Primary Stability Assessment of Conical Implants in Under-Prepared Sites: An In Vitro Study in Low-Density Polyurethane Foams
Abstract
1. Introduction
2. Materials and Methods
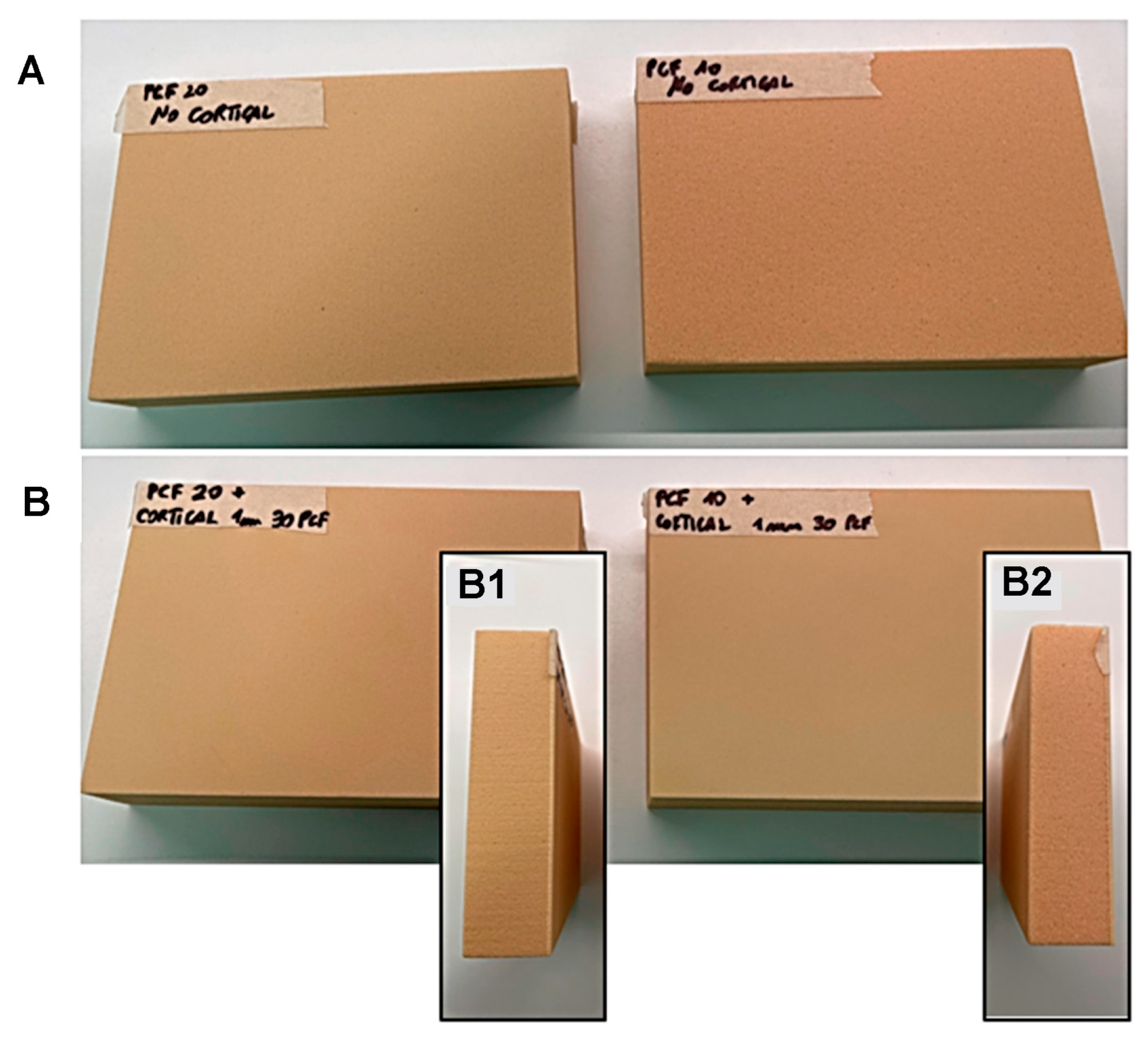
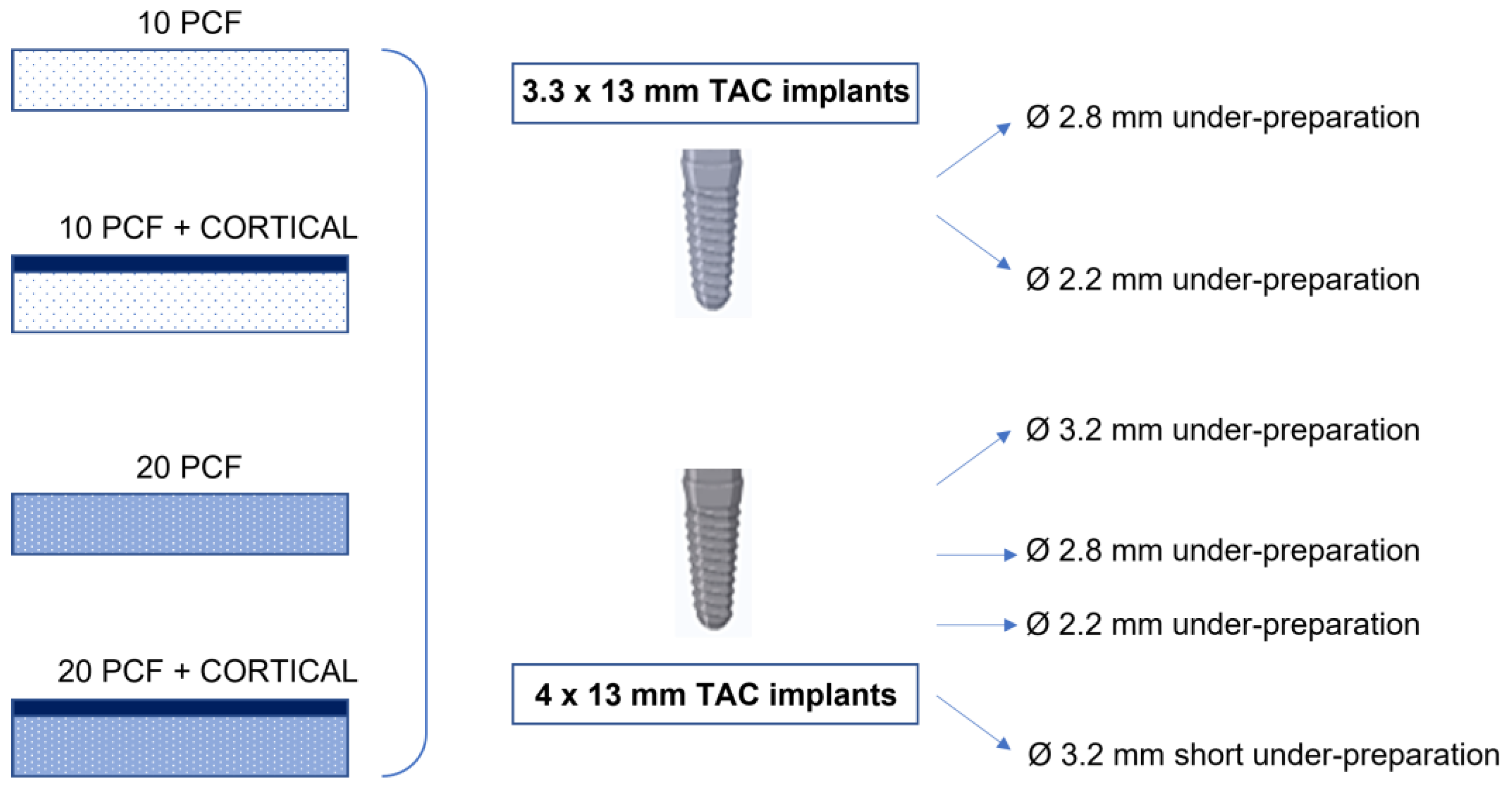
2.1. Polyurethane Foams
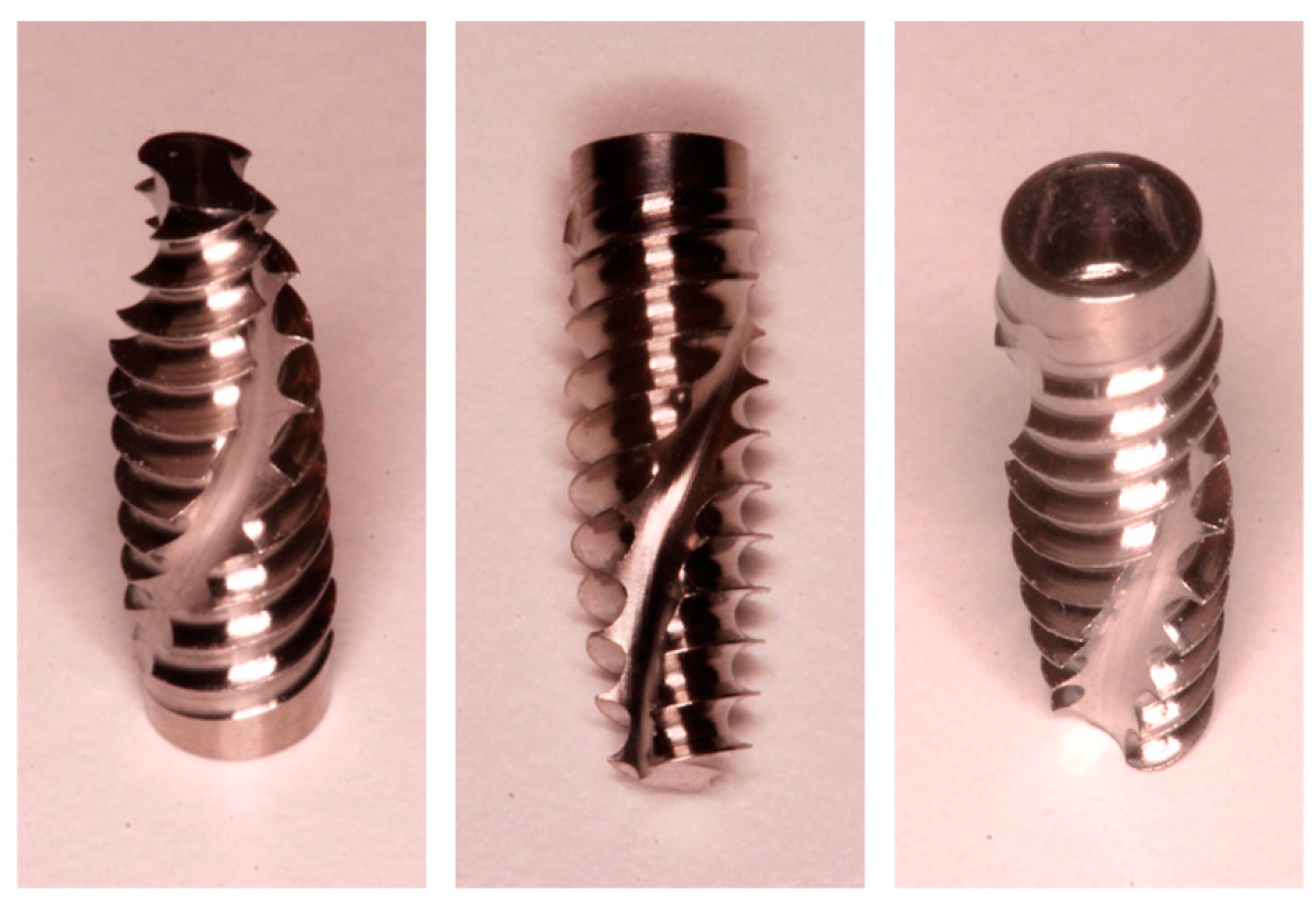
2.2. Implants
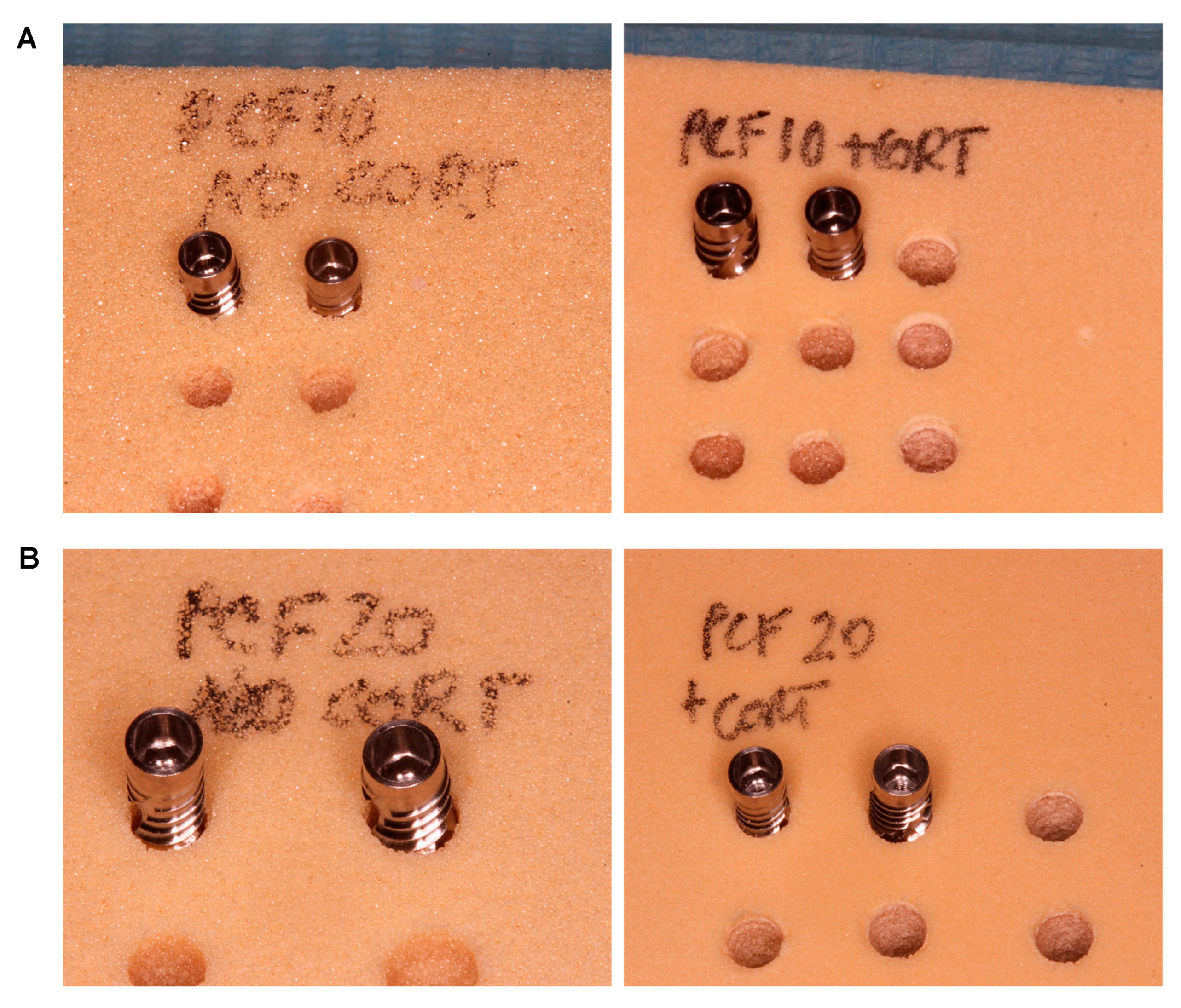
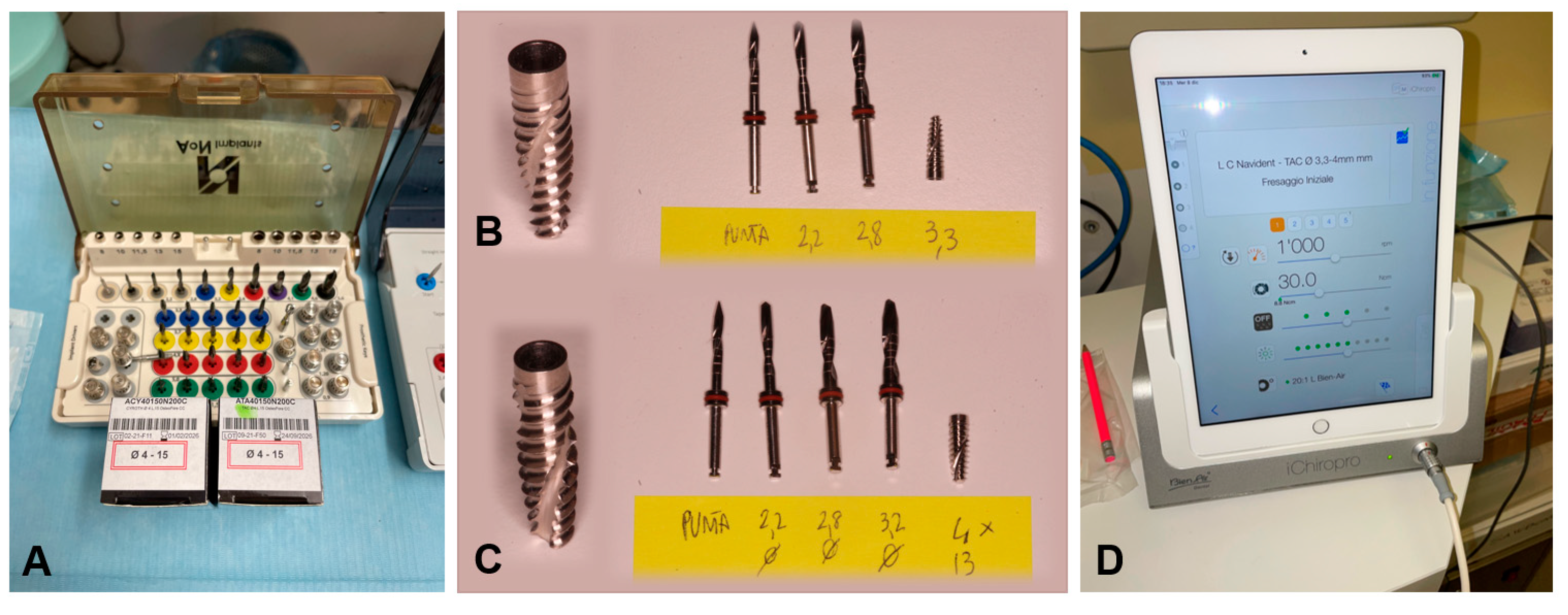
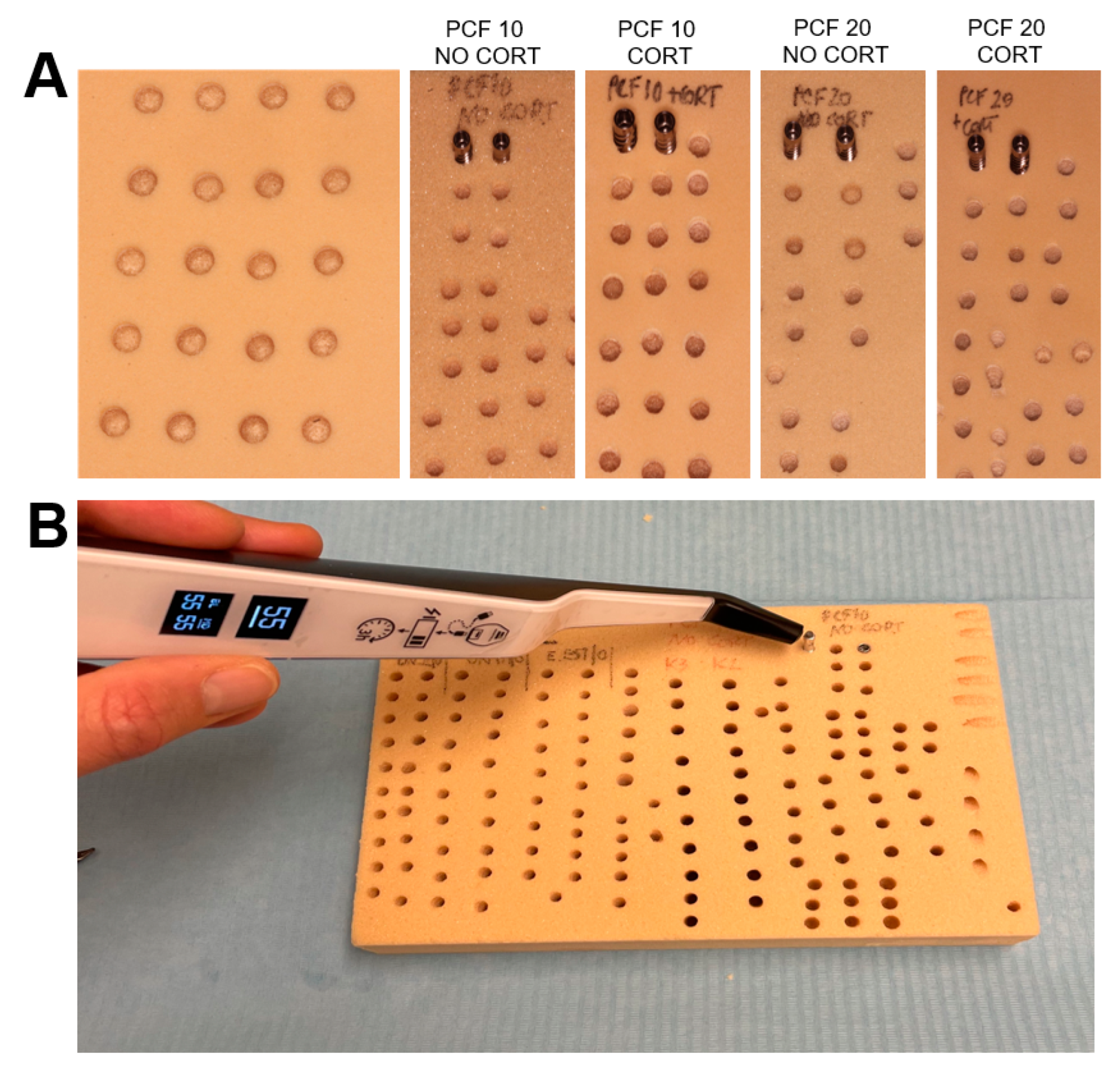
2.3. Drilling Protocol
- A 2.8 mm diameter site (under-preparation size: 0.5 mm);
- A 2.2 mm diameter site (under-preparation size: 1.1 mm).
- A 3.2 mm diameter site (under-preparation size: 0.8 mm);
- A 2.8 mm diameter site (under-preparation size: 1.2 mm);
- A 2.2 mm diameter site (under-preparation size: 1.8 mm);
- A 3.2 mm diameter site (“short”): a 2.8 mm diameter site preparation was performed and, then, another preparation up to a diameter of 3.2 mm only for the first 4–5 mm of the osteotomy (under-preparation size: 1.2 mm in the central and apical parts of the implant and 1.8 mm in the coronal portion of the osteotomy). This protocol was thought to minimize the friction and the stress at the coronal portion of the osteotomy without increasing the whole implant contact with the bone, in case of the presence of a thick cortical bone.
- An initial pointed bur at 300 rpm;
- A 2.2 mm bur at 300 rpm;
- Eventually, a 2.8 mm bur at 300 rpm;
- Eventually, a 3.2 mm bur at 300 rpm;
- Or, a 2.8 mm bur and partially a 3.2 mm bur at 300 rpm, as previously explained in the “short” protocol.
2.4. Statistical Analysis
3. Results
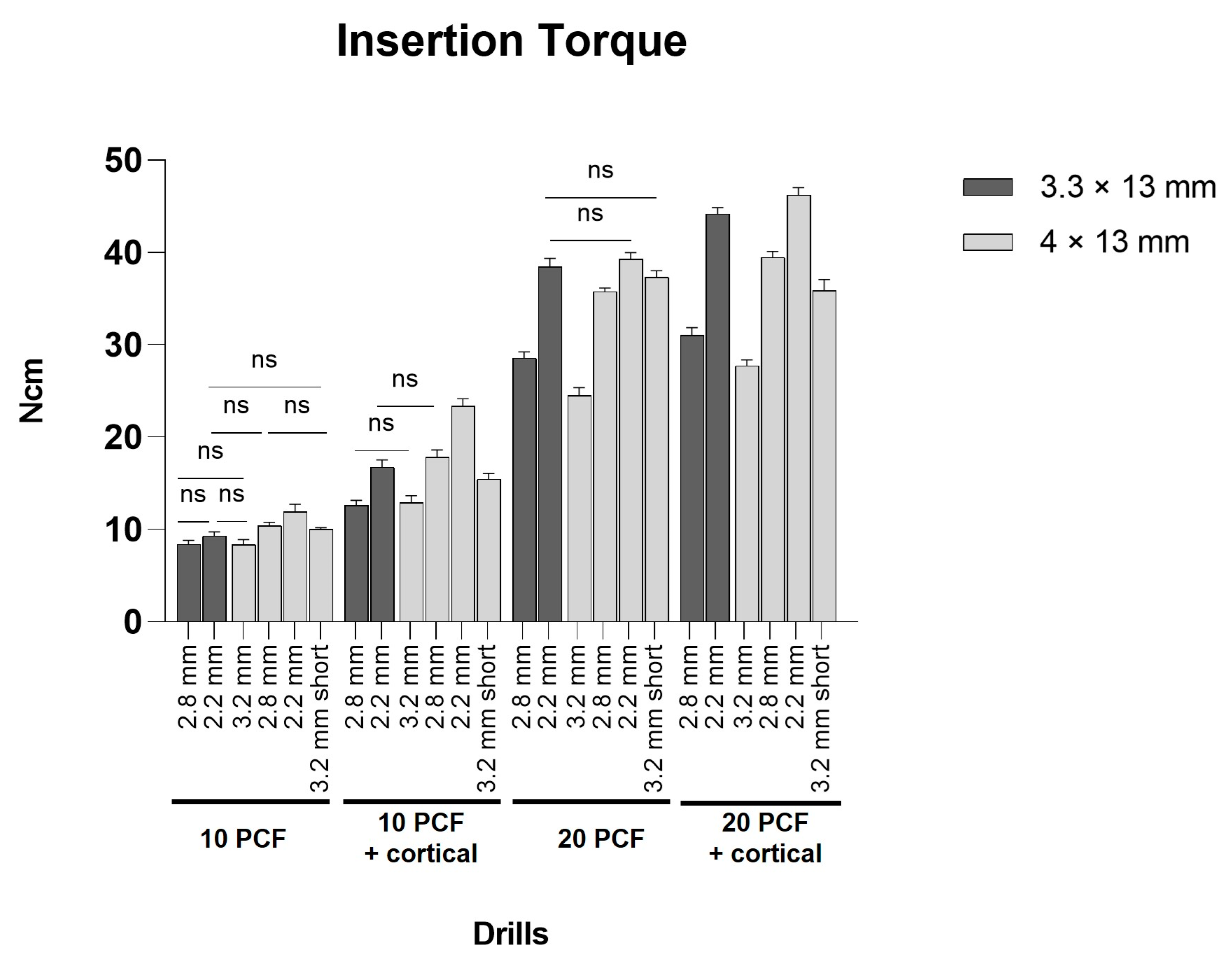
3.1. Evaluation of IT Values
3.2. Evaluation of RT Values
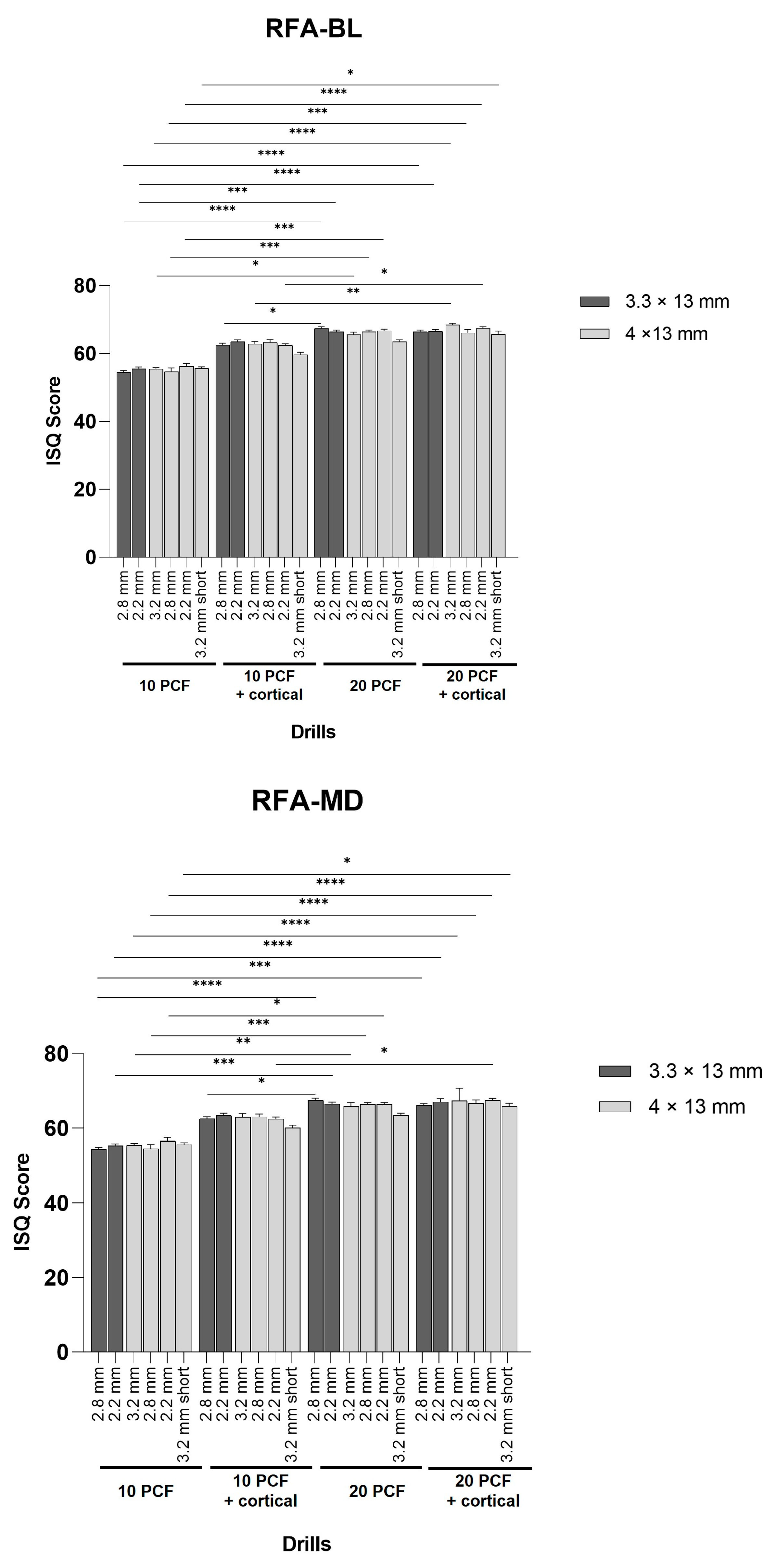
3.3. Evaluation of RFA Values
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Marquezan, M.; Osório, A.; Sant’Anna, E.; Souza, M.M.; Maia, L. Does bone mineral density influence the primary stability of dental implants? A systematic review. Clin. Oral Implant. Res. 2012, 23, 767–774. [Google Scholar] [CrossRef]
