Abstract
Multiple sclerosis (MS) is the most prevalent demyelinating disease of the central nervous system (CNS) with an autoimmune component affecting young adults in their third decade of life. The etiology is still undefined, but myelin damage is mainly due to an aberrant immune response of lymphocyte cells against myelin components. Therefore, inflammation, demyelination, and axonal degeneration represent the major pathologic hallmarks of the disease. There are many risk factors associated with MS, and probably the most relevant is gender-related. Women are up to four times more affected than men are. Although the female prevalence in MS is epidemiologically evident, the identification of key factors involved in this difference is under investigation. On the other side, if women are more affected, men show late onset and worse prognosis. This sexual dimorphism derives from many sources, including sex hormones, different genes on female sex chromosomes, and differences in bacterial species. Indeed, accumulating evidence proves a link among MS and gut microbiota where its dysbiosis could help the immune system to trigger neuroinflammation. In this context, oral biology alteration should be considered, too. This work is intended to explore current knowledge inside MS gender differences with a look towards oral–gut–brain axis involvement.
1. Introduction
Multiple sclerosis (MS) is an inflammatory and degenerative disease of the central nervous system, supposedly of autoimmune etiology [1]. It affects 2.8 million people worldwide, and its prevalence is increasing [2]. MS etiopathogenesis is extremely heterogeneous and multifactorial. Vitamin D deficiency is considered a risk factor of MS [3]. This could partially explain why MS is more frequent in higher latitudes with low sunlight exposure [4]. In addition to geographic location, genetic susceptibility may be involved as well. The more genetic risk variants are shared with a family member of an MS patient, the higher the risk of manifesting the disease. Therefore, monozygotic twins with 100% genetic similarity have 30% to 50% concordance in MS development while dizygotic twins or siblings have a 2–5% concordance compared to a 0.1% risk in the general population [5]. In addition, smoking, obesity, and exposure to viral and bacterial agents such as Epstein Barr virus (EBV) are associated with the onset of MS [6]. Regardless, it is difficult to believe that MS is associated with a single risk factor but is more likely a combination of multiple factors that predispose, initiate, and modify the disease course.
This chronic illness can imply severe physical or cognitive incapacitation, as well as neurological problems, especially in young adults [7]. Clinically, different subtypes have been described and are considered critical not only for prognosis, but also for the treatment choice: relapsing–remitting (RRMS), secondary progressive (SPMS), and primary progressive (PPMS). Of the affected people, 87% show an RR subtype with unexpected attacks followed by remission periods [8]. Within each subtype, a considerable individual variation in the disease course could occur. Biologically, the main cause of myelin destruction is basically due to the infiltration of T-cells and macrophages and their cytokines and due to the death of oligodendrocytes at multifocal zones of inflammation, leading to the CNS plaque formation in white and gray matter [9].
The extreme heterogeneity of the disease and the different responsiveness to treatment have led over time to the formulation of different therapeutic strategies. In general, immunomodulating agents act on the disease progression and reduce some pathological symptoms, such as clinical relapses and the stepwise accumulation of the disease. Instead, immunosuppressive therapies, such as monoclonal antibodies, are directed against immune system cells. Regardless, for treating MS, therapy options are usually based on a personalized approach determined by an individual patient’s prognosis and treatment risks.
In this intricate scenario, gender also plays a critical role in the disease. Females are more affected than males, and the female-to-male ratio varies between 2:1 to 4.1:1 in some countries, such as the Western Pacific and Southeast Asia regions [2]. This sex bias observed in MS epidemiology is still unexplained. However, a variety of factors, such as hormonal and genetic differences, as well as different social, lifestyle, and environmental exposures, have been implicated in the observed increased incidence in females [2].
Furthermore, these gender differences are also reflected in the different disease course. Men show a higher risk of developing PPMS and neurodegeneration while women seem to have more inflammatory lesions [10]. On the other hand, when MS is diagnosed after 50 years of age, men and women have a similar course [10]. In addition, the effect of pregnancy is not totally clarified, as the risk of relapse appears lower during pregnancy than after giving birth, where it seems higher [10]. All of these differences are believed to be mainly due to the sexual hormone effects, but most likely, other factors are involved.
The human gut-associated microbial community, known as the microbiota, consists of 10–100 trillion microbes, including bacteria, viruses, fungi, and parasites, which carry ~100 times more genes than the human genome, the microbiome [10]. The gut microbiota composition influences sex bias in autoimmune diseases, and conversely, sex hormones can influence the gut microbiota composition [10,11].
In this review, we aim to summarize the current knowledge about the link between sex bias and the microbiota in people with MS.
2. Sex Differences in MS Epidemiology
MS is considered the primary cause of non-traumatic neurological disability in young adults [11]. The mean age of onset has progressively increased in the past decades, going from 23.79 ± 10.19 in the 70s to 31.11 ± 9.82 in 2022 [12]. As aforementioned, MS is a multifactorial disease; genetic variants, lifestyle, and environmental factors (e.g., EBV infection, low vitamin D, smoking, and adolescent obesity) are associated with an increased risk of developing the disease [13]. Migration studies highlight the importance of non-genetic factors in modulating the disease risk. The risk of developing MS depends on the destination country and the age at which an individual migrates. Individuals migrating from low-risk countries to high-risk countries before adolescence acquire a high risk, which could suggest environmental factors in the etiology of MS. The existence of a sex bias in MS epidemiology, which increased over the last decade, is also considered further proof of an important environmental influence on disease risk [14,15,16]. A higher incidence of MS in females has been recognized for many years, and the prevalence in females has been increasing in the past decades, leading to a mean female-to-male ratio of 3–4.5:1 [2]. Differences between sexes are also observed in the relapse rate and disease course. Females show a higher relapse rate after the disease onset compared to males. In contrast, males are less susceptible but show a higher accumulation of disability [14]. An increased susceptibility in females compared to males has been described for many autoimmune diseases [15]. Environment–genotype–sex interactions influencing the penetrance of specific gene polymorphisms have been implicated in the increased immunologic susceptibility observed in females [16]. Differences in sex chromosomes, sex hormones, or in the complex interplay between sex and the environment can be advocated to explain the sex bias [14].
