1258. [Google Scholar] [CrossRef]
- Panchmatia, P.M.; Orera, A.; Kendrick, E.; Hanna, J.V.; Smith, M.E.; Slater, P.R.; Islam, M.S. Protonic defects and water incorporation in Si and Ge-based apatite ionic conductors. J. Mater. Chem. 2010, 20, 2766–2772. [Google Scholar] [CrossRef]
- Yashima, M.; Kubo, N.; Omoto, K.; Fujimori, H.; Fujii, K.; Ohoyama, K. Diffusion path and conduction mechanism of protons in hydroxyapatite. J. Phys. Chem. 2014, 118, 5180–5187. [Google Scholar] [CrossRef]
- Haugsrud, R.; Kjølseth, C. Effects of protons and acceptor substitution on the electrical conductivity of La6WO12. J. Phys. Chem. Solids 2008, 69, 1758–1765. [Google Scholar] [CrossRef]
- Amsif, M.; Magrasó, A.; Marrero-López, D.; Ruiz-Morales, J.C.; Canales-Vázquez, J.; Núñez, P. Mo-Substituted lanthanum tungstate La28–yW4+yO54+δ: A competitive mixed electron–proton conductor for gas separation membrane applications. Chem. Mater. 2012, 24, 3868–3877. [Google Scholar] [CrossRef]
- Quarez, E.; Kravchyk, K.V.; Joubert, O. Compatibility of proton conducting La6WO12 electrolyte with standard cathode materials. Solid State Ion. 2012, 216, 19–24. [Google Scholar] [CrossRef]
