Assessment of DPPC Liposome Disruption by Embedded Tocopheryl Malonate
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Liposomes
2.3. Fluorescence Measurements of ANS
2.4. Steady-State Fluorescence Anisotropy of DPH and TMA–DPH
2.5. Differential Scanning Calorimetry (DSC)
2.6. Fitting Procedures
3. Results
3.1. The ANS Emission in DPPC Liposomes
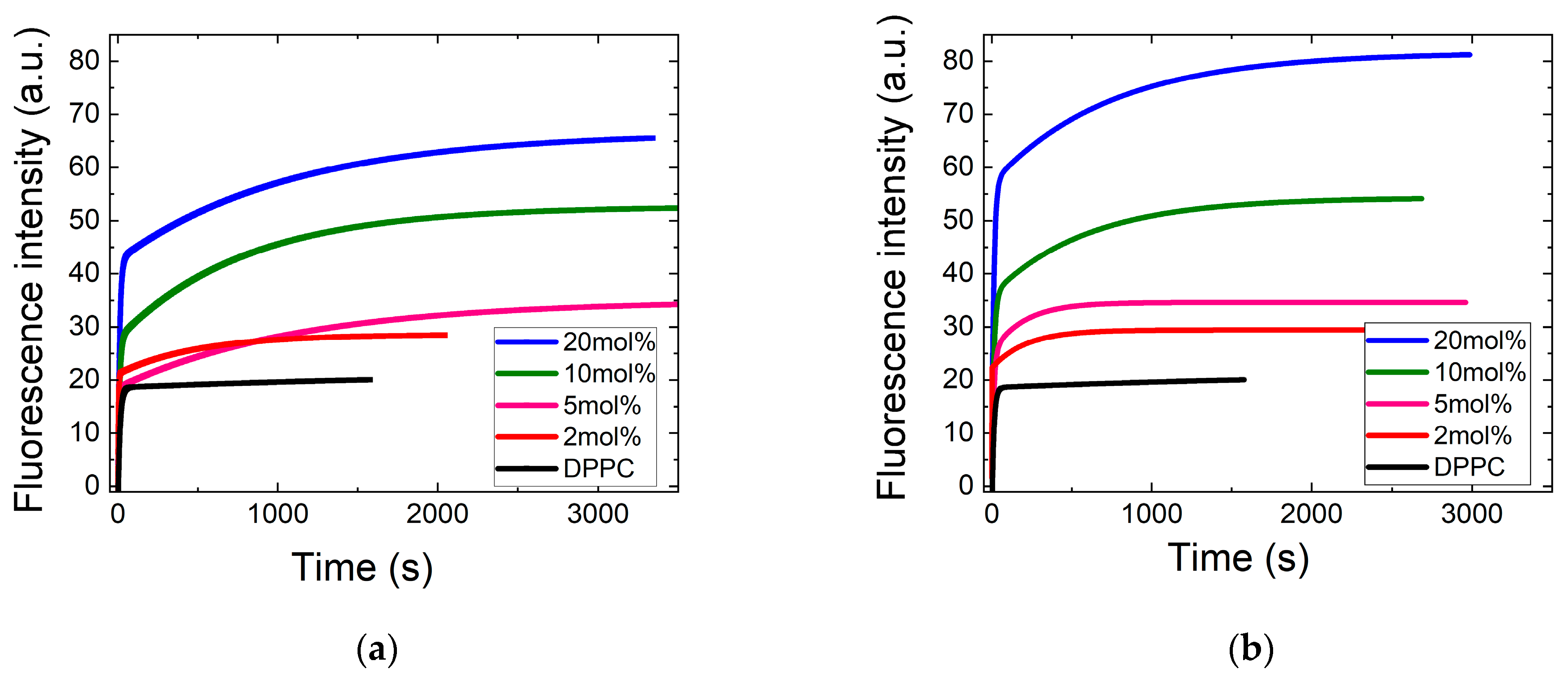
3.1.1. Kinetics of ANS Adsorption on DPPC
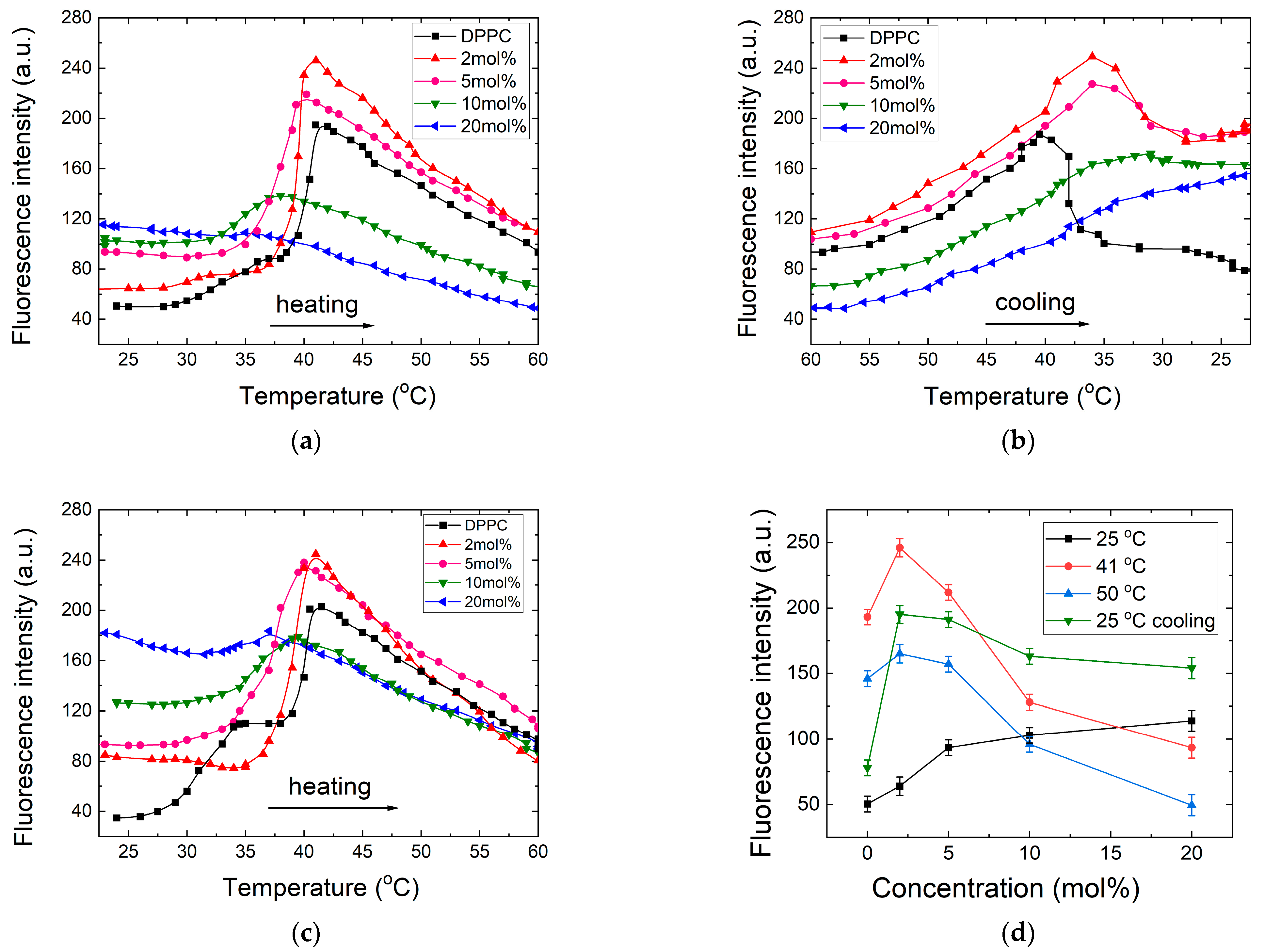
3.1.2. Hydrophobicity and Water Permeability Evaluation in DPPC Using ANS Fluorescence
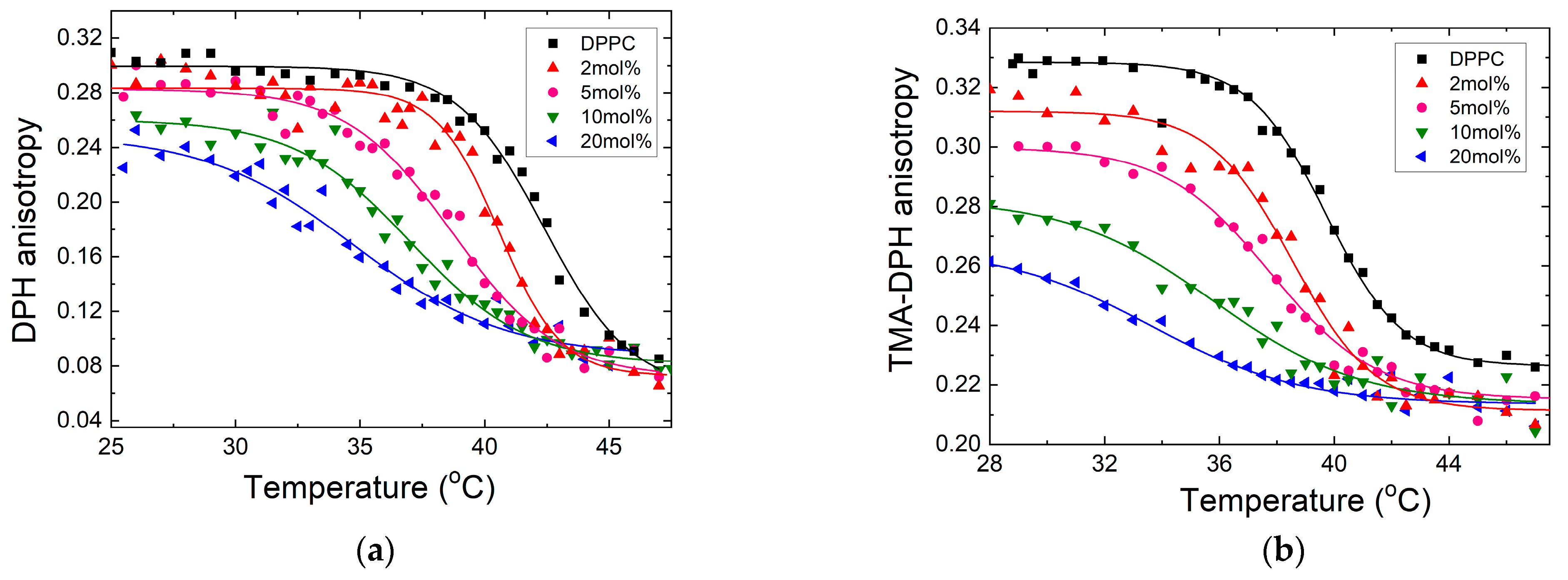
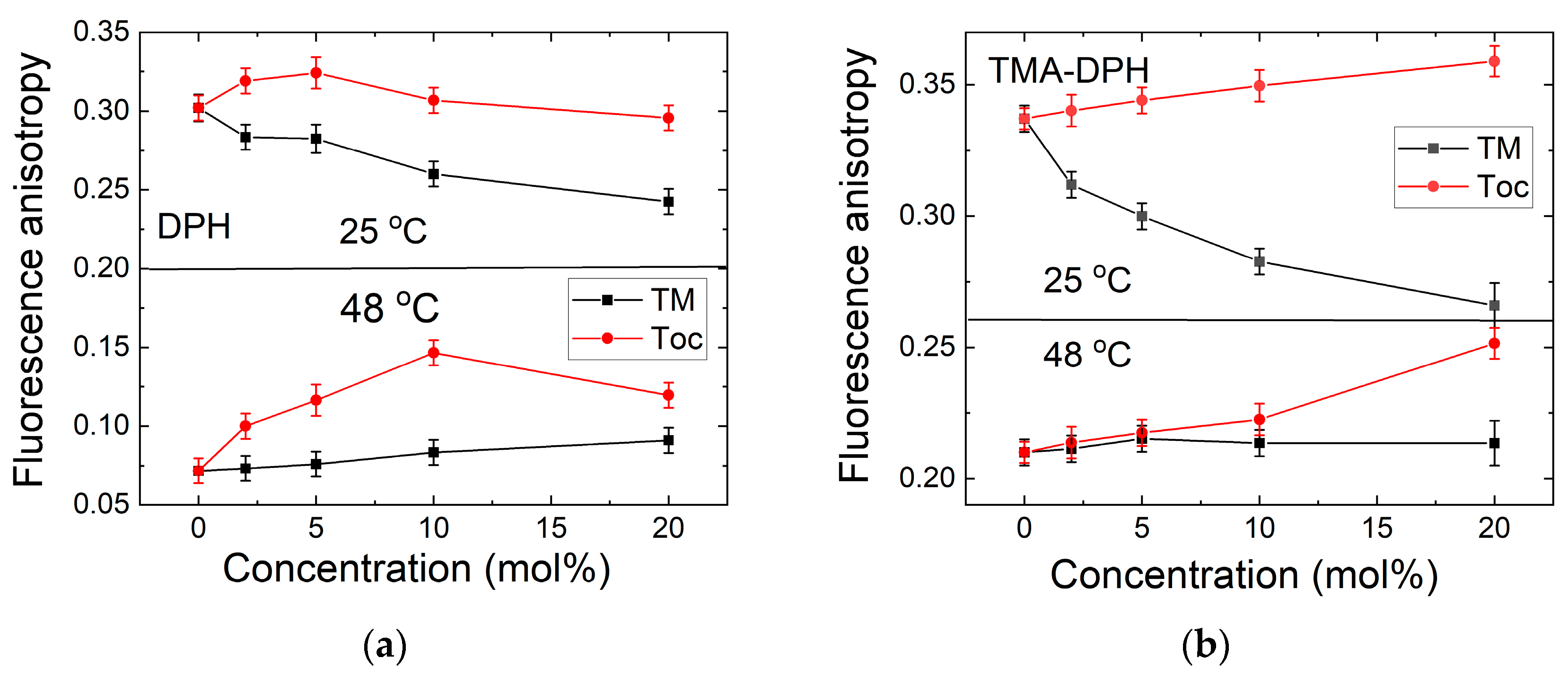
3.2. Fluidity Evaluation—DPH and TMA-DPH Fluorescence Anisotropy
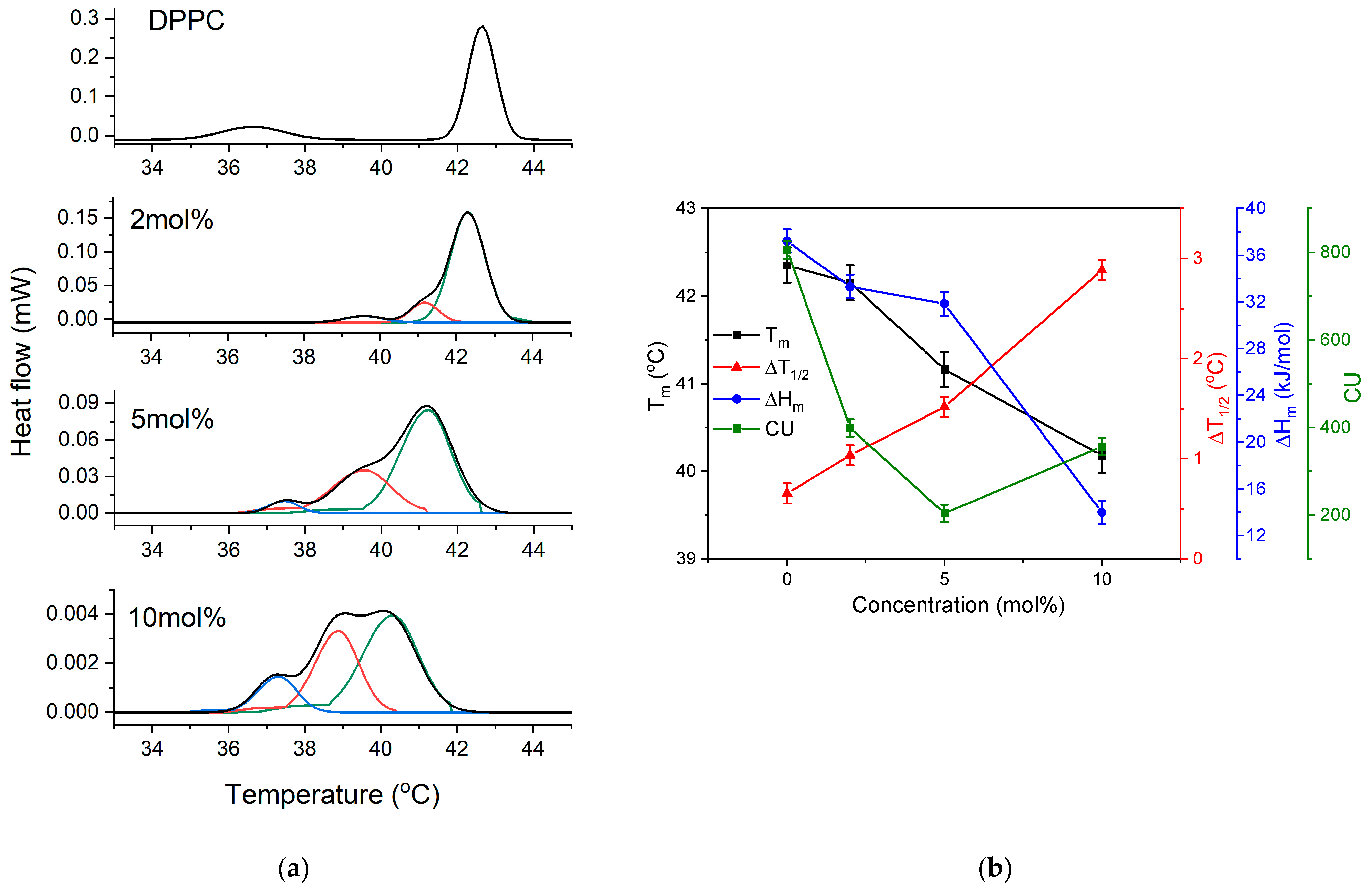
3.3. DSC Measurements
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Azzi, A.; Stocker, A. Vitamin E: Non-antioxidant roles. Prog. Lipid Res. 2000, 39, 231–255. [Google Scholar] [CrossRef] [PubMed]
- Ungurianu, A.; Zanfirescu, A.; Nițulescu, G.; Margină, D. Vitamin E beyond Its Antioxidant Label. Antioxidants 2021, 10, 634. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J. Is the Distribution of α-Tocopherol in Membranes Consistent with Its Putative Functions? Biochemistry 2004, 69, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Atkinson, J.; Epand, R.F.; Epand, R.M. Tocopherols and tocotrienols in membranes: A critical review. Free Radic. Biol. Med. 2008, 44, 739–764. [Google Scholar] [CrossRef]
- Massey, J.B.; She, H.S.; Pownall, H.J. Interaction of vitamin E with saturated phospholipid bilayers. Biochem. Biophys. Res. Commun. 1982, 106, 842–847. [Google Scholar] [CrossRef]
- Wang, X.; Quinn, P.J. Vitamin E and its function in membranes. Prog. Lipid Res. 1999, 38, 309–336. [Google Scholar] [CrossRef]
- Quinn, P.J. The effect of tocopherol on the structure and permeability of phosphatidylcholine liposomes. J. Control. Release 2012, 160, 158–163. [Google Scholar] [CrossRef]