3. Role of Sex Hormones and Chromosomes
Sex hormones have been extensively studied in the past decade. Gender bias is described only in people with MS with an onset during the reproductive age, and there is evidence that the age at menarche may be related to the age at the disease onset [17]. Indeed, early menarche has been associated with an increased risk of developing MS [17]. The possible role of hormones is further supported by the evidence for an effect of pregnancy on MS activity. Studies report a 70% decrease in relapse rates during the third trimester compared with pre-pregnancy levels and increased relapse rates 3–6 months after delivery to levels almost three times higher than pre-pregnancy ones [17]. The effects of pregnancy on disability progression in MS remain controversial. In a cohort of 2466 patients followed for 10 years, more pregnancies were independently associated with lower disability scores [18].
However, although some studies show that women with MS who have been pregnant have less progression of disability and propose that these effects might even be cumulative, other studies have shown no effect on permanent disability scores [19].
Because of the presence of hormone receptors on immune cells, sex hormones, such as estrogens, progesterone, and androgens can influence different aspects of the immune system, potentially modulating the risk, activity, and progression of MS (Figure 1) [20].
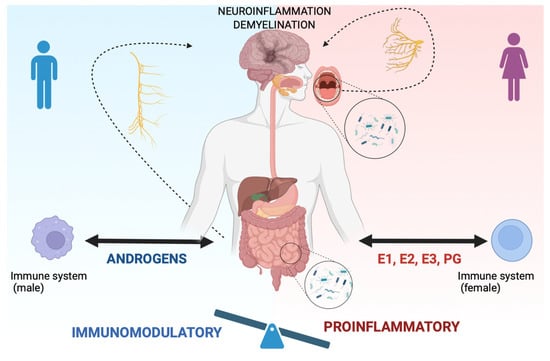
Figure 1.
Sex differences in the oral–gut–brain axis in people with MS. Sex hormones, such as estrogens (E1, E2, E3), progesterone (PG) in females, and androgens in males, can influence different aspects of the immune system, potentially modulating the risk, activity, and progression of MS. In females (right side of the figure), the influence of sex hormones on the immune system can be considered proinflammatory (see balance). In contrast, in males (left side of the figure), the influence of sex hormones shifts the balance versus an immunomodulatory effect. Further, sex hormones have been demonstrated to influence gut microbiota composition. Dysbiotic gut bacteria can lead to systemic inflammation and contribute to neuroinflammation and demyelination through the oral–gut–brain axis, anatomically sustained by the vagus nerve (see arrow in the figure). At the same time, oral bacteria can contribute to neuroinflammation via the trigeminal nerve (see arrow). Created with BioRender.com.
Estrogen receptors (α and β coded by the genes ESR1 and ESR2) are expressed at different levels on peripheral blood mononucleated cells (PBMCs). B cells express the highest levels of ESR1 RNA while CD4+ T cells, CD8+ T cells, natural killer (NK) cells, and plasmacytoid dendritic cells (DCs) express intermediate levels. Monocytes have the lowest levels of ESR1 RNA. ESR2 RNA is expressed at the highest levels in B cells and plasmacytoid DCs and at low levels in other cell types [21].
To further complicate the picture, the expression of estrogen receptors on different immune cells varies based on the sex and life period. Monocytes in premenopausal women contain lower amounts of ESR1 RNA than monocytes isolated from males and postmenopausal women, suggesting that higher estradiol levels correlate with reduced ESR1 expression. In contrast, ESR1 and ESR2 RNA levels did not differ in male and female B and T lymphocytes or plasmacytoid DCs, or in lymphocytes of pre- and postmenopausal women [21].
The effect of estrogens on the immune system depends on the estrogen type and its levels. Endogenous estrogens produced in female mammals include estrone (E1), 17β-estradiol (E2), and estriol (E3, produced only in pregnancy). E2 is the predominant form in premenopausal women. At higher concentrations, E2 exerts mainly anti-inflammatory effects by inhibiting the production and signaling of pro-inflammatory cytokines, such as TNF-α, IL-1, and IL-6, as well as inhibiting NK cell activation, and by inducing expression of anti-inflammatory cytokines, such as IL-4 and IL-10, favoring a Th2 phenotype and transforming growth factor β (TGF-β) expression, and activating Treg cells [17,22].
However, at lower concentrations (equivalent to pre-ovulatory menstrual cycle levels), E2 stimulates TNF-α, IFN-γ, IL-1 production, and NK cell activity, probably promoting a pro-inflammatory environment.
Moreover, EBV infection, the environmental factor more strongly associated with MS risk, is influenced by ESR expression and the host’s sex. In a recent paper, the risk of MS was increased 32-fold in individuals after an EBV infection, and levels of serum neurofilaments, a marker of neurodegeneration, increased after EBV seroconversion [23].
Although both males and females are equally susceptible to EBV, they present different responses to the virus. In people with MS, there is a clear interaction between MS risk genes and EBV latency III infection, with a definite gender effect [24]. EBNA2 expression is negatively correlated with ESR2 in female lymphoblastoid cell lines, but it is not in males. LMP1 is negatively correlated with ESR2 in both males and females, and the EBV DNA copy number was positively correlated with ESR2 expression in females [24].