- Jimbo, R.; Tovar, N.; Marin, C.; Teixeira, H.; Anchieta, R.; Silveira, L.; Janal, M.; Shibli, J.; Coelho, P. The impact of a modified cutting flute implant design on osseointegration. Int. J. Oral Maxillofac. Surg. 2014, 43, 883–888. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.-Y.; Huang, H.-L.; Fuh, L.-J.; Tsai, M.-T.; Hsu, J.-T. The Effects of Insertion Approach on the Stability of Dental Implants. Appl. Bionics Biomech. 2022, 2022, 7188240. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi, Y.; Shiota, M.; FuJii, M.; Sekiya, M.; Ozeki, M. Development and application of a direct method to observe the implant/bone interface using simulated bone. Springerplus 2016, 5, 494. [Google Scholar] [CrossRef] [PubMed]
- Lemos, B.F.; Lopez-Jarana, P.; Falcao, C.; Ríos-Carrasco, B.; Gil, J.; Ríos-Santos, J.V.; Herrero-Climent, M. Effects of Different Undersizing Site Preparations on Implant Stability. Int. J. Environ. Res. Public Health 2020, 17, 8965. [Google Scholar] [CrossRef] [PubMed]
- Krischik, D.; Tokgöz, S.E.; van Orten, A.; Friedmann, A.; Bilhan, H. An In Vitro Evaluation of Primary Stability Values for Two Differently Designed Implants to Suit Immediate Loading in Very Soft Bone. Dent. J. 2021, 9, 5. [Google Scholar] [CrossRef]
- Al-Sabbagh, M.; Eldomiaty, W.; Khabbaz, Y. Can Osseointegration Be Achieved Without Primary Stability? Dent. Clin. N. Am. 2019, 63, 461–473. [Google Scholar] [CrossRef]
- Trisi, P.; Perfetti, G.; Baldoni, E.; Berardi, D.; Colagiovanni, M.; Scogna, G. Implant micromotion is related to peak insertion torque and bone density. Clin. Oral Implant. Res. 2009, 20, 467–471. [Google Scholar] [CrossRef]
- Hsieh, M.-C.; Huang, C.-H.; Hsu, M.-L. Effect of cutting flute design features on primary stability of immediate implant placement and restoration: A dynamic experimental analysis. Med. Biol. Eng. Comput. 2023, 61, 475–484. [Google Scholar] [CrossRef]
- Möhlhenrich, S.; Heussen, N.; Elvers, D.; Steiner, T.; Hölzle, F.; Modabber, A. Compensating for poor primary implant stability in different bone densities by varying implant geometry: A laboratory study. Int. J. Oral Maxillofac. Surg. 2015, 44, 1514–1520. [Google Scholar] [CrossRef]
- Gonzalez-Serrano, J.; Ortega-Aranegui, R.; Lopez-Quiles, J. In vitro comparison of primary stability of two implant designs in D3 bone. Med. Oral Patol. Oral Cir. Bucal. 2017, 22, e473–e477. [Google Scholar] [CrossRef] [PubMed]