- Kogure, K.; Hama, S.; Kisaki, M.; Takemasa, H.; Tokumura, A.; Suzuki, I.; Fukuzawa, K. Structural characteristic of terminal dicarboxylic moiety required for apoptogenic activity of alpha-tocopheryl esters. Biochim. Biophys. Acta-Gen. Subj. 2004, 1672, 93–99. [Google Scholar] [CrossRef]
- Kogure, K.; Manabe, S.; Suzuki, I.; Tokumura, A.; Fukuzawa, K. Cytotoxicity of alpha-tocopheryl succinate, malonate and oxalate in normal and cancer cells in vitro and their anti-cancer effects on mouse melanoma in vivo. J. Nutr. Sci. Vitaminol. 2005, 51, 392–397. [Google Scholar] [CrossRef]
- Kanai, K.; Kikuchi, E.; Mikami, S.; Suzuki, E.; Uchida, Y.; Kodaira, K.; Miyajima, A.; Ohigashi, T.; Nakashima, J.; Oya, M. Vitamin E succinate induced apoptosis and enhanced chemosensitivity to paclitaxel in human bladder cancer cells in vitro and in vivo. Cancer Sci. 2010, 101, 216–223. [Google Scholar] [CrossRef]
- Donapaty, S.; Louis, S.; Horvath, E.; Kun, J.; Sebti, S.M.; Malafa, M.P. RRR-alpha-tocopherol succinate down-regulates oncogenic Ras signaling. Mol. Cancer Ther. 2006, 5, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Malafa, M.P.; Neitzel, L.T. Vitamin E succinate promotes breast cancer tumor dormancy. J. Surg. Res. 2000, 93, 163–170. [Google Scholar] [CrossRef] [PubMed]
- Koudelka, S.; Turanek Knotigova, P.; Masek, J.; Prochazka, L.; Lukac, R.; Miller, A.D.; Neuzil, J.; Turanek, J. Liposomal delivery systems for anti-cancer analogues of vitamin E. J. Control. Release 2015, 207, 59–69. [Google Scholar] [CrossRef] [PubMed]
- Monteiro, N.; Martins, A.; Reis, R.L.; Neves, N.M. Liposomes in tissue engineering and regenerative medicine. J. R. Soc. Interface 2014, 11, 20140459. [Google Scholar] [CrossRef]
- Nsairat, H.; Khater, D.; Sayed, U.; Odeh, F.; Al Bawab, A.; Alshaer, W. Liposomes: Structure, composition, types, and clinical applications. Heliyon 2022, 8, e09394. [Google Scholar] [CrossRef] [PubMed]
