1. Introduction
The initial response after traditional instrument gingivectomy is the formation of a protective blood coagulum. The coagulum is then replaced by granulation tissue. At 24 h, the number of new connective tissue cells, mainly angioblasts, increases beneath the surface layer of inflamed and necrotic tissue. By the third day, many fibroblasts are located in the area. The highly vascularized granulation tissue grows coronally, creating a new free gingival margin and sulcus. The capillaries, formed by the blood cells in the periodontal ligament, migrate into the granulation tissue and connect with the gingival blood vessels within 2 weeks [
1].
After 12 to 24 h, epithelial cells at the edges of the wound begin to migrate onto the granulation tissue, separating it from the contaminated surface layer of the coagulum. Epithelial activity at the edges reaches its maximum at 24 to 36 h.
New epithelial cells are formed by the basal and deep spinal layers of the epithelial ends of the wound, and migrate to the fibrinous layer of the wound, which is later resorbed and replaced by a bed of connective tissue. Epithelial cells bind to the substrate using hemidesmosomes and a new basal lamina [
2].
In 5 to 14 days, the superficial epithelization is complete. During the first 4 weeks after gingivectomy, keratinization is weaker than before resection. Complete recovery of the epithelium takes approximately 1 month. Vasodilatation and vascularity begin to decrease after 4 days of recovery and are closest to normal on day 16. Full connective tissue recovery takes approximately 7 weeks [
3].
The amount of crevicular fluid initially increases after gingivectomy and decreases with the onset of the healing process. The peak in the amount of crevicular fluid is after one week, which coincides with the peak in the inflammatory process [
4].
Although tissue changes that occur in the healing process after gingivectomy are the same in all individuals, the time required for complete recovery varies considerably. It depends on the area of the incised tissue and the interaction with local inflammation and irritation. In patients with physiologically melanotic gingiva, pigmentation is reduced after gingival healing [
5,
6,
7].
Laser gingivectomy is a contemporary alternative to traditional soft tissue surgery. The results from a clinical point of view are competitive and promising. When soft tissues are incised with a laser (with the correct choice of wavelength and settings), healing is referred to as ‘calm’. The need for sutures or periodontal dressings is almost always avoided. Regardless of the wavelength, the healing after laser gingivectomy is per secundam intentionem (from Latin—‘secondary’, literally ‘by secondary intention’); thus, it is impossible to superimpose exactly the shear edges as in their original appearance. It is important to note the phenomenon of the lack of bacterial contamination due to the bactericidal effect of the laser during the procedure [
7,
8]. There is a protective layer of plasma coagulum and blood products—a strong surface film that allows for early healing in the underlying layers. Studies have shown that when longer wavelengths are used, there is no alignment of fibroblasts along the cut line, and there is a reduction in tissue shrinkage during healing. Such findings were also confirmed by clinical observations and are useful in determining the gingival margin level of a veneer or a crown [
7]. The different laser operation modes significantly affect the desired laser–tissue interaction and heat relaxation. If the recommended operating techniques are not followed, charring occurs when the temperature exceeds 200 °C. Carbon absorbs all wavelengths, which leads to local heat accumulation and subsequent damage [
9].
In short waves, heat is conducted and accumulated in the tissue, which leads to collateral unwanted changes, often in the form of edema. This is of particular importance when the thin tissue components lie on a deeper, nontarget tissue (e.g., thin mucosa on the alveolar bone) [
10]. Shorter wavelengths (488–1064 nm) interact with the protein components, causing denaturation and disintegration of long-chain molecules. This leads to tissue shrinkage, retention, and the local conduction of heat, while long lengths cause extensive destruction by water ablation. In cross-sections, the profile of the slit, obtained at cutting, is almost spherical in the short waves and narrow and V-shaped in the long ones [
11,
12,
13,
14]. In long waves, heat distribution is achieved through ablation away from the incision site. There is a lower risk of deep heat effects but a higher risk of tissue rupture due to the fact of water evaporation [
7].
In the case of charring, the deposit should be periodically removed using moisture gauze.
Adequate protection of the tooth or implant is required during any resection procedure that affects the gingival sulcus. For shorter waves, cracking and charring can be expected and for long waves additional hard tissue ablation [
15]. Therefore, with the use of a suitable barrier (plastic instrument or metal matrix) in the gingival sulcus, side effects can be avoided. This is especially important in the noncontact mode, such as when working with CO
2 lasers [
16].
Predictable healing without collateral damage can occur when the proper wavelength and the minimal power for the respective tissue are used. This ensures the thermal rest of the tissue and avoids charring [
17].
Apart from surgical laser use for laser gingivectomy, numerous studies demonstrate the positive effect of adjunctive laser therapy—low-level laser therapy (LLLT) and photoactivated disinfection (PAD)—on gingival healing used separately or combined with traditional or laser gingivectomy [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31].
The aim of the current pilot study was to assess gingival healing after diode laser gingivectomy prior to prosthetic procedures.
2. Materials and Methods
Object of observation
Clinical effectiveness of the healing process of the gingiva after diode laser gingivectomy.
Units of observation
The healing of the cut gingival surface of 41 teeth was examined for the current pilot study.
Parameters of observation:
- -
Recovery time of the gingiva (24th hour, 72nd hour, 1st week, and 2nd week);
- -
Possibility to take the impression at the same visit (i.e., clinical effectiveness);
- -
Duration of the manipulation;
- -
Bleeding during the procedure;
- -
Tissue adherence to the instrument;
- -
Postoperative hemorrhage on probing;
- -
Postoperative pain;
- -
Wound healing in regard to:
- ○
Tissue color;
- ○
Tissue contour;
- ○
Appearance of the wound.
The methodology is a modification of that of Kumar, P. et al.; Amorim JCF et al.; and Ozcelik, O. et al. [
8,
9,
10].
Venue of observation:
Department of Prosthetic Dentistry, Faculty of Dental Medicine, Medical University—Plovdiv, Bulgaria.
Department of Periodontology and Oral Mucosa Diseases, Laser Dental Center, Faculty of Dental Medicine, Research Institute, Medical University—Plovdiv, Bulgaria (RIMUP).
Observation members
For maximum objectivity, four clinicians altogether assessed the results at 24 h, 72 h and at the end of the 1st week and the 2nd week. One was a dentist not familiar with the investigation, and the other three were researchers engaged in performing the study.
Criteria for inclusion in the study:
- -
Patients undergoing soft tissue crown lengthening of front teeth with short clinical crowns and a high smile line—most often due to the fact of passive eruption;
- -
Patients for subgingival finish line exposure;
- -
Patients with deep subgingival carious lesions;
- -
Patients with oblique root fractures in the cervical part;
- -
Patients with short crowns when it is necessary to achieve a ferrule effect;
- -
Patients that need operculectomy;
- -
The Löe and Silness gingival index of the subjects should be 0. The index is used for the assessment of the prevalence and severity of gingivitis in populations, groups, and individuals. A score from 0.1 to 1.0 signifies mild inflammation; 1.1 to 2.0 moderate inflammation, and 2.1 to 3.0 severe inflammation [
1].