- Kohli, N.; Stoddart, J.C.; van Arkel, R.J. The limit of tolerable micromotion for implant osseointegration: A systematic review. Sci. Rep. 2021, 11, 10797. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; Zheng, X.; Zeng, G.; Xu, Y.; Qu, X.; Zhu, M.; Lu, E. Clinical efficacy of early loading versus conventional loading of dental implants. Sci. Rep. 2015, 5, 15995. [Google Scholar] [CrossRef] [PubMed]
- Calandriello, R.; Tomatis, M.; Rangert, B. Immediate functional loading of Brånemark System implants with enhanced initial stability: A prospective 1- to 2-year clinical and radiographic study. Clin. Implant. Dent. Relat. Res. 2003, 55 (Suppl. 1), 10–20. [Google Scholar] [CrossRef]
- Del Giudice, R.; Piattelli, A.; Grande, N.; Cataneo, E.; Crispino, A.; Petrini, M. Implant insertion torque value in immediate loading: A retrospective study. Med. Oral Patol. Oral Cir Bucal. 2019, 24, e398–e403. [Google Scholar] [CrossRef]
- Tsolaki, I.N.; Tonsekar, P.P.; Najafi, B.; Drew, H.J.; Sullivan, A.J.; Petrov, S.D. Comparison of Osteotome and Conventional Drilling Techniques for Primary Implant Stability: An In Vitro Study. J. Oral Implant. 2016, 42, 321–325. [Google Scholar] [CrossRef]
- Gehrke, S.A.; Guirado, J.L.C.; Bettach, R.; Del Fabbro, M.; Martínez, C.P.-A.; Shibli, J.A. Evaluation of the insertion torque, implant stability quotient and drilled hole quality for different drill design: An in vitro Investigation. Clin. Oral Implant. Res. 2018, 29, 656–662. [Google Scholar] [CrossRef]
- Al-Tarawneh, S.; Thalji, G.; Cooper, L. Macrogeometric Differentiation of Dental Implant Primary Stability: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2022, 37, 1110–1118. [Google Scholar] [CrossRef]
- Romanos, G.E.; Bastardi, D.J.; Moore, R.; Kakar, A.; Herin, Y.; Delgado-Ruiz, R.A. In Vitro Effect of Drilling Speed on the Primary Stability of Narrow Diameter Implants with Varying Thread Designs Placed in Different Qualities of Simulated Bone. Materials 2019, 12, 1350. [Google Scholar] [CrossRef]
- Di Stefano, D.A.; Arosio, P.; Gastaldi, G.; Gherlone, E. The insertion torque-depth curve integral as a measure of implant primary stability: An in vitro study on polyurethane foam blocks. J. Prosthet. Dent. 2018, 120, 706–714. [Google Scholar] [CrossRef]
- Romanos, G.E.; Delgado-Ruiz, R.A.; Sacks, D.; Calvo-Guirado, J.L. Influence of the implant diameter and bone quality on the primary stability of porous tantalum trabecular metal dental implants: An in vitro biomechanical study. Clin. Oral Implant. Res. 2018, 29, 649–655. [Google Scholar] [CrossRef]
