Abstract
Even though synthetic colorants can cause side effects such as allergies and pigmentation, they have not been sufficiently researched. Herein, high-performance liquid chromatography, liquid chromatography-tandem mass spectrometry, and liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS) were used to detect 13 banned synthetic colorants in cosmetics and characterize their fragmentation. The developed HPLC method was validated following the International Conference on Harmonisation guidelines (specificity, limit of detection, limit of quantification, recovery, linearity, accuracy, and precision) and applied to 120 distributed cosmetic products, one of which was found to contain three illegal synthetic colorants, namely Basic Blue 26 (0.33 mg/g), Basic Red 2 (0.53 mg/g), and Basic Yellow 28 (31.50 mg/g). Additionally, based on their fragment ions obtained using LC-Q-TOF-MS, the fragmentation pattern of synthetic colorants was predicted. Thus, our work paves the way for the reliable detection of illegal synthetic colorants and may help to prevent the distribution of cosmetics containing the same.
1. Introduction
The reliance of image and personality shaping on physical attractiveness creates a high demand for makeup cosmetics, thus driving the diversification of cosmetics consumers and the growth of the domestic cosmetics market [1,2,3,4,5,6]. In particular, the increased demand for high-persistence cosmetics (i.e., those that are not smeared upon contact with masks) due to the COVID-19 pandemic has inspired the development of new color cosmetics [3,7]. As their name suggests, color cosmetics (e.g., lipsticks, eye shadows, eyeliners, and blushers) contain natural or synthetic colorants and are used to conceal skin defects and create a more attractive appearance [8,9,10]. Compared to natural colorants, synthetic ones exhibit the advantages of long-lasting effect, lower price, and higher stability to light, oxygen, and pH, and are therefore typically the preferred choice [10,11,12,13,14,15,16]. However, most synthetic colorants contain azo functional groups or aromatic rings and can therefore cause allergies, asthma, DNA damage, and even cancer or mutagenesis [11,12,14,15,16,17,18,19,20,21,22]. Therefore, the types and standards of colorants allowed in cosmetics are closely regulated.
The requirements to be met by cosmetics distributed in the European Union are set forth in the Cosmetics Regulation (EC) No. 1223/2009. Annex IV of this regulation allows 153 colorants, prohibits four from use in products applied to eyes and 19 from use in products applied to mucous membranes, and allows 36 to be used only in rinse-off products. Annex II lists the ingredients prohibited from use in cosmetics, while Annex III lists the restricted ingredients. In the United States (US), color additives are regulated by the Food and Drug Administration (FDA; e.g., Federal Food, Drug, and Cosmetic Act, Sec. 721; 21 U.S.C.379e) and must be approved by the same before use in cosmetics. Most color additives must obtain batch certification from the FDA when used in US-marketed cosmetics. The FDA has classified 65 types of color additives as “permissible”. Their availability depends on the eye area and general (including lipstick) and external use. The FDA has also set the usage limit for some color additives.
Although color cosmetics directly contact the skin and must therefore meet all safety requirements, many cosmetics with unusable and unsafe pigments capable of causing side effects such as pigmentation have previously been marketed, which highlights the need for appropriate regulation. In the Republic of Korea, cosmetics manufacturers are required to list all the ingredients of a given product. The Ministry of Food and Drug Safety has listed the colorants allowed for use in color cosmetics as well as the related standards and analytical methods. In particular, the Cosmetics Act lists 129 pigments that can be used in cosmetics. However, the use of these pigments is subject to restrictions depending on the type of cosmetics and/or the targeted skin area. In addition, standards for ingredients that cannot be used in cosmetics and those that can be used only under certain restrictions are also designated and managed through timely notices. The above act also prohibits approximately 90 carcinogenic or harmful colorants from use in color cosmetics.
Previous studies on pigment analysis employed high-performance liquid chromatography (HPLC), ultra-high-performance liquid chromatography (UHPLC), ultra-high-performance supercritical fluid chromatography, and liquid chromatography-electrospray ionization tandem mass spectrometry [12,13,15,21,22,23,24,25,26,27,28,29,30], but have mostly focused on food or textiles and not cosmetics. Guerra et al. used liquid chromatography-tandem mass spectrometry (LC-MS/MS) to simultaneously analyze nine water-soluble pigments found in cosmetics/personal care products and detected seven of these targets at levels of 0.054–580 μg/g [24]. In another work, Guerra et al. analyzed 19 synthetic dyes used in confectionaries and cosmetics using LC-MS/MS [11] and detected nine targets at levels of 0.232–989 μg/g in the 24 tested samples. Chen et al. analyzed 63 pigments in cosmetics (eye shadow, eyeliner, lipstick, blusher, toothpaste) using UHPLC coupled with quadrupole-Orbitrap high-resolution mass spectrometry [10] and detected 11 targets (Basic Violet 1, Basic Violet 10, and Basic Violet 3, etc.) at levels of 0.0190–13.2644 mg/kg in the 26 tested samples.