- Seeger, J.; Ivanova, M.E.; Meulenberg, W.A.; Sebold, D.; Stöver, D.; Scherb, T.; Schumacher, G.; Escolástico, S.; Solís, C.; Serra, J.M. Synthesis and characterization of nonsubstituted and substituted proton-conducting La6-xWO12-y. Inorg. Chem. 2013, 52, 10375–10386. [Google Scholar] [CrossRef]
- Korona, D.V.; Partin, G.S.; Animitsa, I.E.; Sharafutdinov, A.R. Chemical CO2–resistivity of proton conductors on base of Ba4Ca2Nb2O11 and La6WO12. Altern. Energy Ecol. 2018, 10–12, 43–59. [Google Scholar] [CrossRef]
- Shlyakhtina, A.V.; Avdeev, M.; Abrantes, J.C.C.; Gomes, E.; Lyskov, N.V.; Kharitonova, E.P.; Kolbaneva, I.V.; Shcherbakova, L.G. Structure and conductivity of Nd6MoO12-based potential electron–proton conductors under dry and wet redox conditions. Inorg. Chem. Front. 2019, 6, 566–575. [Google Scholar] [CrossRef]
- Sadykov, V.A.; Bespalko, Y.N.; Krasnov, A.V.; Skriabin, P.I.; Lukashevich, A.I.; Fedorova, Y.E.; Sadovskaya, E.M.; Eremeev, N.F.; Krieger, T.A.; Ishchenko, A.V.; et al. Novel proton-conducting nanocomposites for hydrogen separation membranes. Solid State Ion. 2018, 322, 69–78. [Google Scholar] [CrossRef]
