Abstract
Gender-specific medicine studies how sexual biology and gender-related cultural and behavioral differences may influence a person’s health and considers the differences in clinical features, prevention, therapies, prognosis, and psycho-social aspects of diseases with different impacts on women and men. The present work summarizes the main differential impact each risk factor for oral cancer and periodontitis has according to biological sex- and gender-oriented differences. It resulted in differences in epidemiology and the weight of various healthy determinants that may influence the incidence and prognosis of oral cancer and periodontitis. It is desirable to change the methodology of scientific studies with a higher focus on the weight that sexual variables may have on the well-being or the probability of getting ill of each person, thus promoting the development and diffusion of personalized gender dentistry.
Keywords:
gender; sex; oral cancer; periodontitis; oral microbiota; oral microbiome; gender medicine; alcohol; tobacco; smoking; hormones; dysbiosis; epidemiology; risk assessment 1. Introduction
The terms “gender” and “sex” have different meanings. While sex refers to different biological, physical, and physiological attributes of females, males, and intersex persons, such as chromosomes, gene expression, hormones, and reproductive organs [1,2], gender refers to what a given society considers appropriate for men and women, boys and girls, and gender-diverse people, including socially constructed roles, behaviors, activities, and attributes [3].
While sex is usually categorized as female or male, a broader spectrum of gender identities and expressions defines how individuals identify themselves and express their gender. However, both sex and gender are critical determinants of health since they can influence, with different aspects, the subject to be exposed to a disease or a health issue, thus influencing their prevalence and treatment outcomes. Gender identity may be most affected by external factors, such as environment, occupation, access to care, and at-risk behavior, while biological sex is strictly related to differences in pharmacological effects related to different biological effects. In any case, both may influence human well-being and expose each subject to risks specific to every person.
On these bases, gender medicine, or gender-specific medicine, is the study of how sex-based biology and gender-related cultural and socioeconomic differences may influence a person’s health. Furthermore, gender-specific medicine considers the differences in clinical features, prevention, therapies, prognosis, and psycho-social aspects of a disease with the related impact on women and men of every age [4].
Gender medicine is the base for a personalized medicine approach since it studies pathophysiology, the clinical features, and the prognosis of diseases, based on what is expected according to sexual (mainly biological) and gender differences (mainly sociocultural and behavioral) on which the genetic, epigenetic, hormonal, and environmental variables act differently [5].
A wide variety of substances are associated with the onset or worsening of a series of diseases and pathologies of various origins. Alcohol and tobacco are the most frequent risk factors for a series of oral diseases, mainly but not only oral cancer and periodontal diseases.
The present work aims to focus on the sex–gender differences in epidemiology and the sex/gender-related weight of the main risk factors for oral cancer and periodontitis to better understand future research designs and clinical practice strategies.
2. Oral Cancer
Except for rare non-epithelial tumors [6,7,8], oral squamous cell carcinoma (OSCC) is the central and most common oral malignancy. OSCC is a malignant neoplasia of epithelial origin, of the head and neck district, at the seventh place for worldwide prevalence among cancers [9]. OSCC may arise from potentially malignant oral diseases (OPMDs), which are precursor lesions and conditions with an increased risk of malignancy [10]. Furthermore, OSCC recognizes a series of risk factors—mainly alcohol and/or tobacco consumption [11], betel quid chewing [12], chronic traumatism [13], micronutrient deficiencies [14], and infections [15,16,17,18]—which contribute both to keratinocyte derailment toward cancerogenesis and sustain the related chronic inflammation, posing the ideal environment for tumor growth [19,20,21].
In 2020, the lip and oral cavity cancer incidence counted 264,000 new cases in males (70% of total cases) and 113,000 in females, with a male/female ratio equal to 2.3:1; a prevalence in 5 years of over 656,000 and 303,000 and mortality for over 125,000 and 53,000, respectively. Among them, the cases attributable to alcohol were 67,000 for males and 8200 for women [22].
In 2011, Kruse et al. [23] retrospectively reported clinical and demographic differences between 278 patients (159 males and 119 females) with OSCC followed up meanly for 36 months. While the overall median age was similar in both sexes (61 years for males, 65 years for females), their results revealed a slightly higher proportion of females (54%) over men (46%) in the OSCC patients older than 70 years. Smoking was reported in 76% of males and 51% of females; alcohol in 80% and 48%, respectively; conversely, neither alcohol nor tobacco consumption was reported in 14% of males and 39% of females, who were predominantly affected by maxillary OSCC. Furthermore, clinical stage and metastases still seem sex-independent prognostic factors. However, the literature usually did not often correlate the classical prognostic indicators and main outcome with sex [24], merely considering their distribution by sex but not focusing on the differential predictive meaning they could have in different sexes from a statistical point of view [25,26,27].
More recently, Park et al. [28] compared the prevalence by biological sex of head and neck (HN) cancers in 10 million healthy Korean during a 10-year follow-up. In that period, almost 11,000 subjects developed HN cancers, whose 1698 were oral cancers (84% in males and 16% in females). Additionally, in this case, the gap between males/females decreased over 70 years of age. Among men and women who developed oral cancers, the percentages of never smokers were 31% of males and 95% of females; similarly, non-alcohol consumers were 32% of males and 75% of females; both differences were statistically significant. The authors also discussed the higher prevalence of HN cancers in males, also regardless of smoking and drinking, and addressed this evidence to the role of sex hormones, mainly androgens, as associated with higher risks and poor outcomes in men, contrary to estrogen’s protective effects in females [29,30]. Conversely, further gender-specific differences in OSCC survival could also be related to protective gene polymorphisms for males but not for females, which are significantly associated with smoking, as Nagam et al. reported [31].
Furthermore, biological sex differences in the humoral and cell-mediated immune responses also seem to participate in the differences among biological sex on the onset of oral and HN cancers [32], while the detection of HPV, human papillomavirus, was not always investigated, despite its crucial role in a subset of HN cancers, mainly, but not exclusively, of the pharyngeal region [15]. Hence, a more complex interplay among sexual hormones, sex chromosomes, as well as the immune system and metabolism could differentially contribute to establishing sex-specific tumor microenvironments determining the cancer development in biological males and females [33,34].
3. Periodontitis
Periodontitis is a microbially associated periodontal disease characterized by peculiar chronic host-mediated inflammation, resulting in progressive loss of periodontal attachment, alveolar bone resorption, and teeth loss [35].
In detail, as recently defined by Hajishengallis, “Periodontitis is an exemplar of a microbe-driven chronic inflammatory disease that persists in susceptible individuals, in part due to reciprocally reinforced interactions between the dysbiotic microbiome and the host inflammatory response” [36]. Indeed, periodontitis can manifest in predisposed subjects when specific bacteria trigger local inflammation, which becomes chronic and is responsible for progressive periodontal damage. The bacteria associated with periodontitis are well recognized and Socransky et al. grouped and classified them into color-labeled groups according to their periodontopathogen role [37]. According to this classification, each specific bacterium contributes at different stages and with different weights. First, the early colonizers (green complex) adhere to the dental pellicle. Then, the bridge species (yellow and orange complexes) create favorable local conditions for the co-aggregation between different species. Later, in a mature subgingival dental plaque, the most strongly periodontopathogen species (red complex) are the final and directly responsible for the destruction of the periodontium and the triggering of the inflammatory hyper-reactivity of the susceptible host [37,38].
Incidentally, periodontitis determines consequences not only on periodontal health but also extra-orally, mainly by spilling and dissemination to distant organs of bacteria and bacterial toxins from the gingival pockets via ingestion and bloodstream. These events alter gut permeability and perpetuate chronic extra-oral inflammation, which is responsible for systemic effects [39]. The most robust evidence on the role of periodontitis in aggravating or worsening extra-oral morbidities confirms its association with cardiovascular, metabolic, autoimmune, and neurodegenerative diseases, but also with the cancers and health of pregnant women and their infants [40,41].
Periodontitis is the sixth most prevalent condition worldwide in the adulthood and elderly, and its global prevalence is estimated to be between 20 and 50% [42], affecting over 700 million people [43], greater in men, who also show the worst severe degrees than women [44,45]. In detail, while the male-to-female prevalence rate is 1.3 for mild forms of periodontitis, it doubled for severe and aggressive ones, so 28% and 71% more adult males than females suffer mild and severe forms, respectively [46].
Nevertheless, a recent study from Freitag-Wolf et al. [47] reported an earlier onset of periodontitis in young women than in men. The authors addressed this evidence to what they called “genotype-by-sex (GX S) interactions”, which, according to natural genetic variation, could affect the different heritability of periodontitis among sexes; hence they suggested that genes from maternal inheritance could contribute to intersex phenotypic variation in early onset periodontitis. However, that observation was done in a sample of almost 900 subjects from the European region, and it must be considered that the genetic variability among different geographic areas could lead to contrasting results as for other diseases worldwide.