The growing interest in long-lasting cosmetics such as tattoo lipsticks, tattoo eyebrows, and hair tints has increased the incidence of the related side effects. Currently, regulatory authorities manage products by issuing various guidelines; however, no effective analysis methods for illegal colorants in cosmetics have been established. This study contributes to the safety of color cosmetics by developing a method for detecting prohibited colorants therein (Figure 1). Unlike previous investigations, the present work uses HPLC coupled to a diode array detector (HPLC-DAD) and UPLC-MS/MS to develop an analytical method for analyzing illegal colorants and fragmentation of pigments using liquid chromatography-quadrupole time-of-flight mass spectrometry (LC-Q-TOF-MS).
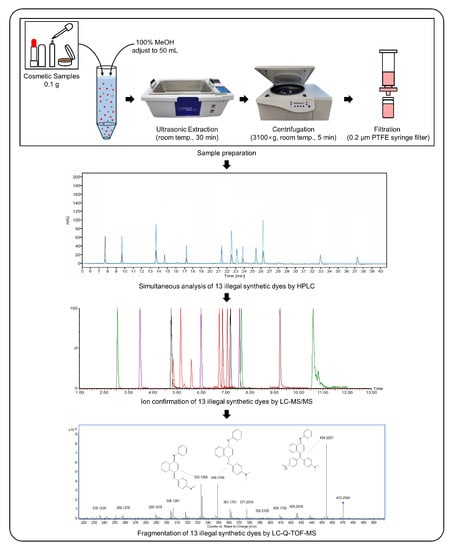
Figure 1.
Experimental process for analysis of 13 illegal synthetic colorants.
2. Materials and Methods
2.1. Chemicals and Reagents
Basic Blue 26, Basic Yellow 28, and Solvent Orange 4 were purchased from BLD Pharmatech, Ltd. (Shanghai, China). Basic Red 2 and HC Yellow No. 5 were purchased from AK Scientific, Inc. (Union City, CA, USA). Disperse Brown 1, Disperse Yellow 3, Solvent Orange 7, Solvent Red 24, and Solvent Yellow 2 were purchased from LGC Standards GmbH (Wesel, Germany). Disperse Orange 3 and Solvent Yellow 1 were purchased from Sigma-Aldrich, Inc. (St. Louis, MO, USA). HC Blue No. 2 was purchased from Tokyo Chemical Industry Co., Ltd. (Tokyo, Japan). HPLC-grade acetonitrile (ACN), methanol (MeOH), and chloroform (CHCl3) were obtained from Merck (Darmstadt, Germany). Ammonium acetate, ammonium formate, and formic acid were purchased from Sigma-Aldrich (St. Louis, MO, USA). Deionized water (18.2 MΩ) was prepared using a Milli-Q system (Millipore, MA, USA).
2.2. Standard Stock Solutions
Stock solutions of Basic Blue 26, Basic Red 2, Disperse Brown 1, Disperse Orange 3, Disperse Yellow 3, HC Blue No. 2, HC Yellow No. 5, Solvent Orange 4, Solvent Yellow 1, and Solvent Yellow 3 were prepared in 100% MeOH. Stock solutions of Solvent Orange 7 and Solvent Red 24 were prepared in 100% CHCl3. Basic Yellow 28 was dissolved in 100% water. All stock solutions were stored in a refrigerator (2–8 °C). Working standard solutions for HPLC-DAD, LC-MS/MS, and LC-Q-TOF-MS analyses were prepared by diluting stock solutions with 100% MeOH.
2.3. Sample Preparation
The samples were purchased through an online store and categorized into tattoo lipstick, tattoo eyebrow, and hair tint. Each sample (100 mg) was placed in a 50 mL corning tube, and the volume was adjusted to 50 mL using 100% MeOH. Ultrasonication-assisted extraction was performed at room temperature for 30 min and was followed by centrifugation at 3100× g for 5 min. Then, the supernatant was filtered with a 0.2 μm PTFE syringe filter and used for HPLC-DAD, LC-MS/MS, and LC-Q-TOF-MS analyses.
2.4. Optimized HPLC-DAD Conditions
The 13 prohibited colorants were detected using an Agilent 1260 Infinity II LC system (Agilent, Santa Clara, CA, USA) equipped with a DAD and separated on a Zorbax Eclipse XDB-C18 (4.6 mm × 150 mm, 5 μm; Agilent, Santa Clara, CA, USA) column maintained at 30 °C. The sample temperature was maintained at 8 °C. The mobile phase consisted of (A) 10 mM ammonium acetate in water containing 0.1% formic acid and (B) ACN–MeOH (80:20, v/v). The flow rate and injection volume equaled 1 mL/min and 10 μL, respectively. The gradient was as follows: 0–15 min (B: 5–50%), 15–17 min (B: 50–52%), 17–19 min (B: 52–60%), 19–29 min (B: 60–90%), 29–34 min (B: 90–100%), 34–39 min (B: 100%), 39–40 min (B: 100–5%), and 40–45 min (B: 5%). Detection was performed at 254, 430, and 590 nm.