Criteria for exclusion from the study:
- -
Patients with severe systemic diseases. They may lead to inflammation and/or bleeding, therefore compromising the results [
32];
- -
Presence of mental illness;
- -
Presence of periodontal diseases;
- -
Presence of inaccurate obturations that can interfere with biological space;
- -
The subjects should not be smokers. This may lead to inflammation and/or bleeding, therefore compromising the results [
33];
- -
The subjects should not drink alcohol during the study (for 15 days). Alcohol consumption may lead to inflammation and/or bleeding, therefore compromising the results [
34,
35].
The study was conducted in accordance with the Declaration of Helsinki and approved by the Committee of Scientific Ethics at Medical University—Plovdiv, reference number: P-7350/01.10.2015. Patients signed an informed consent form and received a document about the study.
Entry of primary data
In order to collect the primary data for the study, a clinical card on the patient was prepared in which the following information was filled with the name, age, sex, date, type of surgical instrument, clinical efficiency (possibility to take the impression in the same visit), duration of the manipulation, bleeding during the procedure, tissue adherence to the instrument, postoperative pain, postoperative hemorrhage, and healing in regard to: tissue color, tissue contour, and appearance of the wound (
Table 1). After careful verification for adequate filling of the protocols, the primary data were entered in spreadsheets for further statistical processing and analysis.
Statistical Analysis Methods
The statistical analysis methods were implemented using the statistical program SPSS version 19, and the results were considered statistically significant at a significance level α < 0.05.
The following methods were applied:
One-dimensional tables of the frequency distribution (structure determination by relative shares) and of the variety of features, characterizing the observed phenomena (in this specific case—the variants of the possible answers to each question asked);
Two-dimensional frequency distribution tables (cross-tabulation) to search for a relationship between two category variables.
Nonparametric analysis—search for statistical dependence between two traits, nominally or ordinally scaled, using the c2 (chi-squared test). Pearson’s agreement criterion (c2—chi-square) was used to evaluate hypotheses;
Graphic analysis to illustrate the obtained results.
Study Design:
Twenty-one patients, subject to fixed prosthetic restorations in the Department of Prosthetic Dentistry, Faculty of Dental Medicine, Medical University—Plovdiv, were examined (a total of 41 teeth). The participants were healthy subjects, who needed crowns, bridges, or post and core restorations. The patients were pre-instructed to maintain good oral hygiene. The first visit included an assessment of the Löe and Silness gingival index, periodontal probing depth (PPD) using a World Health Organization (WHO) periodontal probe, and assessment of the amount of gingival tissue to be removed.
When vital teeth were present, a modified metal matrix was used in order to protect the hard dental tissues from the action of the laser. When performing a soft tissue crown lengthening of front teeth with short clinical crowns, a calibrated Crane–Kaplan pocket marker was used to mark the bleeding points by piercing the tissue to the CEJ level. The aim was to outline the CEJ so that the soft tissue matched its contours, and the root surface was not visible after elongation. In the other resection procedures, the gingival tissue was removed so that the PPD after the manipulation was 3 mm in order to preserve the biologic space. Creating such a distance from the alveolar ridge to the margin of the future restoration led to stable periodontal tissue at this level [
36,
37,
38].
The resection procedure was performed at the same visit using a diode laser with a wavelength of 810 nm (FOX, A.R.C. Lasers GmbH, Nürnberg, Germany) and a maximum power of 8 W. The fiber tip was activated by holding it on a special black paper before the procedure.
The manipulation always started at the lowest laser power. It was set to a continuous mode (cw) with a power of 1.5 W, and depending on the tissue, the power could be increased to 2 W, if necessary. It worked in the contact mode, without water cooling. It was important that the tip slid through the tissue with minimal or no resistance. If this occurred, is had to be cut or replaced. Charring was a side effect that could occur either because too much power was used or because the tip moved slowly. This layer was quickly removed by irrigation with 3% hydrogen peroxide. In the diode laser technique, the tissue was cut coronally at the bleeding points level or to the desired depth in the other gingivectomy procedures. The cutting part of the laser is the tip of the optical fiber; thus, it was directed perpendicular to the surface.
At the end of the procedure, the free gingival margin was beveled by tilting the tip 45 degrees to the root surface in order to eliminate the 90-degree cutting angle and obtain a smooth transition. A sweeping motion from the mesial to the distal of the cementoenamel junction (CEJ) was applied. The PPD was re-measured after the manipulation.
Immediately after the procedure, the clinical efficiency was assessed (i.e., possibility to take the impression at the same visit), and, if necessary, one was taken. The duration of the manipulation was estimated with a stopwatch. The presence of bleeding during the procedure was assessed clinically. The following scale was used:
The tissue adherence to the instrument was evaluated by the following criteria:
None;
Adheres a little, which allows cleaning with damp gauze;
A large amount adheres, which requires mechanical removal of the optical fiber tip or replacement of the tip.