- Liu, P.; Chen, G.; Zhang, J. A Review of Liposomes as a Drug Delivery System: Current Status of Approved Products, Regulatory Environments, and Future Perspectives. Molecules 2022, 27, 1372. [Google Scholar] [CrossRef]
- Krilov, D.; Kosović, M.; Serec, K. Spectroscopic studies of alpha tocopherol interaction with a model liposome and its influence on oxidation dynamics. Spectrochim. Acta-Part A Mol. Biomol. Spectrosc. 2014, 129, 588–593. [Google Scholar] [CrossRef]
- Srivastava, S.; Phadke, R.S.; Govil, G. Magnetic resonance studies on the binding of etomidate to lipid bilayers. Physiol. Chem. Phys. Med. NMR 1987, 19, 241–250. [Google Scholar]
- Wu, R.G.; Wang, Y.R.; Wu, F.G.; Zhou, H.W.; Zhang, X.H.; Hou, J.L. A DSC study of paeonol-encapsulated liposomes, comparison the effect of cholesterol and stigmasterol on the thermotropic phase behavior of liposomes. J. Therm. Anal. Calorim. 2012, 109, 311–316. [Google Scholar] [CrossRef]
- Mason, J.T. Properties of phosphatidylcholine bilayers as revealed by mixed-acyl phospholipid fluorescent probes containing n-(9-anthroyloxy) fatty acids. Biochim. Biophys. Acta (BBA)-Biomembr. 1994, 1194, 99–108. [Google Scholar] [CrossRef]
- Neunert, G.; Tomaszewska-Gras, J.; Witkowski, S.; Polewski, K. Tocopheryl succinate-induced structural changes in DPPC liposomes: DSC and ANS fluorescence studies. Molecules 2020, 25, 2780. [Google Scholar] [CrossRef] [PubMed]
- Quinn, P.J.; Takahashi, H.; Hatta, I. Characterization of complexes formed in fully hydrated dispersions of dipalmitoyl derivatives of phosphatidylcholine and diacylglycerol. Biophys. J. 1995, 68, 1374–1382. [Google Scholar] [CrossRef] [PubMed]