2.5. Method Validation
The HPLC method was validated for specificity, limit of detection (LOD), limit of quantification (LOQ), recovery, linearity, precision, and accuracy according to the guidelines of the International Conference on Harmonisation (ICH) of Technical Requirements for Pharmaceuticals for Human Use [31]. Specificity was confirmed by spiking the blank matrix sample (tattoo lipstick, tattoo eyebrow, and hair tint) with the 13 illegal colorants. The LOD and LOQ were evaluated for the spiked samples using signal-to-noise ratios of 3:1 and 9:1, respectively. The concentration range of linearity was set to six points, considering the analyte LOQ. Calibration curves were obtained in triplicate at each concentration. Recovery was determined by comparing the responses of the colorants in the spiked samples and standard solutions. For precision and accuracy evaluation, the experiment was conducted thrice on the same day (intra-day) and thrice over three days (inter-day) at the three concentrations. The recovery, precision, and accuracy were measured at low, medium, and high concentrations.
2.6. Optimized LC-MS/MS Conditions
For LC-MS/MS, chromatographic separation was performed on a Waters ACQUITY ultra-performance liquid chromatography coupled with a Xevo TQ-XS (Waters, Milford, MA, USA) system. The Waters ACQUITY UPLC BEH C18 (2.1 mm × 100 mm, 1.7 μm; Waters, Milford, MA, USA) column was maintained at 40 °C. The mobile phase consisted of (A) 5 mM ammonium acetate in water containing 0.1% formic acid and (B) ACN–MeOH (80:20, v/v). The flow rate and injection volume equaled 0.3 mL/min and 2 μL, respectively. The gradient was as follows: 0–4 min (B: 5–40%), 4–6 min (B: 40–70%), 6–8 min (B: 70–95%), 8–10 min (B: 95%), 10–10.1 min (B: 95–100%), 10.1–12 min (B: 100%), 12–14 min (B: 100–5%), and 14–17 min (B: 5%). Electrospray ionization (ESI) was operated in both positive- and negative-ion modes. The other MS parameters were as follows: capillary voltage = 2.5 kV, desolvation temperature = 400 °C, desolvation gas (N2) flow = 800 L/h, and cone gas (N2) flow = 150 L/h.
2.7. Optimized LC-Q-TOF-MS Conditions
LC-Q-TOF-MS was performed using an Agilent 1290 Infinity II LC system (Agilent Technologies, Santa Clara, CA, USA) coupled with an Agilent 6545XT Q-TOF-MS system (Agilent Technologies, Santa Clara, CA, USA) to examine analyte fragmentation. The mobile phase consisted of (A) 10 mM ammonium formate in water containing 0.1% formic acid and (B) ACN–MeOH (80:20, v/v). The gradient was as follows: 0–4 min (B: 5–40%), 4–6 min (B: 40–70%), 6–8 min (B: 70–95%), 8–10 min (B: 95%), 10–12 min (B: 95–5%), and 12–15 min (B: 5%). The injection volume and flow rate equaled 1 μL and 0.3 mL/min, respectively. The other MS parameters were as follows: ion source = ESI, capillary voltage = 3500 V, gas temperature = 300 °C, nebulizer = 45 psi, drying gas (N2) = 10 L/min, fragmentor = 175 V, and skimmer = 65 V.
3. Results and Discussion
The detection wavelengths for the simultaneous analysis of the 13 prohibited synthetic colorants by HPLC-DAD were set in consideration of the maximum absorption wavelength (λmax) for each compound. As shown in Figure 2d, three wavelengths were selected to effectively separate the compounds: 254 nm for Basic Red 2, Disperse Yellow 3, HC Blue No. 2, Solvent Orange 7, and Solvent Red 24; 430 nm for Basic Yellow 28, Disperse Brown 1, Disperse Orange 3, HC Yellow No. 5, Solvent Orange 4, Solvent Yellow 1, and Solvent Yellow 3; and 590 nm for Basic Blue 26. The ultraviolet (UV) spectra are shown in Figure 2d.
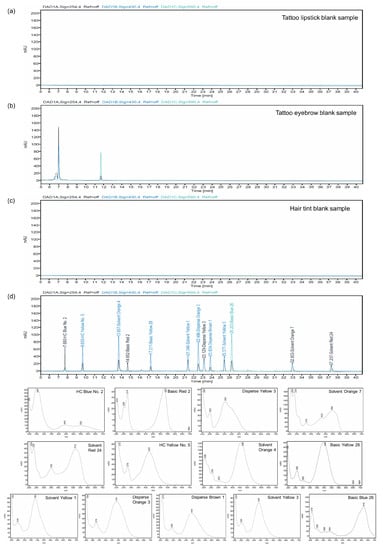
Figure 2.
