The Impact of Water Availability on the Discriminative Status of Nitrogen (N) in Sugar Beet and Celery Using Hyperspectral Imaging Methods
Abstract
1. Introduction
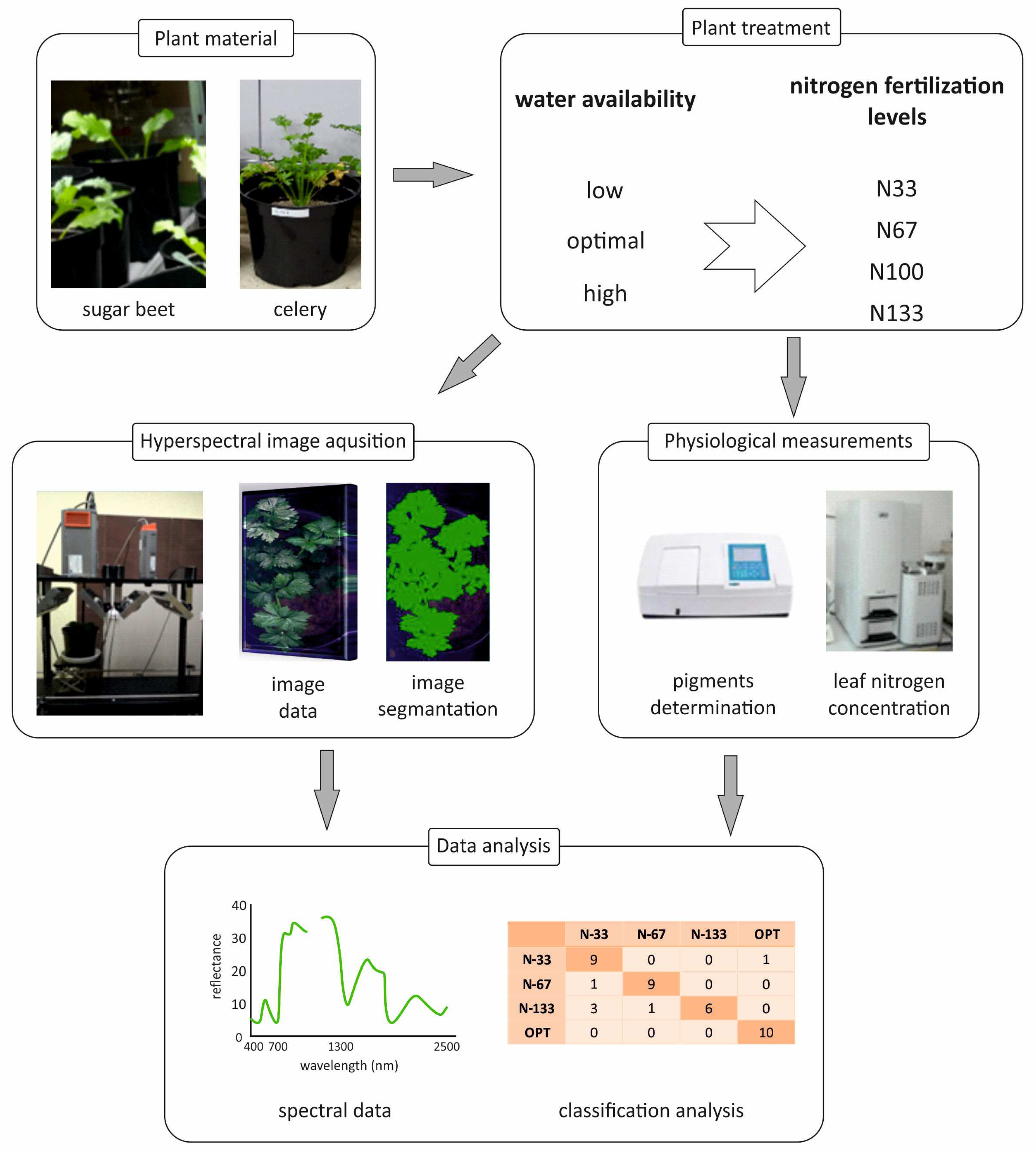
2. Materials and Methods
2.1. Plant Materials
2.2. Hyperspectral Image Acquisition
2.3. Chemical Analysis
2.4. Hyperspectral Image Transformation and Spectral Data Extraction
2.5. Spectral Data Preprocessing
2.6. Statistical Analysis and Model Development
3. Results and Discussion
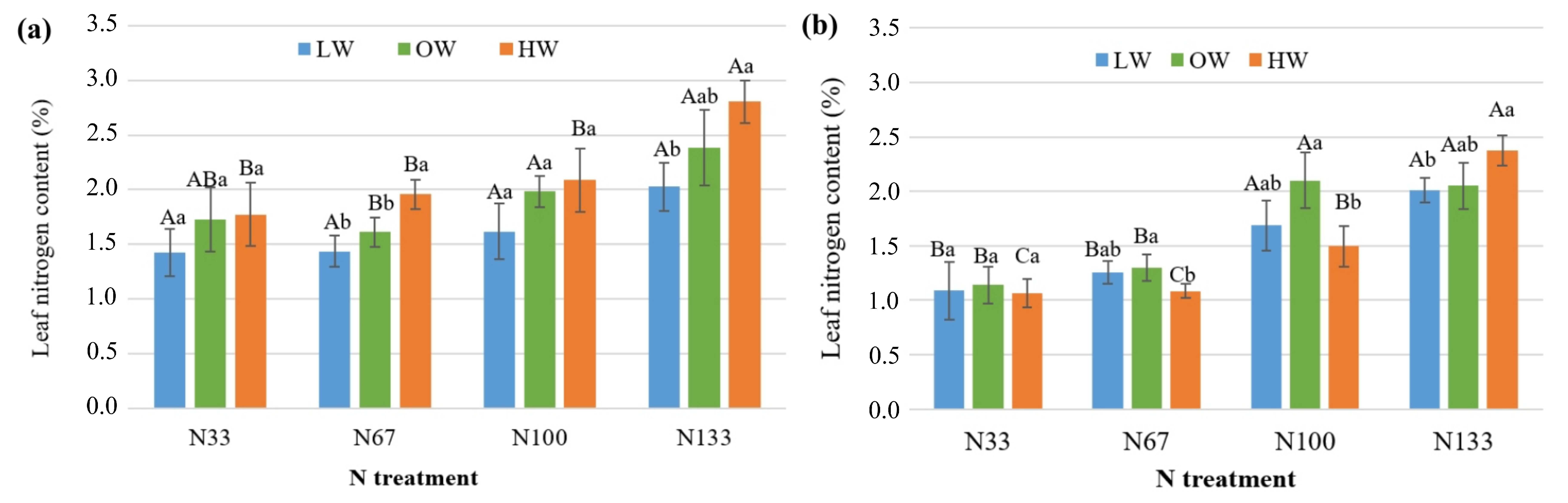
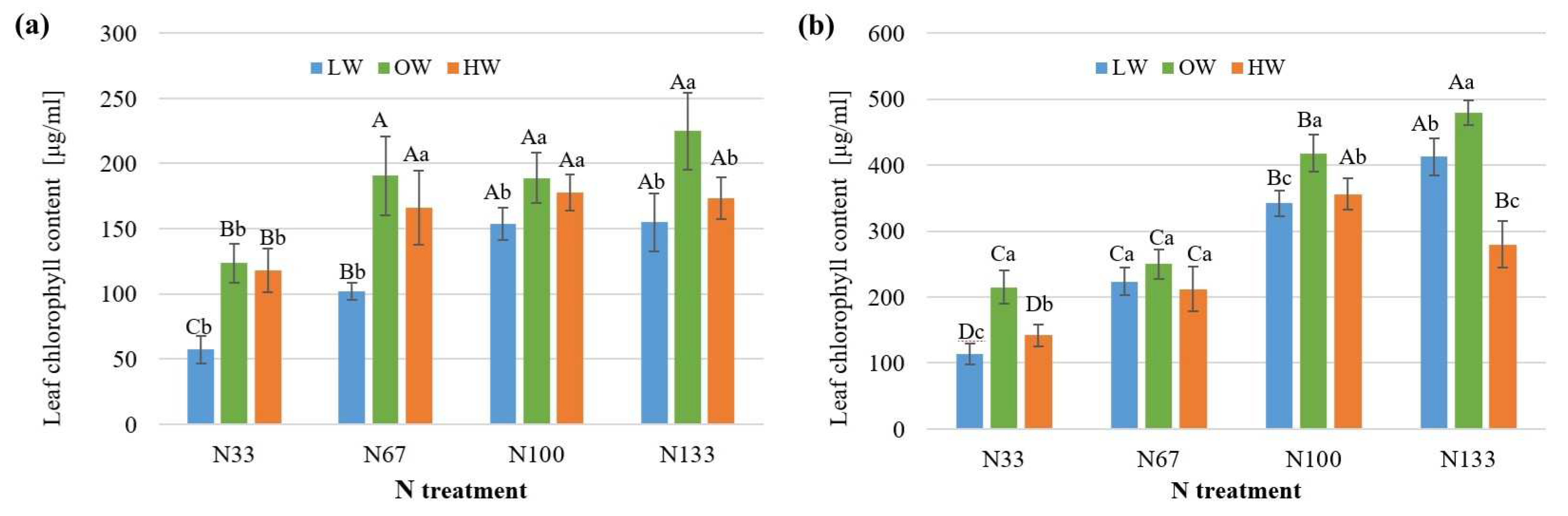
3.1. Nitrogen and Water Stress Impact on Chlorophyll and N Content in Leaves
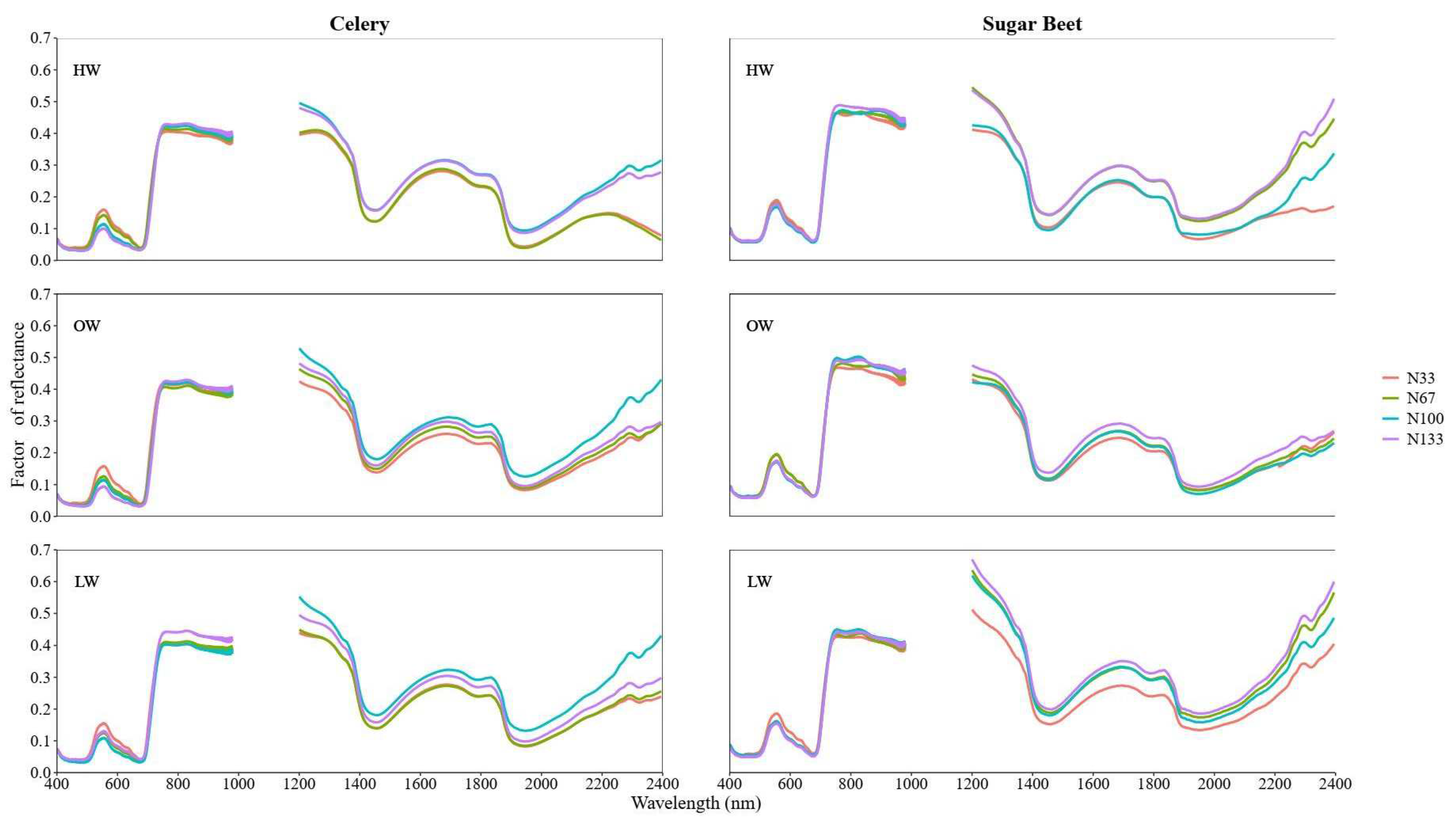
3.2. Spectra Feature
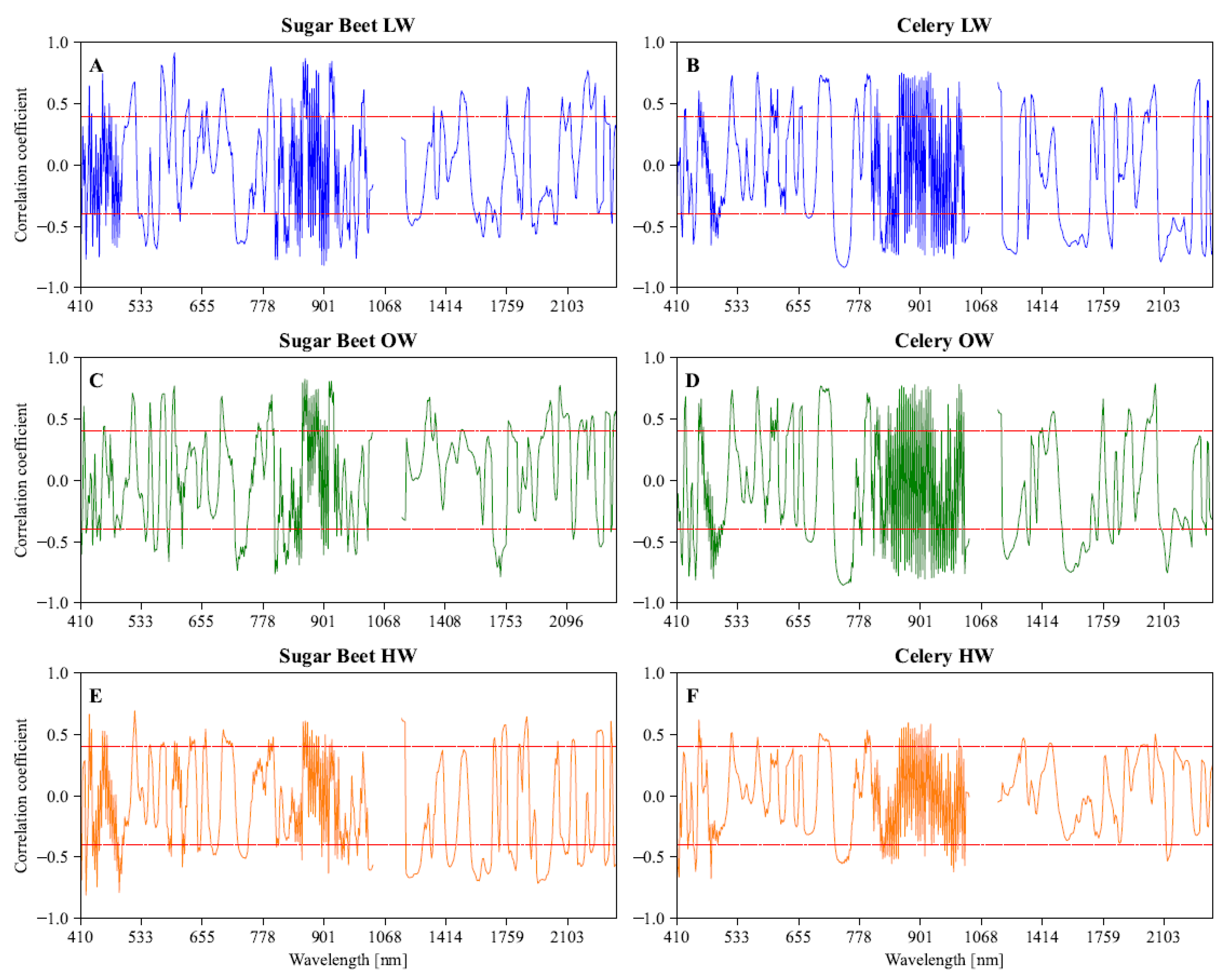
3.3. Effective Wavelengths Selection
3.4. Model Performance
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fageria, N.K.; Baligar, V.C.; Li, Y.C. The role of rutrient efficient plants in pmproving prop yields in the twenty first century. J. Plant Nutr. 2008, 31, 1121–1157. [Google Scholar] [CrossRef]
- Chandini, K.R.; Kumar, R.; Kumar, R.; Prakash, O. The impact of chemical fertilizers on our environment and ecosystem. Res. Trends Environ. Sci. 2019, 35, 69–86. [Google Scholar]
- Mosier, A.; Syers, J.K.; Freney, J.R. Agriculture and the Nitrogen Cycle: Assessing the Impacts of Fertilizer Use on Food Production and the Environment; Island Press: Washington, DC, USA, 2013; ISBN 978-1-59726-743-4. [Google Scholar]
- Savci, S. Investigation of effect of chemical fertilizers on environment. APCBEE Procedia 2012, 1, 287–292. [Google Scholar] [CrossRef]
- Chen, Y.; Guerschman, J.P.; Cheng, Z.; Guo, L. Remote sensing for vegetation monitoring in carbon capture storage regions: A review. Appl. Energy 2019, 240, 312–326. [Google Scholar] [CrossRef]
- Bremner, J.M. Recent research on problems in the use of urea as a nitrogen fertilizer. In Nitrogen Economy in Tropical Soils: Proceedings of the International Symposium on Nitrogen Economy in Tropical Soils, St. Augustine, Trinidad West Indies, 9–14 January 1994; Springer: Dordrecht, The Netherlands, 1995. [Google Scholar]
- Binder, D.L.; Sander, D.H.; Walters, D.T. Maize response to time of nitrogen application as affected by level of nitrogen deficiency. Agron. J. 2000, 92, 1228–1236. [Google Scholar] [CrossRef]
- Alloway, B.J. Micronutrient Deficiencies in Global Crop Production; Springer Science & BusinessMedia: Berlin/Heidelberg, Germany, 2008; ISBN 978-1-4020-6860-7. [Google Scholar]
- Hossain, M.A.; Siddique, M.N.A. Online Fertilizer Recommendation System (OFRS): A Step Towards Precision Agriculture And Optimized Fertilizer Usage By Smallholder Farmers In Bangladesh: Online fertilizer recommendation. Eur. J. Environ. Earth Sci. 2020, 1, 1–9. [Google Scholar] [CrossRef]