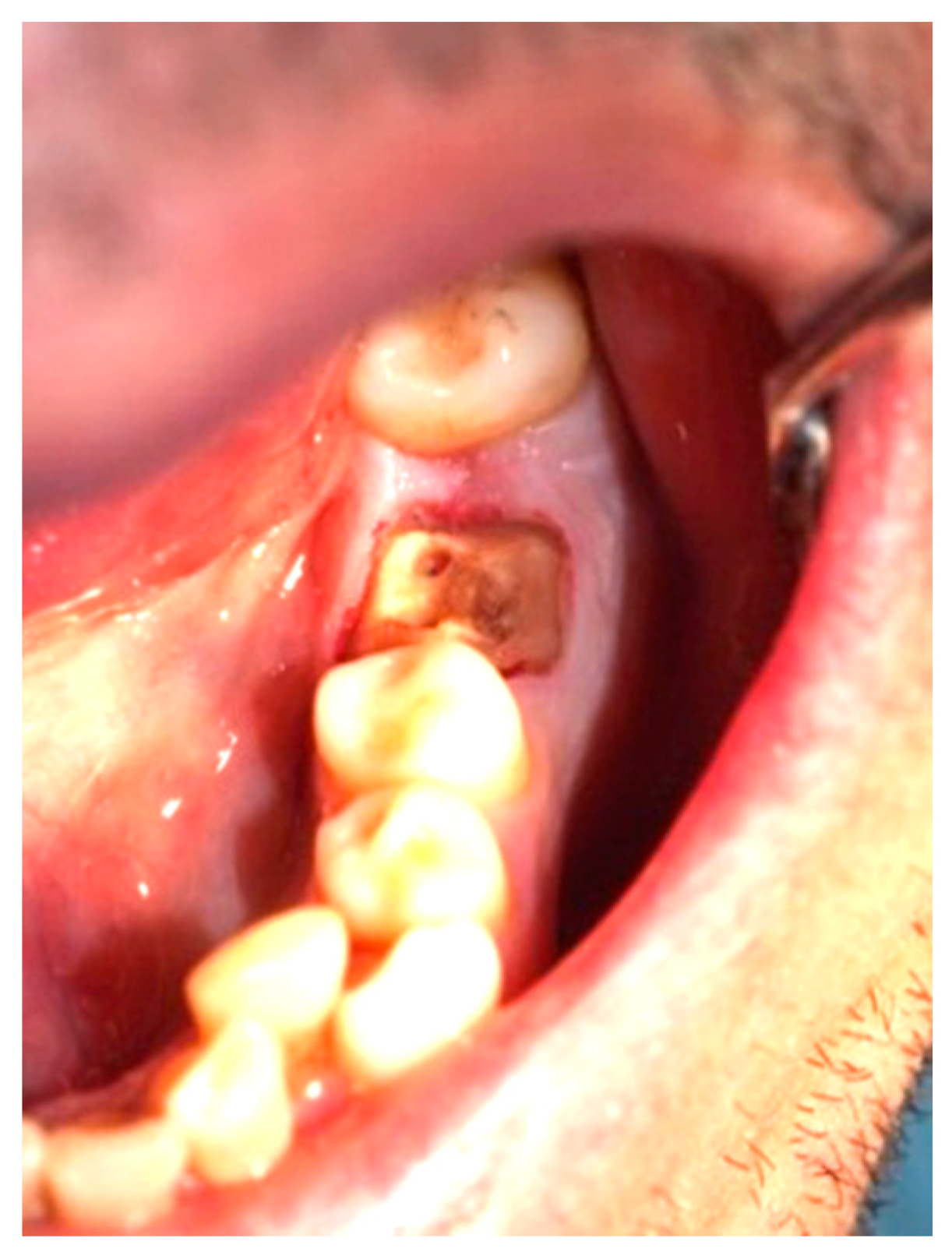
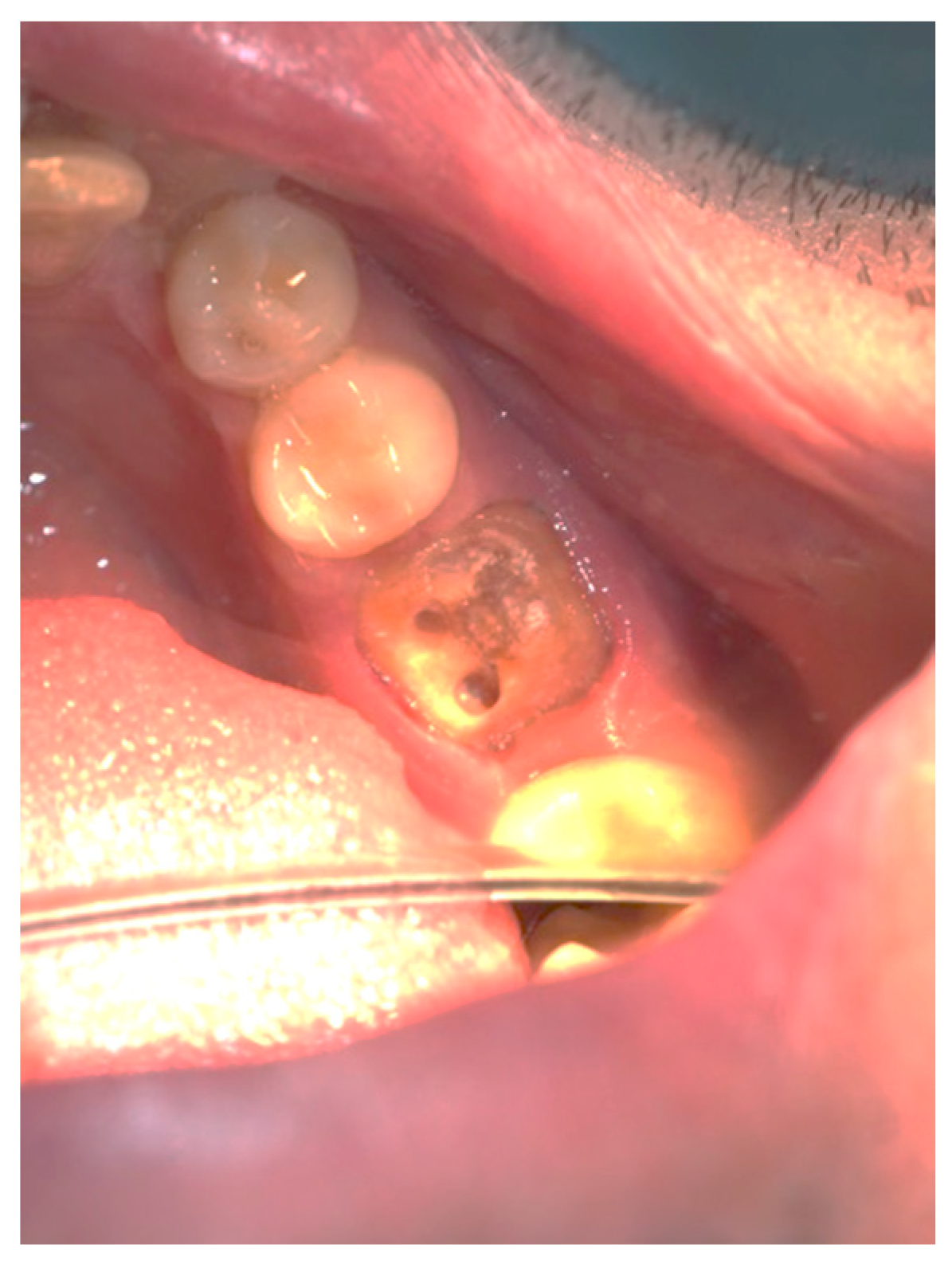
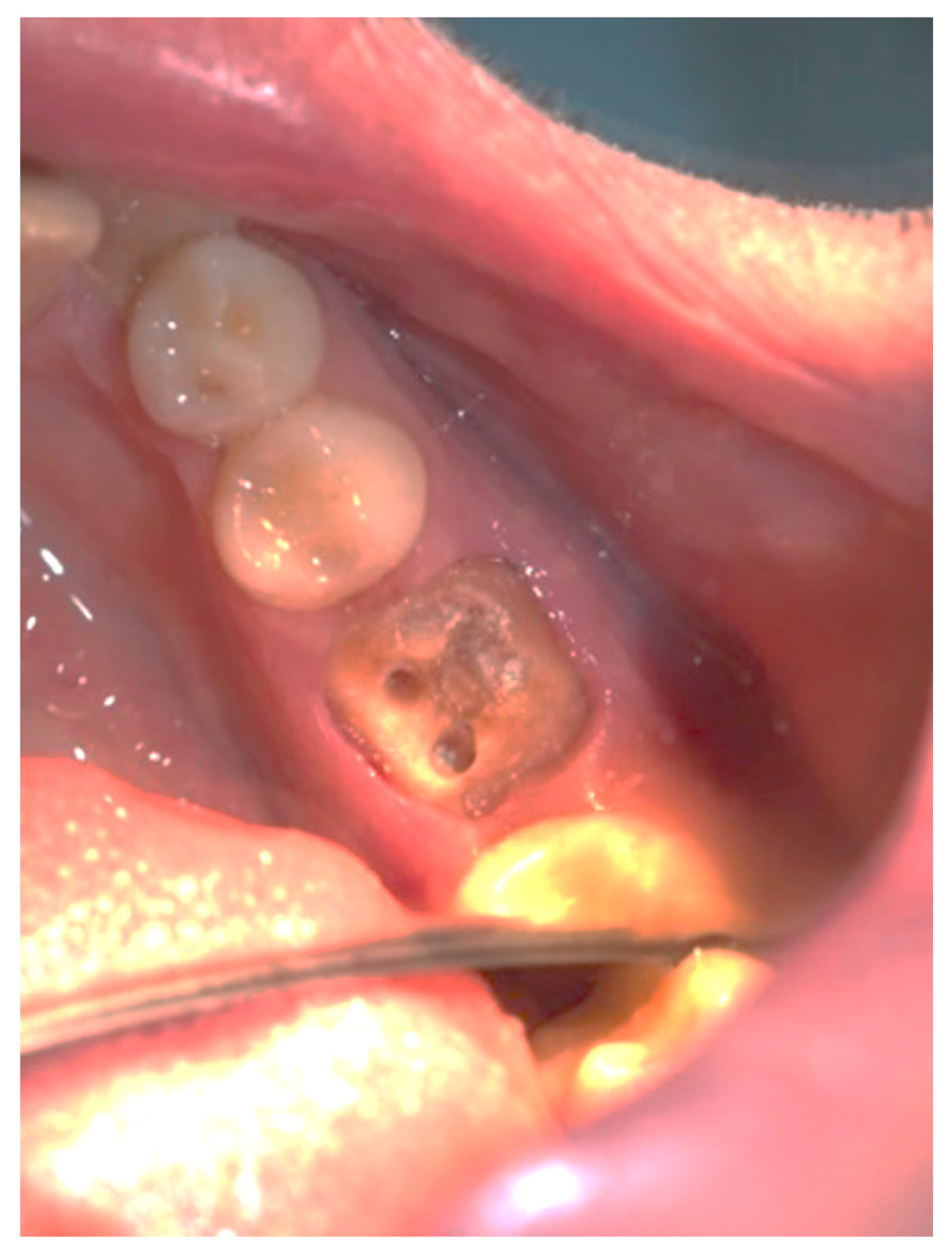
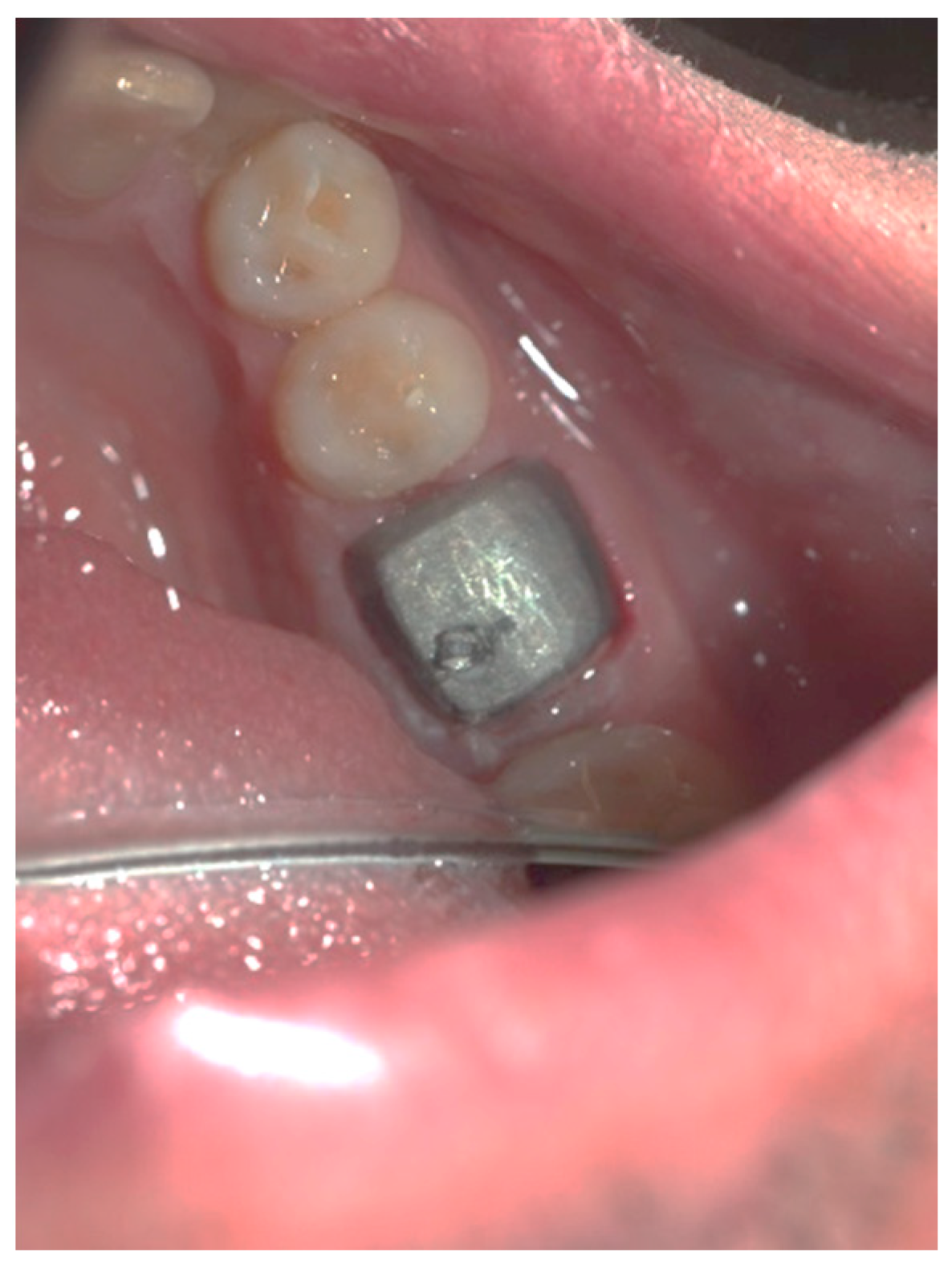