- Neunert, G.; Tomaszewska-Gras, J.; Siejak, P.; Pietralik, Z.; Kozak, M.; Polewski, K. Disruptive effect of tocopherol oxalate on DPPC liposome structure: DSC, SAXS, and fluorescence anisotropy studies. Chem. Phys. Lipids 2018, 216, 104–113. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.Z.; Diizgune, N.; Szoka, F.C. Effects of replacement of the hydroxyl group of cholesterol and tocopherol on the thermotropic behavior of phospholipid membranes. Biochemistry 1985, 24, 1646–1653. [Google Scholar] [CrossRef]
- Gasymov, O.K.; Glasgow, B.J. ANS fluorescence: Potential to augment the identification of the external binding sites of proteins. Biochim. Biophys. Acta-Proteins Proteomics 2007, 1774, 403–411. [Google Scholar] [CrossRef]
- Haynes, D.H.; Staerk, H. 1-Anilino-8-naphthalenesulfonate: A fluorescent probe of membrane surface structure, composition and mobility. J. Membr. Biol. 1974, 17, 313–340. [Google Scholar] [CrossRef]
- Au, S.; Schacht, J.; Weiner, N. Membrane effects of aminoglycoside antibiotics measured in liposomes containing the fluorescent probe, 1-anilino-8-naphthalene sulfonate. Biochim. Biophys. Acta (BBA)-Biomembr. 1986, 862, 205–210. [Google Scholar] [CrossRef]
- Bisby, R.H.; Birch, D.J. A time-resolved fluorescence anisotropy study of bilayer membranes containing alpha-tocopherol. Biochem. Biophys. Res. Commun. 1989, 158, 386–391. [Google Scholar] [CrossRef]
- De Almeida, R.F.M.; Loura, L.M.S.; Fedorov, A.; Prieto, M. Lipid rafts have different sizes depending on membrane composition: A time-resolved fluorescence resonance energy transfer study. J. Mol. Biol. 2005, 346, 1109–1120. [Google Scholar] [CrossRef]
- Jaśkowski, J.; Witkowski, J.; Myśliwski, A.; Zawadzki, H. Effect of air ions on L 1210 cells: Changes in fluorescence of membrane-bound 1,8-aniline-naphthalene-sulfonate (ANS) after in vitro exposure of cells to air ions. Gen. Physiol. Biophys. 1986, 5, 511–515. [Google Scholar]