- Mitra, S. Jackfruit: Botany, Production and Uses; CABI: Warlingford, UK, 2023; ISBN 978-1-80062-229-6. [Google Scholar]
- Tudor, V.C.; Stoicea, P.; Chiurciu, I.-A.; Soare, E.; Iorga, A.M.; Dinu, T.A.; David, L.; Micu, M.M.; Smedescu, D.I.; Dumitru, E.A. The Use of Fertilizers and Pesticides in Wheat Production in the Main European Countries. Sustainability 2023, 15, 3038. [Google Scholar] [CrossRef]
- Ward, G.M.; Johnston, F.B. Chemical method of plant analysis. Publ. Dep. Agric. Can. 1960, 1064, 59. [Google Scholar]
- Marín Del Valle, T.; Jiang, P. Comparison of common classification strategies for large-scale vegetation mapping over the Google Earth Engine platform. Int. J. Appl. Earth Obs. Geoinf. 2022, 115, 103092. [Google Scholar] [CrossRef]
- Joshi, N.; Baumann, M.; Ehammer, A.; Fensholt, R.; Grogan, K.; Hostert, P.; Jepsen, M.R.; Kuemmerle, T.; Meyfroidt, P.; Mitchard, E.T.A. A Review of the application of Optical and Radar remote sensing data fusion to land use mapping and monitoring. Remote Sens. 2016, 8, 70. [Google Scholar] [CrossRef]
- Eberhardt, L.L.; Thomas, J.M. Designing environmental field studies. Ecol. Monogr. 1991, 61, 53–73. [Google Scholar] [CrossRef]
- Asaari, M.S.M.; Mishra, P.; Mertens, S.; Dhondt, S.; Inzé, D.; Wuyts, N.; Scheunders, P. Close-range hyperspectral image analysis for the early detection of stress responses in individual plants in a high-throughput phenotyping platform. ISPRS J. Photogramm. Remote Sens. 2018, 138, 121–138. [Google Scholar] [CrossRef]
- Asaari, M.S.M.; Mertens, S.; Dhondt, S.; Inzé, D.; Wuyts, N.; Scheunders, P. A Analysis of hyperspectral images for detection of drought stress and recovery in maize plants in a high-throughput phenotyping platform. Comput. Electron. Agric. 2019, 162, 749–758. [Google Scholar] [CrossRef]
- Lowe, A.; Harrison, N.; French, A.P. Hyperspectral image analysis techniques for the detection and classification of the early onset of plant disease and stress. Plant Methods 2017, 13, 80. [Google Scholar] [CrossRef]
- El-Hendawy, S.; Al-Suhaibani, N.; Hassan, W.; Tahir, M.; Schmidhalter, U. Hyperspectral reflectance sensing to assess the growth and photosynthetic properties of wheat cultivars exposed to different irrigation rates in an irrigated arid region. PLoS ONE 2017, 12, e0183262. [Google Scholar] [CrossRef]
- Gehards, M.; Schlerf, M.; Mallick, K.; Udelhoven, T. Challenges and Future Perspectives of Multi-/Hyperspectral Thermal Infrared Remote Sensing for Crop Water-Stress Detection: A Review. Remote Sens. 2019, 11, 1240. [Google Scholar] [CrossRef]