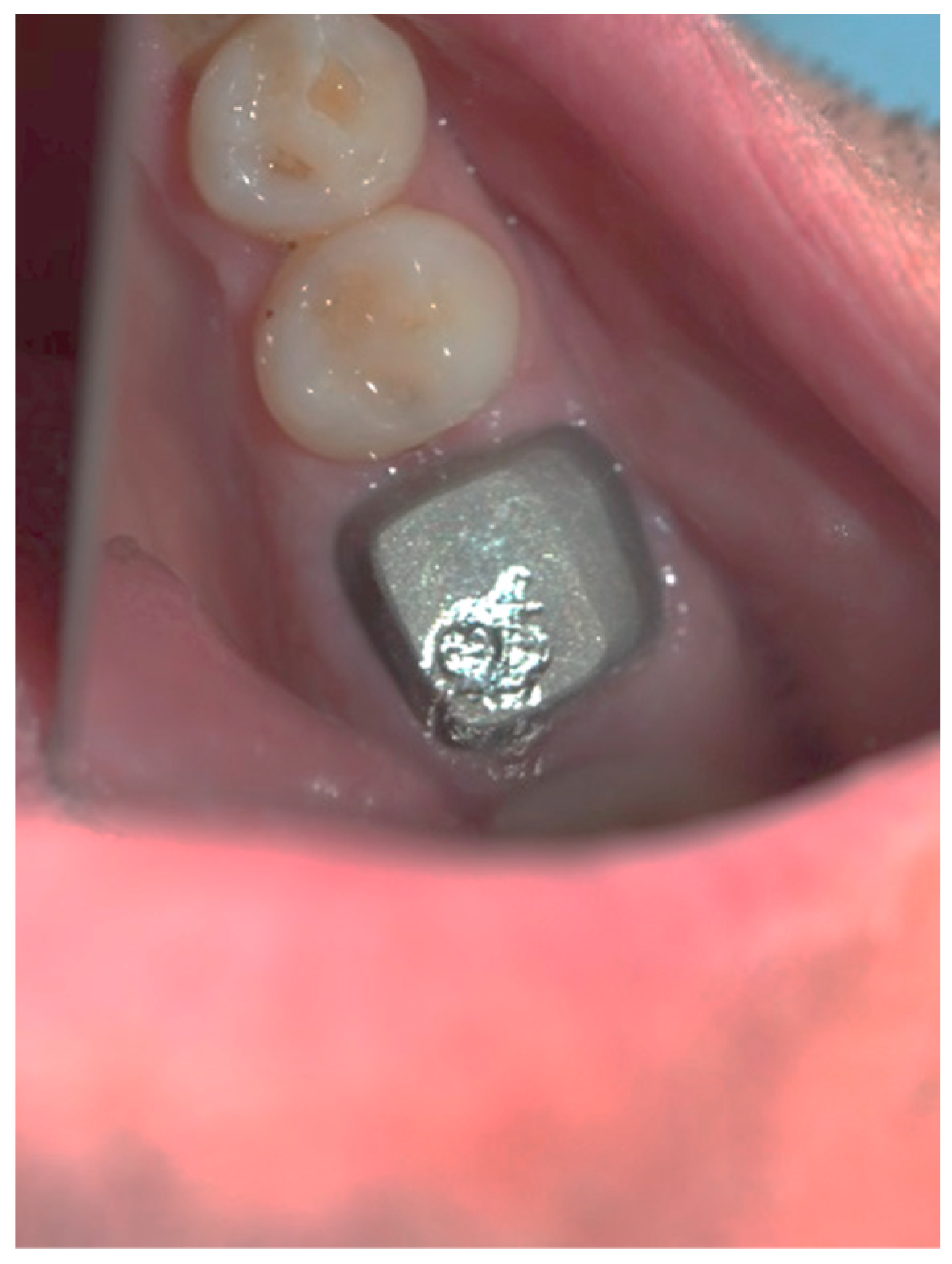
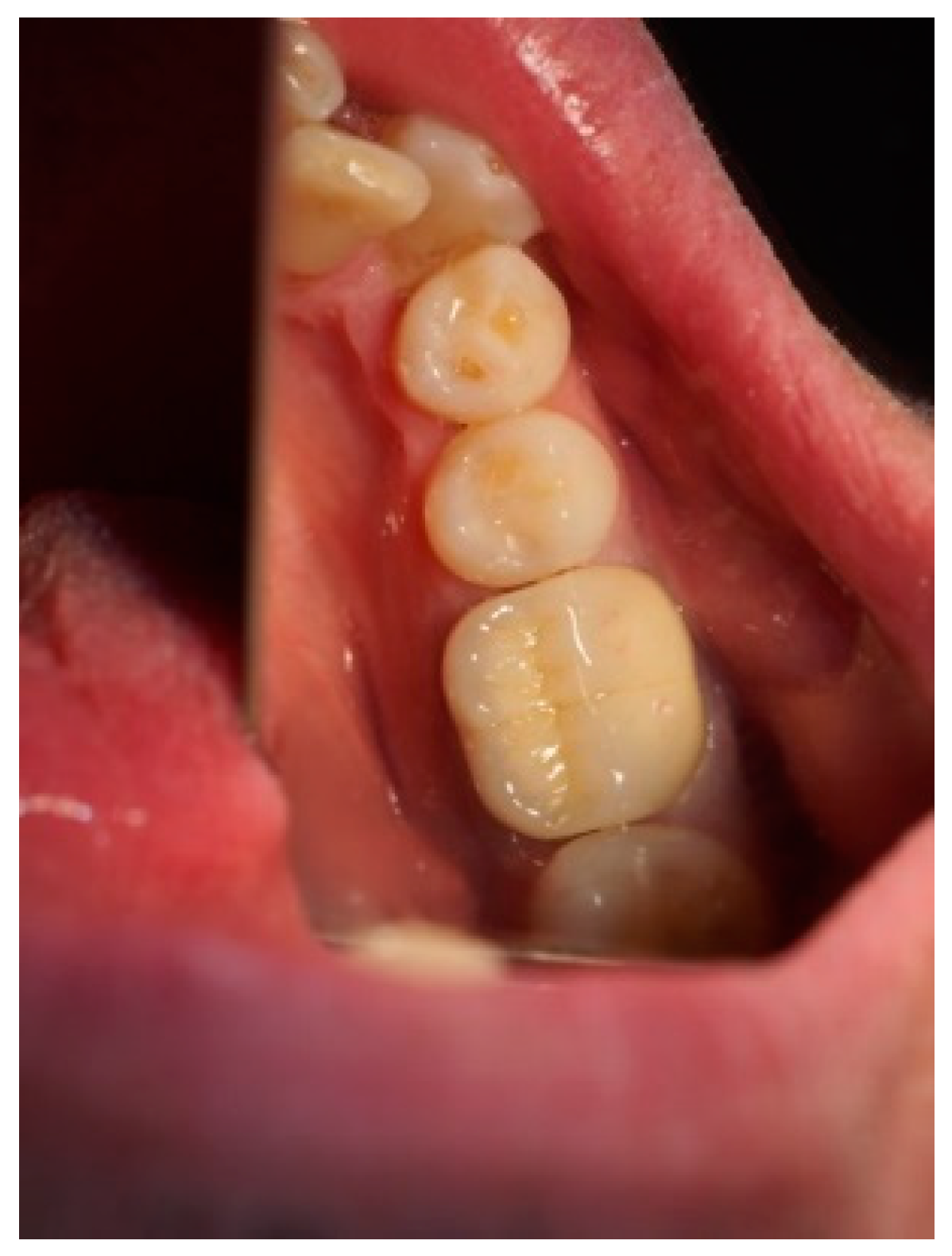
Clinical observation in regard to postoperative pain, postoperative hemorrhage, and healing (tissue color, tissue contour, and appearance of the wound) was performed at the 24th hour, 72nd hour, and, finally, at the 1st week and 2nd week.