- Münch, W.; Seifert, G.; Kreuer, K.D.; Maier, J. A quantum molecular dynamics study of proton conduction phenomena in BaCeO3. Solid State Ion. 1996, 86, 647–652. [Google Scholar] [CrossRef]
- Kendrick, E.; Kendrick, J.; Knight, K.S.; Islam, M.S.; Slater, P.R. Cooperative mechanisms of fast-ion conduction in gallium-based oxides with tetrahedral moieties. Nat. Mater. 2007, 6, 871–875. [Google Scholar] [CrossRef] [PubMed]
- Mather, G.C.; Fisher, C.A.J.; Islam, M.S. Defects, dopants, and protons in LaNbO4. Chem. Mater. 2010, 22, 5912–5917. [Google Scholar] [CrossRef]
- Kendrick, E.; Kendrick, J.; Orera, A.; Panchmatia, P.; Islam, M.S.; Slater, P.R. Novel Aspects of the Conduction Mechanisms of Electrolytes Containing Tetrahedral Moieties. Fuel Cells 2011, 11, 38–43. [Google Scholar] [CrossRef]
- Ferrara, C.; Eames, C.; Islam, M.S.; Tealdi, C. Lattice strain effects on doping, hydration and proton transport in scheelite-type electrolytes for solid oxide fuel cells. Phys. Chem. Chem. Phys. 2016, 18, 29330–29336. [Google Scholar] [CrossRef] [PubMed]