HPLC chromatogram and UV spectra of blank sample and 13 illegal synthetic colorants: (a) tattoo lipstick blank sample; (b) tattoo eyebrow blank sample; (c) hair tint blank sample; (d) 13 illegal synthetic colorants.
The HPLC-DAD method was validated in terms of specificity, LOD, LOQ, linearity, recovery, accuracy, and precision. The specificity was confirmed as peaks of interfering compounds were not observed at the retention times of the colorants in all blank samples. The LODs and LOQs of colorants in tattoo eyebrow samples were 0.10–1.20 μg/mL and 0.30–3.60 μg/mL, respectively, while those for tattoo lipstick and hair tint samples were 0.01–0.30 μg/mL and 0.02–0.90 μg/mL, respectively (Table 1). The calibration curves for all prohibited colorants showed excellent correlation coefficients (R2 > 0.999, Table 1). The recovery was determined at three concentrations (low, medium, and high) based on the LOQ concentration of each compound. Low, medium, and high concentrations were set to the LOQ, 10 times the LOQ, and 50 times the LOQ, respectively. The recoveries of the tattoo lipstick, tattoo eyebrow, and hair tint samples were 86.11–108.39%, 83.32–105.33%, and 93.49–106.48%, respectively, and the corresponding relative standard deviations (RSDs) were <4.66%, 3.58%, and 9.63%, respectively (Table 2, Table 3 and Table 4). The recoveries and RSDs were within the appropriate ranges. Accuracy was evaluated as the average of recovery, and precision was evaluated as RSD% (Table 2, Table 3 and Table 4), with satisfactory results obtained in all cases. For tattoo lipstick samples, the intra- and inter-day accuracies were 85.97–106.50% and 89.22–108.28%, respectively, while the intra- and inter-day precisions were 0.53–8.96% and 0.04–6.96%, respectively. For tattoo eyebrow samples, the intra- and inter-day accuracies were 80.23–103.90% and 80.70–103.74%, respectively, while the intra- and inter-day precisions were 0.14–9.68% and 0.02–7.64%, respectively. For hair tint samples, the intra- and inter-day accuracies were 81.20–107.44% and 86.34–104.02%, respectively, while the intra- and inter-day precisions were 0.08–9.99% and 0.45–9.80%, respectively.

Table 1.
LOD, LOQ, and linearity of colorants in cosmetic samples using HPLC-DAD.

Table 2.
Recovery, accuracy, and precision of colorants in tattoo lipstick sample with different concentrations using HPLC-DAD.

Table 3.
Recovery, accuracy, and precision of colorants in tattoo eyebrow sample with different concentrations using HPLC-DAD.

Table 4.
Recovery, accuracy, and precision of colorants in hair tint sample with different concentrations using HPLC-DAD.
In addition, to check the illegal colorant ions, multiple reaction monitoring (MRM) conditions for each compound were confirmed using LC-MS/MS. Each standard solution was directly infused to find the precursor ion. Then, the product ion was obtained by adjusting the collision energy. Two or more transitions were selected for each analyte. The optimized MRM transitions are listed in Table 5. Disperse Yellow 3 and HC Yellow No. 5 were detected in the negative-ion mode, while the other 11 compounds were detected in the positive-ion mode.

Table 5.
LC-MS/MS MRM transitions of 13 illegal synthetic colorants.
The 120 purchased samples were classified into tattoo lipsticks (40), tattoo eyebrows (41), and hair tints (39). Figure 3 shows the results of illegal pigment detection by HPLC-DAD, revealing that three peaks with retention times and UV spectra matching those of Basic Red 2, Basic Yellow 28, and Basic Blue 26 were detected in a tattoo eyebrow product. The contents of these three components (HPLC-DAD) were determined as 0.53 mg/g (Basic Red 2), 31.50 mg/g (Basic Yellow 28), and 0.33 mg/g (Basic Blue 26). The identities of these three compounds were confirmed by positive mode using LC-MS/MS. The total ion chromatogram (Figure 3) of the tattoo eyebrow sample showed peaks at 5.13, 5.97, and 7.62 min corresponding to the peaks for Basic Red 2, Basic Yellow 28, and Basic Blue 26 standards, respectively. The precursor and product ions of these three colorants were the same as those of the respective standards. Basic Red 2 showed a precursor ion at m/z 315.32 and product ions at m/z 299.30, 210.24, and 237.26. Basic Yellow 28 showed a precursor ion at m/z 322.34 and product ions at m/z 160.24 and 136.18. Basic Blue 26 showed a precursor ion at m/z 470.42 and product ions at m/z 349.30, 454.38, and 333.28. Based on the above, unknown peaks 1, 2, 3 were tentatively identified as Basic Red 2, Basic Yellow 28, and Basic Blue 26, respectively. Hence, the developed method was found to be suitable for the detection and quantitation of illegal synthetic colorants added to cosmetics. In the case of Basic Red 2, several peaks ascribed to impurities were observed and marked as such in the chromatograms of Figure 3 [32].