Postoperative pain was measured using the 10-score visual analog scale (VAS) in which ‘no pain’ equaled 0 and ‘the strongest pain imaginable’ was 10. The patients were not shown their previous results. The presence or absence of postoperative hemorrhage on probing was reported using the WHO periodontal probe. The tissue color was evaluated visually—pink, red, livid, purple, whitened, or burned (in the case of the improper handling of the laser parameters or the slow movement of the tip). Contour assessment is determination of the gingival margin—the level at which the gingival margin is attached to the tooth. Its normal position is at the CEJ level, above that (coronally) is known as proliferation and below (apically) as recession. In the current study, the tissue, depending on its contour, was defined as normal, hyperplastic, and atrophic. The wound can be covered with fibrinous plaque and erythematous halo, granulated, necrotic, or with normal pale pink color.
3. Results
The study evaluated the dynamics of the healing processes after diode laser gingivectomy in 14 women (66.7%) and 7 men (33.4%), altogether 41 teeth. The mean age of the subjects was 39.66 ± 12.87 years (the youngest was 23 years old and the oldest 65 years old). All subjects were Bulgarian, and the gingival changes were examined at the 24th hour, 72nd hour, 1st week, and 2nd week.
Gingival resection was performed on a number of indications, and the specific reasons for the procedure are presented in
Table 2.
The most common cause was the subgingival finish line exposure and short clinical crown lengthening in order to achieve the ferrule effect, 21 (51.2%), followed by soft tissue front teeth crown lengthening, 9 (22.0%), and subgingival finish line and deep carious lesion exposure, 3 (7.3%). It was found that the average periodontal probing depth (PPD) before the manipulation was 5.00 ± 1.342 mm.
Table 3 presents the structural distribution of the teeth that underwent gingivectomy included in the study.
In the study, in all cases, it was possible to take an impression in the same visit. The mean duration of the diode laser gingivectomy manipulations (described in
Table 2) was 12.39 ± 10.98 min.
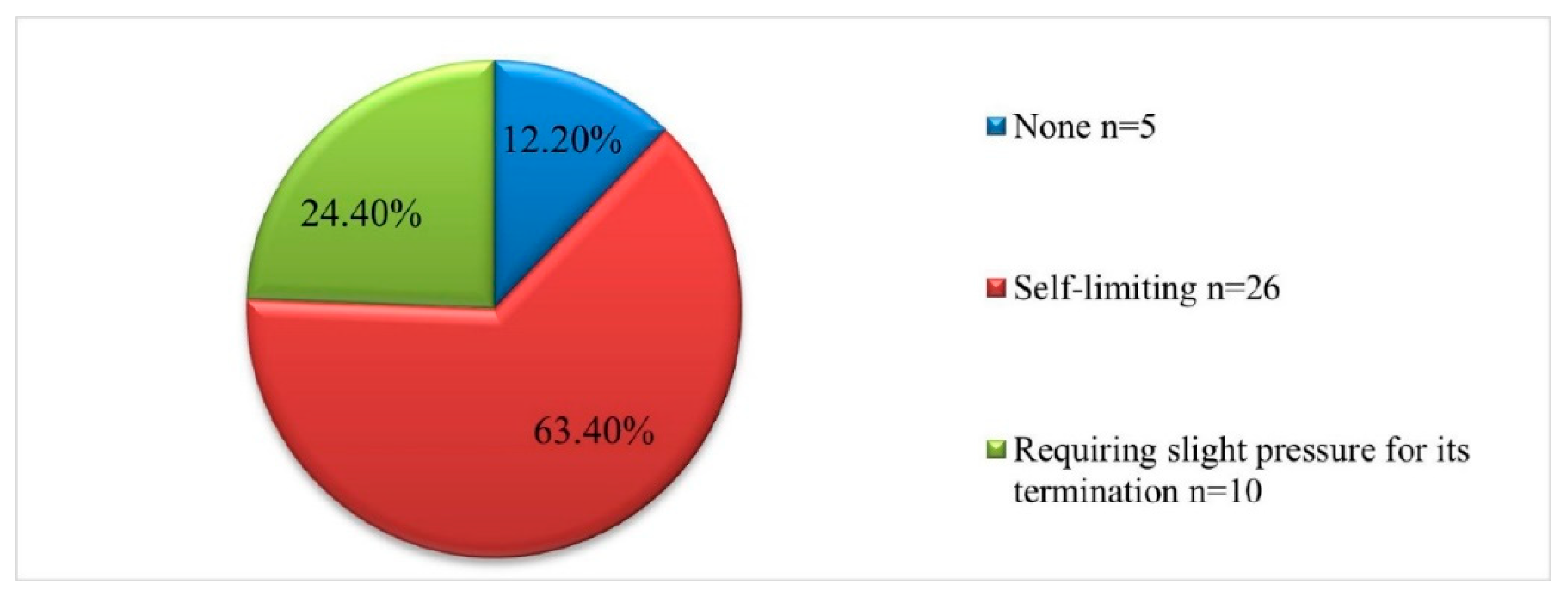
The intraoperative characteristics—bleeding during the procedure and tissue adherence to the instrument—are presented in
Figure 1 and
Figure 2. It should be noted that in 5 of the cases (12.2%) no bleeding was detected at the time of manipulation, in 26 (63.4%) the reported bleeding was self-limiting, and in 10 (24%) slight pressure was required to terminate it (
Figure 1).
During the surgical interventions, a small amount of tissue adherence to the instrument was reported in 36 patients (87.8%) (
Figure 2).
Some postoperative characteristics of the gingivectomy, including postoperative pain, postoperative hemorrhage on probing, tissue color, tissue contour, and appearance of the wound, were also analyzed in dynamics.