Finally, polymorphism in the estrogen receptor binding site has been implicated in the different susceptibility to autoimmunity observed between males and females [20].
Progesterone is another hormone considered immune-modulatory. Progesterone favors Treg cell differentiation and promotes the down-regulation of pro-inflammatory cytokines production and Th2 differentiation with a contemporary downregulation of activation molecules (i.e., CD80, CD86, and MHC-II) [25]. Progesterone can also modulate the remyelination process by increasing the rate of myelin synthesis [26].
A positive correlation between the progesterone and estrogen peak and attenuated clinical activity has been described during the last trimester of pregnancy [26]. Conversely, an increased risk of relapse is typical of the postpartum period when progesterone and estrogen levels dramatically decrease [26].
Androgens are protective against the development of autoimmune diseases of the CNS (Figure 1). It has been demonstrated that androgens upregulate the autoimmune regulator (Aire), thus increasing its thymus expression and enhancing the tolerance mechanism [27].
As outlined above, evidence of a direct effect of sex steroids on the immune system can explain part, but not all, of the sex bias observed in MS incidence and clinical course.
A role for sex chromosome has been less well-studied in both MS and experimental autoimmune encephalomyelitis (EAE). The four core genotypes (FCG) mice have been used to study the role of sex chromosomes without the confounding factor of sex hormones. Chromosome influence has been hypothesized to be correlated to different parental imprinting. In a study by Voskhul and collaborators, they found that maternal imprinting determined a lower methylation and, thus, higher expression of the Foxp3 gene on the X chromosome. This effect is more protective on the XY progeny compared to the XX progeny [14].
Finally, recent reports on the influence of the gut microbiota on gender-based autoimmunity suggest that both bacteria and sex hormones can interact to regulate the immune response and the development of the disease in genetically susceptible individuals.
4. The Role of the Gut Microbiome in MS and the Influence of Sex on the Gut Microbiome Composition
A condition of gut dysbiosis has been described in both animal models and people with autoimmune diseases [28]. Consistent changes observed across multiple studies included a reduced abundance of the Bacteroidaceae family, Faecalibacterium, Clostridium species, and Prevotella strains and an increase in the genus Akkermansia [28,29,30,31,32]. The Clostridium species and Polysaccharide A from the capsule of the human commensal Bacteroides fragilis were shown to exert an immunomodulatory effect, promoting Treg accumulation in the colon and IL-10 secretion in T and B cells [33,34]. Whether these gut microbiota alterations in MS could contribute to the disease pathogenesis or are just a consequence remains unknown. Supporting a real pathogenic role is the finding that gut microbiota or gut-derived molecules obtained from people with MS could modulate EAE when transferred into mice [29,35].
Accumulating evidence shows that the gut microbiota modulate neuroinflammation [36]. Indeed the gut microbiota, acting on the intestinal production of serotonin, can play a role in the activation of the immune system, and it can influence its, interacting directly with T and B cells [36]. Interestingly, mucosal-associated invariant T (MAIT) cells that seem strictly interconnected with intestinal microbiota, which are absent in germ-free mice, localize in the CNS at MS onset and for many years [37]. Furthermore, they express CD103 as resident cells do and release pro-inflammatory cytokines, such as IFNγ and IL-17, with a detrimental effect for the CNS. Indeed, an open question that is relevant for MS is establishing if gut microbiota can interact with the blood-brain barrier (BBB). Some bacterial wall components, such as acid lipoteichoic (LTA), have exchanges with the brain endothelium passing through the BBB. Other species cross the BBB indirectly by taking advantage of peripheral immune cells or simply during BBB damage [38]. Furthermore, LTA and LPS can modulate the BBB permeability, attracting pro-inflammatory cytokines and changing BBB biology [39]. These findings support the hypothesis that gut microbiota could trigger and/or exacerbate neuroinflammation processes, worsening MS disease.
An increasing number of microbiome studies reveals a bidirectional cross-talk between microbiota and the endocrine system with bacteria able to produce, respond to, and regulate the effect of hormones [40]. In detail, sex hormones have been demonstrated to influence gut microbiota composition (Figure 1) [40].
In healthy people, sex influences the gut microbiome composition. Healthy male subjects have a higher abundance of Bacteroides compared to females [41]. Postmenopausal woman gut microbiota did not differ from adult males [41]. In a study comparing male and female gut microbiota, when correcting for other possible confounding factors, such as diet and BMI, women had a lower abundance of Bacteroides, and in women only, obesity determined a more pro-inflammatory gut microbiota composition compared to lean subjects [10].
For many autoimmune disease animal models, gender bias occurs more often in specific pathogen free (SPF) female mice, and the risk can be reversed by transplanting the gut microbiome obtained from SPF male mice [42]. The transplantation of the male gut microbiome at weaning has been associated with increased levels of testosterone and changes in serum metabolic profile [42]. Members of the gut microbiome are reported to interact with steroids, possibly impacting the steroid balance at the intestinal level [42]. Specific taxa have the capacity to metabolize sex steroid hormones. For instance, the intestinal commensal Clostridium expresses enzymes involved in glucocorticoid conversion into androgens [42]. The Slackia species, a common member of the gut microbiome, can exert the interconversion of B-estradiol and estrogen. It is unclear whether or how microbiome-derived sex steroids have an impact on host physiology and immunity. Estrogens can also promote the development of certain types of bacteria [43]. On the other hand, a “favorable” gut microbiota determines a better absorption of estrogens. Estrogens are metabolized by bacteria secreting beta-glucuronidase, an enzyme that deconjugates estrogens, allowing the active metabolite to enter the blood stream and directly bind estrogen receptors [44].
Data on people with MS are lacking, but the evidence that bacteria considered “protective” against autoimmunity in the CNS, like Bacteroides, are more abundant in males than in female subjects supports a possible role of the cross-talk between gut bacteria and hormones also in MS pathogenesis.