4. The Role of Sex Hormones
The specific role of sex hormones in the onset and prognostic features of oral cancer must also be considered, both in association and without tobacco and alcohol as risk factors [48]. Two pieces of evidence exist on the role of estrogen associated with OSCC.
On one side, higher estrogen levels—as in women, women under hormone replacement therapy, pregnant, or given birth before 35 years of age—would play a protective role in cancer development, reinforced by the fact that early menopause onset and estrogen lower levels parallel increase the risk of HN cancers [49]. On the other side, some authors convey that estrogen can increase the risk and worsen the prognosis of OSCC [50,51].
Estrogen receptors (ER) are expressed on oral cancer cells and macrophages associated with the proinflammatory tumoral microenvironment, where estrogen may act with opposite results. Indeed, while estrogen–ER interactions on macrophages lead toward a decrease of proinflammatory cytokine release, with protective effects against oral cancer progression [52], they can also positively promote oral cancer cell migration and proliferation [48]. In detail, Colella et al. [53] reported higher expression of ER (ER-a, and ER-b) and lower androgen receptors (AR) in cancer tissue than in healthy tissue close to cancer. Data have been further detailed in a systematic review by Saranya et al. [54], which confirmed that ER presence on tumoral cells influences the OSCC progression [55]. OSCC with positive ER-α expression was mainly associated with HPV-positive tumors [56] and lesser expressed in men than females [57], while, conversely, 40% of OSCC expressed ER-b and only 26% expressed AR, significantly higher in men [58]. Furthermore, ER-α was majorly present in advanced stages with frequent bone invasion and was associated with significantly lower overall and relapse-free survivals than ER-α negative OSCC [57].
For a clearer understanding of the issue, it must be considered that a peculiar clinical variable of OSCC, early OSCC, affects mainly non-smoker/drinking young women under the age of 45 [59,60]. In this cluster, estrogen could play a crucial role in cancerogenesis more than the extrinsic risk factors, such as alcohol and smoking, probably due to the polymorphism of estrogen and its receptors [61].
Sex hormones may also influence gingival microcirculation, thus impacting the spatial progression of periodontitis. Compared with women, men significantly experience higher vasodilatation in case of inflammation or during wound healing, as well as during active phases of periodontitis [62]. Recently, Vag et al. reported significantly higher gingival blood flow and endothelial reactivity of males, both in healthy and periodontal diseases, and higher nitric oxide—with vasodilating effects—release during periodontitis, thus suggesting an increased destructive inflammatory reaction in males with periodontitis [62,63].
Furthermore, peculiar sex-prevalent comorbidities may also influence and be influenced by periodontitis. Osteoporosis exemplifies this bidirectional relationship and the clinical differences between women and men. Osteoporosis is a systemic disease of the skeletal system, characterized by low mineral density and the deterioration of the micro-architecture of the bone tissue, with a consequent increase in bone fragility mainly linked to aging and sexual hormones [64]. As with any bone in the body, it can also affect the alveolar bone, thus accelerating periodontal destruction during co-occurring periodontitis [65]. Despite osteoporosis doubling the risk of periodontitis in both sexes, around the world, osteoporosis is more prevalent in women (mainly of postmenopausal age) than men [65,66]. This epidemiological prevalence in women could explain the strong association between osteoporosis and periodontitis in women.
5. Oral Dysbiosis
The literature reports evidence of the interrelationship between unbalanced oral microbiota, known as oral dysbiosis, and systemic diseases or therapies [40,41,67,68].
Oral microbiota is what was formerly called “oral bacterial flora”. It is the collection, not only of bacteria but also all the microorganisms [69], including archaea, fungi, and viruses, living in specific ecological niches [70,71] of the oral cavity, which homes over 600 different microorganisms [69,72]. At the same time, the term “microbiome” refers to all their genetic material, which is approximately made up of almost 24 million non-redundant oral genes [73,74]. The more intuitive examples of oral dysbiosis leading to opportunistic oral pathologies occur as the consequences of recurrent use and abuse of antimicrobials, which favors opportunistic infections, such as oral candidiasis, especially in the presence of local and systemic predisposing factors [75,76].
The typical examples of oral diseases induced by peculiar dysbacteriosis are caries [77] and periodontal diseases [35], secondary, respectively, to supragingival and subgingival dental plaque maturation, enriched by a series of cariogenic and periodontopathogen bacteria [37,78], especially in some critic general conditions.
Furthermore, oral dysbiosis may also contribute to extra-oral [79,80] and oral cancer onset [81], supported and not supported by co-occurring risk factors such as smoking and alcohol drinking. Arising studies also connected oral microbiota crosstalk with the host immune system, thus reinforcing the relationships between dysbiosis and poor health [82], as well as the oral–gut axis, which plays a key role in the establishment of systemic diseases [83,84].
Other than genetics, race/ethnicity, socioeconomics, and age [85], the oral microbiota is also influenced by smoking and drinking, and recent studies have discovered substantial differences also according to biological sex [86]. For example, some members of the healthy microbiome are proportionally expressed according to sex hormones and, in women, may also vary according to the reproductive period of life and post-menopause [85,86,87]. Furthermore, people suffering from periodontitis report gender variations in the oral microbiomes, thus suggesting that gender is an essential determinant of different compositions of the subgingival periodontopathogen plaque in men and women [88].
Other infectious diseases exacerbating and sustaining cytokine and other proinflammatory states are still under investigation to understand the role, by sex, of inflammation in the onset and the worsening of periodontitis, as well as the related weight of sex, as emerged during the last few years associated with COVID-19 [89,90,91].
Hence, all these variables can contribute, in various and not yet elucidated ways, to the different degrees of risk of oral diseases in different sexes and genders.
6. Tobacco
Tobacco consumption (smoked and not) represents one of the most significant public health problems worldwide and is one of the major risk factors in the development of chronic non-communicable diseases worldwide (more than 30 diseases, including chronic obstructive pulmonary disease and other chronic lung diseases, lung cancer and other forms of cancer, heart disease, and vascular disease). Tobacco causes more deaths than alcohol, AIDS, drugs, traffic accidents, suicides, and homicides combined [92].
Despite a decreasing prevalence during the last 30 years, both in males (27.5% reduction) and females (37.7% reduction) [93], the total number of smokers has increased as populations have grown [94]. The actual smoking rate is very similar in both sexes (meanly 14%) [95]. To date, tobacco is smoked/used by approximately one billion people worldwide (over 80% living in low- and middle-income countries, where the burden of tobacco-related disease and mortality is heavier) and kills more than 8 million people every year [95].
The GBD 2019 tobacco collaborators [94], in 2019, estimated a prevalence of current use of smoking tobacco of 33% among males and 7% among females aged 15 years and older. The highest smoking prevalence among women was in Greenland, where 42% of women smoke; for males in Georgia and China, one in two men smoke, while in Timor-Leste, the percentage of male smokers was the highest, at 65%.
Smoke-related health issues are proportional to the number of cigarette equivalents smoked daily, with cumulative effects related to years of smoke. Cigarette equivalent smoked per day is an indicator of exposure to smoke and converts the content of tobacco smoked by pipes, cigars, and other products to the equivalent doses of cigarettes. Among over the 700 million smoker males over the age of 29 and the 146 million smoker women of the same age range, respectively, 49% and 33% are higher smokers, with a consumption of over 15 cigarettes or cigarette equivalent per day [94] (details in Figure 1).
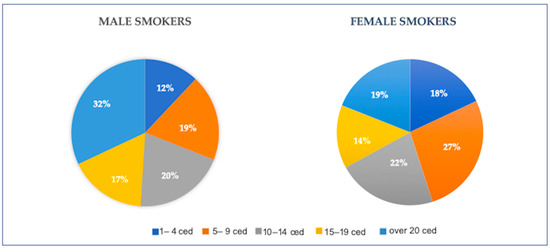
Figure 1.
Percentages of males and females older than 29 years with relative mean cigarette equivalent smoked per day worldwide, in 2019. (Original figures based on extrapolating data from GBD 2019 Tobacco Collaborators [94].) Ced: cigarette equivalent per day.
Apart from smoked tobacco, including cigarettes, cigars, pipes, and other local smoked tobacco products, a wide variety of other tobacco products, such as vaping products, electronic cigarettes (e-cigarettes), or heated tobacco products, have been commercially available in the last few years. Last, tobacco may also be chewed in some regions (smokeless tobacco) [93].