- Fop, S.; McCombie, K.S.; Wildman, E.J.; Skakle, J.M.S.; Irvine, J.T.S.; Connor, P.A.; Savaniu, C.; Ritter, C.; Mclaughlin, A.C. High Oxide Ion and Proton Conductivity in a Disordered Hexagonal Perovskite. Nat. Mater. 2020, 19, 752–757. [Google Scholar] [CrossRef]
- Murakami, T.; Hester, J.; Yashima, M. High Proton Conductivity in Ba5Er2Al2ZrO13, a Hexagonal Perovskite-Related Oxide with Intrinsically Oxygen-Deficient Layers. J. Am. Chem. Soc. 2020, 142, 11653–11657. [Google Scholar] [CrossRef] [PubMed]
- Andreev, R.; Korona, D.; Anokhina, I.; Animitsa, I. Proton and Oxygen-Ion Conductivities of Hexagonal Perovskite Ba5In2Al2ZrO13. Materials 2022, 15, 3944. [Google Scholar] [CrossRef]
- Andreev, R.D.; Korona, D.V.; Anokhina, I.A.; Animitsa, I.E. Novel Nb5+–doped hexagonal perovskite Ba5In2Al2ZrO13 (structure, hydration, electrical conductivity). Chim. Techno Acta 2022, 9, 20229414. [Google Scholar] [CrossRef]
- Shpanchenko, R.; Abakumov, A.; Antipov, E.; Kovba, L. Crystal structure of Ba5In2Al2ZrO13. J. Alloys Compd. 1994, 206, 185–188. [Google Scholar] [CrossRef]