- Crusiol, L.G.T.; Nanni, M.R.; Furlanetto, R.H.; Sibaldelli, R.N.R.; Sun, L.; Gonçalves, S.L.; Foloni, J.S.S.; Mertz-Henning, L.M.; Nepomuceno, A.L.; Neumaier, N. Assessing the sensitive spectral bands for soybean water status monitoring and soil moisture prediction using leaf-based hyperspectral reflectance. Agric. Water Manag. 2023, 277, 108089. [Google Scholar] [CrossRef]
- Mahajan, G.R.; Pandey, R.N.; Sahoo, R.N.; Gupta, V.K.; Datta, S.C.; Kumar, D. Monitoring nitrogen, phosphorus and sulphur in hybrid rice (Oryza sativa L.) using hyperspectral remote sensing. Precis. Agric. 2017, 18, 736–761. [Google Scholar] [CrossRef]
- Singh, H.; Roy, A.; Setia, R.; Pateriya, B. Estimation of chlorophyll, macronutrients and water content in maize from hyperspectral data using machine learning and explainable artificial intelligence techniques. Remote Sens. Lett. 2022, 13, 969–979. [Google Scholar] [CrossRef]
- Jørgensen, R.N.; Christensen, L.K.; Bro, R. Spectral reflectance at sub-leaf scale including the spatial distribution discriminating NPK stress characteristics in barley using multiway partial least squares regression. Int. J. Remote Sens. 2007, 28, 943–962. [Google Scholar] [CrossRef]
- Hongyu, L.; Hanping, M.; Wenjing, Z.; Xiaodong, Z.; Hongyan, G. Rapid diagnosis of tomato NPK nutrition level based on hyperspectral technology. Trans. Chin. Soc. Agric. Eng. 2015, 31, 212–220. [Google Scholar] [CrossRef]
- Mallet, Y.; Coomans, D.; deVel, O. Recent developments in discriminant analysis on high dimensional spectral data. Chemom. Intell. Lab. Syst. 1996, 35, 157–173. [Google Scholar] [CrossRef]
- Ravikanth, L.; Jayas, D.S.; White, N.D.G.; Fields, P.G.; Sun, D.-W. Extraction of spectral information from hyperspectral data and application of hyperspectral imaging for food and agricultural products. Food Bioprocess Technol. 2017, 10, 1–33. [Google Scholar] [CrossRef]
- Shi, Y.; Huang, W.; Luo, J.; Huang, L.; Zhou, X. Detection and discrimination of pests and diseases in winter wheat based on spectral indices and kernel discriminant analysis. Comput. Electron. Agric. 2017, 141, 171–180. [Google Scholar] [CrossRef]
- Munyati, C.; Balzter, H.; Economon, E. Correlating Sentinel-2 MSI-derived vegetation indices with in-situ reflectance and tissue macronutrients in savannah grass. Int. J. Remote Sens. 2020, 41, 3820–3844. [Google Scholar] [CrossRef]
- Oliveira, L.F.R.; Oliveira, M.L.R.; Gomes, F.S.; Santana, R.C. Estimating foliar nitrogen in Eucalyptus using vegetation indexes. Sci. Agric. (Piracicaba Braz.) 2017, 74, 142–147. [Google Scholar] [CrossRef]
- Rodrigues, M.; Cezar, E.; Argenta, J.C.; Barcelos, L.B.; Reis, A.S.; dos Santos, G.L.A.A.; Oliveira, K.M.; Oliveira, R.B.; Rafael Nanni, M. Relationship Between Vegetation Indices, Nutrients Content, and the Biomass Production of Brachiaria (Brachiaria ruziziensis). Commun. Soil Sci. Plant Anal. 2022, 53, 2400–2419. [Google Scholar] [CrossRef]