- Loura, L.M.S.; Fedorov, A.; Prieto, M. Partition of membrane probes in a gel/fluid two-component lipid system: A fluorescence resonance energy transfer study. Biochim. Biophys. Acta-Biomembr. 2000, 1467, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Sengupta, P.; Holowka, D.; Baird, B. Fluorescence resonance energy transfer between lipid probes detects nanoscopic heterogeneity in the plasma membrane of live cells. Biophys. J. 2007, 92, 3564–3574. [Google Scholar] [CrossRef] [PubMed]
- Vladkova, R.; Teuchner, K.; Leupold, D.; Koynova, R.; Tenchov, B. Detection of the metastable rippled gel phase in hydrated phosphatidylcholine by fluorescence spectroscopy. Biophys. Chem. 2000, 84, 159–166. [Google Scholar] [CrossRef]
- Genz, A.; Holzwarth, J.F. Dynamic fluorescence measurements on the main phase transition of dipalmytoylphosphatidylcholine vesicles. Eur. Biophys. J. 1986, 13, 323–330. [Google Scholar] [CrossRef] [PubMed]
- Ortiz, A.; Villalain, J.; Gómez-Fernández, J.C. Interaction of Diacylglycerols with Phosphatidylcholine Vesicles As Studied by Differential Scanning Calorimetry and Fluorescence Probe Depolarization. Biochemistry 1988, 27, 9030–9036. [Google Scholar] [CrossRef] [PubMed]
- Antunes-Madeira, M.C.; Madeira, V.M.C. Membrane fluidity as affected by the insecticide lindane. BBA-Biomembr. 1989, 982, 161–166. [Google Scholar] [CrossRef]
- Wang, S.; Beechem, J.M.; Gratton, E.; Glaser, M. Orientational Distribution of 1,6-Diphenyl-1,3,5-hexatriene in Phospholipid Vesicles As Determined by Global Analysis of Frequency Domain Fluorimetry Data. Biochemistry 1991, 30, 5565–5572. [Google Scholar] [CrossRef]
- Bui, T.T.; Suga, K.; Umakoshi, H. Roles of Sterol Derivatives in Regulating the Properties of Phospholipid Bilayer Systems. Langmuir 2016, 32, 6176–6184. [Google Scholar] [CrossRef]
- Witkowski, S.; Wałejko, P. Synthesis of α-Tocopheryl Glycosides. Z. Naturforsch. 2001, 56b, 411–415. [Google Scholar] [CrossRef]