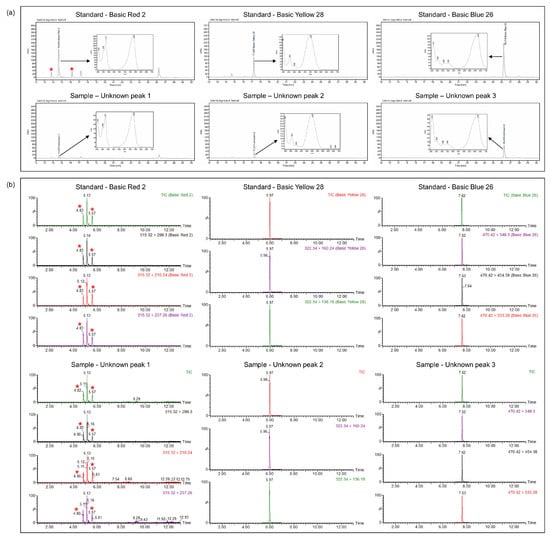
Figure 3.
Chromatograms for 3 illegal synthetic colorants in standards and tattoo eyebrow sample: (a) HPLC chromatograms and UV spectra and (b) total ion chromatograms (TICs) and extracted ion chromatograms by LC-MS/MS (the peaks (*) are observed due to impurities).
The MS/MS fragmentation patterns of the three detected compounds (Basic Blue 26, Basic Red 2, and Basic Yellow 28) were examined via LC-Q-TOF-MS at different collision energies. The optimized MS/MS spectra and the related ion structures are presented in Figure 4, Figure 5 and Figure 6. Basic Blue 26 exhibited a precursor ion at m/z 470.26 (C33H32N3+), and the difference from the theoretical value was estimated to be within 0.64 ppm. Fragment ions were observed at m/z 454.23, 333.14, and 349.17. Basic Red 2 showed a precursor ion at m/z 315.16 (C20H19N4+), and the difference from the theoretical value was estimated to be within 1.90 ppm. Fragment ions at m/z 237.11, 210.10, and 299.13 were observed. Basic Yellow 28 showed a precursor ion at m/z 322.19 (C20H24N3O+), and the difference from the theoretical value was estimated to be within 1.24 ppm. Fragment ions were observed at m/z 160.11 and 136.08. Figures S1–S10 present the fragmentation patterns of 10 colorants other than those detected in cosmetics samples. Disperse Brown 1 exhibited a precursor ion at m/z 433.02 (C16H16Cl3N4O4+) and fragment ions at m/z 356.97, 197.05, and 153.02. Disperse Orange 3 presented a precursor ion at m/z 243.09 (C12H11N4O2+) and fragment ions at m/z 122.02 and 197.09. HC Blue No. 2 showed a precursor ion at m/z 286.14 (C12H20N3O5+) and fragment ions at m/z 210.09 and 241.11. Solvent Orange 4 exhibited a precursor ion at m/z 227.13 (C13H15N4+) and fragment ions at m/z 77.04 and 134.07. Solvent Yellow 1 showed a precursor ion at m/z 198.10 (C12H12N3+) and fragment ions at m/z 77.04, 92.05, and 105.04. Solvent Yellow 3 exhibited a precursor ion at m/z 226.13 (C14H16N3+) and fragment ions at m/z 91.05, 106.07, and 121.08. Disperse Yellow 3 presented a precursor ion at m/z 268.11 (C15H14N3O2−) and fragment ions at m/z 134.06 and 92.05. HC Yellow No. 5 exhibited a precursor ion at m/z 196.07 (C8H10N3O3−) and fragment ions at m/z 166.06 and 137.04. To the best of our knowledge, the fragmentation patterns of the 11 out of the 13 examined dyes have not been reported before, i.e., only the fragmentations of Solvent Orange 7 and Solvent Red 24 have been described [33]. The precursor ions of Solvent Orange 7 and Solvent Red 24 were observed at m/z 277.13 (C18H17N2O+) and 381.17 (C18H17N2O+), respectively, as expected for these analytes. The fragment ions of Solvent Orange 7 were observed at m/z 121.09, 153.04, and 260.13, while those of Solvent Red 24 were observed at m/z 91.05, 106.07, 156.04, 224.12, and 276.11.