5. Oral Microbiome in MS
As outlined above, the interplay between the gut microbiome and host immune system is recently investigated to clarify the pathophysiology of multiple sclerosis (MS). A multi-omics approach to the study of MS revealed that gut microbiome dysbiosis without a unique signature for MS patients can indicate an extra-intestinal pathophysiology associated with an increased circulating proinflammatory marker [45]. In this context, the involvement of the oral microbiome should be considered principally for two reasons: (1) the human oral microbiome harbors the second most abundant, most heterogeneous, and most complex microbial community after the gut one; (2) emerging evidence demonstrates a strictly interconnection between oral and gut microbiome to the extent that oral bacteria can colonize and compete with the gut microbiota (Figure 1) [46,47]. Furthermore, in a dysbiosis scenario, oral microbes could contribute to stimulate the Th1 of the gut-associated lymphoid tissue, driving an aberrant inflammation and activation of the immune response of the GI tract (Figure 1) [48]. This mutual interaction between the oral and gut microbiome in MS is scarcely investigated since evidence of oral dysbiosis linked to neurological diseases are likewise poorly studied. Despite that, mouth dysbiosis may contribute to neuroinflammation indirectly through the spreading of inflammatory mediators or directly by microbes entering the CNS, most likely using the bloodstream [49]. Interestingly, in AD, a Treponema species have been detected in the trigeminal ganglia, suggesting another possible propagating route of oral bacteria to the brain strictly related to MS, as trigeminal neuralgia is common at the early stage of MS [50,51]. Unfortunately, there is a lack of research regarding the role of the oral microbiome in MS. A few articles described a correlation among oral dysbiosis and MS. In particular, periodontitis has been recently linked to a higher risk of developing different neurodegenerative diseases, including MS [52]. In addition, a case control study using a population-based dataset in Taiwan provides evidence on an association between chronic periodontitis and MS in female, but not male, subjects [53]. In contrast of these findings, in a large Norwegian cohort of MS patients, the association between periodontitis and disease was not demonstrated when taking into account the covariate smoking habits of patients versus controls [54]. Smoking is a notorious risk factor of periodontitis, and it should be considered to have more affordable results. Despite that, the link between periodontitis and MS also seems to be found in in vivo MS mice models. Indeed, the ligature-induced periodontitis (LIP) aggravated MS-like symptoms in EAE mice through the expansion of T helper 17 (Th17) cells [55]. Furthermore, fecal microbiota transplantation from EAE mice with LIP also promoted EAE symptoms. These results highlight the role of oral pathogens in EAE [55]. One of the bacteria implicated in periodontal disease is Porphyromonas gingivalis (P. gingivalis) [56]. P. gingivalis levels have been correlated with MS and other neurodegenerative diseases such as Alzheimer’s disease (AD), Parkinson’s disease (PD), frontotemporal dementia, dementia with Lewy Bodies, and Huntington’s disease [56]. Further, P. gingivalis can be found far from the oral cavity as it has been isolated from women with bacterial vaginosis [56,57,58]. This evidence could be in favor of the higher risk of MS in women even though no studies are present in the literature regarding the correlation between P. gingivalis and MS in women. Considering the whole oral microbiome community, the work of Troci and coworkers demonstrated that the oral microbiota composition is altered in patients with MS, and these microbial changes have been linked to MS disease activity and progression [59]. This study showed a specific microbial fingerprint observed in oral swabs in MS patients: the microbial stool changes associated with disease severity at baseline and the long-term reversibility of these compositional differences following B-cell depletion. In conclusion, the study suggests that not only stool, but also oral microbiota composition is altered in MS patients, defining a precise disease signature. Furthermore, treatment with B-cell depleting antibodies in MS patients does not only reduce the overall disease activity, but also associates with specific changes in stool and oral microbiota composition. In the light of these findings, dysbiotic oral bacteria can lead to systemic inflammation and contribute to neuroinflammation acting on: (a) the release of proinflammatory cytokines into the bloodstream, which eventually reach the brain; (b) gut microbiota alterations through the oral–gut–brain axis; and (c) crossing the BBB via the trigeminal nerve. In the brain, microglia can react by regulating the immune response while astrocytes amplify the release of proinflammatory cytokines and neuroinflammation. Importantly, although the intra-subject variability of the oral microbiome is extremely marked, cultures of a person’s saliva share some similarity with the stool coming from the same subject, suggesting a continuum in the individual oral–gut microbiota along the GI tract [60]. Unfortunately, there are not studies on the sex bias in the composition of the oral microbiome in the context of autoimmune diseases. However, this line of research might be particularly interesting to pursue since saliva collection could be the easiest way to detect gut microbiome changes correlated with an increased risk to develop MS.
Furthermore, an oral microbiome signature could be a more specific marker in MS disease progression, and saliva could represent, in perspective, a source of potential analytes monitoring the progression of MS and, more broadly, of neurodegenerative diseases [61]. Saliva is an easy biofluid to study due to its non-invasive and inexpensive collection procedure. In this way, saliva could be helpful in the screening of a broad group of healthy individuals and in the early diagnosis of MS. At the time being, the differential diagnosis of neurodegenerative diseases is complex and relies on the biomarker levels present in the cerebrospinal fluid. Compared with other standard tests and imaging procedures performed to diagnose neurological diseases, saliva could help detect an early specific marker at the earliest time [62].