All forms of tobacco are harmful, and there is no safe level of exposure to tobacco. Tobacco contains nicotine, is responsible for addiction, and has over 70 established carcinogens, varying across products [96]. The main toxic effects of tobacco are related to some properties of its compounds, mainly carcinogenic. Furthermore, the smoke itself, both from smoked and vaped tobacco, is responsible for direct effects on the respiratory and oral epithelial cells and local immune cells, resulting in proinflammatory and immune-suppressive changes [97,98,99].
While nicotine dependence affects 75% of individuals with substance use disorders (SUD), without any significant difference by sex/gender [100]; generally, men tend to use all tobacco products at higher rates than women [101]. In detail, Higgins et al. reported the results from a systematic review on the prevalence of gender differences in tobacco use in the U.S., resulting in a noticeable male prevalence rate exceeding those of females at every age. However, when gender orientation was considered instead of biological sex, the authors found some studies reporting the typical pattern of males smoking at higher rates than females was only seen among heterosexuals. At the same time, overall smoking prevalence was higher among lesbian/gay/bisexuals than heterosexuals, as examples of sex orientations rather than biological sex influencing the choice to smoke [101].
A positive attitude toward tobacco products and brands is reinforced in adolescents and young adults meeting tobacco-related content on social media, which can double the appeal to use tobacco products at any age [102]. Further works have considered the influence of commercials sponsoring tobacco use on different genders. A recent paper by Soneji et al. [103] stratified the tobacco preferences and prevalence according to gender orientation, reporting higher percentages among bisexual (30%) and gay/lesbian (26%) youth than in straight youth (12%). Kasza et al. reported similar data, which estimated preferences of tobacco products consumed in a sample of 46,000 U.S. smokers aged 12 or over, stratified per demographic characteristics [104]. While according to biological sex, 35% of male smokers and 21% of female smokers used any tobacco products, the percentages, and variety of usage changed according to sexual orientation, being lower among those who identified as heterosexual than among those who identified as gay/lesbian or bisexual [104].
In 2019, tobacco was responsible for death in 51% of males and 37% of females with oral cancer [95]. In 2015, a systematic review on smokeless tobacco-associated oral cancers in the Indian population revealed an odds ratio for women 2.5 times higher than men to develop oral cancer [105]. Mu et al. have recently confirmed these data, reporting a doubled relative risk of oral cancer in women than in men [106].
Other than oral cancer, tobacco is equally responsible for a series of oral health issues related to the strict and continuous contact of the oral mucosa with substances other than the heat produced from smoked and/or heated tobacco, resulting in carcinogenic, microbial, immunological, and clinical effects [107]. Tobacco is responsible for half of the cases of periodontitis in the U.S. [107]. Tomar et al. estimated a threefold increased risk of periodontitis in mean smokers and sixfold in heavy smokers so that, in smokers, 74.8% of periodontitis was directly attributable to smoking [107]. Furthermore, smokers have a significantly higher risk (140%) of failure of dental implants [108], which often replace teeth missed for periodontitis [109], this being slightly higher in men than in women [110].
Smoking affects periodontal health both by altering the subgingival plaque and increasing the inflammatory responses, thus resulting, respectively, in the establishment of a microenvironment favorable to the colonization and growth of periodontopathogen microorganisms and the increase of local inflammatory state, sustained by the smoking-related impairment of neutrophil activity and humoral response [111].
7. Alcohol
Alcohol is a toxic and psychoactive substance with dependence-producing properties, responsible for many cancers, such as breast cancer, colorectal cancer, gastric cancer, and oral cancer [112,113,114]. Even though male gender identity and behavior are historically associated with higher use of alcoholic drinking than women [115], the gap is getting closer so that during the last decade, the number of alcohol-addicted increased by 85% in women and was stable at 35% in men [116,117].
The carcinogenic mechanisms of alcohol are mainly related to acetaldehyde, metabolized from ethanol in the liver, gut, and mouth, and ethanol per se is responsible for irreversible DNA methylation and damage, acting together with the increased oxidative stress and folate depletion [118]. Similarly, alcohol influences cell growth by varying estrogen and insulin levels [32].
Globally, alcohol is responsible for almost 3 million deaths every year. It has been estimated a rate of over 2500 deaths per day in the WHO European Region alone (which has the highest levels of alcohol consumption worldwide), where alcohol is responsible for 25% of deaths among young adults (aged 20–24) [119,120]. In 2018, in the same region, alcohol was responsible for 180,000 cancer cases, 110,000 in men and 70,000 in women, contributing to 92,000 cancer deaths [119,120]. If we consider only oral and lip cancers, the number of alcohol-related cases in the same year was approximately 15,000 in men and 7000 in women. The amount of alcohol-related oral and lip cancer death is 40% for men and almost 20% for women.
Every quality, kind, and quantity of alcohol is responsible for cancer risk, and there is no safe amount of alcohol consumption. The “excess” in alcohol consumption has been defined as ≥10 g per day for females and ≥20 g per day for males. Twenty grams of alcohol equals approximately 500 mL of beer, 200 mL of wine, or a super-alcoholic drink (60 mL) [121,122]. Several authors have established that moderate drinking (20 g of pure alcohol per day) is responsible for 9% of alcohol-related oral cancers. In comparison, risky drinking (up to 60 g per day of pure alcohol) and heavy drinking (more than 60 g of pure alcohol per day) are responsible for 30% and 61%, respectively, of alcohol-related oral cancers [122]. In any case, both with the same intake and in the case of a lower intake or for a shorter time, women, compared to men, have a higher risk of developing an alcohol-related disease and oral and other cancers [123].
Higher levels of alcohol consumption are also associated with severe periodontitis [111], especially in the male sex from underdeveloped countries [124].
The combined use—and abuse—of alcohol and tobacco dramatically increase the risk of developing cancers of the oral cavity and other sites of the upper aero-digestive tract and is supra-multiplicative [125,126]. Non-drinker smokers have a mean sevenfold risk of oral and pharyngeal cancer compared to non-smokers [127], and non-smoker drinkers have a mean fivefold risk [121]. In both cases, the risk is proportional to the time and quantity of tobacco/alcohol usage. Users and abusers of both alcohol and tobacco have up to 30-times higher risk of never smoker-drinkers developing oral and pharyngeal cancers, accounting for 74% of oral cancers in men and 57% in women [126].
The cumulative cancerogenic effects of co-occurring alcohol and tobacco consumption are based on the property of alcohol to evaporate and dry the oral mucosa rapidly; it also acts as a solvent for the tobacco carcinogens (mainly formaldehyde), which facilitates their absorption into the oral cells. Bad oral hygiene co-occurring in drinker-smoking addiction and poor diet also increase the risk [127].
8. Discussion
The present work summarized the primary differential measures in which each risk factor for oral cancer and periodontitis impacts according to biological sex and gender-oriented variables. It resulted in differences in the epidemiology and the weight of various determinants that may influence the incidence and prognosis of oral cancer and periodontitis (Figure 2).
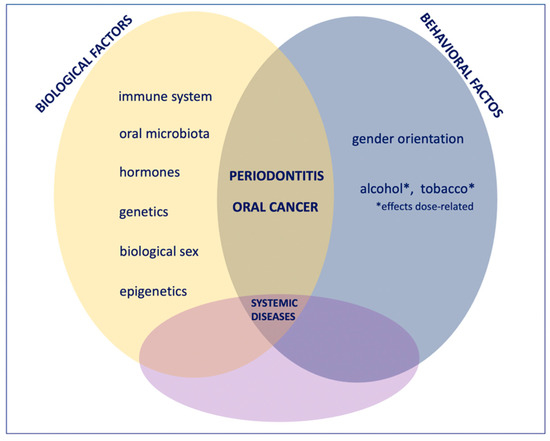
Figure 2.
The onset, course, evolution, prognosis, and therapeutic approach of periodontitis and oral cancer are affected by biological and environmental factors that are inevitably interrelated. In this sharing, each factor is reciprocally influenced by sex-related biological differences (hormones, oral microbiota composition, genetics, inflammatory and immune system, epigenetic factors) and behavioral differences related to gender orientation differing according to the sociocultural matrix in which each person lives. The effects on extra-oral health or, conversely, the influences of extra-oral diseases on oral health give further degrees of complexity and variability so that medicine and dentistry need to move more and more toward a personalized gender perspective to treat each case according to its uniqueness.
As a paradigmatic example, oral cancer—for which more robust studies account for these differences—seems to affect men twofold more than women, with higher alcohol- and tobacco-related deaths in the first [Table 1].

Table 1.
Oral cancer epidemiology by biological sex according to alcohol and tobacco consumption.
Nevertheless, regarding smokeless tobacco, the proportion responsible for oral cancer in women is 2.5 times more than in men, although men are the highest consumer of smokeless products [128]. Hence, these data are not only justifiable by the broader prevalence of smoking and drinking habits among men than women but also influenced by biological, metabolic, microbiological, hormonal, and genetic differences between the biological sexes.