- Herrero-Climent, M.; Lemos, B.F.; Herrero-Climent, F.; Falcao, C.; Oliveira, H.; Herrera, M.; Gil, F.J.; Ríos-Carrasco, B.; Ríos-Santos, J.V. Influence of Implant Design and Under-Preparation of the Implant Site on Implant Primary Stability. An In Vitro Study. Int. J. Environ. Res. Public Health 2020, 17, 4436. [Google Scholar] [CrossRef] [PubMed]
- Turkyilmaz, I.; Tozum, T. Enhancing primary implant stability by undersizing implant site preparation: A human cadaver study. J. Stomatol. Oral Maxillofac. Surg. 2020, 121, 58–62. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of Stepped Osteotomy on Primary Stability of Implants Inserted in Low-Density Bone Sites: An In Vitro Study. Int. J. Oral Maxillofac. Implant. 2017, 32, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Arosio, P.; Greco, G.B.; Zaniol, T.; Iezzi, G.; Perrotti, V.; Di Stefano, D.A. Sinus augmentation and concomitant implant placement in low bone-density sites. A retrospective study on an undersized drilling protocol and primary stability. Clin. Implant. Dent. Relat. Res. 2018, 20, 151–159. [Google Scholar] [CrossRef]
- Boustany, C.M.; Reed, H.; Cunningham, G.; Richards, M.; Kanawati, A. Effect of a Modified Stepped Osteotomy on the Primary Stability of Dental Implants in Low-Density Bone: A Cadaver Study. Int. J. Oral Maxillofac. Implant. 2015, 30, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Comuzzi, L.; Tumedei, M.; Romasco, T.; Petrini, M.; Afrashtehfar, K.I.; Inchingolo, F.; Piattelli, A.; Di Pietro, N. Insertion Torque, Removal Torque, and Resonance Frequency Analysis Values of Ultrashort, Short, and Standard Dental Implants: An In Vitro Study on Polyurethane Foam Sheets. J. Funct. Biomater. 2022, 14, 10. [Google Scholar] [CrossRef]
- Comuzzi, L.; Tumedei, M.; Di Pietro, N.; Romasco, T.; Montesani, L.; Piattelli, A.; Covani, U. Are Implant Threads Important for Implant Stability? An In Vitro Study Using Low-Density Polyurethane Sheets. Eng 2023, 4, 1167–1178. [Google Scholar] [CrossRef]
- Fanali, S.; Tumedei, M.; Pignatelli, P.; Inchingolo, F.; Pennacchietti, P.; Pace, G.; Piattelli, A. Implant primary stability with an osteocondensation drilling protocol in different density polyurethane blocks. Comput. Methods Biomech. Biomed. Eng. 2021, 24, 14–20. [Google Scholar] [CrossRef]