Brain-derived extracellular vesicles (EVs) are also detectable in saliva. EVs secreted from different types of neural cells, such as neurons, astrocytes, microglia, and oligodendrocytes, present on their molecular surface markers almost completely specific to the donor cell [63]. They can cross the blood–brain barrier, and it is possible to speculate that, through the brain–axis consisting of the trigeminal nerve, they may reach the salivary glands where EVs are supposedly secreted with the saliva. Through their cargo, EVs can become a reservoir of potential peripheral biomarkers, mirroring brain pathology. In this regard, MS was, more than 30 years ago, the first neurological disorder identified where the release of EVs is closely related to the disease progression and neuroinflammation [64]. Thus, detecting specific CNS-derived EVs in saliva and other peripheral biofluids could represent a non-invasive and easy way to analyze the CNS status remotely [64]. In this line, EVs should deserve greater attention from the scientific community considering that they provide information not only in the pathogenesis, but also as therapeutic tools due to the intrinsic possibility of EVs carrying disease-modifying drugs back to the CNS donor cell.
6. Conclusions
There is no definitive explanation on why females are more susceptible than males to MS. A complex interaction between environmental, genetic, and epigenetic factors is probably involved. The microbiome is the newest player in MS susceptibility. As outlined above, there is a complex and not fully understood interplay between the microbiome and sex hormones. To further complicate the picture, the microbiome is influenced by many environmental factors, including, but not limited to, diet. Understanding the implications of the observed sex bias in autoimmune diseases is of the utmost importance to better guide patient counseling and therapeutic intervention. Clinical trials trying to use estrogens in people with MS failed to meet primary endpoints, likely because hormonal therapy given later in life cannot mimic hormone and environmental exposure during adolescence, as many lines of evidence point towards adolescence as the specific susceptibility window in which the risk of MS is determined.
Further studies are needed to better explain the sex bias in MS and to fully understand the consequences of pregnancy, hormonal therapy, and menopause on the disease course. The increasing use of multi-omic approaches to unravel the molecular mechanisms underlying the interplay between the environment, the microbiome, the immune system, and neuroinflammation will allow a better understanding of the sex bias observed not only in MS, but also in many autoimmune diseases.
Finally, yet importantly, understanding the sex bias in MS could help the therapeutic approach to be used in a more personalized medicine towards women with MS.
Author Contributions
M.D., F.R.B., D.G. and L.G. writing—original draft preparation; M.D., F.R.B., G.M.T., M.D.F., P.M., E.S., D.G. and L.G. writing—review and editing; F.R.B., figure design and preparation; E.S., D.G. and L.G., supervision. All authors have read and agreed to the published version of the manuscript.
Funding
MD is supported by IMI2-JU project GA No 831434 (3TR).
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Filippi, M.; Bar-Or, A.; Piehl, F.; Preziosa, P.; Solari, A.; Vukusic, S.; Rocca, M.A. Multiple sclerosis. Nat. Rev. Dis. Prim. 2018, 4, 49. [Google Scholar] [CrossRef] [PubMed]
- Mapping multiple sclerosis around the world key epidemiology findings. In Atlas of MS, 3rd ed.; MS International Federation: London, UK, 2020.
- Smolders, J.; Damoiseaux, J.; Menheere, P.; Hupperts, R. Vitamin D as an immune modulator in multiple sclerosis, a review. J. Neuroimmunol. 2008, 194, 7–17. [Google Scholar] [CrossRef]
- Tao, C.; Simpson, S.; van der Mei, I.; Blizzard, L.; Havrdova, E.; Horakova, D.; Shaygannejad, V.; Lugaresi, A.; Izquierdo, G.; Trojano, M.; et al. Higher latitude is significantly associated with an earlier age of disease onset in multiple sclerosis. J. Neurol. Neurosurg. Psychiatry 2016, 87, 1343–1349. [Google Scholar] [CrossRef] [PubMed]
- Willer, C.J.; Dyment, D.A.; Risch, N.J.; Sadovnick, A.D.; Ebers, G.C.; Paty, D.W.; Hashimoto, S.A.; Devonshire, V.; Hooge, J.; Oger, J.; et al. Twin concordance and sibling recurrence rates in multiple sclerosis. Proc. Natl. Acad. Sci. USA 2003, 100, 12877–12882. [Google Scholar] [CrossRef] [PubMed]
- Ascherio, A.; Munger, K.L. Epidemiology of Multiple Sclerosis: From Risk Factors to Prevention—An Update. Semin. Neurol. 2016, 36, 103–114. [Google Scholar] [CrossRef] [PubMed]