With regard to periodontitis, it seems to manifest more severely in men than in women. This difference may be globally explained by the protective role of estrogens in fertile women on one side, and the higher vasodilation levels in men. Conversely, comorbidities more prevalent in the female sex, such as osteoporosis in postmenopausal women or rheumatoid arthritis, may also influence the epidemiological indices of periodontitis.
Last, one cannot ignore the complex and still not fully known metabolic and biological interactions between oral and host microbiota, made even more complex by the variability with which inflammatory, immune, and metabolic mechanisms, which are also affected by hormonal differences, as well as by the sharing of smoke and alcohol.
Furthermore, gender-related cultural and socioeconomic factors must also be considered. As a prominent example, in many countries, being a woman/girl still brings sociocultural disadvantages, manifesting as discrimination and poor access to schooling, cures, and preventive health programs. In this scenario, women do not know or cannot choose healthy behavior or do not have free access to treatment and screening programs. Last, inequities are also related to poverty, an essential barrier to positive health outcomes, which according to WHO, “tends to yield a higher burden on women and girls’ health” [129].
Other than being of the female sex, other groups of discriminated people are those whose gender orientation is different from their biological sex. Bisexuals, gays, lesbians, non-binary, and transgender people still suffer from marginalization and prejudice that directly and indirectly limit their access to treatment and undermine their health, and causing them to incur at-risk habits more or more quickly, such as the abuse of alcohol, smoking, and drugs, with parallels to less access to cures.
9. Conclusions
The relative paucity of sex- and gender-oriented studies limited the global evaluation and definition of the weight that each risk factor and their relationship could impact each person. Apart from biological differences, the access to cures and behavior may also noticeably impact the onset of oral cancer and periodontitis and their course and therapies.
Medicine has historically had an androcentric bias because, until a few years ago and still today, research enrolled predominantly male subjects or failed to establish differences in the studied variables according to the biological sex-related influences, gender orientation, and at-risk behaviors. Nevertheless, all these variables should not be neglected because they significantly impact each disease’s onset, clinical course, severity, and outcome.
Only recently have gender differences been fully acknowledged by more tailored drug trials and scientific research focusing on the noticeable but underestimated biological, hormonal, and genetic differences among sexes, further amplified by the broader variability of gender orientation and behaviors. A growing amount of epidemiological, clinical, and experimental data have shown significant differences in the development, progression, and clinical signs of morbidities that, despite being able to manifest both in men and women, may present differences in the adverse events, outcome, prognosis, and incidence.
The future should move toward personalized sex and gender medicine related to the weighted weight of each risk factor considering all sexual biological and sociocultural variables. At the same time, it is also desirable for “gender dentistry” to consider not only the crude risk factors but also their personalized impact on each person, correlating with their biological and behavioral features.
In conclusion, gender medicine and dentistry should consider the sex and gender differences not to discriminate, but to personalize and support novel medical and dental approaches for each person, making an equal quality of life, independent of their sexual chromosome and orientation, but strictly considering the sex-related impact and variables on their well-being or risk to get ill.
Author Contributions
Conceptualization, M.C., F.D.S. and A.A.; methodology, A.R., G.D. and R.S.; investigation, M.C. and A.B.; data curation, M.C., F.I. and F.D.S.; writing—original draft preparation, A.A. and M.C.; writing—review and editing, M.C., E.X. and A.R.; supervision, M.C., F.I., R.S. and A.B. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- WHO. Gender and Health. Available online: https://www.who.int/news-room/questions-and-answers/item/gender-and-health (accessed on 13 July 2022).
- Heidari, S.; Babor, T.F.; De Castro, P.; Tort, S.; Curno, M. Sex and Gender Equity in Research: Rationale for the SAGER guidelines and recommended use. Res. Integr. Peer Rev. 2016, 1, 2. [Google Scholar] [CrossRef] [PubMed]
- ISTUD. Gender Medicine. A New Approach for Healthcare. Available online: http://service.istud.it/up_media/pwscienziati13/gender_medicine.pdf (accessed on 13 July 2022).
- Baggio, G.; Corsini, A.; Floreani, A.; Giannini, S.; Zagonel, V. Gender medicine: A task for the third millennium. Clin. Chem. Lab. Med. 2013, 51, 713–727. [Google Scholar] [CrossRef] [PubMed]
- Pacifici, L.; Santacroce, L.; Dipalma, G.; Haxhirexha, K.; Topi, S.; Cantore, S.; Altini, V.; Pacifici, A.; De Vito, D.; Pettini, F.; et al. Gender medicine: The impact of probiotics on male patients. Clin. Ter. 2021, 171, e8–e15. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Lo Muzio, L.; Serpico, R.; Maiorano, E. Rhabdomyoma of the head and neck: Clinicopathologic features of two cases. Head Neck 2003, 25, 700–704. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Lo Muzio, L.; Serpico, R.; Maiorano, E. Angiosarcoma of the head and neck with intra-oral presentation. A clinico-pathological study of four cases. Oral Oncol. 2002, 38, 757–762. [Google Scholar] [CrossRef]
- Almeslet, A.S. Pleomorphic Adenoma: A Systematic Review. Int. J. Clin. Pediatr. Dent. 2020, 13, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Warnakulasuriya, S. Oral potentially malignant disorders: A comprehensive review on clinical aspects and management. Oral Oncol. 2020, 102, 104550. [Google Scholar] [CrossRef]
- Mello, F.W.; Melo, G.; Pasetto, J.J.; Silva, C.; Warnakulasuriya, S.; Rivero, E. The synergistic effect of tobacco and alcohol consumption on oral squamous cell carcinoma: A systematic review and meta-analysis. Clin. Oral Investig. 2019, 23, 2849–2859. [Google Scholar] [CrossRef] [PubMed]
- Kumar, M.; Nanavati, R.; Modi, T.G.; Dobariya, C. Oral cancer: Etiology and risk factors: A review. J. Cancer Res. Ther. 2016, 12, 458–463. [Google Scholar] [CrossRef]
- Lazos, J.P.; Piemonte, E.D.; Lanfranchi, H.E.; Brunotto, M.N. Characterization of Chronic Mechanical Irritation in Oral Cancer. Int.J. Dent. 2017, 2017, 6784526. [Google Scholar] [CrossRef] [PubMed]
- Verma, A.; Vincent-Chong, V.K.; DeJong, H.; Hershberger, P.A.; Seshadri, M. Impact of dietary vitamin D on initiation and progression of oral cancer. J. Steroid Biochem. Mol. Biol. 2020, 199, 105603. [Google Scholar]
- Pannone, G.; Santoro, A.; Carinci, F.; Bufo, P.; Papagerakis, S.M.; Rubini, C.; Campisi, G.; Giovannelli, L.; Contaldo, M.; Serpico, R.; et al. Double demonstration of oncogenic high risk human papilloma virus DNA and HPV-E7 protein in oral cancers. Int. J. Immunopathol. Pharmacol. 2011, 24, 95–101. [Google Scholar] [CrossRef] [PubMed]
- Favia, G.; Kanduc, D.; Lo Muzio, L.; Lucchese, A.; Serpico, R. Possible association between HPV16 E7 protein level and cytokeratin 19. Int. J. Cancer 2004, 111, 795–797. [Google Scholar] [CrossRef]
- Hooper, S.J.; Wilson, M.J.; Crean, S.J. Exploring the link between microorganisms and oral cancer: A systematic review of the literature. Head Neck 2009, 31, 1228–1239. [Google Scholar] [CrossRef]
- Castillo, G.D.V.; Blanc, S.L.; Sotomayor, C.E.; Azcurra, A.I. Study of virulence factor of Candida species in oral lesions and its association with potentially malignant and malignant lesions. Arch. Oral Biol. 2018, 91, 35–41. [Google Scholar] [CrossRef]
- Landskron, G.; De la Fuente, M.; Thuwajit, P.; Thuwajit, C.; Hermoso, M.A. Chronic inflammation and cytokines in the tumor microenvironment. J. Immunol. Res. 2014, 2014, 149185. [Google Scholar] [CrossRef]
- Sarkar, P.; Malik, S.; Laha, S.; Das, S.; Bunk, K.; Ray, J.G.; Chatterjee, R.; Saha, A. Dysbiosis of Oral Microbiota during Oral Squamous Cell Carcinoma Development. Front. Oncol. 2021, 11, 614448. [Google Scholar] [CrossRef]
- Perera, M.; Al-Hebshi, N.N.; Speicher, D.J.; Perera, I.; Johnson, N.W. Emerging role of bacteria in oral carcinogenesis: A review with special reference to perio-pathogenic bacteria. J. Oral Microbiol. 2016, 8, 32762. [Google Scholar] [CrossRef]
- International Agency for Research on Cancer. Available online: https://gco.iarc.fr/today/online-analysis-table?v=2020&mode=cancer&mode_population=continents&population=900&populations=900&key=asr&sex=2&cancer=39&type=1&statistic=5&prevalence=0&population_group=0&ages_group%5B%5D=0&ages_group%5B%5D=17&group_cancer=1&include_nmsc=0&include_nmsc_other=1 (accessed on 20 July 2022).