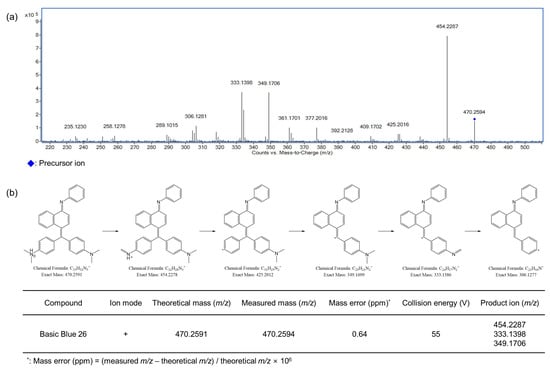
Figure 4.
Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Basic Blue 26 (b) by LC-Q-TOF-MS.
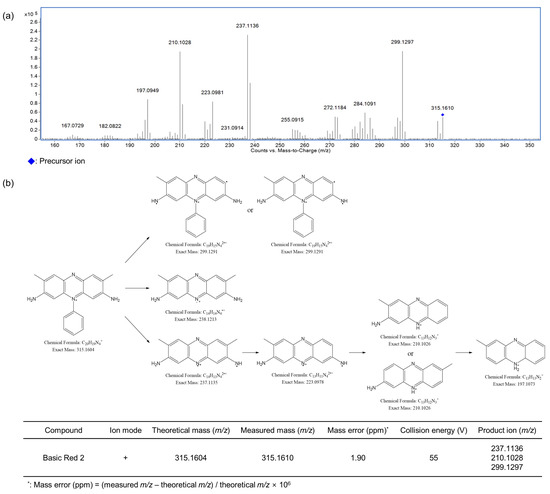
Figure 5.
Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Basic Red 2 (b) by LC-Q-TOF-MS.
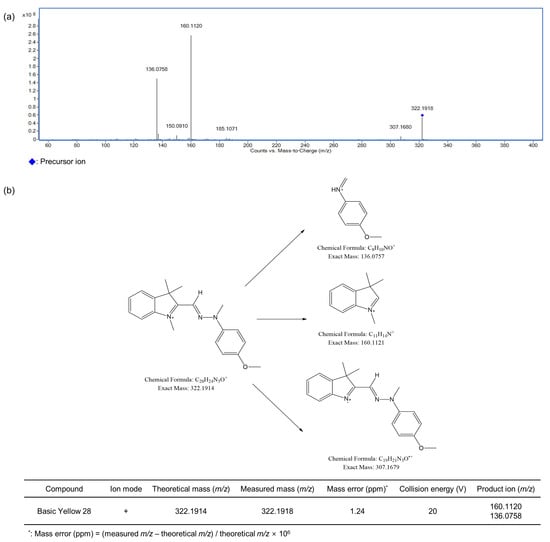
Figure 6.
Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Basic Yellow 28 (b) by LC-Q-TOF-MS.
4. Conclusions
HPLC-DAD and LC-MS/MS were used to simultaneously analyze 13 prohibited colorants in cosmetics (tattoo lipstick, tattoo eyebrow, and hair tint), and the developed HPLC method was validated. The LOQ, recovery, accuracy, and precision were within the range of 0.02–3.60 μg/mL, 83.32–108.39%, 80.23–108.28%, and 0.02–9.99%, respectively. Among the 120 distributed products examined using the newly established procedure, one was found to contain three illegal compounds, namely Basic Blue 26 (0.33 mg/g), Basic Red 2 (0.53 mg/g), and Basic Yellow 28 (31.50 mg/g). The MS fragmentation patterns (confirmed via LC-Q-TOF-MS) of 11 out of 13 species have not been reported before. Thus, our work is expected to hinder the distribution of cosmetics containing illegal synthetic colorants.
Supplementary Materials
The following supporting information can be download at: https://www.mdpi.com/article/10.3390/app13105967/s1, Figure S1: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Disperse Brown 1 (b) by LC-Q-TOF-MS; Figure S2: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Disperse Orange 3 (b) by LC-Q-TOF-MS; Figure S3: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of HC Blue No. 2 (b) by LC-Q-TOF-MS; Figure S4: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Solvent Orange 4 (b) by LC-Q-TOF-MS; Figure S5: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Solvent Yellow 1 (b) by LC-Q-TOF-MS; Figure S6: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Solvent Yellow 3 (b) by LC-Q-TOF-MS; Figure S7: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Disperse Yellow 3 (b) by LC-Q-TOF-MS; Figure S8: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of HC Yellow No. 5 (b) by LC-Q-TOF-MS; Figure S9: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Solvent Orange 7 (b) by LC-Q-TOF-MS; Figure S10: Optimized MS/MS spectrum (a) and proposed fragmentation pattern of Solvent Red 24 (b) by LC-Q-TOF-MS.
Author Contributions
Conceptualization, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C.; data curation, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C.; formal analysis, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C.; investigation, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C.; methodology, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C.; validation, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C.; writing—original draft, K.-M.H., Y.K.K., S.S., J.H.K., J.H.L., H.I.K. and S.C. All authors have read and agreed to the published version of the manuscript.