- Yang, H.; Yin, H.; Li, F.; Hu, Y.; Yu, K. Machine learning models fed with optimized spectral indices to advance crop nitrogen monitoring. Field Crops Res. 2023, 293, 108844. [Google Scholar] [CrossRef]
- Li, H.; Yang, G.; Long, H.; Feng, H.; Xu, B.; Zhao, C. Estimating characteristic coefficient of vertical leaf nitrogen profile within wheat canopy from spectral reflectance. Comput. Electron. Agric. 2023, 206, 107652. [Google Scholar] [CrossRef]
- Filella, I.; Serrano, L.; Serra, J.; Peñuelas, J. Evaluating wheat nitrogen status with canopy reflectance indices and discriminant analysis. Crop Sci. 1995, 35, 1400–1405. [Google Scholar] [CrossRef]
- Sibanda, M.; Mutanga, O.; Rouget, M.; Odindi, J. Exploring the potential of in situ hyperspectral data and multivariate techniques in discriminating different fertilizer treatments in grasslands. JARS 2015, 9, 096033. [Google Scholar] [CrossRef]
- Strachan, I.B.; Pattey, E.; Boisvert, J.B. Impact of nitrogen and environmental conditions on corn as detected by hyperspectral reflectance. Remote Sens. Environ. 2002, 80, 213–224. [Google Scholar] [CrossRef]
- Thomas, S.; Kuska, M.T.; Bohnenkamp, D.; Brugger, A.; Alisaac, E.; Wahabzada, M.; Behmann, J.; Mahlein, A.-K. Benefits of hyperspectral imaging for plant disease detection and plant protection: A technical perspective. J. Plant Dis. Prot. 2018, 125, 5–20. [Google Scholar] [CrossRef]
- Jarecki, W. Production of selected crop plants in Poland over the period of 2010-2019. Acta Agrophysica 2020, 27, 39–45. [Google Scholar] [CrossRef]
- Alam, H.; Khattak, J.Z.K.; Ksiksi, T.S.; Saleem, M.H.; Fahad, S.; Sohail, H.; Ali, Q.; Zamin, M.; El-Esawi, M.A.; Saud, S.; et al. Negative impact of long-term exposure of salinity and drought stress on native Tetraena mandavillei L. Physiol. Plant. 2021, 172, 1336–1351. [Google Scholar] [CrossRef] [PubMed]
- Ikram, M.; Raja, N.I.; Javed, B.; Mashwani, Z.-R.; Hussain, M.; Hussain, M.; Ehsan, M.; Rafique, N.; Malik, K.; Sultana, T. Foliar applications of bio-fabricated selenium nanoparticles to improve the growth of wheat plants under drought stress. Green Process. Synth. 2020, 9, 706–714. [Google Scholar] [CrossRef]
- Ullah, A.; Romdhane, L.; Rehman, A.; Farooq, M. Adequate zinc nutrition improves the tolerance against drought and heat stresses in chickpea. Plant Physiol. Biochem. 2019, 143, 11–18. [Google Scholar] [CrossRef]
- Siedliska, A.; Baranowski, P.; Pastuszka-Woźniak, J.; Zubik, M.; Krzyszczak, J. Identification of plant leaf phosphorus content at different growth stages based on hyperspectral reflectance. BMC Plant Biol. 2021, 21, 28. [Google Scholar] [CrossRef]