The distribution depending on the reported postoperative pain (assessed by the 10-score VAS) in the measured four stages of the study is presented in
Table 3. The highest degree of pain, measured at the 24th hour, was six, as it was reported in nine of the cases (22.0%), and in three (7.3%) no pain was reported after gingivectomy. At the 72nd hour, the highest degree was already three, and this was reported in nine of the cases (22.0%). During the first week, the pain level again in nine of the cases was minimal at −1, and the rest of the patients reported no pain at all. During the second week, no one reported feeling postoperative pain.
The mean level of postoperative pain after diode laser gingivectomy in the four stages of their assessment is presented in
Table 4.
Postoperative hemorrhage on probing is a sign of the formation of a blood coagulum after the procedure and the normal course of the healing process in the gingival sulcus, including resorption of the coagulum and reorganization of the epithelium and the connective tissue. The presence of this indicator during the four stages of this research is presented in
Table 4. At the 24th hour, all 41 observed teeth presented with bleeding on probing, while at the 72nd hour, there were 36 (87.8%). During the 1st week after gingivectomy, postoperative hemorrhage on probing was reported in nine of the cases (22.0%), and during the 2nd week none of the cases (
Table 5).
The gingival tissue color, observed in dynamics during the postoperative period, is described in
Table 6. The whitening of the tissue meant that coagulation had occurred, and the proper laser parameters were used, while a burned color of the tissue was an indicator of excessive power or holding it in one place for too long. During the first stage of observation—the 24th hour—in 31 of the cases (75.6%), the color turned white, and at the 72nd hour, this color was reported in only 2 of the cases (4.9%). During the 1st and 2nd week, there was not a single case with this characteristic. At the 24th hour, a red tissue color was reported in 10 of the examined cases (24.4%), at the 72nd hour this color was present in 37 of the observed gingival surfaces (90.2%), and during the 1st week, it occurred only in 9 (22.0%). During the 2nd week, this tissue color was not reported, and in all cases, only a pink color of the gingiva was reported. In the 1
st week, in 32 of the observed gingivae (78.0%), the reported tissue color was already pink.
It should be noted that in all cases at each stage of the study after diode laser gingivectomy, the tissue contour was normal, and only at the 24th hour a very slight (almost invisible to the naked eye) edematous contour of the incised gingival surface was reported.
The dynamic clinical characteristics of the wound healing process are presented in
Table 7. At the 24th hour, all gingival wounds were covered with fibrinous plaque and an erythematous halo, and at the 72nd hour, in two cases (4.9%) this wound’s characteristic had already passed through to the next healing stage—granulated surface and had normal pale pink color. A granulated surface in the 1st week was reported in 10 (24.4%) of the cases, and in all other 31 (75.6%) complete healing was reported. In the second week, all wound surfaces were completely healed and had a normal pale pink color.
4. Discussion
In the present study, the healing process immediately after gingivectomy with a diode laser with a wavelength of 810 nm was evaluated clinically. The dynamics of the regenerative processes within 14 days was analyzed (i.e., the observation period). In the available literature, publications analyzing the healing processes after the use of this type of laser are extremely scarce, which is why the possibility of comparing our data with the results of other authors is limited.
Healing after gingivectomy is known to be a slow process, and it takes approximately 5 weeks to restore normal gingival epithelization [
39]. According to other authors, the formation of collagen and the organization of new gingival tissue after gingivectomy occurred gradually, within 3–4 weeks, and the inflammation and blood supply of the granulation tissue were reduced to some extent, even if the gingival surface appeared completely restored clinically 2–3 weeks after the surgical procedure. The acceleration of the healing process after laser gingivectomy was probably due to the faster collagen synthesis from the fibroblasts and vascular proliferation in connective tissue, combined with higher mitotic activity in epithelial cells [
40]. According to the data from our study, at the end of the 2nd week (on the 14th day) all gingival resected tissues were completely healed, which demonstrates an accelerated regeneration process after diode laser gingivectomy. In this case, we assume that during the manipulation, the laser beams were absorbed by the surrounding tissues and led to modified biotransformation, accelerating the healing processes [
41].
According to some literature sources, wounds after gingivectomy with traditional instruments healed significantly faster if they were subsequently treated with low-level laser therapy (LLLT). This led to biostimulation and/or biomodulation in the biologic structures as a result of which it was possible to change the cellular behavior [
41]. This effect was also achieved by acting on the mitochondrial respiratory chain and/or on the membrane calcium channels. In vitro and in vivo studies have shown that LLLT and photoactivated disinfection (PAD) aided fibroblast and keratinocyte cell mobility, collagen synthesis, angiogenesis, and the release of growth factors [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
42,
43]. Adjunctive antimicrobial photodynamic therapy displayed excellent results in terms of gingival health and periodontitis healing. In addition to pre-emptive laser analgesia leading to reduced pulpal reactivity and reduction of nociceptive impulse formation, photobiomodulation was also proved to reduce pain levels and aid in the pain level during injection of anesthesia. Another positive effect was bone healing, associated with distraction osteogenesis. Diode lasers at a proper power and wavelength were proved to have bactericidal activity on periodontopathic germs. Photodynamic therapy with diode lasers amplifies the healing effect. Generally, light therapy by itself or as a complementary antibacterial treatment has a positive effect on the whole periodontal complex [
18,
19,
20,
21,
22,
23,
24,
25,
26,
27,
28,
29,
30,
31,
42,
43].
The mean duration of the manipulations in the present study, including resections with a diode laser, was 12.39 ± 10.98 min, which was approximately 4 min shorter than the period of similar manipulations, as reported by Kumar, P. et al. [
9].
Bleeding during the procedure (intraoperative characteristic) demonstrated that in most of our cases (63.4%) it was self-limiting, and in 12.2% there was none. Our data show some similarity with the results of Kumar, P. et al. [
9]. The authors describe that in a group in which the surgery was performed with a diode laser with a wavelength of 980 nm, in 17.6% of the patients there was no bleeding, and it was self-limiting in the rest, 82.4%. It should be noted that the following additional characteristic of bleeding during the procedure was reported in our study—‘requiring slight pressure to terminate it’ (in 10 of the cases—24.4%)—and the abovementioned authors did not cite this variant of the indicator, due to which the comparison of the specific variable was not accurate enough [
7,
8,
9,
10,
41]. Another intraoperative characteristic analyzed in our study was the tissue adherence to the instrument during the manipulation. In 36 of the cases (87.8%), a small amount of tissue adhered to the instrument, which allowed it to be easily cleaned with damp gauze. Completely similar to our data was the result of Kumar, P. et al., where this process was observed in 15 of their cases (88.2%) [
9].