- Kruse, A.L.; Bredell, M.; Grätz, K.W. Oral cancer in men and women: Are there differences? Oral Maxillofac. Surg. 2011, 15, 51–55. [Google Scholar] [CrossRef]
- Migueláñez-Medrán, B.C.; Pozo-Kreilinger, J.J.; Cebrián-Carretero, J.L.; Martínez-García, M.A.; López-Sánchez, A.F. Oral squamous cell carcinoma of tongue: Histological risk assessment. A pilot study. Med. Oral Patol. Oral Cir. Bucal. 2019, 24, e603–e609. [Google Scholar] [CrossRef]
- Contaldo, M.; Di Napoli, A.; Pannone, G.; Franco, R.; Ionna, F.; Feola, A.; De Rosa, A.; Santoro, A.; Sbordone, C.; Longo, F.; et al. Prognostic implications of node metastatic features in OSCC: A retrospective study on 121 neck dissections. Oncol. Rep. 2013, 30, 2697–2704. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Losi-Guembarovski, R.; Menezes, R.P.; Poliseli, F.; Chaves, V.N.; Kuasne, H.; Leichsenring, A.; Maciel, M.E.; Guembarovski, A.L.; Oliveira, B.W.; Ramos, G.; et al. Oral carcinoma epidemiology in Paraná State, Southern Brazil. Cad. Saúde Pública 2009, 25, 393–400. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Chou, H.C.; Lin, H.W.; Yang, J.H.; Lin, P.Y.; Cheng, S.J.; Wu, Y.H.; Kuo, Y.S. Clinical outcomes of oral cancer patients who survive for more than 5 years in Taiwan. J. Formos. Med. Assoc. 2019, 118, 1616–1622. [Google Scholar] [CrossRef]
- Park, J.-O.; Nam, I.-C.; Kim, C.-S.; Park, S.-J.; Lee, D.-H.; Kim, H.-B.; Han, K.-D.; Joo, Y.-H. Sex Differences in the Prevalence of Head and Neck Cancers: A 10-Year Follow-Up Study of 10 Million Healthy People. Cancers 2022, 14, 2521. [Google Scholar] [CrossRef]
- Özdemir, B.C.; Csajka, C.; Dotto, G.P.; Wagner, A.D. Sex Differences in Efficacy and Toxicity of Systemic Treatments: An Undervalued Issue in the Era of Precision Oncology. J. Clin. Oncol. 2018, 36, 2680–2683. [Google Scholar] [CrossRef]
- Fish, E.N. The X-files in immunity: Sex-based differences predispose immune responses. Nat. Rev. Immunol. 2008, 8, 737–744. [Google Scholar] [CrossRef]
- Nagam, S.L.; Katta, S.; Prasad, V.V. Gender specific association of TP53 polymorphisms (EX4 215G>C Arg72Pro, IVS3+40-41ins16, and IVS6+62G>A), with risk of oral cancer subtypes and overall survival of the patients. Mol. Carcinog. 2017, 56, 895–912. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Boccellino, M.; Zannini, G.; Romano, A.; Sciarra, A.; Sacco, A.; Settembre, G.; Coppola, M.; Di Carlo, A.; D’Angelo, L.; et al. Sex Hormones and Inflammation Role in Oral Cancer Progression: A Molecular and Biological Point of View. J. Oncol. 2020, 2020, 9587971. [Google Scholar] [CrossRef]
- Zhang, L.; Liu, Y.; Zheng, H.J.; Zhang, C.P. The Oral Microbiota May Have Influence on Oral Cancer. Front. Cell Infect. Microbiol. 2020, 9, 476. [Google Scholar] [CrossRef]
- Clocchiatti, A.; Cora, E.; Zhang, Y.; Dotto, G.P. Sexual dimorphism in cancer. Nat. Rev. Cancer 2016, 16, 330–339. [Google Scholar] [CrossRef] [PubMed]
- Tonetti, M.S.; Greenwell, H.; Kornman, K.S. Staging and grading of periodontitis: Framework and proposal of a new classification and case definition. J. Periodontol. 2018, 89 (Suppl. 1), S159–S172; [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Interconnection of periodontal disease and comorbidities: Evidence, mech. Microbial complexes in supragingival plaque. Periodontology 2000 2022, 89, 9–18. [Google Scholar]
- Socransky, S.S.; Haffajee, A.D.; Cugini, M.A.; Smith, C.; Kent, R.L., Jr. Microbial complexes in subgingival plaque. J. Clin. Periodontol. 1998, 25, 134–144. [Google Scholar] [CrossRef]
- Ballini, A.; Cantore, S.; Farronato, D.; Cirulli, N.; Inchingolo, F.; Papa, F.; Malcangi, G.; Inchingolo, A.D.; Dipalma, G.; Sardaro, N.; et al. Periodontal disease and bone pathogenesis: The crosstalk between cytokines and porphyromonas gingivalis. J. Biol. Regul. Homeost. Agents 2015, 29, 273–281. [Google Scholar]
- Contaldo, M.; Fusco, A.; Stiuso, P.; Lama, S.; Gravina, A.G.; Itro, A.; Federico, A.; Itro, A.; Dipalma, G.; Inchingolo, F.; et al. Oral Microbiota and Salivary Levels of Oral Pathogens in Gastro-Intestinal Diseases: Current Knowledge and Exploratory Study. Microorganisms 2021, 9, 1064. [Google Scholar] [CrossRef]
- Holmstrup, P.; Damgaard, C.; Olsen, I.; Klinge, B.; Flyvbjerg, A.; Nielsen, C.H.; Hansen, P.R. Comorbidity of periodontal disease:Two sides of the same coin? An introduction for the clinician. J. Oral Microbiol. 2017, 9, 1332710. [Google Scholar] [CrossRef]
- Giordano-Kelhoffer, B.; Lorca, C.; March Llanes, J.; Rábano, A.; Del Ser, T.; Serra, A.; Gallart-Palau, X. Oral Microbiota, Its Equilibrium and Implications in the Pathophysiology of Human Diseases: A Systematic Review. Biomedicines 2022, 10, 1803. [Google Scholar] [CrossRef]
- Nazir, M.; Al-Ansari, A.; Al-Khalifa, K.; Alhareky, M.; Gaffar, B.; Almas, K. Global Prevalence of Periodontal Disease and Lack of Its Surveillance. Sci. World J. 2020, 2020, 2146160. [Google Scholar] [CrossRef]
- Kassebaum, N.J.; Bernabé, E.; Dahiya, M.; Bhandari, B.; Murray, C.J.; Marcenes, W. Global Burden of Severe Tooth Loss: A Systematic Review and Meta-analysis. J. Dent. Res. 2014, 93 (Suppl. 7), 20S–28S. [Google Scholar] [CrossRef]
- Shiau, H.J.; Reynolds, M.A. Sex differences in destructive periodontal disease: A systematic review. J. Periodontol. 2010, 81, 1379–1389. [Google Scholar] [CrossRef] [PubMed]
- Bastos, J.L.; Boing, A.F.; Peres, K.G.; Antunes, J.L.; Peres, M.A. Periodontal outcomes and social, racial and gender inequalities in Brazil: A systematic review of the literature between 1999 and 2008. Cad. Saude Publica 2011, 27 (Suppl. 2), S141–S153. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Albandar, J.M. Global risk factors and risk indicators for periodontal diseases. Periodontology 2000 2002, 29, 177–206. [Google Scholar] [CrossRef]
- Freitag-Wolf, S.; Munz, M.; Junge, O.; Graetz, C.; Jockel-Schneider, Y.; Staufenbiel, I.; Bruckmann, C.; Lieb, W.; Franke, A.; Loos, B.G.; et al. Sex-specific genetic factors affect the risk of early-onset periodontitis in Europeans. J. Clin. Periodontol. 2021, 48, 1404–1413. [Google Scholar] [CrossRef] [PubMed]