- Meredith, N.; Alleyne, D.; Cawley, P. Quantitative determination of the stability of the implant-tissue interface using resonance frequency analysis. Clin. Oral Implant. Res. 1996, 7, 261–267. [Google Scholar] [CrossRef]
- Mish, C.E. Bone Dentistry. A Key Determinant for Clinical Success. Contemp. Implant Dent. 1998, 1, 109–118. [Google Scholar]
- Calvert, K.L.; Trumble, K.P.; Webster, T.J.; Kirkpatrick, L.A. Characterization of commercial rigid polyurethane foams used as bone analogs for implant testing. J. Mater. Sci. Mater. Med. 2010, 21, 1453–1461. [Google Scholar] [CrossRef] [PubMed]
- Nagaraja, S.; Palepu, V. Comparisons of Anterior Plate Screw Pullout Strength Between Polyurethane Foams and Thoracolumbar Cadaveric Vertebrae. J. Biomech. Eng. 2016, 138, 104505. [Google Scholar] [CrossRef] [PubMed]
- ASTM F1839; Standard Specification for Rigid Polyurethane Foam for Use as a Standard Material for Testing Orthopaedic Devices and Instruments. American Society for Testing and Materials; ASTM: West Conshohocken, PA, USA, 2008. [CrossRef]
- Tabassum, A.; Meijer, G.J.; Wolke, J.G.; Jansen, J.A. Influence of surgical technique and surface roughness on the primary stability of an implant in artificial bone with different cortical thickness: A laboratory study. Clin. Oral Implant. Res. 2010, 21, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Degidi, M.; Daprile, G.; Piattelli, A. Influence of Underpreparation on Primary Stability of Implants Inserted in Poor Quality Bone Sites: An In Vitro Study. J. Oral Maxillofac. Surg. 2015, 73, 1084–1088. [Google Scholar] [CrossRef]
- Stocchero, M.; Toia, M.; Jinno, Y.; Cecchinato, F.; Becktor, J.P.; Naito, Y.; Halldin, A.; Jimbo, R. Influence of different drilling preparation on cortical bone: A biomechanical, histological, and micro-CT study on sheep. Clin. Oral Implant. Res. 2018, 29, 707–715. [Google Scholar] [CrossRef]
- Antonacci, D.; Del Fabbro, M.; Bollero, P.; Stocchero, M.; Jinno, Y.; Canullo, L. Clinical effects of conventional and underprepared drilling preparation of the implant site based on bone density: A systematic review and meta-regression. J. Prosthodont. Res. 2023, 67, 23–34. [Google Scholar] [CrossRef]
- Karl, M.; Irastorza-Landa, A. Does Implant Design Affect Primary Stability in Extraction Sites? Quintessence Int. 2017, 48, 219–224. [Google Scholar] [CrossRef]