- McFarland, H.F.; Martin, R. Multiple sclerosis: A complicated picture of autoimmunity. Nat. Immunol. 2007, 8, 913–919. [Google Scholar] [CrossRef]
- Weiner, H.L. A shift from adaptive to innate immunity: A potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008, 255, 3–11. [Google Scholar] [CrossRef]
- Loma, I.; Heyman, R. Multiple Sclerosis: Pathogenesis and Treatment. Curr. Neuropharmacol. 2011, 9, 409–416. [Google Scholar] [CrossRef]
- Dominianni, C.; Sinha, R.; Goedert, J.J.; Pei, Z.; Yang, L.; Hayes, R.B.; Ahn, J. Sex, body mass index, and dietary fiber intake influence the human gut microbiome. PLoS ONE 2015, 10, e0124599. [Google Scholar] [CrossRef]
- Magyari, M.; Sorensen, P.S. The changing course of multiple sclerosis: Rising incidence, change in geographic distribution, disease course, and prognosis. Curr. Opin. Neurol. 2019, 32, 320–326. [Google Scholar] [CrossRef]
- Romero-Pinel, L.; Bau, L.; Matas, E.; León, I.; Muñoz-Vendrell, A.; Arroyo, P.; Masuet-Aumatell, C.; Martínez-Yélamos, A.; Martínez-Yélamos, S. The age at onset of relapsing-remitting multiple sclerosis has increased over the last five decades. Mult. Scler. Relat. Disord. 2022, 68, 104103. [Google Scholar] [CrossRef] [PubMed]
- Olsson, T.; Barcellos, L.F.; Alfredsson, L. Interactions between genetic, lifestyle and environmental risk factors for multiple sclerosis. Nat. Rev. Neurol. 2017, 13, 25–36. [Google Scholar] [CrossRef] [PubMed]
- Voskuhl, R.R.; Sawalha, A.H.; Itoh, Y. Sex chromosome contributions to sex differences in multiple sclerosis susceptibility and progression. Mult. Scler. 2018, 24, 22–31. [Google Scholar] [CrossRef] [PubMed]
- Whitacre, C.C.; Reingold, S.C.; O’Looney, P.A.; Blankenhorn, E.; Brinley, F.; Collier, E.; Duquette, P.; Fox, H.; Giesser, B.; Gilmore, W.; et al. A gender gap in autoimmunity. Science 1999, 283, 1277–1278. [Google Scholar] [CrossRef]
- Palacios, G.; Sola, R.; Barrios, L.; Pietrzik, K.; Castillo, M.J.; González-gross, M. Algorithm for the early diagnosis of vitamin B 12 deficiency in elderly people. Nutr. Hosp. 2013, 28, 1447–1452. [Google Scholar] [CrossRef]
- Ysrraelit, M.C.; Correale, J. Impact of sex hormones on immune function and multiple sclerosis development. Immunology 2019, 156, 9–22. [Google Scholar] [CrossRef]
- Jokubaitis, V.G.; Spelman, T.; Kalincik, T.; Lorscheider, J.; Havrdova, E.; Horakova, D.; Duquette, P.; Girard, M.; Prat, A.; Izquierdo, G.; et al. Predictors of long-term disability accrual in relapse-onset multiple sclerosis. Ann. Neurol. 2016, 80, 89–100. [Google Scholar] [CrossRef]
- D’Amico, E.; Leone, C.; Patti, F. Offspring Number Does Not Influence Reaching the Disability’s Milestones in Multiple Sclerosis: A Seven-Year Follow-Up Study. Int. J. Mol. Sci. 2016, 17, 234. [Google Scholar] [CrossRef]
- Fernandez Lahore, G.; Förster, M.; Johannesson, M.; Sabatier, P.; Lönnblom, E.; Aoun, M.; He, Y.; Nandakumar, K.S.; Zubarev, R.A.; Holmdahl, R. Polymorphic estrogen receptor binding site causes Cd2-dependent sex bias in the susceptibility to autoimmune diseases. Nat. Commun. 2021, 12, 5565. [Google Scholar] [CrossRef]
- Kovats, S. Estrogen receptors regulate innate immune cells and signaling pathways. Cell. Immunol. 2015, 294, 63–69. [Google Scholar] [CrossRef]
- Moulton, V.R. Sex Hormones in Acquired Immunity and Autoimmune Disease. Front. Immunol. 2018, 9, 2279. [Google Scholar] [CrossRef] [PubMed]
- Bjornevik, K.; Cortese, M.; Healy, B.C.; Kuhle, J.; Mina, M.J.; Leng, Y.; Elledge, S.J.; Niebuhr, D.W.; Scher, A.I.; Munger, K.L.; et al. Longitudinal analysis reveals high prevalence of Epstein-Barr virus associated with multiple sclerosis. Science 2022, 375, 296–301. [Google Scholar] [CrossRef] [PubMed]
- Keane, J.T.; Afrasiabi, A.; Schibeci, S.D.; Fewings, N.; Parnell, G.P.; Swaminathan, S.; Booth, D.R. Gender and the Sex Hormone Estradiol Affect Multiple Sclerosis Risk Gene Expression in Epstein-Barr Virus-Infected B Cells. Front. Immunol. 2021, 12, 732694. [Google Scholar] [CrossRef] [PubMed]
- Enninga, E.A.L.; Nevala, W.K.; Creedon, D.J.; Markovic, S.N.; Holtan, S.G. Fetal sex-based differences in maternal hormones, angiogenic factors, and immune mediators during pregnancy and the postpartum period. Am. J. Reprod. Immunol. 2015, 73, 251–262. [Google Scholar] [CrossRef] [PubMed]
- Murgia, F.; Giagnoni, F.; Lorefice, L.; Caria, P.; Dettori, T.; D’Alterio, M.N.; Angioni, S.; Hendren, A.J.; Caboni, P.; Pibiri, M.; et al. Sex Hormones as Key Modulators of the Immune Response in Multiple Sclerosis: A Review. Biomedicines 2022, 10, 3107. [Google Scholar] [CrossRef]