- Yoo, H.J.; Sepkovic, D.W.; Bradlow, H.L.; Yu, G.P.; Sirilian, H.V.; Schantz, S.P. Estrogen Metabolism as a Risk Factor for Head and Neck Cancer. Otolaryngol. Head Neck Surg. 2001, 124, 241–247. [Google Scholar] [CrossRef]
- Hashim, D.; Sartori, S.; Vecchia, C.L.; Serraino, D.; Maso, L.D.; Negri, E.; Smith, E.; Levi, F.; Boccia, S.; Cadoni, G. Hormone Factors Play a Favorable Role in Female Head and Neck Cancer Risk. Cancer Med. 2017, 6, 1998–2007. [Google Scholar] [CrossRef]
- Egloff, A.M.; Rothstein, M.E.; Seethala, R.; Siegfried, J.M.; Grandis, J.R.; Stabile, L.P. Cross-Talk between Estrogen Receptor and Epidermal Growth Factor Receptor in Head and Neck Squamous Cell Carcinoma. Clin. Cancer Res. 2009, 15, 6529–6540. [Google Scholar] [CrossRef]
- Chang, Y.-L.; Hsu, Y.-K.; Wu, T.-F.; Huang, C.-M.; Liou, L.-Y.; Chiu, Y.-W.; Hsiao, Y.-H.; Luo, F.-J.; Yuan, T.-C. Regulation of Estrogen Receptor a Function in Oral Squamous Cell Carcinoma Cells by FAK Signaling. Endocr. Relat. Cancer 2014, 21, 555–565. [Google Scholar] [CrossRef]
- Chanmee, T.; Ontong, P.; Konno, K.; Itano, N. Tumor-Associated Macrophages as Major Players in the Tumor Microenvironment. Cancers 2014, 6, 1670–1690. [Google Scholar] [CrossRef]
- Colella, G.; Izzo, G.; Carinci, F.; Campisi, G.; Lo Muzio, L.; D’Amato, S.; Mazzotta, M.; Cannavale, R.; Ferrara, D.; Minucci, S. Expression of sexual hormones receptors in oral squamous cell carcinoma. Int. J. Immunopathol. Pharmacol. 2011, 24 (Suppl. 2), 129–132. [Google Scholar] [CrossRef]
- Saranya, R.; Chandini, R.; Mohideen, K.; Adtani, P.N.; Subramani, V.; Balasubramaniam, M. Expression of Sex Hormones in Oral Squamous Cell Carcinoma: A Systematic Review on Immunohistochemical Studies. Cureus 2022, 14, e25384. [Google Scholar] [CrossRef]
- Lukits, J.; Remenár, E.; Rásó, E.; Ladányi, A.; Kásler, M.; Tímár, J. Molecular identification, expression and prognostic role of estrogen- and progesterone receptors in head and neck cancer. Int. J. Oncol. 2007, 30, 155–160. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Neto, C.P.D.O.; Brito, H.O.; DA COSTA, R.M.G.; Brito, L.M.O. Is There a Role for Sex Hormone Receptors in Head-and-neck Cancer? Links with HPV Infection and Prognosis. Anticancer Res. 2021, 41, 3707–3716. [Google Scholar] [CrossRef]
- Doll, C.; Bestendonk, C.; Kreutzer, K.; Neumann, K.; Pohrt, A.; Trzpis, I.; Koerdt, S.; Dommerich, S.; Heiland, M.; Raguse, J.-D.; et al. Prognostic Significance of Estrogen Receptor Alpha in Oral Squamous Cell Carcinoma. Cancers 2021, 13, 5763. [Google Scholar] [CrossRef] [PubMed]
- Marocchio, L.S.; Giudice, F.; Corrêa, L.; Pinto Junior Ddos, S.; de Sousa, S.O. Oestrogens and androgen receptors in oral squamous cell carcinoma. Acta Odontol. Scand. 2013, 71, 513–519. [Google Scholar] [CrossRef] [PubMed]
- Kolegova, E.S.; Patysheva, M.R.; Larionova, I.V.; Fedorova, I.K.; Kulbakin, D.E.; Choinzonov, E.L.; Denisov, E.V. Early-onset oral cancer as a clinical entity: Aetiology and pathogenesis. Int. J. Oral Maxillofac. Surg. 2022, 26, S0901-5027(22)00141-2. [Google Scholar] [CrossRef]
- Hanna, G.J.; Woo, S.B.; Li, Y.Y.; Barletta, J.A.; Hammerman, P.S.; Lorch, J.H. Tumor PD- L1 expression is associated with improved survival and lower recurrence risk in young women with oral cavity squamous cell carcinoma. Int. J. Oral Maxillofac. Surg. 2018, 47, 568–577. [Google Scholar] [CrossRef]
- Harris, S.L.; Kimple, R.J.; Hayes, D.N.; Couch, M.E.; Rosenman, J.G. Never-smokers, Never-drinkers: Unique Clinical Subgroup of Young Patients with Head and Neck Squamous Cell Cancers. Head Neck J. Sci. Spec. Head Neck 2010, 32, 499–503. [Google Scholar] [CrossRef]
- Vág, J.; Nagy, T.L.; Mikecs, B. Sex-related differences in endothelium-dependent vasodilation of human gingiva. BMC Oral Health 2022, 22, 177. [Google Scholar] [CrossRef]
- Stănescu, I.; Bulboacă, A.E.; Micu, I.C.; Bolboacă, S.D.; Feștilă, D.G.; Bulboacă, A.C.; Bodizs, G.; Dogaru, G.; Boarescu, P.M.; Popa-Wagner, A.; et al. Gender Differences in the Levels of Periodontal Destruction, Behavioral Risk Factors and Systemic Oxidative Stress in Ischemic Stroke Patients: A Cohort Pilot Study. J. Clin. Med. 2020, 9, 1744. [Google Scholar] [CrossRef]
- Di Naro, E.; Loverro, M.; Converti, I.; Loverro, M.T.; Ferrara, E.; Rapone, B. The Effect of Menopause Hypoestrogenism on Osteogenic Differentiation of Periodontal Ligament Cells (PDLC) and Stem Cells (PDLCs): A Systematic Review. Healthcare 2021, 9, 572. [Google Scholar] [CrossRef] [PubMed]
- Contaldo, M.; Itro, A.; Lajolo, C.; Gioco, G.; Inchingolo, F.; Serpico, R. Overview on Osteoporosis, Periodontitis and Oral Dysbiosis: The Emerging Role of Oral Microbiota. Appl. Sci. 2020, 10, 6000. [Google Scholar] [CrossRef]
- Chou, H.H.; Lu, S.L.; Wang, S.T.; Huang, T.H.; Chen, S.L. The Association between Bone Mineral Density and Periodontal Disease in Middle-Aged Adults. Int. J. Environ. Res. Public Health 2021, 18, 3321. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Liu, Y.; Yang, X.; Li, C.; Song, Z. The Oral Microbiota: Community Composition, Influencing Factors, Pathogenesis, and Interventions. Front. Microbiol. 2022, 13, 895537. [Google Scholar] [CrossRef]
- Di Spirito, F.; Schiavo, L.; Pilone, V.; Lanza, A.; Sbordone, L.; D’Ambrosio, F. Periodontal and Peri-Implant Diseases and Systemically Administered Statins: A Systematic Review. Dent. J. 2021, 9, 100. [Google Scholar] [CrossRef]
- Dewhirst, F.E.; Chen, T.; Izard, J.; Paster, B.J.; Tanner, A.C.; Yu, W.H.; Lakshmanan, A.; Wade, W. The human oral microbiome. J. Bacteriol. 2010, 192, 5002–5017. [Google Scholar]
- Mark Welch, J.L.; Dewhirst, F.E.; Borisy, G.G. Biogeography of the Oral Microbiome: The Site-Specialist Hypothesis. Annu. Rev. Microbiol. 2019, 73, 335–358. [Google Scholar] [CrossRef]