- Lahens, B.; Neiva, R.; Tovar, N.; Alifarag, A.M.; Jimbo, R.; Bonfante, E.A.; Bowers, M.M.; Cuppini, M.; Freitas, H.; Witek, L.; et al. Biomechanical and histologic basis of osseodensification drilling for endosteal implant placement in low density bone. An experimental study in sheep. J. Mech. Behav. Biomed. Mater. 2016, 63, 56–65. [Google Scholar] [CrossRef]
- Gaikwad, A.M.; Joshi, A.A.; Nadgere, J.B. Biomechanical and histomorphometric analysis of endosteal implants placed by using the osseodensification technique in animal models: A systematic review and meta-analysis. J. Prosthet. Dent. 2022, 127, 61–70. [Google Scholar] [CrossRef]
- Yeh, Y.-T.; Chu, T.-M.; Blanchard, S.; Hamada, Y. Effects on Ridge Dimensions, Bone Density, and Implant Primary Stability with Osseodensification Approach in Implant Osteotomy Preparation. Int. J. Oral Maxillofac. Implant. 2021, 36, 474–484. [Google Scholar] [CrossRef] [PubMed]
- Mullings, O.; Tovar, N.; de Bortoli, J.; Parra, M.; Torroni, A.; Coelho, P.; Witek, L. Osseodensification Versus Subtractive Drilling Techniques in Bone Healing and Implant Osseointegration: Ex Vivo Histomorphologic/Histomorphometric Analysis in a Low-Density Bone Ovine Model. Int. J. Oral Maxillofac. Implant. 2021, 36, 903–909. [Google Scholar] [CrossRef] [PubMed]
- El-Kholey, K.E.; Elkomy, A. Does the Drilling Technique for Implant Site Preparation Enhance Implant Success in Low-Density Bone? A Systematic Review. Implant. Dent. 2019, 28, 500–509. [Google Scholar] [CrossRef] [PubMed]
- Bernabeu-Mira, J.C.; Soto-Peñaloza, D.; Peñarrocha-Diago, M.; Camacho-Alonso, F.; Rivas-Ballester, R.; Peñarrocha-Oltra, D. Low-speed drilling without irrigation versus conventional drilling for dental implant osteotomy preparation: A systematic review. Clin. Oral Investig. 2021, 25, 4251–4267. [Google Scholar] [CrossRef] [PubMed]
- Simeone, S.G.; Rios, M.; Simonpietri, J. “Reverse torque of 30 Ncm applied to dental implants as test for osseointegration”—A human observational study. Int. J. Implant. Dent. 2016, 2, 26. [Google Scholar] [CrossRef] [PubMed]
- Ferguson, S.J.; Broggini, N.; Wieland, M.; De Wild, M.; Rupp, F.; Geis-Gerstorfer, J.; Cochran, D.L.; Buser, D. Biomechanical evaluation of the interfacial strength of a chemically modified sandblasted and acid-etched titanium surface. J. Biomed. Mater. Res. A 2006, 2, 291–297. [Google Scholar] [CrossRef]