- Matejovic, I. Total nitrogen in plant material determinated by means of dry combustion: A possible alternative to determination by Kjeldahl digestion. Commun. Soil Sci. Plant Anal. 1995, 26, 2217–2229. [Google Scholar] [CrossRef]
- Abdlaty, R.; Doerwald-Munoz, L.; Farrell, T.J.; Hayward, J.E.; Fang, Q. Hyperspectral imaging assessment for radiotherapy induced skin-erythema: Pilot study. Photodiagnosis Photodyn. Ther. 2021, 33, 102195. [Google Scholar] [CrossRef]
- Li, Y.; Tan, X.; Zhang, W.; Jiao, Q.; Xu, Y.; Li, H.; Zou, Y.; Yang, L.; Fang, Y. Research and application of several key techniques in hyperspectral image preprocessing. Front. Plant Sci. 2021, 12, 627865. [Google Scholar] [CrossRef]
- King, R.L.; Ruffin, C.; LaMastus, F.E.; Shaw, D.R. The analysis of hyperspectral data using Savitzky-Golay filtering-practical issues. 2. In Proceedings of the IEEE 1999 International Geoscience and Remote Sensing Symposium. IGARSS’99 (Cat. No.99CH36293), Hamburg, Germany, 28 June–2 July 1999; Volume 1. pp. 398–400. [Google Scholar]
- Zhang, Y.J.; Xie, Z.K.; Wang, Y.J.; Su, P.X.; An, L.P.; Gao, H. Effect of water stress on leaf photosynthesis, chlorophyll content, and growth of oriental lily. Russ. J. Plant Physiol. 2011, 58, 844–850. [Google Scholar] [CrossRef]
- Kopsell, D.A.; Kopsell, D.E.; Curran-Celentano, J. Carotenoid pigments in kale are influenced by nitrogen concentration and form. J. Sci. Food Agric. 2007, 87, 900–907. [Google Scholar] [CrossRef]
- He, J.; Yang, W.; Qin, L.; Fan, D.-Y.; Chow, W.S. Photoinactivation of Photosystem II in wild-type and chlorophyll b-less barley leaves: Which mechanism dominates depends on experimental circumstances. Photosynth. Res. 2015, 126, 399–407. [Google Scholar] [CrossRef]
- Colovic, M.; Yu, K.; Todorovic, M.; Cantore, V.; Hamze, M.; Albrizio, R.; Stellacci, A.M. Hyperspectral vegetation indices to assess water and nitrogen status of sweet maize crop. Agronomy 2022, 12, 2181. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, X.; Hou, Z.; Ning, J.; Zhang, Z. Discrimination of nitrogen fertilizer levels of tea plant (Camellia sinensis) based on hyperspectral imaging. J. Sci. Food Agric. 2018, 98, 4659–4664. [Google Scholar] [CrossRef]
- Zhao, B.; Ma, B.-L.; Hu, Y.; Liu, J. Characterization of nitrogen and water status in oat leaves using optical sensing approach. J. Sci. Food Agric. 2015, 95, 367–378. [Google Scholar] [CrossRef]
- Zhao, D.; Reddy, K.R.; Kakani, V.G.; Reddy, V.R. Nitrogen deficiency effects on plant growth, leaf photosynthesis, and hyperspectral reflectance properties of sorghum. Eur. J. Agron. 2005, 22, 391–403. [Google Scholar] [CrossRef]