- Segata, N.; Haake, S.K.; Mannon, P.; Lemon, K.P.; Waldron, L.; Gevers, D.; Huttenhower, C.; Izard, J. Composition of the adult digestive tract bacterial microbiome based on seven mouth surfaces, tonsils, throat and stool samples. Genome Biol. 2012, 13, R42. [Google Scholar] [CrossRef]
- Berg, G.; Rybakova, D.; Fischer, D.; Cernava, T.; Vergès, M.C.; Charles, T.; Chen, X.; Cocolin, L.; Eversole, K.; Corral, G.H.; et al. Microbiome definition re-visited: Old concepts and new challenges. Microbiome 2020, 8, 103. [Google Scholar] [CrossRef]
- Deo, P.N.; Deshmukh, R. Oral microbiome: Unveiling the fundamentals. J. Oral Maxillofac. Pathol. 2019, 23, 122–128. [Google Scholar]
- Willis, J.R.; Gabaldón, T. The Human Oral Microbiome in Health and Disease: From Sequences to Ecosystems. Microorganisms 2020, 8, 308. [Google Scholar] [CrossRef] [PubMed]
- Low, A.; Gavriilidis, G.; Larke, N.; B-Lajoie, M.R.; Drouin, O.; Stover, J.; Muhe, L.; Easterbrook, P. Incidence of Opportunistic Infections and the Impact of Antiretroviral Therapy Among HIV-Infected Adults in Low- and Middle-Income Countries: A Systematic Review and Meta-analysis. Clin. Infect. Dis. 2016, 62, 1595–1603. [Google Scholar] [CrossRef] [PubMed]
- Lombardi, A.; Ouanounou, A. Fungal infections in dentistry: Clinical presentations, diagnosis, and treatment alternatives. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2020, 130, 533–546. [Google Scholar] [CrossRef] [PubMed]
- Astasov-Frauenhoffer, M.; Kulik, E.M. Cariogenic Biofilms and Caries from Birth to Old Age. Monogr. Oral Sci. 2021, 29, 53–64. [Google Scholar] [CrossRef] [PubMed]
- Haffajee, A.D.; Socransky, S.S.; Patel, M.R.; Song, X. Microbial complexes in supragingival plaque. Oral Microbiol. Immunol. 2008, 23, 196–205. [Google Scholar] [CrossRef]
- Herremans, K.M.; Riner, A.N.; Cameron, M.E.; McKinley, K.L.; Triplett, E.W.; Hughes, S.J.; Trevino, J.G. The oral microbiome, pancreatic cancer and human diversity in the age of precision medicine. Microbiome 2022, 10, 93. [Google Scholar] [CrossRef]
- Lamont, R.J.; Fitzsimonds, Z.R.; Wang, H.; Gao, S. Role of Porphyromonas gingivalis in oral and orodigestive squamous cell carcinoma. Periodontology 2000 2022, 89, 154–165. [Google Scholar] [CrossRef]
- Li, Z.; Liu, Y.; Zhang, L. Role of the microbiome in oral cancer occurrence, progression and therapy. Microb. Pathog. 2022, 169, 105638. [Google Scholar] [CrossRef]
- Ballini, A.; Dipalma, G.; Isacco, C.G.; Boccellino, M.; Di Domenico, M.; Santacroce, L.; Nguyễn, K.; Scacco, S.; Calvani, M.; Boddi, A.; et al. Oral Microbiota and Immune System Crosstalk: A Translational Research. Biology 2020, 9, 131. [Google Scholar] [CrossRef]
- Santacroce, L.; Sardaro, N.; Topi, S.; Pettini, F.; Bottalico, L.; Cantore, S.; Cascella, G.; Del Prete, R.; Dipalma, G.; Inchingolo, F. The pivotal role of oral microbiota in health and disease. J. Biol. Regul. Homeost. Agents 2020, 34, 733–737. [Google Scholar] [CrossRef]
- Sansores-España, L.D.; Melgar-Rodríguez, S.; Olivares-Sagredo, K.; Cafferata, E.A.; Martínez-Aguilar, V.M.; Vernal, R.; Paula-Lima, A.C.; Díaz-Zúñiga, J. Oral-Gut-Brain Axis in Experimental Models of Periodontitis: Associating Gut Dysbiosis With Neurodegenerative Diseases. Front. Aging. 2021, 2, 781582. [Google Scholar] [CrossRef]
- Krog, M.C.; Hugerth, L.W.; Fransson, E.; Bashir, Z.; Nyboe Andersen, A.; Edfeldt, G.; Engstrand, L.; Schuppe-Koistinen, I.; Nielsen, H.S. The healthy female microbiome across body sites: Effect of hormonal contraceptives and the menstrual cycle. Hum. Reprod. 2022, 37, 1525–1543. [Google Scholar] [CrossRef] [PubMed]
- Benn, A.; Heng, N.; Thomson, W.M.; Sissons, C.H.; Gellen, L.S.; Gray, A.R.; Broadbent, J.M. Associations of sex, oral hygiene and smoking with oral species in distinct habitats at age 32 years. Eur. J. Oral Sci. 2022, 130, e12829. [Google Scholar] [CrossRef] [PubMed]
- Muhleisen, A.L.; Herbst-Kralovetz, M.M. Menopause and the vaginal microbiome. Maturitas 2016, 91, 42–50. [Google Scholar] [CrossRef] [PubMed]
- Ledder, R.G.; Gilbert, P.; Huws, S.A.; Aarons, L.; Ashley, M.P.; Hull, P.S.; McBain, A.J. Molecular analysis of the subgingival microbiota in health and disease. Appl. Environ. Microbiol. 2007, 73, 516–523. [Google Scholar] [CrossRef] [PubMed]
- De, R.; Dutta, S. Role of the Microbiome in the Pathogenesis of COVID-19. Front. Cell Infect. Microbiol. 2022, 12, 736397. [Google Scholar] [CrossRef] [PubMed]
- Amato, A.; Iandolo, A.; Scelza, G.; Spirito, F.; Martina, S. COVID-19: The Patients' Perceived Impact on Dental Care. Eur. J. Dent. 2021, 16, 333–338. [Google Scholar] [CrossRef] [PubMed]
- Di Spirito, F.; Iacono, V.J.; Iandolo, A.; Amato, A.; Sbordone, L. Evidence-based Recommendations on Periodontal Practice and the Management of Periodontal Patients during and after the COVID-19 Era: Challenging Infectious Diseases Spread by Air-borne Transmission. Open Dent. 2021, 15, 325–336. [Google Scholar] [CrossRef]
- Bernardi, A.; Felisatti, V.; Masiero, A.F. Enforcement of Policies Against the Illicit Trade in Tobacco Products in Italy. In Combatting Illicit Trade in Tobacco Products; Tosza, S., Vervaele, J.A.E., Eds.; Springer: Cham, Switzerland, 2022. [Google Scholar] [CrossRef]
- Centers for Disease Control (CDC). Current cigarette smoking among adults in the United States. 2020. Available online: https://www.cdc.gov/tobacco/data_statistics/fact_sheets/adult_data/cig_smoking/index.htm (accessed on 18 July 2022).
- Reitsma, M.B.; Kendrick, P.J.; Ababneh, E.; Abbafati, C.; Abbasi-Kangevari, M.; Abdoli, A.; Abedi, A.; Abhilash, E.S.; Abila, D.B.; Aboyans, V.; et al. Spatial, temporal, and demographic patterns in prevalence of smoking tobacco use and attributable disease burden in 204 countries and territories, 1990-2019: A systematic analysis from the Global Burden of Disease Study 2019. Lancet 2021, 397, 2337–2360. [Google Scholar] [CrossRef]
- Global Burden of Disease [Database]; Institute of Health Metrics: Washington, DC, USA, 2019; Available online: https://vizhub.healthdata.org/gbd-compare/ (accessed on 17 July 2021).