- Zhu, M.L.; Bakhru, P.; Conley, B.; Nelson, J.S.; Free, M.; Martin, A.; Starmer, J.; Wilson, E.M.; Su, M.A. Sex bias in CNS autoimmune disease mediated by androgen control of autoimmune regulator. Nat. Commun. 2016, 7, 11350. [Google Scholar] [CrossRef]
- Ghezzi, L.; Cantoni, C.; Pinget, G.V.; Zhou, Y.; Piccio, L. Targeting the gut to treat multiple sclerosis. J. Clin. Investig. 2021, 131, 143774. [Google Scholar] [CrossRef]
- Cantarel, B.L.; Waubant, E.; Chehoud, C.; Kuczynski, J.; Desantis, T.Z.; Warrington, J.; Venkatesan, A.; Fraser, C.M.; Mowry, E.M. Gut microbiota in multiple sclerosis: Possible influence of immunomodulators. J. Investig. Med. 2015, 63, 729–734. [Google Scholar] [CrossRef]
- Miyake, S.; Kim, S.; Suda, W.; Oshima, K.; Nakamura, M.; Matsuoka, T.; Chihara, N.; Tomita, A.; Sato, W.; Kim, S.W.; et al. Dysbiosis in the Gut Microbiota of Patients with Multiple Sclerosis, with a Striking Depletion of Species Belonging to Clostridia XIVa and IV Clusters. PLoS ONE 2015, 10, e0137429. [Google Scholar] [CrossRef]
- Chen, H.; Ma, X.; Liu, Y.; Ma, L.; Chen, Z.; Lin, X.; Si, L.; Ma, X.; Chen, X. Gut Microbiota Interventions with Clostridium butyricum and Norfloxacin Modulate Immune Response in Experimental Autoimmune Encephalomyelitis Mice. Front. Immunol. 2019, 10, 1662. [Google Scholar] [CrossRef]
- Cosorich, I.; Dalla-Costa, G.; Sorini, C.; Ferrarese, R.; Messina, M.J.; Dolpady, J.; Radice, E.; Mariani, A.; Testoni, P.A.; Canducci, F.; et al. High frequency of intestinal TH17 cells correlates with microbiota alterations and disease activity in multiple sclerosis. Sci. Adv. 2017, 3, e1700492. [Google Scholar] [CrossRef]
- Ochoa-Repáraz, J.; Mielcarz, D.W.; Wang, Y.; Begum-Haque, S.; Dasgupta, S.; Kasper, D.L.; Kasper, L.H. A polysaccharide from the human commensal Bacteroides fragilis protects against CNS demyelinating disease. Mucosal Immunol. 2010, 3, 487–495. [Google Scholar] [CrossRef] [PubMed]
- Ramakrishna, C.; Kujawski, M.; Chu, H.; Li, L.; Mazmanian, S.K.; Cantin, E.M. Bacteroides fragilis polysaccharide A induces IL-10 secreting B and T cells that prevent viral encephalitis. Nat. Commun. 2019, 10, 2153. [Google Scholar] [CrossRef] [PubMed]
- Liu, S.; Rezende, R.M.; Moreira, T.G.; Tankou, S.K.; Cox, L.M.; Wu, M.; Song, A.; Dhang, F.H.; Wei, Z.; Costamagna, G.; et al. Oral Administration of miR-30d from Feces of MS Patients Suppresses MS-like Symptoms in Mice by Expanding Akkermansia muciniphila. Cell Host Microbe 2019, 26, 779–794.e8. [Google Scholar] [CrossRef] [PubMed]
- Correale, J.; Hohlfeld, R.; Baranzini, S.E. The role of the gut microbiota in multiple sclerosis. Nat. Rev. Neurol. 2022, 18, 544–558. [Google Scholar] [CrossRef] [PubMed]
- Held, K.; Beltrán, E.; Moser, M.; Hohlfeld, R.; Dornmair, K. T-cell receptor repertoire of human peripheral CD161hiTRAV1-2+ MAIT cells revealed by next generation sequencing and single cell analysis. Hum. Immunol. 2015, 76, 607–614. [Google Scholar] [CrossRef]
- Coureuil, M.; Lécuyer, H.; Bourdoulous, S.; Nassif, X. A journey into the brain: Insight into how bacterial pathogens cross blood–brain barriers. Nat. Rev. Microbiol. 2017, 15, 149–159. [Google Scholar] [CrossRef]
- Boveri, M.; Kinsner, A.; Berezowski, V.; Lenfant, A.M.; Draing, C.; Cecchelli, R.; Dehouck, M.P.; Hartung, T.; Prieto, P.; Bal-Price, A. Highly purified lipoteichoic acid from gram-positive bacteria induces in vitro blood-brain barrier disruption through glia activation: Role of pro-inflammatory cytokines and nitric oxide. Neuroscience 2006, 137, 1193–1209. [Google Scholar] [CrossRef]
- Rizzetto, L.; Fava, F.; Tuohy, K.M.; Selmi, C. Connecting the immune system, systemic chronic inflammation and the gut microbiome: The role of sex. J. Autoimmun. 2018, 92, 12–34. [Google Scholar] [CrossRef]
- Mueller, S.; Saunier, K.; Hanisch, C.; Norin, E.; Alm, L.; Midtvedt, T.; Cresci, A.; Silvi, S.; Orpianesi, C.; Verdenelli, M.C.; et al. Differences in fecal microbiota in different European study populations in relation to age, gender, and country: A cross-sectional study. Appl. Environ. Microbiol. 2006, 72, 1027–1033. [Google Scholar] [CrossRef]
- Gomez, A.; Luckey, D.; Taneja, V. The gut microbiome in autoimmunity: Sex matters. Clin. Immunol. 2015, 159, 154–162. [Google Scholar] [CrossRef] [PubMed]
- Baker, J.M.; Al-Nakkash, L.; Herbst-Kralovetz, M.M. Estrogen-gut microbiome axis: Physiological and clinical implications. Maturitas 2017, 103, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.L.; Madak-Erdogan, Z. Estrogen and Microbiota Crosstalk: Should We Pay Attention? Trends Endocrinol. Metab. 2016, 27, 752–755. [Google Scholar] [CrossRef] [PubMed]