- Bruning, B.; Liu, H.; Brien, C.; Berger, B.; Lewis, M.; Garnett, T. The Development of Hyperspectral Distribution Maps to Predict the Content and Distribution of Nitrogen and Water in Wheat (Triticum aestivum). Front. Plant Sci. 2019, 10, 1380. [Google Scholar] [CrossRef]
- Hennessy, A.; Clarke, K.; Lewis, M. Hyperspectral classification of plants: A review of waveband selection generalisability. Remote Sens. 2020, 12, 113. [Google Scholar] [CrossRef]
- Liu, J.; Wu, N.; Wang, H.; Sun, J.; Peng, B.; Jiang, P.; Bai, E. Nitrogen addition affects chemical compositions of plant tissues, litter and soil organic matter. Ecology 2016, 97, 1796–1806. [Google Scholar] [CrossRef] [PubMed]
- Loozen, Y.; Rebel, K.T.; deJong, S.M.; Lu, M.; Ollinger, S.V.; Wassen, M.J.; Karssenberg, D. Mapping canopy nitrogen in European forests using remote sensing and environmental variables with the random forests method. Remote Sens. Environ. 2020, 247, 111933. [Google Scholar] [CrossRef]
- Abdel- Rahman, E.M.; Ahmed, F.B.; Ismail, R. Random forest regression and spectral band selection for estimating sugarcane leaf nitrogen concentration using EO-1 Hyperion hyperspectral data. Int. J. Remote Sens. 2013, 34, 712–728. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, T.; Shi, L.; Wang, W.; Niu, Z.; Guo, W.; Ma, X. Combining spectral and texture features of UAV hyperspectral images for leaf nitrogen content monitoring in winter wheat. Int. J. Remote Sens. 2022, 43, 2335–2356. [Google Scholar] [CrossRef]
- Han, N.; Zhang, B.; Liu, Y.; Peng, Z.; Zhou, Q.; Wei, Z. Rapid Diagnosis of Nitrogen Nutrition Status in Summer Maize over Its Life Cycle by a Multi-Index Synergy Model Using Ground Hyperspectral and UAV Multispectral Sensor Data. Atmosphere 2022, 13, 122. [Google Scholar] [CrossRef]
- Yin, C.; Lv, X.; Zhang, L.; Ma, L.; Wang, H.; Zhang, L.; Zhang, Z. Hyperspectral UAV Images at Different Altitudes for Monitoring the Leaf Nitrogen Content in Cotton Crops. Remote Sens. 2022, 14, 2576. [Google Scholar] [CrossRef]
- Olson, M.B.; Crawford, M.M.; Vyn, T.J. Hyperspectral indices for predicting nitrogen use efficiency in maize hybrids. Remote Sens. 2022, 14, 1721. [Google Scholar] [CrossRef]
- Fan, K.; Li, F.; Chen, X.; Li, Z.; Mulla, D.J. Nitrogen Balance Index Prediction of Winter Wheat by Canopy Hyperspectral Transformation and Machine Learning. Remote Sens. 2022, 14, 3504. [Google Scholar] [CrossRef]
- Benmouna, B.; Pourdarbani, R.; Sabzi, S.; Fernandez-Beltran, R.; García-Mateos, G.; Molina-Martínez, J.M. Comparison of Classic Classifiers, Metaheuristic Algorithms and Convolutional Neural Networks in Hyperspectral Classification of Nitrogen Treatment in Tomato Leaves. Remote Sens. 2022, 14, 6366. [Google Scholar] [CrossRef]