- Stepanov, I.; Hatsukami, D. Call to establish constituent standards for smokeless tobacco products. Tob. Regul. Sci. 2016, 2, 9–30. [Google Scholar] [CrossRef]
- Jaspers, I. Cigarette smoke effects on innate immune mechanisms in the nasal mucosa: Potential effects on the microbiome. Ann. Am. Thorac. Soc. 2014, 11 (Suppl. 1), S38–S42. [Google Scholar] [CrossRef] [PubMed]
- Reidel, B.; Radicioni, G.; Clapp, P.W.; Ford, A.A.; Abdelwahab, S.; Rebuli, M.E.; Haridass, P.; Alexis, N.E.; Jaspers, I.; Kesimer, M. E-cigarette use causes a unique innate immune response in the lung, involving increased neutrophilic activation and altered mucin secretion. Am. J. Respir. Crit. Care Med. 2018, 197, 492–501. [Google Scholar] [CrossRef] [PubMed]
- Tomar, S.L.; Hecht, S.S.; Jaspers, I.; Gregory, R.L.; Stepanov, I. Oral Health Effects of Combusted and Smokeless Tobacco Products. Adv. Dent. Res. 2019, 30, 4–10. [Google Scholar] [CrossRef]
- Min, J.Y.; Levin, J.; Weinberger, A.H. Associations of tobacco cigarette use and dependence with substance use disorder treatment completion by sex/gender and race/ethnicity. J. Subst. Abus. Treat. 2022, 140, 108834. [Google Scholar] [CrossRef] [PubMed]
- Higgins, S.T.; Kurti, A.N.; Redner, R.; White, T.J.; Gaalema, D.E.; Roberts, M.E.; Doogan, N.J.; Tidey, J.W.; Miller, M.E.; Stanton, C.A.; et al. A literature review on prevalence of gender differences and intersections with other vulnerabilities to tobacco use in the United States, 2004–2014. Prev. Med. 2015, 80, 89–100. [Google Scholar] [CrossRef] [PubMed]
- Donaldson, S.I.; Dormanesh, A.; Perez, C.; Majmundar, A.; Allem, J.P. Association Between Exposure to Tobacco Content on Social Media and Tobacco Use: A Systematic Review and Meta-analysis. JAMA Pediatr. 2022. [Google Scholar] [CrossRef]
- Soneji, S.; Knutzen, K.E.; Tan, A.S.L.; Moran, M.B.; Yang, J.; Sargent, J.; Choi, K. Online tobacco marketing among US adolescent sexual, gender, racial, and ethnic minorities. Addict. Behav. 2019, 95, 189–196. [Google Scholar] [CrossRef]
- Kasza, K.A.; Ambrose, B.K.; Conway, K.P.; Borek, N.; Taylor, K.; Goniewicz, M.L.; Cummings, K.M.; Sharma, E.; Pearson, J.L.; Green, V.R.; et al. Tobacco-Product Use by Adults and Youths in the United States in 2013 and 2014. N. Engl. J. Med. 2017, 376, 342–353. [Google Scholar] [CrossRef]
- Sinha, D.N.; Abdulkader, R.S.; Gupta, P.C. Smokeless tobacco-associated cancers: A systematic review and meta-analysis of Indian studies. Int. J. Cancer 2016, 138, 1368–1379. [Google Scholar] [CrossRef]
- Mu, G.; Wang, J.; Liu, Z.; Zhang, H.; Zhou, S.; Xiang, Q.; Cui, Y. Association between smokeless tobacco use and oral cavity cancer risk in women compared with men: A systematic review and meta-analysis. BMC Cancer 2021, 21, 960. [Google Scholar] [CrossRef]
- Tomar, S.L.; Asma, S. Smoking-attributable periodontitis in the United States: Findings from NHANES III. National Health and Nutrition Examination Survey. J. Periodontol. 2000, 71, 743–751. [Google Scholar] [CrossRef] [PubMed]
- Mustapha, A.D.; Salame, Z.; Chrcanovic, B.R. Smoking and Dental Implants: A Systematic Review and Meta-Analysis. Medicina 2021, 58, 39. [Google Scholar] [CrossRef] [PubMed]
- Caton, J.G.; Armitage, G.; Berglundh, T.; Chapple, I.; Jepsen, S.; Kornman, K.S.; Mealey, B.L.; Papapanou, P.N.; Sanz, M.; Tonetti, M.S. A new classification scheme for periodontal and peri-implant diseases and conditions—Introduction and key changes from the 1999 classification. J. Periodontol. 2018, 89 (Suppl. 1), S1–S8. [Google Scholar] [CrossRef] [PubMed]
- Chrcanovic, B.R.; Albrektsson, T.; Wennerberg, A. Dental implants inserted in male versus female patients: A systematic review and meta-analysis. J. Oral Rehabil. 2015, 42, 709–722. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.S. Interventions to prevent periodontal disease in tobacco-, alcohol-, and drug-dependent individuals. Periodontology 2000 2020, 84, 84–101. [Google Scholar] [CrossRef] [PubMed]
- Rehm, J.; Shield, K.D.; Weiderpass, E. Alcohol consumption: A leading risk factor for cancer. Chem. Biol. Interact. 2020, 331, 109280. [Google Scholar] [CrossRef]
- IARC Working Group on the Evaluation of Carcinogenic Risks to Humans. Alcohol consumption and ethyl carbamate. IARC Monogr. Eval. Carcinog. Risks Hum. 2010, 96, 3–1383. [Google Scholar]
- World Cancer Research Fund International, American Institute for Cancer Research. Diet, Nutrition, Physical Activity and Cancer: A Global Perspective. Continuous Update Project Expert Report 2018; World Cancer Research Fund International: London, UK, 2018; Available online: https://www.wcrf.org/dietandcancer (accessed on 15 July 2022).
- Keyes, K.M.; Martins, S.S.; Blanco, C.; Hasin, D.S. Telescoping and gender differences in alcohol dependence: New evidence from two national surveys. Am. J. Psychiatry 2010, 167, 969–976. [Google Scholar] [CrossRef]
- McKee, S.A.; McRae-Clark, A.L. Consideration of sex and gender differences in addiction medication response. Biol. Sex Differ. 2022, 13, 34. [Google Scholar] [CrossRef]
- Grant, B.F.; Chou, S.P.; Saha, T.D.; Pickering, R.P.; Kerridge, B.T.; Ruan, W.J.; Huang, B.; Jung, J.; Zhang, H.; Fan, A.; et al. Prevalence of 12-month alcohol use, high-risk drinking, and DSM-IValcohol use disorder in the United States, 2001–2002 to 2012–2013:results from the national epidemiologic survey on alcohol and related conditions. JAMA Psychiat. 2017, 74, 911–923. [Google Scholar] [CrossRef]
- Żukowski, P.; Maciejczyk, M.; Waszkiel, D. Sources of free radicals and oxidative stress in the oral cavity. Arch. Oral Biol. 2018, 92, 8–17. [Google Scholar] [CrossRef] [PubMed]
- World Health Organization. Alcohol and Cancer in the WHO European Region: An Appeal for Better Prevention; Licence: CC BY-NC-SA 3.0 IGO; WHO Regional Office for Europe: Copenhagen, Denmark, 2020. [Google Scholar]
- World Health Organization. Making the WHO European Region SAFER: Developments in Alcohol Control Policies Across the WHO European Region; WHO Regional Office for Europe: Copenhagen, Denmark, 2020. [Google Scholar]
- Bagnardi, V.; Rota, M.; Botteri, E.; Tramacere, I.; Islami, F.; Fedirko, V.; Scotti, L.; Jenab, M.; Turati, F.; Pasquali, E.; et al. Alcohol consumption and site-specific cancer risk: A comprehensive dose-response meta-analysis. Br. J. Cancer 2015, 112, 580–593. [Google Scholar] [CrossRef] [PubMed]
- Alcohol and Cancer Risks: A Guide for Health Professionals. Edinburgh: Scottish Health Action on Alcohol Problems (SHAAP). 2019. Available online: https://www.shaap.org.uk/images/Alcohol_and_Cancer_Guide.pdf (accessed on 2 September 2022).
- National Institute of Alcohol Abuse and Alcoholism (NIAAA). Women and Alcohol. 2017. Available online: https://www.niaaa.nih.gov/publications/brochures-and-fact-sheets/women-and-alcohol (accessed on 2 September 2022).
- Oliveira, L.M.; da Silva Pilecco, K.; de Oliveira, C.A.; Antoniazzi, R.P.; Demarco, F.F.; Zanatta, F.B. Alcohol Intake Influences the Occurrence and Progression of Periodontitis Differently According to Sex and Country Sociodemographic Development: A Two-Stage Systematic Review. Alcohol Alcohol. 2022, agac023. [Google Scholar] [CrossRef] [PubMed]
- Pelucchi, C.; Gallus, S.; Garavello, W.; Bosetti, C.; La Vecchia, C. Cancer risk associated with alcohol and tobacco use: Focus on upper aero-digestive tract and liver. Alcohol Res. Health 2006, 29, 193–198. [Google Scholar] [PubMed]
- Hashibe, M.; Brennan, P.; Chuang, S.-C.; Boccia, S.; Castellsagué, X.; Chen, C.; Curado, M.P.; Maso, L.D.; Daudt, A.W.; Fabianova, E.; et al. Interaction between Tobacco and Alcohol Use and the Risk of Head and Neck Cancer: Pooled Analysis in the International Head and Neck Cancer Epidemiology Consortium. Cancer Epidemiol. Biomark. Prev. 2009, 18, 541–550. [Google Scholar] [CrossRef]
- Talamini, R.; La Vecchia, C.; Levi, F.; Conti, E.; Favero, A.; Franceschi, S. Cancer of the oral cavity and pharynx in nonsmokers who drink alcohol and in nondrinkers who smoke tobacco. J. Natl. Cancer Inst. 1998, 90, 1901–1903. [Google Scholar] [CrossRef][Green Version]
- Singh, P.K.; Singh, N.; Jain, P.; Sinha, P.; Kumar, C.; Singh, L.; Singh, A.; Yadav, A.; Singh Balhara, Y.P.; Kashyap, S.; et al. Mapping the triple burden of smoking, smokeless tobacco and alcohol consumption among adults in 28,521 communities across 640 districts of India: A sex-stratified multilevel cross-sectional study. Health Place 2021, 69, 102565. [Google Scholar] [CrossRef]
- World Organization Health. Women’s Health. Available online: https://www.who.int/health-topics/women-s-health (accessed on 2 September 2022).
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).