- Witkowski, S.; Maciejewska, D.; Wawer, I. A solution and solid state conformations of chromanol esters, 13C MAS NMR and d-NMR study. J. Chem. Soc. Perkin Trans. 2000, 2, 1471–1476. [Google Scholar] [CrossRef]
- Slavík, J. Anilinonaphthalene sulfonate as a probe of membrane composition and function. BBA-Rev. Biomembr. 1982, 694, 1–25. [Google Scholar] [CrossRef] [PubMed]
- Neunert, G.; Tomaszewska-Gras, J.; Baj, A.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Phase transitions and structural changes in DPPC liposomes induced by a 1-carba-alpha-tocopherol analogue. Molecules 2021, 26, 2851. [Google Scholar] [CrossRef] [PubMed]
- Tsong, T.Y. Effect of phase transition on the kinetics of dye transport in phospholipid bilayer structures. Biochemistry 1975, 14, 5409–5414. [Google Scholar] [CrossRef] [PubMed]
- Jacobson, K.; Papahadjopoulos, D. Effect of a phase transition on the binding of 1-anilino-8-naphthalenesulfonate to phospholipid membranes. Biophys. J. 1976, 16, 549–560. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Böckmann, R.A. Membrane phase transition during heating and cooling: Molecular insight into reversible melting. Eur. Biophys. J. 2018, 47, 151–164. [Google Scholar] [CrossRef]
- do Canto, A.M.T.M.; Robalo, J.R.; Santos, P.D.; Carvalho, A.J.P.; Ramalho, J.P.P.; Loura, L.M.S. Diphenylhexatriene membrane probes DPH and TMA-DPH: A comparative molecular dynamics simulation study. Biochim. Biophys. Acta-Biomembr. 2016, 1858, 2647–2661. [Google Scholar] [CrossRef]
- Massey, J.B. Interfacial properties of phosphatidylcholine bilayers containing vitamin E derivatives. Chem. Phys. Lipids 2001, 109, 157–174. [Google Scholar] [CrossRef]
- Neunert, G.; Hertmanowski, R.; Witkowski, S.; Polewski, K. Effect of Ester Moiety on Structural Properties of Binary Mixed Monolayers of Alpha-Tocopherol Derivatives with DPPC. Molecules 2022, 27, 4670. [Google Scholar] [CrossRef]