- de FátimadaSilva, F.; Luz, P.H.C.; Romualdo, L.M.; Marin, M.A.; Zúñiga, A.M.G.; Herling, V.R.; Bruno, O.M. A diagnostic tool for magnesium nutrition in maize based on image analysis of different leaf sections. Crop Sci. 2014, 54, 738–745. [Google Scholar] [CrossRef]
- Sun, Y.; Gao, J.; Wang, K.; Shen, Z.; Chen, L. Utilization of machine vision to monitor the dynamic responses of rice leaf morphology and colour to nitrogen, phosphorus, and potassium deficiencies. J. Spectrosc. 2018, 2018, e1469314. [Google Scholar] [CrossRef]
- Culman, M.A.; Gomez, J.A.; Talavera, J.; Quiroz, L.A.; Tobon, L.E.; Aranda, J.M.; Garreta, L.E.; Bayona, C.J.A. A novel application for identification of nutrient deficiencies in oil palm using the internet of things. In Proceedings of the 2017 5th IEEE International Conference on Mobile Cloud Computing, Services, and Engineering (MobileCloud), San Francisco, CA, USA, 6–7 April 2017; pp. 169–172. [Google Scholar]
| Calibration Set | Validation Set | ||||
|---|---|---|---|---|---|
| Overall Accuracy (%) | Kappa Coefficient | Overall Accuracy (%) | Kappa Coefficient | ||
| HW | BNN | 98 | 0.98 | 83 | 0.78 |
| Logistic | 66 | 0.54 | 78 | 0.70 | |
| RF | 100 | 1 | 86 | 0.81 | |
| SVM | 83 | 0.78 | 75 | 0.67 | |
| kNN | 100 | 1 | 78 | 0.70 | |
| OW | BNN | 96 | 0.95 | 78 | 0.70 |
| Logistic | 83 | 0.78 | 80 | 0.74 | |
| RF | 100 | 1 | 83 | 0.78 | |
| SVM | 0.85 | 0.80 | 80 | 0.74 | |
| kNN | 100 | 1 | 78 | 0.70 | |
| LW | BNN | 92 | 0.89 | 69 | 0.59 |
| Logistic | 81 | 0.75 | 72 | 0.63 | |
| RF | 100 | 1 | 81 | 0.74 | |
| SVM | 78 | 0.70 | 72 | 0.63 | |
| kNN | 100 | 1 | 67 | 0.56 | |
| Calibration Set | Validation Set | ||||
|---|---|---|---|---|---|
| Overall Accuracy (%) | Kappa Coefficient | Overall Accuracy (%) | Kappa Coefficient | ||
| HW | BNN | 93 | 0.91 | 78 | 0.70 |
| Logistic | 86 | 0.81 | 75 | 0.67 | |
| RF | 100 | 1 | 81 | 0.74 | |
| SVM | 68 | 0.58 | 69 | 0.59 | |
| kNN | 100 | 1 | 75 | 0.67 | |
| OW | BNN | 97 | 0.96 | 72 | 0.63 |
| Logistic | 86 | 0.81 | 78 | 0.70 | |
| RF | 100 | 1 | 81 | 0.74 | |
| SVM | 86 | 0.81 | 75 | 0.67 | |
| kNN | 100 | 1 | 67 | 0.56 | |
| LW | BNN | 94 | 0.93 | 67 | 0.56 |
| Logistic | 88 | 0.84 | 72 | 0.63 | |
| RF | 100 | 1 | 78 | 0.70 | |
| SVM | 84 | 0.79 | 69 | 0.59 | |
| kNN | 100 | 1 | 75 | 0.67 | |
| Class | TP Rate | FP Rate | Precision | Recall | F1-Score | Accuracy | |
|---|---|---|---|---|---|---|---|
| Sugar Beet LW | All | 0.806 | 0.065 | 0.861 | 0.806 | 0.797 | |
| N33 | 0.667 | 0.000 | 1.000 | 0.667 | 0.800 | ||
| N67 | 1.000 | 0.148 | 0.692 | 1.000 | 0.818 | 0.81 | |
| N100 | 0.556 | 0.000 | 1.000 | 0.556 | 0.714 | ||
| N133 | 1.000 | 0.111 | 0.750 | 1.000 | 0.857 | ||
| Sugar Beet OW | All | 0.833 | 0.056 | 0.835 | 0.833 | 0.833 | |