- Shpanchenko, R.; Abakumov, A.; Antipov, E.; Nistor, L.; van Tendeloo, G.; Amelinckx, S. Structural study of the new complex oxides Ba5-ySryR2-xAl2Zr1+xO13+x/2 (R = Gd-Lu, Y, Sc). J. Solid State Chem. 1995, 118, 180–192. [Google Scholar] [CrossRef]
- Shpanchenko, R.V.; Antipov, E.V.; Paromova, M.V.; Kovba, L.M. Crystal structure of Ba7Sc6Al2O19. J. Inorg. Chem. 1991, 36, 1402–1407. [Google Scholar]
- Shpanchenko, R.V.; Nistor, L.; van Tendeloo, G.; Amelinckx, S.; Antipov, E.V.; Kovba, L.M. High–resolution electron microscopic study of Ba7Sc6Al2O19 and related phases. J. Solid State Chem. 1994, 113, 193–204. [Google Scholar] [CrossRef]
- Irvine, J.; Sinclair, D.; West, A. Electroceramics: Characterization by Impedance Spectroscopy. Adv. Mater. 1990, 2, 132–138. [Google Scholar] [CrossRef]
- Su, C.; Wang, W.; Shao, Z. Cation-Deficient Perovskites for Clean Energy Conversion. Acc. Mater. Res. 2021, 2, 477–488. [Google Scholar] [CrossRef]
- Kovalevsky, A.V.; Yaremchenko, A.A.; Populoh, S.; Weidenkaff, A.; Frade, J.R. Effect of A Site Cation Deficiency on the Thermoelectric Performance of Donor-Substituted Strontium Titanate. J. Phys. Chem. C 2014, 118, 4596–4606. [Google Scholar] [CrossRef]