- Barry, J.A.; Gawrisch, K. Effects of ethanol on lipid bilayers containing cholesterol, gangliosides, and sphingomyelin. Biochemistry 1995, 34, 8852–8860. [Google Scholar] [CrossRef]
- Reeves, M.D.; Schawel, A.K.; Wang, W.; Dea, P. Effects of butanol isomers on dipalmitoylphosphatidylcholine bilayer membranes. Biophys. Chem. 2007, 128, 13–18. [Google Scholar] [CrossRef]
- Vierl, U.; Löbbecke, L.; Nagel, N.; Cevc, G. Solute effects on the colloidal and phase behavior of lipid bilayer membranes: Ethanol-dipalmitoylphosphatidylcholine mixtures. Biophys. J. 1994, 67, 1067–1079. [Google Scholar] [CrossRef] [PubMed]
- Suga, K.; Umakoshi, H. Detection of nanosized ordered domains in DOPC/DPPC and DOPC/CH binary lipid mixture systems of large unilamellar vesicles using a TEMPO quenching method. Langmuir 2013, 29, 4830–4838. [Google Scholar] [CrossRef] [PubMed]
- Ausili, A.; Torrecillas, A.; De Godos, A.M.; Corbalán-García, S.; Gómez-Fernández, J.C. Phenolic Group of α-Tocopherol Anchors at the Lipid-Water Interface of Fully Saturated Membranes. Langmuir 2018, 34, 3336–3348. [Google Scholar] [CrossRef]
- Fukuzawa, K.; Ikebata, W.; Shibata, A.; Kumadaki, I.; Sakanaka, T.; Urano, S. Location and dynamics of α-tocopherol in model phospholipid membranes with different charges. Chem. Phys. Lipids 1992, 63, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Makino, K.; Yamada, T.; Kimura, M.; Oka, T.; Ohshima, H.; Kondo, T. Temperature- and ionic strength-induced conformational changes in the lipid head group region of liposomes as suggested by zeta potential data. Biophys. Chem. 1991, 41, 175–183. [Google Scholar] [CrossRef] [PubMed]
- Tenchov, R.; Bird, R.; Curtze, A.E.; Zhou, Q. Lipid Nanoparticles—From Liposomes to mRNA Vaccine Delivery, a Landscape of Research Diversity and Advancement. ACS Nano 2021, 15, 16982–17015. [Google Scholar] [CrossRef] [PubMed]