Funding
This study was supported by a Grant [No. 20181MFDS461 and No. 22231MFDS271] of the Ministry of Food and Drug Safety, Republic of Korea.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
The data presented in this study are available on request from the first author.
Acknowledgments
We would like to thank all companies who contributed to this study.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Kim, Y.E.; Kim, J.D. A study on the use of color cosmetics according to women’s appearance interest. Korean Soc. Cosmet. Cosmetol. 2020, 10, 289–305. [Google Scholar]
- Ahn, J.J. A study on the consumption propensity of men’s color cosmetics. Asian J. Beauty Cosmetol. 2020, 18, 533–547. [Google Scholar] [CrossRef]
- Lee, J.E.; Kim, J.D. A study on women’s appearance interest and cosmetics purchasing behavior due to Corona 19. Korean Soc. Cosmet. Cosmetol. 2021, 11, 231–242. [Google Scholar]
- Lee, H.; Kang, W. Effects of makeup motivation and implicit self-theory on beauty product use and purchase. Asian J. Beauty Cosmetol. 2021, 19, 209–221. [Google Scholar] [CrossRef]
- Ha, J.K. Clothing and make-up behavior by appearance management motive. Korean J. Community Living Sci. 2009, 20, 385–396. [Google Scholar]
- Park, E.H.; Ku, Y.S. Lifestyle, fast fashion attitude, and cosmetics behavior according to college students’ pursuit of clothing benefits typology. J. Fash. Bus. 2012, 16, 121–136. [Google Scholar] [CrossRef]
- Ahn, J.J. A study on cosmetics purchasing trends after COVID-19. J. Humanit. Soc. Sci. 2022, 30, 210–231. [Google Scholar]
- Park, A.R.; Lee, J.N. The influence of beauty influencer’s characteristics on makeup behavior and color cosmetics purchase intention in young female consumers aged 20–30s. J. Korean Appl. Sci. Technol. 2021, 38, 1093–1106. [Google Scholar]
- Weisz, A.; Milstein, S.R.; Scher, A.L.; Hepp, N.M. Colouring agents in cosmetics: Regulatory aspects and analytical methods. In Analysis of Cosmetic Products; Salvador, A., Chisvert, A., Eds.; Elsevier: Boston, MA, USA, 2018; Chapter 7; pp. 123–157. [Google Scholar]
- Chen, M.; Bai, H.; Zhai, J.; Meng, X.; Guo, X.; Wang, C.; Wang, P.; Lei, H.; Niu, Z.; Ma, Q. Comprehensive screening of 63 coloring agents in cosmetics using matrix solid-phase dispersion and ultra-high-performance liquid chromatography coupled with quadrupole-Orbitrap high-resolution mass spectrometry. J. Chromatogr. A 2019, 1590, 27–38. [Google Scholar] [CrossRef]
- Guerra, E.; Llompart, M.; Garcia-Jares, C. Miniaturized matrix solid-phase dispersion followed by liquid chromatography-tandem mass spectrometry for the quantification of synthetic dyes in cosmetics and foodstuffs used or consumed by children. J. Chromatogr. A 2017, 1529, 29–38. [Google Scholar] [CrossRef]
- Zhao, S.; Yin, J.; Zhang, J.; Ding, X.; Wu, Y.; Shao, B. Determination of 23 dyes in chili powder and paste by high-performance liquid chromatography–electrospray ionization tandem mass spectrometry. Food Anal. Methods 2012, 5, 1018–1026. [Google Scholar] [CrossRef]
- Cooper, J.; Marchand, J. Improving the speed and quantitative performance for the analysis of allergenic and carcinogenic dyes in industrial, cosmetics, personal care and consumer products. Consum. Prod. Test. 2016, 32. Available online: https://lcms.labrulez.com/labrulez-bucket-strapi-h3hsga3/720004492en_672f2e9680/720004492en.pdf (accessed on 25 March 2020).