- Gottlow, J.; Dard, M.; Kjellson, F.; Obrecht, M.; Sennerby, L. Evaluation of a New Titanium-Zirconium Dental Implant: A Biomechanical and Histological Comparative Study in the Mini Pig. Clin. Implant. Dent. Relat. Res. 2012, 14, 538–545. [Google Scholar] [CrossRef]
- Thurzo, A.; Gálfiová, P.; Nováková, Z.V.; Polák, Š.; Varga, I.; Strunga, M.; Urban, R.; Surovková, J.; Leško, Ľ.; Hajdúchová, Z.; et al. Fabrication and In Vitro Characterization of Novel Hydroxyapatite Scaffolds 3D Printed Using Polyvinyl Alcohol as a Thermoplastic Binder. Int. J. Mol. Sci. 2022, 23, 14870. [Google Scholar] [CrossRef]


| Density | Compression | Shear | ||||
|---|---|---|---|---|---|---|
| PCF | g/cc | Strength (MPa) | Modulus (MPa) | Strength (MPa) | Modulus (MPa) | |
| Sawbones Europe AB (Malmö, Sweden) | 10 | 0.16 | 2.2 | 58 | 1.6 | 19 |
| 20 | 0.32 | 8.4 | 210 | 4.3 | 49 | |
| 30 | 0.48 | 18 | 445 | 7.6 | 87 | |
| ASTM F1839-08 required values [34] | 10 | 0.14–0.18 | 1.745–2.820 | 45.75–71.70 | 1.225–2.010 | 15.15–22.75 |
| 20 | 0.29–0.35 | 6.630–10.45 | 167.5–257.5 | 3.395–5.275 | 40.75–59.25 | |
| 30 | 0.43–0.53 | 14.30–22.70 | 355.5–548.5 | 7.460–11.95 | 71.70–105.0 | |
| IT | 10 PCF | 10 PCF + Cortical | 20 PCF | 20 PCF + Cortical | ||||||||||||||||||||
| a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | |
| Mean | 8.38 | 9.31 | 8.36 | 10.38 | 11.88 | 10.03 | 12.60 | 16.73 | 12.89 | 17.80 | 23.34 | 15.43 | 28.58 | 38.45 | 24.47 | 35.75 | 39.28 | 37.33 | 31.02 | 44.19 | 27.74 | 39.47 | 46.21 | 35.89 |
| SD | 0.43 | 0.41 | 0.52 | 0.38 | 0.86 | 1.57 | 0.55 | 0.77 | 0.75 | 0.81 | 0.79 | 0.64 | 0.63 | 0.89 | 0.86 | 0.39 | 0.68 | 0.68 | 0.84 | 0.67 | 0.61 | 0.65 | 0.79 | 1.17 |
| RT | 10 PCF | 10 PCF + cortical | 20 PCF | 20 PCF + cortical | ||||||||||||||||||||
| a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | |
| Mean | 7.39 | 8.31 | 6.81 | 9.30 | 10.41 | 8.42 | 11.28 | 14.45 | 11.11 | 14.79 | 17.24 | 11.16 | 17.92 | 27.55 | 14.87 | 24,90 | 36.65 | 23.75 | 19.46 | 32.38 | 23.84 | 34.42 | 39.09 | 28.67 |
| SD | 0.34 | 0.43 | 0.82 | 0.43 | 0.43 | 0.40 | 0.92 | 0.53 | 0.89 | 0.76 | 0.74 | 1.29 | 0.64 | 0.80 | 0.75 | 0.49 | 1.06 | 0.81 | 0.68 | 0.68 | 0.97 | 0.71 | 0.72 | 0.84 |
| RFA-BL | 10 PCF | 10 PCF + cortical | 20 PCF | 20 PCF + cortical | ||||||||||||||||||||