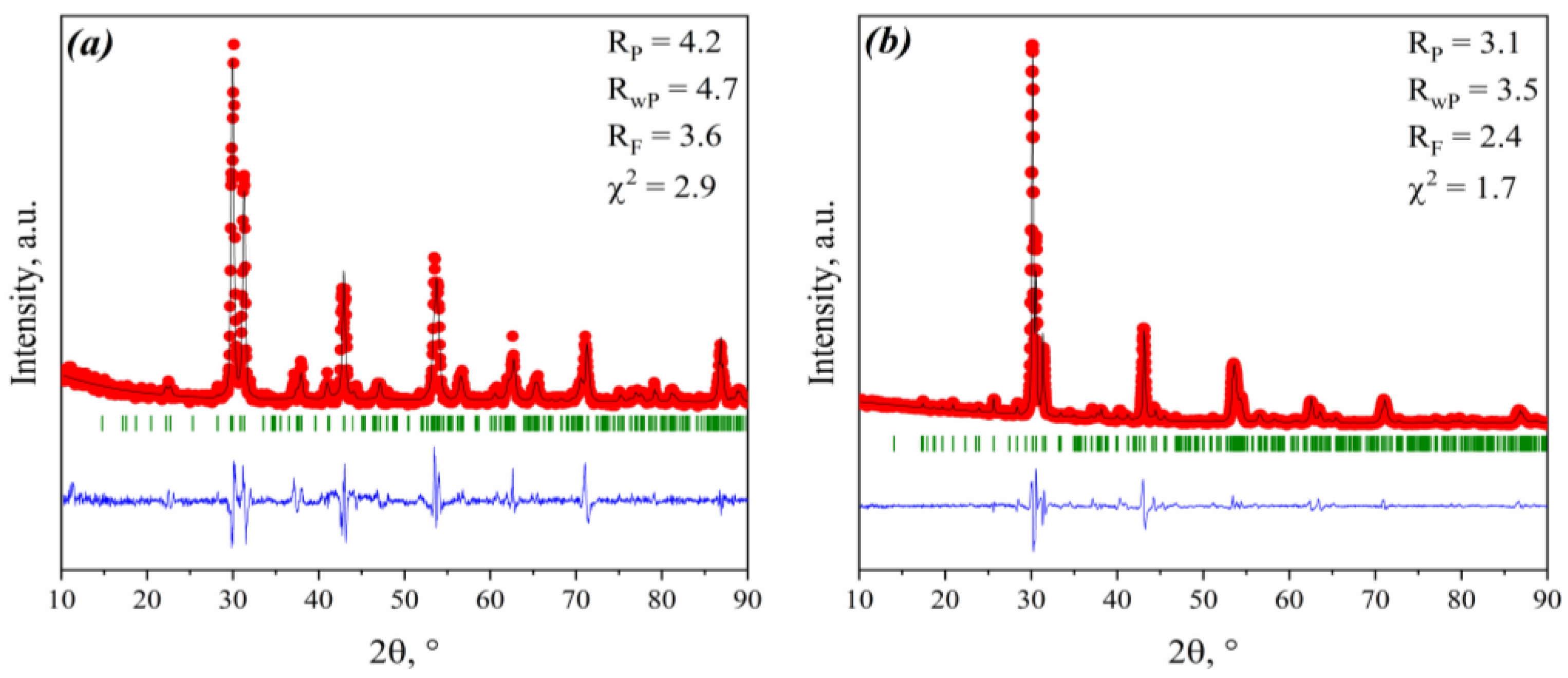



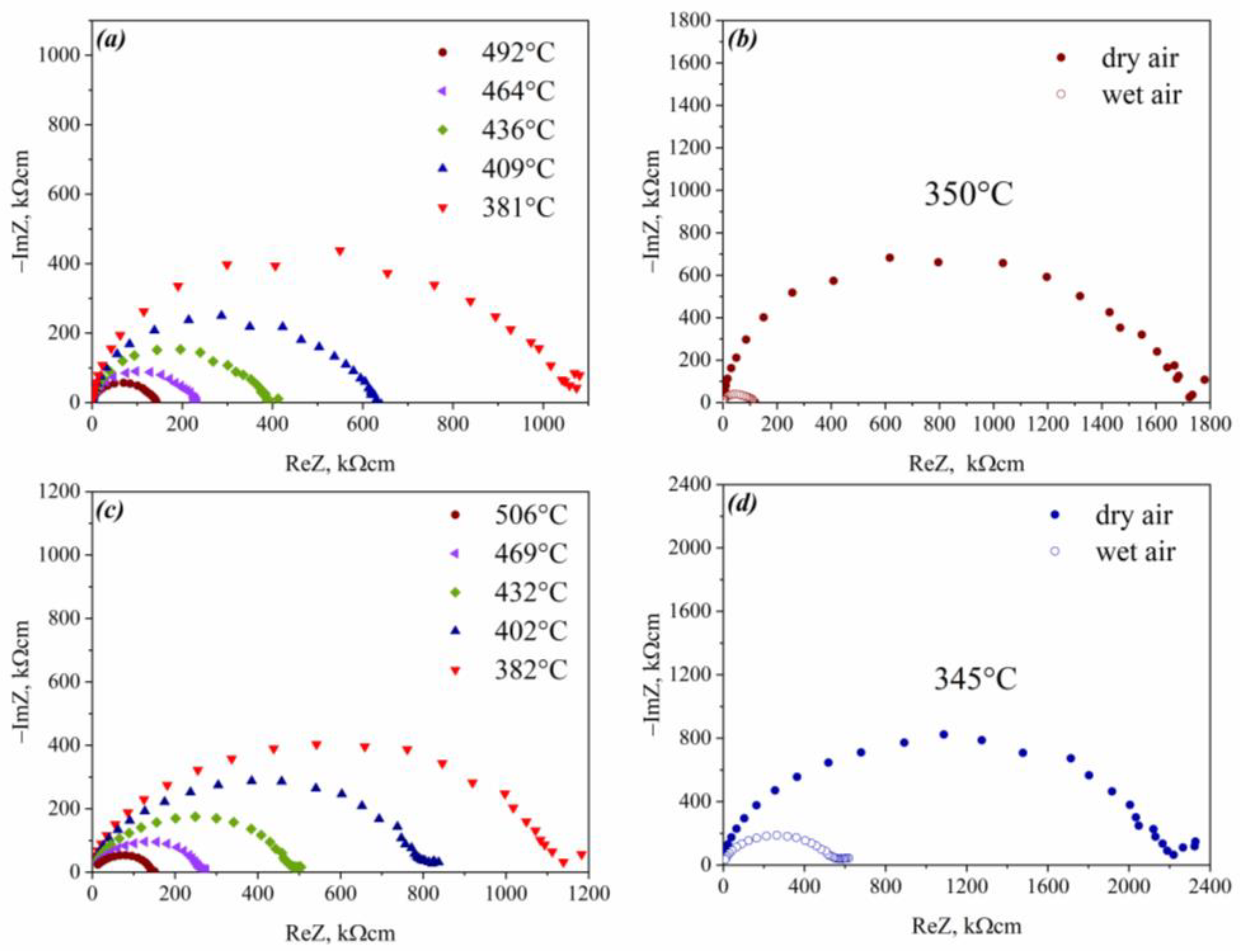


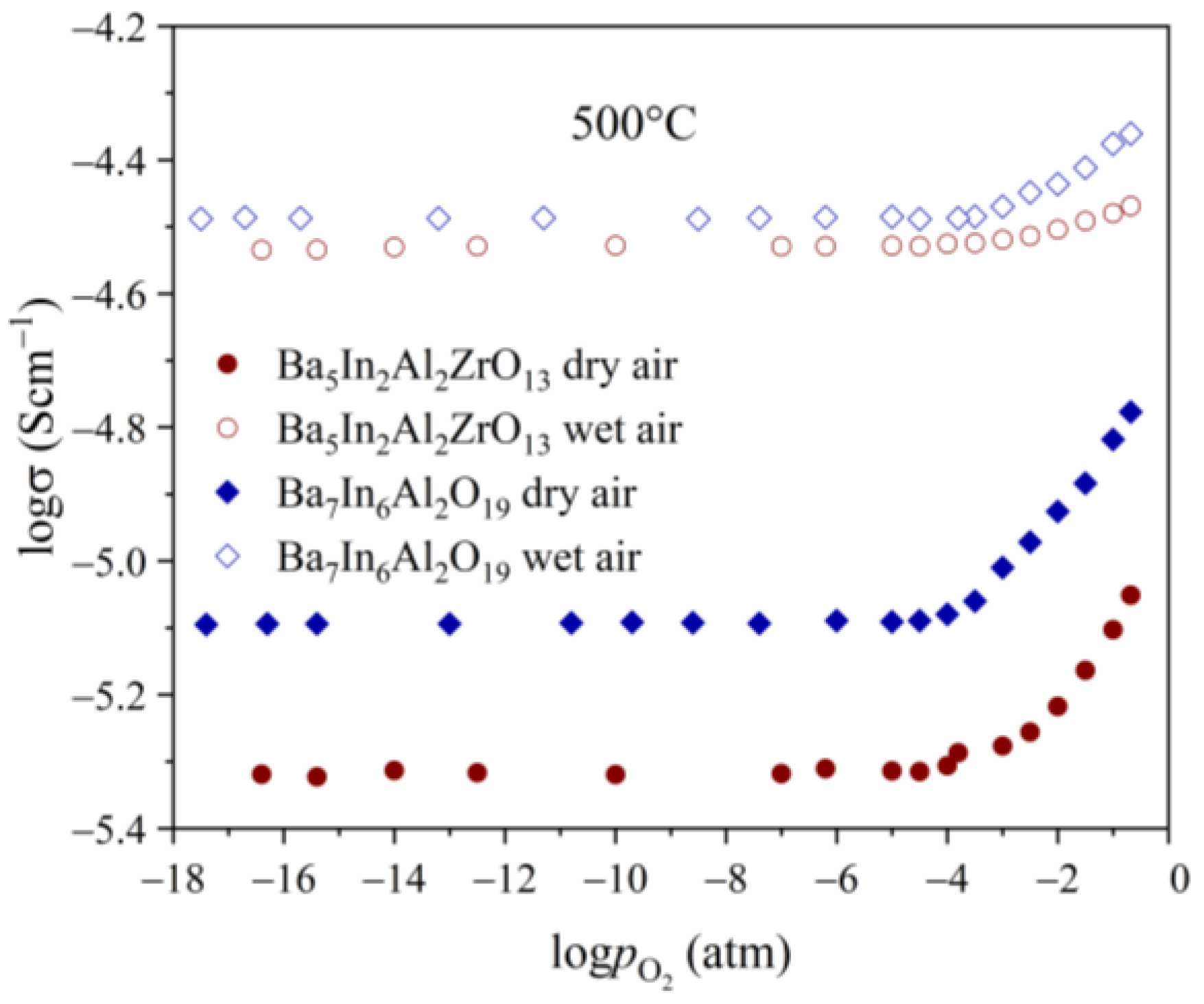

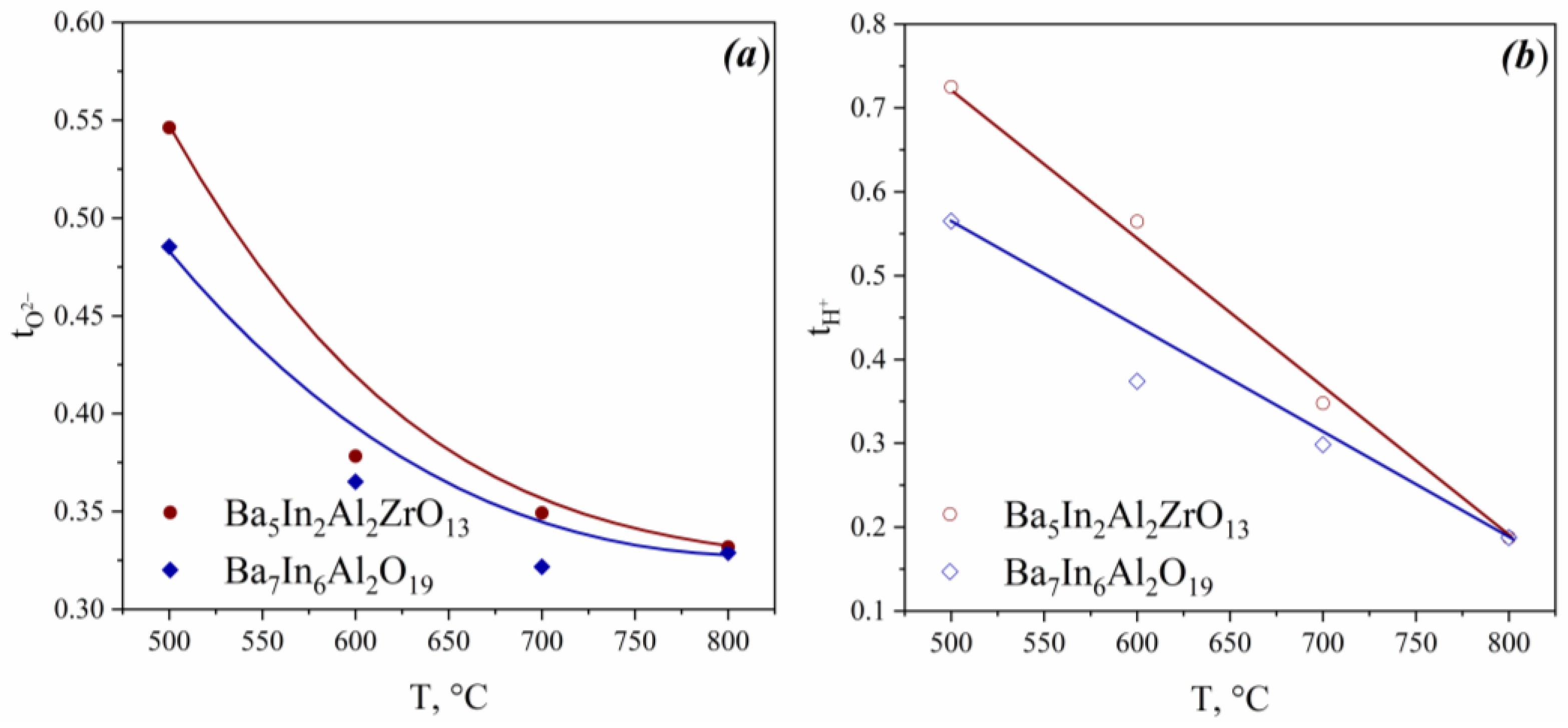
| Composition | a (Å) | c (Å) |
|---|---|---|
| Ba5In2Al2ZrO13 | 5.967(2) | 24.006(8) |
| Ba7In6Al2O19 | 5.921(2) | 37.717(4) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andreev, R.; Animitsa, I. Transport Properties of Intergrowth Structures Ba5In2Al2ZrO13 and Ba7In6Al2O19. Appl. Sci. 2023, 13, 3978. https://doi.org/10.3390/app13063978
Andreev R, Animitsa I. Transport Properties of Intergrowth Structures Ba5In2Al2ZrO13 and Ba7In6Al2O19. Applied Sciences. 2023; 13(6):3978. https://doi.org/10.3390/app13063978
Chicago/Turabian StyleAndreev, Roman, and Irina Animitsa. 2023. "Transport Properties of Intergrowth Structures Ba5In2Al2ZrO13 and Ba7In6Al2O19" Applied Sciences 13, no. 6: 3978. https://doi.org/10.3390/app13063978
APA StyleAndreev, R., & Animitsa, I. (2023). Transport Properties of Intergrowth Structures Ba5In2Al2ZrO13 and Ba7In6Al2O19. Applied Sciences, 13(6), 3978. https://doi.org/10.3390/app13063978