- Filiz, Z.; Oymak, T.; Dural, E. Determination of synthetic colorants in cosmetic products by reversed-phase high-performance liquid chromatography coupled with diode-array detector. J. Res. Pharm. 2019, 23, 1048–1059. [Google Scholar] [CrossRef]
- García-Lavandeira, J.; Blanco, E.; Salgado, C.; Cela, R. Fast throughput, highly sensitive determination of allergenic disperse dyes in textile products by use of sample composition. Talanta 2010, 82, 261–269. [Google Scholar] [CrossRef] [PubMed]
- Ardila-Leal, L.D.; Poutou-Pinales, R.A.; Pedroza-Rodriguez, A.M.; Quevedo-Hidalgo, B.E. A Brief History of Colour, the Environmental Impact of Synthetic Dyes and Removal by Using Laccases. Molecules 2021, 26, 3813. [Google Scholar] [CrossRef] [PubMed]
- Pas, A.; Carballo, J.; Perez, M.J.; Dominguez, J.M. Biological treatment of model dyes and textile wastewaters. Chemosphere 2017, 181, 168–177. [Google Scholar] [CrossRef]
- Zucca, P.; Cocco, G.; Sollai, F.; Sanjust, E. Fungal laccases as tools for biodegradation of industrial dyes. Biocatalysis 2015, 1, 82–108. [Google Scholar] [CrossRef]
- Mpountoukas, P.; Pantazaki, A.; Kostareli, E.; Christodoulou, P.; Kareli, D.; Poliliou, S.; Mourelaos, C.; Lambropoulou, V.; Lialiaris, T. Cytogenetic evaluation and DNA interaction studies of the food colorants amaranth, erythrosine and tartrazine. Food Chem. Toxicol. 2010, 48, 2934–2944. [Google Scholar] [CrossRef]
- Benigni, R.; Passerini, L. Carcinogenicity of the aromatic amines: From structure–activity relationships to mechanisms of action and risk assessment. Mutat. Res. Rev. Mutat. Res. 2002, 511, 191–206. [Google Scholar] [CrossRef]
- Wang, L.; Zheng, J.; Zhang, Z.; Wang, T.; Che, B. Determination of eight Sudan dyes in chili powder by UPLC-MS/MS. Engineering 2013, 5, 154–157. [Google Scholar] [CrossRef]
- Chew, Y.L.; Xing, J.; Lim, L.G.S.; Zhan, Z. Development of LC/MS/MS Method for Screening and Quantitation of 47 Synthetic Dyes Under Restricted Substance List in Textiles. Available online: https://www.shimadzu.com (accessed on 26 November 2020).
- Jia, W.; Chu, X.; Ling, Y.; Huang, J.; Lin, Y.; Chang, J. Simultaneous determination of dyes in wines by HPLC coupled to quadrupole Orbitrap mass spectrometry. J. Sep. Sci. 2014, 37, 782–791. [Google Scholar] [CrossRef]
- Guerra, E.; Celeiro, M.; Lamas, J.P.; Llompart, M.; Garcia-Jares, C. Determination of dyes in cosmetic products by micro-matrix solid phase dispersion and liquid chromatography coupled to tandem mass spectrometry. J. Chromatogr. A 2015, 1415, 27–37. [Google Scholar] [CrossRef] [PubMed]
- Khalikova, M.A.; Šatínský, D.; Solich, P.; Nováková, L. Development and validation of ultra-high performance supercritical fluid chromatography method for determination of illegal dyes and comparison to ultra-high performance liquid chromatography method. Anal. Chim. Acta 2015, 874, 84–96. [Google Scholar] [CrossRef]
- Tang, B.; Xi, C.; Zou, Y.; Wang, G.; Li, X.; Zhang, L.; Chen, D.; Zhang, J. Simultaneous determination of 16 synthetic colorants in hotpot condiment by high performance liquid chromatography. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2014, 960, 87–91. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Liu, J.; Zhang, Y.; Chen, Y.; Yang, X.; Duan, L.; Dharmarajan, R.; Wang, X.; Li, L. Simultaneous determination of 20 disperse dyes in foodstuffs by ultra high performance liquid chromatography–tandem mass spectrometry. Food Chem. 2019, 300, 125183. [Google Scholar] [CrossRef]
- Yang, Y.; Yin, J.; Shao, B. Simultaneous determination of five aluminum lake dyes in chewing gum by HPLC with photodiode array detection. Food Addit. Contam. Part A 2011, 28, 1159–1167. [Google Scholar] [CrossRef] [PubMed]
- Yoshioka, N.; Ichihashi, K. Determination of 40 synthetic food colors in drinks and candies by high-performance liquid chromatography using a short column with photodiode array detection. Talanta 2008, 74, 1408–1413. [Google Scholar] [CrossRef] [PubMed]
- Guerra, E.; Alvarez-Rivera, G.; Llompart, M.; Garcia-Jares, C. Simultaneous determination of preservatives and synthetic dyes in cosmetics by single-step vortex extraction and clean-up followed by liquid chromatography coupled to tandem mass spectrometry. Talanta 2018, 188, 251–258. [Google Scholar] [CrossRef]
- ICH Harmonised tripartite guideline. In Validation of Analytical Procedures: Text and Methodology Q2(R1); International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use: Geneva, Switzerland, 2005.
- Wan, H.; Chen, H.; Chu, Y.; Ju, X.; Jiang, H. Structure characterization and optical properties investigation of the four main components of the classical phenazinium dye Safranin O. Analyst 2019, 144, 7149–7156. [Google Scholar] [CrossRef]
- Di Donna, L.; De Nino, A.; Maiuolo, L.; Mazzotti, F.; Napoli, A.; Salerno, R.; Sindona, G. High–throughput mass spectrometry: The mechanism of sudan azo dye fragmentation by ESI tandem mass spectrometry and extensive deuterium labeling experiments. J. Mass Spectrom. 2007, 42, 1057–1061. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).