- Cantoni, C.; Lin, Q.; Dorsett, Y.; Ghezzi, L.; Liu, Z.; Pan, Y.; Chen, K.; Han, Y.; Li, Z.; Xiao, H.; et al. Alterations of host-gut microbiome interactions in multiple sclerosis. eBioMedicine 2022, 76, 103798. [Google Scholar] [CrossRef]
- Verma, D.; Garg, P.K.; Dubey, A.K. Insights into the human oral microbiome. Arch. Microbiol. 2018, 200, 525–540. [Google Scholar] [CrossRef]
- Li, B.; Ge, Y.; Cheng, L.; Zeng, B.; Yu, J.; Peng, X.; Zhao, J.; Li, W.; Ren, B.; Li, M.; et al. Oral bacteria colonize and compete with gut microbiota in gnotobiotic mice. Int. J. Oral Sci. 2019, 11, 10. [Google Scholar] [CrossRef]
- Atarashi, K.; Suda, W.; Luo, C.; Kawaguchi, T.; Motoo, I.; Narushima, S.; Kiguchi, Y.; Yasuma, K.; Watanabe, E.; Tanoue, T.; et al. Ectopic colonization of oral bacteria in the intestine drives TH1 cell induction and inflammation. Science 2017, 358, 359–365. [Google Scholar] [CrossRef]
- Narengaowa; Kong, w.; Lan, F.; Awan, U.F.; Qing, H.; Ni, J. The Oral-Gut-Brain AXIS: The Influence of Microbes in Alzheimer’s Disease. Front. Cell. Neurosci. 2021, 15, 633735. [Google Scholar] [CrossRef]
- Riviere, G.; Riviere, K.H.; Smith, K.S. Molecular and immunological evidence of oral Treponema in the human brain and their association with Alzheimer’s disease. Oral Microbiol. Immunol. 2002, 17, 113–118. [Google Scholar] [CrossRef]
- Mills, R.J.; Young, C.A.; Smith, E.T. Central trigeminal involvement in multiple sclerosis using high-resolution MRI at 3 T. Br. J. Radiol. 2010, 83, 493–498. [Google Scholar] [CrossRef]
- Li, X.; Kiprowska, M.; Kansara, T.; Kansara, P.; Li, P. Neuroinflammation: A Distal Consequence of Periodontitis. J. Dent. Res. 2022, 101, 1441–1449. [Google Scholar] [CrossRef] [PubMed]
- Sheu, J.J.; Lin, H.C. Association between multiple sclerosis and chronic periodontitis: A population-based pilot study. Eur. J. Neurol. 2013, 20, 1053–1059. [Google Scholar] [CrossRef] [PubMed]
- Gustavsen, M.W.; Celius, E.G.; Moen, S.M.; Bjølgerud, A.; Berg-Hansen, P.; Nygaard, G.O.; Sandvik, L.; Lie, B.A.; Harbo, H.F. No association between multiple sclerosis and periodontitis after adjusting for smoking habits. Eur. J. Neurol. 2015, 22, 588–590. [Google Scholar] [CrossRef] [PubMed]
- Zhou, L.-J.; Lin, W.-Z.; Liu, T.; Chen, B.-Y.; Meng, X.-Q.; Li, Y.-L.; Du, L.-J.; Liu, Y.; Qian, Y.-C.; Zhu, Y.-Q.; et al. Oral Pathobionts Promote MS-like Symptoms in Mice. J. Dent. Res. 2022, 102, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Fiorillo, L.; Cervino, G.; Laino, L.; D’Amico, C.; Mauceri, R.; Tozum, T.F.; Gaeta, M.; Cicciù, M. Porphyromonas gingivalis, Periodontal and Systemic Implications: A Systematic Review. Dent. J. 2019, 7, 114. [Google Scholar] [CrossRef] [PubMed]
- Cassini, M.A.; Pilloni, A.; Condò, S.G.; Vitali, L.A.; Pasquantonio, G.; Cerroni, L. Periodontal Bacteria in the Genital Tract: Are They Related to Adverse Pregnancy Outcome? Int. J. Immunopathol. Pharmacol. 2013, 26, 931–939. [Google Scholar] [CrossRef] [PubMed]
- Africa, C.W.J.; Nel, J.; Stemmet, M. Anaerobes and Bacterial Vaginosis in Pregnancy: Virulence Factors Contributing to Vaginal Colonisation. Int. J. Environ. Res. Public Heal 2014, 11, 6979–7000. [Google Scholar] [CrossRef] [PubMed]
- Troci, A.; Zimmermann, O.; Esser, D.; Krampitz, P.; May, S.; Franke, A.; Berg, D.; Leypoldt, F.; Stürner, K.H.; Bang, C. B-cell-depletion reverses dysbiosis of the microbiome in multiple sclerosis patients. Sci. Rep. 2022, 12, 3728. [Google Scholar] [CrossRef]
- Ding, T.; Schloss, P.D. Dynamics and associations of microbial community types across the human body. Nature 2014, 509, 357–360. [Google Scholar] [CrossRef]
- Goldoni, R.; Dolci, C.; Boccalari, E.; Inchingolo, F.; Paghi, A.; Strambini, L.; Galimberti, D.; Tartaglia, G.M. Salivary biomarkers of neurodegenerative and demyelinating diseases and biosensors for their detection. Ageing Res. Rev. 2022, 76, 101587. [Google Scholar] [CrossRef]
- Buccellato, F.R.; Galimberti, D.; Tartaglia, G.M. Beyond dentistry: Could the prevention and screening for neurodegenerative diseases start at dentist practice? Neural Regen. Res. in press.
- D’Anca, M.; Fenoglio, C.; Serpente, M.; Arosio, B.; Cesari, M.; Scarpini, E.A.; Galimberti, D. Exosome determinants of physiological aging and age-related neurodegenerative diseases. Front. Aging Neurosci. 2019, 11, 232. [Google Scholar] [CrossRef] [PubMed]
- D’Anca, M.; Fenoglio, C.; Buccellato, F.R.; Visconte, C.; Galimberti, D.; Scarpini, E. Extracellular Vesicles in Multiple Sclerosis: Role in the Pathogenesis and Potential Usefulness as Biomarkers and Therapeutic Tools. Cells 2021, 10, 1733. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).