The examination of the postoperative pain level (VAS scale scores) in our study showed slightly lower levels of pain during the observed periods (
Table 4) than for the above cited authors. We assume that this difference was due to the fact that the diode laser with which the other researchers work was with a higher wavelength of 980 nm, compared to ours, which was 810 nm. It is known that the longer the wavelength, the more the damage to the surrounding tissues (i.e., thermal destruction), and low-frequency lasers mainly have a regenerating effect on the treated biological structures. When comparing the level of pain after moderate chronical periodontitis treatment with an Er:YAG laser and Gracey curettes, it was found that the pain in the laser-treated group was two times lower than in the control group. Extremely low pain levels were also found in other studies [
12,
17].
Another indicator characterizing the postoperative condition of the treated area was postoperative hemorrhage on probing. Reduction of postoperative hemorrhage on probing after treatment with different kinds of lasers was reported in several scientific studies [
14]. Schwarz, F. et al. found that it was more significantly reduced in the laser-treated group than in conventionally treated cases [
44]. The researchers concluded that the Er:YAG laser was as effective in nonsurgical periodontal treatment of chronic periodontitis as in conventional scaling and root planing with manual instruments. Schwarz et al. demonstrated that the positive results after the Er:YAG laser therapy could be maintained for a period of 2 years [
44].
In a clinical (randomized controlled) trial with a split mouth design, covering 12 months of follow-up, the clinical efficacy of an Er:YAG laser and Gracey curettes in the treatment of moderate chronical periodontitis was examined. The author found that ‘Instrumentation with an Er:YAG laser results in a significant reduction of the pocket depth on probing, bleeding on probing, as well as clinical attachment gain.’ The obtained results remained significantly lower a year after the therapy, compared to their values in the initial clinical trial [
17]. In the present study, postoperative hemorrhage on probing after diode laser gingivectomy was examined at a much earlier and shorter stage after treatment, and in this case, it was not a sign of inflammation but reflected its faster and better therapeutic efficacy (i.e., accelerated tissue regenerative capacity).
The next analyzed postsurgical characteristic, evaluating the healing process, was gingival tissue color. The results from our study demonstrate that at the 24th hour, 10 of the gingival surfaces (24.4%) were red and 31 of them (75.6%) whitened—indicating coagulation—an indirect characteristic that the intervention was performed with the correct parameters. At the 72nd hour, in almost all cases—37 (90.2%)—the gingiva was red; there were 2 cases (4.9%) with pink and 2 cases (4.9%) with a whitened color. At the end of the 1st week, almost 80% of the observed surfaces were pink, and at the end of the 2nd week, all gingivae already had the quoted color. It should be explicitly noted that in our work with the diode laser with a wavelength 810 nm, a continuous mode, and a power of 1.5 W, there was not a single case of burned gingiva, which means that the correct parameters and appropriate speed were applied. The study by Kumar, P. et al. in which the authors assessed whether the laser had advantages over electrosurgery when performing gingivectomy, there was burned gingiva in both compared groups, and it was significantly more in three of the patients after laser gingivectomy. We assume that their result was a consequence of the applied operating mode of the diode laser used (wavelength of 980 nm and power of 5 W), which quite logically led to more serious damage to the surrounding tissues [
9].
Another postsurgical indicator from our study characterizing the regenerative processes after laser surgery was tissue contour. Except for the registered very slight edematous contour of the cut gingival surface at the 24th hour, during the other three stages of observation—at the 72nd hour and during the 1st week and the 2nd week—the gingival contour was normal. Confirmation of these good results is found in the study by Amorim et al. on 20 patients with periodontal disease, who underwent bilateral conventional gingivectomy of the maxillary and mandibular premolars [
8]. The study evaluated the recovery after gingivectomy with additional low-level laser therapy. The authors used a diode laser with a wavelength of 685 nm, in a continuous mode, and the treatment areas were irradiated immediately after surgery at the 24th hour and on the 3rd and 7th days. It was found that in the control group (in which low-level laser therapy was not applied), the gingival contour was edematous and redness was present, whereas in the laser group, the gingival contour was well formed and the color was pink, which demonstrates better recovery than the control group [
8].
The last postsurgical characteristic that we analyzed in our study was the appearance of the wound, which again showed a significantly accelerated healing process. At the end of the first week, in more than two-thirds of the cases observed by us, 31 of the wounds (75.6%) already had a normal pale pink color and only 10 (24.4%) had a granulated surface. At the end of the second week, all cases had a normal pink color. According to the literature, this recovery requires a minimum of 3–4 weeks, and in most of the cases it took 4–5 weeks. Similar results for accelerating the wound healing process after laser treatment were observed in other studies, such as in [
8]. The mechanism of LLLT has not been fully examined, but some studies attempted to explain the effect of laser radiation on the biological structures. Numerous LLLT and PAD mechanisms that can improve wound healing have been described. They include ATP synthesis, fibroblasts proliferation, collagen synthesis, macrophages phagocytosis, and acceleration of the inflammatory phase of healing. All of these mechanisms can lead to cell proliferation and accelerate the healing process [
39,
40,
41,
42].
Some authors did not support the above statements on the positive effect of lasers on biological structures. For example, Damante et al., in their study, found that LLLT did not accelerate oral mucosa healing after gingivoplasty [
45]. Treated and untreated gingiva did not show a statistically significant difference in terms of epithelization, inflammatory cells, collagen fibers, or number of fibroblasts [
45,
46,
47]. In the context of this claim, it is well known that many parameters should be considered, such as wavelength, power, and distance from the source to the tissue, as well as clinical factors, such as radiation dose, exposure time, intensity, method, and number of exposures.
Lione, R. et al., in 2020, compared the use of diode lasers with conventional surgery, evaluating the effectiveness of gingivectomy as an adjunct to nonsurgical periodontal treatment in the management of gingival enlargement (GE) during orthodontic treatment [
48]. They discovered that adjunct use of both scalpel gingivectomy and laser gingivectomy was more effective in controlling gingival inflammation than nonsurgical periodontal treatment alone at 1, 3, and 6 months. In the control group, greater improvement in the periodontal parameters were observed within 3 months, depending on a self-care approach for the management of GE. Bhasker et al., in 2021, described a clinical case where they compared scalpel and diode laser use for treatment of GE [
49]. Diode laser provided adequate hemostasis and accurate incision margins. The authors reported a lack of pain, swelling, less scar tissue formation, and good and uneventful wound healing [
49]. The wounds induced by laser heal via reparative matrix proteins synthesis. The wound contraction in laser-treated areas was less compared to the scalpel areas [
49]. The duration of the procedure was shorter than that of a scalpel [
49].
As a summary of our results and after a comparison with the literature, it can be concluded that gingivectomies performed with a diode laser are characterized by simplified procedure techniques and significantly accelerated and improved healing processes (lack of infectious complication) in the postsurgical period. Madi et al. stated that ‘Low-level laser as an adjunctive therapy to gingivectomy procedure can be used to decrease patients’ pain perception following surgery and accelerate the healing process especially in the early phases’ [
47].