- Augustyńska, D.; Burda, K.; Jemioła-Rzemińska, M.; Strzałka, K. Temperature-dependent bifurcation of cooperative interactions in pure and enriched in β-carotene DPPC liposomes. Chem. Biol. Interact. 2016, 256, 236–248. [Google Scholar] [CrossRef]
- Timoszyk, A. Dynamics of Model Membranes by NMR. In Spectroscopic Analyses—Developments and Applications; IntechOpen: London, UK, 2017; ISBN 978-953-51-3628-6. [Google Scholar]
- Willumeit, R.; Kumpugdee, M.; Funari, S.S.; Lohner, K.; Navas, B.P.; Brandenburg, K.; Linser, S.; Andrä, J. Structural rearrangement of model membranes by the peptide antibiotic NK-2. Biochim. Biophys. Acta-Biomembr. 2005, 1669, 125–134. [Google Scholar] [CrossRef]
- Lorincz, A.; Mihály, J.; Németh, C.; Wacha, A.; Bóta, A. Effects of ursolic acid on the structural and morphological behaviours of dipalmitoyl lecithin vesicles. Biochim. Biophys. Acta 2015, 1848, 1092–1098. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Neunert, G.; Tomaszewska-Gras, J.; Gauza-Włodarczyk, M.; Witkowski, S.; Polewski, K. Assessment of DPPC Liposome Disruption by Embedded Tocopheryl Malonate. Appl. Sci. 2023, 13, 6219. https://doi.org/10.3390/app13106219
Neunert G, Tomaszewska-Gras J, Gauza-Włodarczyk M, Witkowski S, Polewski K. Assessment of DPPC Liposome Disruption by Embedded Tocopheryl Malonate. Applied Sciences. 2023; 13(10):6219. https://doi.org/10.3390/app13106219
Chicago/Turabian StyleNeunert, Grażyna, Jolanta Tomaszewska-Gras, Marlena Gauza-Włodarczyk, Stanislaw Witkowski, and Krzysztof Polewski. 2023. "Assessment of DPPC Liposome Disruption by Embedded Tocopheryl Malonate" Applied Sciences 13, no. 10: 6219. https://doi.org/10.3390/app13106219
APA StyleNeunert, G., Tomaszewska-Gras, J., Gauza-Włodarczyk, M., Witkowski, S., & Polewski, K. (2023). Assessment of DPPC Liposome Disruption by Embedded Tocopheryl Malonate. Applied Sciences, 13(10), 6219. https://doi.org/10.3390/app13106219