| a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | |
| Mean | 54.50 | 55.50 | 55.40 | 54.60 | 56.20 | 55.6 | 62.50 | 63.50 | 62.80 | 63.30 | 62.40 | 59.70 | 67.40 | 66.40 | 65.60 | 66.40 | 66.70 | 63.50 | 66.40 | 66.60 | 68.40 | 66.10 | 67.40 | 65.70 |
| SD | 0.53 | 0.53 | 0.52 | 1.17 | 0.92 | 0.52 | 0.53 | 0.53 | 0.79 | 0.82 | 0.52 | 0.67 | 0.52 | 0.52 | 0.70 | 0.52 | 0.48 | 0.53 | 0.52 | 0.52 | 0.52 | 0.99 | 0.52 | 0.95 |
| RFA-MD | 10 PCF | 10 PCF + cortical | 20 PCF | 20 PCF + cortical | ||||||||||||||||||||
| a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | a | b | c | d | e | f | |
| Mean | 54.50 | 55.40 | 55.50 | 54.60 | 56.60 | 55.7 | 62.60 | 63.50 | 63.00 | 63.10 | 62.50 | 60.10 | 67.60 | 66.50 | 65.90 | 66.40 | 66.40 | 63.50 | 66.20 | 67.10 | 67.40 | 66.70 | 67.50 | 65.80 |
| SD | 0.52 | 0.52 | 0.53 | 1.08 | 0.97 | 0.48 | 0.52 | 0.53 | 0.94 | 0.74 | 0.53 | 0.74 | 0.52 | 0.53 | 0.99 | 0.52 | 0.52 | 0.53 | 0.42 | 0.88 | 3.34 | 0.95 | 0.53 | 0.92 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Comuzzi, L.; Tumedei, M.; Covani, U.; Romasco, T.; Petrini, M.; Montesani, L.; Piattelli, A.; Di Pietro, N. Primary Stability Assessment of Conical Implants in Under-Prepared Sites: An In Vitro Study in Low-Density Polyurethane Foams. Appl. Sci. 2023, 13, 6041. https://doi.org/10.3390/app13106041
Comuzzi L, Tumedei M, Covani U, Romasco T, Petrini M, Montesani L, Piattelli A, Di Pietro N. Primary Stability Assessment of Conical Implants in Under-Prepared Sites: An In Vitro Study in Low-Density Polyurethane Foams. Applied Sciences. 2023; 13(10):6041. https://doi.org/10.3390/app13106041
Chicago/Turabian StyleComuzzi, Luca, Margherita Tumedei, Ugo Covani, Tea Romasco, Morena Petrini, Lorenzo Montesani, Adriano Piattelli, and Natalia Di Pietro. 2023. "Primary Stability Assessment of Conical Implants in Under-Prepared Sites: An In Vitro Study in Low-Density Polyurethane Foams" Applied Sciences 13, no. 10: 6041. https://doi.org/10.3390/app13106041
APA StyleComuzzi, L., Tumedei, M., Covani, U., Romasco, T., Petrini, M., Montesani, L., Piattelli, A., & Di Pietro, N. (2023). Primary Stability Assessment of Conical Implants in Under-Prepared Sites: An In Vitro Study in Low-Density Polyurethane Foams. Applied Sciences, 13(10), 6041. https://doi.org/10.3390/app13106041