| N33 | 1.000 | 0.000 | 1.000 | 1.000 | 1.000 | ||
| N67 | 0.778 | 0.111 | 0.700 | 0.778 | 0.737 | 0.83 | |
| N100 | 0.889 | 0.037 | 0.889 | 0.889 | 0.889 | ||
| N133 | 0.667 | 0.074 | 0.750 | 0.667 | 0.706 | ||
| Sugar Beet HW | All | 0.861 | 0.046 | 0.863 | 0.861 | 0.855 | |
| N33 | 1.000 | 0.074 | 0.818 | 1.000 | 0.900 | ||
| N67 | 1.000 | 0.037 | 0.900 | 1.000 | 0.947 | 0.86 | |
| N100 | 0.667 | 0.037 | 0.857 | 0.667 | 0.750 | ||
| N133 | 0.778 | 0.037 | 0.875 | 0.778 | 0.824 |
| Class | TP Rate | FP Rate | Precision | Recall | F1-Score | Accuracy | |
|---|---|---|---|---|---|---|---|
| Celery LW | All | 0.778 | 0.074 | 0.797 | 0.778 | 0.781 | |
| N33 | 0.778 | 0.000 | 1.000 | 0.778 | 0.875 | ||
| N67 | 0.889 | 0.074 | 0.800 | 0.889 | 0.842 | 0.81 | |
| N100 | 0.677 | 0.074 | 0.750 | 0.667 | 0.706 | ||
| N133 | 0.778 | 0.148 | 0.636 | 0.778 | 0.781 | ||
| Celery OW | All | 0.806 | 0.065 | 0.841 | 0.806 | 0.810 | |
| N33 | 0.667 | 0.000 | 1.000 | 0.667 | 0.800 | ||
| N67 | 0.778 | 0.148 | 0.636 | 0.778 | 0.700 | 0.81 | |
| N100 | 0.889 | 0.000 | 1.000 | 0.889 | 0.941 | ||
| N133 | 0.889 | 0.111 | 0.727 | 0.889 | 0.800 | ||
| Celery HW | All | 0.806 | 0.065 | 0.819 | 0.806 | 0.808 | |
| N33 | 0.778 | 0.000 | 1.000 | 0.778 | 0.875 | ||
| N67 | 0.778 | 0.111 | 0.700 | 0.778 | 0.737 | 0.81 | |
| N100 | 0.778 | 0.074 | 0.778 | 0.778 | 0.778 | ||
| N133 | 0.889 | 0.074 | 0.800 | 0.889 | 0.842 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Siłuch, M.; Siedliska, A.; Bartmiński, P.; Kociuba, W.; Baranowski, P.; Krzyszczak, J. The Impact of Water Availability on the Discriminative Status of Nitrogen (N) in Sugar Beet and Celery Using Hyperspectral Imaging Methods. Appl. Sci. 2023, 13, 6072. https://doi.org/10.3390/app13106072
Siłuch M, Siedliska A, Bartmiński P, Kociuba W, Baranowski P, Krzyszczak J. The Impact of Water Availability on the Discriminative Status of Nitrogen (N) in Sugar Beet and Celery Using Hyperspectral Imaging Methods. Applied Sciences. 2023; 13(10):6072. https://doi.org/10.3390/app13106072
Chicago/Turabian StyleSiłuch, Marcin, Anna Siedliska, Piotr Bartmiński, Waldemar Kociuba, Piotr Baranowski, and Jaromir Krzyszczak. 2023. "The Impact of Water Availability on the Discriminative Status of Nitrogen (N) in Sugar Beet and Celery Using Hyperspectral Imaging Methods" Applied Sciences 13, no. 10: 6072. https://doi.org/10.3390/app13106072
APA StyleSiłuch, M., Siedliska, A., Bartmiński, P., Kociuba, W., Baranowski, P., & Krzyszczak, J. (2023). The Impact of Water Availability on the Discriminative Status of Nitrogen (N) in Sugar Beet and Celery Using Hyperspectral Imaging Methods. Applied Sciences, 13(10), 6072. https://doi.org/10.3390/app13106072









